Abstract
Greater activation of glia, a key component of neuroinflammation, is an important process to target in neuropsychiatric illnesses. However, the magnitude of gliosis varies across cases so low-cost predictors are needed to stratify subjects for clinical trials. Here, several such blood serum measures were assessed in relation to TSPO VT, an index of translocator protein density, measured with positron emission tomography. Blood serum concentration of several products known to be synthesized by activated microglia (and to some extent astroglia) [prostaglandin E2 (PGE2), prostaglandin F2 alpha (PGF2α), and tumor necrosis factor alpha (TNFα)], controlled by an index of peripheral inflammation [C-reactive protein (CRP)] and TSPO VT were measured in 3 cohorts: prefrontal cortex TSPO VT of 20 subjects with major depressive episodes (MDEs) from major depressive disorder (MDD); and 56 subjects with treatment resistant MDEs from MDD; and dorsal caudate TSPO VT of 20 subjects with obsessive-compulsive disorder. Ln(PGE2/CRP) and ln(TNFα/CRP) consistently correlated with TSPO VT (R2 = 0.36 to 0.11, p = 0.0030 to p = 0.0076). Assessment of threshold serum values to predict highly elevated TSPO VT, demonstrated that a positive predictive value (PPV) of 80% was possible while retaining 40% of participant samples and that receiver operating curves (ROC) ranged from 75 to 81%. Post-hoc selection of ln(CRP) was more predictive (R2 = 0.23 to 0.39, p = 0.0058 to p = 0.00013; ROC > 80%). Systematic assessment of selected peripheral inflammatory markers is promising for developing low cost predictors of TSPO VT. Marker thresholds with high PPV will improve subject stratification for clinical trials of glial targeting therapeutics.
Subject terms: Anxiety, Predictive markers, Depression
Introduction
Psychiatric and neurological diseases are burdensome to society since they affect one in four people and are often treatment resistant [1]. Neuroinflammation, usually in response to neuronal damage is an important cluster of processes that occurs across many neuropsychiatric illnesses including major depressive disorder (MDD), obsessive-compulsive disorder (OCD), and neurodegenerative diseases [2–8]. Such responses typically include microglial and/or astroglial activation, which involve morphological changes of an enlarged cell body and thickened dendrites or, for microglia, possibly an ameboid shape. Increased gliosis is a promising target for immune modulating treatments to alter microglial and/or astroglial function away from potentially harmful roles like producing reactive oxygen species, prostaglandins, and proteinases. Immune modulating treatments may also alter glia cells towards more curative roles such as enhancing release of neurotrophic factors, promoting vascularization, and phagocytosing cellular debris [6, 9]. However, a critical barrier for human clinical trials of investigational treatments targeting gliosis is that there is heterogeneity in the neuroinflammatory response among individuals with neuropsychiatric diseases. Heterogeneity is attributable to multiple factors including stage of illness, comorbid disease and, most likely, multiple etiological phenotypes [2, 3]; thereby limiting optimal matching of cases to treatment.
No easily applicable, replicable, low cost measure indicative of microglial activation has been developed. Presently positron emission tomography (PET) imaging of translocator protein (TSPO) binding, is the most established in vivo marker although there are nuances for its interpretation: In health, binding of TSPO in brain is considered mainly attributable to binding to endothelial cells [10]. After inflammatory stimuli, brain TSPO binding is elevated and this TSPO radiotracer binding closely parallels the magnitude of greater TSPO expression in microglia, with a modest contribution from greater TSPO expression in activated astrocytes [11, 12] (for further discussion see Supplementary Information). Other PET radiotracers targeting P2X7, P2Y12 and CSF 1 receptors [13, 14] are being advanced, but all of these methods are expensive, require scarce resources, and typically need arterial blood sampling for kinetic modeling because microglia are ubiquitous throughout the brain. Another direction is cerebrospinal fluid concentration of products consequent to greater indoleamine 2,3-dioxygenase activity and/or cytokines but the necessary lumbar puncture is difficult for many patients [15, 16]. In regards to magnetic resonance imaging (MRI) methods with paramagnetic probes like Cd-bis-5-HT-DTPA that measure myeloperoxidase activity across activated microglia, neutrophils and monocytes, their sensitivity to detect microglial activation in neuropsychiatric disease is not yet established and it is possible that their application will be restricted to disease states with substantial blood brain barrier breakdown [17]. Moreover, to date, no blood marker has been demonstrated to consistently predict the level of microglial activation in brain.
For the present study, to design low cost blood markers, composite measures were created. Activated microglia in brain were considered an important source of inflammatory markers. Several products of activated microglia that are also actively transported out of the central nervous system into blood were measured, including serum prostaglandin E2 (PGE2), prostaglandin F2 alpha (PGF2α), and tumor necrosis factor alpha (TNFα) [18–22]. PGE2 was given the highest priority since it is arguably the most commonly measured product of activated microglia among in vitro studies [18]. Since PGE2, PGF2α, and TNFα are also produced by the immune response of peripheral tissues [23] and microglial activation in brain is influenced by peripheral inflammation [9], a controlling measure of a well-accepted index of peripheral inflammation, serum C-reactive protein (CRP) concentration was applied. Hence, our primary hypothesis is that PGE2/CRP will predict TSPO total distribution volume (VT) in brain and PGF2α/CRP and TNFα/CRP are our secondary candidates. While the issue could be raised that during inflammatory states astrogliosis may also contribute to the measure of TSPO VT, to some extent this issue is mitigated because PGE2, PGF2α and TNFα are not selective products of microglial activation and are also produced by astrogliosis.
In the present study, the relationship of these serum markers to brain TSPO VT is assessed in three groups: medication free MDD; antidepressant treated, treatment resistant MDD (TRD); and medication free OCD subjects, illnesses for which TSPO VT is elevated yet there is also substantial variability across subjects [2–8] (for further review see the Supplementary Information). In medication free MDD and TRD we prioritized the prefrontal cortex (PFC) because subregions of the PFC are often adversely affected in these illnesses [24–26], and it is a large portion of brain tissue. In OCD, the caudate nucleus was prioritized because it is the region with the most abnormally elevated TSPO VT [2], and is strongly implicated in the pathophysiology of OCD, having the greatest convergence of neurochemical abnormalities across investigations in OCD [27–29].
Materials and methods
Participants
Three cohorts were recruited to assess the relationship between PET and peripheral markers. Having three cohorts provides the ability to assess the generalizability of this relationship across these disease conditions and the extent to which it replicates across these disease conditions. The cohorts included: 20 with medication free MDE [7]; 56 TRD (31 previously described [3]); and 20 medication free OCD (19 previously described [2]) were recruited from the Greater Toronto Area and the Centre for Addiction and Mental Health between July 2010 and October 2018 (Table 1). To assess the diagnosis, all participants underwent the Structured Clinical Interview for DSM-IV with confirmation by a consultation with a psychiatrist (JHM). All participants were currently experiencing significant symptoms. For MDE and TRD participants, a minimum of 17 on the 17-item Hamilton Depressive Rating Scale (17-item HDRS) [30] was required because this is a commonly applied minimum threshold to verify a current MDE. Greater TSPO VT is commonly found during an MDE, but this may not necessarily occur during recovery [5, 8]. For OCD participants, scores on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) [31] reflected moderate to severe illness. All participants were aged 18–72 years, non-cigarette smoking, and otherwise in good physical health (Table 1). All TRD participants were taking at least one antidepressant medication at a standard clinical dose for a minimum of 4 weeks prior to PET scanning (detailed list of medications provided in Supplementary Information).
Table 1.
Demographic characteristics
| Characteristics | Medication free major depressive episode (n = 20) | Treatment resistant depression (n = 56) | Medication free obsessive-compulsive disorder (n = 20) | P-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Female | 12 | 60 | 36 | 64 | 11 | 55 | 0.76a |
| TSPO Genotypeb | 0.67a | ||||||
| HAB | 15 | 75 | 36 | 64 | 13 | 65 | |
| MAB | 5 | 25 | 20 | 36 | 7 | 35 | |
| Previous Antidepressant Trial | 9 | 45 | 56 | 100 | NA | NA | <0.0001a |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age, year | 34.5 | 11.2 | 34.7 | 11.2 | 27.8 | 7.0 | 0.040c |
| BMI | 23.4 | 5.4 | 25.2 | 4.2 | 23.8 | 5.0 | 0.28c |
| 17-Item HDRS Scored | 20.0 | 3.8e | 21.6 | 4.2 | NA | NA | 0.16c |
| Age at First Episode, Yearf | 15.7 | 5.2 | 15.9 | 7.0 | 13.1 | 7.4 | 0.26e |
| Y-BOCS Score | NA | NA | NA | NA | 22.5 | 6.1 | NA |
aPearson chi-squared test
bSingle nucleotide polymorphism rs6971 of the TSPO gene known to influence [18F]FEPPA binding: HAB, high affinity binders; MAB, mixed affinity binders
cAnalysis of variance
d17-item Hamilton Depression Rating Scale (HDRS); scores derived on the day of scanning
eMissing data in one medication free MDE subject
fMDE for MDD; or first episode of OCD symptoms for OCD cases
BMI body mass index, MDD major depressive disorder, MDE major depressive episode, N number, NA not applicable, OCD obsessive compulsive disorder, SD standard deviation, TSPO translocator protein, Y-BOCS Yale-Brown Obsessive-Compulsive Scale, % percentage
All participants provided written informed consent after all procedures were fully explained. The study, protocol, and informed consent forms were approved by the Research Ethics Board at the Centre for Addiction and Mental Health.
Image acquisition and analysis
As previously described [2, 3, 7], each participant underwent one [18F]FEPPA PET (HRRT; CPS/Siemens, Knoxville, TN, USA) and one MRI scan at the Research Imaging Centre at the Centre for Addiction and Mental Health (see the online Supplementary Information for additional detail). [18F]FEPPA was administered intravenously as a bolus (mean 184.6 MBq [SD 13.0]). [18F]FEPPA was of high radiochemical purity (>96%) and high specific activity (mean 83.1 TBq/mmol [SD 73.0]). Manual and automatic blood samplings (ABSS, Model #PBS-101; Veenstra Instuments, Joure, The Netherlands) were obtained to determine the unmetabolised parent radioligand in plasma which was the input function for the kinetic analysis. A brain MRI was acquired for each participant for the anatomical delineation of regions of interest (ROIs) generated using the semi-automated software (ROMI, Toronto, Ontario) [32]. A two-tissue compartment model was applied to the time–activity curves from regions of interest to measure TSPO VT, which is the optimal model for [18F]FEPPA PET [33] (see the online Supplementary Information for additional detail).
Peripheral inflammatory marker measurements
PGE2 and PGF2α concentrations were determined using the competitive immunoassay, Prostaglandin E2 Assay (Parameter, R&D Systems Inc) and Prostaglandin F2α ELISA Kit (MyBioSource), respectively. TNFα level was analyzed using the Human Adipokine Magnetic Bead Panel 2 (Milliplex MAP, EMD Millipore Corp) and CRP was analyzed with the CRPHS Assay (Cobas C, Roche Diagnostics). PGE2 and TNFα levels were measured twice in the same sample and the mean value was applied, and all other markers were measured in singleton. Further details regarding sample collection are described in the online supplemental methods.
Statistical analyses
For the main analyses, TSPO VT was the dependent variable and the natural logarithm of the blood markers (PGE2/CRP, PGF2α/CRP, and TNFα/CRP) were each assessed separately as predictor variables in a linear regression in each cohort. The natural logarithm was applied to produce a normal distribution of the predictor variables. To address for the effect of the rs6971 genotype on TSPO VT, a corrective adjustment was applied to the mixed-affinity binder (MAB) TSPO VT values based on a linear regression assessing the effect of genotype with regional TSPO VT as the dependent variable such that the differential effect of genotype was added to the TSPO VT of the MAB subjects (see the online Supplementary Information for further description). Since PGE2/CRP was the primary hypothesized predictor, the threshold for significance for the linear regression was set at 0.05 for each cohort. For the other two main predictor markers being assessed (PGF2α/CRP and TNFα/CRP), the threshold for significance of the linear regression was set at P < 0.017 since inclusion of these two measures resulted in a total of three high priority measures. For statistical analysis, the adjusted R2 was assessed and reported.
Also, receiver operator characteristic (ROC) curves were generated to evaluate the performance of consistently predictive biomarkers. For this, TSPO VT values from all subjects were treated as dichotomous variables with PFC TSPO VT ≥ 13.5 for MDE and TRD, and dorsal caudate TSPO VT ≥ 9.4 for OCD (after adjusting MAB TSPO VT as described in the preceding paragraph). These thresholds correspond to ~30% greater than healthy controls representing ~2 standard deviation difference. All analyses were performed using IBM SPSS Statistics (version 21).
Results
Linear regression assessing relationship of blood markers to TSPO VT
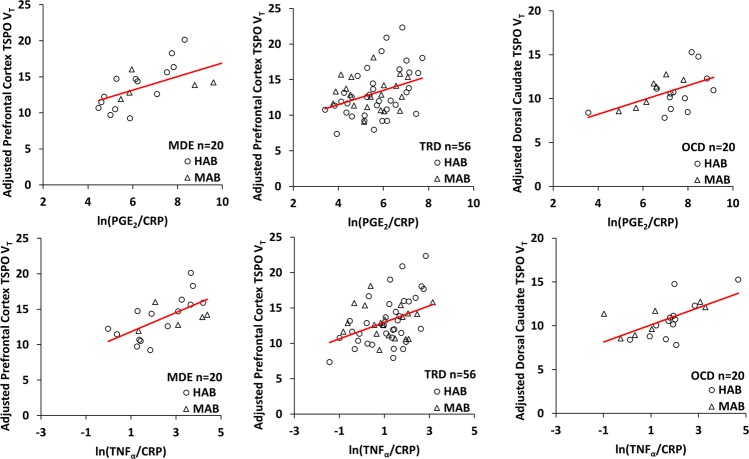
Ln(PGE2/CRP) and ln(TNFα/CRP) were consistent and highly significant correlates of TSPO VT for all three cohorts (Fig. 1, Table 2; ln(PGE2/CRP): MDE, F1,19 = 10.3 to 11.8, P = 0.0030 to 0.0048; TRD F1,55 = 7.7 to 10.0, P = 0.0026 to 0.0076; OCD, F1,19 = 7.0 to 11.3, p = 0.0035 to 0.016), especially in the MDE and OCD cohorts in which they accounted for 24.1 to 36.2% of the variance. Ln(PGF2α/CRP) was only significantly predictive in the MDE and OCD cohorts (Table 2; MDE, F1,19 = 9.1, P = 0.0093; OCD, F1,19 = 25.5, P < 0.0001); accounting for 35.0 and 56.3% of the variance respectively (see Figure S1 in the online Supplementary Information). Since TSPO VT tended to be highly correlated across regions, the predictors tended to also be correlated with TSPO VT values in other brain regions (see Table S2 in the online Supplementary Information).
Fig. 1.
Blood Serum Markers Significantly Correlated with Translocator Protein Distribution Volume in all Three Cohorts. Ln(PGE2/CRP) and ln(TNFα/CRP) were highly significant correlates of PFC TSPO VT in MDE and TRD cohorts, and dorsal caudate TSPO VT in OCD cohort. MDE cohort n = 20 [ln(PGE2/CRP) R2 = 33.0, P = 0.0048; ln(TNFα/CRP) R2 = 36.2, P = 0.0030]. TRD cohort n = 56 [ln(PGE2/CRP) R2 = 10.8, p = 0.0076; ln(TNFα/CRP) R2 = 14.0, P = 0.0026]. OCD cohort n = 20 [ln(PGE2/CRP) R2 = 24.1, p = 0.016; ln(TNFα/CRP) R2 = 35.1, P = 0.0035]. To address the effect of the rs6971 genotype on TSPO VT, the differential effect of genotype in a linear regression was found for TSPO VT. Then, the MAB TSPO VT values were adjusted by adding the differential effect of genotype to the TSPO VT values. (Adjusted prefrontal cortex TSPO VT = unadjusted TSPO VT + b1*genotype, where b1 = 4.939 in MDE cohort and b1 = 4.678 in TRD cohort. Adjusted dorsal caudate TSPO VT = unadjusted TSPO VT + b1*genotype, where b1 = 4.230 in OCD cohort). CRP c-reactive protein, HAB high-affinity binders, MAB mixed-affinity binders, MDE medication free major depressive episodes secondary to major depressive disorder, OCD obsessive compulsive disorder, PFC prefrontal cortex; PGE2 prostaglandin E2, TNFα tumor necrosis factor alpha, TRD treatment resistant major depressive episodes secondary to major depressive disorder, TSPO VT translocator protein distribution volume
Table 2.
Relationship of serum predictor markers to TSPO VT
| Clinical Group | Region | Predictor markera | Contribution to variance (Adjusted R2 × 100%) | P-value |
|---|---|---|---|---|
| MDE (n = 20) | Prefrontal Cortex TSPO VT | ln(PGE2/CRP) | 33.0 | 0.0048 |
| ln(PGF2α/CRP)b | 35.0 | 0.0093 | ||
| ln(TNFα/CRP) | 36.2 | 0.0030 | ||
| TRD (n = 56) | Prefrontal Cortex TSPO VT | ln(PGE2/CRP) | 10.8 | 0.0076 |
| ln(PGF2α/CRP) | 2.6 | 0.12 | ||
| ln(TNFα/CRP) | 14.0 | 0.0026 | ||
| OCD (n = 20) | Dorsal Caudate TSPO VT | ln(PGE2/CRP) | 24.1 | 0.016 |
| ln(PGF2α/CRP) | 56.3 | <0.0001 | ||
| ln(TNFα/CRP) | 35.1 | 0.0035 |
aLinear regression with genotyped adjusted TSPO VT as dependent variable with predictor listed as independent
bPGF2α data missing from 4 medication free MDE subjects
CRP c-reactive protein, MDE major depressive episode, n number, OCD obsessive compulsive disorder, PGE2 prostaglandin E2, PGF2α prostaglandin F2 alpha, TNFα tumor necrosis factor alpha, TRD treatment resistant depression, TSPO VT translocator protein distribution volume
Receiver operating characteristic curve analysis with A priori hypothesized blood markers
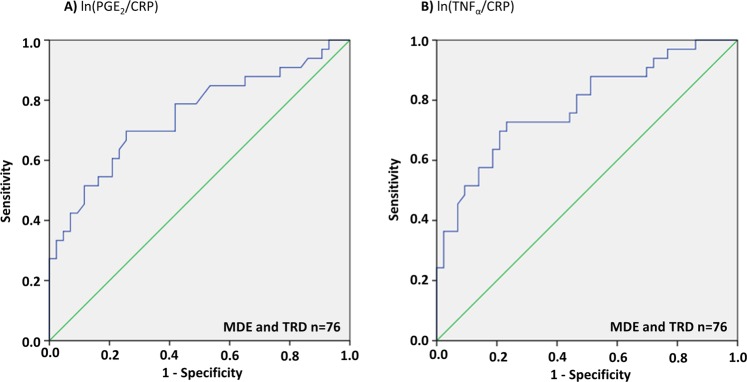
Receiver operating characteristic (ROC) curve analyses were applied for the collective depressed sample (MDE and TRD n = 76) for ln(PGE2/CRP) and ln(TNFα/CRP), assessing revealed an accuracy of 74.9% (P = 0.00021, 95% confidence interval [63.5, 86.4]) and 78.1% (P < 0.0001, 95% confidence interval [67.5, 88.7]) in the ability of the blood marker to correctly classify those with and without elevated PFC TSPO VT (Fig. 2). ROC curve analyses were applied for the OCD cohort for ln(PGE2/CRP), ln(PGF2α/CRP), and ln(TNFα/CRP), assessing revealed an accuracy of 80.2% (P = 0.029, 95% confidence interval [58.4, 100.0]), 79.1% (P = 0.036, 95% confidence interval [57.5, 100.0]), and 81.3% (P = 0.024, 95% confidence interval [60.0, 100.0]), respectively, in the ability of the blood marker to correctly classify those with and without elevated dorsal caudate TSPO VT (see Table S3 in the online Supplementary Information). Although the a priori region is different in OCD, ROC curve analyses were applied for the whole sample (n = 96) to assess the ability of the blood markers to correctly classify those with and without elevated PFC TSPO VT (see Fig. S8 in the online Supplementary Information).
Fig. 2.
Receiver Operating Characteristic Curve Analyses in Collective Depressed (MDE and TRD) Cohorts. Receiver operating characteristic (ROC) curve analyses in the collective depressed sample (MDE and TRD n = 76) for a) ln(PGE2/CRP) and b ln(TNFα/CRP). ROC curve analyses revealed accuracies of 74.9% (P = 0.00021, 95% confidence interval [63.5, 86.4]) and 78.1% (p < 0.0001, 95% confidence interval [67.5, 88.7]) in the ability of ln(PGE2/CRP) and ln(TNFα/CRP) biomarkers to correctly classify those with and without elevated prefrontal cortex TSPO VT, respectively. To address the effect of the rs6971 genotype on TSPO VT, the differential effect of genotype in a linear regression was found for TSPO VT. Then, the MAB TSPO VT values were first adjusted by adding the differential effect of genotype to the TSPO VT values. (Adjusted prefrontal cortex TSPO VT = unadjusted TSPO VT + b1*genotype, where b1 = 4.939 in MDE cohort and b1 = 4.678 in TRD cohort). CRP c-reactive protein, MDE medication free major depressive episodes secondary to major depressive disorder, PGE2 prostaglandin E2, TNFα tumor necrosis factor alpha, TRD treatment resistant major depressive episodes secondary to major depressive disorder, TSPO VT translocator protein distribution volume
Relationship of positive predictive value and sample retention
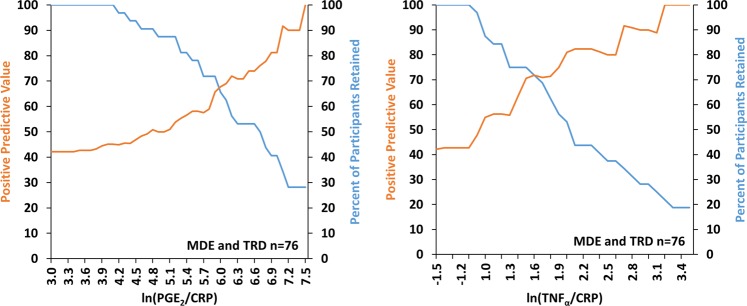
In the collective MDE and TRD sample, varying thresholds of ln(PGE2/CRP) and ln(TNFα/CRP) were assessed in relation to the positive predictive value and proportion of participants retained as these are key practical values for practical use of blood markers in clinical trials. With cut-offs of ln(PGE2/CRP) = 6.9 and ln(TNFα/CRP) = 2.1 respectively in the depressed sample, positive predictive values greater than 80% for elevated prefrontal cortex TSPO VT were possible while retaining 40% of the participants (Fig. 3). Similarly, a threshold serum value of 7.3 for ln(PGE2/CRP) or 1.9 for ln(TNFα/CRP) in OCD subjects selects ~50% of cases of which 85% have dorsal caudate TSPO VT values >9.4.
Fig. 3.
Relationship of Positive Predictive Value and Proportion of Participants Retained in Collective Depressed (MDE and TRD) Cohorts. Relationship of positive predictive value and proportion of participants retained with varying thresholds of ln(PGE2/CRP) and ln(TNFα/CRP) in the collective depressed sample (MDE and TRD n = 76). Cut-offs of ln(PGE2/CRP) = 6.9 and ln(TNFα/CRP) = 2.1 reveal positive predictive values of ~80% for prefrontal cortex TSPO VT ≥ 13.5 while retaining 40% of cases. To address the effect of the rs6971 genotype on TSPO VT, the differential effect of genotype in a linear regression was found for TSPO VT. Then, the MAB TSPO VT values were first adjusted by adding the differential effect of genotype to the TSPO VT values. (Adjusted prefrontal cortex TSPO VT = unadjusted TSPO VT + b1*genotype, where b1 = 4.939 in MDE cohort and b1 = 4.678 in TRD cohort). CRP c-reactive protein, PGE2 prostaglandin E2, TNFα tumor necrosis factor alpha, TSPO VT translocator protein distribution volume
Post-Hoc Analyses
Since the ln(PGE2/CRP) or ln(TNFα/CRP) may be equivalently expressed as ln(PGE2) minus ln(CRP) or ln(TNFα) minus ln(CRP) respectively, it is feasible to test different linear combinations of ln(PGE2) and ln(TNFα) with ln(CRP) with linear regression (see Table S4 in the online Supplementary Information). The most optimal predictor was ln(CRP) alone which resulted in higher levels of significance in predicting PFC TSPO VT in MDE and TRD participants and dorsal caudate TSPO VT in OCD participants (MDE: F1,19 = 13.0, R2 = 0.39, P = 0.0020; TRD: F1,55 = 17.0, R2 = 0.23, P = 0.00013; OCD: F1,19 = 9.8, R2 = 0.32, P = 0.00058). The ROC curve analysis of ln(CRP) in the OCD cohort revealed an accuracy of 81.3% (P = 0.024, 95% confidence interval [61.5, 100.0]) (see Table S3 in the online Supplementary Information). Interestingly, predictiveness increased further in the collective depressed sample (MDE and TRD n = 76) when body mass index (BMI) was included, leading to a ROC curve accuracy of 85.3% (see the online Supplementary Information for more detail and Figures S2–S7).
Discussion
We found that composite blood serum measures of products previously established to be synthesized by activated microglia (and to some extent astroglia), that are also known to have strong efflux from the central nervous system, controlled for by peripheral inflammation are consistently significantly correlated with TSPO VT. The predictiveness of two such measures created a priori, ln(PGE2/CRP) and ln(TNFα/CRP), replicate across three cohorts. However, it was also noted that ln(PGF2α/CRP) accounted for a high proportion of variance of TSPO VT in the OCD sample and that in post-hoc analyses that the ln(CRP) was, on average, a stronger predictor of TSPO VT across the three cohorts. Low cost predictive markers of TSPO VT have major implications for subject stratification for clinical trials of therapeutics targeting activated microglia.
The present study demonstrates that either serum ln(PGE2/CRP) or ln(TNFα/CRP) consistently identifies a subsample with a high TSPO VT phenotype. Figure 3, indicates that a threshold serum value of 6.9 for ln(PGE2/CRP) or 2.1 for ln(TNFα/CRP) in the combined MDE and TRD samples selects a subsample of ~40% of cases of which 80% have PFC TSPO VT values more than 13.5, which corresponds to 2 standard deviations above the mean of health. Similarly, a threshold serum value of 7.3 for ln(PGE2/CRP) or 1.9 for ln(TNFα/CRP) in OCD subjects (n = 20) selects ~50% of cases of which 85% have dorsal caudate TSPO VT values >9.4. Since the heterogeneity of TSPO VT elevation ranges from 0% to over 100% in primary regions of interest for MDE and OCD [2–4], these serum thresholds represent a practical low-cost option to stratify subjects. Moreover, there will be opportunities to assess these thresholds in clinical trials, particularly for MDD and TRD. For example, therapeutic development to target microglial activation and/or gliosis is actively occurring with P2X7 antagonists for microglial proliferation, and indolamine 2,3 dioxygenase inhibitors to promote conversion of tryptophan towards serotonin rather than the kyurenine pathway. In addition, repurposed medications like minocycline and the simvastatin that influence major histocompatibility protein II to reduce adverse functions of gliosis [34, 35] are currently being investigated.
A consistent, positive relationship between blood markers of inflammation and TSPO VT in brain has not been previously reported. In a sample of 30 subjects (14 cases with schizophrenia and 16 healthy), no significant relationships were found between TSPO VT and plasma IL-6, TNFα, plasma interferon gamma (IFNγ), plasma IL-10, or cerebrospinal fluid IL-6 levels [36]. In a sample of 8 healthy subjects exposed to lipopolysaccharide, Sandiego et al. demonstrated elevations in TSPO VT as well as TNFα, plasma IFNγ, IL-6, IL-8, and IL-10, but there was no correlation between change among brain and peripheral blood measures [37]. Similarly no relationship between blood CRP level and TSPO VT was reported in a sample of 10 MDD subjects some of whom were currently in a MDE, nor in our previous sample of 20 MDE subjects [7, 38]. In a sample of 48 MDD subjects with concurrent TSPO imaging and plasma samples, among 8 plasma measures including CRP, Richards et al. reported one positive correlation of plasma adiponectin, at an exploratory uncorrected significance [4]. As compared to the present study, there were a number of differences in the study by Richards et al. which examined several different peripheral inflammatory markers (IL-2, IL-5, IL-6, IL-8, and INF-γ) and did not look at the ln transformation of CRP in relation to TSPO VT.
There were several additional predictors that merit further study, due to the high level of variance in TSPO VT accounted for by the markers. Although all our cohorts included those with neuropsychiatric disease, the diseases were not identical. Hence, ln(PGF2α/CRP) which accounted for 56% of the variance in TSPO VT in the dorsal caudate in medication free OCD but was not predictive in the TRD sample, should be reassessed in future samples of medication free OCD subjects since this finding may be specific for OCD. Similarly, since ln(CRP) and BMI collectively, albeit with most of the impact from ln(CRP), accounted for 32% of the variance and 85.3% of the area under the ROC to predict elevated TSPO VT in the collective MDE and TRD cohorts; and that the relationship between BMI and TSPO VT was also reported in a separate sample from ours [4] suggest that this combination should receive further study in MDE and TRD samples.
There are several limitations in the present study. First, there is some lack of selectivity for gliosis across the markers tested. For example, when elevated TSPO VT is present, it is associated with microglial activation but TSPO overexpression is not fully selective for microglial activation since TSPO is detectable in other cells such as astroglia and endothelial cells [11, 12]. Also, some of the serum markers measured like PGE2, PGF2α and TNFα, while synthesized by activated microglia are not specific to such, for example, they may also be produced by activated astrocytes [39, 40]. Second, when greater TSPO expression often occurs after exposure to inflammatory stimuli, it is associated with the morphological changes of an activated state, predominantly in microglia but there is a range of cellular functions that may occur in the activated state. Third, we did not assess the relationship of the prioritized blood markers to elevated brain TSPO VT in healthy subjects because in health, TSPO VT has a limited range. In addition, in neuroinflammatory states the elevation in TSPO VT is generally viewed as reflecting variable levels of gliosis whereas the range in health is likely attributable to variation in other aspects of TSPO binding like binding to endothelial cells [10]. Fourth, it could be questioned as to which peripheral blood markers were decided a priori, a challenging issue since disclosing these prior to patenting in a public database invalidates the patent and then limits the translational impact of such markers. We can largely address this question by our emerging patenting order of peripheral blood markers which demonstrates initial selection of PGE2/CRP and later selection of our post-hoc parameter ln(CRP), which in some examples both alone and in combination with BMI value yields even greater levels of predictiveness for TSPO VT (see Figures S5–S7 in the online Supplementary Information).
In addition, should the serum markers of the present study be applied to other studies, it is important that the sampling protocol is highly similar to ours. One important component of our sampling protocol is that to avoid potentially rapid degradation of PGE2 and TNFα, blood samples taken were converted into serum over 30 min, placed in a chilled centrifuge, and stored quickly at −80 °C (see the online Supplementary Information). Also, to apply the relationship of the serum measures tested to TSPO VT, it is also important to address whether participants sampled had recent infections since our participant sample reported no recent infections in the four weeks prior to scanning which may have lowered variability in the TSPO VT measure.
In summary, we assessed a general strategy of testing the serum ratio of products synthesized by activated microglia that are also actively removed from the central nervous system, controlled for by markers of peripheral inflammation, identified the natural logarithm of serum PGE2/CRP and TNFα/CRP, as highly significant correlates of TSPO VT in brain in three separate samples. We also noted in post-hoc analyses several linear combinations of markers that were also highly predictive including ln(CRP) itself as well as in combination with BMI. These measures may be applied at thresholds with high positive predictive value to select subjects with elevated TSPO VT thereby addressing the heterogeneity of the TSPO VT marker when recruiting subjects for clinical trials of therapeutics targeting gliosis. Moreover, systematically assessing collective predictors in relation to TSPO VT has intriguing potential to be developed further towards more individualized clinical care of neuropsychiatric illnesses with elevated microglial activation and gliosis.
Funding and Disclosures
This study received funding support from the Canadian Institutes of Health Research [Canada Research Chair and Operating Grant (MOP-136955), JHM; Doctoral Award (GSD-157948), SA; Fellowship Award, ES; Canada Research Chair, NV], the Brain and Behavior Foundation, and the neuroscience catalyst fund (from the Government of Ontario and Janssen). None of the funding sources participated in the execution of the project, generation of results, interpretation of data nor drafting of the manuscript. Funding for infrastructure was from the Azrieli Foundation, the Canadian Foundation for Innovation and the Ontario Ministry for Innovation. All other authors are paid employees of the Centre for Addiction and Mental Health. AAW, SH, and JHM have received operating grant funds from Janssen in the past 5 years. JHM has been a consultant to Lundbeck and Takeda, in the past 5 years. JHM is an inventor on five patents of blood and/or clinical markers to predict brain inflammation or to diagnose affective disorders (including those in the present submission), and a dietary supplement to reduce depressed mood post-partum. JHM is arranging collaborations with nutraceutical companies for the dietary supplement to prevent post-partum depression. SK has operating grant funds from US National Institutes of Health National Institute on Drug Abuse (DA04066) to measure microglial status in the brains of methamphetamine users, and from Jazz Pharmaceuticals for an unrelated study.
Supplementary information
Figure S1. Correlation of Ln(PGF2α/CRP) with Dorsal Caudate Translocator Protein Distribution Volume in Obsessive Compulsive Disorder
Figure S2. Negative Ln(CRP) Serum Marker Significantly Correlated with Translocator Protein Distribution Volume in Collective Depressed (MDE and TRD) Cohorts
Figure S3. Receiver Operating Characteristic Curve Analysis of Ln(CRP) Serum Marker in Collective Depressed (MDE and TRD) Cohorts
Figure S4. Relationship of Positive Predictive Value of Ln(CRP) Serum Marker and Proportion of Participants Retained in Collective Depressed (MDE and TRD) Cohorts
Figure S5. Linear Combination of Ln(CRP) and Body Mass Index Significantly Predictive of Translocator Protein Distribution Volume in Collective Depressed (MDE and TRD) Cohorts
Figure S6. Receiver Operating Characteristic Curve Analysis of Linear Combination of Ln(CRP) and Body Mass Index in Collective Depressed (MDE and TRD) Cohorts
Figure S7. Relationship of Positive Predictive Value of Linear Combination of Ln(CRP) and Body Mass Index and Proportion of Participants Retained in Collective Depressed (MDE and TRD) Cohorts
Figure S8. Receiver Operating Characteristic Curve Analyses in Entire Sample
Acknowledgements
AN, LN, and CC worked as study PET technicians. AK, NK, AS, and RM provided medical coverage for the PET scans. JP, AG, and Michael Harkness of Research Imaging Centre, served as PET chemistry staff. AR, GED, and HB, worked as study MRI technicians. All other contributors are paid employees of the Centre for Addiction and Mental Health.
Author contributions
Study concept and design: JHM, ES, AAW, SH. Acquisition, analysis, or interpretation of data: SA, ES, PMR, LM, CX, CH, SK, JHM. Drafting of the manuscript: SA, JHM. Critical revision of the manuscript for important intellectual content: SA, Setiawan, Wilson, PMR, LM, CX, CH, MIH, SK, NV, SH, JHM. Statistical analysis: SA, JHM. Obtained funding: SA, JHM, ES, AAW, SH. Administrative, technical, or material support: AAW, PMR, LM, SK, NV, SH, JHM. Study supervision: JHM.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0561-y).
References
- 1.WHO. 169 (World Health Organization, Geneva, Switzerland, 2001).
- 2.Attwells S, Setiawan E, Wilson AA, Rusjan PM, Mizrahi R, Miler L, et al. Inflammation in the neurocircuitry of obsessive-compulsive disorder. JAMA Psychiatry. 2017;74:833–40. doi: 10.1001/jamapsychiatry.2017.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setiawan E, Attwells S, Wilson AA, Mizrahi R, Rusjan PM, Miler L, et al. Association of translocator protein total distribution volume with duration of untreated major depressive disorder: a cross-sectional study. Lancet Psychiatry. 2018;5:339–47. doi: 10.1016/S2215-0366(18)30048-8. [DOI] [PubMed] [Google Scholar]
- 4.Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8:57. doi: 10.1186/s13550-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Sagar AP, Keri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog neuro-Psychopharmacol Biol Psychiatry. 2018;83:1–7. doi: 10.1016/j.pnpbp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–69. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol Psychiatry. 2018;83:61–69. doi: 10.1016/j.biopsych.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 10.Betlazar C, Harrison-Brown M, Middleton RJ, Banati R, Liu GJ. Cellular sources and regional variations in the expression of the neuroinflammatory marker translocator protein (TSPO) in the normal brain. Int J Mol Sci. 2018;19:pii: E2707. doi: 10.3390/ijms19092707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banati RB, Myers R, Kreutzberg GW. PK (‘peripheral benzodiazepine’)–binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. doi: 10.1023/A:1018567510105. [DOI] [PubMed] [Google Scholar]
- 12.Martin A, Boisgard R, Theze B, Van Camp N, Kuhnast B, Damont A, et al. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 2010;30:230–41. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanaswami V, Dahl K, Bernard-Gauthier V, Josephson L, Cumming P, Vasdev N. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol imaging. 2018;17:1536012118792317. doi: 10.1177/1536012118792317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horti AG, Naik R, Foss CA, Minn I, Misheneva V, Du Y, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proceedings of the National Academy of Sciences of the United States of America. 2019. [DOI] [PMC free article] [PubMed]
- 15.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophrenia Bull. 2018;44:75–83. doi: 10.1093/schbul/sbx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulli B, Chen JW. Imaging neuroinflammation - from bench to bedside. J Clin Cell Immunol. 2014;5:pii: 226. doi: 10.4172/2155-9899.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minghetti L, Polazzi E, Nicolini A, Creminon C, Levi G. Up-regulation of cyclooxygenase-2 expression in cultured microglia by prostaglandin E2, cyclic AMP and non-steroidal anti-inflammatory drugs. Eur J Neurosci. 1997;9:934–40. doi: 10.1111/j.1460-9568.1997.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 19.Akanuma S, Higuchi T, Higashi H, Ozeki G, Tachikawa M, Kubo Y, et al. Transporter-mediated prostaglandin E(2) elimination across the rat blood-brain barrier and its attenuation by the activation of N-methyl-D-aspartate receptors. Drug Metab pharmacokinetics. 2014;29:387–93. doi: 10.2133/dmpk.DMPK-14-RG-004. [DOI] [PubMed] [Google Scholar]
- 20.Tachikawa M, Hosoya K, Terasaki T. Pharmacological significance of prostaglandin E2 and D2 transport at the brain barriers. Adv Pharm. 2014;71:337–60. doi: 10.1016/bs.apha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Banks WA. The blood-brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. doi: 10.1016/j.bbi.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagen AA, Gerber JN, Sweeley CC, White RP, Robertson JT. Levels and disappearance of prostaglandin F2alpha in cerebral spinal fluid: a clinical and experimental study. Stroke. 1977;8:672–5. doi: 10.1161/01.STR.8.6.672. [DOI] [PubMed] [Google Scholar]
- 23.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21–8. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–80. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams KH, Hansen ES, Pinborg LH, Hasselbalch SG, Svarer C, Holm S, et al. Patients with obsessive-compulsive disorder have increased 5-HT2A receptor binding in the caudate nuclei. Int J Neuropsychopharmacol. 2005;8:391–401. doi: 10.1017/S1461145705005055. [DOI] [PubMed] [Google Scholar]
- 28.Wong DF, Brasic JR, Singer HS, Schretlen DJ, Kuwabara H, Zhou Y, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2008;33:1239–51. doi: 10.1038/sj.npp.1301528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br. J. Psychiatry. 1998;35:26–37. [PubMed]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The yale-brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 32.Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S, et al. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab. 2011;31:1807–16. doi: 10.1038/jcbfm.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 35.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, et al. In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry. 2016;6:e777. doi: 10.1038/tp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA. 2015;112:12468–73. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I, et al. The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun. 2013;33:131–8. doi: 10.1016/j.bbi.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito O, Svensson CI, Buczynski MW, Wegner K, Hua XY, Codeluppi S, et al. Spinal glial TLR4-mediated nociception and production of prostaglandin E(2) and TNF. Br J Pharmacol. 2010;160:1754–64. doi: 10.1111/j.1476-5381.2010.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation of Ln(PGF2α/CRP) with Dorsal Caudate Translocator Protein Distribution Volume in Obsessive Compulsive Disorder
Figure S2. Negative Ln(CRP) Serum Marker Significantly Correlated with Translocator Protein Distribution Volume in Collective Depressed (MDE and TRD) Cohorts
Figure S3. Receiver Operating Characteristic Curve Analysis of Ln(CRP) Serum Marker in Collective Depressed (MDE and TRD) Cohorts
Figure S4. Relationship of Positive Predictive Value of Ln(CRP) Serum Marker and Proportion of Participants Retained in Collective Depressed (MDE and TRD) Cohorts
Figure S5. Linear Combination of Ln(CRP) and Body Mass Index Significantly Predictive of Translocator Protein Distribution Volume in Collective Depressed (MDE and TRD) Cohorts
Figure S6. Receiver Operating Characteristic Curve Analysis of Linear Combination of Ln(CRP) and Body Mass Index in Collective Depressed (MDE and TRD) Cohorts
Figure S7. Relationship of Positive Predictive Value of Linear Combination of Ln(CRP) and Body Mass Index and Proportion of Participants Retained in Collective Depressed (MDE and TRD) Cohorts
Figure S8. Receiver Operating Characteristic Curve Analyses in Entire Sample