Abstract
Intermittent fasting (IF) has been reported to have beneficial effects on improving gut function via lowering gut inflammation and altering the gut microbiome diversity. In this study, we aimed to investigate the differential effects of three different common IF treatments, alternate day fasting (ADF), time-restricted fasting (TRF), and intermittent energy restriction (IER), on a dextran sodium sulfate (DSS)-induced colitis mouse model. The results indicated that TRF and IER, but not ADF improved the survival rates of the colitis mice. TRF and IER, but not ADF, reversed the colitis pathological development by improving the gut barrier integrity and colon length. Importantly, TRF and IER suppressed the inflammatory responses and oxidative stress in colon tissues. Interestingly, TRF and IER also attenuated colitis-related anxiety-like and obsessive-compulsive disorder behavior and alleviated the neuroinflammation and oxidative stress. TRF and IER also altered the gut microbiota composition, including the decrease of the enrichments of colitis-related microbes such as Shigella and Escherichia Coli, and increase of the enrichments of anti-inflammatory-related microbes. TRF and IER also improved the short chain fatty acid formation in colitis mice. In conclusion, the TRF and IER but not ADF exhibited the protective effects against colitis and related behavioral disorders, which could be partly explained by improving the gut microbiome compositions and preventing gut leak, and consequently suppressing the inflammation and oxidative damages in both colon and brain. The current research indicates that proper IF regimens could be effective strategies for nutritional intervention for the prevention and treatment of colitis.
Keywords: Intermittent fasting, Colitis, Anxiety-like behavior, Gut microbes, Oxidative stress, Inflammation
Graphical abstract
Highlights
-
•
Intermittent fasting regimens TRF and IER but not ADF attenuate DSS-induced colitis.
-
•
TRF and IER protect the gut barrier and the mucosal layer integrity from colitis.
-
•
TRF and IER alleviate colitis-related anxiety and obsessive-compulsive behaviors.
-
•
TRF and IER suppress inflammation and oxidative stress in both gut and brain.
-
•
TRF and IER balance gut microbiota composition and metabolites production.
1. Introduction
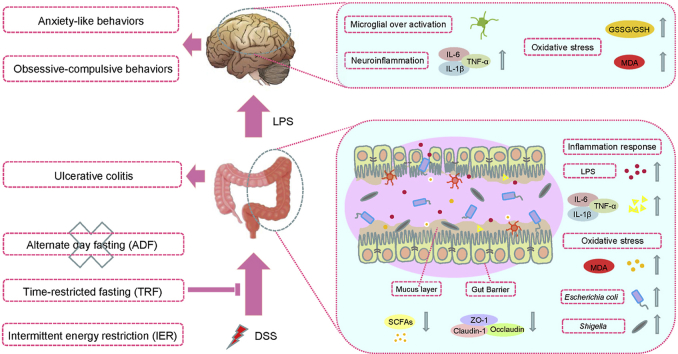
Inflammatory bowel disease (IBD), including Crohn's disease (CD) and Ulcerative colitis (UC), is a group of chronic inflammatory diseases of the gastrointestinal tract [1]. The prevalence of IBD has been continually increased in the last decades in global [2]. UC is characterized with mucosa damages and ulceration in the colon and rectum [3]. Moreover, recent research also showed that UC is a high risk for mental health problems, including depression and anxiety-like behaviors [4,5]. Oxidative stress is one of the triggering factors for UC development [6], which is highly associated with the inflammatory responses including over-release of cytokines in gut barriers [7]. However, the underlying mechanism of UC-induced behavioral disorders is not fully understood. One possible mechanism explained how colitis correlates with anxiety is that the inflammatory responses leads to gut barrier damage, charactering with damages of mucosal layer and loss of conjunctions protein such as Claudin-1 and ZO-1 [8]. Consequently the leaky gut leads to penetration of liposaccharide (LPS), a component of the outer membrane of gram-negative bacteria, to blood and further triggers neuro-inflammation and oxidative damages in the brain [9,10].
Although the etiopathogenesis of UC is still unclear, recent studies indicated that the balance of gut microbial environment plays an important role in regulating the inflammation and oxidative damages of colon [11]. It has been found that the composition of gut microbes and microbial metabolites are enormously altered in UC patients and colitis mouse models, including the increase of the enrichments of Shgella, and Escherichia Coli and the decrease of the enrichment of Lactobacillus [12,13]. It has been reported that the transplantation of microbiota from healthy donors could reversed the colitis symptoms [14]. Supplementation of Lactobacillus significantly alleviated the inflammatory responses in a murine colitis model [15].
Gut microbiota diversity is critical for linking the diet and host physiology and pathology, and are influenced by dietary composition and patterns [16]. Intermittent fasting (IF) are a group of periodic energy restriction dietary patterns, including alternate-day fasting (ADF), time-restricted fasting (TRF), and intermittent energy restriction (IER) [17,18]. Previous research has reported that ADF, TRF, and IER had beneficial regulatory effects on the compositions of gut microbes in various animal models and human trials [[19], [20], [21]]. Our recent research demonstrated that IF significantly improved the gut function in a diabetic mouse model by balancing gut microbes and enhancing formation of microbial metabolites [22]. The study also found that IF reversed the anxiety-like behaviors and cognitive function [22]. Recently, several studied reported that IF prompted recovery from colitis by decreasing inflammatory responses in animal models [23,24]. However, the differential efficacy of these IF regimens on chronic colitis is still unclear and the roles of gut microbiota involved need to be further investigated.
In this study, a dextran sodium sulfate (DSS)-induced chronic colitis mouse model was used to evaluate the different effects of the ADF, TRF, and IER regimens on the survival rate and colitis development. The behavioral tests were performed to investigate the beneficial effects of IF on colitis-related anxiety-like behaviors. The mucosal damages and conjunctions protein expressions were also determined to examine the gut barrier integrity. It has been found that the TRF and IER, but not ADF, improved the survival rates and alleviated colitis development. TRF and IER prevented DSS-induced behavioral disorders. The TRF and IER also suppressed the inflammatory responses and oxidative stress in both gut and brain. Importantly, the TRF and IER altered the gut microbiota diversity and microbial metabolites short chain fatty acids production in colitis mice. Based on these results we concluded the proper IF regimens for colitis prevention are the TRF and IER but not ADF.
2. Materials and methods
2.1. Animals, DSS-induced colitis model, and intermittent fasting
C57BL/6 mice (Male, aged 7–8 weeks) were purchased from Xi'an Jiaotong University (Xi'an, Shanxi, China). Dextran sodium sulfate (M/Wt 36,000–50,000; MP Biomedicals, Solon, OH, USA) was stored at room temperature and added to drinking water at a final concentration of 2%. All mice were housed in a temperature and humidity-controlled environment (25 ± 2 °C temperature, 50% ± 5% humidity) with a 12 h light/dark cycle. All mice were fed with a standard diet (AIN-93 M, purchased from TROPHIC Animal Feed High-tech Co., Ltd Nantong, China).
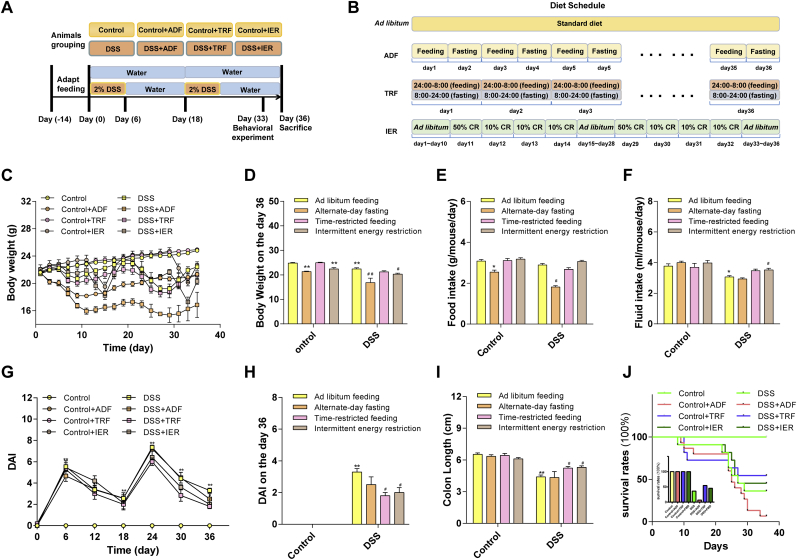
The grouping and schedule of the animal experiments were illustrated in Fig. 1A and B. One week of adaptive feeding before the start of the formal experiment. Then, the mice were randomly divided into 8 groups (n = 12/group) as follows: (1) The control group: water and food were provided ad libitum; (2) The control-ADF group: water and food were provided according to alternate-day fasting (ADF); (3) the control-TRF group: water and food were provided according to time-restricted feeding (TRF); (4) the control-IER group: water and food were provided according to intermittent energy fasting (IER); (5–8), in the DSS treatment groups (DSS, DSS-ADF, DSS-TRF and DSS-IER), DSS water was provided instead of normal water compared to control groups (Fig. 1A, S1A). In the control group and the control + IF (ADF, TRF, IER) groups, distilled water was provided ad libitum during the experiment. And the mice in DSS group and the DSS + IF (ADF, TRF, IER) group were received water with DSS (2% w/w) for 6 consecutive days, followed by 12 consecutive days of water. This cycle lasts twice in total. The mice in the ADF group were fed standard diet on feeding days, while food was removed on fasting days, in a 24 h feeding/fasting circle. The mice in the TRF group were fed for only 8 h per day, who were fed at 24:00 p.m. and started fasting at 8:00 a.m. in the next morning. The mice in the IER group were provided with two cycles of four days of IER diet from day 11–14 and day 29–32. Our experimental IER diet is based on previous research [25]. In brief, the mice in the control group consumed an average of 3.2 g/day. On the first day of IER, mice were provided with 50% of their normal calorie intake at 9:00 a.m. From the second through fourth days of IER, mice were provided with 10% of their normal calorie intake every day at 9:00 a.m. and non-fasted mice were given free access to standard feed every day. The litter of mice was disposed daily. Body weight, food intake (energy intake), and water intake were measured and recorded on a daily basis. The animal experiment protocol was approved by the Animal Ethics Committee of Xi'an Jiaotong University. The experimental procedures were performed under animal anesthesia to minimize pain. After behavioral tests, the mice were anesthetized with 3.5% Chloral hydrate i. p. injection before sacrificed.
Fig. 1.
Effects of ADF, TRF and IER on the survival rates and colitis development in DSS-treated mice. (A) Experimental schedule of animal treatments (n = 12 mice per group). (B) Timeline depicting the diet of ad libitum, ADF, TRF and IER. (C) Body weight and (D) Body weight of mice in each group on day 36. (E) Food intake of mice in each group. (F) Fluid intake of mice in each group. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group). (G) DAI values in 36-day treatment. (H) Disease activity index (DAI) score on the day 36. (I) Colon length in each group of the ADF, TRF and IER regimens. (J) Survival rate of mice in each group during the 36-day treatment. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
Considering the survival rates and animal numbers in DSS + ADF group were low at the end of the study, the behavioral tests and biochemical studies, except the gut microbes composition analysis, were only performed on the TRF and IER groups.
2.2. Behavioral tests
2.2.1. Marble burying test (MBT)
The inside of test cages of MBT was covered with 5 cm deep wood chip cushion, and 20 glass marbles with a diameter of 15 mm were arranged on the wood chip in a 4 × 5 design. The mice were placed in a test cage and record the number of marbles buried in the 30 min (two-thirds of the height).
2.2.2. Elevated plus maze (EPM)
The elevated plus maze is connected by two opposing open arms (30 cm × 8 cm) and two opposing closed arms (30 cm × 8 cm × 15 cm) to form a central area of 8 cm × 8 cm. Mice were placed in the center of the EPM, faced with an open arm, and each test last for 5 min. Within 5 min, the number of times each mouse entered the open and closed arms and the dwell time on each arm were recorded. We calculated the number of times the mouse entered the open arm and the ratio of the time spent on the open arm.
2.3. Assessment of disease activity index (DAI)
The temporary weight loss caused by fasting is due to a decrease in energy intake, which may interfere with the exact severity of the disease. Therefore, in this experiment, the sum of stool consistency and rectal bleeding was recorded as the total disease activity index (DAI) score [23]. The DAI score assesses the severity of the disease by recording the extent of weight loss, the consistency of the stool, and the amount of blood in the feces. In the two cycles of colitis induction, a DAI of all mice was recorded every 6 days. Stool consistency was determined as follows: score 0, solid granular feces; score 2, loose stools, as a sign of thickening; score 4, bloody diarrhea. The blood in the feces was evaluated using Fecal Occult Blood Reagent (Pyramidon semi-quantitative Method) and following the manufacturer's agreement. The score was determined as follows: Score 0, no signs of bleeding; Score 1, weak positive occult blood; Score 2, occult blood positive; Score 3, occult blood positive with visible bleeding; Score 4, occult blood positive with massive visible bleeding.
2.4. Sample collection and biochemical analysis
Orbital blood was drawn from the mice under anesthesia. Serum was obtained after centrifugation and stored at −80 °C for subsequent experiments. All mice were sacrificed by cervical dislocation before the brains were quickly removed. The hippocampus was separated and then stored in a 4% paraformaldehyde solution or −80 °C environment for further use. At the same time, the colon was removed and rinsed with saline. Small amount of tissue of the colon was used for section staining. Meanwhile, the remaining tissue was frozen at −80 °C for subsequent experiments. The ELISA kit was used to determine the level of lipopolysaccharide (LPS) in the colon and serum, and the expression levels of TNF-α and IL-1β cytokines in the colon (Xinle Biotechnology, Shanghai, China). MDA, GSH, and GSSG levels in the colon and cerebral cortex were determined using a commercial kit (Nanjing Jiancheng Technology Co., Ltd., Nanjing, China).
2.5. Hematoxylin and eosin (H&E) staining and assessment of histological injury
After the mice were sacrificed, the colon tissue was immersed in a 4% paraformaldehyde solution for fixation, then rinsed with PBS and dehydrated with a gradient of 75%, 80%, 95%, and 100% ethanol. Then, the tissue was embedded in paraffin after all ethanol was removed. Tissue sections were stained with H&E and observed with an optical microscope. Referring to previous reports, all tissue sections were scored based on cellular infiltration and epithelial damage of inflammatory cells [26].
2.6. Real-time polymerase chain reaction (RT-PCR)
The total RNA of brain and colon were extracted by TRIzol (Jingcai Biotechnology, Xi'an, China). The HiFiScript cDNA Synthesis Kit was then used to configure the RNA reverse transcription system. After reverse transcription, the samples were diluted 5-fold and then subjected to PCR. All PCR reactions were performed in a mixed system containing 2 μL of the sample, 1 μL of upstream primer, 1 μL of downstream primer, 6 μL of ddH2O, and 10 μL of the mixture. The primer sequences are shown in Table 1.
Table 1.
Primer Sequences Used for qRT-PCR Analysis.
| Forward Primer | Reverse Primer | |
|---|---|---|
| Occludin | ACGGACCCTGACCACTATGA | TCAGCAGCAGCCATGTACTC |
| Claudin-1 | AGCTGCCTGTTCCATGTACT | CTCCCATTTGTCTGCTGCTC |
| ZO-1 | ACCCGAAACTGATGCTGTGGATAG | AAATGGCCGGGCAGAACTTGTGTA |
| TNF-α | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| IL-6 | CTCTGGCGGAGCTATTGAGA | AAGTCTCCTGCGTGGAGAAA |
| IFN-γ | AGCTCTTCCTCATGGCTGTT | GGTCAACCAACCACAAGCAT |
| IL-1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| MUC-2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG |
| Gapdh | TGGAGAAACCTGCCAAGTATGA | TGGAAGAATGGGAGTTGCTGT |
2.7. Immunohistochemistry
The tissue sections were prepared, dewaxed with xylene, and then hydrated in a graded alcohol series. After 15 min of infiltration with 0.5% Triton-X 100, antigen retrieval was performed in citrate buffer. The slices were then treated with 3% H2O2 for 15 min to eliminate the endogenous peroxidase. To prevent non-specific staining, goat serum was added to the sections for blocking. Then the mixture was incubated for 10 min before antibodies were added. After washed with PBS, they were treated with 3, 3′-diaminobenzidine tetrahydrochloride (DAB) for 15 min. The stained tissue was observed with an optical microscope (Olympus, Tokyo, Japan).
2.8. Transmission electron microscopy (TEM)
Colonic tissues of mice were collected, fixed with 2.5% glutaraldehyde (v/v) for more than 6 h, and then rinsed with 0.1 M/L phosphate buffer (PBS) at pH of 7.2. Next, the tissue was fixed in 1% osmium tetroxide for 1 h, then dehydrated with 30%, 50%, 70%, 80%, 90%, 100% ethanol (15 min/time). Finally, the LR-WHITE was embedded and dried in an oven at 60 °C. A transmission electron microscope (H-7650, Hitachi, Japan) was used to observe the samples stained with uranyl acetate and lead citrate.
2.9. Alcian blue staining
Alcian blue is a fluoride salt with a positive charge and forms an insoluble complex with anionic groups (acidic mucus substances with carboxyl and sulfate groups) contained in the tissue. Therefore, Alcian blue staining has been widely used to visualize mucin-producing goblet cells and mucus layers [27]. Colonic tissue containing feces was fixed in a methanol-Carnot solution (60% methanol, 30% chloroform, 10% glacial acetic acid) for 24 h. The tissue was then embedded vertically in paraffin and cut into 5 μm sections. Sections were stained with Alcian-Blue/Nuclear-Fast-Red. The colon tissue sections were observed at 200X magnification. The Image J software (National Institutes of Health, Bethesda, MD, USA) can be used for further quantitative analysis of mucin-producing goblet cells.
2.10. 16S rRNA sequencing analysis
After the mice were sacrificed, the colon contents were quickly collected in a sterile environment. Sample DNA was extracted using the EZNA Stool DNA Kit (Omega, Norcross, GA, USA). The V3–V4 region of the 16S rDNA gene was then amplified by PCR and quantified on a QuantiFluor-ST fluorometer (Promega, USA) using primer: 341_F: 5′-CCTACGGGNGGCWGCAG-3′ and 802_R: 5′ TACNVGGGTATCTAATCC-3′. Samples were sequenced on the Illumina MiSeq platform. According to previously published methods for the library preparation. For microbiome analysis, the raw reads were merged and trimmed, then chimeras were removed and zero-radius Opera Tional Taxonomic Units (zOTU) were constructed by implementing UNOISE in Vsearch (v2.6.0). The green genes (13.8) 16S rRNA gene database was used as a reference for annotation. A Venn diagram and heatmap were generated using the R package vegan. PLS-DA analysis via R (v3.2.1) mixOmics package for analysis of differences between groups. The linear discriminant analysis effect size (LEfSe) was used to analyze biomarkers with LDA scores higher than 2. The Wilcox test was conducted to screen for differential flora, and the alpha value of the Wilcoxon test was set to 0.1.
2.11. Content detection of SCFAs
Mouse feces (0.2 g) were placed in a 2 mL centrifuge tube before 1 mL of ultrapure water was added, and then it was sealed with paraffin parafilm before vortexed for 10 min H2SO4 solution (0.15 mL, 50%) and 1.6 mL of ether were added into the tube, which was shaken at 4 °C for 20 min and then centrifuged at 12,000 rpm for 10 min 1 mL of the upper ether clear solution was added into a concentrating tube and gently blew with a nitrogen blower to concentrate the liquid layer volume to 0.2 mL. Finally, the liquid was aspirated with a disposable syringe and placed it in the sample vial to be tested. The concentration of SCFA (acetic acid, propionic acid, isobutyric acid, butyric acid, valeric acid, and isovaleric acid) in the samples was measured by GC (Shimadzu Corporation, Kyoto, Japan).
2.12. Statistical analysis
The sequencing data of 16S rDNA was analyzed by Wilcoxon rank sum test to assess significant differences between different groups. All biochemical parameters and RT-PCR data were analyzed using GraphPad 7.0 (GraphPad Software Inc, San Diego, CA, USA). Significant differences between the means were determined by two-way ANOVA followed by Newman-Keuls multiple comparisons test. Data are reported as mean ± SEM, and difference at p < 0.05 is considered as a statistical significance.
3. Results
3.1. Effects of IF on the survival rates and colitis development in the DSS-treated mice
The experiments were designed as described in Materials and methods section (Fig. 1A and B). The ADF and IER, but not the TRF, decreased the bodyweight of the control mice after the treatment (p < 0.01). DSS treatment also significantly reduced the bodyweight (p < 0.01). The ADF, but not the TRF and IER treatment substantially reduced the bodyweight in colitis mice (p < 0.01) (Fig. 1C and D). As for the food intake, DSS treatment did affect the energy intake, but the ADF treatment significantly decreased the energy intake in both control and DSS-treated mice (p < 0.05) (Fig. 1E and Fig. S1A). The IER, but not the ADF and TRF elevated the decreased water intake in the DSS-treated mice (p < 0.05) (Fig. 1F). Moreover, the DSS treatment significantly elevated the DAI values (p < 0.01) (Fig. 1G and H) and shortened the colon length (p < 0.01) compared with the control group (Fig. 1I and Fig. S1B). However, the TRF and IER, but not the ADF significantly improved the colon length and reduced the DAI values in DSS-treated mice (p < 0.05) (Fig. 1G–I and Fig. S1B).
The survival rates of the control with different IF regimens were 100%. However, DSS significantly decreased the survival rate after the first and second treatment cycles (Fig. 1J). On the day 36, at the end of the treatment, the survival rate of the DSS group was 36.4%. The ADF treatment lowered the survival rate to 6.7%, but the IER and TRF improved the survival rate to 45.5% and 54.5%, respectively (Fig. 1J).
These results indicated that the ADF treatment had no beneficial effects on the colon damages and even lower the survival rate of the colitis mice. However, the TRF and IER treatment are effective interventions on DSS-induced colitis of the mice. Therefore, we focus on the preventive effects of the TRF and IER in the following study.
3.2. Effects of IF on the histopathological changes in the colon of the DSS-induced colitis mice
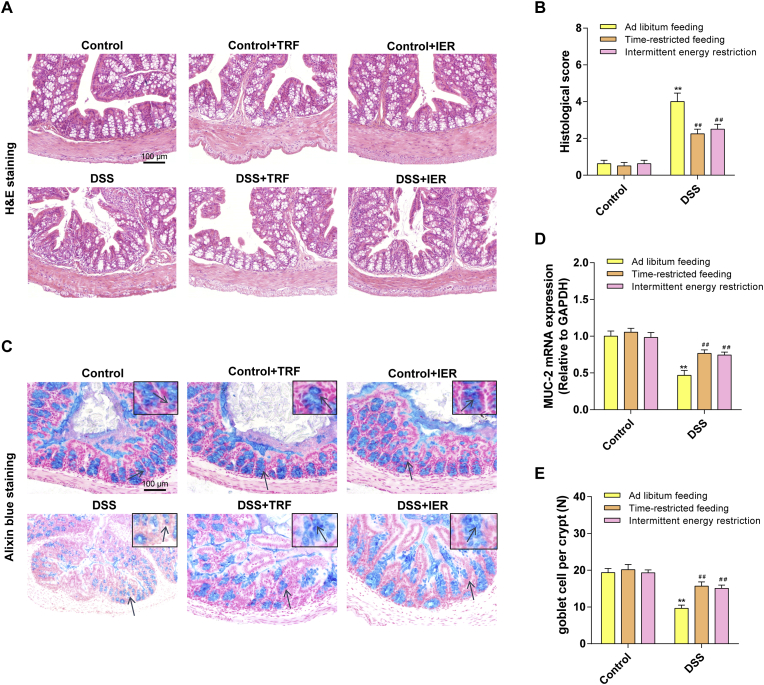
As shown in Fig. 2A and B and Figs. S1C and D, the DSS treatment triggered severe ulceration in the colon with damaged crypts and infiltrated neutrophils and monocytes, which were typical colitis symptoms (p < 0.01). Consistent with DAI examination, the TRF and IER regimens, but not the ADF, significantly reversed the colitis development in the DSS-treated mice (p < 0.01). Moreover, the Alcian blue staining were employed to detect the mucosa status and the number of goblet cells (Fig. 2C). The TRF and IER significantly suppressed the DSS-induced mucosal damage, improved the down-regulated MUC-2 mRNA expression (p < 0.01), and attenuated the loss of goblet cells (p < 0.01) (Fig. 2D and E). These data indicate that the TRF and IER treatment protected the mucosal layer in the DSS-treated mice.
Fig. 2.
Effects of ADF, TRF and IER on DSS-induced histopathological changes in DSS-induced colitis mice. (A) Representative images of H&E staining of colon for each group of the TRF and IER regimens. (B) Histological score based on H&E-stained sections per mouse (n = 6). (C) Representative images of Alcian blue staining of colon for each group of the TRF and IER regimens. (D) The mRNA expression of MUC-2 in colon for each group of the TRF and IER regimens. (E) Quantification of goblet cells based on Alcian blue -stained sections by ImageJ software. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Effects of TRF and IER on the gut barrier integrity loss, inflammatory response, and oxidative stress in the colitis mice
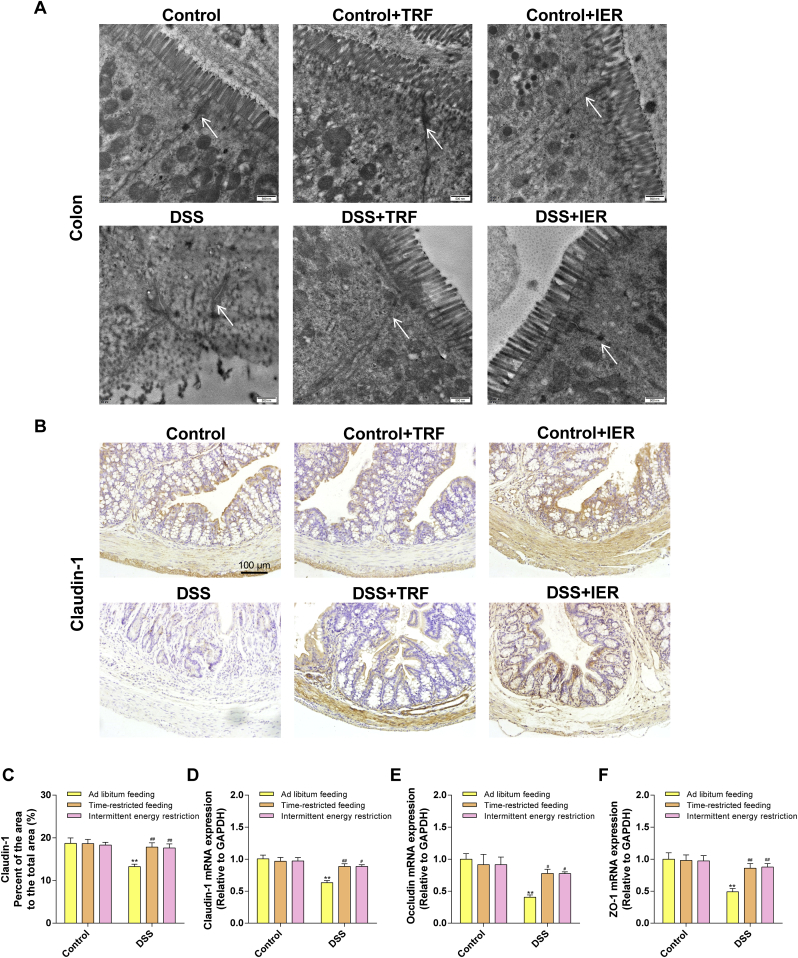
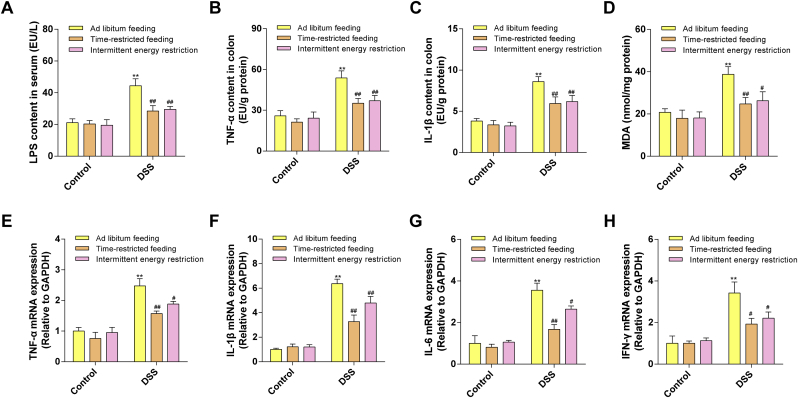
To determine the gut barrier integrity, we detected the ultra-structure of the colon tissue and the expressions of tight junction proteins. As shown in Fig. 3A, the transmission electron microscopy images demonstrated that DSS treatment significantly damaged the tight junction structures of the colon, compared with the control mice. However, the TRF and IER treatment protected the ultra-structure of the tight junction. Consistently, immunohistochemical staining also indicated that the TRF and IER treatment significantly improved the expression of Claudin-1, a tight junction protein [28], in the colon tissue of the colitis mice (Fig. 3B and C). The mRNA expressions of tight junction proteins including Claudin-1, Occludin, and ZO-1, were significantly improved in TRF- and IER-treated colitis mice (p < 0.01) (Fig. 3D–F). Moreover, the serum level of LPS, a composition of the outer membrane of gram-negative bacteria, was significantly increased in the DSS-treated mice compared with the control group (p < 0.01). However, TRF and IER significantly suppressed the penetration of LPS into the serum from gut (p < 0.01), which also indicated that the integrity of the colon tissue was protected by these two IF regimens from the DSS-induced damages (p < 0.01) (Fig. 4A).
Fig. 3.
Effects of TRF and IER on the gut barrier integrity DSS-induced colitis mice. (A) Representative images of transmission electron microscopy of colonic ultrastructure in epithelial cells for each group of the TRF and IER regimens. (B) Representative images of immunohistochemistry of Claudin-1 in colon for each group of the TRF and IER regimens. (C) Quantitative immunohistochemical analysis of claudin-1 protein in colon: the positive region was identified by ImageJ software and the area ratio with the colon wall region was calculated. (D–F) The mRNA expression of Claudin-1, ZO-1, Occludin in colon for each group of the TRF and IER regimens. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
Fig. 4.
Effects of TRF and IER on inflammatory responses and oxidative stress in the colon of DSS-induced colitis mice. (A) LPS levels in serum. (B–C) Protein levels of inflammatory cytokines TNF-α and IL-6 in mice colon. (D) MDA level in the colon. (E–H) The mRNA expression of TNF-α, IL-1β, IL-6, IFN-γ in colon for each group of the TRF and IER regimens. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
The inflammatory responses in the colon tissue were also evaluated. The TRF and IER treatment significantly down-regulated the DSS-increased levels of TNF-α and IL-1β (p < 0.01) (Fig. 4B and C), in line with the preventive effects on the elevated mRNA levels of cytokines including TNF-α, IL-1β, IL-6, and IFN-γ, in the colon tissues when compared with control group (Fig. 4E–H). The level of MDA in colon tissues, as a biomarker of oxidative stress, has also been detected [29]. The DSS treatment significantly enhanced the MDA levels in the colon tissues compared with control mice (p < 0.01). However, the TRF (p < 0.01) and IER (p < 0.05) treatment significantly suppressed the MDA levels in the colon of colitis mice (Fig. 4D).
3.4. Effects of TRF and IER on the behavioral disorders in the colitis mice
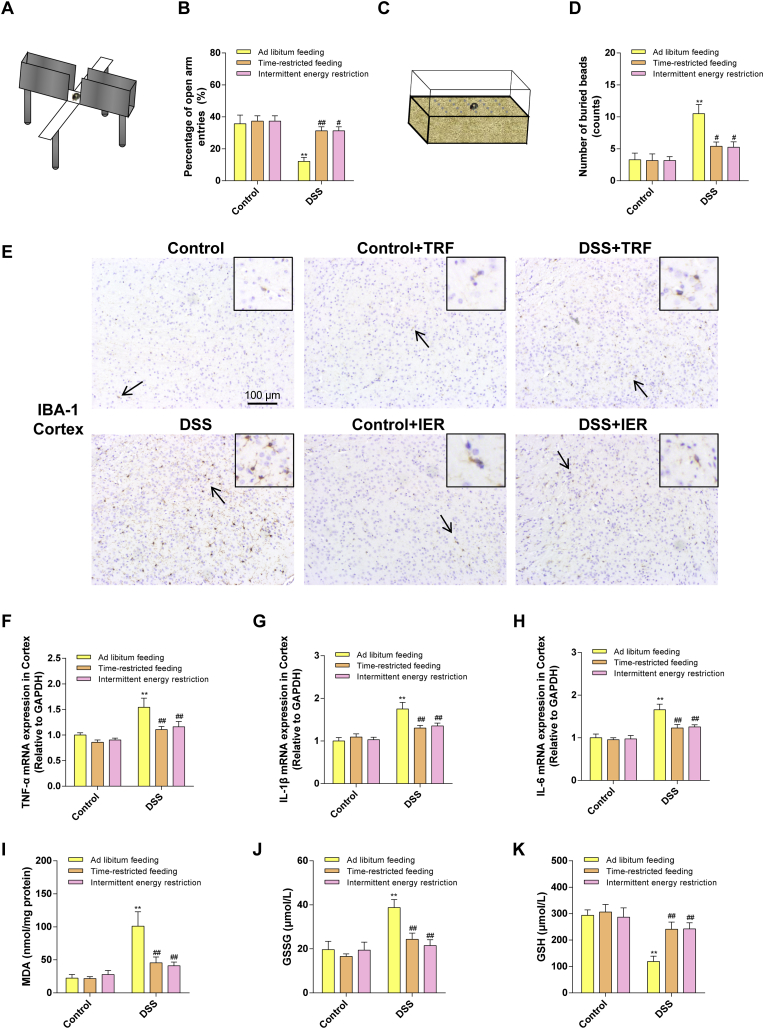
To determine the behavioral changes in the colitis mice, the elevated plus maze test and the marble burying test were employed in the current study. The EPM test is a behavioral test to detect the anxiety-like behaviors in animal models [30](Fig. 5A). These results indicated that DSS treatment significantly decreased the percentage of the mice moved into the open arms, compared with the control group (p < 0.01), which indicated that the mice had an anxiety-like behavior. However, the TRF and IER treatment significantly prevented such behavioral disorders (p < 0.01) (Fig. 5B). The MBT is a test to examine the obsessive-compulsive behaviors of the mice [31](Fig. 5C). As illustrated in Fig. 5D, the mice buried more beads in the DSS group than the control group. However, the TRF and IER treatment significantly attenuated such obsessive-compulsive behavior (p < 0.05) (Fig. 5D). These results indicated that TRF and IER attenuated colitis-related anxiety-like and obsessive-compulsive behavioral disorders.
Fig. 5.
Effects of TRF and IER on behavioral disorders, Inflammation and oxidative stress in the brain of DSS-induced colitis. (A) Diagram of an elevated plus maze (EPM). (B) The result of elevated plus maze test. (C) Diagram of a marble burying test (MBT). (D) The result of marble burying test. (E) Representative images of immunohistochemistry of IBA-1 in cortex region for each group of the TRF and IER regimens. (F–H) The mRNA expression of TNF-α, IL-1β, IL-6 in cortex for each group of the TRF and IER regimens. (I–K) The levels of MDA, GSSG and GSH in cortex. Data are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 12 mice per group, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
3.5. Effects of TRF and IER on the inflammatory responses and oxidative stress in the brain
The expressions of IBA-1, a biomarker of microglia activation, reflects the neuroinflammation status [32]. In the current research, the IBA-1 expressions were significantly increased in the cortex and hippocampus of the DSS-treated mice, compared with control group mice (p < 0.01). However, the TRF and IER prevented the overexpression of IBA-1 in the brain (Fig. 5E and Figs. S2A–C) (p < 0.01). Consistently, the up-regulated expressions of cytokines including TNF-α, IL-1β, and IL-6, were also significantly inhibited by the TRF and IER treatment in the DSS-treated mice (Fig. 5F–H) (p < 0.01). Furthermore, it has been found that the MDA levels were significantly increased in brain of colitis mice, accompanied by the increased level of GSSG and the decreased level of GSH (p < 0.01). However, the TRF and IER treatment substantially reduced the MDA and GSSG levels and improved the GSH levels in the colitis mice brain (Fig. 5I–K) (p < 0.01). Moreover, one of the biomarkers indicating the oxidized status in the tissues [33], the GSSG/GSH level was also detected in the brain of colitis mice. Compared with control group, the GSSG/GSH ratio in brain was significantly increased in colitis mice. However, the TRF and IER treatment significantly reduced this ratio (Fig. S2D) (p < 0.01). These data indicated that the TRF and IER significantly alleviated the neuroinflammation and oxidative stress in the brain of the DSS-induced colitis mice.
3.6. Effects of IF regimens on the gut microbiome diversity and short chain fatty acids generation in DSS-induced colitis mice
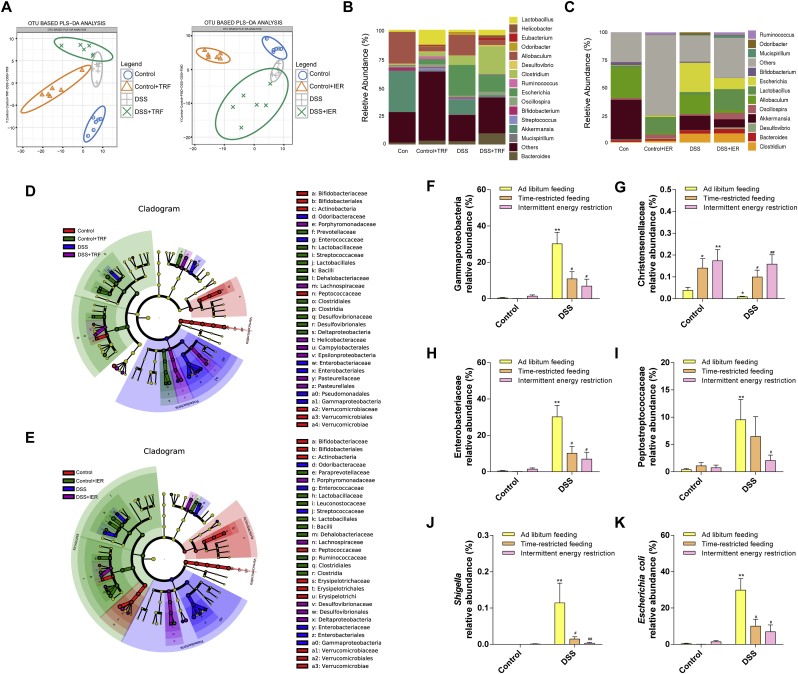
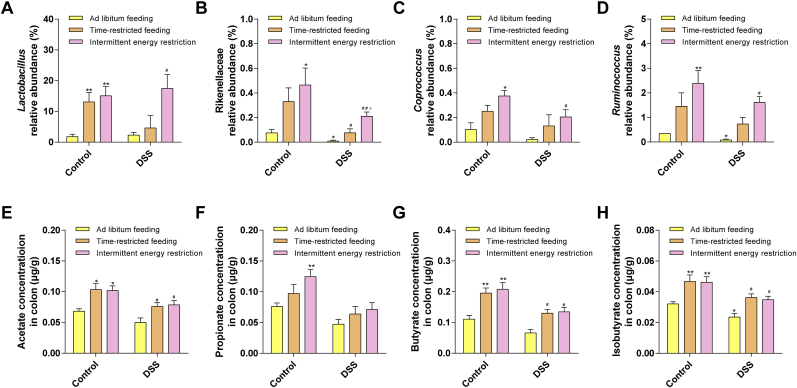
The gut microbiome diversity changes were detected by 16S rRNA sequencing as described in the Material and methods section. The species cumulative curve reflects the effect of the number of samples on species diversity. In this study, the upward trend at the end of the species cumulative curve has flattened, indicating that the sample size is sufficient (Fig. S3A). The results indicated that the ADF, the TRF, and the IER, all affected the gut microbiota compositions in both control and DSS-treated mice (Fig. 6A and Figs. S3B and D). As shown in Fig. 6D–E, Fig. S3E and Fig. S4, the LDA and LEfSe analysis indicated that the enrichment of some bacteria was altered by the DSS and/or IF treatment. On genus level, the enrichment of Escherichia was significantly increased in DSS-treated mice, compared with the control group. However, TRF and IER treatment significantly suppressed the levels of Escherichia in colitis mice (Fig. 6B–C and Fig. S3C). Specifically, the TRF and IER, but not the ADF significantly inhibited the enrichment of Gammaproteobacteria and Enterobacteriaceae in colitis mice (p < 0.05) (Fig. 6F, H and Figs. S5A and C). The TRF (p < 0.05) and IER (p < 0.01), but not ADF, treatment significantly improved the enrichment of Christensenellaceae in both control and DSS-treated mice (Fig. 6G and Fig. S5B). The IER but not TRF and ADF significantly reduced the enrichment of Peptostreptococcaceae (Fig. 6I and Fig. S5D) (p < 0.05). In the genus level, the enrichments of Shigella and Escherichia_coli were significantly down-regulated by the TRF and IER, but not ADF, in the colitis mice (p < 0.05) (Fig. 6J–K and Figs. S5E–F). Moreover, the generation of SCFAs and related microbes were also determined. As shown in Fig. 7A–D, the enrichments of SCFAs generation-related microbes including Rikenellaceae, Lactobacillus, Coproccus, and Ruminococcus were partly improved by the TRF and IER treatment (p < 0.05). Consistently, the generation of SCFAs including acetate, butyrate, and isobutyrate were significantly improved by the treatment of TRF and IER, but not ADF, in the DSS-treated mice (Fig. 7E–H and Figs. S5G–H) (p < 0.05). These results indicated that the IF regimens significantly altered the gut microbiome structure, and the TRF and IER treatment up-regulated SCFAs generation and related microbes enrichment in colitis mice gut.
Fig. 6.
Effects of TRF and IER on the gut microbiome structure in DSS-induced colitis mice. (A) Partial least squares discrimination analysis (PLS-DA) of each group of the TRF and IER regimens. (B) and (C) Relative abundance of the gut microbiota in genus levels in the TRF and IER regimens. (D) and (E) Linear discriminant analysis of the TRF and IER regimens. (F) The relative abundance of Gammaproteobacteria. (G) The relative abundance of Christensenellaceae. (H) The relative abundance of Enterobacteriaceae. (I) The relative abundance of Peptostreptococcaceae. (J) The relative abundance of Shigella. (K) The relative abundance of Escherichia coli. Microbiome sequencing was performed by data of control group, Control-ADF group, Control-TRF group, Control-IER group, DSS group, DSS-ADF group, DSS -TRF group, DSS-IER group (n = 6 per group). The data of intestinal flora are presented as mean ± SEM and statistical significance was determined by Wilcox test, n = 6, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
Fig. 7.
Effects of TRF and IER on gut microbiome and corresponding metabolites production in DSS-induced colitis mice. (A) The relative abundance of Lactobacillus. (B) The relative abundance of Rikenellaceae. (C) The relative abundance of Coprococcus. (D) The relative abundance of Ruminococcus. The data of intestinal flora are presented as mean ± SEM and statistical significance was determined by Wilcox test, n = 6, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group, $p < 0.05, $$p < 0.01, compared with the DSS-TRF group). (E) The concentration of acetate. (F) The concentration of propionate. (G) The concentration of butyrate. (H) The concentration of isobutyrate. The data of short-chain fatty acids are presented as mean ± SEM and statistical significance was determined by two-way ANOVA with Newman-Keuls multiple comparisons test, n = 6, (*p < 0.05, **p < 0.01, compared with the Control group, #p < 0.05, ##p < 0.01, compared with the DSS group).
4. Discussion
The current study found that the TRF and IER, but not the ADF, has beneficial alternate-day fasting, time-restricted fasting and intermittent energy restriction on DSS-induced colitis and behavioral disorders. TRF and ADF significantly improved the survival rate and bodyweight of the colitis mice. However, ADF has negative effects on the development of colitis. TRF and IER substantially attenuated the inflammatory responses, oxidative damages, and the barrier leak in the gut of DSS-treated mice. Moreover, TRF and ADF significantly prevented anxiety-like and obsessive-compulsive behaviors and suppressed neuroinflammation and oxidative stress in the brain of colitis mice. Importantly, we found that the TRF, IER, and ADF also reshaped the gut microbes in DSS-treated mice. TRF and IER also enhanced SCFAs generation, which provided an explanation of how they positively affect the function of gut.
4.1. TRF and IER, but not ADF, improved the survival rates of the colitis mice
Our previous research found that alternate-day fasting could attenuate diabetes-related cognitive disorders by regulating “gut-brain axis”. ADF significantly improved the gut barrier integrity by increasing the Claudin-1 expression and altering gut microbes/metabolites composition [22]. There are several reports indicated that fasting or intermittent fasting has beneficial effects on the development of colitis. Sävendahl et al. found that a 2-day fasting process prevents DSS-induced colitis in a mouse model. Okada et al. reported that a one-time fasting/refeeding intermittent fasting also improved the recovery of the epithelial cells and reduced inflammation. However, in the current work, ADF treatment aggravated DSS-induced gut damages. It has been observed that the DSS-treated mice had abnormal eating behaviors, which is consistent with a previous colitis mouse model research [34]. It has been also found that the ADF treatment, as an extreme dietary restriction, leading to less food intake and bodyweight loss, indicated that the mice experienced starvation and malnutrition during the 36 days ADF cycles and DSS treatment. Recent research reported that starvation, in contrast to short-term fasting, could compromise antimicrobial immunity and tissue repair. These results and reference could partly explain why ADF induced higher death rate and aggravated the colitis progress and provide a clue that multiple alternate day fasting cycles could worsen the gut damages.
Nevertheless, in the current work, the other two kinds of moderate IF treatment, i.e. TRF and IER has benefits on improving the survival rates in colitis mice. It has been reported the timing of calorie restriction impacts its beneficial effects on the gut barrier function [35,36] and microbiota composition. As a kind of time-restricted fasting, the Islamic fasting or Ramadan fasting has also been reported to altering the gut microbiota diversity [37]. A short-term 2-day fed:5-day fasted IF regime significantly improved the gut barrier integrity of Drosophila melanogaster [38]. Consistently, it has been found that a fasting-mimicking diet, i.e. an IER-like fasting but with specific formula diet, reversed the intestinal pathology, altered the microbiota composition, and improved the immune cell profile in a DSS-induced mouse model [23,39].
4.2. TRF and IER alleviated the inflammation and oxidative stress in the gut
DSS colitis mouse model is one of the widely used chemical induced colitis models, as its simplicity and has similarities with human UC [40]. The possible mechanism by which DSS triggers gut inflammation results in epithelial monolayer lining disruption, inducing bacteria invading into the mucosa and leading to inflammatory responses [41]. It has been found that the IBD patients had mucin depletion, which could also be observed in DSS-treated murine models [42]. The loss of mucin is related to the neutrophil infiltration as a result of oxidative stress [43,44]. Increased oxidative stress is associated with mucosal inflammation in ulcerative colitis, and it may be a contributing factor to the progression to malignancy associated with this disorder [45]. Excessive ROS can irreversibly or irreversibly destroy oxidizable biomolecules, including membrane molecules, to form lipid peroxidation products that disrupt cell membrane function and structure. MDA is an important indicator of lipid peroxidation and ROS-induced damage [46]. It has been found that the antioxidant defense system was also defected during the IBD development [44]. Here, we found that the TRF and IER significantly rescued the mucosal layer and improved goblet cell numbers in the DSS-treated mice, accompanied with the increasing expressions of MUC-2. Of note, the gut barrier integrity was also protected by the TRF and IER treatment by enhancing Claudin-1 expression. Importantly, the level of the MDA was also down-regulated by the TRF and IER treatment. These results indicated that the TRF and IER treatment might boost the antioxidant defense system in the gut barrier, and further study could focus on the redox related signaling changes in the gut of colitis during IF.
4.3. TRF and IER prevented the colitis-related behavioral disorders and neuro-inflammation and oxidative damages
Furthermore, the TRF and IER attenuated the anxiety-like and obsessive-compulsive behaviors in current experiment. It has been reported that colitis is related to depression and anxiety-like mental behavioral changes [47]. Some colitis animal studies demonstrated that the overexpressed proinflammatory mediators and overactivated microglia were observed and could be the explanations of behavioral disorders [48]. It has been also reported that the increased levels of TNF-α, IL-1β, and IL-6 were detected in the cerebral cortex of individuals with anxiety and depression-like behavior [49]. In line with these reports, we also found that microglial activation and cytokines expression were elevated in DSS group. However, the TRF and IER treatment significantly suppressed the neuroinflammation in the brain of colitis mice. Moreover, the leaking gut also results in increased levels of LPS in the serum [50]. Previous of our research indicated that the leaking LPS could be related to neuroinflammation and subsequent depression and/or anxiety-like behavioral disorders, and IF could attenuate the inflammatory responses and behavioral disorders [22,49]. Interestingly, in the current study, it has also been found that the TRF and IER lowered the serum LPS levels in colitis, which could partly explain how these two IF regimens alleviated the behavioral disorders. On the other side, the oxidative stress in the brain of colitis mice was also suppressed by the IF treatments by up-regulating GSH level and down-regulating GSSG and MDA levels. The mental behavioral disorders were also strongly associated with oxidative damages. The cytokines including TNF-α and IL-1β could also stimulate oxidative damages by activating ROS and RNS producing enzymes [51]. Previous research also found that IF and calorie restriction has preventive effects on the oxidative stress in aging or stroke animal models [52,53]. In the future study, the IF could be a promising strategy to improve cognitive function and alleviate depression-related central nervous system diseases.
4.4. The roles of gut microbes involved during the IF regimens
The etiological causality of the gut microbiome disorder during the colitis develop is still unclear at the current stage. However, amount of research indicates that the gut microbiota composition altered in the UC patients. It has been found that the enrichment of Gammaproteobacteria, especially Escherichia coli, was significantly increased in the colitis patients [54]. Consistently, the enrichment of Gammaproteobacteria and Escherichia coli were also enhanced in DSS-treated mice. However, the TRF and IER, but not ADF, significantly inhibited these microbes' enrichment. Similarly, it has been reported that the enriched Enterobacteriaceae and Peptostreptococcaceae could aggravate the colitis and colon cancer development [55,56]. Nonetheless, TRF and IER, but not ADF, also significantly down-regulated the levels of these two bacteria in the gut of colitis mice. The enrichment of Shigella, another inflammatory enterophaogen producing bacteria [57], has also been found suppressed by the TRF and IER. Controversially, the enrichment of the probiotics including Lactobacillus and Eubacterium, was decreased in colitis patients’ gut [58,59]. However, we found that the enrichment of several probiotics including Parabacteroides distasonis, Eubacterium, Akkermansia muciniphila, and Lactobacillus was significantly increased by the TRF and IER regimens. These bacteria have been reported to and be the most promising probiotics in preventing UC by enhancing the production of SCFAs [11,[60], [61], [62], [63]].
SCFAs are a group of metabolites mainly generated by gut microbes from fermenting dietary fibers [64]. It has been found that the SCFAs levels were decreased in the UC patients and animal models [65,66]. Dietary fiber supplementation could be an effective treatment for preventing colitis and colon cancer [67]. Clinical trials indicated that supplementation of SCFAs could effectively attenuate mild to moderate distal ulcerative colitis with little side effects [68]. Several human trials of fecal microbiota transplantation also suggest that the transplanted feces from healthy donors significantly increase the level of SCFAs biosynthesis and the generation of secondary bile acids in UC patient. A probiotic therapy study also indicates that the increased level serum bile acids is also associated with the UC restoring. Our previous research also found that the IF regimen substantially improved several beneficial microbial metabolites including SCFAs, bile acids, and indole-3-propionic acid, which is highly correlated with the gut microbiota composition alteration and gut function. These results indicate the protective effects of IF against colitis could be partly explained by its regulating role in gut microbes/metabolites. The composition of gut microbiota plays an essential role in mediating colitis development [69]. It has been reported that transplantation of gut microbiota UC mice into the healthy germ-free mice triggers serves inflammatory responses in the colon [1]. Although our previous research reports found that IF can significantly remodel the intestinal microbes of mice, in the future studies, the causality of the gut microbiota changes and colitis/behavior improvements in IF regimens should be investigated by germ-free and/or fecal transplantation animal model. And the metabolome analysis of the feces and serum and causality verification experiments could provide more evidence for the mechanism how IF attenuating colitis.
5. Conclusion
The current study found that TRF and IER, but not ADF, are effective treatments in alleviating DSS-induced colitis and behavioral disorders in a mouse model. TRF and IER suppressed the inflammatory responses and oxidative stress in both gut and brain, which could be partly explained by the mediating effects on gut microbiota composition and SCFAs generation. The efficiency of different IF regimens should be evaluated by more clinical trials. Once validated, IF can be translated into a novel ecological approach for colitis and other inflammatory bowel diseases.
Author contribution
XZ and ZL designed the experiments; XZ, QZ, BZ, JW and ZL performed and analyzed the experiments; XZ, ZL, interpreted the data; XZ, WZ, YL, RL and ZL wrote the manuscript; ZL, XL, and RL supervised the work. All authors approved the final version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Nos. 81871118 and 81803231), a General Financial Grant from China Postdoctoral Science Foundation (No. 2016M602867), a Special Financial Grant from China Postdoctoral Science Foundation (No. 2018T111104), the China Postdoctoral Science Foundation (No. 2019M653768), and the Innovative Talent Promotion Program-Technology Innovation Team (2019TD-006). Dr. Zhigang Liu are also funded by the Tang Cornell-China Scholars Program from Cornell University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101535.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Roy U., Gálvez E.J.C., Iljazovic A., Lesker T.R., Błażejewski A.J., Pils M.C., Heise U., Huber S., Flavell R.A., Strowig T. Distinct microbial communities trigger colitis development upon intestinal barrier damage via innate or adaptive immune cells. Cell Rep. 2017;21:994–1008. doi: 10.1016/j.celrep.2017.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Tawil A.M. Epidemiology and inflammatory bowel diseases. World J. Gastroenterol. 2013:6–8. doi: 10.3748/wjg.v19.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams S.M., Bornemann P.H. Ulcerative colitis. Am. Fam. Physician. 2013;87:699–705. [PubMed] [Google Scholar]
- 4.Navabi S., Gorrepati V.S., Yadav S., Chintanaboina J., Maher S., Demuth P., Stern B., Stuart A., Tinsley A., Clarke K., Williams E.D., Coates M.D. Influences and impact of anxiety and depression in the setting of inflammatory bowel disease. Inflamm. Bowel Dis. 2018;24:2303–2308. doi: 10.1093/ibd/izy143. [DOI] [PubMed] [Google Scholar]
- 5.Neuendorf R., Harding A., Stello N., Hanes D., Wahbeh H. Depression and anxiety in patients with Inflammatory Bowel Disease: a systematic review. J. Psychosom. Res. 2016;87:70–80. doi: 10.1016/j.jpsychores.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Rezaie A., Parker R.D., Abdollahi M. Oxidative stress and pathogenesis of inflammatory bowel disease: an epiphenomenon or the cause? Dig. Dis. Sci. 2007;52:2015–2021. doi: 10.1007/s10620-006-9622-2. [DOI] [PubMed] [Google Scholar]
- 7.Ohkusa T., Yoshida T., Sato N., Watanabe S., Tajiri H., Okayasu I. Commensal bacteria can enter colonic epithelial cells and induce proinflammatory cytokine secretion: a possible pathogenic mechanism of ulcerative colitis. J. Med. Microbiol. 2009;58:535–545. doi: 10.1099/jmm.0.005801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poritz L.S., Garver K.I., Green C., Fitzpatrick L., Ruggiero F., Koltun W.A. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007;140 doi: 10.1016/j.jss.2006.07.050. 0-19. [DOI] [PubMed] [Google Scholar]
- 9.Szebeni B., Veres G., Dezsõfi A., Rusai K., Arató A. Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin. Exp. Immunol. 2008;151:34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horst S., Chao A., Rosen M., Nohl A., Duley C., Wagnon J.H., Beaulieu D.B., Taylor W., Gaines L., Schwartz D.A. Treatment with immunosuppressive therapy may improve depressive symptoms in patients with inflammatory bowel disease. Dig. Dis. Sci. 2015;60:465–470. doi: 10.1007/s10620-014-3375-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang W., Li Q., Chai W., Sun C., Zhang T., Zhao C., Yuan Y., Wang X., Liu H., Ye H. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci. Nutr. 2019;7:2636–2646. doi: 10.1002/fsn3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C.-S.-E., Li W.-B., Wang H.-Y., Ma Y.-M., Zhao X.-H., Yang H., Qian J.-M., Li J.-N. VSL#3 can prevent ulcerative colitis-associated carcinogenesis in mice. World J. Gastroenterol. 2018;24:4254–4262. doi: 10.3748/wjg.v24.i37.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilarczyk-Zurek M., Strus M., Adamski P., Heczko P.B. The dual role of Escherichia coli in the course of ulcerative colitis. BMC Gastroenterol. 2016;16:128. doi: 10.1186/s12876-016-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narula N., Kassam Z., Yuan Y., Colombel J.-F., Ponsioen C., Reinisch W., Moayyedi P. Systematic review and meta-analysis: fecal microbiota transplantation for treatment of active ulcerative colitis. Inflamm. Bowel Dis. 2017;23:1702–1709. doi: 10.1097/MIB.0000000000001228. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Gu Y., Fang K., Mao K., Dou J., Fan H., Zhou C., Wang H. Lactobacillus acidophilus and Clostridium butyricum ameliorate colitis in murine by strengthening the gut barrier function and decreasing inflammatory factors. Benef. Mirbobes. 2018;9:775–787. doi: 10.3920/BM2017.0035. [DOI] [PubMed] [Google Scholar]
- 16.Crawford P.A., Crowley J.R., Sambandam N., Muegge B.D., Costello E.K., Hamady M., Knight R., Gordon J.I. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11276–11281. doi: 10.1073/pnas.0902366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinsley G.M., La Bounty P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015;73:661–674. doi: 10.1093/nutrit/nuv041. [DOI] [PubMed] [Google Scholar]
- 18.Patterson R.E., Laughlin G.A., LaCroix A.Z., Hartman S.J., Natarajan L., Senger C.M., Martínez M.E., Villaseñor A., Sears D.D., Marinac C.R. Intermittent fasting and human metabolic health. J. Acad. Nutr. Diet. 2015;115:1203–1212. doi: 10.1016/j.jand.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L., Patel D., Ma Y., Brocker C.N., Yan T., Krausz K.W., Xiang R., Gavrilova O., Patterson A.D., Gonzalez F.J. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabol. 2017;26:672–685. doi: 10.1016/j.cmet.2017.08.019. e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson R.E., Sears D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017;37:371–393. doi: 10.1146/annurev-nutr-071816-064634. [DOI] [PubMed] [Google Scholar]
- 21.Wei S., Han R., Zhao J., Wang S., Huang M., Wang Y., Chen Y. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores beta cells and reconstructs gut microbiota in mice. Nutr. Metab. 2018;15:80. doi: 10.1186/s12986-018-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z., Dai X., Zhang H., Shi R., Hui Y., Jin X., Zhang W., Wang L., Wang Q., Wang D., Wang J., Tan X., Ren B., Liu X., Zhao T., Wang J., Pan J., Yuan T., Chu C., Lan L., Yin F., Cadenas E., Shi L., Zhao S., Liu X. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020;11:855. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rangan P., Choi I., Wei M., Navarrete G., Guen E., Brandhorst S., Enyati N., Pasia G., Maesincee D., Ocon V. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019;26:2704–2719. doi: 10.1016/j.celrep.2019.02.019. e2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada T., Otsubo T., Hagiwara T., Inazuka F., Kobayashi E., Fukuda S., Inoue T., Higuchi K., Kawamura Y.I., Dohi T. Intermittent fasting prompted recovery from dextran sulfate sodium-induced colitis in mice. J. Clin. Biochem. Nutr. 2017;61:100–107. doi: 10.3164/jcbn.17-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G., Dubeau L., Yap L.P., Park R., Vinciguerra M., Di Biase S., Mirzaei H., Mirisola M.G., Childress P., Ji L., Groshen S., Penna F., Odetti P., Perin L., Conti P.S., Ikeno Y., Kennedy B.K., Cohen P., Morgan T.E., Dorff T.B., Longo V.D. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metabol. 2015;22:86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirtz S., Neufert C., Weigmann B., Neurath M.F. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 27.Alipour M., Zaidi D., Valcheva R., Jovel J., Martínez I., Sergi C., Walter J., Mason A.L., Wong G.K., Dieleman L.A., Carroll M.W., Huynh H.Q., Wine E. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J. Crohns Colitis. 2016;10:462–471. doi: 10.1093/ecco-jcc/jjv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin S., Yang H., Tao Y., Wei S., Li L., Liu M., Li J. Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response. Inflammation. 2020 doi: 10.1007/s10753-019-01164-1. 10.1007/s10753-10019-01164-10751. [DOI] [PubMed] [Google Scholar]
- 29.Samarghandian S., Azimi-Nezhad M., Farkhondeh T., Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 2017;87:223–229. doi: 10.1016/j.biopha.2016.12.105. [DOI] [PubMed] [Google Scholar]
- 30.Reimer A.E., de Oliveira A.R., Diniz J.B., Hoexter M.Q., Chiavegatto S., Brandão M.L. Rats with differential self-grooming expression in the elevated plus-maze do not differ in anxiety-related behaviors. Behav. Brain Res. 2015;292:370–380. doi: 10.1016/j.bbr.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Kedia S., Chattarji S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci. Methods. 2014;233:150–154. doi: 10.1016/j.jneumeth.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Calcia M.A., Bonsall D.R., Bloomfield P.S., Selvaraj S., Barichello T., Howes O.D. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology. 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruzat V.F., Bittencourt A., Scomazzon S.P., Leite J.S., de Bittencourt P.I., Jr., Tirapegui J. Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia. Nutrition. 2014;30:602–611. doi: 10.1016/j.nut.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Wirtz S., Popp V., Kindermann M., Gerlach K., Weigmann B., Fichtner-Feigl S., Neurath M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017;12:1295–1309. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Xue X., Zhai R., Yang X., Li H., Zhao L., Zhang C. Timing of calorie restriction in mice impacts host metabolic phenotype with correlative changes in gut microbiota. mSystems. 2019;4 doi: 10.1128/mSystems.00348-19. 00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Merwe M., Sharma S., Caldwell J.L., Smith N.J., Gomes C.K., Bloomer R.J., Buddington R.K., Pierre J.F. Time of feeding alters obesity-associated parameters and gut bacterial communities, but not fungal populations, in C57bl/6 male mice. Curr. Dev. Nutr. 2020;4 doi: 10.1093/cdn/nzz145. nzz145-nzz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Özkul C., Yalınay M., Karakan T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: a preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019;30:1030–1035. doi: 10.5152/tjg.2019.19185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catterson J.H., Khericha M., Dyson M.C., Vincent A.J., Callard R., Haveron S.M., Rajasingam A., Ahmad M., Partridge L. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol. 2018;28:1714–1724. doi: 10.1016/j.cub.2018.04.015. e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei S., Han R., Zhao J., Wang S., Huang M., Wang Y., Chen Y. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr. Metab. 2018;15 doi: 10.1186/s12986-018-0318-3. 80-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 41.Chassaing B., Aitken J.D., Malleshappa M., Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Im. 2014;104:15. doi: 10.1002/0471142735.im1525s104. 25.11-15.25.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCormick D.A., Horton L.W., Mee A.S. Mucin depletion in inflammatory bowel disease. J. Clin. Pathol. 1990;43:143–146. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikkhah-Bodaghi M., Maleki I., Agah S., Hekmatdoost A. Zingiber officinale and oxidative stress in patients with ulcerative colitis: a randomized, placebo-controlled, clinical trial. Compl. Ther. Med. 2019;43:1–6. doi: 10.1016/j.ctim.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 44.Buffinton G.D., Doe W.F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic. Biol. Med. 1995;19:911–918. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh M., Kapoor A., Bhatnagar A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem. Biol. Interact. 2015;234:261–273. doi: 10.1016/j.cbi.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M.C., Jung Y.S., Song Y.S., Lee J.I., Park J.H., Sohn C.I., Choi K.Y., Park D.I. Factors associated with anxiety and depression in Korean patients with inactive inflammatory bowel disease. Gut Liver. 2016;10:399–405. doi: 10.5009/gnl15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dempsey E., Abautret-Daly Á., Docherty N.G., Medina C., Harkin A. Persistent central inflammation and region specific cellular activation accompany depression- and anxiety-like behaviours during the resolution phase of experimental colitis. Brain Behav. Immun. 2019;80:616–632. doi: 10.1016/j.bbi.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Wang Q., Jia M., Zhao Y., Hui Y., Pan J., Yu H., Yan S., Dai X., Liu X., Liu Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019;67:12441–12451. doi: 10.1021/acs.jafc.9b03652. [DOI] [PubMed] [Google Scholar]
- 50.Li T., Gao J., Du M., Mao X. Bovine α-lactalbumin hydrolysates ameliorate obesity-associated endotoxemia and inflammation in high-fat diet-fed mice through modulation of gut microbiota. Food Funct. 2019;10:3368–3378. doi: 10.1039/c8fo01967c. [DOI] [PubMed] [Google Scholar]
- 51.Fischer R., Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid. Med. Cell Longev. 2015;2015 doi: 10.1155/2015/610813. 610813-610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y., Zhang M., Chen Y., Yang Y., Zhang J.-J. Postoperative intermittent fasting prevents hippocampal oxidative stress and memory deficits in a rat model of chronic cerebral hypoperfusion. Eur. J. Nutr. 2019;58:423–432. doi: 10.1007/s00394-018-1606-4. [DOI] [PubMed] [Google Scholar]
- 53.Fann D.Y.-W., Santro T., Manzanero S., Widiapradja A., Cheng Y.-L., Lee S.-Y., Chunduri P., Jo D.-G., Stranahan A.M., Mattson M.P., Arumugam T.V. Intermittent fasting attenuates inflammasome activity in ischemic stroke. Exp. Neurol. 2014;257:114–119. doi: 10.1016/j.expneurol.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Scales B.S., Dickson R.P., Huffnagle G.B. A tale of two sites: how inflammation can reshape the microbiomes of the gut and lungs. J. Leukoc. Biol. 2016;100:943–950. doi: 10.1189/jlb.3MR0316-106R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garrett W.S., Gallini C.A., Yatsunenko T., Michaud M., DuBois A., Delaney M.L., Punit S., Karlsson M., Bry L., Glickman J.N., Gordon J.I., Onderdonk A.B., Glimcher L.H. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y., Jobin C. Novel insights into microbiome in colitis and colorectal cancer. Curr. Opin. Gastroenterol. 2017;33:422–427. doi: 10.1097/MOG.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DuPont H.L. Approach to the patient with infectious colitis. Curr. Opin. Gastroenterol. 2012;28:39–46. doi: 10.1097/MOG.0b013e32834d3208. [DOI] [PubMed] [Google Scholar]
- 58.Tang C., Kamiya T., Liu Y., Kadoki M., Kakuta S., Oshima K., Hattori M., Takeshita K., Kanai T., Saijo S., Ohno N., Iwakura Y. Inhibition of dectin-1 signaling ameliorates colitis by inducing lactobacillus-mediated regulatory T cell expansion in the intestine. Cell Host Microbe. 2015;18:183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Hirano A., Umeno J., Okamoto Y., Shibata H., Ogura Y., Moriyama T., Torisu T., Fujioka S., Fuyuno Y., Kawarabayasi Y., Matsumoto T., Kitazono T., Esaki M. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2018 doi: 10.1111/jgh.14129. 10.1111/jgh.14129. [DOI] [PubMed] [Google Scholar]
- 60.Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 61.Lyu M., Wang Y.-F., Fan G.-W., Wang X.-Y., Xu S.-Y., Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.02146. 2146-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bunesova V., Lacroix C., Schwab C. Mucin cross-feeding of infant bifidobacteria and Eubacterium hallii, microb. Ecol. 2018;75:228–238. doi: 10.1007/s00248-017-1037-4. [DOI] [PubMed] [Google Scholar]
- 63.Shen Z.-H., Zhu C.-X., Quan Y.-S., Yang Z.-Y., Wu S., Luo W.-W., Tan B., Wang X.-Y. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holscher H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microb. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumari R., Ahuja V., Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J. Gastroenterol. 2013;19:3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parada Venegas D., De la Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-Mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00277. 277-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian Y., Xu Q., Sun L., Ye Y., Ji G. Short-chain fatty acids administration is protective in colitis-associated colorectal cancer development. J. Nutr. Biochem. 2018;57:103–109. doi: 10.1016/j.jnutbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Kim Y.I. Short-chain fatty acids in ulcerative colitis. Nutr. Rev. 1998;56:17–24. doi: 10.1111/j.1753-4887.1998.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 69.Bhan A.K., Mizoguchi E., Smith R.N., Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol. Rev. 1999;169:195–207. doi: 10.1111/j.1600-065x.1999.tb01316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.