Abstract
Cognitive control regulates cognitive and emotional systems to facilitate goal-directed behavior in the context of task-irrelevant distractors. Cognitive control deficits contribute to residual functional impairments across psychiatric disorders and represent a promising novel treatment target. Translational evidence suggests that modafinil may enhance performance in executive functions; however, differential effects on regulatory control in cognitive and emotional domains have not been examined. The present pre-registered randomized-controlled pharmacological fMRI trial examined differential effects of modafinil (single-dose, 200 mg) on cognitive and emotional conflict processing. To further separate objective cognitive enhancing effects from subjective performance perception, a metacognitive paradigm was employed. Results indicated that modafinil specifically enhanced cognitive conflict performance and concomitantly increased activation in the inferior frontal gyrus and its functional communication with the dorsomedial prefrontal cortex. Exploratory analysis further revealed modafinil-enhanced basolateral amygdala reactivity to cognitive conflict, with stronger reactivity being associated with higher cognitive conflict performance. Whereas modafinil enhanced cognitive performance in the metacognitive paradigm, confidence indices remained unaffected. Overall, the present results suggest that modafinil has the potential to enhance cognitive conflict processing while leaving emotional conflict processing unaffected. On the neural level modafinil enhanced the recruitment of a network engaged in general conflict and regulatory control processes, whereas effects on the amygdala may reflect improved arousal-mediated attention processes for conflicting information. The pattern of cognitive enhancing effects in the absence of effects on affective processing suggests a promising potential to enhance cognitive control in clinical populations.
Subject terms: Cognitive control, Human behaviour
Introduction
Cognitive control regulates cognitive and emotional systems to facilitate goal-directed behavior despite distracting and conflicting information [1, 2]. Impairments in this cardinal cognitive function have been observed across major psychiatric disorders [3] and have been associated with core symptoms in attention deficit/hyperactivity disorder [4, 5], addiction [6] and depression [7]. Cognitive control deficits have been increasingly considered as promising transdiagnostic treatment target [8]; however, the currently established pharmacological interventions primarily target affective dysregulations [9]. Cognitive impairments therefore often persist despite remission of affective symptoms [10, 11] and considerably contribute to long-term functional impairments [7] with deficient cognitive control additionally contributing to impairments in a range of other cognitive domains [12]. Against this background pharmacological cognitive enhancers (neuroenhancers) have been increasingly advocated as a novel treatment strategy to specifically enhance cognitive functioning [13].
Cognitive control incorporates the ability to monitor conflicting task-irrelevant interferences and to inhibit prepotent motor responses [1, 2]. Both processes strongly rely on the prefrontal cortex (PFC) [14], particularly the ventrolateral PFC (vlPFC) located on the inferior frontal gyrus (IFG) [2, 15]. With respect to conflict processing, the interference-related neural systems vary as a function of context [2]. Initial studies employing affective Stroop paradigms reported that conflicts arising from cognitive distractors engage lateral prefrontal systems, whereas emotional conflict primarily recruits more medial prefrontal regions, particularly the rostral anterior cingulate cortex (rACC) and—to a lesser extend—the amygdala [2, 16, 17]. A recent meta-analysis covering fMRI studies that employ classical emotion-word Stroop paradigms further confirmed consistent recruitment of the vlPFC and additionally suggested robust recruitment of the dorsomedial prefrontal cortex (dmPFC) during conflict processing [18]. Furthermore, network-level approaches emphasize the interplay between these regions, such that increased functional communication between the vlPFC and dmPFC has been observed during cognitive control [19, 20].
Converging translational evidence suggests that psychostimulants may enhance cognitive control, particularly inhibitory motor control, in healthy individuals and neuropsychiatric patients [21, 22]. Compared with typical amphetamine-type stimulants, the wakefulness-promoting agent modafinil (MOD), which has been approved for the treatment of narcolepsy, has a relatively low risk for abuse, cardiovascular [23, 24] and anxiogenic side effects [25] and thus represents a particular promising candidate to enhance cognitive control. On the neurochemical level, MOD increases extracellular levels of dopamine [26], norepinephrine [27], glutamate [28] and serotonin [29], and attenuates GABAergic neurotransmission in the PFC [30], with effects on catecholaminergic neurotransmission being considered as primary mechanism for improving prefrontal cognitive functions [9, 22].
Previous studies reported beneficial effects of a single dose of MOD on cognitive control in the domain of inhibitory motor control in healthy non-sleep deprived subjects [31] and neuropsychiatric patients [32, 33], whereas studies examining effects on cognitive conflict revealed inconsistent results [31, 34]. However, previous studies on cognitive conflict employed classical (non-affective) word-color Stroop paradigms [35, 36] with near ceiling performance in the placebo groups reflecting a low sensitivity to determine neuroenhancing effects.
Compared to the vast literature on the cognitive effects of MOD, only few studies examined effects on emotional processing and findings remain controversial. Several single-dose administration studies did not observe MOD effects on self-reported affective states [25], while others reported increased general mood [37] and anxiety [22] with two fMRI studies reporting either decreased or increased amygdala threat reactivity following repeated (100 mg/7 days) or high-dosage (600 mg) MOD, respectively [38, 39]. Underlying effects of MOD on emotional processes may in turn interfere with emotional conflict processing, which is essential for everyday functioning, such that threatening or social-emotional stimuli including facial expressions convey important information to redirect attention and adapt behavior. MOD effects in this domain could thus interfere with functioning in affectively remitted patients or could exaggerate dysregulated emotional conflict processing during symptomatic states [40, 41].
Against this background the present preregistered randomized double-blind placebo-controlled pharmaco-fMRI experiment in n = 72 healthy subjects aimed at determining distinct effects of a single dose of 200 mg MOD on cognitive and emotional conflict processing and the underlying neural mechanisms. To account for subjective enhancing effects in the absence of objective performance improvements as reported for other psychostimulants [42], a metacognitive paradigm [43] was administered to disentangle effects on subjective confidence from objective performance. Based on previous single-dose MOD administration studies suggesting facilitated cognitive control in the context of enhanced neural activation [39], we hypothesized that MOD would promote conflict processing, particularly cognitive conflict processing. On the neural level we expected that facilitated performance would be accompanied by (1) enhanced neural activation, specifically IFG or dmPFC activation during cognitive conflict processing or rACC activation during emotional conflict processing, respectively, and (2) facilitated functional communication within this network.
Materials and methods
Participants
Seventy-four healthy, right-handed male participants (undergraduate/graduate students) were enrolled after providing written informed consent. In line with previous studies examining the effects of MOD in healthy subjects [32, 44] and to reduce variance related to sex-differences and menstrual cycle-related hormonal variations on emotional processing [45, 46], only male subjects were enrolled. Participants were free from current or a history of psychiatric or neurological disorders, regular use of psychotropic substances including nicotine, visual or motor impairments that may interfere with performance. Subjects with contraindications for MOD (i.e. allergies, cardiovascular or sleeping disorders) or MRI were excluded. One subject did not understand the paradigm and was excluded after the pre-fMRI training session; following initial data quality assessment, data from one subject was excluded due to excessive head motion during fMRI (>3 mm or 3°). Consequently, data from 72 subjects (MOD, n = 35; placebo, n = 37) were included in the final analyses (age, 21.51 ± 2.58 years).
Study procedures were approved by the local ethics committee and adhered to the latest revision of the Declaration of Helsinki. The study was preregistered on clinicaltrials.gov (https://www.clinicaltrials.gov/ct2/show/NCT03426202).
Experimental procedures and assessment of potential confounders
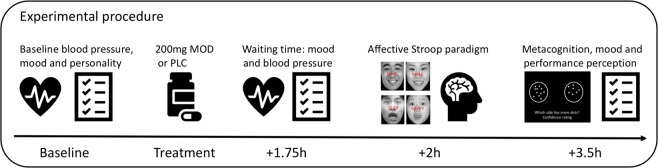
A randomized double-blind, placebo-controlled, between-subject pharmaco-fMRI design was employed with subjects being randomly assigned to the p.o. administration of either MOD (200 mg) or placebo (PLC). Dose selection was consistent with previous studies [24, 32]. In line with the pharmacodynamic profile of MOD [47, 48], the experimental paradigms started 2 h after administration. Participants underwent an affective Stroop paradigm followed by a reinforcement learning task during fMRI, which lasted approximately 20 min and will be reported in a separate publication. Mood and traits were assessed before treatment, and cardiovascular indices (blood pressure and pulse rate) were measured at baseline and before scanning (+1.75 h). Affective states were additionally monitored at the end of the experiment (assessed at baseline, +1.75 h and after experiment, +3.5 h). In a post-scanning session, a metacognitive paradigm was employed to disentangle MOD effects on subjective confidence from objective performance, and task-specific ratings of subjective perception of performance [42] and task enjoyment [24] were assessed using visual analog scales (VAS) (Fig. 1, Supplementary Materials).
Fig. 1. Experimental timeline.
Experimental timeline of the randomized placebo-controlled double-blind between-subject experiment. Informed consent for the publication of the image has been obtained. MOD modafinil, PLC placebo.
fMRI cognitive and emotional conflict paradigm
A validated affective Stroop paradigm [2, 16, 17] incorporating emotional and non-emotional (cognitive) conflict was employed during fMRI. The stimuli consisted of facial stimuli from 20 individuals (10 males) with happy or fearful expressions, with words (“happy/fear”, emotional condition or “male/female”, cognitive condition) written in prominent red letters over the faces (stimuli from [49]). Facial and written stimuli were either congruent (c) or incongruent (i). Participants were instructed to ignore the irrelevant word distracters and to judge the expression or gender of each face as fast and accurately as possible. All subjects underwent two runs per experimental condition (emotional, cognitive) encompassing 80 trials. The trials were interspersed by a jittered inter-trial interval of 4 s (3–5 s) and stimuli were presented for 1 s (total duration for each run was 426 s). Facial stimuli were validated in an independent sample (emotion intensity and gender ratings using 9-point Likert scales) and counterbalanced across trial types and response buttons. Order of the conditions was counterbalanced across treatment groups.
Metacognition paradigm
Metacognitive performance was assessed using a validated paradigm combining dot-density discrimination and confidence ratings (available from https://github.com/metacoglab/meta_dots).
Briefly, during each trial, two circles with different numbers of dots were presented and subjects had to indicate which circle contained more dots and subsequently rate their subjective confidence on a 1–6 scale [43, 50, 51]. Performance was individually adjusted (70.7% accuracy) using a staircase procedure. During the subsequent main paradigm, eight blocks each encompassing 25 trials were presented (Supplementary Materials).
Data analysis approach
Behavioral data analysis
To examine whether MOD treatment increased behavioral performance, mixed-ANOVAs were conducted on the dependent variables accuracy and reaction time (RT) for correct responses with treatment (MOD, PLC) as between-subject factor, congruence (i, c) and task (emotional, cognitive) as within-subject factors. Metacognitive efficiency was measured by the correspondence between trial-by-trial performance and confidence using a hierarchical meta-d′ model [50]. Based on signal detection theory, meta-d′ characterizes the sensitivity of individuals’ confidence reports to correct or incorrect responses (http://www.columbia.edu/~bsm2105/type2sdt); d′ reveals the objective task performance with respect to perceptual detection, and c measures response bias [51]. Analyses were employed in SPSS 22 (Armonk, NY: IBM Corp.) with p < 0.05.
MRI data acquisition, preprocessing and analysis
MRI data were collected on a 3.0 Tesla system (GE MR750, General Electric Medical System) and preprocessed using evaluated procedures in SPM12 (Supplementary Materials). On the first level, the two experimental conditions, “i”, “c” and “error” for incorrect responses were modeled for each run using a stick function convolved with the standard hemodynamic response function and including six head-motion parameters. Contrast images between incongruent and congruent conditions (“i > c”) were created separately for the emotional and non-emotional task. In line with the behavioral analyses, for the second-level analysis, the primary hypotheses of the study were tested by examining the three-way interaction between treatment, congruence and task. To further disentangle interaction effects, parameter estimates were extracted using MarsBaR [52]. Based on our a priori regional-specific hypothesis [2, 16–18], the analysis focused on the conflict network as identified by a single mask encompassing the (atlas [53] defined) bilateral IFG, rACC and dmPFC (Supplementary Materials). Within a priori regions, results were thresholded at p < 0.05 family-wise error (FWE) corrected at peak level with small volume correction (SVC).
Given the distinct neural systems underlying cognitive and emotional conflict processing, an additional exploratory analysis examined the differences between emotional and cognitive conflict processing [cognitivei-c > emotionali-c] within treatment groups using paired t tests on the whole-brain level (pFWE-cluster < 0.05). For anatomical mapping of the effects, probabilistic maps from the Anatomy toolbox 3.0 [54] were employed.
Conflict-dependent functional connectivity
To explore MOD effects on the functional communication between conflict-related brain regions, functional connectivity analyses (gPPI) [55] were performed for emotional and cognitive conflict processing separately. The corresponding first-level models included identical regressors as the BOLD activation design matrix and respective psychophysiological interaction terms. Based on the analysis of conflict-dependent neural activation patterns (Supplementary Materials), the IFG and rACC served as seed regions for the seed-to-voxel analyses. Corresponding seed regions in the conflict-general bilateral IFG and the emotional-conflict-specific rACC were defined by initially examining the whole-brain main effect of congruence across groups and placing 6-mm-radius spheres centered at peak voxel of the activated clusters. Treatment effects on context-dependent connectivity within the conflict network were determined by two-sample t tests (“i > c”, emotional and cognitive respectively). Results were thresholded at pSVC-FWEpeak < 0.05, employing SVC within the mask encompassing the IFG, rACC and dmPFC.
Results
Potential confounders
Pretreatment data revealed comparable age, weight, pathological symptom load and mood between groups (Table 1; ps > 0.05). Examining affective and cardiovascular indices did not reveal significant differences between groups (ps > 0.05; Supplementary Materials, Supplementary Fig. S1). Postexperiment assessments of subjective performance perception and task enjoyment indicated no significant differences (ps > 0.05). No group differences were observed with respect to guessing the administered treatment (χ2 = 2.69, p > 0.05) confirming successful double blinding.
Table 1.
Group difference on pretreatment assessments.
| MOD (M ± SD) | PLC (M ± SD) | t70 | p | |
|---|---|---|---|---|
| Age | 21.11 ± 2.44 | 21.89 ± 2.70 | −1.28 | 0.20 |
| Weight (kg) | 64.46 ± 8.92 | 64.92 ± 7.95 | −0.23 | 0.82 |
| PANAS-positive | 27.77 ± 5.42 | 28.62 ± 4.15 | −0.75 | 0.46 |
| PANAS-negative | 15.09 ± 5.38 | 15.59 ± 5.52 | −0.40 | 0.69 |
| STAI-state | 36.49 ± 8.28 | 35.57 ± 5.08 | 0.57 | 0.57 |
| STAI-trait | 39.43 ± 9.32 | 36.95 ± 4.70 | 1.44 | 0.16 |
| BDI II | 7.40 ± 6.69 | 6.54 ± 5.52 | 0.60 | 0.55 |
| BIS | 15.60 ± 3.04 | 16.22 ± 2.77 | −0.90 | 0.37 |
| BAS | 23.60 ± 4.72 | 25.30 ± 4.40 | −1.58 | 0.12 |
| ASQ | 20.97 ± 6.35 | 21.65 ± 5.35 | −0.49 | 0.63 |
M mean, SD standard deviation, PANAS Positive And Negative Affect Score, STAI State Trait Anxiety Inventory, BDI II Beck Depression Inventory II, BIS Behavioral Inhibition system scale, BAS Behavioral Activation system scale, ASQ Autism Spectrum Quotient.
Effects of MOD on conflict processing performance
Examining conflict processing accuracy by means of a treatment (MOD vs. PLC) × congruence (i vs. c) × task (emotional vs. cognitive), mixed-ANOVA revealed a main effect of congruence (F(1, 70) = 104.72, p < 0.001, partial η2 = 0.599), indicating that participants responded with higher accuracy during congruent trials (t71 = 10.19, p < 0.001, d = 1.201, d indicates effect size for t test in terms of Cohen’s d) and a significant task × congruence interaction effect (F (1, 70) = 15.79, p < 0.001, partial η2 = 0.184) documenting that accuracy was higher for incongruent trials in the cognitive compared to the emotional task (t71 = 2.44, p < 0.05, d = 0.289). Importantly, the task by treatment interaction effect reached marginal significance (F (1, 70) = 3.91, p = 0.052, partial η2 = 0.053), demonstrating that following MOD participants exhibited higher accuracy during cognitive compared to emotional conflict (t34 = 2.19, p < 0.05, d = 0.369), which was not observed in the PLC group. No other main effects or interaction effects reached significance (all ps > 0.05).
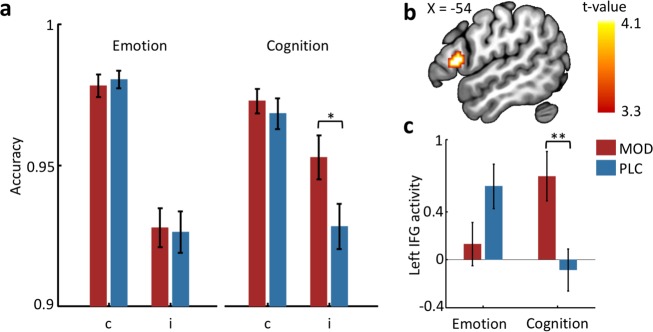
Conflict-dependent treatment effects were further disentangled by separate treatment × congruence ANOVAs for the emotional and cognitive domains. Main effects of congruence were significant for both, emotional and cognitive conflict (ps < 0.001). In the emotional domain, no significant effects involving treatment were observed (main effect, F(1, 70) = 0.001, p = 0.970; interaction effect with congruence, F (1, 70) = 0.17, p = 0.678), whereas the treatment main (F(1, 70) = 3.42, p = 0.069, partial η2 = 0.047) and interaction effect with congruence (F (1, 70) = 4.12, p < 0.05, partial η2 = 0.056) reached (marginal) significance in the cognitive domain. Post-hoc analysis revealed that MOD specifically improved accuracy during cognitive conflict (nonemotional incongruent trials) compared to PLC (t70 = 2.20, p < 0.05, d = 0.519, Fig. 2a).
Fig. 2. MOD selectively improved cognitive conflict processing and associated IFG activation.
a MOD selectively enhanced behavioral performance towards cognitive incongruent distractors, b which was concomitantly observed in the increased IFG activation and c specifically in the cognitive but not emotional conflict domain. *p < 0.05; **p < 0.01. MOD modafinil, PLC placebo, c congruent, i incongruent, IFG inferior frontal gyrus.
Examining response times revealed significant main effects of congruence (F(1, 70) = 323.43, p < 0.001, partial η2 = 0.822) and task (F(1, 70) = 25.51, p < 0.001, partial η2 = 0.267), demonstrating faster responses in the congruent condition (t71 = 18.08, p < 0.001, d = 2.131) and the cognitive task (t71 = 4.95, p < 0.001, d = 0.584). No significant treatment main or interaction effects were observed (ps > 0.05; Supplementary Table S1).
Effects of MOD on brain activation during conflict processing
The main effects of congruence, task and treatment were examined via whole-brain analyses (Supplementary Materials, Supplementary Tables 2–4). Notably, exploring conflict-dependent neural activation patterns independent of treatment revealed that the IFG was recruited during both emotional and cognitive conflict, whereas the rACC was specifically engaged during emotional but not cognitive conflict (all pFWE-cluster < 0.05) confirming previous studies suggesting that the lateral PFC, specifically IFG represents a conflict-general region [15] and that the conflict-specific rACC specifically contributes to emotional conflict [2, 17] (Supplementary Materials).
The regional-specific a priori hypotheses were evaluated using a treatment × congruence × task mixed-ANOVA, which revealed a significant three-way interaction effect located in the left IFG (Fig. 2b; peak MNI coordinates: [−57, 18, 9], k = 42, t70 = 4.08, pSVC-FWEpeak < 0.05). Consistent with the behavioral findings, extraction of parameter estimates from a 6-mm-radius sphere centered at the peak coordinate further revealed that MOD specifically increased left IFG activity (i > c) during cognitive (t70 = 2.91, p < 0.01, d = 0.686), but not emotional conflict (p > 0.05; Fig. 2c) relative to PLC. Probabilistic mapping demonstrated that the cluster was located in the pars opercularis of the IFG (Brodmann area 44, 73.1% probability).
Examining effects on rACC activation revealed no significant results (even at a liberal puncorrected < 0.001, or exploratory parameter estimate extraction from the rACC), confirming the absence of MOD effects on emotional conflict processing and associated rACC activation.
Effects of MOD on conflict-dependent functional connectivity
During cognitive conflict (i > c), MOD increased right IFG coupling strength with the bilateral dmPFC relative to PLC (Fig. 3; peak MNI coordinates: [6, 24, 51], k = 86, t70 = 4.10, pSVC-FWEpeak < 0.05). No treatment effects were observed during emotional conflict or for left IFG or rACC seeds.
Fig. 3. The contribution of MOD treatment to facilitating functional coupling during cognitive conflict.
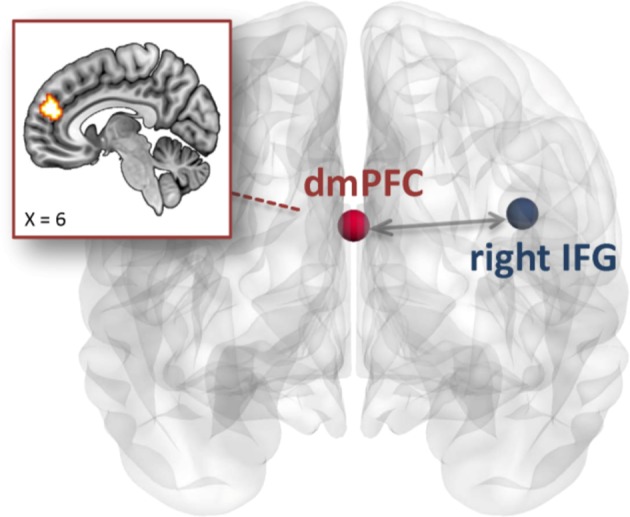
MOD treatment specifically enhanced right IFG connectivity strength with the bilateral dmPFC compared to PLC during cognitive but not emotional conflict. MOD modafinil, PLC placebo, IFG inferior frontal gyrus, dmPFC dorsomedial prefrontal cortex.
Exploratory analysis within treatment groups
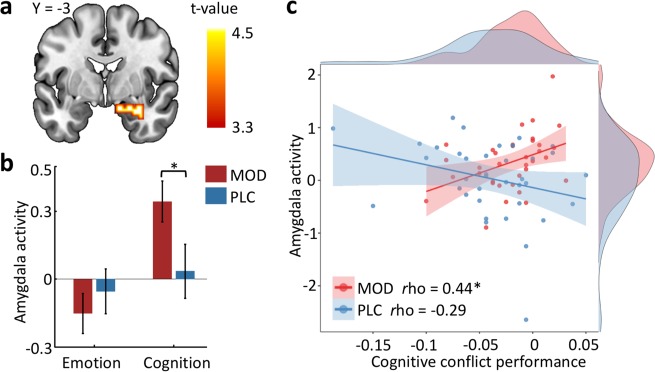
An additional exploratory analysis examined differences in brain activation between emotional and cognitive conflict processing in the separate groups (contrast [cognitivei-c > emotionali-c], whole-brain analysis, pFWE-cluster < 0.05). Results revealed no significant neural differences following PLC, whereas increased activation during cognitive relative to emotional conflict was observed in a broad limbic network, including the fusiform gyrus and amygdala and posterior frontal and parietal networks, following MOD (pFWE-cluster < 0.05; Supplementary Table S5). Given previous inconsistent findings on the involvement of the amygdala in both, conflict processing [2, 16–18] as well as MOD effects [38, 39], we specifically followed up the amygdala finding. The amygdala activation mapped specifically on the basolateral subregion (Fig. 4a; peak MNI coordinates: [33, −3, −18], k = 116, t34 = 4.42, pFWE-cluster < 0.05), and extraction of parameter estimates from a 6-mm-radius amygdala sphere centered at the corresponding cluster confirmed that MOD enhanced amygdala reactivity to cognitive (t70 = 2.03, p < 0.05, d = 0.479) yet not emotional conflict (p > 0.05; Fig. 4b). Excluding one participant following PLC with relatively low amygdala responses (lower than 3 SD from the mean value) indicated stable MOD enhancing effects on cognitive conflict (t69 = 1.76, pone-sided < 0.05, d = 0.417), arguing against eliminated amygdala activity in the PLC group being driven by outliers. In subsequent analyses we explored brain−behavior associations between cognitive conflict accuracy and corresponding neural activity using Shapiro−Wilk tests (due to non-normal distribution). Following MOD accuracy was positively associated with right amygdala reactivity during cognitive conflict (rho = 0.44, p < 0.01), whereas following PLC no association was observed (rho = −0.29, p = 0.078; significant correlation differences between groups, Fisher’s z = 2.21, p < 0.05; Fig. 4c). The correlation between conflict performance and amygdala activity in PLC was not significant even after excluding one PLC-treated participant with low amygdala reactivity (cognition: rho = −0.26, p = 0.122; emotion: rho = −0.29, p = 0.09). Further analysis revealed no significant associations during emotional conflict or with IFG activation.
Fig. 4. Exploratory analysis of MOD effects on amygdala reactivity and the association with cognitive conflict performance.
a Examining distinct conflict processes within separate groups revealed increased right amygdala activation following MOD treatment during cognitive relative to emotional conflict, and b enhancing effects were observed during cognitive but not emotional conflict. c In the MOD group accuracy was positively correlated with right amygdala activity during cognitive but not emotional conflict processing, whereas no association was observed in the PLC group. The filled curves show standard errors for each treatment group and the distribution curves in marginals indicate statistical densities of cognitive conflict performance and amygdala activity respectively. *p < 0.05. MOD modafinil, PLC placebo, IFG inferior frontal gyrus.
Effects of MOD on metacognition
Examining differences between the treatment groups revealed no significant MOD effects on confidence reports (meta-d′) or response bias (c) (ps > 0.05), whereas MOD enhanced discrimination sensitivity (d′; t70 = 2.23, p < 0.05, d = 0.527; Supplementary Fig. S2) reflecting that MOD specifically facilitated cognitive performance rather than subjective perception of performance. No treatment effects were observed on reaction times for dot discrimination or confidence ratings (ps > 0.05).
Discussion
The present study aimed at determining differential effects of MOD on cognitive and emotional conflict processing while strictly controlling for effects on cardiovascular indices, affective state, experienced task enjoyment and subjective performance perception. In nonsleep-deprived subjects, MOD selectively enhanced accuracy in the context of cognitive but not emotional distractors. In line with the behavioral findings, MOD specifically enhanced IFG activation and its functional communication with the dmPFC during cognitive conflict, while emotional conflict and corresponding rACC activation remained unaffected. Further exploratory analysis provided evidence for MOD-enhanced amygdala reactivity in response to cognitive conflict, with stronger MOD-induced amygdala activation being associated with higher accuracy during cognitive conflict. Examining subjective ratings and metacognitive performance revealed no significant treatment effects on affective states, perceived task performance or metacognitive confidence while MOD improved discrimination sensitivity further emphasizing its selective effect on cognitive performance.
Whereas accumulating evidence suggests that MOD can enhance cognitive performance particularly in the domain of PFC-dependent executive functions, including working memory [9, 48], cognitive flexibility [24, 32] and inhibitory control [32, 44], effects on cognitive conflict processing assessed by the classical color-word Stroop paradigm remained inconsistent [31, 34]. The sensitivity of the classical Stroop paradigm for evaluating pharmacological neuroenhancers is limited due to low error rates in healthy individuals [16] and lack of concomitant assessment of emotional distractors [56]. Previous studies propose that MOD selectively facilitates executive control at the most challenging task levels [9, 24] and complex task demands [31], suggesting that the present affective Stroop paradigm may have more efficiently determined MOD effects on conflict processing with a higher sensitivity. Despite the generally lower accuracy for emotional relative to cognitive distractors, no MOD effects on emotional conflict were observed, indicating that MOD specifically enhanced cognitive conflict performance.
On neural level the present paradigm engaged conflict-general and -specific networks, confirming the role of IFG as a conflict-general region which is recruited during both cognitive and emotional conflict [15] and the specific engagement of the rACC in emotional conflict [2, 17]. In accordance with conflict-dependent contribution of the PFC and our a priori hypothesis, enhanced cognitive conflict performance following MOD was accompanied by increased IFG activation, particularly in the pars opercularis (Brodmann area 44), a subregion of the IFG strongly engaged in cognitive control, particularly inhibitory motor control [57, 58]. Previous pharmacological fMRI studies reported that enhancement of inhibitory control following single-dose administration of MOD was accompanied by increased neural activation in the PFC [39, 59]. Successful cognitive control in the domain of inhibitory motor control specifically relies on noradrenaline—rather than serotonin [60] or dopamine [61]—signaling. Improved response inhibition following the noradrenaline reuptake inhibitor atomoxetine has been observed with concomitantly enhanced IFG activation [21], which together suggests that noradrenergic effects of MOD may explain the beneficial effects of MOD on cognitive conflict. In accordance with the domain-specific enhancement of cognitive conflict performance, no MOD effects on the rACC, a region strongly involved in emotional conflict regulation, were observed.
Further exploratory analyses revealed that the beneficial effect of MOD on cognitive conflict processing was additionally accompanied by increased right IFG-dmPFC coupling and right amygdala activation. The right IFG plays a critical role in the implementation of cognitive control, including inhibiting prepotent responses [14, 15], and facilitates error detection, performance monitoring and encoding task-oriented goals during response preparation [62, 63]. In a rodent model selectively inactivating the dmPFC led to specific deficits in inhibiting incorrect responses [64]. Moreover, both the IFG and dmPFC as well as their functional interaction contribute to domain-general cognitive conflict processing [19, 65], suggesting that the cognitive-conflict-enhancing effects of MOD are moderated by region-specific effects on increased communication within the domain-general conflict network, specifically the right IFG and the dmPFC.
Exploratory whole-brain analyses further revealed that—relative to emotional conflict—the MOD-treated group exhibited enhanced amygdala reactivity to cognitive conflict, with a positive association between the amygdala activation and cognitive conflict accuracy being observed. The role of the amygdala in context-specific conflict processing remains a matter of debate, such that initial studies reported that activation of the amygdala signals the amount of emotional conflict [16], whereas subsequent studies suggest comparable engagement during cognitive conflict [2] or no robust engagement of the amygdala during emotional conflict [65]. Although the amygdala has been considered as emotional, specifically fear-specific region [66], accumulating evidence suggests a broader role of the amygdala in general arousal and salience signaling [67], attention orientation [68] and response monitoring [69]. Early animal models reported increased activation and serotonergic neurotransmission in the amygdala following MOD [30]. On the other hand, converging translational evidence suggests a stronger involvement of noradrenergic rather than serotonergic signaling in cognitive control [60]. The basolateral amygdala receives dense noradrenergic projections from the locus coeruleus [70] and these noradrenergic pathways play an important role in mediating the effects of arousal on cognition, including facilitation of inhibitory avoidance retention [71]. Moreover, the basolateral amygdala shows a high sensitivity and partly opposing effects to pharmacological modulation of noradrenergic β- and α-receptors [72]. A previous study suggested that MOD-enhanced arousal and neural activity was attenuated by the β-adrenergic antagonist propranolol [22], which may together point to a β-adrenergic-mediated mechanism of MOD on amygdala activation during cognitive conflict. In contrast to the increased conflict-dependent amygdala reactivity, previous studies indicated either enhanced or attenuated threat-related amygdala activity following high (600 mg) or repeated (100 mg/7 days) MOD [38, 39]. Most previous studies examined MOD effects on cognitive performance employing single 200 mg dosages [24, 32] and did not observe robust effects on subjective emotional processing, suggesting that the divergent effects may—at least partly—be explained by different dosage schedules.
The present findings confirm the potential contribution of a single dose of 200 mg MOD to enhance executive control in healthy, nonsleep-deprived individuals [24]. Importantly, the effects were selectively observed in the cognitive but not emotional conflict domain and in the absence of impact on subjective performance perception and affective states. Initial studies have demonstrated cognitive enhancing effects of MOD in remitted depression [9], schizophrenia [30] and addiction [33]. These disorders are characterized by residual cognitive impairments even after affective recovery [73]. The present findings did not reveal robust effects of MOD on affective processing or emotional conflict-related rACC activation, a region critical for emotion regulation [74] and reward learning [75], which suggests a promising cognitive enhancer candidate (MOD) for facilitating cognitive conflict processing in clinical populations.
Despite these promising findings the present study needs to be interpreted with some limitations, such that only young male participants were examined. To this end subsequent studies need to determine (1) generalization of MOD effects to female participants and across age ranges, (2) the potential of MOD in patients with cognitive control deficits or populations with age-related decline in cognitive conflict processing, and (3) compare MOD effects with other pharmacological modulators to determine emotional conflict-specific targets. Finally, the marginal significant behavioral and exploratory neural effects of MOD need to be interpreted with corresponding caution.
Funding and disclosure
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0701400), National Natural Science Foundation of China (NSFC, No 91632117, 31530032); Fundamental Research Funds for Central Universities (ZYGX2015Z002), Science, Innovation and Technology Department of the Sichuan Province (2018JY0001). The authors declare no competing interests.
Supplementary information
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-0625-z).
References
- 1.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Rev. 2001;108:624. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 2.Egner T. Multiple conflict-driven control mechanisms in the human brain. Trends Cogn Sci. 2008;12:374–80. doi: 10.1016/j.tics.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 3.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark L, Blackwell AD, Aron AR, Turner DC, Dowson J, Robbins TW, et al. Association between response inhibition and working memory in adult ADHD: a link to right frontal cortex pathology? Biol Psychiatry. 2007;61:1395–401. doi: 10.1016/j.biopsych.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 6.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–94. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Rock P, Roiser J, Riedel W, Blackwell A. Cognitive impairment in depression: a systematic review and meta-analysis. Psychological Med. 2014;44:2029–40. doi: 10.1017/S0033291713002535. [DOI] [PubMed] [Google Scholar]
- 8.Schumann G, Binder EB, Holte A, de Kloet ER, Oedegaard KJ, Robbins TW, et al. Stratified medicine for mental disorders. Eur Neuropsychopharmacol. 2014;24:5–50. doi: 10.1016/j.euroneuro.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Kaser M, Deakin JB, Michael A, Zapata C, Bansal R, Ryan D, et al. Modafinil improves episodic memory and working memory cognition in patients with remitted depression: a double-blind, randomized, placebo-controlled study. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2017;2:115–22. doi: 10.1016/j.bpsc.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bora E, Harrison BJ, Yücel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychological Med. 2013;43:2017–26. doi: 10.1017/S0033291712002085. [DOI] [PubMed] [Google Scholar]
- 11.Hasselbalch BJ, Knorr U, Kessing LV. Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord. 2011;134:20–31. doi: 10.1016/j.jad.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn, Affect, Behav Neurosci. 2012;12:241–68. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahakian BJ, Morein-Zamir S. Pharmacological cognitive enhancement: treatment of neuropsychiatric disorders and lifestyle use by healthy people. Lancet Psychiatry. 2015;2:357–62. doi: 10.1016/S2215-0366(15)00004-8. [DOI] [PubMed] [Google Scholar]
- 14.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 17.Egner T, Etkin A, Gale S, Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb Cortex. 2007;18:1475–84. doi: 10.1093/cercor/bhm179. [DOI] [PubMed] [Google Scholar]
- 18.Feng C, Becker B, Huang W, Wu X, Eickhoff SB, Chen T. Neural substrates of the emotion-word and emotional counting Stroop tasks in healthy and clinical populations: a meta-analysis of functional brain imaging studies. NeuroImage. 2018;173:258–74. doi: 10.1016/j.neuroimage.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Cai W, Ryali S, Chen T, Li C-SR, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. J Neurosci. 2014;34:14652–67. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bos DJ, Oranje B, Achterberg M, Vlaskamp C, Ambrosino S, de Reus MA, et al. Structural and functional connectivity in children and adolescents with and without attention deficit/hyperactivity disorder. J Child Psychol Psychiatry. 2017;58:810–8. doi: 10.1111/jcpp.12712. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–5. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 23.Deroche-Gamonet V, Darnaudery M, Bruins-Slot L, Piat F, Le Moal M, Piazza P. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–95. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- 24.Müller U, Rowe J, Rittman T, Lewis C, Robbins T, Sahakian B. Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology. 2013;64:490–5. doi: 10.1016/j.neuropharm.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolder PC, Müller F, Schmid Y, Borgwardt SJ, Liechti ME. Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects. Psychopharmacology. 2018;235:467–79. doi: 10.1007/s00213-017-4650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TL, Tian YH, You IJ, Lee SY, Jang CG. Modafinil‐induced conditioned place preference via dopaminergic system in mice. Synapse. 2011;65:733–41. doi: 10.1002/syn.20892. [DOI] [PubMed] [Google Scholar]
- 27.de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–7. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- 28.Dawson N, Thompson RJ, McVie A, Thomson DM, Morris BJ, Pratt JA. Modafinil reverses phencyclidine-induced deficits in cognitive flexibility, cerebral metabolism, and functional brain connectivity. Schizophrenia Bull. 2010;38:457–74. doi: 10.1093/schbul/sbq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraro L, Fuxe K, Agnati L, Tanganelli S, Tomasini MC, Antonelli T. Modafinil enhances the increase of extracellular serotonin levels induced by the antidepressant drugs fluoxetine and imipramine: a dual probe microdialysis study in awake rat. Synapse. 2005;55:230–41. doi: 10.1002/syn.20111. [DOI] [PubMed] [Google Scholar]
- 30.Scoriels L, Jones PB, Sahakian B. Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacology. 2013;64:168–84. doi: 10.1016/j.neuropharm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Battleday RM, Brem A-K. Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol. 2015;25:1865–81. doi: 10.1016/j.euroneuro.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology. 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 33.Schmaal L, Goudriaan AE, Joos L, Krüse AM, Dom G, van den Brink W, et al. Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol Psychiatry. 2013;73:789–95. doi: 10.1016/j.biopsych.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Franke AG, Gränsmark P, Agricola A, Schühle K, Rommel T, Sebastian A, et al. Methylphenidate, modafinil, and caffeine for cognitive enhancement in chess: a double-blind, randomised controlled trial. Eur Neuropsychopharmacol. 2017;27:248–60. doi: 10.1016/j.euroneuro.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Rattray B, Martin K, Hewitt A, Cooper G, McDonald W. Effect of acute modafinil ingestion on cognitive and physical performance following mental exertion. Hum Psychopharmacol. 2019;34:1–9. doi: 10.1002/hup.2700. [DOI] [PubMed] [Google Scholar]
- 36.Randall DC, Viswanath A, Bharania P, Elsabagh SM, Hartley DE, Shneerson JM, et al. Does modafinil enhance cognitive performance in young volunteers who are not sleep-deprived? J Clin Psychopharmacol. 2005;25:175–9. doi: 10.1097/01.jcp.0000155816.21467.25. [DOI] [PubMed] [Google Scholar]
- 37.Taneja I, Haman K, Shelton RC, Robertson D. A randomized, double-blind, crossover trial of modafinil on mood. J Clin Psychopharmacol. 2007;27:76–8. doi: 10.1097/jcp.0b013e31802eb7ea. [DOI] [PubMed] [Google Scholar]
- 38.Rasetti R, Mattay VS, Stankevich B, Skjei K, Blasi G, Sambataro F, et al. Modulatory effects of modafinil on neural circuits regulating emotion and cognition. Neuropsychopharmacology. 2010;35:2101–9. doi: 10.1038/npp.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt A, Müller F, Dolder PC, Schmid Y, Zanchi D, Liechti ME, et al. Comparative effects of methylphenidate, modafinil, and MDMA on response inhibition neural networks in healthy subjects. Int J Neuropsychopharmacol. 2017;20:712–20. doi: 10.1093/ijnp/pyx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fournier JC, Chase HW, Greenberg T, Etkin A, Almeida JR, Stiffler R, et al. Neuroticism and individual differences in neural function in unmedicated major depression: findings from the EMBARC Study. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2017;2:138–48. doi: 10.1016/j.bpsc.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 42.Ilieva I, Boland J, Farah MJ. Objective and subjective cognitive enhancing effects of mixed amphetamine salts in healthy people. Neuropharmacology. 2013;64:496–505. doi: 10.1016/j.neuropharm.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 43.Allen M, Frank D, Schwarzkopf DS, Fardo F, Winston JS, Hauser TU, et al. Unexpected arousal modulates the influence of sensory noise on confidence. Elife. 2016;5:1–17. doi: 10.7554/eLife.18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rycroft N, Hutton S, Clowry O, Groomsbridge C, Sierakowski A, Rusted J. Non-cholinergic modulation of antisaccade performance: a modafinil-nicotine comparison. Psychopharmacology. 2007;195:245–53. doi: 10.1007/s00213-007-0885-x. [DOI] [PubMed] [Google Scholar]
- 45.Cservenka A, Stroup ML, Etkin A, Nagel BJ. The effects of age, sex, and hormones on emotional conflict-related brain response during adolescence. Brain Cognition. 2015;99:135–50. doi: 10.1016/j.bandc.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Z, Yao S, Li K, Sindermann C, Zhou F, Zhao W, et al. Real-time functional connectivity-informed neurofeedback of amygdala-frontal pathways reduces anxiety. Psychother Psychosom. 2019;88:5–15. doi: 10.1159/000496057. [DOI] [PubMed] [Google Scholar]
- 47.Wong YN, King SP, Simcoe D, Gorman S, Laughton W, McCormick GC, et al. Open‐label, single‐dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol. 1999;39:281–8. [PubMed] [Google Scholar]
- 48.Müller U, Steffenhagen N, Regenthal R, Bublak P. Effects of modafinil on working memory processes in humans. Psychopharmacology. 2004;177:161–9. doi: 10.1007/s00213-004-1926-3. [DOI] [PubMed] [Google Scholar]
- 49.Xu X, Li J, Chen Z, Kendrick KM, Becker B. Oxytocin reduces top-down control of attention by increasing bottom-up attention allocation to social but not non-social stimuli—a randomized controlled trial. Psychoneuroendocrinology. 2019;108:62–9. [DOI] [PubMed]
- 50.Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. Relating introspective accuracy to individual differences in brain structure. Science. 2010;329:1541–3. doi: 10.1126/science.1191883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cognition. 2012;21:422–30. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 52.Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain, Vol. 16, Abstract 497 (Sendai, Japan, 2002).
- 53.Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–26. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 55.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song S, Zilverstand A, Song H, Uquillas FdO, Wang Y, Xie C, et al. The influence of emotional interference on cognitive control: a meta-analysis of neuroimaging studies using the emotional Stroop task. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-02266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson TK, Lenartowicz A, Berkman ET, Ji D, Poldrack RA, Knowlton BJ. Putting the brakes on the brakes: negative emotion disrupts cognitive control network functioning and alters subsequent stopping ability. Exp Brain Res. 2016;234:3107–18. doi: 10.1007/s00221-016-4709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Hum Brain Mapp. 2009;30:2821–33. doi: 10.1002/hbm.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmaal L, Goudriaan A, Joos L, Dom G, Pattij T, van den Brink W, et al. Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychological Med. 2014;44:2787–98. doi: 10.1017/S0033291714000312. [DOI] [PubMed] [Google Scholar]
- 60.Chamberlain SR, Müller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–3. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bensmann W, Zink N, Arning L, Beste C, Stock A-K. The presynaptic regulation of dopamine and norepinephrine synthesis has dissociable effects on different kinds of cognitive conflicts. Mol Neurobiol. 2019:56:8087–100. [DOI] [PubMed]
- 62.Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson B. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. J Neurosci. 2011;31:16208–16. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taren AA, Venkatraman V, Huettel SA. A parallel functional topography between medial and lateral prefrontal cortex: evidence and implications for cognitive control. J Neurosci. 2011;31:5026–31. doi: 10.1523/JNEUROSCI.5762-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Wit S, Kosaki Y, Balleine BW, Dickinson A. Dorsomedial prefrontal cortex resolves response conflict in rats. J Neurosci. 2006;26:5224–9. doi: 10.1523/JNEUROSCI.5175-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen T, Becker B, Camilleri J, Wang L, Yu S, Eickhoff SB, et al. A domain-general brain network underlying emotional and cognitive interference processing: evidence from coordinate-based and functional connectivity meta-analyses. Brain Struct Funct. 2018;223:3813–40. doi: 10.1007/s00429-018-1727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becker B, Mihov Y, Scheele D, Kendrick KM, Feinstein JS, Matusch A, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72:70–7. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 67.LeDoux J. The amygdala. Curr Biol. 2007;17:868–74. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 69.Pagliaccio D, Pine DS, Leibenluft E, Monte OD, Averbeck BB, Costa VD. Cross-species convergence in pupillary response: understanding human anxiety via non-human primate amygdala lesion. Soc Cogn Affect Neurosci. 2019;14:591–9. doi: 10.1093/scan/nsz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic Study Brain Res. 1977;127:23–53. [PubMed] [Google Scholar]
- 71.Liang K, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–33. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 72.Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of α-2 and β receptor activation. J Neurosci. 2007;27:12358–66. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conradi H, Ormel J, De Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychological Med. 2011;41:1165–74. doi: 10.1017/S0033291710001911. [DOI] [PubMed] [Google Scholar]
- 74.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11:413–8. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Zhou F, Zimmermann K, Xin F, Scheele D, Dau W, Banger M, et al. Shifted balance of dorsal versus ventral striatal communication with frontal reward and regulatory regions in cannabis‐dependent males. Hum Brain Mapp. 2018;39:5062–73. doi: 10.1002/hbm.24345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.