Abstract
In our institution, between January 2010 and December 2017, 15 140 peripherally inserted central catheters (PICCs) were inserted in 12 314 patients. Using time-series analysis to evaluate the annual historical trend (AHT), we observed a significant increase in bloodstream infections (BSIs; AHT = 24; p < 0.001) and associated deaths (AHT = 3; p 0.02) in patient with PICCs. The risk of experiencing a BSI was significantly higher in patients with PICCs (odds ratio = 9.6; 95% confidence interval, 9.08–10.18; p < 0.001). To reduce PICC-related BSIs and their related mortality, it is important to limit the overuse of PICCs and to implement a ‘no PICC’ policy by limiting the insertion of PICCs to situations without other available options.
Keywords: Bacteraemia, bloodstream infection, hospital-acquired infection, mortality, PICC Line
Introduction
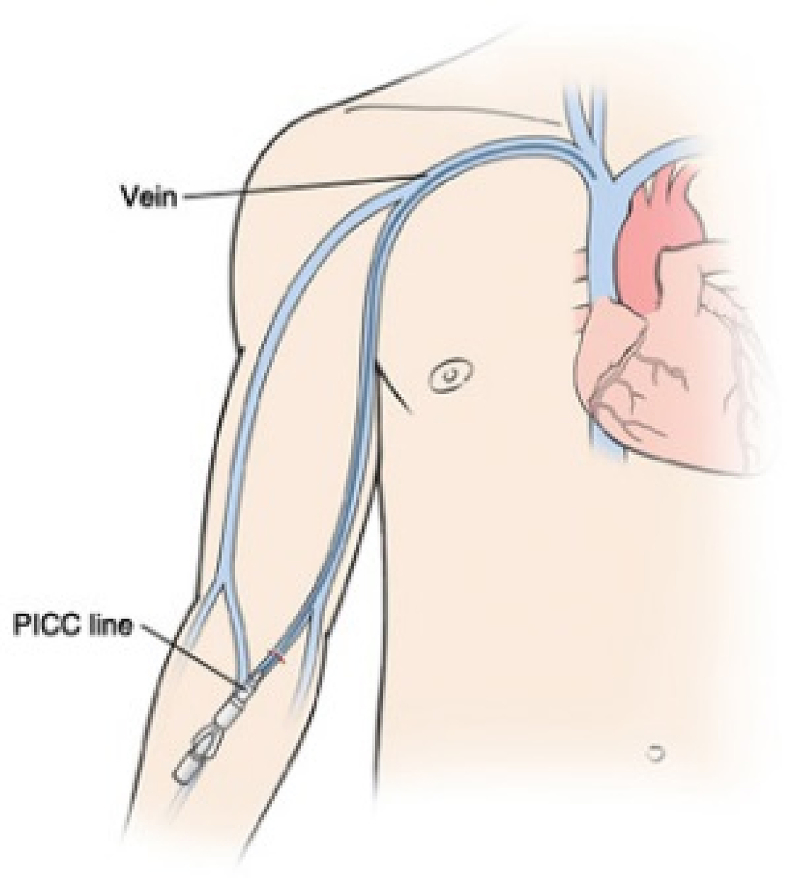
Peripherally inserted central catheters (PICCs) are central catheters that were first used in 1975 [1]. PICC enter the body through the skin (percutaneously) at a peripheral site, extending to the superior vena cava (a central venous trunk) and remaining in place (dwelling within the veins) for days or weeks (Fig. 1). They are currently used in both inpatients and outpatients for several indications, mainly for delivery of intravenous antibiotics, parenteral nutrition and chemotherapy [2,3]. However, PICCs are associated with various complications, particularly thrombosis and bloodstream infections (BSIs) [[4], [5], [6]].
Fig. 1.
Peripherally inserted central catheter line enters body through skin (percutaneously) at peripheral site, extends to superior vena cava (central venous trunk) and stays in place (dwells within veins) for days or weeks.
BSIs are life-threatening conditions associated with high morbidity and hospital costs. Indeed, the US Centers for Disease Control and Prevention (CDC) estimate that 250 000 BSIs and 80 000 catheter-related BSIs (CRBSIs) occur annually in the United States, classifying it as the 12th cause of death in 2007 [7,8]. Moreover, associated costs are estimated in the United States to range from $7288 to $29 156 per episode [9].
A previous study performed at our institution in 2013 found that 1033 patients experienced healthcare-associated BSIs in the 48 hours after admission, leading to a mortality rate of 11% at 15 days [10]. Globally, in the literature, the incidence of CRBSIs associated with PICCs ranges between 0.5% and 12.7% [11,12].
PICCs have been used in the four university hospitals in Marseille since 2007, and routine monitoring of BSIs in hospitalized patients with PICCs began in 2010. The aim of our study was to compare BSI and mortality rates in patients with PICCs compared to patients without PICCs who were hospitalized from our hospital centre between 2010 and 2017.
Materials and methods
We retrospectively studied the positive blood culture data from our hospital centre (Marseille, France) between January 2010 and December 2017. During this period, PICC lines were inserted by the radiology department only. All PICCs were implanted by the same team (interventional radiology), and only by trained senior or senior-supervised residents following national protocols that had been edited by the French society for hygiene, and with the use of alcohol-based antiseptic techniques. The recommended vein to use was the humeral vein. Maintenance of the PICC line was performed according to the nursing protocols in each department. We used single light catheters, but in 2011 a system was implemented with a pressure check valve, which has been reported to be associated with a lower risk of contamination and bacteraemia [13].
We defined BSI events according to the CDC as at least two positive blood cultures growing commensal bacteria (coagulase-negative staphylococci, Micrococcus spp., Corynebacterium spp., Propionibacterium acnes and Bacillus spp.), or at least one culture growing pathogenic bacteria, without any cultures with the same bacterium from another kind of sample within a 14-day period. The BSI events were then deduplicated according to patient identity number, sampling date and bacterial identification, and were cross-checked against the list of PICCs installed in our institution over the study period. We considered a BSI to have occurred in a patient with a PICC if the date of insertion of the PICC was before the date of the BSI event. Finally, we checked whether patients who had experienced a BSI event had died within the next 30 days in our centre.
All statistical analyses were performed by R software (https://www.r-project.org/). Proportional comparisons were performed by Pearson chi-square tests, and odds ratios (ORs) and confidence intervals (CIs) were calculated to evaluate the risk associated with the compared conditions. Finally, linear models were built to analyse the annual historical trends (AHTs) (i.e. the annual trend in the mean number of patients in different conditions). Classic linear regression models were applied to estimate the trends in the annual mean number of patients through years. The trends for each condition were defined as the estimated slopes of each model and tested to zero (no trends). p ≤ 0.05 was considered to be statistically significant.
All the methods were carried out in accordance with the European General Data Protection Regulation. The study was a retrospective analysis of patients' biological and registry data issued from the hospital information system, which is an authorized healthcare database. Access to the registry was approved by the data protection committee of our institution (Assistance publique des hôpitaux de Marseille, APHM) and was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019-73. The study was supervised by a person who was fully aware of the confidentiality requirements.
Results
Between January 2010 and December 2017, a total of 15 140 PICCs were inserted in 12 314 patients. Globally, 11 890 BSI events occurred in 10 942 patients (1.08 BSI events per patient) (Table 1). The annual number of patients with BSI increased significantly over time (AHT = 30; p 0.04), in parallel with an increase in the number of PICC-associated CRBSIs (AHT = 24; p < 0.001). The annual increase in the number of patients hospitalized in our setting, as well as the number of patients with BSIs, was not statistically significant (AHT = 12, p 0.98; and AHT = 9, p 0.5, respectively). Interestingly, over the study period, the number of installed PICCs did not increase significantly; this was likely related to the decrease in the number of PICCs inserted during the last 2 years (AHT = 12; p 0.7). When we considered the total number of CRBSIs occurring in patients with PICCs (1477 BSI events, 9.8% of all the PICCs used) and the total number of BSIs in patients without PICCs (10 413 BSI events), the calculated risk of experiencing a BSI was significantly higher in patients with PICCs (OR = 9.6; 95% CI, 9.08–10.18; p < 0.001).
Table 1.
AHTs of number of patients with BSIs with and without PICCs over 7 years of follow-up (2010–2017)
| Year | No. of hospitalized patients | No. of installed PICCs |
BSIs with PICCs |
BSIs without PICCs |
Total |
pa | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of events | No. of patients | No. of eventsb | No. of patients | No. of events | No. of patients | No. of events | ||||
| 2010 | 114 171 | 1 293 | 1 638 | 78 | 88 | 1 263 | 1 308 | 1 341 | 1 396 | <0.01 | 4.8 (3.87–6.03) |
| 2011 | 120 893 | 1 520 | 1 902 | 99 | 112 | 1 248 | 1 314 | 1 347 | 1 426 | <0.01 | 5.6 (4.59–6.83) |
| 2012 | 120 339 | 1 529 | 1 884 | 121 | 135 | 1 174 | 1 254 | 1 295 | 1 389 | <0.01 | 7.2 (6.00–8.67) |
| 2013 | 121 806 | 1 605 | 1 959 | 129 | 151 | 1 097 | 1 167 | 1 226 | 1 318 | <0.01 | 8.5 (7.13–10.13) |
| 2014 | 120 498 | 1 751 | 2 128 | 171 | 195 | 1 170 | 1 236 | 1 341 | 1 431 | <0.01 | 9.6 (8.17–11.19) |
| 2015 | 118 427 | 1 769 | 2 144 | 200 | 237 | 1 284 | 1 391 | 1 484 | 1 628 | <0.01 | 10.3 (8.88–11.87) |
| 2016 | 117 664 | 1 577 | 1 946 | 249 | 297 | 1 259 | 1 320 | 1 508 | 1 617 | <0.01 | 15.6 (13,64–17.86) |
| 2017 | 117 626 | 1 270 | 1 539 | 218 | 262 | 1 303 | 1 423 | 1 521 | 1 685 | <0.01 | 16.5 (14.33–19.07) |
| Total | 951 424 | 12 314 | 15 140 | 1 265 | 1 477 | 9 798 | 10 413 | 11 063 | 11 890 | <0.01 | 9.6 (9.08–10.18) |
| AHT (p) | 12 (0.98) | 12 (0.7) | 24 (<0.01) | 9 (0.5) | 30 (0.04) | ||||||
AHTs were calculated using linear models, with each value demonstrating annual trend of mean number of patients in different conditions.
AHT, annual historical trend; BSI, bloodstream infection; CI, confidence interval; OR, odds ratio; PICC, peripherally inserted central catheter.
Uncorrected bilateral chi-square tests, ORs, CIs and AHTs were calculated by R software (https://www.r-project.org/); p ≤ 0.05 was considered statistically significant. Comparisons were made between number of BSI events.
Event is number of PICCs; number of PICCs can be larger than number of patients.
Over the study period, 255 (21.4%) of 1265 patients with a PICC-related CRBSI and 1410 (14.4%) of 9798 patients with BSIs without PICCs died within the first 30 days of follow-up (Table 2). The 30-day mortality rate associated with PICC-related CRBSIs increased significantly from 2010 to 2017 (AHT = 3; p 0.02), while it did not increase for patients with BSIs without PICCs (AHT = 2; p 0.6). The risk of dying after PICC-related CRBSIs was 1.5-fold higher than that in patients with BSIs but without PICCs (OR = 1.5; 95% CI, 1.30–1.74; p < 0.001).
Table 2.
AHTs of 30-day mortality in patients with BSIs with and without PICCs over 7 years of follow-up (2010–2017)
| Year | Total no. of deaths | With PICCs, n (%) |
Without PICCs, n (%) |
pa | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Alive at 1 month | Dead at 1 month | Alive at 1 month | Dead at 1 month | ||||
| 2010 | 2 651 | 57 (78.1) | 21 (26.9) | 1 060 (83.9) | 203 (16.1) | 0.01 | 1.9 (1.14–3.24) |
| 2011 | 2 593 | 68 (68.7) | 31 (31.3) | 1 096 (87.8) | 152 (12.2) | <0.01 | 3.3 (2.08–5.19) |
| 2012 | 2 719 | 100 (82.6) | 21 (17.4) | 1 003 (85.4) | 171 (14.6) | 0.4 | 1.2 (0.75–2.03) |
| 2013 | 2 858 | 97 (75.2) | 32 (24.8) | 9 380 (85.5) | 159 (14.5) | <0.01 | 1.9 (1.26–3.00) |
| 2014 | 2 694 | 146 (85.4) | 25 (14.6) | 1 008 (86.2) | 162 (13.8) | 0.8 | 1.1 (0.68–1.68) |
| 2015 | 2 812 | 165 (82.5) | 35 (17.5) | 1 099 (85.6) | 185 (14.4) | 0.3 | 1.3 (0.85–1.87) |
| 2016 | 2 842 | 201 (80.7) | 48 (19.3) | 1 069 (84.9) | 190 (15.1) | 0.1 | 1.3 (0.95–1.91) |
| 2017 | 2 946 | 176 (80.7) | 42 (19.3) | 1 115 (85.6) | 188 (14.4) | 0.1 | 1.4 (0.98–2.05) |
| Total | 22 115 | 1 010 (78.6) | 255 (21.4) | 8 388 | 1 410 (14.4) | <0.01 | 1.5 (1.30–1.74) |
| AHT (p) | 41 (<0.01) | 21 (<0.01) | 3 (0.02) | 7 (0.5) | 2 (0.6) | ||
AHTs were calculated using linear models, with each value demonstrating annual trend of mean number of patients in different conditions.
AHT, annual historical trend; BSI, bloodstream infection; CI, confidence interval; OR, odds ratio; PICC, peripherally inserted central catheter.
Uncorrected bilateral chi-square tests, ORs, CIs and AHTs were calculated by R software (https://www.r-project.org/); p ≤ 0.05 was considered statistically significant.
The main microorganisms associated with BSIs were coagulase-negative staphylococci (46.51%), followed by Enterobacteriaceae (23.25%), then Staphylococcus aureus and Enterococcus spp. (11.24% and 5.42% respectively). No correlation was established between microbiology and mortality [14].
Discussion
This time-series study revealed an increasing number of BSIs among patients with PICCs, with a global 9.6-fold higher risk of BSIs in this population. Moreover, we identified that BSIs due to PICCs were associated with a risk of death that was 1.5 times higher than the risk in patients experiencing BSIs without PICCs. While patients with PICCs might have greater comorbidity rates, which were not recorded in this study (especially cancer), the increasing use of PICCs is particularly concerning, especially because it is known that in the United States, approximately 41 000 BSI events occur each year in hospitalized patients with a central venous catheter [7]. A national French survey performed in 2012 and 2017 identified an increase (+169%; range, 0.38–1.3%; 95% CI, 1.59–3.31) in the use of PICC lines compared to the use of other catheters [15,16]. Of 1620 patients who had experienced at least one hospital-acquired BSI, 3.4% of cases occurred in patients with PICCs [16]. In our study, an infection control intervention in 2014 may have resulted in a decrease in PICC insertion, followed by a nonsignificant trend of PICC-related CRBSI reduction.
In France, PICCs are especially indicated in outpatients and neonates who need venous access for more than 7 days and less than 3 months. In our experience, because they are easier to manipulate (interventional radiology) than other central venous catheters such as the Port-a-Cath, PICCS are often inserted for unjustified reasons such as to provide prolonged antimicrobial therapy while an oral option is available, easy access for regular blood sampling, rehydration in elderly patients when subcutaneous infusion is feasible and, in the worst situation, for no reason (‘just in case’). Moreover, PICCs are not regularly removed as soon as indicated (after the end of antimitotic or antimicrobial chemotherapy), leading to late and life-threatening infectious and thromboembolic complications. Sengupta et al. [17] found an increased risk of central line–associated BSIs of 33% per day after day 36 of PICC insertion.
To reduce BSIs (and subsequently mortality) associated with PICCs, we believe that it is important to limit the overuse of PICCs and to implement a ‘no PICC’ policy that limits the insertion of PICCs to situations where other options are not possible. In these situations, the risk of infectious complications—including both patient factors (neutropenia, haematologic diseases or other underlying conditions) and device factors (number of prior PICC insertions, number of lumens and duration of PICC placement, right-sided line insertion)—should be taken into consideration [2]. Finally, central catheter care practice bundles should be implemented, including regular staff training to learn correct insertion practices; to address the appropriate handling and maintenance of a central line; and to emphasize the importance of promptly removing unnecessary PICCs. This can be achieved by using either innovative electronic tools [18] or new management approaches such as designated nursing teams [19]. Similarly, to address the electronic monitoring of hygiene and surveillance systems, new approaches to enhance the traceability of catheters are being developed, with the goal of helping caregivers monitor catheter duration and to ensure catheter removal as soon as possible [20,21].
Although there are limitations to our study (particularly the fact that we were unable to assess the CRBSI incidence per day of PICC use, the result of a lack of data), our results emphasize the importance of reconsidering the use of PICCs in hospital settings and of ensuring infection prevention. Our study also highlights the need to perform case–control studies to better evaluate the incidence and mortality rate of BSIs in patients with PICCs, as well as in patients with implantable ports and peripheral venous access devices.
Conflict of Interest
None declared.
Acknowledgements
Supported by the ‘Programme d'investissement d'avenir’ from the French government (ANR, IHU, Mediterranée infection 10-IAHU-03). English-language editorial services were provided by American Journal Expert (CR574X5Z).
References
- 1.Hoshal V.L., Jr. Total intravenous nutrition with peripherally inserted silicone elastomer central venous catheters. Arch Surg. 1975;110:644–646. doi: 10.1001/archsurg.1975.01360110190032. [DOI] [PubMed] [Google Scholar]
- 2.Chopra V., Anand S., Krein S.L., Chenoweth C., Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125:733–741. doi: 10.1016/j.amjmed.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi R.S., Noritomi D.T., Degaspare N.V., Munoz G.O.C., Porto A.P.M., Costa S.F. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: a propensity-adjusted analysis. Intensive Care Med. 2017;43:1097–1104. doi: 10.1007/s00134-017-4852-7. [DOI] [PubMed] [Google Scholar]
- 4.Chopra V., Anand S., Hickner A., Buist M., Rogers M.A., Saint S. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311–325. doi: 10.1016/S0140-6736(13)60592-9. [DOI] [PubMed] [Google Scholar]
- 5.Maki D.G., Kluger D.M., Crnich C.J. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 6.Fallouh N., McGuirk H.M., Flanders S.A., Chopra V. Peripherally inserted central catheter–associated deep vein thrombosis: a narrative review. Am J Med. 2015;128:722–738. doi: 10.1016/j.amjmed.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Vital signs: central line–associated blood stream infections—United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60:243–248. [PubMed] [Google Scholar]
- 8.O’Grady N.P., Alexander M., Burns L.A., Dellinger E.P., Garland J., Heard S.O. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl. 1):S1–S34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Douglas Scott R., II The direct medical costs of healthcare-associated infections in US hospitals and the benefits of prevention. Natl Cent Preparedness, Detect Control Infect Dis. 2009 https://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf Available at: [Google Scholar]
- 10.Richet H., Carrieri P., Loffeier V., Cassir N., La S.B., Obadia Y. Health-care–associated bloodstream infections in France. Lancet Infect Dis. 2013;13:656. doi: 10.1016/S1473-3099(13)70189-0. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Liu Y., Ma X., Wei L., Chen W., Song L. The incidence and risk factors of peripherally inserted central catheter–related infection among cancer patients. Ther Clin Risk Manag. 2015;11:863–871. doi: 10.2147/TCRM.S83776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto Y., Fukuta T., Maruyama J., Omura H., Tanaka T. Experience of peripherally inserted central venous catheter in patients with hematologic diseases. Intern Med. 2017;56:389–393. doi: 10.2169/internalmedicine.56.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yebenes J.C., Delgado M., Sauca G., Serra-Prat M., Solsona M., Almirall J. Efficacy of three different valve systems of needle-free closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med. 2008;36:2558–2561. doi: 10.1097/CCM.0b013e318183effb. [DOI] [PubMed] [Google Scholar]
- 14.Bessis S., Cassir N., Meddeb L., Remacle A.B., Soussan J., Vidal V. Early mortality attributable to PICC-lines in 4 public hospitals of Marseille from 2010 to 2016 (revised V3) Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000018494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desenclos J.C. RAI Euro Surveill. 2009;14(46) [PubMed] [Google Scholar]
- 16.Sante Publique France . Santé Publique France; September 1, 2019. Enquete nationale de prevalence des infections nosocomiales et des traitements anti-infectieux en etablissement de sante Mai–Juin 2017.https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-associees-aux-soins-et-resistance-aux-antibiotiques/infections-associees-aux-soins/documents/enquetes-etudes/enquete-nationale-de-prevalence-des-infections-nosocomiales-et-des-traitements-anti-infectieux-en-etablissements-de-sante-mai-juin-2017-synthese Available at: [Google Scholar]
- 17.Sengupta A., Lehmann C., Diener-West M., Perl T.M., Milstone A.M. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics. 2010;125:648–653. doi: 10.1542/peds.2009-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim-Saechao S.J., Almario E., Rubin Z.A. A novel infection prevention approach: leveraging a mandatory electronic communication tool to decrease peripherally inserted central catheter infections, complications, and cost. Am J Infect Control. 2016;44:1335–1345. doi: 10.1016/j.ajic.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Krein S.L., Kuhn L., Ratz D., Chopra V. Use of designated nurse PICC teams and CLABSI prevention practices among US hospitals: a survey-based study. J Patient Saf. 2019;15:293–295. doi: 10.1097/PTS.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 20.Dufour J.C., Reynier P., Boudjema S., Soto A.A., Giorgi R., Brouqui P. Evaluation of hand hygiene compliance and associated factors with a radio-frequency-identification–based real-time continuous automated monitoring system. J Hosp Infect. 2017;95:344–351. doi: 10.1016/j.jhin.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Brouqui P., Boudjema S., Soto A.A., Chabriere E., Florea O., Nguyen H. New approaches to prevent healthcare-associated infection. Clin Infect Dis. 2017;65(Suppl. 1):S50–S54. doi: 10.1093/cid/cix433. [DOI] [PubMed] [Google Scholar]