Abstract
During scientific investigations, the explanation of remarkably interesting phenomena must often await development of new methods or accrual of new observations that in retrospect can lead to lucid answers to the initial problem. A case in point is the control of genetic recombination during meiosis, which leads to crossovers between chromosomes critical for production of healthy offspring. Crossovers must be properly placed along meiotic chromosomes for their accurate segregation. Here, we review observations on two aspects of meiotic crossover control – crossover interference and repression of crossovers near centromeres, both observed more than 85 years ago. Only recently have relatively simple molecular mechanisms for these phenomena become clear through advances in both methods and understanding the molecular basis of meiotic recombination.
Keywords: crossover interference, DNA break-hotspot clusters, linear element proteins, centromeric repression, heterochromatin, sister chromatid cohesins
Meiotic Chromosome Segregation and Crossingover
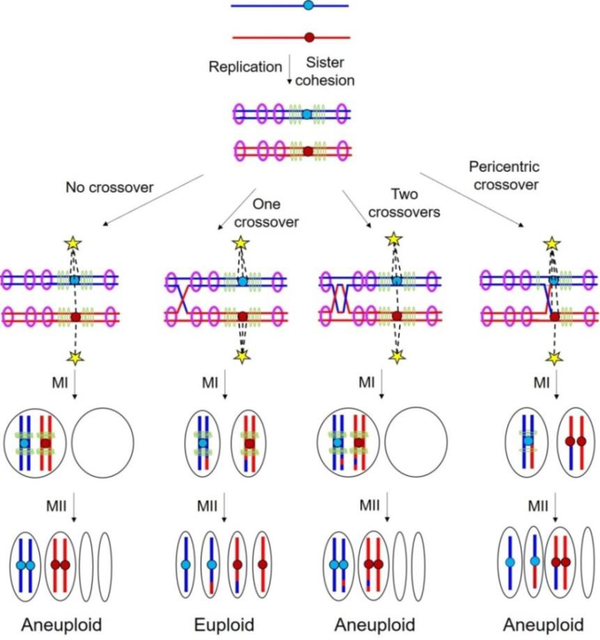
Meiosis is the specialized cell division that converts diploid cells (see glossary) of the body into haploid cells for reproduction (gametes, called eggs and sperm in animals such as mammals and insects; eggs and pollen in flowering plants; and spores in ferns and fungi such as yeasts). This reduction in chromosome number results from one round of DNA replication (to form two pairs of sister chromatids) followed by two nuclear divisions with segregation of the chromosomes led by microtubule “strings” attached to each chromosomal centromere (Figure 1). In the first meiotic division (MI, reductional), the pair of centromeres from one parent segregates from the other parental pair. In the second meiotic division (MII, equational), the paired (sister) centromeres segregate. At each division, all of the chromosomal material follows the centromere to which it is attached.
Figure 1. Chromosomal segregation during meiosis.
After replication, sister chromatids of each parent are held together by cohesin complexes deposited at points across the chromosomal arms (purple rings) and especially densely at the pericentric regions (green rings). One or more crossovers between homologs in the chromosomal arms and sister chromatid cohesion are required in most species for proper reductional division at meiosis I (MI) (second panel from the left), but a crossover too near another may leave too little cohesion to be effective (third panel from the left). A crossover near the telomere (not shown) also leaves too little cohesion distal to the crossover to be effective [11]. In the absence of crossovers, there is no tension generated between homologs, which results in missegregation and aneuploidy (left-most panel). Pericentric crossovers may lead to misorientation of the kinetochores or loss of sister chromatid cohesion at the centromeres, which also results in missegregation and aneuploidy (right-most panel). Each solid line depicts double-stranded DNA of a chromatid; red and blue indicate a representative homolog pair (one chromosome from each parent); central circles indicate the centromeres; dashed lines depict microtubules that pull the chromosomes toward the spindle pole bodies (yellow stars) at the opposite poles.
Proper chromosomal segregation at the first division requires in most species tested, crossovers between the two parental chromosomes (homologs). Crossovers provide tension between homologs when their centromeres begin to be pulled toward opposite poles of the cell, as required for reductional division (Figure 1, second panel from the left). Tension appears to be the signal that segregation is proceeding normally [1–3], as segregation is delayed until tension is generated on each homolog pair [4]. Without tension, for example in the absence of crossovers, homologs eventually segregate at random (Figure 1, far left) and frequently give rise to abnormal gametes with a set of chromosomes that cannot support healthy life. In humans, an estimated one-third of conceptions end in spontaneous abortion, many of which appear to be caused by abnormal chromosome numbers in the gametes [5, 6]. Down syndrome is caused by chromosome missegregation that results in two copies, not one, of chromosome 21 in one of the gametes (usually the egg). Although crossovers are nearly universally critical for correct chromosome segregation, they must also be appropriately placed along chromosomes to aid segregation. Aberrant recombination levels, including absence of crossovers, or positions of crossovers, e.g., too near the centromere or telomeres, are major contributors to the generation of meiotic segregation errors in gametes [7–9]. Although this problem was recognized for decades, molecular bases for appropriate crossover placement have been reported only recently. In this review, we describe molecular mechanisms that properly place crossovers for generation of viable gametes to assure a healthy next-generation.
Crossover interference to properly space crossovers
Crossovers are formed by genetic recombination between homologs (reviewed by Cromie and Smith [10]). An early step in recombination is formation of DNA double-strand breaks (DSBs) in one chromatid by the Spo11 complex. Resection of one DNA strand produces single-stranded DNA with a free 3’ end; this “tail” is bound by Rad51 and other proteins, which promote invasion of the tail into the other homolog or sister chromatid at a point of extensive nucleotide-sequence identity. Further reactions, including the formation of cross-strand structures such as Holliday junctions and resolution of these structures into linear duplexes, generate chromosomes with exchange of flanking DNA (a crossover) or without exchange (a non-crossover). Crossovers, but not non-crossovers, provide a physical connection between homologs that allow tension leading to correct segregation. Cohesion between the two copies of each homolog, provided by sister chromatid cohesins, is also required for tension generation (Figure 1). If crossovers occur too close to each other (Figure 1, second panel from the right) or only near a telomere, there may be insufficient cohesion to allow tension generation [11]. Thus, crossovers are crucial but must be an appropriate distance from each other.
The phenomenon of crossovers being farther apart than expected from their random distribution is called crossover interference and was first reported by Sturtevant in 1915 [12]. Studying fruit flies (Drosophila melanogaster), he saw that chromosomes with two crossovers in adjacent intervals were observed less frequently than predicted from independence of crossovers in the two intervals; i.e., double crossovers were less frequent than the product of the crossover frequencies in the individual intervals. Interference (I) can be defined quantitatively as I = 1 – CoC (the coefficient of coincidence), where CoC = RD/(R1 • R2); RD is the frequency of double crossovers, and R1 and R2 are the frequencies in intervals 1 and 2, respectively. Thus, if RD = 0, there are no double crossovers and interference is complete (I = 1). If RD = R1 • R2, double crossovers occur at the frequency expected from independence, and I = 0. In Sturtevant’s studies, I ranged from 0.77 to 0.20. Later studies showed that I is a function of the sizes of the intervals: I is nearly 1 for small intervals and approaches 0 when the intervals are large [approximately the size of the interval in which one crossover per cell occurs on average in the population of diploid meiotic cells, or 50 centiMorgans (cM) in the population of haploid gametes] (reviewed by Foss et al. [13]). Similar observations were made in other species, including the filamentous fungus Neurospora crassa [13]. Interference is also observed in the budding yeast Saccharomyces cerevisiae [14] but at a lower level than in D. melanogaster or N. crassa.
A molecular mechanism of crossover interference remained unknown until recently. Indeed, the first model of which we are aware was published only in 1990, three-quarters of a century after discovery of the phenomenon. King and Mortimer [15] postulated that after one crossover was formed, some substance diffused along the chromosomes in each direction and prevented any further crossovers from occurring; non-crossovers could still occur, however. Foss et al. [13] proposed that once one crossover was formed, a set number of non-crossovers to each side of the crossover must be made before another could be formed. Experiments showed this model did not apply to S. cerevisiae [16]. Fujitani et al. [17] postulated that a crossover-promoting factor diffuses randomly along a chromosome until it forms a crossover and remains there. However, when another copy of the crossover-promoting factor encounters a similar diffusing factor or another at a crossover point, it is inactivated, resulting in interference. Recent studies in the roundworm Caenorhabditis elegans describe the meiosis-specific synaptonemal complex (SC) between homologs as having liquid-crystal-like properties that could facilitate diffusion of interference-related factors along the chromosome, as described in the above models [18]; the SC proteins Zhp-1 to Zhp-4 appear to be such diffusible proteins [19], but their mode of action is unclear. Kleckner et al. [20] viewed paired homologs as a rigid beam coated with a brittle film; as the beam is bent, tension builds locally in the film. They postulated that tension on the homologs is required to form a crossover, but crossover formation relieves the tension locally, much as bending the rigid beam would produce tension and subsequently a crack in the brittle film with loss of nearby tension. Thus, a second crossover could form at some distance but not nearby. Hulten [21] suggested that homologs approach each other and undergo separate wave-like oscillatory movements. Where homologs approach each other closely, a crossover forms; an additional crossover is unlikely nearby because the wave-like shape of the chromosomes keeps the homologs apart nearby. Except for the C. elegans SC proteins Zhp-1 to Zhp-4 noted above [19], these models did not state the nature of the critical substances involved, their rates or mode of diffusion (active or passive), or the source of tension and its role in crossover formation. Thus, testing most of these models genetically has been problematic.
Recently, a model incorporating identified molecules with known properties has been proposed in the fission yeast Schizosaccharomyces pombe (Figure 2). Fowler et al. [22] proposed this model based on their previous identification of three linear element proteins that bind chromosomal sites at which DSBs are made at high frequency (DSB hotspots) in S. pombe; these proteins are also required for formation of most of the DSBs at most hotspots [23]. In this model for crossover interference, nearby hotspots bound by their determinant proteins form a cluster in which a single DSB is made, resulting in at most a single crossover in the chromosomal interval corresponding to the DSB hotspot-clustered interval.
Figure 2. Clustering model for crossover interference [22].
DSB hotspots (green circles) on each pair of sister chromatids (one homolog; panel A) or on both homologs (panel B) are gathered into a cluster in a limited region (bounded by dotted grey lines). Within each cluster, one DSB (yellow lightning bolt) is formed and activates the Tel1 DNA damage protein kinase, which phosphorylates and inactivates some component of the DSB-forming complex (not shown). Consequently, at most only one crossover (CO) is made in each limited region. DSBs, and thus crossovers, are made independently on each homolog in panel A; two crossovers can occur close together more frequently than in panel B, and interference is stronger in species represented by panel B than in those represented by panel A.
Several observations in S. pombe support this clustering model. The DSB hotspot protein determinants (Rec25, Rec27, and Mug20) are meiosis-specific and form mutually dependent nuclear foci seen by light microscopy [24, 23]. A modified form of chromosomal conformation capture (3C) technique shows that DSB-hotspot-bound Rec27 physically interacts with other hotspots to form a cluster but preferentially with those located less than ~200 kb away; this distance corresponds to about 35 cM, about the distance corresponding to one crossover (as previously discussed). In addition, introduction of a novel Rec27-dependent hotspot reduces DSB formation at nearby hotspots (a phenomenon called DSB hotspot competition) [22]. Furthermore, formation of doubly-broken DNA molecules between nearby hotspots occurs much less frequently than expected from independent breakage at the two sites (DSB interference) [22].
The strength of these three phenomena (DSB hotspot clustering, DSB hotspot competition, and DSB interference) in S. pombe decreases with distance between hotspots and becomes negligible at ~200 kb [22]. DSB interference depends on the DNA damage-response protein kinase Tel1 (homolog of the human ATM protein associated with ataxia telangiectasia) [22]. Crossover interference also depends on Tel1 [22]. In fact, both types of interference become negative in the absence of Tel1 – more close double events, for both DSBs and crossovers, arise than expected from independence [22]. These features are expected if formation of the first DSB in a cluster activates Tel1, which then phosphorylates and inactivates some component of the DSB-forming complex. In the absence of Tel1, the complex could make multiple DSBs in one cluster, frequently giving rise to doubly-broken DNA and double crossovers; such coordinated action readily accounts for the observed negative interference. As expected, negative DSB interference is strongest for close DSB hotspots and becomes negligible at ~200 kb. The similar distances over which hotspot clustering, DSB competition, and both positive and negative DSB interference act support this model.
Crossover interference in S. pombe is weaker than that reported in other species: I = 0.26 ± 0.051 for the region most studied [22]. This low value is consistent with the observation that DSB competition acts only in cis (i.e., on one homolog) in S. pombe [22]; DSB interference is defined, and thus measured, only in cis. If clusters also form only in cis, as postulated (Figure 2, left), then DSBs would arise independently on the two homologs and give rise to double crossovers but not as frequently as independence would predict because of the absence of nearby DSB pairs on each homolog. In species with strong interference, such as D. melanogaster or N. crassa, clusters may encompass both homologs (Figure 2, right). DSB interference and competition also occur in S. cerevisiae [25–30]. In S. cerevisiae, DSB interference depends on Tel1 and becomes negative in its absence [30]; crossover interference is also reduced in the absence of Tel1 [31]. Crossover interference in this species is stronger than that in S. pombe but not complete; it may have a mixture of clusters encompassing one or both homologs. Additional or alternative mechanisms may apply in some species. For example, in S. cerevisiae cytological interference, based on the positions of microscopic foci of certain SC proteins such as the potential SUMO E3 ligase Zip3, extends over limited distances along chromosomes; it is a distinct phenomenon related to crossover interference, but its molecular mechanism remains to be determined, although topoisomerase II is partially required [32–34]. Further studies in a variety of species are required to determine the generality of the clustering model, a molecular mechanism that explains well the observations in S. pombe and may be applicable to other species as well.
The molecular mechanism of crossover interference presented here (DSB hotspot clustering) was conceived only with the findings of DSB precursors to crossovers, DSB hotspots and their protein determinants, and the clustering of nearby hotspots via these determinants. In addition, the basic finding that DNA is the genetic material [35], made 30 years after the discovery of crossover interference, was essential for proposing any material basis for interference. The advent of 3C analysis [36] made possible the detailed study of DSB hotspot interactions at a distance (clusters), an essential feature of the mechanism. While these discoveries were not aimed at elucidating interference, they were necessary for this scientific advance.
Centromeric repression of deleterious crossovers
Correct chromosome segregation requires not only that crossovers be sufficiently far apart but also that they not be too close to the centromere. Presumably, a crossover near the centromere disrupts kinetochore orientation or sister chromatid cohesion, or both, and thus proper chromosome movement (Figure 1, far right). The region around the centromere (the pericentric region) has a reduced crossover density in all species tested. In humans, Down syndrome trisomy 21 as well as maternally-derived sex chromosome trisomies (47 total chromosomes instead of the normal 46) are often associated with meiotic crossovers at or near the centromeres [37, 38]. Only recently has a molecular basis for this long-known effect been elucidated.
The reduced frequency of meiotic crossovers near centromeres was first noted in 1932 by Beadle [39] in studies of chromosomal translocations in D. melanogaster: a genetic interval had fewer crossovers when it was moved close to a centromere. About three-quarters of a century later, Westphal and Reuter [40] found that crossovers in the interval spanning a pericentric region were more abundant in several mutants with altered heterochromatin, most notably in Su(var)3–9 mutants lacking a histone H3 lysine 9 (H3 K9) methyltransferase. Peng and Karpen [41] found that this mutant had more foci of the DNA damage-induced phosphorylated histone γ-H2Av. They interpreted these foci as DNA double-strand breaks (DSBs), which in budding and fission yeasts are demonstrated initiators of meiotic recombination [42–45] and may be in other species with Spo11-dependent recombination (see below). The studies of Peng and Karpen [41] indicate that heterochromatin blocks the formation of DSBs and thus crossovers near the centromeres of D. melanogaster.
In the fission yeast S. pombe, heterochromatin prevents essentially all pericentric meiotic crossovers. Using markers closely flanking the pericentric heterochromatic region, Ellermeier and Smith [46] found up to 100-fold more pericentric crossovers, approaching the genome median density, in mutants lacking Clr4 (H3 K9 methyltransferase) or components of RNA interference (RNAi) needed for heterochromatin establishment. DSBs were present in mutants with increased pericentric recombination but were not detectable in wild-type. Thus, this work showed directly that DSB repression by RNAi and heterochromatin is the mechanism of reduced pericentric meiotic recombination.
Nambiar and Smith [47] further elucidated the molecular mechanism responsible for pericentric DSB and crossover repression. Swi6 (homolog of heterochromatin protein HP1 of D. melanogaster, mice, and humans) binds to methylated H3 K9 in heterochromatin and plays both negative and positive roles in pericentric DSB and crossover formation (Figure 3). In its negative role, Swi6 binds and recruits to the pericentric region the sister chromatid cohesin subunit Psc3, which then excludes its meiosis-specific paralog Rec11 from the pericentric region. In chromosomal arms Rec11, after its phosphorylation by a casein kinase homolog, recruits Rec10, a protein essential for activation of the DSB-forming complex, whose active site is in Spo11 (called Rec12 in S. pombe) [45, 48–50]. Thus, Swi6 indirectly blocks DSB formation in the pericentric region. The positive role of Swi6 involves Rec8, a meiosis-specific cohesin subunit associated with Rec11 and essential for most DSB and crossover formation in chromosomal arms [48]; Rec8 binds Psc3 in pericentric regions but Rec11 in chromosomal arms [51]. Thus, removing Swi6 leads to low levels of (or no) Rec8 in pericentric regions; therefore, both Rec11 and DSBs are lacking in pericentric regions in swi6 mutants. The dual roles of Swi6 may reflect a fail-safe mechanism to ensure absence of pericentric crossovers, which prevent accurate chromosome segregation and gamete viability.
Figure 3. Mechanism for pericentric repression in S. pombe [47].
In euchromatic DNA, the major form in chromosomal arms, the meiosis-specific cohesin complex contains Rec8 and Rec11 subunits along with Smc1-Smc3. Rec11 recruits the linear element protein Rec10, which activates Spo11(Rec12)-dependent DSB formation. Other linear element proteins Rec25, Rec27, and Mug20 are recruited along with Rec10 at DSB hotspots. However, in heterochromatic DNA, the major form in pericentric regions, the cohesin complex contains Psc3 instead of Rec11; Psc3 does not bind Rec10 or activate DSB formation. Rec8-Psc3 specific loading is mediated by Swi6, an HP1 homolog, which specifically binds to H3 K9 methylated histones present in heterochromatin. This model is for S. pombe but is likely conserved even in mammals (see text). Each solid line indicates the double-stranded DNA of a chromatid.
In a recent study, Hartmann et al. [52] attempted to elucidate further the mechanism of centromere-proximal crossover repression in D. melanogaster. In accord with the above studies, they concluded that both heterochromatin and the “centromere effect,” completely or in a centromere distance-dependent manner, respectively, contribute to pericentric crossover repression. A marginal effect of removal of the Blm helicase led them to propose that this DNA helicase is involved, in an unstated way, in the “centromere effect.” The heterochromatin effect may be similar to that described here for S. pombe.
Features of meiotic recombination and pericentric regions in other species suggest that the molecular mechanism elucidated in S. pombe is wide-spread. Clearly, heterochromatin plays a repressive role in S. pombe and D. melanogaster. STAG3, the Rec11 homolog of mice and humans, is meiosis-specific, phosphorylated, localized to chromosomal arms, and required for fertility [53, 54]. In mice, Bhattacharyya et al. [55] recently showed that the meiotic chromosomal axis-associated cohesin complexes containing STAG3 and the hotspot determinant PRDM9 activate SPO11 for DSB formation in chromosomal arms. This result parallels the role of cohesin subunits Rec8 and Rec11 in meiotic DSB formation in S. pombe and is consistent with mouse pericentric recombination also being limited by the absence of STAG3. In D. melanogaster, the cohesin-like, meiosis-specific protein C(2)M encodes a cohesin subunit (kleisin)-related protein and promotes formation of crossovers in chromosomal arms [56]. These observations show that the molecular mechanism of pericentric crossover repression is likely conserved in diverse species.
A different molecular scenario operates in S. cerevisiae, which lacks methylated H3 K9-containing heterochromatin. Vincenten et al. [57] showed that removing components of the kinetochore, which binds the centromere for chromosomal segregation, increases DSB frequency ~5-fold in a short (~10 kb) interval encompassing the centromere. In addition, they proposed that the kinetochore deposits in the pericentric region cohesins that guide DSB repair with the sister chromatid and thus produce no genetic crossovers; this feature can account for the ~20-fold higher crossover frequency in this pericentric region in kinetochore mutants relative to that in wild type. S. cerevisiae appears to lack a meiosis-specific cohesin subunit comparable to Rec11 or STAG3 and may, therefore, have adopted a different strategy for preventing deleterious pericentric crossovers. Nevertheless, it is interesting that cohesins are at the basis of pericentric recombination control in the several species examined.
As for crossover interference, elucidation of the molecular basis of pericentric crossover repression required many advances between the observation and knowing the mechanism. Knowledge of the proteins required for DSB and crossover formation, and of their interactions, was essential to direct artificial recruitment of Rec10 to the pericentric region and thus high crossover frequency there [47]. Knowledge of the critical role of methylated H3 K9 in heterochromatin and its binding to Swi6, as well as the role of Swi6 in depositing pericentric-specific cohesin complexes, was also essential. These advances were independent and came together in a surprising way to help elucidate the molecular mechanisms to prevent deleterious crossovers.
Concluding Remarks and Future Perspectives
There was a long time between the observations of crossover interference and pericentric repression and determination of their molecular mechanisms. Thus, it may be a long time before the molecular mechanisms are completely known and, perhaps, recapitulated with purified components. Meiosis is one of the most diverse features of biology [54], making it likely that additional mechanisms remain to be described in various species. Having specific molecular mechanisms (Figures 2 and 3; [53]) will enable tests of these models and modifications as necessary. These mechanisms provide a basis for studying these phenomena in other species and finding them similar, or not, to those proposed.
Numerous questions remain even with these models in hand, as discussed in the “Outstanding questions” box. Although many critical proteins have been identified, their additional features must be determined. For example, it is still unknown what loads the S. pombe linear element proteins onto DSB hotspots over a limited region to effect DSB and crossover interference and what prevents Rec11 cohesin subunit from being loaded in pericentric regions to prevent DSBs and crossovers. Knowing the identities of these key proteins, however, will allow seeking the answers with a combination of methods in genetics, cell biology, and biochemistry. This knowledge will lead to a better understanding of how viable gametes are formed to continue a healthy lineage.
Highlights.
Meiotic crossovers are important for proper chromosome segregation to form viable haploid gametes from diploid precursors.
Crossovers too near each other or too near the centromere block proper segregation and may lead to birth defects and infertility.
Crossovers are spaced far apart by “interference,” first observed in 1915, and are kept away from the centromere by “pericentric repression,” observed in 1932.
Crossover interference in S. pombe stems from DNA double-strand break (DSB) interference at hotspots, which form clusters through DSB hotspot-determinant proteins; both types of interference are regulated by Tel1 protein kinase.
Pericentric repression stems from DSB inhibition due to absence of a key activator, the meiosis-specific sister chromatid cohesin subunit Rec11 in S. pombe and its homolog STAG3 in mammals, by complex circuitry now elucidated.
Outstanding questions.
How are DSB hotspot clusters formed over limited regions? Crossover interference acts over chromosomal regions approximately the size in which, on average, one crossover occurs (50 centiMorgans), corresponding to about 250 kb in S. pombe. Possibly, some “machine”, such as cohesin or condensin, loads linear element proteins over a limited region and gathers them into a single cluster.
Do clusters form between sister chromatids in some species and between homologs (all four chromatids) in others? Clustering, and thus DSB interference, over both homologs would account for strong crossover interference in some species, such as Drosophila, whereas clustering over only sister chromatids would allow two DSBs in a genetic interval and thus incomplete crossover interference in other species, such as S. pombe. Species with a mixture would have intermediate levels of crossover interference, as appears to be the case in S. cerevisiae.
How are additional DSBs blocked after the first DSB is formed? In S. pombe and distantly related species, Tel1 (homolog of the human ATM protein kinase activated by DNA damage) is required for full crossover interference. Identifying the substrate(s) for Tel1 would further illuminate the molecular basis of crossover interference.
How is the meiosis-specific cohesin subunit (Rec11 or STAG3) excluded from the pericentric region? In S. pombe the mitotic cohesin subunit Psc3 is loaded at the pericentric region by the heterochromatin protein Swi6 (homolog of Drosophila and mammalian HP1). Presumably, this excludes loading by Swi6 of its paralog Rec11, which is required for full DSB formation in chromosomal arms, but how the complex that loads Rec11 in arms is prevented from loading Rec11 in pericentric regions remains unknown.
Acknowledgments
We are grateful to Sue Amundsen, Randy Hyppa, and Nancy Maizels for helpful comments on the manuscript. This work was supported by research grant R35 GM118120 to GRS from the National Institute of General Medical Sciences of the United States of America.
Glossary
- aneuploid
a cell with an abnormal set of chromosomes, such as a trisome (a diploid with an extra copy of a chromosome) or a monosome (a diploid lacking one copy of a chromosome).
- centiMorgan (cM)
0.01 Morgans; a Morgan is the genetic distance along a chromosome generated from one crossover in a meiotic cell; since only two of the four chromatids recombine in one event, one crossover generates 50 cM in the population of cells.
- centromere
the point on a chromosome to which microtubule “strings” attach to segregate chromosomes.
- chromosomal conformation capture (3C) technique
a method employing ligation of DNA fragments from sites far apart on a chromosome (generally more than about 5 kb) or on separate chromosomes, to show they are close in 3-dimensional space in the nucleus.
- chromosome segregation
movement of each copy of a duplicated chromosome to opposite sides of the cell to incorporate each into one daughter cell or nucleus.
- coefficient of coincidence (CoC)
observed frequency of double crossovers divided by the frequency expected from independence. CoC = RD/(R1 • R2); I = 1 – CoC. I = 0 if double crossovers occur independently (no interference). I = 1 if there are no double crossovers (complete interference). I < 0 indicates negative interference, or the occurrence of more double crossovers than independence predicts.
- cohesin
a large ring-shaped protein complex that holds sister chromatids together after replication.
- crossover
reciprocal recombination between homologs; e.g., formation of ++ and ab from a+ and +b parental homologs.
- diploid
a cell, such as those of the mammalian body, with two copies of each chromosome.
- DNA double-strand break (DSB)
a chromosome with each DNA strand cut at close positions, producing two chromosomal fragments; DSB repair with an intact homolog can lead to crossovers.
- DSB hotspot
a point or small region on a chromosome at which DSBs are made at a frequency higher than the genome average; these may range from about a hundred to several thousand bp in width and be cut as much as a thousand times the genome average.
- haploid
a cell, such as an egg or sperm, with only one copy of each chromosome.
- heterochromatin
a chromosomal region, such as the pericentric region, containing modified histones (often taken to be histone H3 methylated at lysine 9, or H3 K9Me).
- Holliday junction
a DNA intermediate between DSB formation and crossover formation; it contains two ds DNA molecules connected by two single DNA strands exchanged between the nearly identical ds DNA molecules.
- homolog (protein)
proteins with closely related amino acid sequences occurring in different species.
- homologs (chromosome)
in eukaryotes two nearly identical chromosomes, one inherited from each parent.
- interference (I)
occurrence of two crossovers on one homolog pair at a frequency (RD) less than the product of the frequencies of the individual crossovers (R1 and R2).
- kinetochore
a large protein complex that connects the centromere and microtubules.
- linear element proteins
S. pombe proteins resembling proteins of the synaptonemal complex of other species; Rec10 is required for all meiotic DSB formation and recombination, and three small partner proteins (Rec25, Rec27, and Mug20) bind to DSB hotspots and promote DSB formation.
- meiosis I and II (MI and MII)
the two nuclear divisions of meiosis; in MI homologs are replicated and recombine, and the paired homolog centromeres separate from each other; in MII, without any further replication, paired sister centromeres separate from each other.
- meiosis
special nuclear divisions that form one to four haploid cells from a diploid cell.
- paralog
proteins with closely related amino acid sequences occurring in the same species, often with different but related functions.
- pericentric region
region around the centromere, often more than 100 kb of DNA.
- recombination
formation of a chromosome containing parts of two interacting chromosomes; homologous recombination preserves the overall structure of the two participating homologs.
- sister chromatids
the two copies of a homolog after its replication.
- synaptonemal complex
a large, meiosis-specific proteinaceous structure holding homologs together in alignment and extending the length of the homologs.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jang JK, et al. (1995) Induction of metaphase arrest in Drosophila oocytes by chiasma-based kinetochore tension. Science 268, 1917–1919 [DOI] [PubMed] [Google Scholar]
- 2.Li X and Nicklas RB (1995) Mitotic forces control a cell-cycle checkpoint. Nature 373, 630–632 [DOI] [PubMed] [Google Scholar]
- 3.Koehler KE, et al. (1996) Recombination and nondisjunction in humans and flies. Human Molecular Genetics 5, 1495–1504 [DOI] [PubMed] [Google Scholar]
- 4.Marston AL and Wassmann K (2017) Multiple Duties for Spindle Assembly Checkpoint Kinases in Meiosis. Front Cell Dev Biol 5, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassold T and Chiu D (1985) Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70, 11–17 [DOI] [PubMed] [Google Scholar]
- 6.Capalbo A, et al. (2017) Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update 23, 706–722 [DOI] [PubMed] [Google Scholar]
- 7.Nagaoka SI, et al. (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13, 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou Y, et al. (2013) Genome analyses of single human oocytes. Cell 155, 1492–1506 [DOI] [PubMed] [Google Scholar]
- 9.Ottolini CS, et al. (2015) Genome-wide maps of recombination and chromosome segregation in human oocytes and embryos show selection for maternal recombination rates. Nat Genet 47, 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cromie GA and Smith GR (2008) Meiotic recombination in Schizosaccharomyces pombe: A paradigm for genetic and molecular analysis In Recombination and meiosis: Models, means, and evolution (Egel R and Lankenau D-H, eds), pp. 195–230, Springer-Verlag; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nambiar M, et al. (2019) Distributing meiotic crossovers for optimal fertility and evolution. DNA Repair (Amst) 81, 102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sturtevant AH (1915) The Behavior of the Chromosomes as Studied through Linkage. Z. Indukt. Abstammungs. Vererbungsl 13, 234–287 [Google Scholar]
- 13.Foss E, et al. (1993) Chiasma interference as a function of genetic distance. Genetics 133, 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaback DB, et al. (1999) Chromosome size-dependent control of meiotic reciprocal recombination in Saccharomyces cerevisiae: the role of crossover interference. Genetics 152, 1475–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King JS and Mortimer RK (1990) A polymerization model of chiasma interference and corresponding computer simulation. Genetics 126, 1127–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foss EJ and Stahl FW (1995) A test of a counting model for chiasma interference. Genetics 139, 1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujitani Y, et al. (2002) A reaction-diffusion model for interference in meiotic crossing over. Genetics 161, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rog O, et al. (2017) The synaptonemal complex has liquid crystalline properties and spatially regulates meiotic recombination factors. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. (2018) A compartmentalized signaling network mediates crossover control in meiosis. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleckner N, et al. (2004) A mechanical basis for chromosome function. Proc. Natl. Acad. Sci. USA 101, 12592–12597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulten MA (2011) On the origin of crossover interference: A chromosome oscillatory movement (COM) model. Mol Cytogenet 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fowler KR, et al. (2018) Physical basis for long-distance communication along meiotic chromosomes. Proc Natl Acad Sci U S A 115, E9333–E9342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler KR, et al. (2013) Protein determinants of meiotic DNA break hotspots. Mol. Cell 49, 983–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis L, et al. (2008) Rec25 and Rec27, novel components of meiotic linear elements, link cohesin to DNA breakage and recombination in fission yeast. Current Biology 18, 849–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu TC and Lichten M (1995) Factors that affect the location and frequency of meiosis-induced double-strand breaks in Saccharomyces cerevisiae. Genetics 140, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L and Kleckner N (1995) Sequence non-specific double-strand breaks and interhomolog interactions prior to double-strand break formation at a meiotic recombination hot spot in yeast. EMBO Journal 14, 5115–5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan QQ, et al. (1997) Competition between adjacent meiotic recombination hotspots in the yeast Saccharomyces cerevisiae. Genetics 145, 661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jessop L, et al. (2005) Infrequent co-conversion of markers flanking a meiotic recombination initiation site in Saccharomyces cerevisiae. Genetics 169, 1353–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robine N, et al. (2007) Genome-wide redistribution of meiotic double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 27, 1868–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia V, et al. (2015) Tel1(ATM)-mediated interference suppresses clustered meiotic double-strand-break formation. Nature 520, 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson CM, et al. (2015) Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM. PLoS Genet 11, e1005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SY, et al. (2008) Global analysis of the meiotic crossover landscape. Development Cell 15, 401–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, et al. (2014) Topoisomerase II mediates meiotic crossover interference. Nature 511, 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyatnitskaya A, et al. (2019) Crossing and zipping: molecular duties of the ZMM proteins in meiosis. Chromosoma 128, 181–198 [DOI] [PubMed] [Google Scholar]
- 35.Avery OT, et al. (1944) Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types : Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii. J Exp Med 79, 137–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dekker J, et al. (2002) Capturing chromosome conformation. Science 295, 1306–1311 [DOI] [PubMed] [Google Scholar]
- 37.May KM, et al. (1990) The parental origin of the extra X chromosome in 47,XXX females. American journal of human genetics 46, 754–761 [PMC free article] [PubMed] [Google Scholar]
- 38.Lamb NE, et al. (1996) Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet 14, 400–405 [DOI] [PubMed] [Google Scholar]
- 39.Beadle GW (1932) A Possible Influence of the Spindle Fibre on Crossing-Over in Drosophila. Proc Natl Acad Sci U S A 18, 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westphal T and Reuter G (2002) Recombinogenic effects of suppressors of position-effect variegation in Drosophila. Genetics 160, 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng JC and Karpen GH (2009) Heterochromatic genome stability requires regulators of histone H3 K9 methylation. PLoS Genet 5, e1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Game JC, et al. (1989) Use of a ring chromosome and pulsed-field gels to study interhomolog recombination, double-strand DNA breaks and sister-chromatid exchange in yeast. Genetics 123, 695–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun H, et al. (1989) Double-strand breaks at an initiation site for meiotic gene conversion. Nature 338, 87–90 [DOI] [PubMed] [Google Scholar]
- 44.Cao L, et al. (1990) A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell 61, 1089–1101 [DOI] [PubMed] [Google Scholar]
- 45.Cervantes MD, et al. (2000) Meiotic DNA breaks associated with recombination in S. pombe. Mol. Cell 5, 883–888 [DOI] [PubMed] [Google Scholar]
- 46.Ellermeier C, et al. (2010) RNAi and heterochromatin repress centromeric meiotic recombination. Proc. Natl. Acad. Sci. USA 107, 8701–8705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nambiar M and Smith GR (2018) Pericentromere-Specific Cohesin Complex Prevents Meiotic Pericentric DNA Double-Strand Breaks and Lethal Crossovers. Mol Cell 71, 540–553 e544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellermeier C and Smith GR (2005) Cohesins are required for meiotic DNA breakage and recombination in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. USA 102, 10952–10957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phadnis N, et al. (2015) Casein kinase 1 and phosphorylation of cohesin subunit Rec11 (SA3) promote meiotic recombination through linear element formation. Plos Genetics 11, e1005225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakuno T and Watanabe Y (2015) Phosphorylation of cohesin Rec11/SA3 by casein kinase 1 promotes homologous recombination by assembling the meiotic chromosome axis. Developmental cell 32, 220–230 [DOI] [PubMed] [Google Scholar]
- 51.Kitajima TS, et al. (2003) Rec8 cleavage by separase is required for meiotic nuclear divisions in fission yeast. The EMBO journal 22, 5643–5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartmann M, et al. (2019) Centromere-Proximal Meiotic Crossovers in Drosophila melanogaster Are Suppressed by Both Highly Repetitive Heterochromatin and Proximity to the Centromere. Genetics 213, 113–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prieto I, et al. (2001) Mammalian STAG3 is a cohesin specific to sister chromatid arms in meiosis I. Nat. Cell Biol 3, 761–766 [DOI] [PubMed] [Google Scholar]
- 54.Fukuda T, et al. (2012) Phosphorylation of chromosome core components may serve as axis marks for the status of chromosomal events during mammalian meiosis. PLoS Genet 8, e1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhattacharyya T, et al. (2019) Prdm9 and Meiotic Cohesin Proteins Cooperatively Promote DNA Double-Strand Break Formation in Mammalian Spermatocytes. Curr Biol 29, 1002–1018 e1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manheim EA and McKim KS (2003) The Synaptonemal complex component C(2)M regulates meiotic crossing over in Drosophila. Curr Biol 13, 276–285 [DOI] [PubMed] [Google Scholar]
- 57.Vincenten N, et al. (2015) The kinetochore prevents centromere-proximal crossover recombination during meiosis. Elife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]