Abstract
Facet joints are the only synovial joints in the spine and can be involved in a large number of pathological processes including arthropathy, infection, inflammation, trauma and tumour.
In this review article, we present a spectrum of pathologies that arise from or involve facet joints that we have encountered in our tertiary orthopaedic and spinal centre. The objective of this review is to create an aide memoire for the general radiologist who may encounter facet joint pathology, which they may not be familiar with.
Keywords: Facet, Anatomy, Arthritis, Tumour, Infection
1. Introduction
Facet joints are the only synovial joints in the spine and can be involved in an array of processes. Isolated facet joint disease can be overlooked on spinal imaging either due to incomplete imaging or due to unfamiliarity with the spectrum of diseases that can involve the facet joints.
In this article, we will review the spectrum of diseases, both common and rarely encountered, which have presented to our tertiary referral centre for spinal surgery. Trauma to the facet joints is excluded from this review article.
2. Facet joint functional anatomy
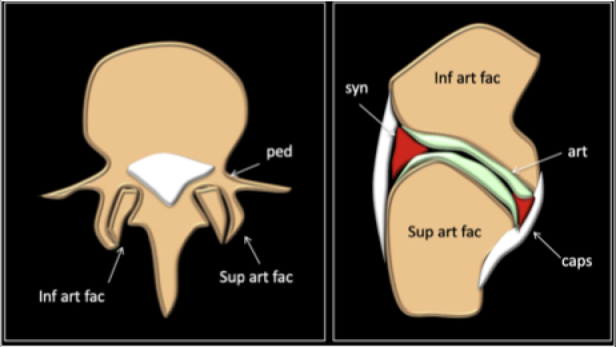
Facet joints are symmetrical synovial-lined joints with a fibrous capsule that connect the articular facets of the vertebrae. The superior facet of the lower vertebra articulates with the inferior i facet of the vertebra above (Fig. 1).
Fig. 1.
Animation of anatomy of facet joint.
The facet joints are situated between the pedicle and lamina of the same vertebra and form the articular pillars that act to provide structural stability to the vertebral column as a whole.
The functional unit of the vertebral column consists of two adjacent vertebral bodies, an intervertebral disc and two facet joints. This unit is called the motion segment.1
The posterior portion of the spinal motion segment guides spinal movement, with the type of motion determined by the plane of facet articulation. The coronal orientation of the thoracic facet joints minimises extension but allows rotation, while the sagittal oblique orientation of the lumbar articular facets minimises rotation.1
The posterior portion of the motion segment contains the posterior ligamentous complex (PLC), which plays a critical stabilizing role. The PLC is composed of the supraspinous ligament, interspinous ligament, articular facet capsules, and ligamentum flavum.1
3. Imaging techniques of facet joints
Radiographs are often used for initial assessment of facet mediated back pain. Although oblique views are better at visualising the facet joints than AP or lateral views, their utilisation has been replaced by cross sectional studies such as CT and MRI.2
Both CT and MRI are equally useful in assessing the morphology of the facet joints. However, MRI has the advantage of depicting bone marrow and soft tissue oedema.
In addition, Single photon emission computed tomography (SPECT) is utilised for detecting abnormal activity or inflammation.2 SPECT CT has been shown to be useful in selecting patients for facet joint injection where a positive uptake may predict a better outcome.2
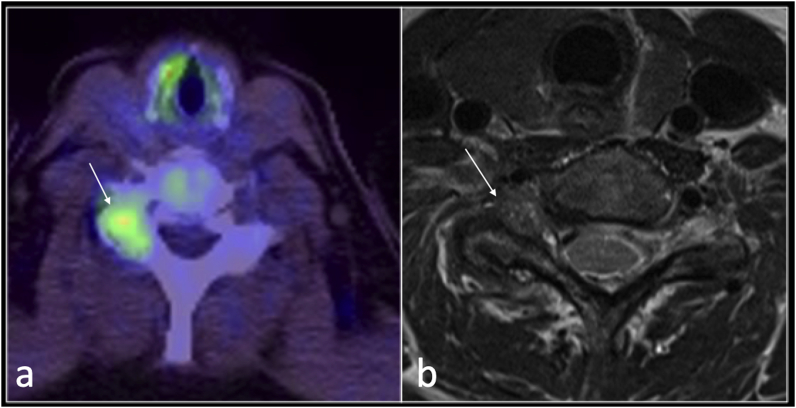
Other functional imaging, such as PET CT, may depict pain generator such as in this case of non-specific synovitis was demonstrated incidentally on PET CT (Fig. 2).
Fig. 2.
PET CT axial (a) and T2 axial (b) showing increased uptake in right facet joint on PET-CT with florid synovitis (b) in patient with gout.
4. Spectrum of facet joint diseases
4.1. Facet joint effusion
Several studies have indicated that facet joint effusions can be related to spinal instability and a degenerate spondylolisthesis.3,4
It is well recognised that supine lumbar MRI can underestimate the degree of spinal instability and that, to date, a standing lumbar spine radiograph or a standing lateral flexion extension (SLFE) radiograph is essential for assessing instability before any surgical intervention.
Upright MRI has gained popularity in recent years with the advances in technology and the increasing availability of open MRI scanners. This is particularly helpful for dynamic assessment of spinal canal stenosis and lumbar radiculopathy. With upright position, disc pathologies, foraminal stenosis and facet joint cysts may become more conspicuous.5
In a retrospective study of 139 patients, focussed on the L4/L5 level, Chaput and co-workers have proposed that a facet joint effusion on MRI is defined as curvilinear CSF like high T2 signal which is > 1 mm in depth.3
The authors suggested that an effusion equal to or more than 1.5 mm should prompt a dynamic radiographic assessment as it can be associated with degenerate spondylolisthesis even in the absence of measurable translation on supine MRI.3 Furthermore, in the absence of facet joint effusions and a detectable listhesis on supine MRI, a flexion and extension radiograph is unlikely to show significant listhesis.3Subsequently, there have been increasing concerns amongst spinal surgeons that the presence of a facet joint effusion may indicate potential instability even with normal standing radiographs, and therefore alter the surgical approach from decompression only to fusion and decompression given that the radiograph is only a two-dimensional assessment of the three-dimensional nature of spinal instability.6 Koji Tamai and co-workers suggest that facet joint effusions do not alter the surgical outcome of minimally invasive lumbar decompression in degenerative spondylolisthesis and dynamic radiographs remain the gold standard to assess instability.6
4.2. Facet joints synovial cysts
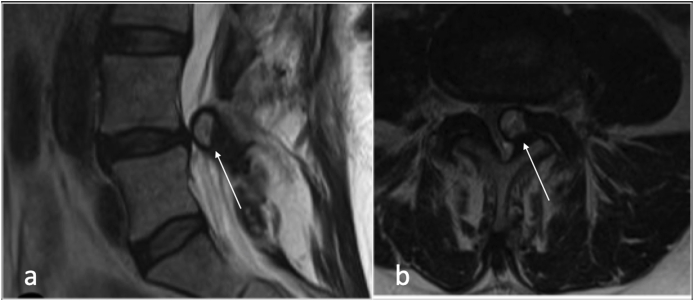
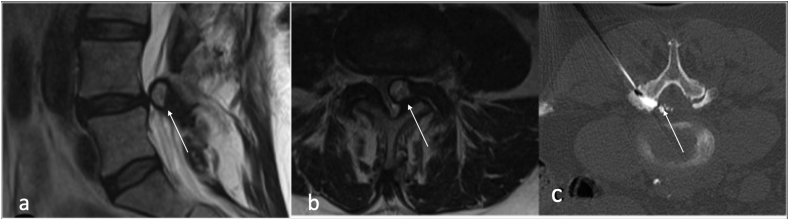
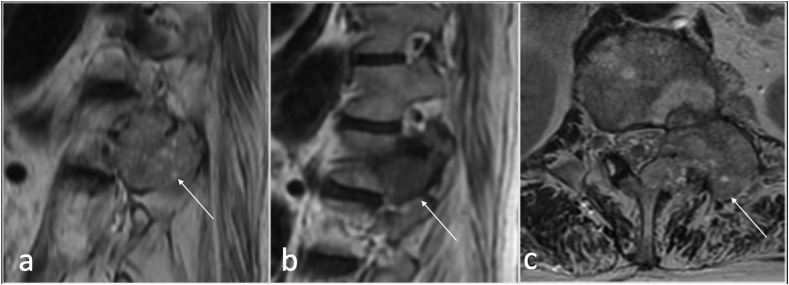
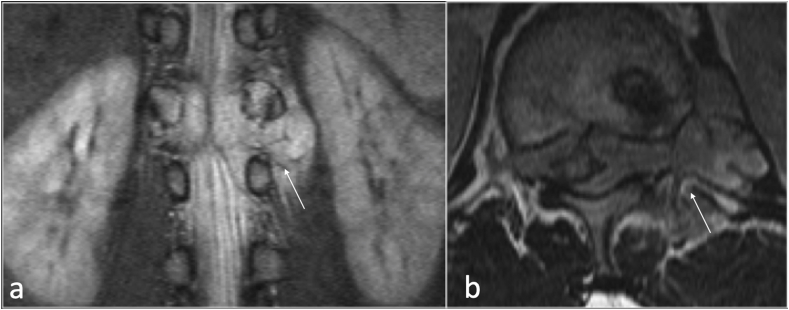
Synovial cysts (SCs) are fluid -filled cysts arising from a defect in the synovial membrane, with or without identifiable communication with the joint itself.7,8 (Fig. 3).
Fig. 3.
Sagittal T2(a), and axial (b) showing left facet joint synovial cyst(arrow).
SCs have a synovial lining while ganglia do not. However, these have similar imaging characteristics.7 Other differential diagnoses for synovial cysts would include conjoint nerve root, sequestered disc segments, intra-spinal cyst and cystic neurofibroma.7 Facet joint cysts maybe found incidentally and be completely asymptomatic or cause radiculopathy, neurogenic claudication, sensory deficits and, to a lesser extent, motor deficits dependents on their location.7, 8, 9 The lumbar spine is the commonest location for synovial cysts, L4/L5 being the most affected level, with rare incidence in the cervical or thoracic segments.7,8
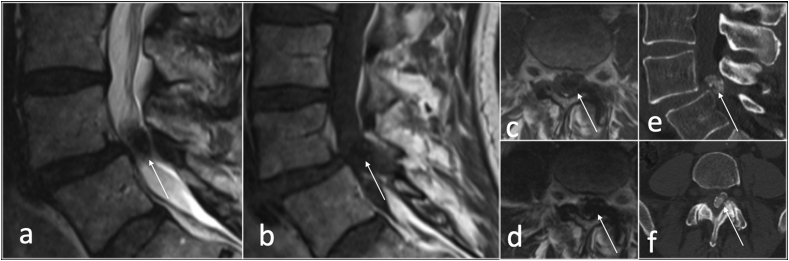
On MRI, the SC is usually a rounded cystic lesion, filled with fluid, in communication with a degenerate facet joint, and typically smaller than 22 mm.8The MRI signal characteristics of synovial cysts varies according to its content, whether it is clear, proteinaceous fluid, haemorrhage or calcification. Calcification within cyst wall appears low signal intensity on both T1 and T2 weighted images whereas haemorrhagic cysts display increased intensity compared to CSF likely due to T1 shortening from methaemoglobin (Fig. 4, Fig. 5).
Fig. 4.
Sagittal T2 (a) and T1(b), axial T1(c,d) and CT sagittal (e) and axial (f) showing calcified left synovial cyst (arrow).
Fig. 5.
Axial T2 (a,b) and T1(c) showing right facet joint cyst with haemorrhage (arrow).
Synovial cysts can on rare occasion haemorrhage into surrounding soft tissues and/or the spinal canal causing acute compression of the spinal cord.9 Facet joint cysts can be treated percutaneously using CT to aspirate or rupture the cyst with or without steroid injection.7
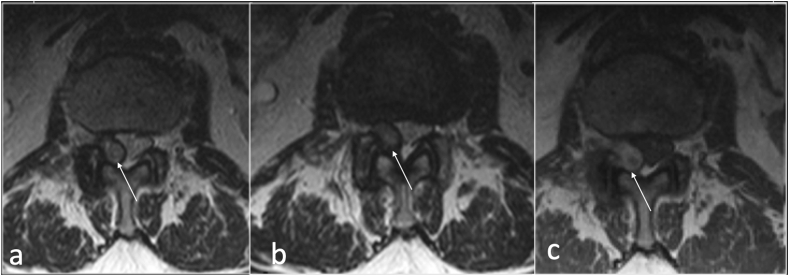
The presence of T2 hyperintense or intermediate signal intensity has been shown to correlate with more successful outcome in percutaneous aspiration.8, 9, 10 (Fig. 6).
Fig. 6.
Sagittal T2(a), axial T2(b) showing left facet joint synovial cyst(arrow) who underwent CT guided aspiration of the cyst (c).
5. Arthritis
5.1. Osteoarthritis of the facet joints
The development of facet OA usually begins with degenerative changes in the articular cartilage and extends to the synovium, joint capsule, subchondral bone, ligaments, and musculature, leading to failure of the entire joint.11 Facet OA is a very common entity. According to a cadaveric study based on the presence of osteophytes in the lumbar facets, OA is a universal finding in adults older than 60 years, and severity increases with age.12
The lumbar spine is the most common site for facet OA, particularly at L4–L5 and L5–S1.
Despite the reported prevalence of facet degeneration, the relationship between facet degeneration and back pain is unclear.11 Although facet arthropathy was believed to be secondary to intervertebral disc disease, a cadaveric study has shown facet OA to be a common finding in people younger than 30 years of age despite the absence of significant disc degeneration.12 Typical radiographic features of OA include joint space narrowing from cartilage thinning, osteophytes, subchondral cysts, articular process hypertrophy, and subchondral bone sclerosis. On MRI, thinning of the articular cartilage, osteophytes narrowing the neural foramina, ligamentous thickening/buckling and joint space effusion are shown. Periarticular oedema and enhancement may exist which can be confusing for inflammatory or infective aetiology.
5.1.1. Role of SPECT for percutaneous injection
SPECT has been shown to be more precise in identifying pain generator in lower back pain and therefore is a very helpful tool in patient's selection for imaging guided facet joint injection.2
5.2. Rheumatoid arthritis (RhA)
The spinal involvement in RhA is predominantly seen at the cervical segment and particularly the atlantoaxial portion which is very crucial to detect early to prevent instability and cord compression.13,14 Progressive synovial inflammation causes fibrovascular proliferation (pannus formation) which leads to bone erosion and ligamentous laxity with subsequent subluxation and instability.15 While the cervical spine is frequently involved in RhA (the second most common site after peripheral joints with prevalence up to 80%), thoracic and lumbar involvement is rare.15
The most common pattern of involvement is that of peridental pannus formation with anterior atlantoaxial subluxation and/or vertical subluxation (basilar invagination). The second most common findings of RhA in the cervical spine is subaxial subluxation secondary to erosive changes in the facet and uncovertebral joints which can extend to the intervertebral disc and be associated with ligamentous laxity. The latter may lead to subluxation in one or multiple levels.15 Multilevel involvement causes a stepladder/staircase deformity.14,15
The imaging appearances of the affected facet joints in RhA are similar to other peripheral synovial joints. The MRI may detect early changes of bone marrow oedema and enhancement before erosive changes occur.13 Performing cervical spine radiographs (Lateral neutral, flexion and extention with an open mouth views) are essential in patients with RhA with duration more than 2 years, to detect early subluxation.14,15
5.3. Seronegative spondyloarthropathy
Seronegative spondylarthritis (SpA) is a term used for arthritidies that are not associated with rheumatoid factors and cause inflammatory back pain that involve the spine and the sacroiliac joints (SIJ). These distinct but related disorders encompass ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis (PsA), enteropathic arthritis (in crohn disease or ulcerative colitis), Juvenile arthritis and undifferentiated spondylarthritis.16,17 Unlike in rheumatoid arthritis, the most common axial skeleton involvement is the SIJs and thoracolumbar spine with a combination of osteodestructive (erosive changes and discovertebral lesions) and osteoproliferative (subchondral sclerosis, syndesmophytoses, bone bridging and ankylosis) changes.16,17 In all types of SpAs, the facet joints, being the only synovial joints in the spine, may be affected with erosive changes, joint effusion, synovitis, bone marrow oedema and leading to ankylosis in the later stages.16In addition, periarticular inflammation in the interspinous and supraspinous ligaments as well as bone marrow oedema in the posterior elements are frequent findings.14On radiographs, arthritic changes of the facet joints are depicted by blurring of the joint clefts, whereas in ankylosis, the joint is no longer delineated.16 MRI remains the gold standard imaging modality to detect early changes such as osteitis, synovitis or early structural lesions such as erosions.
5.4. SAPHO
SAPHO is an abbreviation for synovitis, acne, pustulosis, hyperostosis and osteitis. This chronic remitting inflammatory disease may affect all parts of the spine including vertebral bodies, intervertebral disc, facet joints, ligamentous structures and surrounding soft tissue.
The absence of intradiscal fluid signal and enhancement may be a clue to differentiate SAPHO from spinal infection. Contiguous involvement of vertebral bodies is much more common in SAPHO in contrast to spondyloarthropathy.19 If facet joints are involved, MRI may show T2 hyperintensity and enhancement. This may be unilateral or bilateral and usually involving multiple levels.19
5.5. Crystal pyrophosphate dihydrate deposition (CPPD) arthropathy
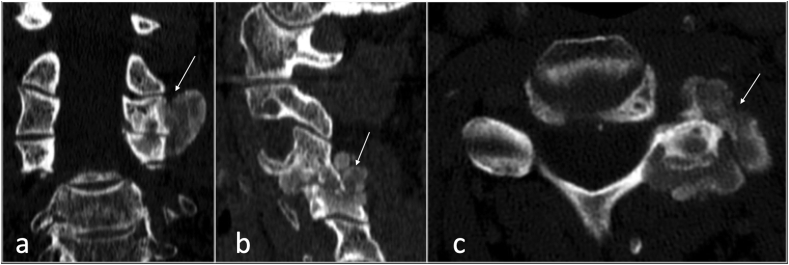
The spinal involvement of CPPD is uncommon and is often seen incidentally in patients with widespread peripheral CPPD arthropathy.20 The cervical and lumbar regions are most involved, in particular, the atlantoaxial articulation, facet joints and ligamentum flavum.20,21 It is usually present in patients above 50 years of age and the prevalence increases with age. CPPD is often associated with history of trauma or spinal surgery.20,21 CPPD can be found in isolation or in association with many underlying disorders such as, osteoarthritis, gout, hemochromatosis, rheumatoid arthritis, diabetes mellitus or thyroid dysfunction to name a few. The CPPD deposits are best shown on CT and radiographs as punctate, linear or mass-like calcifications and located in the facet joint capsule, hyaline cartilage, ligamentous structures or intervertebral discs.
Calcification may be missed on MRI due to the low signal intensity on all sequences, similar to the ligaments and discs (Fig. 7, Fig. 8).
Fig. 7.
CT coronal (a), sagittal (b) and axial (c) showing crystal deposition involving left facet joint (arrow) of CPPD.
Fig. 8.
Sagittal T1 (a), coronal T1 fat suppressed post contrast, T2 axial (c), axial T1 fat suppressed post contrast showing lesion involving left fact joint in keeping with CPPD.
5.6. Gout
Despite many case reports and case series on spinal gout in the literature, the prevalence of spinal gout is difficult to ascertain and is probably under diagnosed.24,25 One prospective study by Konatalpalli and co-workers claimed that spinal erosive changes and/or tophi were found in 35% of established, poorly controlled gout.24 Earlier retrospective studies indicated a smaller incidence of 14%.24Axial gout has nonspecific clinical presentation and varied radiological features that can mimic other disorders that are more prevalent in the spine such as infection especially when imaged with MRI.25
This is further complicated by the fact that acute onset of spinal gout can be associated with constitutional symptoms and raised inflammatory markers.26 The lack of a previous history of gout at the point of MRI imaging, and the unfamiliarity of the masquerading potential of axial gout and tophaceous lesions, may make diagnosis challenging. All levels of the spine can be involved in gout. However, the lumbar spine is most affected, followed by cervical spine, thoracic spine and SIJs.24The facet joints are the most common site of involvement in spinal gout with erosive changes.24,25 Other radiological findings include lobular juxta-articular masses related to facet joints with attenuation higher than adjacent muscles and relative preservation of the synovial joint spaces.24
However, gout can involve any part of the spinal column including the intradural space, extradural space, neural foramina, ligamentum flavum, spinous processes, pedicles, filum terminale and discovertebral junction.26 Although MRI can detect tophi, the appearances are not pathognomonic, therefore, CT is superior to MRI in depicting the characteristic appearances of gout changes with a sclerotic margin and surrounding masses with attenuation higher than adjacent muscle owing to the presence of calcium and sodium urate crystal deposition.26 In the right clinical setting with a strong history of previous or active gout, the presence of juxta-articular facet masses on MRI with low T1 and low to intermediate signal on T2/STIR can be a clue to the diagnosis of axial gout .
Dual energy CT (DECT) is a novel technique that is increasingly being used to detect early gout and aid treatment and follow up patients. The DECT basic principle is to differentiate materials based on their x-ray absorbability in different photon energy.27
5.7. Hydroxyapatite deposition disease (HADD)
Spinal involvement of HAD disease is shown as nonspecific tendinous and ligamentous calcification most commonly in the cervical spine affecting the longus colli muscles. Longitudinal ligaments, intervertebral discs and facet joints may also be involved.28
5.8. Synovial chondromatosis (SC)
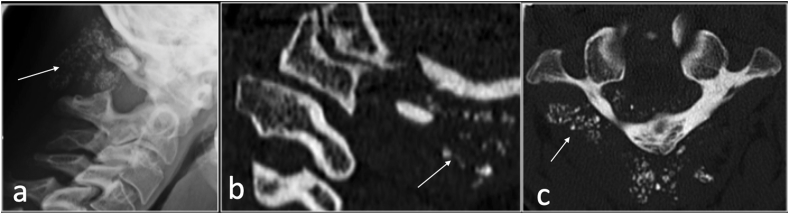
SC is an idiopathic metaplastic monoarticular condition which is characterised by synovial proliferation and metaplasia resulting in calcified or ossified cartilaginous loose bodies.
This intra-articular or extra-articular extension to the soft tissue results in mechanical symptoms.29 The spine involvement of SC is rare and mostly reported in the cervical spine and arising from the facet joints. SC can be primary which is idiopathic metaplastic synovial process or secondary to underlying arthritidies. Typically, classification/ossification is seen on radiographs, although it can be absent in 5%–30% of cases due to the lack of a mineralised matrix.29 CT has improved sensitivity in the detection of calcifications. MRI reveals nodules which are intermediate to isointense on T1 and high on T2, typical of cartilage.29 (Fig. 9).
Fig. 9.
Lateral radiograph(a), CT sagittal(b) and axial (c) showing synovial osteochondromatosis (arrow) involving the articulation of C1/2.
5.9. Septic arthritis of the facet joint
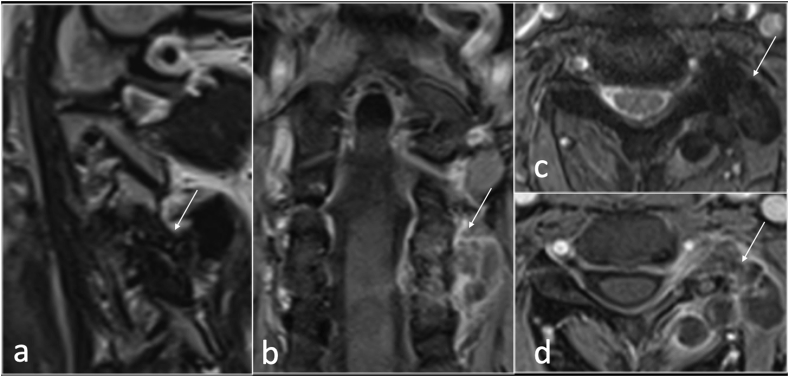
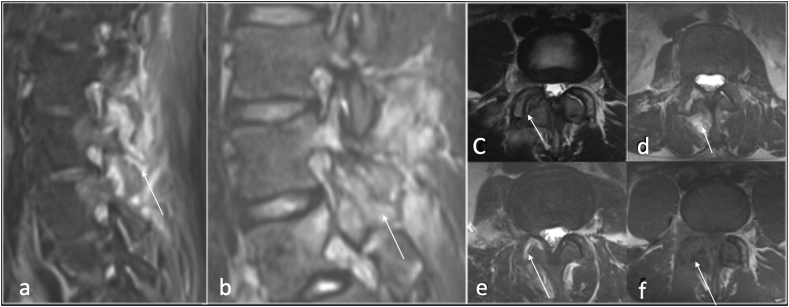
Septic arthritis of the facet joint is an uncommon entity with a similar clinical presentation to spondylodiscitis in which a delayed diagnosis may lead to significant morbidity and mortality resulting from local or systemic spread of infection.30 Approximately two-thirds of facet joint infection are caused by Staphylococcus aureus.31 More indolent infections resulting from fungal or mycobacterial pathogens have also been reported. Predisposing factors include age, diabetes mellitus, immunosuppressed patients, rheumatoid arthritis, skin infection, IV drug use, and previous joint manipulation including joint prosthesis, recent joint surgery and intraarticular corticosteroid injections.30, 31, 32 Septic arthritis is caused by hematogenous spread, direct inoculation of the joint from corticosteroid injection, surgery or trauma, or from spread of adjacent infection into the joint space.32 MRI is the imaging modality of choice for diagnosing facet joint infection due to its high sensitivity and specificity, (Fig. 10).
Fig. 10.
Sagittal STIR (a and b), axial T2(c,d,e) and T1(f) showing destruction, oedema and effusion right facet joint septic arthritis (arrow).
MRI is key for assessing the extent of infection and the presence of any complication such as epidural and paraspinal collection. Soft tissue enhancement may be seen on MRI within 2 days from the onset of symptoms.30 The early MRI features of infection show bone marrow and periarticular soft tissue oedema, facet joint fluid and enhancement following gadolinium administration. Radiographs are likely to be falsely reassuring. CT may demonstrate erosive changes in relation to facet joint but could be normal in the early stage of an infectious process. CT is also used for biopsy and facet joint aspiration (Fig. 11). The MRI findings are sometimes very subtle with non-specific swelling of the joint capsule and periarticular soft tissue related to the facet joint which may mimic degenerative joint disease.31 Diffusion weighted imaging (DWI) maybe helpful to detect a small abscess or pus collection in the epidural space or surrounding soft tissue adjacent to the facet joint.31 Other differential diagnosis considerations include non-pyogenic infection (such as Tuberculosis), degenerative or inflammatory arthritis and malignancy.
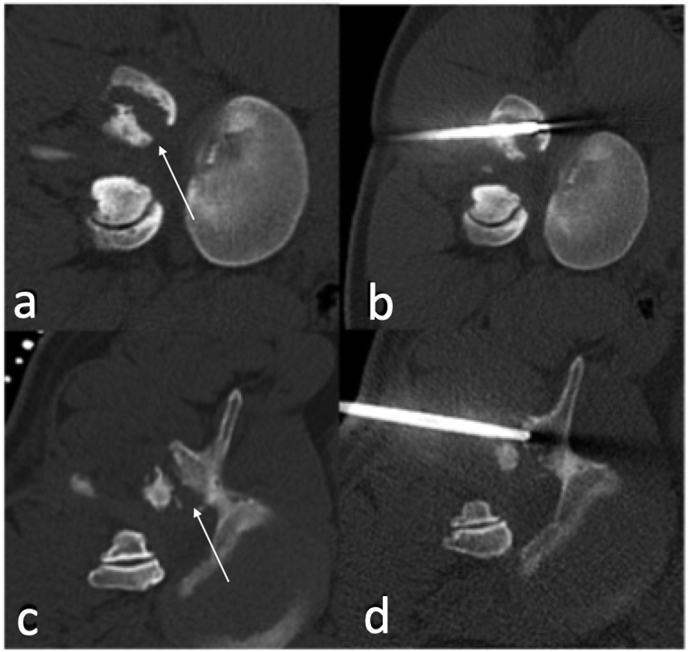
Fig. 11.
CT axial (a and c) showing destruction of right facet joint (arrow) with CT guided biopsy (c and d) of septic arthritis of right facet joint.
5.10. Tumours
Metastatic disease, myeloma, and lymphoproliferative tumours of the spine commonly cause multiple lesions, which usually allow the diagnosis to be easily made.
In contrast, primary spinal tumours must be considered in cases of a solitary spinal lesion.
Benign tumours, such as osteoid osteoma, osteoblastoma, osteochondroma and ABC are commonly located in the posterior elements. Other tumours encountered in our tertiary centre includes chondrosarcoma, Ewing sarcoma and aggressive haemangioma.
5.11. Osteoid osteoma/Osteoblastoma
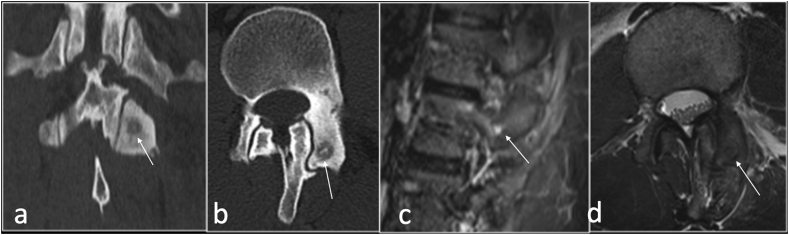
Osteoid osteoma (OO) is a benign osteoblastic tumour that occurs most frequently in patients between 7 and 25 years old with male predominance.33 Most patients experience pain that worsens at night and is promptly relieved by the administration of Non-steroidal anti-inflammatory medications. This is more commonly seen in long bone diaphysis. The typical radiographic and CT finding is that of radiolucent nidus, with or without central mineralization, associated with surrounding reactive sclerosis ranging from mild cancellous sclerosis to florid periosteal reaction and bone formation.33 The most common location of spinal OO is the lumbar, followed by the cervical and the thoracic segments. The sacrum is the least commonly affected spinal region. In most cases, the nidus is located in the neural arch. Patients with spinal OO present with radicular pain, gait disturbance, limb atrophy, and painful scoliosis, when scoliosis is present, the nidus typically is on the concave side of the lumbar curvature. Radiograph may simply show scoliosis. CT is the best modality to detect and confirm the presence of the nidus. MRI will show nonspecific oedema but may miss the presence of the nidus (Fig. 12). The presence of oedema in the pedicle and lamina extending anteriorly to involve one-to two-thirds of the posterolateral vertebral body with sparing of the intervertebral disk space should raise suspicion for the diagnosis.34
Fig. 12.
CT coronal (a), axial (b), sagittal STIR(c) and T2 axial (d) showing osteoid osteoma (arrow) involving the superior articular facet of the left facet joint.
Osteoblastoma (OB) is histologically similar to OO but with distinct clinical and imaging features.34 Clinically, OB can be asymptomatic or may cause a localised dull pain.
Approximately 40% of OBs arise in the spine with posterior element being, by far, the most common origin. OB often extends to vertebral body in approximately 40% of cases.34
OB differs from OO by its progressive clinical course and is usually more than 2 cm in size.
OB has a variable imaging appearance and may appear aggressive on MRI with marked reactive osseous oedema and soft tissue component. It is often lytic and expansile (Fig. 13). The signal intensity varies according to the amount of osteoid matrix present which may be variable. The presence of adjacent sclerosis on radiograph and CT is a common finding.
Fig. 13.
Sagittal T1(a), STIR (b), CT(c) and axial CT showing bone forming tumour involving the right facet joint in keeping with osteoblastoma.
OB can be associated with secondary aneurysmal bone cyst in 10% of cases.
5.12. Aneurysmal bone cyst (ABC)
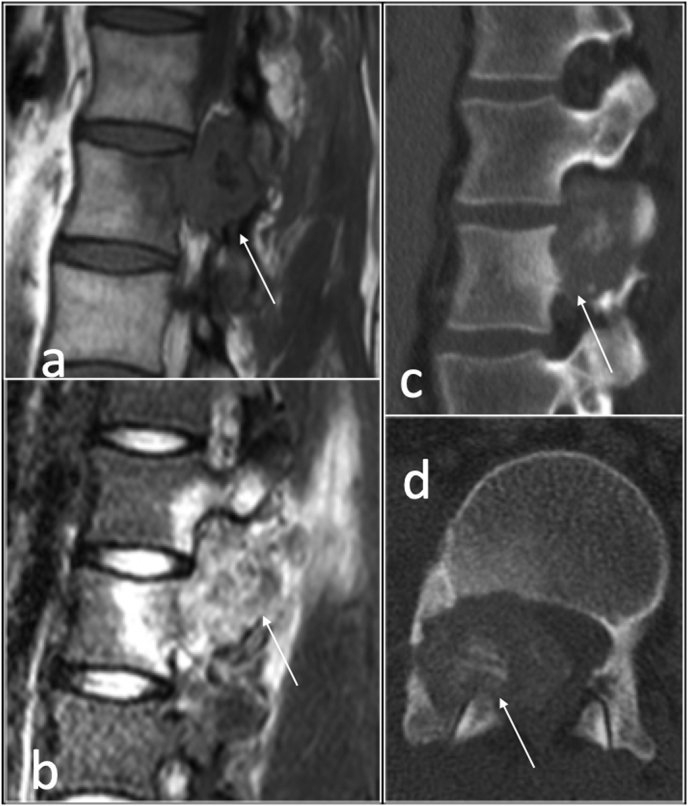
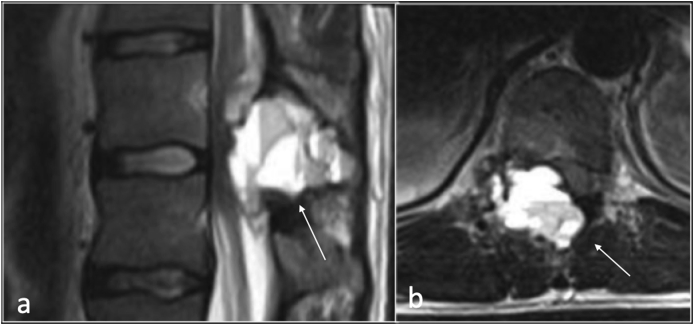
ABC is a rare benign tumour accounting for approximately 2% of primary bone tumours. It is more common in young patients, typically between 5 and 20 years of age. Approximately 20% of ABCs present in the spine, typically situated in the posterior elements although expansion into the vertebral body is seen in two thirds of the cases.34 Dependent on the size of the lesion, ABCs can be symptomatic with pain and swelling and may cause cord and/or nerve root compression (Fig. 14). CT and MRI demonstrate a well define lesion with thin wall, internal septations and fluid fluid levels. MRI depicts high T1 signal intensity within the fluid fluid levels indicative of haemorrhage (Fig. 14). The presence of solid component on MRI is helpful in differentiating primary ABC from an underlying tumour with secondary ABCs such as in giant cell tumour or osteoblastoma.34
Fig. 14.
T2 sagittal (a) and axial)b) showing fluid-fluid level involving right facet joint in keeping with aneurysmal bone cyst.
5.13. Osteochondroma
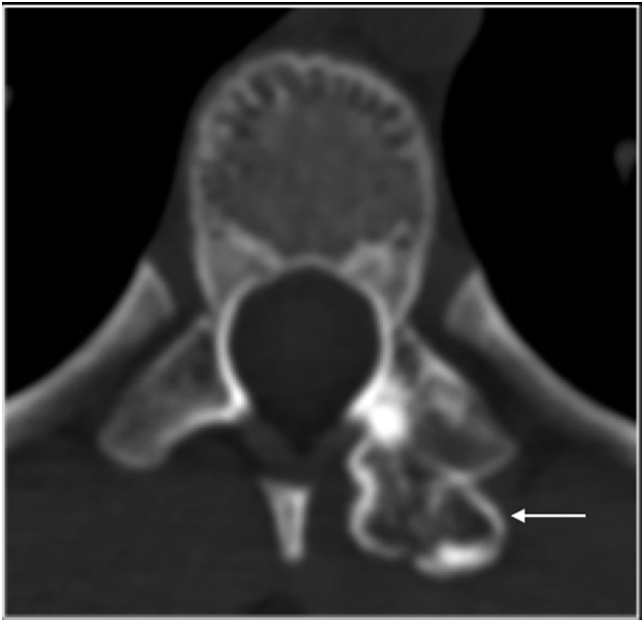
Osteochondroma is the most common bone tumour and less than 5% of solitary lesions are present in the spine.34 The spinal involvement is increased to approximately 9% in multiple exostosis.34 () Osteochondroma may be radiotherapy induced and usually solitary arising in the periphery of the radiotherapy field in childhood malignancy with approximately 12% prevalence.34 Osteochondroma may arise anywhere in the spine including the posterior element (Fig. 15). However, Osteochondroma is rare in the facet articular process.34
Fig. 15.
Axial CT showing osteochondroma (arrow) arising from superior articular facet of left facet joint.
MRI is the imaging modality of choice to assess the cartilage cap, but CT is superior to MRI in depicting the typical features of cortical and medullary bone continuity to confirm diagnosis.34 A cartilage cap that is thicker than 2 cm in adult is suspicious for malignant transformation of the underlying osteochondroma.
5.14. Aggressive haemangioma
Haemangioma is a common benign bone tumour with approximately 11% of all haemangioma present in the spine, particularly in the thoracic segment. Haemangioma is classified as typical, atypical or aggressive (compressive) depends on its imaging features and its fatty and vascular components. 30% of spinal haemangiomata are multiple.
Haemangioma is usually found incidentally, confined to the vertebral body.34 Aggressive haemangioma typically shows bone expansion, extraosseous extension and may cause neural element compression or vertebral collapse.35 Although less frequent, haemangioma may arise from the posterior elements (Fig. 16). Aggressive haemangioma can be mistaken for metastasis. Other differentials include hemangioblastoma, lymphangioma or Ewing sarcoma.34
Fig. 16.
Sagittal T2 (a), T1(b) and axial T2(c) showing large tumour (arrow) involving the left facet joint with epidural component. Biopsy showed this to be an aggressive haemangioma.
5.15. Chondrosarcoma
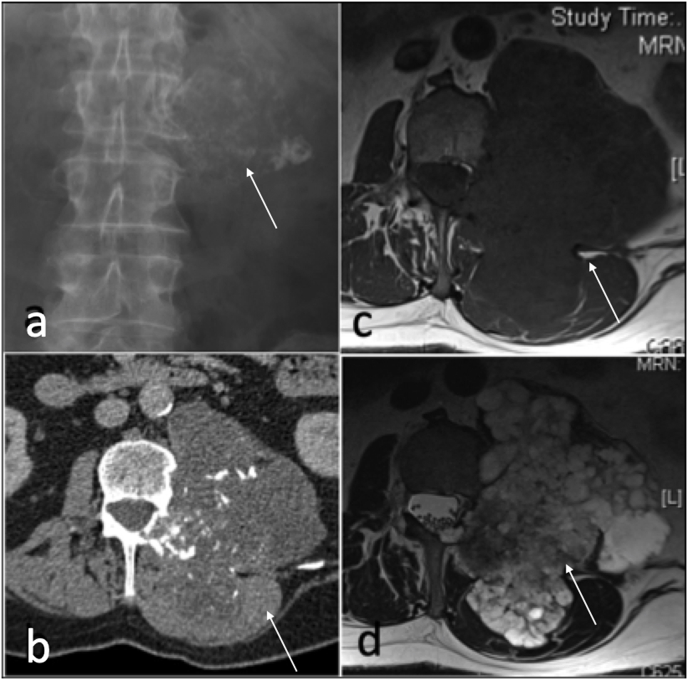
Chondrosarcoma present as a calcific mass with bone destruction, typically affects adults older than 30 years of age and can be primary or secondary. CT is superior in depicting mineralised matrix with arcs and rings appearances. MRI demonstrates lobular masses with a very high signal intensity in T2 weighted sequences due to the very high water content of hyaline cartilage.34 (Fig. 17) The presence of true ossification corresponds to residual osteochondroma and secondary malignant transformation.34
Fig. 17.
Anteroposterior radiograph (a), axial T1 (b), CT(c) and T2(d) showing large chondrosarcoma(arrow) arising from the left facet joint.
5.16. Ewing sarcoma
Ewing sarcoma is a small round blue cell tumour and the most common tumour in children. It is typically seen in 10–30 years of age. 3–10% of Ewing sarcoma are present in the spine with increasing frequency from cranial to caudal, being most common in the sacrum and least common in the cervical spine.34 The imaging appearances are usually of lytic permeative bone destruction and disproportionately large soft tissue component with Intermediate T1 and intermediate to high T2 signal on MRI. The posterior elements may also be the origin site of Ewing sarcoma with large soft tissue and epidural extension (Fig. 18).
Fig. 18.
STIR coronal (a) and axial T2(b) showing extensive Ewings sarcoma (arrow) involving the facet joint with epidural component.
6. Conclusion
In this study, we have reviewed a variety of pathologies arising from or involving the facet joints that have presented to our spinal tertiary centre. Radiologists require to keep an open mind to the various pathological entities that affect facet joints. Degenerative process is by far the most common finding in imaging the spine. However, infective and inflammatory processes may affect the facet joints either in isolation or as part of multifocal disease and requires a prompt recognition for early treatment. Other entities such as benign and malignant tumours involving the facet joints can be encountered and cause some diagnostic challenges.
Declaration of competing interest
No conflicts of interest.
References
- 1.Jaumard N.V., Welch W.C., Winkelstein B.A. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J England Biomech. 2011;133(7) doi: 10.1115/1.4004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perolat R., Kastler A., Nicot B. Facet joint syndrome: from diagnosis to interventional management. Insights Imaging. 2018;9(5):773–789. doi: 10.1007/s13244-018-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaput C., Padon D., Rush J., Lenehan E., Rahm M. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine. 2007;32(17):1883–1887. doi: 10.1097/BRS.0b013e318113271a. [DOI] [PubMed] [Google Scholar]
- 4.Rihn J.A., Lee J.Y., Khan M. Does lumbar facet fluid detected on magnetic resonance imaging correlate with radiographic instability in patients with degenerative lumbar disease? pine (Phila Pa 1976) 2007;32(14):1555–1560. doi: 10.1097/BRS.0b013e318067dc55. [DOI] [PubMed] [Google Scholar]
- 5.Botchu R., Bharath A., Davies A.M., Butt S., James S.L. Current concept in upright spinal MRI. Eur Spine J. 2018;27(5):987–993. doi: 10.1007/s00586-017-5304-3. [DOI] [PubMed] [Google Scholar]
- 6.Tamai K., Kato M., Konishi S., Matsumura A., Hayashi K., Nakamura H. Facet effusion without radiographic instability has No effect on the outcome of minimally invasive decompression surgery. Global Spine J. 2017;7(1):21–27. doi: 10.1055/s-0036-1583173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolaki E., Davies A.M., Evans N., Cassar-Pullicino V.N. MR imaging of lumbar facet joint synovial cysts. Eur Radiol. 2000;10(4):615–623. doi: 10.1007/s003300050973. [DOI] [PubMed] [Google Scholar]
- 8.Neto N., Nunnes P. Spectrum of MRI features of ganglion and synovial cysts [published correction appears in Insights Imaging. Insights Imaging. 2016 Jun;7(3):461. doi: 10.1007/s13244-016-0463-z. 2016;7(2):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan A.M., Girardi F. Spinal lumbar synovial cysts. Diagnosis and management challenge. Eur Spine J. 2006;15(8):1176–1182. doi: 10.1007/s00586-005-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cambron S.C., McIntyre J.J., Guerin S.J., Li Z., Pastel D.A. Lumbar facet joint synovial cysts: does T2 signal intensity predict outcomes after percutaneous rupture? Am J Neuroradiol. 2013;34(8):1661–1664. doi: 10.3174/ajnr.A3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Leary S.A., Paschos N.K., Link J.M., Klineberg E.O., Hu J.C., Athanasiou K.A. Facet joints of the spine: structure–function relationships, problems and treatments, and the potential for regeneration. Annu Rev Biomed Eng. 2018;20:145–170. doi: 10.1146/annurev-bioeng-062117-120924. [DOI] [PubMed] [Google Scholar]
- 12.Eubanks J.D., Lee M.J., Cassinelli E., Ahn N.U. Does lumbar facet arthrosis precede disc degeneration? A postmortem study. Clin Orthop Relat Res. 2007;464:184–189. doi: 10.1097/BLO.0b013e3181583d4e. [DOI] [PubMed] [Google Scholar]
- 13.Gillick J.L., Wainwright J., Das K. Rheumatoid arthritis and the cervical spine: a review on the role of surgery. Int J Rheumatol. 2015;2015:252456. doi: 10.1155/2015/252456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurik G.A. Imaging the spine in arthritis-a pictorial review. Insights imag. 2011;2(2):177–191. doi: 10.1007/s13244-010-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joaquim A.F., Appenzeller S. Cervical spine involvement in rheumatoid arthritis - a systematic review. Autoimmun Rev. 2014;13(12):1195–1202. doi: 10.1016/j.autrev.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Hermann K.G.A., Althoff C.E., Schneider U. Spinal changes in patients with spondyloarthritis: comparison of MR imaging and radiographic appearances. Radiographics. 2005;25(3):559–569. doi: 10.1148/rg.253045117. [DOI] [PubMed] [Google Scholar]
- 17.Carmona R., Harish S., Linda D.D., Ioannidis G., Matsos M., Khalidi N.A. MR imaging of the spine and sacroiliac joints for spondyloarthritis: influence on clinical diagnostic confidence and patient management. Radiology. 2013;269(1):208–215. doi: 10.1148/radiol.13121675. [DOI] [PubMed] [Google Scholar]
- 19.McGauvran A.M., Kotsenas A.L., Diehn F.E., Wald J.T., Carr C.M., Morris J.M. SAPHO syndrome: imaging findings of vertebral involvement. Am J Neuroradiol. 2016;37(8):1567–1572. doi: 10.3174/ajnr.A4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moshrif A., Laredo J.D., Bassiouni H. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: a series of 37 patients. Semin Arthritis Rheum. 2019;48(6):1113–1126. doi: 10.1016/j.semarthrit.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro T., Nabeshima Y., Yasui S., Fujita I., Yoshiya S., Fujii H. Pseudogout attack of the lumbar facet joint: a case report. Spine (Phila Pa 1976) 2002;27(17):E396–E398. doi: 10.1097/00007632-200209010-00028. [DOI] [PubMed] [Google Scholar]
- 24.Konatalpalli R.M., Demarco P.J., Jelinek J.S. Gout in the axial skeleton. J Rheumatol. 2009;36(3):609–613. doi: 10.3899/jrheum.080374. [DOI] [PubMed] [Google Scholar]
- 25.Yoon J.W., Park K.B., Park H. Tophaceous gout of the spine causing neural compression. Korean J Spain. 2013;10(3):185–188. doi: 10.14245/kjs.2013.10.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumezanu E., Konatalapalli R.M., Weinstein A. Axial (spinal) gout. Curr Rheumatol Rep. 2012;14(2):161–164. doi: 10.1007/s11926-012-0236-8. [DOI] [PubMed] [Google Scholar]
- 27.Chou H., Chin T.Y., Peh W.C.G. Dual energy CT in gout – a review of current concepts and applications. J Radiat Med Sci. 2017;64(1):41–51. doi: 10.1002/jmrs.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia G.M., McCord G.C., Kumar R. Hydroxyapatite crystal deposition disease. Semin Muscoskel Radiol. 2003;7(3):187–193. doi: 10.1055/s-2003-43229. [DOI] [PubMed] [Google Scholar]
- 29.Ghorpade R.S., Lokanath Y.K. Synovial chondromatosis of dorsal spine: case report of rare pathological entity and review. Korean J Spine. 2016;13(4):196–199. doi: 10.14245/kjs.2016.13.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stecher J.M., El-Khoury G.Y., Hitchon P.W. Cervical facet joint septic arthritis: a case report. Orthop J. 2010;30:182–187. [PMC free article] [PubMed] [Google Scholar]
- 31.Moritani T., Kim J., Capizzano A.A., Kirby P., Kademian J., Sato Y. Pyogenic and non-pyogenic spinal infection:emphysis on diffusion-weighted imaging for detection of abscesses and pus collection. Br J Radiol. 2014;87(1041) doi: 10.1259/bjr.20140011. 20140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diehn F.E. Imaging of spine infection. Radiol Clin. 2012;50(4):777–798. doi: 10.1016/j.rcl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Chai J.W., Hong S.H., Choi J.Y. Radiologic diagnosis of osteoid osteoma: from simple to challenging findings. Radiographics. 2010;30(3):737–749. doi: 10.1148/rg.303095120. [DOI] [PubMed] [Google Scholar]
- 34.Rodallec M.H., Feydy A., Larousserie F. Diagnostic imaging of solitary tumors of the spine: what to do and say. Radiographics. 2008;28(4):1019–1041. doi: 10.1148/rg.284075156. [DOI] [PubMed] [Google Scholar]
- 35.Pinto D.S., Hoisala V.R., Gupta P., Sarkar P. Aggressive vertebral body hemangioma causing compressive myelopathy - two Case Reports. J Orthop Case Rep. 2017;7(2):7–10. doi: 10.13107/jocr.2250-0685.724. [DOI] [PMC free article] [PubMed] [Google Scholar]