Abstract
Background
Microcirculatory defects in diabetes are linked with neuropathy and the onset of diabetic foot syndrome. In this study we quantify pressure- and posture-dependent changes of plantar temperatures as a surrogate of tissue perfusion in healthy volunteers versus diabetes patients diagnosed with neuropathy in the absence of macroangiopathy.
Methods
Healthy volunteers (n = 31) as well as patients with diabetes diagnosed with severe polyneuropathy (n = 30) were enrolled in a clinical study to test for plantar temperature changes in the feet during extended episodes of standing. These lasted between 5 and 20 min each over 95 min, in between the participants were asked to take a seated position for 5 min and release the pressure from the feet. Major macroangiopathy was excluded before study enrolment. Custom-made insoles harbored temperature and pressure sensors positioned at eight preselected positions for recording.
Findings
In both subgroups a significant plantar temperature downshift occurred within 10 min of standing, which was especially detected during the initial 45 min of the study protocol. Comparisons between healthy volunteers and patients with diabetes revealed no differences in the magnitude of temperature downshifts during stance episodes. Pressure sensor recordings revealed that healthy volunteers intermittently released pressure during the longer stance episodes due to discomfort, whereas the patients with diabetes and polyneuropathy did not.
Interpretation
Our findings demonstrate a tight plantar temperature regulation following pressure exposure. In patients with diabetes and peripheral sensoric neuropathy the temperature drop is similar to healthy volunteers. Potentially, prolonged stance periods resulting in less perfused plantar tissue may remain unrecognized with polyneuropathy, whereas discomfort develops in healthy controls.
Funding
The study was supported by EFRE Förderung der Europäischen Union und Landesmittel des Ministeriums für Wirtschaft, Wissenschaft und Digitalisierung Sachsen-Anhalt (Vorhabennummer: ZS/2016/05/78,615 and ZS/2018/12/95,325). JK and PRM were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - project ID 97,850,925 - SFB854, AM by the Chinese Scholarship Council (CSC).
Keywords: Diabetes, Diabetic neuropathy, Plantar temperature, Plantar pressure, Posture-dependent plantar temperature changes
Research in context.
Evidence before this study
Patients with diabetes are at high risk for micro- and macroangiopathy, as well as polyneuropathy. The diabetic foot syndrome is a frequent sequela, mostly due to impaired sensation. There are evidences toward a microcirculatory defect in patients with diabetes and polyneuropathy that may pave the way toward tissue damage. Once tissue injuries are incited in the feet, they may remain unnoticed and repeated insults may thereafter aggravate tissue destruction. By searching PubMed and other medical databases no studies were identified that have systematically recorded temperature changes in the plantar areas of the feet during extended standing episodes (search performed 02/2020). Our hypothesis was that plantar temperatures decrease over time and that the temperature changes differ between healthy volunteers versus patients with diabetes and severe polyneuropathy.
Added value of this study
Healthy volunteers (n = 31) as well as patients with diabetes diagnosed with severe polyneuropathy (n = 30) were enrolled in a clinical study to test for plantar temperature changes in the feet during extended episodes of standing. These lasted between 5 and 20 min each over 95 min, in between the participants were asked to take a seated position for 5 min and release the pressure from the feet. Major macroangiopathy was excluded before study enrolment. Custom-made insoles harbored temperature and pressure sensors positioned at eight preselected positions for recording. The results revealed that in both subgroups a significant plantar temperature downshift occurred within 10 min of standing, which was especially detected during the initial 45 min of the study protocol. Comparisons between healthy volunteers and patients with diabetes revealed no differences in the magnitude of temperature downshifts during stance episodes. Pressure sensor recordings revealed that healthy volunteers intermittently released pressure during the longer stance episodes due to discomfort, whereas the patients with diabetes and polyneuropathy did not.
Implications of all the available evidence
The study results provide key insights into posture-dependent plantar temperature changes. Our findings suggest that notwithstanding any alterations of the microvasculature in patients with diabetes the underlying blood supply is similarly regulated as in healthy controls and that the plantar temperature is not disturbed with diabetes. Our results highlight that timely temperature changes occur in stance position. These may lay the groundwork for approaches to identify pressure and temperature patterns that are indicatory of emerging microcirculatory foot problems with diabetes in the future.
Alt-text: Unlabelled box
1. Introduction
Up to 25% of all patients suffering from diabetes will develop foot ulcerations during their lifetime [1] with many affected individuals facing amputations in the next four years [2]. More than 85% of foot amputations relate to foot ulcers [3,4]. The risk of foot amputations among these individuals is 17 to 40 times higher than in the general population [5] with markedly reduced expectation of life [6]. Most predisposing is a peripheral sensoric neuropathy, followed by arterial occlusive disease [7]. With sensoric neuropathy harmful events and injuries remain unnoticed and perpetuated insults may aggravate tissue destruction. The understanding of underlying pathomechanisms of tissue destruction in the absence of traumata however is limited. The (micro-)circulation in the lower extremities has been analyzed in patients suffering from diabetes versus healthy controls to causally link diminished blood supply with diabetic foot syndrome onset. The precapillary resistance in the skin of the foot rises in the supine position, thereby preventing excessive elevation of capillary pressure due to the vertical blood column [8], [9], [10]. Such a change in resistance may prevent orthostatic hypotensive episodes, a finding especially common in patients with sympathetic dysfunction. Early impairment of systemic vascular tone results in orthostatic hypotension which has been described in diabetes patients suffering from autonomic neuropathy with myogenic dysregulation [11,12]. Furthermore, blood supply abnormalities characterized by reduced capillary diameters and basement membrane thickening have been detected in diabetes patients [13,14]. Earlier studies performed with microinjection techniques demonstrated that the capillary pressure is higher in diabetes patients when compared to healthy controls, similarly is the foot swelling rate determined by mercury strain gage plethysmography [15,16].
Beyond vascular tone regulation there is scarce knowledge on plantar temperature fine-tuning, especially when changes of the posture (standing/seating) occurs. A recent study revealed remarkable plantar temperature elevations by approximately 3 °C during a 45 min exercise [17]. A tight regulation of foot temperatures with long-lasting changes may be anticipated from studies focusing on early detection of foot ulcers [18]. A regional plantar temperature rise of up to 2.2 °C was seen about 5 days before onset of tissue destruction and overt ulcer formation [18]. These findings have been confirmed in independent studies and some patients even developed temperature rises up to 5 weeks before ulcer formation [19].
Our hypothesis was that plantar and environmental temperatures as well as the body position (standing/seating) are relevant interlinked parameters. Alterations of plantar temperatures may be of relevance in respect to the interpretation of rapid as well as long-lasting temperature changes in diabetes patients [20]. In our earlier work, we showed that patients differ from healthy participants in their patterns of plantar pressure distribution [21]. Since the plantar pressure distribution may affect plantar temperatures, our primary aim in the pilot study was to determine the extent of temperature changes in two different cohorts, without and with sensoric neuropathy.
2. Materials and methods
2.1. Infrared imaging of plantar temperatures
A setup was chosen that allows sitting on a chair with positioning of both feet on a transparent mesh. An infrared thermographic device (Optris PI 400, Optris GmbH, Berlin, Germany) was situated below the mesh. Changes of plantar temperatures were recorded by infrared spectroscopy at defined intervals. Measurements were commenced without additional force on the feet. Thereafter a 20 kg weight was placed on the front of both upper thighs. The weight was released at the end of the observation period of 30 min (setup of infrared temperature recording is depicted in Fig. 1 top left).
Fig. 1.
Regional changes of temperatures visualized by thermography. Infrared images with pseudo-colors for a temperature range from 29 to 34 °C were obtained with a healthy volunteer in a seated position without application of pressure to the feet (before) and following positioning of a 20 kg weight on both front thighs. A time-dependent decrease of temperature was detected predominantly in the forefoot, visualized by yellow color during the pressure load. Within 1 min of pressure release a rapid temperature rise was detected.
2.2. Clinical study protocol with sensor-bearing insoles
The study protocol was set up to address the issue of positioning-dependent plantar temperature changes over time. The protocol was designed with our knowledge of time-dependent decrease of temperatures seen by infrared spectroscopy over 30 min. Our study was performed with two different environmental temperatures. The participants followed a simple protocol of standing and seating and utilized customized sensor-equipped insoles (8 pressure + 8 temperature + 1 ambient temperature sensors). The sensors recorded with high frequency and data were transferred via Bluetooth to a data base. The study participants were instructed to perform 6 stance episodes with repeated seating over 95 min. The study protocol received approval from the local institutional ethics board at the University of Magdeburg (EUDAMED CIV-13-06011441) [22]. The study protocol was exempted from approval for medical devices due to low safety risk, which was certified by the German Federal Institute for Drugs and Medical Devices. All participants in the study provided written informed consent. Overall 31 healthy volunteers and 30 diabetes patients with severe polyneuropathy (vibration perception <2/8 with graduated tuning fork for both feet) were enrolled into the study. Inclusion criteria for healthy volunteers were (i) absent sensomotoric neuropathy, (ii) no history of foot ulcerations, (iii) absent macroangiopathy tested by ABI, (iv) absence of skin defects in the lower extremities, (v) exclusion of diabetes in past medical history, (vi) lack of amputations of limbs or deformations of the spine, and (vii) no past medical history for heart failure or myocardial infarctions. Inclusion criteria for diabetes patients with neuropathy were (i) type 1 or 2 diabetes with HbA1c levels above 6.5%, (ii) peripheral sensoric neuropathy with impaired proprioception (vibration perception <2/8 with graduated tuning fork for both feet), (iii) absence of (current) neuropathic ulcerations, other skin defects and absence of major macroangiopathies (ABI>0,9), (iv) positive patellar and ankle jerk reflexes, (v) positive muscle strength for plantar pressure and flexor/extensor muscle strengths, (vi) no major movement impairment, (vii) no paralysis of lower extremities, (viii) no heart failure stages III and IV, (ix) no amputations of limbs or deformations, or myocardial infarction within the preceding 12 weeks. To test for changes of plantar temperatures over time within a simple protocol of standing and seating a custom-made sensor-equipped insole was constructed (Thorsis Technologies GmbH and Medixmind GmbH, Magdeburg, Germany; Supplementary Fig. 1a).
2.3. Positioning of temperature and pressure sensors within insole
To record plantar pressure and temperature changes over time, a dedicated insole prototype was used [23]. The insole comprised of a measurement system to which pressure and temperature sensors were connected. The sensor positioning was based on the knowledge of critical regions exposed to high pressure loads [19,24]. The insole consisted of eight distinct pressure and temperature sensors each, the latter placed on its upper surface to allow for close contact to the participant's foot (scheme on positioning of sensors is depicted in Supplement Fig. 1b). Furthermore, an ambient temperature sensor was placed at the bottom of the insole to capture the in-shoe ambient temperature. The insole recorded temperature and pressure values and wirelessly transmitted these to a smartphone via Bluetooth at the end of the experiment. The insoles consisted of a plastic core (ethylene vinyl acetate) and was divided into two components, an upper sole (shore hardness A 35) and a body (shore hardness A 55). Eight pressure sensors, eight temperature sensors and a temperature sensor in vicinity of the processing unit at the bottom of the insole for ambient temperature recordings were incorporated into the insole. The pressure sensors were of type TTForce A01 (Thorsis Technologies GmbH, Magdeburg, Germany) with detection range of 0 to 50 N/cm² and a sensing surface diameter of 10 mm. The flat temperature sensors were of type NTC 805 (TE Connectivity Ltd, Schaffhausen, Suisse) with a detection range from −55 °C to 125 °C and a sensing surface diameter of 10 mm. Indentations in the insole allowed to install the sensors without protrusions.
The insoles were positioned into closed protection shoes specifically developed for diabetes patients. The participants of the study all wore the same make of socks and utilized a “closed” shoe specifically designed for diabetes patients. Within such shoes the temperature increases over time due to exchange with the body temperature of the user and is also affected by the environmental temperature. To closely monitor the in-shoe temperature changes one sensor was placed at the bottom of the insole without contact to the feet, which was denoted “ambient temperature sensor”. The data acquisition and recording started immediately following putting on the shoes. The participants were asked to follow a pre-specified sequence of actions, that are alternating postures of standing (stance episode) and seating (pause). The sessions consisted of six stance episodes, lasting 5, 10, 20, 5, 10 and 20 min each, separated by seating episodes lasting 5 min each. The participants were instructed to equally apply pressure to both feet while standing. No immediate feedback of the actual pressure application was provided to the patients during the sessions, however the participants were verbally encouraged to keep the pressure without release while standing, In the seated position the participants were instructed to release pressure for 5 min, while still keeping contact to the insole. The participants were explicitly asked to adhere to these instructions, e.g. they should not temporarily release pressure during a stance episode. The study protocol furthermore encompassed that the measurements were performed twice, once at room temperature of ~22C° and once outdoors with an ambient temperature of ~16 °C. The two measurements were performed at two independent days.
2.4. Data pre-processing
For all participants recordings of both feet were available and used independently. Since each participant completed the protocol at two different environmental temperatures, there were a total of 244 data sets available (2 sessions x 2 feet x (31 healthy individuals + 30 diabetic patients)). Data were omitted when one of the following criteria was fulfilled: at least one of the temperature sensors was faulty; the duration of the recordings was less than 70 min; less than six distinct stance episodes were identified from the pressure recordings; at least one sensor with more than 10 outlier temperature measurements of <0 °C or >40 °C was present. To account for significant differences between controls and diabetes patients with respect to confounders (see Table 1), the datasets were stratified via one-to-one nearest neighbor propensity score matching. Propensity score matching was applied as post-hoc statistical method that estimates the manifestation of a target variable by accounting for the covariates that potentially predicts this characteristic. Logistic regression was performed to calculate the propensity score of the individuals that were either in the diabetes group or the healthy control group. Nearest neighbor matching then selected the best control match for each individual in the diabetes group based on the propensity score. The considered covariates were age, sex, and room temperature. The characteristics of the stratified trial data sets are depicted in Table 1. Wilcoxon rank sum test was used to quantify between-group differences, except for sex differences where the chi-square test was performed.
Table 1.
Bibliography of the study population and propensity score adjusted trial data.
| Controls | Diabetes patients | p-value | |
|---|---|---|---|
| Study participants | 31 | 30 | |
| Age [years] | 46.3 ± 0.3 | 63.6 ± 2.3 | <0.001 |
| Sex [% male] | 58% | 73% | 0.325 |
| Matched data | |||
| #trial datasets | 57 | 57 | — |
| Age [years] | 59.0 ± 15.8 | 61.8 ± 13.1 | 0.544 |
| Sex [% male] | 54% | 67% | 0.250 |
| Room temp. [°C] | 18.4 ± 2.7 | 18.6 ± 4.1 | 0.889 |
Sensor temperatures demonstrated a gradual upward shift that paralleled the ambient in-shoe temperatures. The “in-shoe milieu” comprises of the temperature that is detected by a sensor positioned below the insole with no immediate contact to the foot but with positioning within the “closed” compartment of the shoe. To more easily visualize differences in plantar temperatures that are distinct from the ambient in-shoe milieu an adjusted temperature Tnorm was calculated as the difference of sensor and in-shoe ambient temperatures. For each of the six stance episodes and eight plantar sensors, the change in temperature ∆T between the last and first recording of that episode was expressed as difference in adjusted temperature Tnorm.
Results are expressed as means ± standard deviation (SD). To examine whether there was a significant difference in mean ∆T between diabetes patients and healthy volunteers, two-sided t-tests were carried out between the two groups with a significance level of α=0.05. Furthermore, it was tested whether the temperature downshift was significant (∆T<0 °C) by applying a left tailed t-test with a significance level of α=0.05. Normality of the ∆T score distribution was checked using Shapiro-Wilk test (α=0.05).
2.5. Discomfort testing while seating
Given that our study protocol did not anticipate discomfort feelings during the extended stance episodes these were not recorded systematically. Some participants expressed strong discomfort in their feet and the pressure recording revealed that short episodes of pressure releases occurred. To obtain a rough estimate on discomfort in the feet with impaired movements an independent setup was chosen. 119 volunteers without known diabetes from past medical history were instructed to remain in a seated position in a room with an ambient temperature of 21 °C with both feet on the ground and to establish their feet position following an acoustic signal. In the case that the participants felt an irresistible urge to move one of their feet they should lift their hands and indicate so. The intervals from start of feet positioning to “strong discomfort” was recorded.
3. Results
3.1. Plantar temperatures following pressure application
In our quest to visualize plantar temperature changes over time with application of moderate pressure we took advantage of infrared spectroscopy. The setup of the imaging is depicted in Fig. 1. The pseudocolors revealed a wide range of temperatures between 29 and 34 °C. The pseudocolors changed considerably following exposure of a 20 kg weight positioned on the front of both upper thighs. There was a time-dependent decrease of plantar temperatures, predominantly in the forefoot region (Fig. 1). After 30 min the weight was released and within 1 min a temperature rise was detected. At this time point the infrared images resembled the one before pressure application. From these results we conclude that pressure application impairs the microcirculation of the foot and results in significant downshift of plantar tissue temperatures.
3.2. Changes of the ambient in-shoe temperatures over time
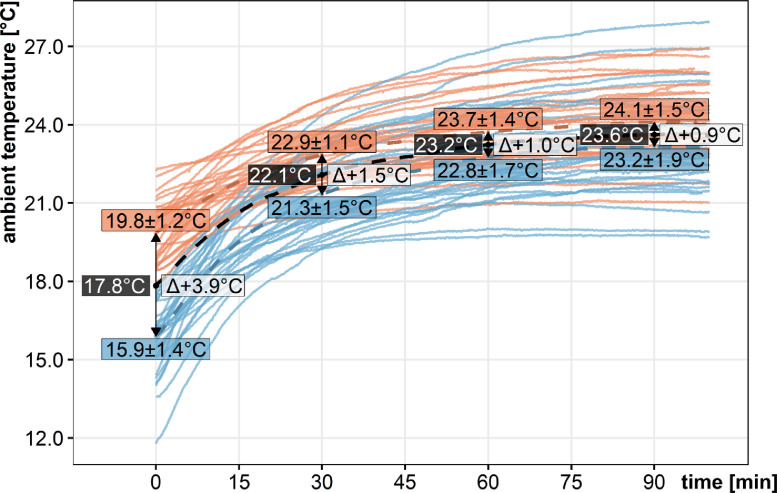
The temperature changes were recorded over time at two different environmental temperatures that are 17.8 to 22.8 °C and 11.2 to 17.7 °C, with all participants, yielding two different data sets. There was an asymptotic rise of the ambient temperatures under both conditions that reached a mean of 24.1 ± 1.5 °C with the higher environmental temperatures and 23.2 ± 1.9 °C with the lower environmental temperatures. These are depicted for all healthy volunteers (Fig. 2) and were similarly found in diabetes patients. Thus, even after 90 min a difference of 0.9 °C for the two distinct environment temperatures persisted due to the thermophilic exchange. Notably, there was a considerable overlap of in-shoe ambient temperatures reached in the individuals for both datasets. Our data set demonstrates that temperature changes within a shoe occur over an extended time period of 90 min. In the following, plantar temperature recordings were “normalized” for ambient in-shoe temperatures to yield Tnorm values.
Fig. 2.
Ambient temperature changes in the study dataset from healthy controls. All participants performed recordings at two different environmental temperatures, above (brown color) and below (blue color) 17.8 °C. The ambient temperature recordings are depicted for healthy volunteers over 95 min. Mean temperatures are visualized as dashed lines. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Plantar temperatures are lowered following pressure application
The stance episodes extended over 5, 10, 20, 5, 10 and 20 min, with 5 min of seating in-between. The protocol included a repetition of the different positions. The overall time of continuous measurement extended over 95 min, with two recordings at low and high environmental temperatures, respectively. Overall 31 healthy volunteers and 30 diabetes patients were enrolled (see Table 1 for bibliographic data). Each dataset was cleared for incomplete values and outliers. A total of 114 datasets (57 for each cohort) fulfilled the high quality criteria.
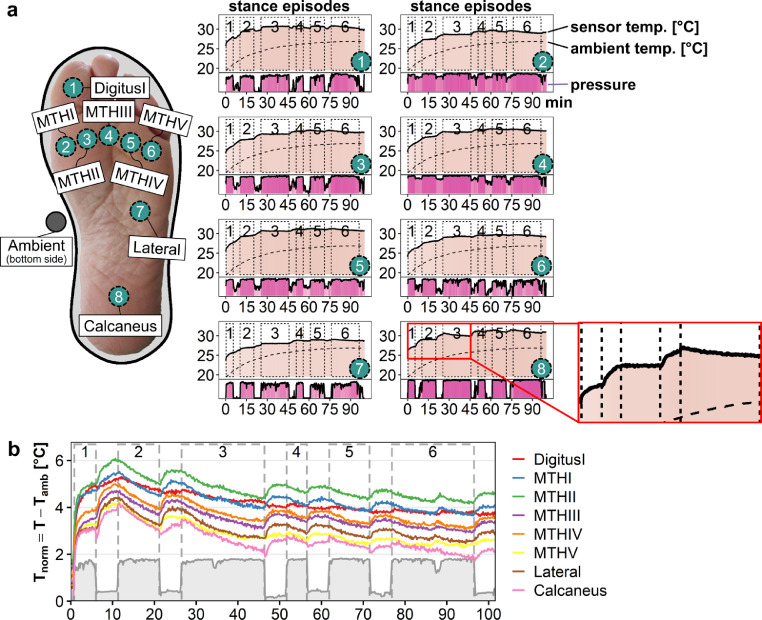
To obtain an overview on temperature changes over time pressure and temperature data were visualized within a composite graph. A representative result on temperature and pressure time curves is depicted for a healthy volunteer, revealing the asymptotic rise of plantar temperatures over 95 min (light color, Fig. 3a), while the pressure recordings are indicated in dark color. In contrast to the recordings of the ambient sensor (dashed line) with rising of temperature recordings over time, a closer look reveals indentations of the temperature curves with transient temperature downshifts, especially for the intervals with extended standing. In the shown example the indentations are especially prominent for sensors located at MTHI (2) and MTHV (6). The temperature downshifts were recorded during the stance episodes 1 through 3, but to a much lesser degree in the stance episodes 4 to 6.
Figs. 3.
a and b. Temperature changes with study protocol recorded by sensor-equipped insole. a. Cartoon illustrating the sensor positioning in relation to foot placement (left). Pressure and temperature recordings corresponding to the different sensors are depicted as time curves generated from six sensors with a healthy volunteer (right). Note indentations of temperature curve especially detected with sensors placed at MTHI (2) and MTHV (6). Ambient temperature recordings are indicated by dashed line. b. The “normalization” of sensor temperatures was performed by subtracting the ambient in-shoe temperatures from the sensor values obtained at each insole position. In the depicted example obtained with a healthy volunteer the resultant Tnorm values demonstrates marked downshifts at stance episodes 2 and 3 and to a lesser extent thereafter.
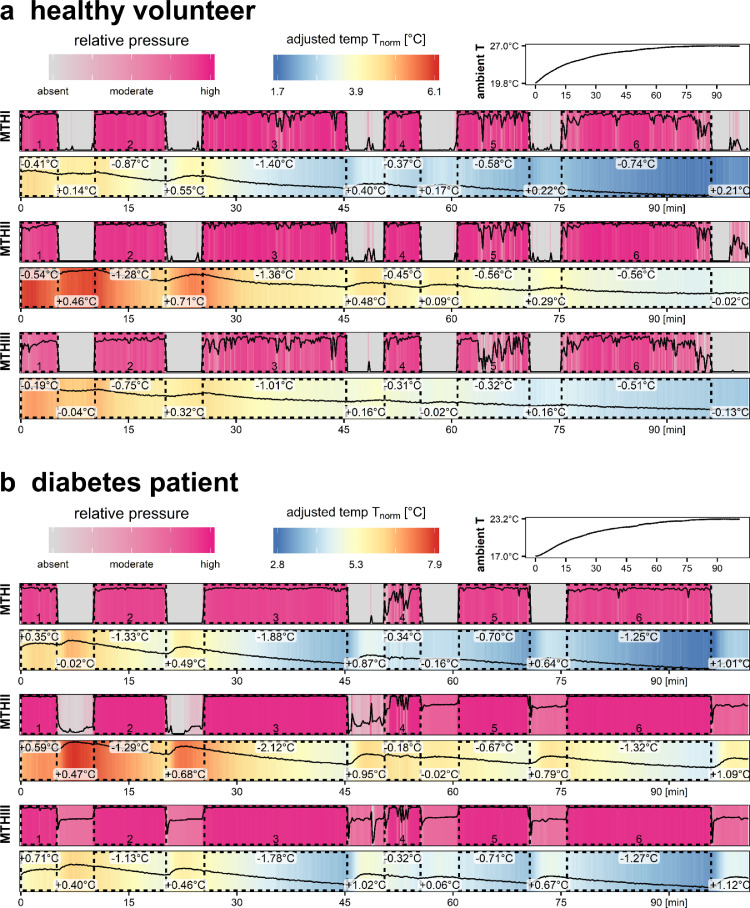
Given that our hypothesis was a pressure-dependent plantar temperature downshift and with our knowledge about the confounding factor of time-dependent rising ambient in-shoe temperatures we thereafter chose to subtract the ambient in-shoe temperatures from the sensor recordings. An example of recordings with a healthy volunteer is depicted in Fig. 3b for all temperature sensors and the pressure recording at MTHIII. In another graphical workup of the data we visualized the normalized temperature values with corresponding pressure values. Representative findings for a healthy volunteer (Fig. 4a) and a diabetes patient with diagnosed sensoric neuropathy (Fig. 4b) exemplify the findings. The examples reveal a maximal downshift of 1.36 °C and 2.12 °C, respectively, in stance episode 3 with 20 min of standing. In the second half of the study protocol such a prominent temperature downshift was not seen. Temperature downshifts were seen in all study participants. Notably, the recordings on the applied pressure reveal that the healthy volunteer depicted releases the applied pressure after approximately 10 min, while the diabetes patient was able to apply the pressure throughout the whole stance episodes.
Figs. 4.
a. and b. Juxtaposition of temperature changes for example patients. For a representative healthy volunteer (a.) and a diabetes patient (b.) temperature recordings at MTH1, 2 and 3 are provided with pseudo-colors ranging from blue to red (see top color legend). In these graphs corresponding pressure recordings are depicted as low to high (purple color). Time intervals with the participant in stance position and pressure application are highlighted by dotted rectangles. The stance episodes lasted over 5, 10, 20, 5, 10 and 20 min, respectively. Temperature differences from start versus end of stance episode as well as for the periods of seating are calculated. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Plantar temperatures change significantly while performing the study protocol
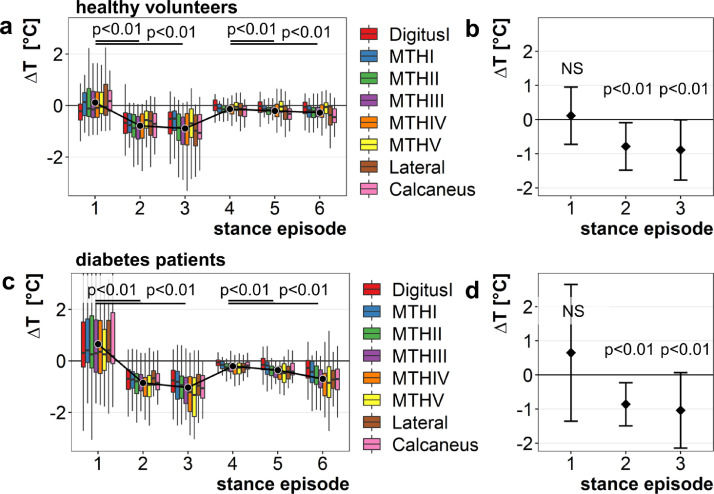
Data are provided for healthy controls and diabetes patients (Fig. 5a and b), all with adjustment for ambient in-shoe temperature recordings. Beginning from the second stance episode, mean stance-dependent temperature downshifts were significant (p < 0.01; Fig. 5a and b) with ∆T = −0.8 ± 0.6 °C (stance episode 2), ∆T = −1.0 ± 0.8 °C (stance episode 3), ∆T = −0.3 ± 0.4 °C (stance episode 5), and ∆T = −0.5 ± 0.6 °C (stance episode 6) for healthy controls (Fig. 5a and b; Table 2). The respective values for diabetes patients are ∆T = −0.9 ± 0.6 °C (stance episode 2), ∆T = −1.0 ± 0.7 °C (stance episode 3), ∆T = −0.4 ± 0.3 °C (stance episode 5), and ∆T = −0.7 ± 0.5 °C (stance episode 6; Fig. 5c and d; Table 2). These results indicate that there is a significant temperature downshift within 10 min of standing which is more prominent within the first 45 min of the protocol.
Fig. 5.
a–d. Temperature changes over time during the study protocol in healthy controls and diabetes patients. The distribution of temperature change ∆T for each stance episode and for all sensors was calculated and visualized for healthy volunteers (a and b) and diabetes patients (c and d). Points represent average ∆T for each stance episode over all sensors. A significant temperature decline compared to the one in seated position is observed for stance episodes 2/3 and 5/6. Means +/- SD are calculated with levels of significance for the different stance episodes.
Table 2.
Means ± standard deviation of ∆T for each stance episode per group. Student's t-test is performed to examine intra-group and inter-group differences. The null hypothesis of the intra-group test is that ∆T is equal to or larger than 0. The null hypothesis of the inter-group test is that the difference between ∆T of the control group and ∆T of the diabetic group is equal to 0. NS not significant, *p < 0.05, ⁎⁎p < 0.01.
| Inter-group difference |
||||
|---|---|---|---|---|
| Stance Episode | Controls 57 datasets | Diabetes patients 57 datasets | p-value | 95% CI |
| 1. 5 min | 0.10NS ± 0.81 | 0.63NS ± 1.79 | 0.04* | [−1.06,−0.02] |
| 2. 10 min | −0.80⁎⁎ ± 0.60 | −0.86⁎⁎ ± 0.55 | 0.59NS | [−0.16, 0.27] |
| 3. 20 min | −0.91⁎⁎ ± 0.76 | −1.04⁎⁎ ± 0.71 | 0.34NS | [−0.14, 0.41] |
| 4. 5 min | −0.13⁎⁎ ± 0.24 | −0.21⁎⁎ ± 0.23 | 0.08NS | [−0.01, 0.17] |
| 5. 10 min | −0.21⁎⁎ ± 0.28 | −0.36⁎⁎ ± 0.28 | < 0.01⁎⁎ | [0.05, 0.26] |
| 6. 20 min | −0.27⁎⁎ ± 0.34 | −0.69⁎⁎ ± 0.54 | < 0.01⁎⁎ | [0.25, 0.59] |
3.5. Temperature downshifts do not differ between healthy controls and diabetes patients
One of the hypotheses tested with the study design is that the autoregulation of the plantar temperatures differs between healthy controls and diabetes patients diagnosed with peripheral sensoric neuropathy. In all participants peripheral arterial occlusive disease was excluded by ABI measurements and Doppler ultrasound examinations. Comparisons between the two enrolled groups were performed, yielding no differences in the magnitude of average temperature downshifts following standing (Fig. 6a). The maximum temperature downshift from all stance episodes did not differ between both groups (Fig. 6b). The changes of temperature within each group for each of the stance episodes and foot regions were calculated (Table 3). Significant differences in mean ∆T were only observed for the fifth metatarsal region (MTHV), whereas all other comparisons yielded non-significant differences between both groups. The most prominent temperature downshift was detected in different sensors. The contribution of each sensor to the maximum temperature downshift in the different cohorts is visualized in Fig. 6c. Whereas for healthy volunteers 1/3 of maximum temperature downshifts was detected with DigitusI, no single sensor was identified with diabetes patients.
Fig. 6.
Diabetes patients with sensoric neuropathy have similar down-regulation of plantar temperatures upon pressure load. a. Comparison of maximum temperature change (∆T) distribution over all sensors between healthy controls (blue) and diabetes patients with neuropathy (red). b. Maximum temperature changes over all stance episodes are identified and added up to calculate the average maximum temperature change for both groups. c. For all stance episodes sensors were identified with the maximum temperature difference when comparing data sets before and after standing. The relative contribution of each sensor to the ∆T values is expressed for both study groups as percentage. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Means ± standard deviation of ∆T for each stance episode and sensor per group. Student's t-test is performed to examine intra-group and inter-group differences. The null hypothesis of the intra-group test is that ∆T is equal to or larger than 0. The null hypothesis of the inter-group test is that the difference between ∆T of the control group and ∆T of the diabetic group is equal to 0. NS not significant, *p < 0.05, ⁎⁎p < 0.01.
| Inter-group difference |
|||||
|---|---|---|---|---|---|
| Stance Episode | Sensor | Controls 57 datasets | Diabetes patients57 datasets | p-value | 95% CI |
| 1. 5 min | DigitusI | −0.07NS ± 0.92 | 0.54NS ± 2.04 | 0.05NS | [−1.23, 0.01] |
| MTHI | 0.18NS ± 0.85 | 0.82NS ± 2.00 | 0.06NS | [−1.30, 0.03] | |
| MTHII | 0.12NS ± 0.87 | 0.53NS ± 2.21 | 0.21NS | [−1.05, 0.23] | |
| MTHIII | 0.09NS ± 0.84 | 0.44NS ± 2.33 | 0.29NS | [−1.02, 0.31] | |
| MTHIV | 0.08NS ± 0.81 | 0.76NS ± 1.93 | 0.02* | [−1.26,−0.10] | |
| MTHV | 0.12NS ± 0.73 | 0.63NS ± 1.73 | 0.04* | [−1.01,−0.02] | |
| Lateral | 0.26NS ± 0.90 | 0.74NS ± 1.89 | 0.09NS | [−1.04, 0.07] | |
| Calcaneus | 0.26NS ± 0.75 | 0.78NS ± 1.92 | 0.10NS | [−1.14, 0.10] | |
| 2. 10 min | DigitusI | −0.61⁎⁎ ± 0.69 | −0.70⁎⁎ ± 0.59 | 0.47NS | [−0.15, 0.33] |
| MTHI | −0.75⁎⁎ ± 0.54 | −0.79⁎⁎ ± 0.59 | 0.75NS | [−0.22, 0.31] | |
| MTHII | −0.90⁎⁎ ± 0.69 | −0.87⁎⁎ ± 0.59 | 0.82NS | [−0.27, 0.21] | |
| MTHIII | −0.91⁎⁎ ± 0.71 | −0.87⁎⁎ ± 0.63 | 0.78NS | [−0.29, 0.22] | |
| MTHIV | −0.86⁎⁎ ± 0.71 | −1.03⁎⁎ ± 0.73 | 0.23NS | [−0.11, 0.44] | |
| MTHV | −0.66⁎⁎ ± 0.68 | −1.00⁎⁎ ± 0.70 | 0.01* | [0.08, 0.60] | |
| Lateral | −0.76⁎⁎ ± 0.73 | −0.73⁎⁎ ± 0.60 | 0.85NS | [−0.27, 0.23] | |
| Calcaneus | −0.83⁎⁎ ± 0.69 | −0.86⁎⁎ ± 0.59 | 0.87NS | [−0.29, 0.34] | |
| 3. 20 min | DigitusI | −0.60⁎⁎ ± 0.96 | −0.83⁎⁎ ± 0.71 | 0.15NS | [−0.09, 0.55] |
| MTHI | −0.65⁎⁎ ± 0.78 | −0.87⁎⁎ ± 0.71 | 0.23NS | [−0.14, 0.58] | |
| MTHII | −0.92⁎⁎ ± 0.85 | −1.03⁎⁎ ± 0.66 | 0.47NS | [−0.18, 0.39] | |
| MTHIII | −0.98⁎⁎ ± 0.88 | −1.10⁎⁎ ± 0.65 | 0.40NS | [−0.17, 0.41] | |
| MTHIV | −1.01⁎⁎ ± 0.94 | −0.97⁎⁎ ± 2.49 | 0.91NS | [−0.78, 0.70] | |
| MTHV | −0.85⁎⁎ ± 0.91 | −1.37⁎⁎ ± 0.90 | < 0.01⁎⁎ | [0.18, 0.86] | |
| Lateral | −1.04⁎⁎ ± 0.78 | −0.97⁎⁎ ± 0.69 | 0.63NS | [−0.34, 0.21] | |
| Calcaneus | −0.99⁎⁎ ± 0.80 | −1.10⁎⁎ ± 0.73 | 0.55NS | [−0.26, 0.48] | |
| 4. 5 min | DigitusI | −0.02NS ± 0.27 | −0.04NS ± 0.31 | 0.75NS | [−0.09, 0.13] |
| MTHI | −0.16⁎⁎ ± 0.24 | −0.21⁎⁎ ± 0.24 | 0.35NS | [−0.06, 0.17] | |
| MTHII | −0.16⁎⁎ ± 0.27 | −0.27⁎⁎ ± 0.25 | 0.03* | [0.01, 0.21] | |
| MTHIII | −0.17⁎⁎ ± 0.27 | −0.25⁎⁎ ± 0.26 | 0.09NS | [−0.01, 0.19] | |
| MTHIV | −0.16⁎⁎ ± 0.27 | −0.15NS ± 0.93 | 0.92NS | [−0.28, 0.26] | |
| MTHV | −0.05NS ± 0.33 | −0.29⁎⁎ ± 0.31 | < 0.01⁎⁎ | [0.12, 0.36] | |
| Lateral | −0.19⁎⁎ ± 0.36 | −0.26⁎⁎ ± 0.25 | 0.21NS | [−0.04, 0.19] | |
| Calcaneus | −0.25⁎⁎ ± 0.33 | −0.17⁎⁎ ± 0.25 | 0.28NS | [−0.22, 0.07] | |
| 5. 10 min | DigitusI | −0.08* ± 0.36 | −0.14⁎⁎ ± 0.39 | 0.43NS | [−0.09, 0.20] |
| MTHI | −0.20⁎⁎ ± 0.33 | −0.30⁎⁎ ± 0.36 | 0.24NS | [−0.07, 0.26] | |
| MTHII | −0.26⁎⁎ ± 0.32 | −0.41⁎⁎ ± 0.29 | 0.01* | [0.03, 0.26] | |
| MTHIII | −0.24⁎⁎ ± 0.32 | −0.44⁎⁎ ± 0.23 | < 0.01⁎⁎ | [0.09, 0.30] | |
| MTHIV | −0.22⁎⁎ ± 0.36 | −0.32* ± 0.96 | 0.51NS | [−0.19, 0.38] | |
| MTHV | −0.11* ± 0.43 | −0.51⁎⁎ ± 0.42 | < 0.01⁎⁎ | [0.24, 0.56] | |
| Lateral | −0.30⁎⁎ ± 0.39 | −0.43⁎⁎ ± 0.33 | 0.06NS | [−0.01, 0.27] | |
| Calcaneus | −0.31⁎⁎ ± 0.33 | −0.33⁎⁎ ± 0.40 | 0.86NS | [−0.16, 0.18] | |
| 6. 20 min | DigitusI | −0.13* ± 0.50 | −0.36⁎⁎ ± 0.57 | 0.03* | [0.02, 0.43] |
| MTHI | −0.25⁎⁎ ± 0.39 | −0.50⁎⁎ ± 0.56 | 0.03* | [0.02, 0.46] | |
| MTHII | −0.30⁎⁎ ± 0.39 | −0.64⁎⁎ ± 0.59 | < 0.01⁎⁎ | [0.16, 0.53] | |
| MTHIII | −0.32⁎⁎ ± 0.41 | −0.75⁎⁎ ± 0.59 | < 0.01⁎⁎ | [0.23, 0.61] | |
| MTHIV | −0.27⁎⁎ ± 0.44 | −0.89⁎⁎ ± 0.99 | < 0.01⁎⁎ | [0.31, 0.92] | |
| MTHV | −0.13* ± 0.50 | −0.90⁎⁎ ± 0.77 | < 0.01⁎⁎ | [0.52, 1.01] | |
| Lateral | −0.43⁎⁎ ± 0.48 | −0.78⁎⁎ ± 0.64 | < 0.01⁎⁎ | [0.14, 0.56] | |
| Calcaneus | −0.41⁎⁎ ± 0.49 | −0.74⁎⁎ ± 0.79 | 0.03* | [0.04, 0.62] | |
3.6. In contrast to healthy controls diabetes patients strictly adhered to the study protocol
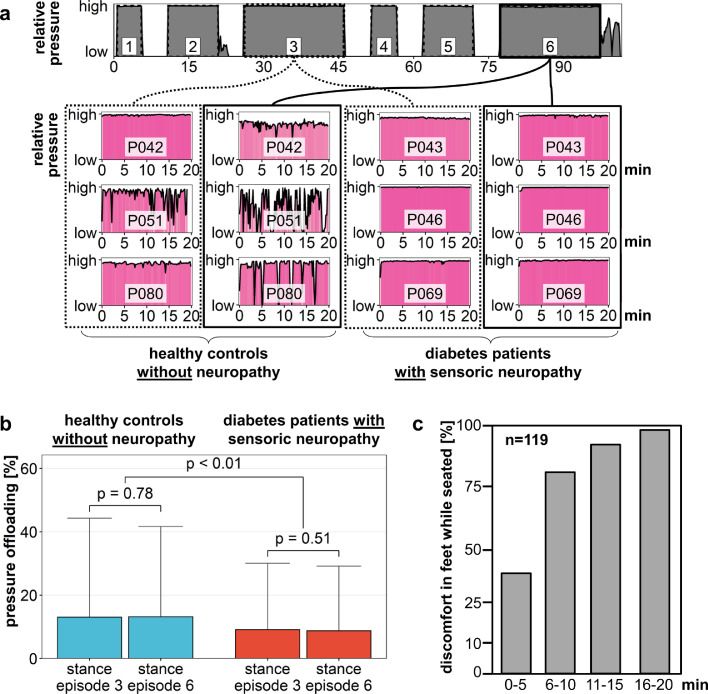
All study participants were instructed to relentlessly apply pressure to their feet during each stance episode. Pressure release was only permitted during the intermittent seating episodes. It was expected that this would be easier during the short stance episodes 1 and 4 than the longer ones. Indeed, pressure recordings showed that healthy volunteers briefly released pressure during the longer stance episodes, as is visualized in Fig. 7a where the pressure time curves of stance episodes 3 and 6 for three diabetes patients with polyneuropathy and three healthy controls are visualized. The pressure sensors detected maximum values for all the shown participants, however there are short releases of pressure especially with healthy volunteers in the last stance episodes. To quantify these changes for the whole study cohort, statistical analysis was performed by calculating the percentage of recordings where the participants released pressure during stance episodes 3 and 6 (Fig. 7b). No significant differences between stance episodes 3 and 6 were found within the different groups. However, mean percentage of pressure release recordings were significantly different between healthy volunteers and diabetes patients with sensoric polyneuropathy. To obtain additional data on the time-to-discomfort for feet that are exposed to “normal pressure” applied in a seated position we asked a cohort of healthy volunteers that had no history of diabetes to keep their feet at a given position. The 119 healthy volunteers were asked to indicate when they felt discomfort in their feet and imperative urge to reposition their legs. Already 38% reported discomfort within the first 5 min, more than 90% within 15 min (Fig. 7c).
Fig. 7.
Pressure exposure is not continuous in all recordings. a. Representative pressure recordings for three healthy controls and three diabetes patients with polyneuropathy (NP) are provided. Whereas most healthy controls show intermittent discontinuation of pressure application during the longer stance episodes pressure curves of diabetes patients reveal a higher degree of adherence to the study protocol. b. Intermittent pressure release in diabetes patients with neuropathy occurs less often than in healthy controls. The average proportion of recordings without pressure application was calculated for the two 20 min stance episodes 3 and 6. c. To obtain additional data on the time-to-discomfort in volunteers not diagnosed with diabetes a cohort of 119 participants were asked to report on an imperative urge to move legs when seated. The share of volunteers reporting discomfort are depicted over time.
4. Discussion
Our study was designed to test for temperature changes with pressure load in a stance position over a predefined time of up to 20 min. Auto-regulatory mechanisms linked to the autonomous nerve system may be operative under these circumstances with constriction of the arterioles, thus contributing to the counter-regulation of blood pressure drops [25]. Indeed, we observed a down-shift of plantar temperatures that did not become obvious within the first 5 min of stance, however with extended pressure load of 10 min and more. The downshift was pronounced in areas of maximal pressure load, arguing against a generalized regulation by the autonomous nervous system. The downshift was independent of the ambient in-shoe temperature, lasted transiently, and immediately resolved following pressure release. In this context one has to emphasize that the ambient in-shoe temperatures on average increased asymptotic by 5.8 °C over the course of 90 min, from 17.8 ± 2.4 °C at the start to 23.6 ± 1.8 °C at the end of recording. The curves’ asymptotic shape may be explained by a gradual energy exchange of the body and the environment in a closed compartment of the shoe. The in-shoe ambient temperature changes were similar in healthy volunteers as well as diabetes patients with diagnosed sensoric polyneuropathy, as were the pressure-dependent downshifts following pressure load. Of note, the temperature downshifts when standing became evident when the ambient in-shoe temperatures were subtracted from the actual plantar sensor values.
Our findings suggest that notwithstanding differences in the microvasculature of diabetes patients the underlying temperature autoregulation is not disturbed with diabetes [26]. The study protocol focused on temporal dynamics of plantar temperature changes, whereas Nagase et al. [27]. employed a static thermography setup to visualize temperature differences of the plantar surface in healthy volunteers versus non-ulcer diabetes patients. The extent of temperature drops over time were similar in the absence and presence of diabetes, reaching about −1.0 ± 0.8 °C over a period of 20 min (Fig. 6a and b). To our knowledge, such transient temperature downshifts have not been reported before. Unexplainably, the downshifts were of lower magnitude when the study participants repeatedly performed the pressure load for more than 45 min. Potentially, there is an intact counter-autoregulation in place, independent of the polyneuropathy [28]. Another putative explanation is the intermittent release of body weight on the insoles due to discomfort in healthy volunteers which may result in less prominent temperature changes over time.
All participants were similarly instructed to expose their weight to the insoles over the whole stance episodes. Nevertheless there were marked differences in the application of weight and pressure application between the groups. In healthy volunteers the pressure sensors detected brief episodes of pressure relief during the longer stance episodes. In accord with this observation, most healthy controls claimed discomfort in their feet during the study whereas the diabetes patients did not. In a survey we asked a cohort of 119 seated healthy volunteers to keep their feet in a given position. The time-to-discomfort was recorded and each participant indicated when he felt an imperative need to change the foot position. More than 90% of the volunteers reported discomfort within 15 min.
One may speculate that the discomfort in the feet experienced by healthy volunteers are not present to the same degree in diabetes patients with sensoric impairment, which is also supported by the data on intermittent pressure release. Given the relevance of tissue blood supply one may envision that diabetes patients may undergo critical tissue ischemia when exposed to their own body weight for extended periods of time. Especially those working in an upright position may suffer from repetitive plantar microcirculatory impairments, ultimately skewing the tissue homeostasis [20].
Our findings clearly need to be extended to larger cohorts, possibly with more strict adherence to the study protocol, and recording of time-to-discomfort within the groups. Temperature recordings in the plantar regions of the feet will possibly close the gap toward early detection of tissue damage and ulcer formation. Our findings highlight that not only long-term changes but also timely changes in stance position occur. The detection and visualization of plantar foot temperature changes has gained attention since clinical studies demonstrated a predictive value of elevated temperatures for the onset of diabetic ulcer disease [18,29,30]. Notably, such changes may exceed more than 2 °C, even days to weeks before overt ulcer formation [20]. Transiently lowered plantar temperatures may conversely incite injuries when prolonged stance episodes are performed.
Declaration of Competing Interest
SK and PRM filed a patent on sensor-equipped insoles: WO2012084814A1.
TS is CEO at Thorsis Technologies GmbH.
Acknowledgments
Acknowledgments
We are grateful for expert IT support by F. Samland (Thorsis).
Funding
The study was supported by EFRE Förderung der Europäischen Union und Landesmittel des Ministeriums für Wirtschaft, Wissenschaft und Digitalisierung Sachsen-Anhalt (Vorhabennummer: ZS/2016/05/78615 and ZS/2018/12/95325). JK and PRM were supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - project ID 97850925 - SFB854, AM by the Chinese Scholarship Council (CSC). The funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Footnotes
Clinical Trial Registration Number: DRKS00005683.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102712.
Contributor Information
Uli Niemann, Email: uli.niemann@ovgu.de.
Peter R. Mertens, Email: peter.mertens@med.ovgu.de.
Appendix. Supplementary materials
References
- 1.Boulton A.J., Vileikyte L., Ragnarson-Tennvall G., Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Kumari M.J.S.J., Jagdish S. How to prevent amputation in diabetic patients. Int J Nurs Educ. 2014;6:40–44. [Google Scholar]
- 3.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014;370(16):1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 4.Volmer-Thole M., Lobmann R. Neuropathy and diabetic foot syndrome. Int J Mol Sci. 2016;17(6) doi: 10.3390/ijms17060917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frykberg R.G., Zgonis T., Armstrong D.G. Diabetic foot disorders. A clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45(5 Suppl):S1–66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 6.Walsh J.W., Hoffstad O.J., Sullivan M.O., Margolis D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diab Med. 2016;33(11):1493–1498. doi: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 7.Boyko E.J., Seelig A.D., Ahroni J.H. Limb- and Person-Level risk factors for lower-limb amputation in the prospective Seattle Diabetic Foot Study. Diab Care. 2018;41(4):891–898. doi: 10.2337/dc17-2210. [DOI] [PubMed] [Google Scholar]
- 8.Van Battum P., Schaper N., Prompers L. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diab Med. 2011:199–205. doi: 10.1111/j.1464-5491.2010.03192.x. [DOI] [PubMed] [Google Scholar]
- 9.Rayman G., Hassan A., Tooke J.E. Blood flow in the skin of the foot related to posture in diabetes mellitus. Br Med J (Clin Res Ed) 1986;292(6513):87–90. doi: 10.1136/bmj.292.6513.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rayman G., Williams S.A., Spencer P.D., Smaje L.H., Wise P.H., Tooke J.E. Impaired microvascular hyperaemic response to minor skin trauma in type I diabetes. Br Med J (Clin Res Ed) 1986;292(6531):1295–1298. doi: 10.1136/bmj.292.6531.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun P.C., Chen C.S., Kuo C.D. Impaired microvascular flow motion in subclinical diabetic feet with sudomotor dysfunction. Microvasc Res. 2012;83(2):243–248. doi: 10.1016/j.mvr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Fard A.S., Esmaelzadeh M., Larijani B. Assessment and treatment of diabetic foot ulcer. Int J Clin Pract. 2007;61(11):1931–1938. doi: 10.1111/j.1742-1241.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaper N.C., Van Netten J.J., Apelqvist J., Lipsky B.A., Bakker K. International Working Group on the Diabetic F. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diab Res Clin Pract. 2017;124:84–92. doi: 10.1016/j.diabres.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Rayman G., Malik R.A., Sharma A.K., Day J.L. Microvascular response to tissue injury and capillary ultrastructure in the foot skin of type I diabetic patients. Clin Sci (Lond) 1995;89(5):467–474. doi: 10.1042/cs0890467. [DOI] [PubMed] [Google Scholar]
- 15.Rayman G., Williams S.A., Gamble J., Tooke J.E. A study of factors governing fluid filtration in the diabetic foot. Eur J Clin Invest. 1994;24(12):830–836. doi: 10.1111/j.1365-2362.1994.tb02027.x. [DOI] [PubMed] [Google Scholar]
- 16.Arts M.L., de Haart M., Waaijman R. Data-driven directions for effective footwear provision for the high-risk diabetic foot. Diab Med. 2015;32(6):790–797. doi: 10.1111/dme.12741. [DOI] [PubMed] [Google Scholar]
- 17.Reddy P.N., Cooper G., Weightman A., Hodson-Tole E., Reeves N.D. Walking cadence affects rate of plantar foot temperature change but not final temperature in younger and older adults. Gait Posture. 2017;52:272–279. doi: 10.1016/j.gaitpost.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Frykberg R.G., Gordon I.L., Reyzelman A.M. Feasibility and efficacy of a smart mat technology to predict development of diabetic plantar ulcers. Diabetes Care. 2017;40(7):973–980. doi: 10.2337/dc16-2294. [DOI] [PubMed] [Google Scholar]
- 19.Bharara M., Schoess J., Armstrong D.G. Coming events cast their shadows before: detecting inflammation in the acute diabetic foot and the foot in remission. Diab Metab Res Rev. 2012;28(Suppl 1):15–20. doi: 10.1002/dmrr.2231. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 21.Niemann U., Spiliopoulou M., Szczepanski T. Comparative clustering of plantar pressure distributions in diabetics with polyneuropathy may be applied to reveal inappropriate biomechanical stress. PLoS ONE. 2016;11(8) doi: 10.1371/journal.pone.0161326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bus S.A., Haspels R., Busch-Westbroek T.E. Evaluation and optimization of therapeutic footwear for neuropathic diabetic foot patients using in-shoe plantar pressure analysis. Diab Care. 2011;34(7):1595–1600. doi: 10.2337/dc10-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waaijman R., Arts M.L., Haspels R., Busch-Westbroek T.E., Nollet F., Bus S.A. Pressure-reduction and preservation in custom-made footwear of patients with diabetes and a history of plantar ulceration. Diab Med. 2012;29(12):1542–1549. doi: 10.1111/j.1464-5491.2012.03700.x. [DOI] [PubMed] [Google Scholar]
- 24.Bus S.A. Innovations in plantar pressure and foot temperature measurements in diabetes. Diab Metab Res Rev. 2016;32(Suppl 1):221–226. doi: 10.1002/dmrr.2760. [DOI] [PubMed] [Google Scholar]
- 25.Midttun M., Sejrsen P., Paaske W.P. Blood flow rate during orthostatic pressure changes in the pulp skin of the first toe. Eur J Vasc Endovasc Surg. 1997;13(3):278–284. doi: 10.1016/s1078-5884(97)80099-8. [DOI] [PubMed] [Google Scholar]
- 26.Vilcahuaman L., Harba R., Canals R. Detection of diabetic foot hyperthermia by infrared imaging. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4831–4834. doi: 10.1109/EMBC.2014.6944705. [DOI] [PubMed] [Google Scholar]
- 27.Nagase T., Sanada H., Takehara K. Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: novel classification using angiosome concept. J Plastic Reconstr Aesthetic Surg. 2011;64(7):860–866. doi: 10.1016/j.bjps.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Tansey E.A., Roe S.M., Johnson C.J. The sympathetic release test: a test used to assess thermoregulation and autonomic control of blood flow. Adv Physiol Educ. 2014;38(1):87–92. doi: 10.1152/advan.00095.2013. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong D.G., Holtz-Neiderer K., Wendel C., Mohler M.J., Kimbriel H.R., Lavery L.A. Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med. 2007;120(12):1042–1046. doi: 10.1016/j.amjmed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Houghton V.J., Bower V.M., Chant D.C. Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? a systematic review and meta-analysis. J Foot Ankle Res. 2013;6(1):31. doi: 10.1186/1757-1146-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.