Abstract
Although vivid memories of drug experiences are prevalent within clinical contexts and addiction folklore (“chasing the first high”), little is known about the relevance of cognitive processes governing memory retrieval to substance use disorder. Drawing on recent work that identifies episodic memory’s influence on decisions for reward, we propose a framework in which drug choices are biased by selective sampling of individual memories during two phases of addiction: (i) downward spiral into persistent use and (ii) relapse. Consideration of how memory retrieval influences the addiction process suggests novel treatment strategies. Rather than try to break learned associations between drug cues and drug rewards, treatment should aim to strengthen existing and/or create new associations between drug cues and drug-inconsistent rewards.
Subject terms: Addiction, Human behaviour
Introduction
Human choices are shaped by awareness of past experiences and anticipation of future possibilities. Faced with choices in the here and now, we draw on memories to imagine the different paths before us depending on which way we choose. These memories drive us forward one way or another, as we seek to avoid prior mistakes or relive past experiences. Such memory-based decision-making is familiar from our own lives and a core topic in the cognitive neuroscience of memory. It is also a prevalent theme within clinical contexts and addiction folklore, as demonstrated by the trope of “chasing the first high”:
“The first time I took a drink it was like the black and white world became Technicolor… The first time I smoked a cigarette, I can act it out for you, but then you can't record that. It felt like this. (Demonstrates – sighs.) […]. It relaxed my mind, my body, my breathing, everything. And that is what I was continuing to search for every time I smoked a cigarette after that” [1].
Yet despite empirical data showing that hippocampus and adjacent structures, which in humans are tightly linked to episodic memory, are critical to addiction-related behaviors [2–4], and recent work highlighting the role of drugs in memory encoding [5, 6], the role of memory retrieval remains less well-understood, and potentially crucial [7–10].
In the field of decision making, there is emerging consensus that processes occurring during memory retrieval play a distinct role in biasing choice [11–13]. Our aim in this article is to situate memory retrieval within the theory of addiction mechanisms. Drawing on recent work that identifies a key role for episodic memory in decisions for reward [14–20], we propose a framework in which drug choices are biased by selective sampling of individual memories during two key phases of addiction: (i) downward spiral into persistent use and (ii) relapse. Existing mechanistic accounts that posit addiction as a disorder of value learning [21–24] or as uniquely determined by incentive sensitization and cue-induced craving [25–27] or habit-like compulsion [28–30] have difficulty explaining both phases. Memory sampling offers a framework for value-based choice which incorporates features of these mechanisms, allowing flexibility in choice while explaining the outsized influence of particular past experiences (Box 1). In essence, the core idea is that episodic memories of highly rewarding past experiences [31–33] can be elicited by associated cues and contexts and come to bias present choices, as opposed to these choices either being determined by a static summary of past outcomes or driven by inflexible compulsion; importantly, these memories and processes can be conscious or unconscious (Box 2). Memory sampling as a mechanism is therefore distinct from, but complementary to, incentive sensitization and craving [25–27] in functioning as a causal intermediary between drug-associated cues and contexts on the one hand, and drug choices and behavior on the other.
Box 1: Choice variability and decision time in memory sampling and reinforcement learning.
The dominant approach to modeling value-based choice captures value learning and action selection using models derived from reinforcement learning (RL). In these models, values of choice options are learned incrementally, over repeated experience, and updated by the degree to which received reward deviates from expectations (Eq. 1). Actions are selected with probability proportional to the difference in expected values (Eq. 2). This type of model is fruitfully applied to explaining behavioral and neural signatures of simple choices [129, 130], complex plans [131–133], and aberrant choices such as in substance use disorder (SUD) [21, 22, 24].
| 1 |
| 2 |
Formally, learning involves comparing the reward experienced (R) as resulting from action (a) against the value the agent expected to result (Q(a)). Expected value is adjusted by the difference between these two quantities—the Reward Prediction Error (RPE)—scaled by a learning rate parameter, α. A foundational finding is that this difference term closely matches firing rates of neurons in the dopaminergic midbrain [129, 134, 135], suggesting that one role of dopamine is to signal this quantity.
Recent work shows that the average behavioral patterns captured by these models can also be captured by a different approach, in which expectations are not determined by incremental learning, but according to memories of individual past choices, recalled at the time of decision in proportion to the similarity of past states s’ to current state s [13, 15–17, 20] (Eq. 3). This inherently probabilistic value-setting guides choice dynamically, integrating “samples” of action values (Qi) computed on the basis of remembered choice outcomes i (Eq. 4) until achieving a decision threshold (z) (Eq. 5). The model also predicts the time necessary to reach a decision (response time; RT), as a function of the memory-derived values (Eq. 6).
| 3 |
| 4 |
| 5 |
| 6 |
As typically formulated, both models capture the fact that choices for rewarded options are likely to be repeated, and that, over time, the more rewarding option is likely to be chosen more often. But there is a critical difference relevant to SUD. In RL, the tendency to choose an option is a function of its learned value, which is maintained as a static quantity. Action selection uses a function of the form in Eq. 2 (but see Shteingart et al. [81] for discussion of alternative approaches) that scales the probability of a given action a with the ratio of the value of this option to the total value to be gained among all options available. The key feature of this model for our discussion is that any variability in choices beyond learned values is treated as “noise”—that is, symmetric and independent. This makes it difficult for the model to capture choices in SUD, which can both persist in ignorance of recent reward and reinstate long-past preferences.
Equation 3 describes how action selection differs in memory sampling. As in RL, the probability of choosing action a is proportional to the reward value experienced from that action in the past. However, these memories of past states (s’ ) are weighted by their sampling priority with respect to the current mental state (s; Eq. 3). Memory selection thus determines the likelihood that a given experience will be retrieved, based on similarity between elements of the current mental context, and the context of the past experience. Selection has been shown to be influenced by recency [17], goal congruence [88], mood-congruence [85], and an incrementally-learned estimate of the contingencies between states in the environment [136]. These influences are likely non-exclusive, with time-varying impact on the memory retrieval process, meaning that factors that alter the time available to make a decision, or the time it takes for memories to be recalled, can meaningfully affect choices [13] (Eqs. 4–6).
The introduction of memory sampling therefore explains seemingly erratic shifts in preference, which are usually treated as unmodeled variance or strategic exploration [137], as instead driven by systematic influences of past experiences. This re-conceptualization of choice variability underpins our proposed explanations for the puzzles of addiction.
Beyond the question of how well the models quantitatively account for the data, memory sampling differs from adapted RL accounts of drug choice in the type of explanation it offers. RL as described in Eq. 1 reflects a normatively-motivated solution to the well-formed problem of learning rewards in sequential choice environments; it is proven to converge to an optimal solution, under explicitly stated constraints. Extending the framework with additional machinery to account for behavioral and biological signatures of SUD necessarily departs from this normative basis. For RL to explain SUD behaviors, additions are required; however, these additional mechanisms are motivated only by these stylized findings. As a consequence the altered RL models do not share the explanatory status of the original: they can only describe the data, not the underlying generative process. The challenge therefore remains: to identify the computational problem, and to propose a model that solves it in a way that captures the observed data. Memory sampling does so by proposing that sequential choice is treated as non-parametric estimation, an optimal approach in situations where the underlying state space and value function cannot be compactly represented. Therefore, memory sampling offers a normative foundation that explains behavior in both healthy populations and SUD sufferers without need for additional mechanisms nor, critically, additional optimality criteria. From the perspective of memory sampling, healthy and SUD individuals are solving the same computational problem via the same mechanism but with different behavioral outcomes that can be accounted for, quantitatively, via distinctions in the content and character of experience representations and/or environmental influences on their access.
Box 2: Episodic memory in humans and animals.
“Episodic memory” was initially characterized by Tulving [138] as representations of specific past autobiographical events, distinct from semantic and procedural memories. Within this tradition, episodic memory has been conceived as in essence both declarative and conscious. However, theoretical [32, 33, 139] and empirical [140] work has repeatedly suggested that this conception is needlessly limiting. An alternative is to characterize episodic memory by operationally tractable features, namely: it is acquired in a “single-shot” manner, and it is pervasively associative, linking both (i) items incidentally present within a single event and (ii) multiple events with overlapping setting (“context”). These features yield a unique retrieval profile: episodic retrieval can be spurred by incidental associations experienced only once before, and can result in immediate, involuntary, but nonetheless flexible, subsequent retrieval of related information from the same or other events. Memory sampling builds on this profile to capture the specific and outsized influence that current retrieval of a particular past event has on decision-making (Box 1). Importantly, memories thus characterized may be both declarative and conscious, but they are not required to possess these features.
One key consequence of treating episodic memories as inclusive of unconscious retrieval is the bridge constructed between studies of memory in humans and animal models. There is increasing evidence that various animal species are capable of retrieving particular past event representations for use in decision-making [141–145]. Yet, absent declarative report, the question of the nature and sophistication of animal consciousness is theoretically and empirically vexed [146]. Operationalizing episodic memory enables integration of animal and human studies within a unified decision-making framework, despite persistent controversy surrounding determinations of consciousness in both animals [147] and humans [148].
Nonetheless, the status of any particular episodic memory as conscious or unconscious has obvious ramifications. For example, only conscious episodic memories are available for declarative report; relatedly, deliberations that depend on consciously-retrieved memories may result in choices less susceptible to post-hoc confabulation (“choice blindness”) [149]. In addition, it is conscious memories that prototypically figure in the construction of a self-narrative. Engagement with each other, including people who suffer with SUD, as conscious subjects capable of self-reflection on the role of our past in shaping present choices, therefore offers distinctive ways to intervene and influence those choices (see Box 3) [150]. But this insight need not obscure the possibility that episodic memories and their influence on decision-making may also be unconscious.
The puzzle of addiction
All addiction originates in non-addictive psychoactive drug consumption. In humans, this consumption is goal-directed: a means to achieve represented outcomes, such as their anticipated effects on mental states [8, 34]. These effects may be intrinsically valuable, e.g., hedonia, or relief from boredom, pain, stress and psychological suffering; or they may facilitate valued behaviors, e.g., sociability, sex, and task performance. Regardless of the goal of consumption, the majority of users do not transition to addiction [35–37], maintaining patterns of consumption that exploit drug benefits while incurring minimal costs.
The transition from non-addictive use to addiction occurs when this balance tips and drug costs come to outweigh drug benefits [38]. However, this immediately complicates demarcating non-addicted and addicted use. Costs and benefits must be weighed relative to values, which vary between individuals, including people with SUDs and observers; moreover, contingent, environmental factors, e.g., status and wealth, can protect against costs [38, 39]. This individuality and complexity is reflected in the DSM-5 [40] diagnostic criteria for SUD that is both polythetic and graded from mild to severe. These criteria include: (i) cravings and failures to limit use as intended, as (ii) drugs come to occupy increasing time and attentional focus, despite (iii) incurring severe risks and negative consequences, including e.g., drug-related mental and physical health problems, and loss of important relationships, social standing, employment, or housing. In countries that criminalize drug possession and stigmatize drug users, there is also risk of criminal sanction and social ostracization.
Continued consumption at the expense of other goods and despite costs is central to the construct of addiction and defines what is so puzzling about it as a form of behavior. Even though drug choices are initially goal-directed, they come to appear to have features not in keeping with purposive behavior, in so far as they contradict first-person reports of desired abstinence and incur costs that, at least from an observer’s vantage point, ought to tip the balance and disincentivize use. Put crudely, the puzzle of addiction is to understand why individuals keep using when drugs no longer appear worth it [38].
This general puzzle of addiction is particularly striking in relation to two key phases of the addiction process:
(i) Spiraling into addiction. Initial stages of consumption prior to addiction typically involve highly rewarding drug experiences. By contrast, the spiral into addiction is characterized not only by increasing negative consequences but by diminishing drug returns: as tolerance increases, hedonia is often claimed to decrease [25, 27]. This behavior is frustrating for standard RL accounts of value-based choice (RL; Box 1), which base decisions on a “running average” of experienced reward (and punishment) outcomes. Taken alone, these models predict that drug choice would eventually cease of its own accord. This gap can be accounted for by introducing disordered learning signals that result in persistent drug preference [21]; however, this leaves abstinence to be explained via “unlearning”, which compromises the model’s capacity to explain relapse [41]. Further, while this model succeeds in predicting drug choice behavior that is sensitive to the rational tradeoff against other rewards [42], it fails to capture the rich repertoire of flexible, goal-directed drug-seeking behaviors evident in human drug choice [24, 43].
(ii) Relapse. Quit attempts often occur when individuals hit “rock bottom” [44] and cannot avoid facing the most severe negative consequences of consumption [45–47]. Although medical management can ease the effects of withdrawal, it typically remains physically and psychologically painful to endure. Post-withdrawal, ex-users must maintain resolve and fashion a drug-free life. Successful quit attempts therefore not only indicate awareness of negative consequences and motivation to abstain; they also involve sunk costs. Why, if an ex-user is aware of drug costs and has lived through the pain of withdrawal and its aftermath, would they suddenly and spontaneously choose to use again? This puzzle is similarly frustrating for standard learning accounts of value-guided decisions, as they predict that recent experience should be the primary determining factor in choice; instead, people with SUD appear suddenly to reinstate past preferences without any apparent learning in the intervening period, consistent with the view that extinction is itself a process of new learning, rather than overwriting previous associations [48–50].
RL accounts can be extended to incorporate atypically persistent preferences, for instance by adding a term to the standard value update calculation (Box 1) that reflects a dopamine “surge” at the time of consumption [21]. Because dopamine is thought to signal the difference between reward expected and reward received, based on which expectation of future reward is updated, additional dopamine distorts the signal, causing expectations to remain high even as outcomes diminish. However, these approaches suffer from three related problems. First, a focus on dopamine leaves the model underspecified in its account of non-dopaminergic drugs. Many non-dopaminergic drugs are known to indirectly cause dopamine release [51], but this predicts a quantitative asymmetry in addictive potential, yet to be substantiated by empirical findings [52]. More importantly, this focus also fails to address the second puzzle of relapse after abstinence. This second puzzle is often explained as a form of context-dependent preference [22–24]. But this raises two additional problems for RL accounts that must in turn be answered, namely: where these context-dependent preferences are stored, and how they come to be reinstated after long periods of dormancy.
Redish et al. [22, 23] identified these challenges and proposed a “situation recognition” component, realized by interactions between hippocampus and PFC, that reinstates past preferences on the basis of similarity to current context. Our proposal is consistent with this idea and connects it with known mechanisms of memory-guided decision-making. Specifically, we link this “situation recognition” component to the episodic memory system, and place particular emphasis on the dynamics of situation recognition that are entailed by context-guided memory sampling. We further predict that this system is instrumental not only in relapse, but in persistent use as well. Notably, our proposed mechanism does not exclude the possibility of atypical dopamine response. Indeed, dopamine release is associated with enhanced memory encoding [53, 54], which may explain why some early drug memories are often vivid and persistent. However, in our framework, atypical dopamine is not required to explain behaviors of interest, consistent with the failure to find outsized addiction potential for directly-dopaminergic drugs.
Cue-induced craving
Research into cue-induced craving contributes to the explanation of both puzzles of (i) downward spiral and (ii) relapse. In animal models, drug-associated cues, similarly to drug priming, reinstate drug-seeking behavior after extinction and forced abstinence [55–57]. Although an association between craving and consumption in human addiction studies is contested and remains far from well established [58, 59], there is nonetheless evidence that in human laboratory settings, stress and drug-associated cues predict first-person reports of craving, which is associated with subsequent relapse in cocaine ex-users [60]; and that, outside of the laboratory, craving is associated with consumption in smokers [61] and cocaine users [62]. Cue-induced craving not only characterizes periods of active use but endures for months, possibly years, post-cessation [25–27]. Crucially, craving requires effort to resist, which entails both costs (that can be rationally traded off against benefits [63]) and the likelihood of failure due to simple mechanistic fallibility [64]. Together, these predict that there will be occasions when consumption results from stress or encounters with drug-associated cues. This is part of the explanation of both puzzles.
Nonetheless, cue-induced craving alone cannot provide a complete explanation of the puzzles, for the simple reason that it does not necessitate drug-taking: craving may be associated with consumption, but it does not compel it. This is demonstrated by converging lines of evidence from animal models and human addiction studies establishing that the majority of animals and humans alike respond to context-specific contingencies and choose non-drug alternatives across multiple choice settings, cues and craving notwithstanding [38, 65, 66].
Research in laboratory animals has demonstrated that although rodents will escalate drug self-administration in deprived settings where no alternative rewards are available, the vast majority will choose food or social rewards over drugs in forced-choice studies [65, 66]. Recent research suggests that social reward in particular is a potent alternative for rodents, with 100% choosing it over drug reward in the presence of drug-associated cues and irrespective of sex, drug class, dose, training conditions, abstinence duration, social housing, or “addiction score” based on a DSM-style model; only delay or punishment of social reward choices affects choice [67].
Human studies demonstrate similar flexibility in drug choice and behavior. Although drug choices in SUD have features that resemble habitual behavior [28], there is nonetheless limited evidence in support of a habit theory of addiction [68]: in the majority of cases, consumption appears to remain goal-directed [69]. In forced-choice studies offering the immediate opportunity to use crack cocaine or receive money reward, individuals with SUD frequently choose money over drug reward [42]. In addition, contingency management treatment is highly effective [70] and offers positive reward contingent on drug-free urine samples in the form of money, prizes, and most recently and successfully, employment [71]; rates of use are cost-sensitive [72]; and correctional services and courts can succeed in establishing abstinence by imposing costs for failure [73]. Lastly, epidemiological data suggests that the majority of people with SUD (including those with physical dependence) recover without clinical intervention by their late 20 s or early 30s [35].
These lines of evidence converge to establish that on the whole, individuals with SUD choose non-drug alternatives in the presence of drug options across multiple choice settings. Together, they underscore that drug consumption, even in addiction, typically remains purposive [74] and involves choice, as opposed to being compelled by craving alone. Hence, although craving is part of the solution to the puzzles of downward spiral and relapse, it cannot be the whole of it. Given the evidence demonstrating flexibility in behavior and responsiveness to incentives, why do individuals choose to use drugs in both stages of the addiction process, despite the evident costs?
The answer in part lies in a multi-factorial approach to how choice can be biased in addiction. Individuals with SUD display a range of decision-making anomalies, including reflective impulsivity, risk and ambiguity tolerance [75], impatience in delay discounting [76, 77], reduced insight and self-awareness [78], and denial of the severe consequences of use [79]. These may influence subjective expected utility of drug and non-drug choices, such that immediate, certain drug reward is strongly preferred to the delayed, uncertain rewards of abstinence, while drug risks are minimized and drug harms fail to be processed at all. We propose that memory sampling is a further previously unexplored factor that interacts with—and potentially provides a cohesive mechanistic framework giving rise to [80]—many of these decision-making anomalies. The selective sampling of individual episodic memories of rewarding past drug use to anticipate future possibilities can bias present choices towards drugs; and, like incentive salience and craving, is affected by cues and context. This mechanism can lead to individuals “chasing the first high” when exposed to drug-associated cues and context: imagining future possibilities of use that are ripe with rewards of the sorts previously experienced and elaborated—even when recent outcomes should encourage a more sober outlook.
The memory sampling framework
The standard account of value-based choice assumes that choices are based on values learned incrementally, over repeated experience. Therefore, it cannot account for addictive choices, where preferences persist despite repeated counterevidence, or change suddenly after long periods of stability.
Memory sampling offers an alternative account, in which values are estimated at the time of choice, guided by selected memories of similar past experience [13–18, 20]. This model treats outcomes of previous choices as “samples” of what may result from the current decision. When these memories are reinstated, they serve as evidence for the favorability of one option or another. Therefore, choices depend on which memories come to mind at the time of decision. In this way, memory sampling can explain puzzling choices as a consequence of known cognitive and neurobiological mechanisms of memory retrieval.
There are two critical relevant differences between memory sampling and incremental RL:
Choice variability
In the standard incremental-learning model there is a degree of “noise”—choices sometimes go against what would be predicted based on learned values alone. But it has difficulty capturing choices that consistently deviate from recent learning, e.g., appearing to “underweight” (ignore) or “overweight” (fixate) on recent experiences [13, 17, 80, 81]. Memory sampling attributes these variations to situational and environmental factors which naturally persist between decisions, predicting consistent deviations from RL of the sort observed in addiction (see Box 1 for a further contrast of choice variability in both models).
Learning history
In the standard incremental-learning model, values are learned as a running average, a single summary representation of past rewards. In essence, previous experiences are lost, reflected only in the learned value. Once a behavior is abandoned, the model predicts that a return ought to be preceded by re-learning the values that guided it. This is inconsistent with recent work demonstrating that incidental reminders of individual past decision outcomes and contexts meaningfully affect choice [17, 18, 82–84]. Memory sampling explains these effects because the full history of individual past outcomes and contexts is available for sampling.
Memories of past experiences can be brought to mind by incidental reminders, such as external stimulus (or internal stimulus [85, 86] e.g., stress [6]). At a neural level, partial reactivation of activity patterns formed during the initial experience can lead to reactivation of all of the pattern. This function, pattern completion [87], has particular relevance for memory-guided decisions, because it predicts that memories of past rewards can be reinstated on the basis of stimuli only fleetingly associated with a given valuable outcome. Crucially, this means that decisions are guided by memoranda recalled at the time of choice (Box 1).
The extended set of associations and activity patterns that follow memory retrieval are referred to as context memory [88, 89]. Computational and neurobiological models of context memory emphasize the influence that context has not just on how memories are organized, but on how they are retrieved [90]: memories which share context tend to be retrieved together. For instance: When trying to decide where to eat, we may recall a night at a particular restaurant; context theories suggest that the next recollection will likely be another restaurant nearby in space, or an evening nearby in time. A recent study demonstrated that which context is retrieved can have sizable and specific impact on decisions for reward, separate from that of the initial retrieved memory [18]. Therefore, factors that influence the process or content of context retrieval—such as acute stress [91, 92]—should also influence choice. A more direct analogy to drug relapse can be seen in another study showing that acute stress prior to the presentation of a conditioned fear cue can preferentially diminish recall of the extinction context—though not the cue itself, nor the associated fear response [93].
Importantly, this context-guided “clustering” in memory retrieval is additive: speed and consistency is enhanced when retrieved context is consistent with other internally-guided factors, such as goals, intentions, and mood [85, 86, 94]. This is critical because the dynamics of memory retrieval are instrumental in adjudicating its influence on behavior [13, 95–97]: a memory that comes to mind more quickly has a better chance of influencing choice, and of overcoming competing values based on representations other than individual episodic memories [13].
Lastly, memory’s role in decision-making is not simply to bind us to the past. Extensive research demonstrates a role for memory retrieval in imagining novel future scenarios [98, 99], in particular the possible outcomes of decisions about the future being made in the current moment [100–103]. Suggestively, these imagined future scenarios may have an optimistic bias [104].
Solving the puzzles
Memory sampling addresses both puzzles of (i) downward spiral and (ii) relapse where previous models fail by introducing flexibility to capture both persistent and also reawakened preferences.
Spiraling into addiction:
“Crystallized Molly you can snort it and shoot it. My personal choice was to inject it. I have injected crystal meth and everything but literally the first time with Molly was so intense. Everyone says the first time with anything is intense but for me I overdosed twice in the same week. That was because I was chasing that first high. It was so intense and I just wanted to feel it again. It was just way overwhelming. You just want to chase that first high and that’s why people just keep doing it especially where I’m from.” (from an interview published on the forum Inspirations Youth).
Relapse:
“I was several years sober. Walking past a museum cafe at lunch, I saw white table cloths, nice silverware, attractively dressed people, perfectly framed by goblets of red wine. I was gripped by the sight of a handsome couple, and overcome by the powerful thought that I could and should be doing that—sipping red wine like a gentleman with a beautiful woman in an elegant restaurant. All the possibilities were alive—romance, love, joyful, generous conversation—and required, almost as a necessity, the red wine. The experience repeats when I observe that sort of scene. No craving. But my entire being is sucked into that experience… I don’t believe I ever kissed a girl — let alone fell in love, etc—without spirits being involved until I was over 45. My whole upbringing and history taught me that alcohol made love possible.” (anonymous individual in recovery, personal communication)
These reports describe the subjective experience of memory in both phases of addiction. With respect to the downward spiral, the person wants to re-experience the intensity of the first high. With respect to relapse, they report being swayed not simply by craving for alcohol itself, but by the allure of associated goods, e.g., love, sex, and intimacy. Memory sampling allows us to model the effect of this subjective experience, offering new understanding of both puzzles.
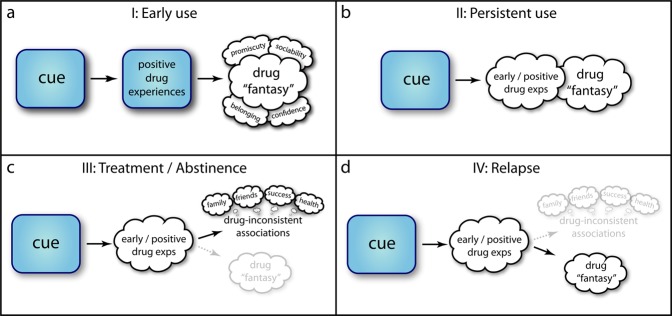
We propose that memory for drug choices develops over four distinct stages (Fig. 1a–d). We illustrate this development via the progressive cognitive implications of an initial drug-associated cue. This cue can be an external stimulus previously experienced during consumption (e.g., a physical object or location, or a social setting); or an internal stimulus that previously led to spontaneous drug-seeking (e.g., a mood or an emotion). Importantly, drug-associated cues can be multitudinous as well as highly contingent: incidental, ephemeral associations can reinstate entire patterns of experience, even after long periods of dormancy.
Fig. 1. The four stages of memory-guided drug choice.
a During early use, various memoranda (“cues”—such as people, places, things, or even internal signals) are bound to the memory of positive drug experiences and also to drug-related “fantasies”—episodes which did not occur, but which are imagined on the basis of the material provided by drug experiences and associated rewards. b Over persistent use, these associations between cues, experience memories, and fantasies are strengthened, leading to rapid, involuntary reinstatement of the latter when presented with the former. These signals carry positive reward, thereby encouraging consumption and explaining persistent use despite lessening actual rewards and/or increasing negative consequences. c During treatment or other forms of abstinence, drug memories and fantasies persist and can be recalled, but now have competition in their effect on action selection, in the form of “drug-inconsistent” mental context: memories and imagined scenarios of alternative goods, e.g., of relationships, employment, health. Persistent abstinence suggests these memories have become bound to widely available cues, or are otherwise easily recalled. d However, the original, drug-consistent, memories are still available to recall. If a momentary influence—such as a shift in the environment, or an acute stressor—disrupts recall of drug-inconsistent associations, their effect on behavior can reduce, allowing drug-related memories to prevail.
The first stage occurs during early and highly rewarding experiences, when drugs become associated with the cue in question (Fig. 1a). In this stage, memory is critical not only to the formation of the association between cue and intrinsic drug reward, but equally to the formation of the association between drug and other rewards present during these early experiences. These associated rewards can be contingent upon or coincidental with consumption, and may include e.g., increased confidence, sociability, promiscuity, or feelings of safety. We call these drug-associated rewards the drug “fantasy” as they are components of both the memory of previous drug experiences and the episodic simulation of future drug experiences. As drug choice and consumption persist over time, these associations are elaborated and strengthened, leading to the near-simultaneous retrieval of memory and fantasy.
The second stage occurs when drug choice and consumption become less rewarding (Fig. 1b). Though use may no longer be reinforced by high reward, the early memories and associated fantasies nonetheless persist and are triggered by the same drug-associated cues. These associations between cues, early memories, and drug fantasies evoke reward signals that affect decisions similarly to externally-provided reward [13, 105]. In this way, memory sampling explains the first puzzle of drug choice: the downward spiral into addiction. Individuals with SUD may persist in using drugs despite decreasing drug returns because they are basing their choice on episodic memories of early highly rewarding drug experience and associated drug fantasies.
Importantly, memory and fantasy can also play a role in abstinence (Fig. 1c). A core aim of various therapies for SUD is to instill a vivid sense of the value of a drug-free life: the clear recognition of goods and rewards that are contingent upon abstinence, such as improved personal relationships, employment opportunities, health, and other drug-inconsistent personally meaningful pursuits. This is a feature of twelve-step fellowships [106, 107], group therapy [108], as well as harm reduction approaches [109]; in addition, experience of life meaning has been shown to predict better treatment outcomes for alcohol dependence [110]; and, intriguingly, a recent study suggests that meaning interventions reduce alcohol-cue interference in Stroop tasks [111]. When faced with a drug-associated cue, people with SUD must learn to recall and act to secure this alternative rewarding future, by choosing against present consumption. Over time, drug cues may as a result become associated not only with drug memories and drug fantasies, but with the rewards of a drug-free life (“drug-inconsistent associations” in Fig. 1c, d), as abstinence persists and a series of choices are made against consumption, and in favor of this alternative.
However, drug memories and fantasies do not disappear simply because a competing association between drug cue and a drug-free life is well established. Memory sampling therefore offers a model for explaining the puzzle of relapse: relapse occurs precisely when drug memories and drug fantasies– as opposed to non-drug associations– are retrieved when cued thereby once again exerting an influence on present decisions (Fig. 1d). A crucial issue to preventing relapse is therefore to understand the factors that disrupt the retrieval of cue-associated non-drug alternatives and to design interventions accordingly.
Of the many factors that influence memory retrieval, acute stress may be particularly important in explaining drug choices post abstinence. Acute stress (i) triggers relapse (a process correlated with atypicality in the hippocampal response to drug-associated cues [3]); (ii) shifts reward decisions from goal-directed to seemingly habit-like [112, 113]; and (iii) preferentially lessens retrieval of contextually-linked memories [91–93] e.g., the drug-inconsistent associations illustrated above. The precise mechanisms by which stress exposure yields each of the above effects remains an open question. But a tantalizing possibility is that the effects of stress on choice are mediated by the effects of stress on memory retrieval. When context memories are not recalled, decisions may be swayed by the first memory that comes to mind [18]. Suggestively, recent work has shown that memory recall prioritizes events with highly salient, surprising rewards [114–116]–which may describe early, positive drug experiences; and that stress may cue retrieval of past stressful occasions—which, in SUD populations, may include or cause drug use [6, 85]. Together, these findings support two routes by which acute stress may influence memory retrieval and yield relapse: (a) diminishing retrieval of drug-inconsistent context memories, leaving initial drug-related memories to guide behavior; and (b) promoting retrieval of drug-consistent context memories and hence drug memories themselves.
Translational implications
There are two routes by which research into the mechanisms of addiction can inform treatment. It can illuminate why existing psychological and pharmacological interventions are successful; or it can suggest new interventions. Recognizing the potential role of memory sampling in explaining drug choices promises both.
Complementing various therapies (see above), qualitative sociological research has long suggested that overcoming SUD is facilitated by the capacity to vividly and realistically imagine an alternative drug-free life of personal value [46, 117–119]. In addition, recent experimental studies converge on an emerging theoretical framework that posits memory-guided simulation as a core component of intertemporal choices in all domains [100, 101, 120, 121]. A set of targeted studies [102, 103] probing the effect of episodic future thinking about drug-inconsistent future goods on rates of delay discounting in smokers is concordant with this outlook; as is recent work on orbitofrontal deficits and targeted neural activation in animal models of addiction [122]. These findings suggest that individuals with SUD can be motivated to abstain not simply because they recognize that drugs have negative consequences, but because they recognize that abstinence has positive consequences, in so far as it brings personally meaningful drug-inconsistent rewards into reach [7].
Much existing treatment uses psychological and pharmacological interventions to block the association between drugs and positive reward, e.g., naltrexone and opiate-replacement therapy aim to do so directly by acting on opioid receptors, while cognitive-behavioral therapy and techniques such as motivational interviewing aim to refocus attention away from drug reward onto the negative consequences of use. But once established via real-world experience, memories are difficult, if not impossible, to extinguish [41, 93, 123–126]. Evidence for the idea that extinction is mediated by creation of new memories, rather than unlearning of previous associations, is found in recent observations that extinction and fear memories are supported by parallel engrams [127, 128]. The memory sampling framework presented here is consistent with these findings from neurobiology, and also supports the clinical and sociological insight that it can be effective to focus treatment on the development of the capacity to imagine an alternative drug-free life when confronted with drug cues: to harness what has been called “meaning in life” [111] or “a stake in conventional life” [118] to recovery. Rather than try to break the association between drug cues and drug reward, treatment should aim to strengthen existing and/or create new associations between drug cues and drug-inconsistent rewards (Fig. 1c), which can compete with and over time potentially dominate drug reward in memory retrieval.
Box 3: Implications for treatment.
Memory sampling not only offers a theoretical framework for existing interventions but, by drawing these together, suggests the following novel treatment strategy:
(1) Delivery of a short course of therapy designed to help people with SUDs produce a vivid and realistic image of an alternative drug-free life, containing a clear set of personally meaningful and genuinely available drug-inconsistent rewards.
(2) Training to voluntary recall the image.
(3) Once (2) is established, conditioning to develop an association between the image and two kinds of stimuli: (i) drug-associated cues, so as to increase the probability of spontaneous retrieval when opportunities to consume are present; (ii) personal reminders such as smells [151] and app-based individualized photos and pictures [108] (for a real-world example see www.sobergrid.com), which can be voluntarily self-cued to facilitate retrieval.
(4) Clear instructions to retrieve the image using voluntary recall or self-cueing as a strategy to support non-drug choices when risk of drug choices is high.
(5) The use of pharmacological memory enhancers, such as acetylcholinesterase inhibitors, may also be considered during the short course of therapy (1) and/or the conditioning phase (2).
Box 4: Limitations and directions for future research.
• The treatment strategy outlined in Box 3 is speculative and the clinical protocol for implementing each stage 1–5 needs to be made precise; similarly, studies exploring memory sampling within SUD population groups have not yet been conducted.
• Though the phenomenon of “chasing the first high” is widely documented, rigorous qualitative work probing recollections of early and motivating drug experiences is required. Recent technological advances in momentary experience reporting [61, 152] and large-scale text analysis [153] promise new possibilities in this direction [154, 155]. A firmer classification of the types, valence, and behavioral relevance of memories and associated drug fantasies may provide valuable material for implementation of individualized treatment.
• The role of acute stress requires further investigation. Is there a distinct role at retrieval as opposed to encoding? What are the mechanistic consequences on memory retrieval—does acute stress cease extended recall, change the content of what is recalled, or slow all memory retrieval, leaving the first to dominate choices made under time pressure? Each of these mechanisms has support from the memory literature, and may coexist. Variation in the prevalence of one or other mechanism may be a valuable individual difference measure, predicting susceptibility to kind of relapse trigger. Finally, does the impact of stress on memory retrieval change with the adapted cortisol response often observed in chronic drug users 156?
• While many studies suggest that drug-related memories are strongly encoded and persistent [156–159], little is known about the precise ways in which drugs alter memory encoding. The prominence of surprising rewards in memory recall [114, 116] may arise from event boundaries introduced at encoding that establish the surprising reward memories as the primary event in a new context [115], a feature known to promote resistance to extinction [160]. However, the link between these findings and drug experiences has yet to be firmly established.
• Memory sampling predicts that aspects of memory encoding and retrieval should be instrumental to relapse. Neural circuits known to support episodic and relational memory and those identified as critical for multiple forms of relapse show considerable overlap, particularly in medial temporal lobe (MTL) structures such as subiculum, entorhinal cortex, and dentate gyrus, as well as ventral and dorsal medial prefrontal cortex [161]. It is unknown whether these structures support functions common to both sets of behaviors. A fruitful avenue for further research may be to integrate the extensive sets of findings in both literatures, in particular the detailed subparcellation of function of MTL regions in memory encoding and retrieval [162].
Conclusion
We propose a framework for explaining drug choices in addiction as guided by memories of individual past experiences of drug use. The memory sampling model predicts that such choices can exhibit persistence beyond that captured by incremental learning models, consistent with known drug choice behavior in the downward spiral into addiction and relapse alike.
This framework offers a new perspective on existing, but undertheorized, treatment strategies, as well as suggesting new approaches that center on the life narrative of the person who is suffering. Ultimately, this may facilitate targeting of individual contextual influences, by providing a mechanism for their effect on choice.
The idea that memories can guide choices toward undesirable outcomes might seem cause for despair: if memory can never fully be extinguished, what hope is there for fully overcoming its effects? But the model presented here offers an opportunity for rethinking conceptual frameworks that sometimes threaten to equate people who struggle with addiction with automatons. Specifically, by linking drug choices to the retrieval of memories in the moment, it suggests that the effect of the past on the present is always dynamic—and so potentially subject to interventions leading towards a more desirable future.
Acknowledgements
The authors wish to thank Keiland W. Cooper for help with figure design, and Brenda Curtis, David Epstein, John Fedota, Elizabeth Goldfarb, Ian Phillips, Yavin Shaham, and Elliot Stein for helpful discussions.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Aaron M. Bornstein, Hanna Pickard
Contributor Information
Aaron M. Bornstein, Email: aaron.bornstein@uci.edu
Hanna Pickard, Email: h.pickard@jhu.edu.
References
- 1.Hammer RR, Dingel MJ, Ostergren JE, Nowakowski KE, Koenig BA. The experience of addiction as told by the addicted: incorporating biological understandings into self-story. Cult Med Psychiatry. 2012;36:712–34. doi: 10.1007/s11013-012-9283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–47. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, et al. Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology. 2005;183:171–80. doi: 10.1007/s00213-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 4.Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–39. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–8. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb EV, Sinha R. Drug-induced glucocorticoids and memory for substance use. Trends Neurosci. 2018;41:853–68. doi: 10.1016/j.tins.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krank MD, Wall AM. Context and retrieval effects on implicit cognition for substance use. In: Handbook of implicit cognition and addiction. California: Sage Publications, Inc.; 2006. p. 281–92.
- 8.Müller CP, Schumann G. Drugs as instruments: a new framework for non-addictive psychoactive drug use. Behav Brain Sci. 2011;34:293–310. doi: 10.1017/S0140525X11000057. [DOI] [PubMed] [Google Scholar]
- 9.Schwabe L, Dickinson A, Wolf OT. Stress, habits, and drug addiction: a psychoneuroendocrinological perspective. Exp Clin Psychopharmacol. 2011;19:53–63. doi: 10.1037/a0022212. [DOI] [PubMed] [Google Scholar]
- 10.Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2013;226:659–72. doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shohamy D, Daw ND. Integrating memories to guide decisions. Curr Opin Behav Sci. 2015;5:85–90. [Google Scholar]
- 12.Pezzulo G, Donnarumma F, Maisto D, Stoianov I. Planning at decision time and in the background during spatial navigation. Curr Opin Behav Sci. 2019;29:69–76. [Google Scholar]
- 13.Wang S, Bornstein AM. Mixing memory and desire. Wiley Interdiscip Rev Cogn Sci. 2019. [DOI] [PMC free article] [PubMed]
- 14.Lengyel M. Dayan P. Hippocampal contributions to control: the third way. In: Platt JC, Koller D, Singer Y, Roweis ST, editors. Advances in neural information processing systems 20. Curran Associates, Inc.; 2008. p. 889–96.
- 15.Bornstein AM, Daw ND. Cortical and hippocampal correlates of deliberation during model-based decisions for rewards in humans. PLoS Comput Biol. 2013;9:e1003387. doi: 10.1371/journal.pcbi.1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shadlen MN, Shohamy D. Decision making and sequential sampling from memory. Neuron. 2016;90:927–39. doi: 10.1016/j.neuron.2016.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bornstein AM, Khaw MW, Shohamy D, Daw ND. Reminders of past choices bias decisions for reward in humans. Nat Commun. 2017;8:15958. doi: 10.1038/ncomms15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornstein AM, Norman KA. Reinstated episodic context guides sampling-based decisions for reward. Nat Neurosci. 2017;20:997–1003. doi: 10.1038/nn.4573. [DOI] [PubMed] [Google Scholar]
- 19.Ritter S, Wang JX, Kurth-Nelson Z, Botvinick MM. Episodic control as meta-reinforcement learning. 2018; bioRxiv, 360537. 10.1101/360537
- 20.Gershman SJ, Daw ND. Reinforcement learning and episodic memory in humans and animals: an integrative framework. Annu Rev Psychol. 2017;68:101–28. doi: 10.1146/annurev-psych-122414-033625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–7. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- 22.Redish AD, Jensen S, Johnson A, Kurth-Nelson Z. Reconciling reinforcement learning models with behavioral extinction and renewal: implications for addiction, relapse, and problem gambling. Psychol Rev. 2007;114:784–805. doi: 10.1037/0033-295X.114.3.784. [DOI] [PubMed] [Google Scholar]
- 23.Redish DA, Jensen S, Johnson A. Addiction as vulnerabilities in the decision process. Behav Brain Sci. 2008;31:461–87. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon DA, Daw ND, Dual-system learning models and drugs of abuse. Comput Neurosci Drug Addic. 2012;145–61. 10.1007/978-1-4614-0751-5_5
- 25.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 26.Holton R, Berridge K. Addiction between compulsion and choice. Addic Self-Control. 239–68; 2013. 10.1093/acprof:oso/9780199862580.003.0012
- 27.Robinson MJF, Robinson TE, Berridge KC. The current status of the incentive sensitization theory of addiction. In: Pickard H, Ahmed S, editors. The philosophy and science of addiction. Routledge; 2018. p. 351–61.
- 28.Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 29.Charland LC. Cynthia’s dilemma: consenting to heroin prescription. Am J Bioeth. 2002;2:37–47. doi: 10.1162/152651602317533686. [DOI] [PubMed] [Google Scholar]
- 30.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 31.Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. J Exp Psychol Learn Mem Cogn. 1985;11:501–18. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- 32.Henson RN, Gagnepain P. Predictive, interactive multiple memory systems. Hippocampus. 2010;20:1315–26. doi: 10.1002/hipo.20857. [DOI] [PubMed] [Google Scholar]
- 33.Wang JX, Cohen NJ, Voss JL. Covert rapid action-memory simulation (CRAMS): a hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiol Learn Mem. 2015;117:22–33. doi: 10.1016/j.nlm.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed SH, Badiani A, Miczek KA, Müller CP. Non-pharmacological factors that determine drug use and addiction. Neurosci Biobehav Rev. (2018). 10.1016/j.neubiorev.2018.08.015 [DOI] [PMC free article] [PubMed]
- 35.Heyman GM. Addiction: a disorder of choice. Harvard University Press; 2009.
- 36.Zinberg NE. Drug, set, and setting: the basis for controlled intoxicant use. New York: Yale University Press; 1984.
- 37.Substance Abuse Mental Health Services Administration. Results from the 2005 National survey on drug use and health: national findings. http://www.oas.samhsa.gov/nsduh/2k5nsduh/2k5Results.pdf (2006).
- 38.Pickard H. The puzzle of addiction. In: The routledge handbook of philosophy and science of addiction. Routledge; 2018. p. 29–42.
- 39.Martin CS, Langenbucher JW, Chung T, Sher KJ. Truth or consequences in the diagnosis of substance use disorders. Addiction. 2014;109:1773–8. doi: 10.1111/add.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasin DS, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–51. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–86. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 42.Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Hart CL, Krauss RM. Human drug addiction is more than faulty decision-making. Behav Brain Sci. 2008;31:448–9. [Google Scholar]
- 44.Kirouac M, Frohe T, Witkiewitz K. Toward the operationalization and examination of ‘hitting bottom’ for problematic alcohol use: a literature review. Alcohol Treat Q. 2015;33:312–27. [Google Scholar]
- 45.Waldorf D. Natural recovery from addiction: some social-psychological processes of untreated recovery. J Drug Issues. 1983;13:237–80. [Google Scholar]
- 46.McKeganey JMN, McIntosh J, McKeganey N. Identity and recovery from dependent drug use: the addict’s perspective. Drugs: Educ, Prev Policy. 2001;8:47–59. [Google Scholar]
- 47.Mackintosh V, Knight T. The notion of self in the journey back from addiction. Qual Health Res. 2012;22:1094–101. doi: 10.1177/1049732312450325. [DOI] [PubMed] [Google Scholar]
- 48.Flaherty CF. Animal learning and cognition. Distributed by Random House. 1985.
- 49.Robbins SJ. Mechanisms underlying spontaneous recovery in autoshaping. J Exp Psychol Anim Behav Process. 1990;16:235. [Google Scholar]
- 50.Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 51.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pettit HO, Ettenberg A, Bloom FE, Koob GF. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology. 1984;84:167–73. doi: 10.1007/BF00427441. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi T, et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature. 2016;537:357–62. doi: 10.1038/nature19325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNamara CG, Dupret D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017;40:383–4. doi: 10.1016/j.tins.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Girardeau P, et al. Relapse to cocaine use persists following extinction of drug-primed craving. Neuropharmacology. 2019;155:185–93. doi: 10.1016/j.neuropharm.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 57.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology. 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- 59.Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15:1167–82. doi: 10.1093/ntr/nts268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 61.Serre F, Fatseas M, Swendsen J, Auriacombe M. Ecological momentary assessment in the investigation of craving and substance use in daily life: a systematic review. Drug Alcohol Depend. 2015;148:1–20. doi: 10.1016/j.drugalcdep.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 62.Preston KL, et al. Cocaine craving and use during daily life. Psychopharmacology. 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–40. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sripada C. Addiction and fallibility. J Philos. 2018;115:569–87. [Google Scholar]
- 65.Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–84. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–7. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 67.Venniro M, et al. Volitional social interaction prevents drug addiction in rat models. Nat Neurosci. 2018;21:1520–9. doi: 10.1038/s41593-018-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogarth L. A critical review of habit theory of drug dependence. Psychol Habit. 2018;325–41. 10.1007/978-3-319-97529-0_18
- 69.Hogarth L. Controlled and automatic learning process in addiction. In: Pickard H, Ahmed SA, editors. The philosophy and science of addiction. Routledge; 2018. p. 325–38.
- 70.Zajac K, Alessi SM, Petry NM. Contingency management approaches. In: Pickard H, Ahmed SA, editors. The philosophy and science of addiction. Routledge; 2018. p. 455–63.
- 71.Silverman K, Holtyn AF, Morrison R. The therapeutic utility of employment in treating drug addiction: science to application. Transl Issues Psychol Sci. 2016;2:203–12. doi: 10.1037/tps0000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ainslie G. A research-based theory of addictive motivation. Law Philos. 2000;19:77–115. [Google Scholar]
- 73.Hawken A, Kleiman M. Managing drug involved probationers with swift and certain sanctions: evaluating Hawaii’s HOPE: Executive summary. Washington, DC: National Criminal Justice Reference Services; 2009. [Google Scholar]
- 74.Pickard H. The purpose in chronic addiction. AJOB Neurosci. 2012;3:40–9. doi: 10.1080/21507740.2012.663058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verdejo-Garcia A. Decision-making dysfunctions in addiction. In Pickard H, Ahmed SA, editors. The philosophy and science of addiction. Routledge; 2018. p. 339–50.
- 76.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 77.Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–77. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–41. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pickard H. Denial in addiction. Mind Lang. 2016;31:277–99. [Google Scholar]
- 80.Biele G, Erev I, Ert E. Learning, risk attitude and hot stoves in restless bandit problems. J Math Psychol. 2009;53:155–67. [Google Scholar]
- 81.Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant learning. J Exp Psychol Gen. 2013;142:476–88. doi: 10.1037/a0029550. [DOI] [PubMed] [Google Scholar]
- 82.Ludvig EA, Madan CR, Spetch ML. Priming memories of past wins induces risk seeking. J Exp Psychol Gen. 2015;144:24–9. doi: 10.1037/xge0000046. [DOI] [PubMed] [Google Scholar]
- 83.Murty VP, FeldmanHall O, Hunter LE, Phelps EA, Davachi L. Episodic memories predict adaptive value-based decision-making. J Exp Psychol: Gen. 2016;145:548–58. doi: 10.1037/xge0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duncan KD, Shohamy D. Memory states influence value-based decisions. J Exp Psychol Gen. 2016;145:1420–6. doi: 10.1037/xge0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Talmi D, Lohnas LJ, Daw ND. A retrieved context model of the emotional modulation of memory. Psychol Rev. 2019;126:455–85. doi: 10.1037/rev0000132. [DOI] [PubMed] [Google Scholar]
- 86.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24:6979–85. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 88.Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46:269–99. [Google Scholar]
- 89.Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychological Rev. 2008;115:893–912. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Folkerts S, Rutishauser U, Howard MW. Human episodic memory retrieval is accompanied by a neural contiguity effect. J Neurosci. 2018;38:4200–11. doi: 10.1523/JNEUROSCI.2312-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwabe L, Böhringer A, Wolf OT. Stress disrupts context-dependent memory. Learn Mem. 2009;16:110–3. doi: 10.1101/lm.1257509. [DOI] [PubMed] [Google Scholar]
- 92.Goldfarb EV, Tompary A, Davachi L, Phelps EA. Acute stress throughout the memory cycle: diverging effects on associative and item memory. J Exp Psychol Gen. 2019;148:13–29. doi: 10.1037/xge0000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raio CM, Brignoni-Perez E, Goldman R, Phelps EA. Acute stress impairs the retrieval of extinction memory in humans. Neurobiol Learn Mem. 2014;112:212–21. doi: 10.1016/j.nlm.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lohnas LJ, Polyn SM, Kahana MJ. Expanding the scope of memory search: Modeling intralist and interlist effects in free recall. Psychological Rev. 2015;122:337–63. doi: 10.1037/a0039036. [DOI] [PubMed] [Google Scholar]
- 95.Redish AD. Vicarious trial and error. Nat Rev Neurosci. 2016;17:147–59. doi: 10.1038/nrn.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buzsáki G, Tingley D. Space and time: the hippocampus as a sequence generator. Trends Cogn Sci. 2018;22:853–69. doi: 10.1016/j.tics.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bornstein AM, et al. Perceptual decisions result from the continuous accumulation of memory and sensory evidence. bioRxiv. 2018;10:186817. Preprint at:10.1101/186817
- 98.Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 99.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104:1726–31. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–48. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 101.Palombo DJ, Keane MM, Verfaellie M. The medial temporal lobes are critical for reward-based decision making under conditions that promote episodic future thinking. Hippocampus. 2015;25:345–53. doi: 10.1002/hipo.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stein JS, et al. Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology. 2016;233:3771–8. doi: 10.1007/s00213-016-4410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stein JS, Tegge AN, Turner JK, Bickel WK. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med. 2018;41:269–76. doi: 10.1007/s10865-017-9908-1. [DOI] [PubMed] [Google Scholar]
- 104.Sharot T. The optimism bias. Curr Biol. 2011;21:R941–5. doi: 10.1016/j.cub.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 105.Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alcoholics Anonymous World Services, Anonymous, A., Hazelden. Alcoholics anonymous, large print. Chicago: Alcoholics Anonymous World Services, Incorporated; 2001.
- 107.Kelly JF, Cristello J, Bergman B. Integrated 12-Step facilitation to promote adolescent mutual-help involvement. In: Brief interventions for adolescent alcohol and substance abuse 380. New York: Guilford Press; 2018.
- 108.Frings D, Albery IP. The social identity model of cessation maintenance: formulation and initial evidence. Addict Behav. 2015;44:35–42. doi: 10.1016/j.addbeh.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 109.Fernandez J. Power over addiction: a harm reduction workbook for changing your relationship with drugs. Invisible Work Press; 2018.
- 110.Waisberg JL, Porter JE. Purpose in life and outcome of treatment for alcohol dependence. Br J Clin Psychol. 1994;33:49–63. doi: 10.1111/j.2044-8260.1994.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 111.Ostafin BD, Feyel N. The effects of a brief meaning in life intervention on the incentive salience of alcohol. Addict Behav. 2019;90:107–11. doi: 10.1016/j.addbeh.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 112.Schwabe L, Wolf OT. Stress prompts habit behavior in humans. J Neurosci. 2009;29:7191–8. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences, 2013;110:20941-20946. [DOI] [PMC free article] [PubMed]
- 114.Rouhani N, Norman KA, Niv Y. Dissociable effects of surprising rewards on learning and memory. J Exp Psychol Learn Mem Cogn. 2018;44:1430–43. doi: 10.1037/xlm0000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rouhani N, Norman KA, Niv Y, Bornstein AM. Reward prediction errors create event boundaries in memory. Cognition. 2019 doi: 10.1101/725440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jang AI, Nassar MR, Dillon DG, Frank MJ. Positive reward prediction errors during decision-making strengthen memory encoding. Nat Hum Behav. 2019;3:719–32. doi: 10.1038/s41562-019-0597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waldorf D, Biernacki P. The natural recovery from opiate addiction: some preliminary findings. J Drug Issues. 1981;11:61–74. [Google Scholar]
- 118.Waldorf D, Reinarman C, Murphy S. Cocaine changes: the experience of using and quitting. Philadelphia, PA: Temple University Press; 1992.
- 119.Pickard H. Addiction and the Self. Noûs.
- 120.Gabaix X, Laibson D. Myopia and Discounting (No. w23254). National bureau of economic research. 2017. 10.3386/w23254.
- 121.Hunter LE, Bornstein AM, Hartley CA. A common deliberative process underlies model-based planning and patient intertemporal choice. 2018; 499707. 10.1101/499707
- 122.Schoenbaum G, Chang C-Y, Lucantonio F, Takahashi YK. Thinking outside the box: orbitofrontal cortex, imagination, and how we can treat addiction. Neuropsychopharmacology. 2016;41:2966–76. doi: 10.1038/npp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–4. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 124.Chan WYM, Leung HT, Westbrook RF, McNally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–21. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–66. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- 126.Chan, JCK., Lapaglia JA. Does Reconsolidation Happen for Episodic Memory in Humans? PsycEXTRA Dataset. 2012. 10.1037/e502412013-124
- 127.Warren BL, et al. Distinct Fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci. 2016;36:6691–703. doi: 10.1523/JNEUROSCI.0140-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Warren BL. et al. Separate vmPFC ensembles control cocaine self-administration versus extinction in rats. J Neurosci. 2019. 10.1523/JNEUROSCI.0918-19.2019 [DOI] [PMC free article] [PubMed]
- 129.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 130.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 131.Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–11. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 132.Gläscher J, Daw N, Dayan P, O’Doherty JP. States versus rewards: dissociable neural prediction error signals underlying model-based and model-free reinforcement learning. Neuron. 2010;66:585–95. doi: 10.1016/j.neuron.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–15. doi: 10.1016/j.neuron.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Barto AG, Houk JC, Davis JL, Beiser DG. Models of information processing in the basal ganglia. Cambridge, MA: 1995.
- 135.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–47. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gershman SJ, Moore CD, Todd MT, Norman KA, Sederberg PB. The successor representation and temporal context. Neural Comput. 2012;24:1553–68. doi: 10.1162/NECO_a_00282. [DOI] [PubMed] [Google Scholar]
- 137.Daw ND, O’Doherty JP, Dayan P, Seymour B, Dolan RJ. Cortical substrates for exploratory decisions in humans. Nature. 2006;441:876–9. doi: 10.1038/nature04766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tulving E, et al. Episodic and semantic memory. Organ Mem. 1972;1:381–403. [Google Scholar]
- 139.Schacter, D. L. (1989). On the relation between memory and consciousness: Dissociable interactions and conscious experience. In H. L. Roediger III & F. I. M. Craik (Eds.), Varieties of memory and consciousness: Essays in honour of Endel Tulving (p. 355–389). Lawrence Erlbaum Associates, Inc.
- 140.Slotnick SD, Schacter DL. Conscious and nonconscious memory effects are temporally dissociable. Cogn Neurosci. 2010;1:8–15. doi: 10.1080/17588920903474263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eichenbaum H, Fortin NJ. Bridging the gap between brain and behavior: cognitive and neural mechanisms of episodic memory. J Exp Anal Behav. 2005;84:619–29. doi: 10.1901/jeab.2005.80-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- 143.Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proc Natl Acad Sci USA. 2009;106:9525–9. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Templer VL, Hampton RR. Episodic memory in nonhuman animals. Curr Biol. 2013;23:R801–6. doi: 10.1016/j.cub.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Allen TA, Fortin NJ. The evolution of episodic memory. Proc Natl Acad Sci USA. 2013;110( Suppl 2):10379–86. doi: 10.1073/pnas.1301199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Andrews K, Beck J (Eds.). The Routledge handbook of philosophy of animal minds. Oxford: Taylor & Francis; 2017.
- 147.Colin A, Trestman M. Animal consciousness. In: Edward NZ editor. Stanford encyclopedia of philosophy (Winter 2017 Edition), 2017.
- 148.Peters MAK, Kentridge RW, Phillips I, Block N. Does unconscious perception really exist? Continuing the ASSC20 debate. Neurosci Consciousness. 2017; 2017. [DOI] [PMC free article] [PubMed]
- 149.Johansson P, Hall L, Sikström S, Olsson A. Failure to detect mismatches between intention and outcome in a simple decision task. Science. 2005;310:116–9. doi: 10.1126/science.1111709. [DOI] [PubMed] [Google Scholar]
- 150.Lane RD, Ryan L, Nadel L, Greenberg L. Memory reconsolidation, emotional arousal, and the process of change in psychotherapy: new insights from brain science. Behav Brain Sci. 2015;38:e1. doi: 10.1017/S0140525X14000041. [DOI] [PubMed] [Google Scholar]
- 151.Zernig G, et al. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction. 2008;103:2024–31. doi: 10.1111/j.1360-0443.2008.02348.x. [DOI] [PubMed] [Google Scholar]
- 152.Epstein DH, et al. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mikolov T, Chen K, Corrado G, Dean J. Efficient estimation of word representations in vector space. arXiv [cs.CL]. 2013.
- 154.Nguyen T, et al. Estimation of the prevalence of adverse drug reactions from social media. Int J Med Inform. 2017;102:130–7. doi: 10.1016/j.ijmedinf.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 155.Sidani JE, et al. I wake up and hit the JUUL: Analyzing Twitter for JUUL nicotine effects and dependence. Drug Alcohol Depend. 2019;204:107500. doi: 10.1016/j.drugalcdep.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wemm SE, Sinha R. Drug-induced stress responses and addiction risk and relapse. Neurobiol Stress. 2019;10:100148. doi: 10.1016/j.ynstr.2019.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Franken IHA, Rosso M, van Honk J. Selective memory for alcohol cues in alcoholics and its relation to craving. Cogn Ther Res. 2003;27:481–8. [Google Scholar]
- 158.Klein AA, Nelson LM, Anker JJ. Attention and recognition memory bias for alcohol-related stimuli among alcohol-dependent patients attending residential treatment. Addict Behav. 2013;38:1687–90. doi: 10.1016/j.addbeh.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 159.Fridrici C, et al. Investigating biases of attention and memory for alcohol-related and negative words in alcohol-dependents with and without major depression after day-clinic treatment. Psychiatry Res. 2014;218:311–8. doi: 10.1016/j.psychres.2014.03.041. [DOI] [PubMed] [Google Scholar]
- 160.Nevin JA. Behaviorpal momentum and the partial reinforcement effect. Psychol. Bull. 103; 44–56.
- 161.Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, Shaham Y. Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology. 2019;44:465–77. doi: 10.1038/s41386-018-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carr VA, Rissman J, Wagner AD. Imaging the human medial temporal lobe with high-resolution fMRI. Neuron. 2010;65:298–308. doi: 10.1016/j.neuron.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]