Abstract
Carbon (C) plays a vital role in regulating soil nutrient cycling and increasing soil microbial community, but there is still limited information on how C source utilization characteristics responds to soil physical and chemical properties changes under double-cropping rice (Oryza sativa L.) paddy field in southern China. Therefore, the effects of different short-term manure nitrogen (N) input rate managements on C source utilization characteristics in rice rhizosphere and non-rhizosphere soils under double-cropping rice field in southern China were studied by using 18O-H2O method. Therefore, a field experiment were established in Ningxiang city of Hunan Province, and five different fertilizer treatments were applied: (1) 100% N of chemical fertilizer (M0), (2) 30% N of organic manure and 70% N of chemical fertilizer (M30), (3) 50% N of organic manure and 50% N of chemical fertilizer (M50), (4) 100% N of organic manure (M100), and (5) without N fertilizer input as control (CK). The results showed that soil microbial biomass C content, soil microbial growth rate, and soil microbial basal respiration with application of organic manure treatments (M30, M50, M100) were significantly higher (p < 0.05) than that of CK treatment. And the soil C utilization efficiency with M0 treatment were significantly higher (p < 0.05) than that of M100 treatment. Compared with CK and M0 treatments, the metabolic capacity of soil microorganisms to exogenous C sources with M30, M50 and M100 treatments were increased. The largest types of exogenous C source was carboxylic acids, followed by amino acid and carbohydrate, and complex compounds was the smallest. The RDA analysis results indicated that fertilizer treatments significantly changed the utilization characteristics of soil microorganisms to exogenous C sources. As a result, this study found that characteristics of soil C source utilization were significantly affected by different short-term manure N input rate managements.
Subject terms: Ecology, Environmental impact
Introduction
Soil microorganisms play a vital role in regulating soil physical and chemical properties, increasing soil microbial community and diversity, maintaining soil quality and soil fertility, and contributing to crop growth1. Soil organic carbon (SOC) content were strongly affected by soil microorganisms2. And rhizosphere processes were important for the functioning of paddy ecosystems for that rhizosphere processes were regulated nutrient cycling, such as soil organic matter (SOM) decomposition and nutrient dynamics, which were closely related to crop root and their related rhizosphere processes3. Meanwhile, the non-rhizosphere processes were also play an important role in biogeochemical cycling and crop growth. The soil microbial carbon (C) use efficiency (CUE) were usually considered as the proportion of growth and organic C taken up in organic C, and it was important comprehensive characteristics of soil microbial community metabolism4,5. In the previous studies, it was found that types of exogenous carbon sources mainly utilized by soil microorganism were included carboxylic acids, amino acid, carbohydrate, complex compounds, and so on6,7. Although the soil microbial community metabolism was important for C cycling, there is still limited information on the ecophysiology of microbial C cycling and the effects of different factors on shape soil microbial CUE8.
Soil microbial CUE were affected by different agriculture managements, such as the crop types, tillage, fertilizer regime, irrigation patterns, and so on. Soil biogeochemical characteristics and nutrient cycle were changed under application of different fertilizer conditions, which in return change soil microbial biomass stoichiometry and microorganisms9. As a result, soil microbial CUE were affected by taken different fertilizer practices. Manzoni et al.4 results showed that soil microbial CUE achieved maximum with low C:N ratio. Meanwhile, SOC concentration and quality, microbial community compositions were also major factors in changing soil microbial CUE and soil microbial biomass content10. In the previous studies, it was found that CUE of soil microbial communities were closely related with decomposing complex compounds, respiration rate of unit C assimilation11. Don et al.12 results indicated that organic C mineralization were decreased with decrease of C concentration. Tang et al.13 research found that C source utilization of soil microorganisms in paddy field were increased under combined application of organic manure or crop residue with inorganic fertilizer conditions. Fang et al.14 results showed that soil microbial biomass and C-use efficiencies were increased by applied with organic amendment and inorganic fertilizer. But other study indicated that soil microbial CUE were decreased under combined application of plant residue with inorganic fertilizer conditions15. Therefore, soil microbial CUE were closely related to soil organic C quality and contents. Furthermore, some studies indicated that soil microbial CUE of fungi were higher than that of bacteria10. However, other studies indicated that there was no significant difference in soil fungal and bacterial CUE16.
The method for investigated soil microbial CUE in paddy ecosystem were accepted by more and more researchers5. Previous studies showed that soil microbial CUE were calculated by soil microbial incorporation and respiration of specific 13C-labeled substrates17. However, this method found it was very different soil microbial CUE estimates for that this determination method confuses soil microbial CUE with the specific substrate5. To overcome these problems, a novel method was suggested based on the incorporation of 18O from water into DNA during soil microbial biomass growth. That is, during the incubation time, the increase of soil microbial biomass C was investigated according to the 18O-DNA9. Therefore, the soil microbial CUE were calculated according to both the soil microbial growth rate and the basal respiration rate.
Rice (Oryza sativa L.) is one of the main crops in Asia, and double-cropping rice system (early rice and late rice) is the main land use in southern China18. It is benefit practices for maintain the paddy soil quality and fertility by application of fertilizer managements (organic fertilizer, inorganic fertilizer, and so on). And the manure N input rate managements may profound effects on soil physical and chemical characteristics such as pH, soil bulk density, SOC content, which in return affect soil microbiological properties and characteristics of carbon utilization. We hypothesized that: (i) soil microbial CUE were increased under application chemical fertilizer conditions; (ii) metabolic capacity of soil microorganisms to exogenous carbon sources would be higher with organic manure input conditions than that of with chemical fertilizer and without N fertilizer input conditions. Therefore, a short-term field experiment with different fertilizer treatments were conducted in a double-cropping rice system in the southern China. Hence, the objective of this study was (1) to investigate the microbial CUE in rice rhizosphere and non-rhizosphere soils under different manure N input rate fertilization conditions; (2) to evaluate metabolic capacity of soil microorganisms to exogenous carbon sources with different manure N input rate practices in a double-cropping rice system.
Results
Soil microbial carbon utilization efficiency
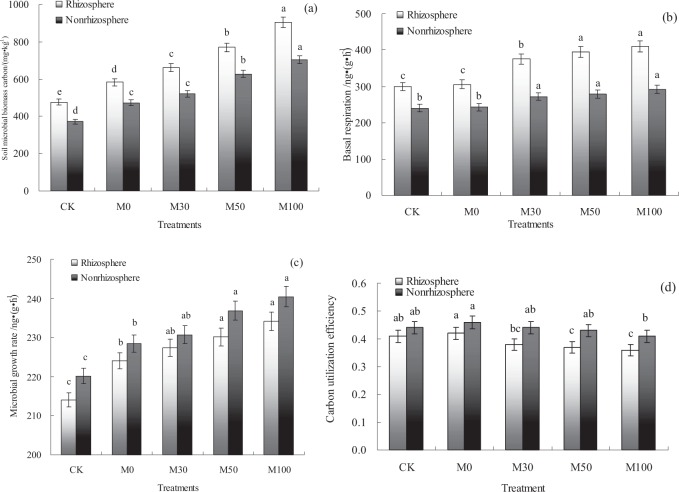
The effects of different manure N input rate treatments on soil microbial carbon utilization efficiency (CUE) in the double-cropping rice paddy field were shown in Fig. 1. The results showed that range of soil microbial biomass carbon (MBC) content was 371.61 to 904.83 mg kg−1 in rhizosphere and non-rhizosphere soils with different fertilizer treatments. And the soil MBC content in rhizosphere soils were higher (p < 0.05) than that of non-rhizosphere soils with different fertilizer treatments. In rhizosphere and non-rhizosphere soils, the lowest soil MBC content were observed with the CK treatment, while the highest soil MBC content were observed with the M100 treatment. The results showed that soil MBC content with M50 and M100 treatments were higher (p < 0.05) than that of M0 and CK treatments in the double-cropping rice paddy field (Fig. 1a). Compared with application of chemical fertilizer and without N fertilizer input practices, the soil MBC content were increased with application of organic manure practices.
Figure 1.
Soil microbial distribution in rhizosphere and non-rhizosphere soils with different fertilizer treatments. M0: 100% N of chemical fertilizer, M30: 30% N of organic manure and 70% N of chemical fertilizer, M50: 50% N of organic manure and 50% N of chemical fertilizer, M100: 100% N of organic manure, CK: without N fertilizer input as a control. (a) is soil microbial biomass carbon, (b) is basal respiration of soil microorganism, (c) is soil microbial growth rate, (d) is soil microbial carbon utilization efficiency. Error bars represent standard error of mean. Different letters are significantly different at P < 0.05 level. The same as below.
The range of soil microorganism basal respiration was 240.32 to 410.36 ng (g·h)−1 in rhizosphere and non-rhizosphere soils with different fertilizer treatments. And the basal respiration of soil microorganism in rhizosphere soils were higher (p < 0.05) than that of non-rhizosphere soils with different fertilizer treatments. In rhizosphere and non-rhizosphere soils, the basal respiration of soil microorganism with M30, M50 and M100 treatments were higher (p < 0.05) than that of M0 and CK treatments, but there was no significant difference (p > 0.05) in basal respiration of soil microorganism between M50 and M100 treatments (Fig. 1b).
The soil microbial growth rate (CGrowth) were calculated based on the equation as mentioned above. The results showed that range of CGrowth was 214.06 to 240.47 ng (g·h)−1 in rhizosphere and non-rhizosphere soils with different fertilizer treatments. And the CGrowth in non-rhizosphere soils were higher (p < 0.05) than that of rhizosphere soils with different fertilizer treatments. In rhizosphere and non-rhizosphere soils, the CGrowth with M50 and M100 treatments were higher (p < 0.05) than that of M0 and CK treatments, but there was no significant difference (p > 0.05) in CGrowth between M50 and M100 treatments (Fig. 1c).
The range of soil microbial CUE were 0.36 to 0.46 ng (g·h)−1 in rhizosphere and non-rhizosphere soils with different fertilizer treatments. And the CUE of soil microbial in non-rhizosphere soils were higher (p < 0.05) than that of rhizosphere soils with different fertilizer treatments. In rhizosphere soils, the CUE of soil microbial with M0 and CK treatments were higher (p < 0.05) than that of M50 and M100 treatments. In non-rhizosphere soils, the CUE of soil microbial with M0 treatment were higher (p < 0.05) than that of M100 treatment, but there was no significant difference (p > 0.05) in CUE of soil microbial among M0, M30, M50 and CK treatments (Fig. 1d).
Characteristics of utilization of different types of exogenous carbon sources
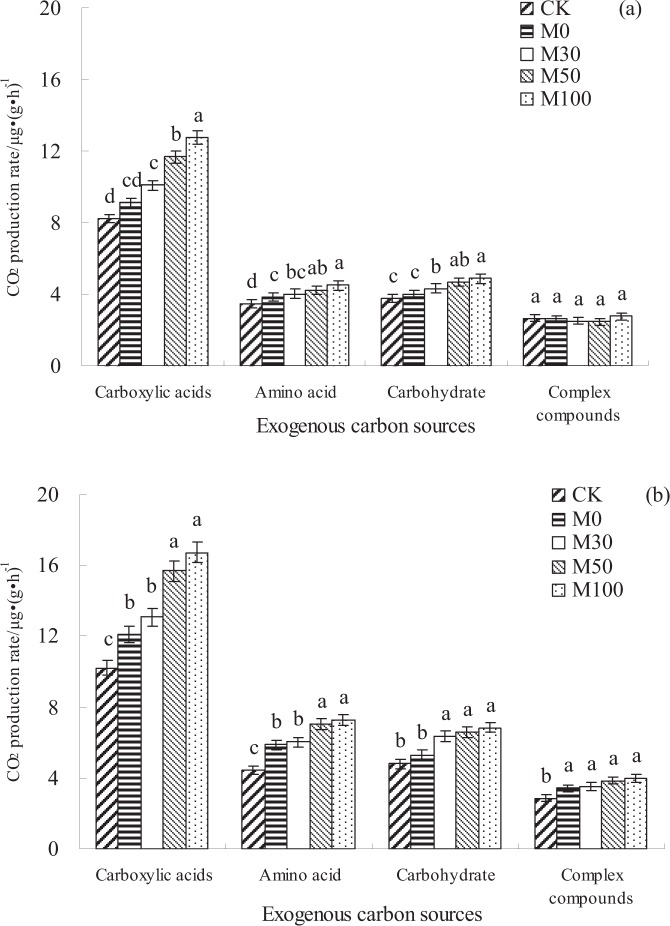
The metabolic capacity of soil microorganisms to the types of exogenous carbon sources (carboxylic acids, amino acid, carbohydrate, complex compounds) in non-rhizosphere soils were higher (p < 0.05) than that of rhizosphere soils with different fertilizer treatments (Fig. 2). Compared with CK and M0 treatments, the metabolic capacity of soil microorganisms to exogenous carbon sources in rhizosphere soils were increased with application of organic manure managements (M30, M50, and M100 treatments). In rhizosphere and non-rhizosphere soils, there was no significant difference (p > 0.05) in microbial utilization of complex compounds among different fertilizer treatments (M0, M30, M50, and M100 treatments) (Fig. 2). In different types of exogenous carbon sources, the average utilization rate of carboxylic acids were higher (p < 0.05) than that of complex compounds with different fertilizer treatments.
Figure 2.
Characteristics of utilization of different types of exogenous carbon sources by rhizosphere and non-rhizosphere soils microorganisms with different fertilizer treatments. (a,b) indicate rhizosphere and non-rhizosphere soils, respectively.
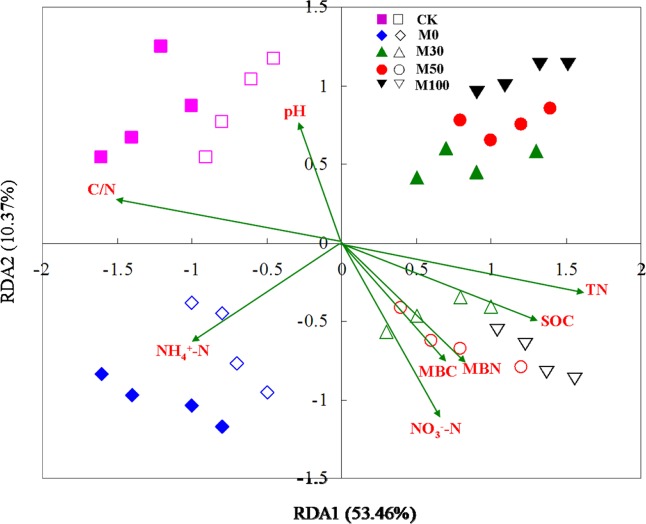
Considering the whole soil microbial carbon source utilization rate, the results revealed there was a significant correlation between soil microbial carbon source utilization rate and soil chemical properties (Fig. 3). In addition, the soil chemical properties can explain the variation (53.46%) in soil microbial carbon source utilization rate between the M0, M30, M50, M100 and CK treatments. Under different fertilizer treatments, M0 and CK treatments were separated from application of organic manure treatments (M30, M50 and M100 treatments), indicating that fertilizer treatments significantly changed the utilization characteristics of soil microorganisms to exogenous carbon sources. The soil chemical properties was significantly correlated with the utilization characteristics of soil microorganisms to exogenous carbon sources including the C/N, NH4+-N, NO3−-N, SOC, and TN contents.
Figure 3.
Redundancy analysis of microbial carbon source utilization rate and soil chemical properties. Solid icon and hollow icon indicate rhizosphere and non-rhizosphere soils, respectively. SOC: soil organic carbon, TN: soil total nitrogen, MBC: soil microbial biomass carbon, MBN: soil microbial biomass nitrogen.
Disscussion
In the present study, the results indicated that soil MBC content in rhizosphere and non-rhizosphere soils were increased with application of organic manure practices, compared with without N fertilizer input practices. The reason maybe that soil available nutrients increased by application of organic materials, which provided carbon substrates and nutrients for the growth and reproduction of soil microorganisms, and then increased soil microbial growth rate and microbial biomass19, compared with without N fertilizer input practices. Meanwhile, the soil MBC content with organic manure treatments were higher than that with chemical fertilizer alone treatment, the reason maybe that decomposition of organic manure, soil C/N and slow-acting nutrients were increased under input organic manure conditions, which promote the growth of soil microorganisms20. And the basal respiration of soil microorganism were increased under application of organic manure conditions, the reason maybe that decomposable organic carbon is the main carbon source for microbial utilization, and there are significant differences in the content of decomposable organic carbon under different manure N input fertilizer treatments21. On the other hand, rice root exudates can induce a positive stimulating effect of soil organic carbon decomposition, which in return increases soil microbial respiration22. And the amount of root exudates were affected by application of different fertilization practices, root biomass and root exudates were increased under application of organic manure conditions9. In this studies, the CGrowth with fertilizer treatments were higher (p < 0.05) than with without N fertilizer input treatment, the reason maybe that there are significant differences in soil organic carbon content among different fertilizer treatments, which may lead to significant differences in available carbon sources of soil microorganisms23.
In this study, the results showed that CUE of soil microbial in rhizosphere soils were lower than that of non-rhizosphere soils, the reason maybe that value of CRespiration were higher in rhizosphere soils, thus the distribution of respiration carbon by rhizosphere soils microorganisms were higher than that of growth carbon. On the other hand, carbon content in rhizosphere soils were increased in the process of root system absorbs nutrients, and the soil microbial CUE were decreases with the increase of C content9. There is no significant difference in CUE of non-rhizosphere soils among different fertilizer treatments, the reason may be that physiological environment of non-rhizosphere soils is relatively stable, which was consistent with previous research24. In this study, the higher CUE of soil microbial with chemical fertilizer alone treatment (M0) than that of with organic manure treatments (M30, M50 and M100) consistent with our Hypothesis 1 that soil microbial CUE were increased under application chemical fertilizer conditions (Fig. 1), the main reason was that soil carbon nutrient ratio and carbon dioxide (CO2) through overflow respiration were increased, soil carbon nutrient ratio was benefit to meet the nutritional needs of soil microorganisms10, and thus lower soil microbial CUE under application of organic manure conditions9. However, the oxidase involved in the degradation of aromatic compounds in N were inhibited, the energy demand of soil microorganisms were reduced with chemical NPK fertilizer input, therefore, the soil microbial CUE were increased under application of chemical fertilizer conditions9.
The characteristics of soil microbial communities on carbon metabolism can reflect the bioavailability and functional diversity of soil microorganisms7. The average CO2 production rate was usually used as an effective indicator of soil microbial activity, and also reflects the ability of soil microbial communities to utilize carbon sources5. In the present study, the metabolic capacity of soil microorganisms to exogenous carbon sources with application of organic manure treatments were higher than with chemical fertilizer alone and without N fertilizer input treatments, the reason maybe that organic manure contains a large number of living microorganisms and active organic carbon sources, and thus the metabolism of microorganisms to carbon sources were promoted when application of organic manure. As a result, the higher metabolism of exogenous carbon sources with organic manure treatments than that of with chemical fertilizer alone and without N fertilizer input treatments consistent with our Hypothesis 2 that metabolic capacity of soil microorganisms to exogenous carbon sources would be higher with organic manure input conditions than that of with chemical fertilizer alone and without N fertilizer input conditions. And the metabolic capacity of soil microorganisms to exogenous carbon sources with chemical fertilizer alone treatment were higher than without N fertilizer input treatment, indicating that soil C/N were increased, soil microbial activity and decomposition rate were decreased, and thus soil microbial metabolic ability to carbon source were decreased under without N fertilizer input conditions19. Our results indicated that average utilization rate of carboxylic acids were higher than that of complex compounds, which suggested that carboxylic acid carbon sources account for only a small proportion of soil dissolved organic carbon, but they are important energy sources for the growth and metabolism of soil microorganisms25. On the other hand, degradation of complex compounds requires the interaction of various extracellular enzymes, more carbon sources and energy were invested in the synthesis of extracellular enzymes during soil microorganisms growth, and thus the utilization rate of complex compounds were reduced3,26. In this study, the RDA analysis results showed that there was obvious difference in metabolism of soil microorganisms to exogenous carbon sources between without N fertilizer input, chemical fertilizer alone and organic manure treatments, indicating that organic manure treatments has greatly changed the soil environment, heterogenous organic manure and microorganisms were introduced, and then significantly changed the utilization characteristics of soil microorganisms to exogenous carbon sources.
In the present study, the soil microbial growth rate were increased, but the soil microbial CUE were decreased under application of organic manure condition. These results were also confirmed in the previous research by using MicroRespTM method, the soil microbial metabolism ability to exogenous carbon (release to CO2) were significantly increased with organic manure practices27. In addition, the metabolic capacity of soil microorganisms to exogenous carbon sources in non-rhizosphere soils were higher than that of rhizosphere soils (Fig. 2), which was inconsistent with the results of soil basic respiration based on 18O-H2O method. The reason may be that MicroRespTM method mainly monitors microbial decomposition of exogenous carbon, and it doesn’t differentiate between excitation effects, but 18O-H2O method mainly monitors microbial decomposition of soil carbon. On the other hand, rhizosphere soils were adaptability to exogenous carbon source input (root exudates) conditions. Meanwhile, the positive stimulation effect of decomposition of original organic carbon in non-rhizosphere soils were induced with input of exogenous carbon sources, and thus microbial mineralization of organic carbon were enhanced26.
Conclusions
In this study, carbon source utilization characteristics in rice rhizosphere and non-rhizosphere soils under different short-term manure N input rate management conditions using a novel substrate-independent method were performed. The results indicated that characteristics of carbon source utilization in rhizosphere and non-rhizosphere soils were significantly affected by the short-term manure N input rate managements in a double-cropping rice paddy field. In summary, our results indicated that application of organic manure practices promotes soil microbial biomass carbon content and soil microbial growth rate in both rhizosphere and non-rhizosphere soils, whereas application of inorganic fertilizers promotes soil microbial carbon utilization efficiency. The results also showed that application of organic manure practices reduces carbon utilization efficiency in both rhizosphere and non-rhizosphere soils. The metabolic capacity of soil microorganisms to exogenous carbon sources in non-rhizosphere soils were higher than that of rhizosphere soils with different fertilizer treatments. And the utilization rate of carboxylic acids, amino acids and carbohydrates by soil microorganisms were increased with application of organic manure managements. There is an obvious difference in characteristic of carbon source metabolism between application of inorganic fertilizers and organic manure treatments. However, future studies were needed to investigate how changes of carbon source utilization soil microbial structure under different manure N input practices influence on ecological functions of rhizosphere microorganism.
Materials and methods
Sites and cropping system
The experiment was begun in 2017. It was located in Ningxiang County (28°07′N, 112°18′E) of Hunan Province, China. At beginning of this experiment, the surface soil characteristics (0–20 cm) were as follows: pH 6.80, soil organic carbon (SOC) 22.75 g kg−1, total N 2.24 g kg−1, total phosphorous (P) 0.66 g kg−1, total potassium (K) 14.45 g kg−1, available N 178.90 mg kg−1, available P 18.45 mg kg−1, and available K 69.50 mg kg−1. The climate condition (annual mean precipitation and evapotranspiration, monthly mean temperature) of this region, soil types and soil texture, and crop system were described by Tang et al.13.
Experimental design
The experiment including five fertilizer treatments: (1) 100% N of chemical fertilizer (M0), (2) 30% N of organic manure and 70% N of chemical fertilizer (M30), (3) 50% N of organic manure and 50% N of chemical fertilizer (M50), (4) 100% N of organic manure (M100), and (5) without N fertilizer input as control (CK). There were three replications and each plot size was 88.0 m2 (10.0 m × 8.0 m). The M0, M30, M50 and M100 treatments received the same total amount of N, phosphorus pentoxide (P2O5), potassium oxide (K2O) (the total amount of N, P2O5, K2O were included chemical fertilizer and that from organic manure) during early rice and late rice growth period, respectively. During early rice and late rice growing season, the applied total amount of N were 135.0 and 165.0 kg ha−1 (60%, 30% and 10% at basal, tillering and full heading stages), 54.0 and 45.0 kg ha−1 of P2O5, 67.5 and 90.0 kg ha−1 of K2O, respectively. All the P2O5 and K2O fertilizers were applied at tillage and then transplanting rice seedling. During early rice growth period, the total quantity of organic manure added to paddy field for the M30, M50 and M100 treatments were 828.0, 1380.0 and 2760.0 kg ha−1, respectively. During late rice growth period, the total quantity of organic manure added to paddy field for the M30, M50 and M100 treatments were 1012.5, 1687.5 and 3375.0 kg ha−1, respectively. During early rice and late rice growing season, the N, P2O5 and K2O content of organic manure were 48.9 g kg−1, 17.3 g kg−1, and 15.1 g kg−1. Before transplanting rice seedling, organic manure were added to paddy field and incorporated into 0–20 cm soil layer with tillage.
Soil sampling and sample preparation
Soil samples were collected at tillering stage of late rice in 2018. The rhizosphere and non-rhizosphere soils were collected and preprocessed according to the method described by Tang et al.13. Briefly, rhizosphere soil was operationally defined as soil adhering to the total roots after gentle shaking. The whole plant with their roots were extracted from soil and after shaking off the loosely adhering soil, the tightly adhering soil (i.e. rhizosphere soil) was carefully collected. In order to obtain the enough rhizosphere soil for multiplicating, twenty plants were randomly selected from each plot, and these rhizosphere soils were pooled to form one composite sample. Non-rhizosphere soil was defined as unvegetated soil adjacent to the rice plants. The unvegetated soil cores (5 cm diameter) adjacent to the rice plants (i.e. non-rhizosphere soil) were sampled at depth 0–20 cm. Correspondingly, one composite non-rhizosphere soil consisting of twenty cores was taken from each plot. Thus, three composite samples of rhizosphere and non-rhizosphere soils with each fertilizer treatment were collected at sampling time. The fresh samples were placed immediately on ice box and transported to the laboratory. Plant roots were removed by passing the sample through a 2-mm mesh sieve, and aliquots of the samples were then stored at room temperature until soil chemical properties analysis (for pH, C/N, NH4+-N, NO3−-N, SOC, and total nitrogen (TN)), at −20 °C until molecular analysis the types of exogenous carbon source (carboxylic acids, amino acid, carbohydrate, complex compounds). The samples were pre-incubated at 15 °C in aerated polyethylene bags for a total duration of six days before the beginning of the incubation, during which soil microbial carbon utilization efficiency (CUE) and microbial biomass turnover was determined. The pre-incubation was performed to allow the respiration to reach basal rate after sieving. Since the soils were at approximately 45% field capacity the soil water content was not manipulated.
Soil laboratory analysis
Soil chemical properties analysis
Soil chemical properties were measured according to the methods described by Bao28 and Wu et al.29. Briefly, soil pH was measured with a compound electrode (PE-10, Sartorious, Germany) using a soil to water rate of 1: 2.5. SOC and TN content were determined by an elemental analyzer (Carlo Erba 1110, CE Instruments) coupled to a Delta Plus isotope ratio mass spectrometer (Finnigan MAT) via a Conflo III (Thermo Fisher)28. Soil NH4+-N and NO3−-N concentrations in the extracts were determined by flow injection analysis28. Soil microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN) contents were determined by chloroform fumigation-extraction method29.
Determination of soil CUE
After the pre-incubation period, soil microbial CUE were determined based on incorporation of 18O from 18O-labeledwater into microbial genomic DNA following Spohn et al.9. Briefly, the respiration flux (CRespiration) were calculated based on the amount of carbon dioxide (CO2)-C produced during the incubation and the duration of the incubation period. The flux of C allocated to biomass production (CGrowth) were calculated by dividing the amount of microbial biomass carbon produced during the incubation by the duration of the incubation. It should be noted that production of microbial biomass carbon (growth) does not necessarily imply a net change in the pool size (net growth)9.
Based on the steady-state assumption, the amount of C taken up by the soil microbial biomass (CUptake) were calculated as
| 1 |
where CGrowth was the flux of C allocated to biomass production (growth), and CRespiration was the flux of C allocated to the production of CO2 (respiration).
Soil microbial CUE were then calculated by the following equation9.
| 2 |
Characteristics of utilization of different types of exogenous carbon sources
The MicroRespTM approach as described by Campbell et al.27 were used to measure exogenous carbon sources (carboxylic acids, amino acid, carbohydrate, and complex compounds), with the modifications described in detail in Lalor et al.6 and Banning et al.7. Briefly, the indicator dye with the gel detector plate consisted of 20 ppm cresol red dye, 240 mM potassium chloride and 4 mM sodium bicarbonate set into a 1% gel of noble agar (150 μL per well). Soil (300 μL total volume) was added to the 96-well microtiter deep well plates after 30 μL of each substrate had been dispensed (three replicate wells per substrate plus nine water controls per plate). This gave a final average water content of 60% of water holding capacity (WHC). Substrates were supplied at 30 mg ml−1 soil water or at 7.5 mg ml−1 soil water for those not readily water soluble. Substrate solutions were adjusted to pH 5.5–6.0. Incubations were run for 4 h at 25 °C. Absorbance of the detector plates was determined using a microplate reader at 590 nm (ASYS Expert 96, Biochrom, UK)6,7,27. Microbial carbon metabolism was investigated according to the method described by Campbell et al.27. Briefly, based on the calibration curve of CO2 production rate and light absorption value of specific wavelength by indicator, the exponential attenuation model was fitted to calculate the metabolic capacity of soil microorganisms to different carbon sources (CO2 production rate) within 4 hours.
CO2 production rate [μg (g·h)−1] were then calculated by the following equation:
| 3 |
where T was the incubation temperature (25.0 °C), L was the volume of per hole deep orifice plate (945 μL), M was dry weight of soil (g), and t was incubation time (4 h).
Statistical analysis
The statistical analyses of this manuscript were conducted by using SAS 9.3 software package30. To study the relationship between soil chemical properties and soil microbial carbon source utilization rate, we used a redundancy analysis. The redundancy analysis were performed using “vegan” packages in the R v3.20 statistical environment. The data of different treatments means in this manuscript were compared by using one-way analysis of variance (ANOVA) following standard procedures at the p < 0.05 probability level.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31872851), Innovative Research Groups of the Natural Science Foundation of Hunan Province (2019JJ10003).
Author contributions
Tang Haiming and Xiao Xiaoping designed this study; Li Chao, Pan Xiaochen and Cheng Kaikai performed the experiments; Li Weiyan and Wang Ke analyzed the data, Tang Haiming wrote and finalized the manuscript. Pan Xiaochen, and Wang Ke prepared Figures 1 and 2, and Li Weiyan, Cheng Kaikai prepared Figure 3. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang XM, et al. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014;73:42–48. doi: 10.1016/j.soilbio.2014.01.021. [DOI] [Google Scholar]
- 2.Schmidt MWI, et al. Persistence of soil organic matter as an ecosystem property. Nature. 2011;478:49–56. doi: 10.1038/nature10386. [DOI] [PubMed] [Google Scholar]
- 3.Cheng WX. Rhizosphere priming effect: Its functional relationships with microbial turnover, evapotranspiration, and C–N budgets. Soil Biol. Biochem. 2009;41:1795–1801. doi: 10.1016/j.soilbio.2008.04.018. [DOI] [Google Scholar]
- 4.Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- 5.Sinsabaugh RL, Manzoni S, Moorhead DL, Richter A. Carbon use efficiency of microbial communities: stoichiometry, methodology and modelling. Ecol. Lett. 2013;16:930–939. doi: 10.1111/ele.12113. [DOI] [PubMed] [Google Scholar]
- 6.Lalor BM, Cookson WR, Murphy DV. Comparison of two methods that assess soil community level physiological profiles in a forest ecosystem. Soil Biol. Biochem. 2007;39:454–462. doi: 10.1016/j.soilbio.2006.08.015. [DOI] [Google Scholar]
- 7.Banning NC, Lalor BM, Cookson WR, Grigg AH, Murphy DV. Analysis of soil microbial community level physiological profiles in native and post-mining rehabilitation forest: Which substrates discriminate? Appl. Soil Ecol. 2012;56:27–34. doi: 10.1016/j.apsoil.2012.01.009. [DOI] [Google Scholar]
- 8.Ekschmitt K, Liu M, Vetter S, Fox O, Wolters V. Strategies used by soil biota to overcome soil organic matter stabilitydwhy is dead organic matter left over in the soil? Geoderma. 2005;128:167–176. doi: 10.1016/j.geoderma.2004.12.024. [DOI] [Google Scholar]
- 9.Spohn M, et al. Soil microbial carbon use efficiency and biomass turnover in a long-term fertilization experiment in a temperate grassland. Soil Biol. Biochem. 2016;97:168–175. doi: 10.1016/j.soilbio.2016.03.008. [DOI] [Google Scholar]
- 10.Keiblinger KM, et al. The effect of resource quantity and resource stoichiometry on microbial carbon-use-efficiency. FEMS Microbiol. Ecol. 2010;73:430–440. doi: 10.1111/j.1574-6941.2010.00912.x. [DOI] [PubMed] [Google Scholar]
- 11.van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundstroom US. The carbon we do not see e the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol. Biochem. 2005;37:1–13. doi: 10.1016/j.soilbio.2004.06.010. [DOI] [Google Scholar]
- 12.Don A, Roodenbeck C, Gleixner G. Unexpected control of soil carbon turnover by soil carbon concentration. Environ. Chem. Lett. 2013;11:407–413. doi: 10.1007/s10311-013-0433-3. [DOI] [Google Scholar]
- 13.Tang HM, et al. Utilization of carbon sources in the rice rhizosphere and non-rhizosphere soils with different long-term fertilization management. J. Basic Microb. 2019;59:621–631. doi: 10.1002/jobm.201800736. [DOI] [PubMed] [Google Scholar]
- 14.Fang YY, et al. Nutrient stoichiometry and labile carbon content of organic amendments control microbial biomass and carbon-use efficiency in a poorly structured sodic-subsoil. Biol. Fert. Soils. 2020;56:219–233. doi: 10.1007/s00374-019-01413-3. [DOI] [Google Scholar]
- 15.Saifullah KK, Georg GR. Stoichiometry of the soil microbial biomass in response to amendments with varying C/N/P/S ratios. Biol. Fert. Soils. 2019;55:265–274. doi: 10.1007/s00374-019-01346-x. [DOI] [Google Scholar]
- 16.Thiet RK, Frey SD, Six J. Do growth yield efficiencies differ between soil microbial communities differing in fungal, bacterial ratios? Reality check and methodological issues. Soil Biol. Biochem. 2006;38:837–844. doi: 10.1016/j.soilbio.2005.07.010. [DOI] [Google Scholar]
- 17.Frey SD, Lee J, Melillo JM, Six J. The temperature response of soil microbial efficiency and its feedback to climate. Nat. Clim. Change. 2013;3:395–398. doi: 10.1038/nclimate1796. [DOI] [Google Scholar]
- 18.Yang XY, Ren WD, Sun BH, Zhang SL. Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma. 2012;177–178:49–56. doi: 10.1016/j.geoderma.2012.01.033. [DOI] [Google Scholar]
- 19.Masto RE, Chhonkar PK, Singh D, Patra AK. Changes in soil biological and biochemical characteristics in a long-term field trial on a sub-tropical inceptisol. Soil Biol. Biochem. 2006;38:1577–1582. doi: 10.1016/j.soilbio.2005.11.012. [DOI] [Google Scholar]
- 20.Spohn M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences. 2015;12:817–823. doi: 10.5194/bg-12-817-2015. [DOI] [Google Scholar]
- 21.Riggs CE, Hobbie SE, Bach EM, Hofmockel KS, Kazanski CE. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry. 2015;125:203–219. doi: 10.1007/s10533-015-0123-2. [DOI] [Google Scholar]
- 22.Kuzyakov Y. Review: factors affecting rhizosphere priming effects. J. Plant Nut. Soil Sci. 2002;165:382–396. doi: 10.1002/1522-2624(200208)165:4<382::AID-JPLN382>3.0.CO;2-#. [DOI] [Google Scholar]
- 23.Tong CL, et al. Long-term fertilizer effects on organic carbon and total nitrogen and coupling relationships of C and N in paddy soils in subtropical China. Soil Till. Res. 2009;106:8–14. doi: 10.1016/j.still.2009.09.003. [DOI] [Google Scholar]
- 24.Dijkstra P, et al. High carbon use efficiency in soil microbial communities is related to balanced growth, not storage compound synthesis. Soil Biol. Biochem. 2015;89:35–43. doi: 10.1016/j.soilbio.2015.06.021. [DOI] [Google Scholar]
- 25.Klimek B, Chodak M, Jazwa M, Niklinska M. Functional diversity of soil microbial communities in boreal and temperate scots pine forests. Eur. J. Forest Res. 2016;135:731–742. doi: 10.1007/s10342-016-0968-5. [DOI] [Google Scholar]
- 26.van Groenigen KJ, et al. Using metabolic tracer techniques to assess the impact of tillage and straw management on microbial carbon use efficiency in soil. Soil Biol. Biochem. 2013;66:139–145. doi: 10.1016/j.soilbio.2013.07.002. [DOI] [Google Scholar]
- 27.Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microb. 2003;69:3593–3599. doi: 10.1128/AEM.69.6.3593-3599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S.D. Soil and Agricultural Chemistry Analysis. China Agriculture Press, Beijing, pp 49–56 (2000).
- 29.Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC. Measurement of soil microbial biomass C by fumigation-extractiondan automated procedure. Soil Biol. Biochem. 1990;22:1167–1169. doi: 10.1016/0038-0717(90)90046-3. [DOI] [Google Scholar]
- 30.SAS. SAS Software of the SAS System for Windows. SAS Institute Inc, Cary, NC, USA. (2008).