Abstract
Background
We developed a novel risk scoring system for urothelial cancer (UC) patients receiving immune checkpoint inhibitors (ICI).
Methods
We conducted a retrospective review of 67 UC patients treated with ICI at Winship Cancer Institute of Emory University from 2015 to 2018. Using stepwise variable selection in Cox proportional hazard model and Sullivan's weighting schema, baseline platelet‐to‐lymphocyte ratio (PLR), presence of liver metastasis, baseline albumin, and baseline Eastern Cooperative Oncology Group performance status (ECOG PS) were used for risk scoring. Patients were categorized into good risk (risk score 0‐1), intermediate risk (risk score 2‐3), and poor risk (risk score 4‐6). Univariable (UVA) and multivariable analysis (MVA) and Kaplan‐Meier method were used to assess overall survival (OS) and progression free survival (PFS).
Results
The Emory Risk Scoring System had C‐statistics of 0.74 (Standard Error = 0.047) in predicting OS and 0.70 (Standard Error = 0.043) in predicting PFS. Compared to good risk patients, poor risk patients had significantly shorter OS and PFS in both UVA and MVA (all P < .001), and intermediate risk patients had significantly shorter OS and PFS in both UVA and MVA (all P < .03).
Conclusions
Risk scoring using baseline PLR, presence of liver metastasis, baseline albumin, and baseline ECOG PS may effectively predict OS and PFS in UC patients receiving ICI.
Keywords: cancer risk factors, immunology, risk assessment, urological oncology
Novel Emory risk scoring system may predict clinical outcomes in urothelial cancer patients receiving immunotherapy. Increased baseline PLR, low baseline albumin, metastasis to the liver, and higher ECOG PS associated with decreased survival.

1. BACKGROUND
Immune checkpoint inhibitors (ICI) have emerged as promising treatment options for patients with various primary cancer histologies including melanoma, lung cancer, renal cell carcinoma, and urothelial cancer (UC). ICI agents have a tolerable toxicity profile and offer the promise of durable responses.1, 2, 3 Several ICI agents have been approved over the past 4 years by the FDA for treatment of patients with metastatic UC, including nivolumab, atezolizumab, pembrolizumab, avelumab, and durvalumab.4Unfortunately, a subset of patients still does not respond to ICI, and immune‐related adverse events, although rare, can significantly affect patients’ quality of life.3, 5 Hence, it is very important to find biomarkers of response for UC patients treated with ICI.
At this point, there is no universally accepted risk stratification system for UC patients receiving ICI. Sonpavde et al (2016) showed that albumin, hemoglobin, performance status, presence of liver metastasis, and time from previous chemotherapy were significant prognosticators of overall survival (OS) in UC patients treated with salvage systemic therapy.6 However, patients treated with ICI likely require unique risk stratification given that ICI rely on the reaction of the host immune system for their response.7
In this study, we investigated the factors most predictive of clinical outcomes in UC patients treated with ICI at our institution. We developed a novel risk stratification system, the Emory Risk Scoring System for UC patients treated with ICI, using four risk factors: platelet‐to‐lymphocyte ratio (PLR), Eastern Cooperative Oncology Group performance status (ECOG PS), presence of liver metastasis, and albumin. Using these aforementioned four variables as proxies for systemic inflammation, clinical presentation, tumor microenvironment, and nutritional status, respectively, we categorized the patients into three risk groups with regard to relevant clinical outcomes.
2. MATERIALS AND METHODS
2.1. Data collection
We conducted a retrospective review of 67 UC patients treated with PD‐1 or PD‐L1 inhibitors at Winship Cancer Institute of Emory University between 2015 and 2018. OS and progression free survival (PFS) were measured from start of ICI to date of death or hospice referral and clinical or radiographic progression, respectively.8 Several variables at baseline were collected from electronic medical records including demographic information, monocyte‐to‐lymphocyte ratio (MLR), neutrophil‐to‐lymphocyte ratio (NLR), PLR, albumin level, hemoglobin level, ECOG PS, number and sites of distant metastases, and body mass index (BMI). Sites of metastasis were collected from radiology reports and clinical notes. BMI was used to represent body composition.
2.2. Statistical methods
All data analyses were done in SAS 9.4 with summary reports generated by SAS macros.9 Summary statistics were applied to all variables of interest. Univariable analysis (UVA) of the association between collected variables and OS and PFS used Cox proportional hazard model. For continuous biomarkers, their nonlinear relationship with OS was examined by martingale residual plot and an optimal cutoff (OC) that maximizes the separation between the two groups was searched by a bias adjusted log rank test.10, 11
Using the significant prognostic factors per UVA, a stepwise variable selection was implemented in Cox proportional hazard model regarding OS with entering P < .3 and staying P < .1. Based on the final prediction model, a score was assigned according to the Sullivan's weighting schema, where the regression coefficient (RC) for each predictor was divided into the smallest absolute RC for all predictors and rounded to the nearest integer.12, 13
The Emory Risk Scoring System for UC patients treated with ICI is shown in Table 1. The final variables selected for the risk scoring system were baseline PLR, presence of liver metastasis, baseline albumin level, and ECOG PS. The optimal cut for PLR and albumin were 301.87 and 3.9 g/dL, respectively. We rounded the PLR optimal cut value of 301.87‐302 for ease in using the risk scoring system. The variables baseline PLR ≥302 and presence of liver metastasis each counted as 1 point in the risk score, while baseline albumin ≤3.9 g/dL and baseline ECOG PS ≥2 counted as 2 points each. Based on the 6‐month and 12‐month survival rates and sample size distribution for each individual score of 0 through 6, patients were further stratified into good risk (risk score 0‐1), intermediate risk (risk score 2‐3), and poor risk (risk score 4‐6). The Cox proportional hazard model was used for the related survival analysis for OS in the univariable and multivariable models. Kaplan‐Meier method was applied to determine the median OS and PFS for each risk group. The discrimination power by the Emory Risk Scoring System in predicting survival was measured by Uno's C‐statistics.14
Table 1.
Emory risk scoring system for UC patients treated with immune checkpoint inhibitors
| Variable | Points |
|---|---|
| PLR ≥302 | 1 |
| PLR <302 | 0 |
| Liver metastasis | 1 |
| No liver metastasis | 0 |
| Albumin <3.9 g/dL | 2 |
| Albumin ≥3.9 g/dL | 0 |
| ECOG PS ≥2 | 2 |
| ECOG PS <2 | 0 |
| Total possible | 6 |
3. RESULTS
3.1. Patient characteristics
Descriptive statistics of this patient cohort are presented in Table 2. The median patient age was 69 and most (79%) were male. Many patients (42%) had received two or more prior lines of systemic therapy before receiving ICI. Sites of metastasis collected were lymph node (n = 49), lung (n = 21), bone (n = 20), liver (n = 14), and brain (n = 1). Most patients (88%) had ECOG PS of 0 or 1 at baseline. Patient BMI ranged from 15.8 to 49.5, with a mean of 26.3. Patients were on treatment for an average of 28.1 weeks, with a range of 2.9 to 131 weeks. Median baseline NLR was 4.00, median baseline MLR was 0.58, and median baseline PLR was 193.08.
Table 2.
Baseline patient characteristics
| Variable | N (%) = 67 |
|---|---|
| Median age | 69 (range: 32‐93) |
| Sex | |
| Male | 53 (79) |
| Female | 14 (21) |
| Race | |
| White/Asian | 54 (81) |
| Black | 13 (19) |
| Eastern cooperative oncology group performance status | |
| 0 | 45 (67) |
| 1 | 14 (21) |
| 2‐3 | 8 (12) |
| Smoker | |
| Yes | 33 (49) |
| No | 34 (51) |
| Number of metastatic sites | |
| 0‐1 | 27 (40) |
| 2 | 24 (36) |
| 3‐5 | 16 (24) |
| Site of metastasis | |
| Lymph node | 49 (73) |
| Lung | 21 (31) |
| Bone | 20 (30) |
| Liver | 14 (21) |
| Brain | 1 (2) |
| Number of prior systemic therapies | |
| 0‐1 | 39 (58) |
| 2 | 14 (21) |
| 3‐5 | 14 (21) |
| Type of immunotherapy | |
| Atezolizumab | 50 (75) |
| Pembrolizumab | 12 (18) |
| Nivolumab | 3 (4) |
| Nivolumab + experimental agent | 2 (3) |
3.2. Emory risk group analysis
All collected variables were examined for their association with OS and PFS using Cox proportional hazard model. Results of the UVA of a sample of the variables we explored are shown in Table 3. The UVA and multivariable analysis (MVA) of the association between the Emory risk groups and survival is presented in Table 4. In UVA, poor risk patients had significantly shorter OS (HR: 169.39, CI: 34.94‐821.24, P < .001) and significantly shorter PFS (HR: 43.65, CI: 13.65‐139.60, P < .001) compared to good risk patients. In MVA, poor risk patients had significantly shorter OS (HR: 230.79, CI: 44.26‐1203.52, P < .001) and significantly shorter PFS (HR: 38.46, CI: 11.93‐123.99, P < .001) compared to good risk patients. Intermediate risk patients also had significantly shorter OS and PFS compared to good risk patients in both UVA and MVA (all P < .03).
Table 3.
UVA of explored covariates with survival
| Variable | OS | PFS | |||
|---|---|---|---|---|---|
| HR (CI) | P‐value | HR (CI) | P‐value | ||
| ECOG PS | 0‐1 (n = 59) | 0.28 (0.12‐0.69) | .005 * | 0.34 (0.15‐0.76) | .009 * |
| 2‐3 (n = 8) | — | — | — | — | |
| Number of metastatic sites | 0‐1 (n = 27) | 0.41 (0.16‐1.02) | .055 | 0.49 (0.23‐1.04) | .064 |
| 2 (n = 24) | 1.22 (0.55‐2.72) | .628 | 0.99 (0.50‐1.98) | .980 | |
| 3‐5 (n = 16) | — | — | — | — | |
| Prior lines of therapy | 0‐1 (n = 39) | 1.34 (0.57‐3.13) | .504 | 0.94 (0.47‐1.89) | .871 |
| 2 (n = 14) | 0.99 (0.35‐2.83) | .983 | 0.67 (0.28‐1.62) | .373 | |
| 3‐6 (n = 14) | — | — | — | — | |
| Sites of metastasis | No lymph mets (n = 18) | 0.94 (0.45‐1.95) | .873 | 0.70 (0.36‐1.37) | .296 |
| Lymph mets (n = 49) | — | — | — | — | |
| No bone mets (n = 47) | 0.38 (0.19‐0.73) | .004 * | 0.46 (0.25‐0.82) | .009 * | |
| Bone mets (n = 20) | — | — | — | — | |
| No liver mets (n = 53) | 0.41 (0.20‐0.85) | .017 * | 0.56 (0.29‐1.11) | .096 | |
| Liver mets (n = 14) | — | — | — | — | |
| No brain mets (n = 66) | 0.67 (0.09‐4.94) | .696 | 1.36 (0.19‐9.87) | .762 | |
| Brain mets (n = 1) | — | — | — | — | |
| No lung mets (n = 46) | 1.08 (0.53‐2.19) | .825 | 0.93 (0.51‐1.68) | .803 | |
| Lung mets (n = 21) | — | — | — | — | |
| Baseline albumin | ≥3.9 g/dL (n = 46) | 0.23 (0.11‐0.46) | <.001 * | 0.36 (0.20‐0.65) | <.001 |
| <3.9 g/dL (n = 21) | — | — | — | — | |
| Baseline Hgb | ≥10 g/dL (n = 53) | 0.45 (0.22‐0.93) | .030 * | 0.54 (0.28‐1.02) | .057 |
| <10 g/dL (n = 14) | — | — | — | — | |
| Baseline BMI | <25 (n = 27) | 1.19 (0.62‐2.31) | .603 | 1.05 (0.59‐1.85) | .873 |
| ≥25 (n = 40) | — | — | — | — | |
| Sex | Female (n = 14) | 0.91 (0.40‐2.07) | .816 | 0.93 (0.47‐1.87) | .849 |
| Male (n = 53) | — | — | — | — | |
| Baseline PLR at optimal cut (301.87) | Below (n = 49) | 0.30 (0.15‐0.58) | <.001 * | 0.45 (0.24‐0.81) | .008 * |
| Above (n = 18) | — | — | — | — | |
| Baseline NLR at optimal cut (4.66) | Below (n = 38) | 0.29 (0.15‐0.58) | <.001 * | 0.52 (0.30‐0.91) | .023 * |
| Above (n = 29) | — | — | — | — | |
| Baseline MLR at optimal cut (0.55) | Below (n = 33) | 0.40 (0.20‐0.79) | .008 * | 0.64 (0.37‐1.13) | .128 |
| Above (n = 34) | — | — | — | — | |
Bold values are statistically significant with α < 0.05.
Abbreviations: BMI, body mass index; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; Hgb, hemoglobin; HR, hazard ratio; Mets, metastasis; MLR, monocyte‐to‐lymphocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; OS, overall survival; PFS, progression free survival; PLR, platelet‐to‐lymphocyte ratio; UVA, univariable analysis.
Statistical significance at α < 0.05.
Table 4.
UVA and MVAa of risk group and survival
| Risk groups | UVA | MVA | ||||||
|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | |||||
| HR (CI) | P‐value | HR (CI) | P‐value | HR (CI) | P‐value | HR (CI) | P‐value | |
|
Poor risk (score = 4‐6) n = 9 |
169.39 (34.94‐821.24) | <.001 * | 43.65 (13.65‐139.60) | <.001 * | 230.79 (44.26‐1203.52) | <.001 * | 38.46 (11.93‐123.99) | <.001 * |
|
Intermediate risk (score = 2‐3) n = 33 |
4.24 (1.70‐10.59) | .002 * | 2.42 (1.24‐4.72) | .010 * | 3.64 (1.43‐9.28) | .007 * | 2.15 (1.09‐4.27) | .028 * |
|
Good risk (score = 0‐1) n = 25 |
1 | 1 | 1 | 1 | ||||
Bold values are statistically significant with α < 0.05.
Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression free survival; UVA, univariable analysis.
MVA controlled for age, race, sex, number of prior lines of therapy, number of sites of metastasis and smoking status.
Statistical significance at α < 0.05 by Chi‐square test.
The median OS (Figure 1) and PFS (Figure 2) were significantly shorter for poor risk patients than intermediate risk and good risk patients per Kaplan‐Meier estimation. The median OS and PFS were 0.8 months and 0.4 months for poor risk patients, respectively, compared to the median OS of 9.1 months and median PFS of 3.3 months for intermediate risk patients. Median OS was not reached for good risk patients and median PFS was 8 months (all P < .0001). The Uno's C‐statistics for the final 3‐level risk group was 0.74 (Standard Error = 0.047) for OS and 0.70 (Standard Error = 0.043) for PFS.
Figure 1.
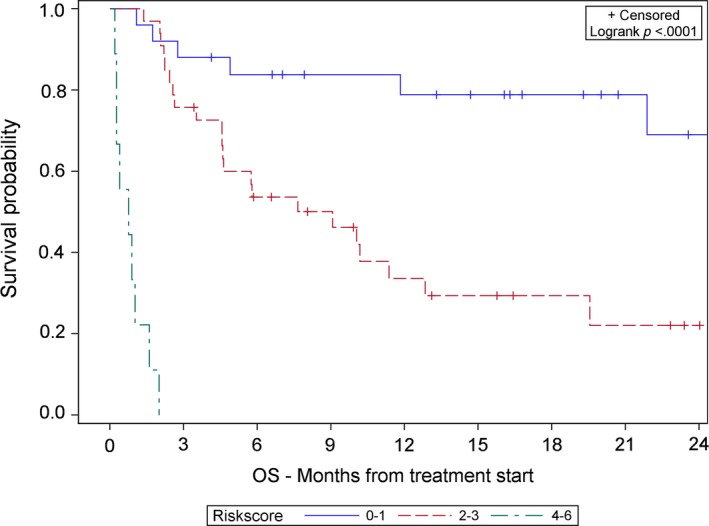
Kaplan‐Meier association of risk score and overall survival
Figure 2.
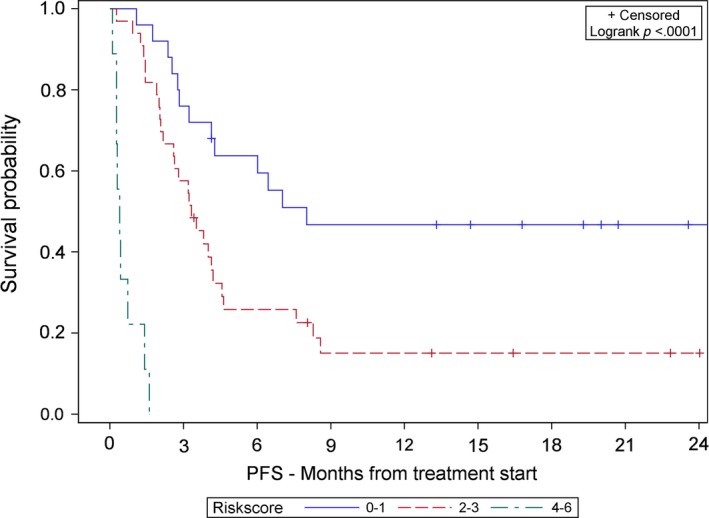
Kaplan‐Meier association of risk score and progression free survival
High NLR, MLR, and PLR were associated with worse clinical outcomes in UVA and furthermore were correlated with one another (all Pearson correlation coefficients ≥0.45, all P ≤ .0001) (Figure S1). Given the correlation between these markers of inflammation, supplemental risk groups were created using NLR or MLR as a risk factor. Supplemental Kaplan‐Meier estimates for NLR‐based risk groups and association with OS and PFS are given in Figure [Link], [Link]A,B, respectively. The optimal cut for NLR was 4.67. Supplemental Kaplan‐Meier estimates for MLR‐based risk groups and association with OS and PFS are given in Figure [Link], [Link]A,B, respectively. The optimal cut for MLR was 0.545.
4. DISCUSSION
The ability to predict survival of UC patients is helpful in guiding management and treatment options. As the number of FDA‐approved ICI treatments for UC patients grows, the urological oncology field would benefit from an all‐encompassing risk scoring system for UC patients receiving ICI. In this study, we developed a hypothesis‐generating risk scoring system based on four variables to represent systemic inflammation (PLR), tumor microenvironment (liver metastasis), nutritional status (albumin), and clinical presentation (ECOG PS). Each of these variables is readily available in the clinical setting, thus, increasing the practicality and usefulness of the risk scoring system.
Bellmunt et al (2010) developed a scoring system for UC patients who experience treatment failure with platinum‐based regimens.15 Their scoring system included three factors: ECOG PS, hemoglobin level, and liver metastasis. Sonpavde et al (2016) later proposed five factors for prognosis of UC patients receiving salvage therapy: ECOG PS, liver metastasis, hemoglobin, time from prior chemotherapy, and albumin. Our scoring system validates three of these factors, adds a factor for assessing systemic inflammation, and is novel in its application to patients receiving ICI rather than chemotherapy.
Our finding that high PLR predicts poor clinical outcomes is consistent with and builds upon previous studies investigating the significance of markers of inflammation in patients receiving ICI. Baseline NLR, MLR, and PLR and early change in these variables have proven to be significantly associated with clinical outcomes in patients receiving ICI across several primary malignancies.16, 17, 18, 19, 20, 21 In this study, markers of inflammation are highly correlated with one another. With stepwise variable selection in building our risk scoring system, PLR was found to affect clinical outcomes most significantly, but with such high correlation among biomarkers of inflammation, PLR could be substituted by NLR or MLR. One hypothesis as to why these markers are effective in prediction of survival is that these variables may reveal an inability to increase lymphocytes as part of host immune activation. The host immune system plays a key role in the success of ICI.22 High NLR, MLR, and PLR indirectly reflect immune dysregulation in these patients and suggest poor response to ICI treatment. The results of our analysis support the inclusion of an inflammatory biomarker in a prognostic model for UC patients treated with ICI.
Metastasis to the liver has long been established as a predictor for decreased survival in cancer patients receiving chemotherapy.23, 24 Several studies have also implicated liver metastasis in decreased response to ICI‐based therapies in cancers such as melanoma, lung cancer, colorectal cancer, and bladder cancer.25, 26, 27, 28, 29, 30 The continued significance of liver metastasis in cancer patients is provocative. This finding may be explained by the liver's role as a tolerogenic organ.31, 32, 33 Tumeh et al (2017) found that melanoma and lung cancer patients with liver metastases treated with anti‐PD‐1 agents had decreased CD8+T‐cell density at the liver metastatic margin and furthermore that these patients had decreased response rate and shortened PFS.34 The density of CD8+T‐cells in the liver may, therefore, affect response to ICI. Metastasis to the liver may disrupt the organ's immune‐modulatory role, thereby restricting the host's immune response to ICI‐based treatments. The physiology of the tumor microenvironment with liver metastasis should be further explored.
Our study found that both liver and bone metastases were poor prognostic factors in UC patients receiving ICI. Patients with bone or liver metastases had significantly shorter OS in UVA and patients with bone metastases also had significantly shorter PFS in UVA. Previous studies have shown that cancer patients of various primary malignancies with bone metastases have decreased survival.35, 36, 37, 38 The effect of bone metastases on survival of cancer patients receiving ICI is not well studied. One explanation for why bone metastases do not respond well to immunotherapy may be that transforming growth factor‐beta (TGF‐β) released from bone suppresses T‐cell proliferation and activity.39 Furthermore, TGF‐β production and the pool of regulatory T‐cells may increase under the influence of cancer cells.40, 41 The bone microenvironment in the setting of metastases, as a result, may inhibit the potential T‐cell antitumor responses that are essential for the efficacy of immunotherapy.42, 43 Although we did not include presence of bone metastasis as a risk factor in our model, updated prognostic models may consider including bone metastasis.
Our finding that low baseline albumin predicts shorter OS and PFS is consistent with previous data. Albumin transports hydrophobic species that are not otherwise soluble in plasma, including hormones, fatty acids, bilirubin, metals, and lipopolysaccharides.44, 45, 46 Albumin also binds to drugs, increasing their bioavailability.47 Levels of this protein reflect nutritional status and liver function. Low albumin has been correlated with incidence of morbidity and mortality in hospitalized patients 48 and baseline albumin has been explored as a predictor of survival in cancer patients, including in UC patients.49, 50, 51, 52, 53 Consequently, albumin levels may be an indicator of illness severity. Moreover, albumin may play an important regulatory role in the immune system.54, 55
The final prognostic factor included in the Emory Risk Scoring System for UC patients is ECOG PS, which is a widely used method for assessing functional status of cancer patients. A patient's ECOG PS can be used to predict their ability to tolerate therapy and has consistently shown to be highly correlated with survival, including in patients receiving ICI.56, 57, 58 Determining a UC patient's ECOG PS requires no more than an assessment of their ability to perform activities of daily living (ADLs) and should be determined prior to treatment initiation. It stands to reason that a patient who cannot perform ADLs will not tolerate treatment as well as patients who are fully active, and therefore, patients with higher ECOG PS scores have worse clinical outcomes.
While the results of this study are clinically relevant, there are some limitations that should be mentioned. First, this is a retrospective study, which is vulnerable to selection bias. We tried to lessen the effects of this potential bias by including all UC patients who received at least one dose of ICI at our site, regardless of the treatment regimen or other baseline characteristics. Second, it is possible that metastasis to another site that we did not analyze could be more predictive of clinical outcomes than liver metastases. To minimize this problem, we categorized the five most common sites of metastasis in initial data collection and analyzed each site independently. Finally, the size of our cohort limits the statistical power of our findings. Future studies are necessary to validate the results of this study and to elucidate the underlying physiology explaining how systemic inflammation, nutritional status, clinical presentation and metastatic sites impact host immune response to ICI. Further efforts to develop prognostic models would likely be strengthened by the incorporation of tumor genomic data as well.
5. CONCLUSION
In this study, we developed a novel risk scoring system to predict clinical outcomes in UC patients receiving ICI therapy. We showed that increased baseline PLR, low baseline albumin, metastasis to the liver, and higher ECOG PS were associated with decreased survival in UC patients treated with ICI.
The results of this study reveal an important area for improvement in ICI‐based therapies for UC patients who fall into the poor risk group. Advancements in treating these patients are needed. Categorization into the poor risk group should not necessarily preclude patients from receiving ICI‐based therapies. Rather, this stratification may be useful in guiding treatment for these UC patients and may suggest that these patients should receive novel combination therapy, for example, more than one ICI or ICI with monoclonal antibodies targeting costimulatory molecules.59, 60
This is an important study in determining risk factors that affect clinical response of UC patients receiving ICI. This study is hypothesis‐generating and these findings warrant external validation with a larger, prospective study before being incorporated into clinical practice.
CONFLICT OF INTEREST
BCC has a consulting/advisory role with Astellas Medivation, Pfizer, and Blue Earth Diagnostics and receives travel accommodations from Bristol‐Myers Squibb. MAB has a consulting/advisory role with Exelixis, Nektar, and Sanofi and receives research funding from Bayer, Bristol‐Myers Squibb, Genentech/Roche, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton, and Pfizer. MAB has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Genomic Health, Nektar, and Sanofi and has received grants to his institution from Xencor, Bayer, Bristol‐Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Peleton Therapeutics, and Pfizer for work performed as outside of the current study. All other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
JMS was involved in conceptualization, data curation, formal analysis, methodology, project administration, writing‐original draft, and writing‐review and editing. DJM was involved in data curation, formal analysis, writing‐original draft, and administration. YL was involved data curation, formal analysis, project administration, validation, visualization, and writing‐review and editing. MAB was involved in conceptualization, formal analysis, funding acquisition, investigation, project administration, and writing‐ review and editing. DR, JB, EEH, GAR, SC, HK, MA, KO, WBH, VAM, OK, and BCC were involved in investigation, resources, and writing‐ review and editing. All authors reviewed and approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
ACKNOWLEDGMENTS
Preliminary data on the effects of inflammatory markers and sites of metastasis on survival outcomes in urothelial cancer patients at our institution was presented at the 2019 GU ASCO Meeting in San Francisco, California.
Shabto JM, Martini DJ, Liu Y, et al. Novel risk group stratification for metastatic urothelial cancer patients treated with immune checkpoint inhibitors. Cancer Med. 2020;9:2752–2760. 10.1002/cam4.2932
Funding information
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Martini DJ, Hamieh L, McKay RR, et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue PD‐1/PD‐L1 therapy for immune‐related adverse events. Cancer Immunol Res. 2018;6(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 2. McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol. 2015;33(18):2013‐2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158‐168. [DOI] [PubMed] [Google Scholar]
- 4. Seront E, Catala G, Dermine A,Lejeune S, Rysselinck S. Immune checkpoint inhibitors as a real hope in advanced urothelial carcinoma. Future Sci OA. 2018;4(10):FSO341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baxi S, Yang A, Gennarelli RL, et al. Immune‐related adverse events for anti‐PD‐1 and anti‐PD‐L1 drugs: systematic review and meta‐analysis. BMJ. 2018;360:k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sonpavde G, Pond GR, Rosenberg JE, et al. Improved 5‐factor prognostic classification of patients receiving salvage systemic therapy for advanced urothelial carcinoma. J Urol. 2016;195(2):277‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069‐1086. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros. F1000Research. 2018;7:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mandrekar JN, Mandrekar SJ, Cha SS. Cutpoint determination methods in survival analysis using SAS. Proceedings of the 28th SAS Users Group International Conference (SUGI) 2003:261‐328.
- 11. Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30(3):253‐270. [Google Scholar]
- 12. Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631‐1660. [DOI] [PubMed] [Google Scholar]
- 13. Mehta HB, Mehta V, Girman CJ, Adhikari D, Johnson ML. Regression coefficient‐based scoring system should be used to assign weights to the risk index. J Clin Epidemiol. 2016;79:22‐28. [DOI] [PubMed] [Google Scholar]
- 14. Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C‐statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum‐containing regimens. J Clin Oncol. 2010;28(11):1850‐1855. [DOI] [PubMed] [Google Scholar]
- 16. Bilen MA, Martini DJ, Liu Y, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced‐stage cancer treated with immunotherapy. Cancer. 2019;125(1):127‐134. [DOI] [PubMed] [Google Scholar]
- 17. Lalani A‐K, Xie W, Martini DJ, et al. Change in neutrophil‐to‐lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer. 2017;106:1‐7. [DOI] [PubMed] [Google Scholar]
- 19. Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil‐to‐lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2016;27(4):732‐738. [DOI] [PubMed] [Google Scholar]
- 20. Diem S, Schmid S, Krapf M, et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176‐181. [DOI] [PubMed] [Google Scholar]
- 21. Bilen MA, Dutcher GMA, Liu Y, et al. Association between pretreatment neutrophil‐to‐lymphocyte ratio and outcome of patients with metastatic renal‐cell carcinoma treated with nivolumab. Clin Genitourin Cancer. 2018;16(3):e563‐e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spitzer MH, Carmi Y, Reticker‐Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487‐502.e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood CB, Gillis CR, Blumgart LH. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976;2(3):285‐288. [PubMed] [Google Scholar]
- 24. Hoe AL, Royle GT, Taylor I. Breast liver metastases–incidence, diagnosis and outcome. J R Soc Med. 1991;84(12):714‐716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pires da Silva I, Lo S, Gonzalez M, Guminski AD, Long GV, Menzies AM. Distinct patterns of response and toxicity (tox) by sites of metastases (mets) in patients (pts) treated with ipilimumab combined with PD‐1 antibodies (ipi+PD1). J Clin Oncol. 2018;36(15_suppl):9553‐9553. [Google Scholar]
- 26. Bilen MA, Shabto JM, Martini DJ, et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer. 2019;19(1):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee J, Mehdizadeh S, Tsai K, et al. Immunological Insights Into Liver Metastasis Associated Resistance To Checkpoint Blockade Cancer Immunotherapy. J Immunol. 2018;200(1 Suppl):122‐126. [Google Scholar]
- 28. Pond GR, Niegisch G, Rosenberg JE, et al. New 6‐factor prognostic model for patients (pts) with advanced urothelial carcinoma (UC) receiving post‐platinum atezolizumab. J Clin Oncol. 2018;36(6_suppl):413. [Google Scholar]
- 29. Shabto JM, Martini DJ, Liu Y, et al. Sites of metastases (mets) and their association with clinical outcomes (CO) in urothelial cancer patients (pts) treated with immunotherapy (IO). J Clin Oncol. 2019;37(7_suppl):473. [Google Scholar]
- 30. Tamiya M, Tamiya A, Inoue T, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non‐small cell lung cancer: a retrospective multicenter trial. PLoS ONE. 2018;13(2):e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mazzolini G, Ochoa MC, Morales‐Kastresana A, Sanmamed MF, Melero I. The liver, liver metastasis and liver cancer: a special case for immunotherapy with cytokines and immunostimulatory monoclonal antibodies. Immunotherapy. 2012;4(11):1081‐1085. [DOI] [PubMed] [Google Scholar]
- 32. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147‐163. [DOI] [PubMed] [Google Scholar]
- 33. Abe M, Thomson AW. Antigen processing and presentation in the liver In: Gershwin ME, Vierling JM, Manns MP. ed. Liver Immunology Principles and Practice. Totowa, NJ: Humana Press Inc; 2007:49‐59. [Google Scholar]
- 34. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with Anti‐PD‐1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614‐1627. [DOI] [PubMed] [Google Scholar]
- 36. Ishikawa S, Soloway MS, Van der Zwaag R, Todd B. Prognostic factors in survival free of progression after androgen deprivation therapy for treatment of prostate cancer. J Urol. 1989;141(5):1139‐1142. [DOI] [PubMed] [Google Scholar]
- 37. Bajorin DF, Dodd PM, Mazumdar M, et al. Long‐term survival in metastatic transitional‐cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173‐3181. [DOI] [PubMed] [Google Scholar]
- 38. Svensson E, Christiansen CF, Ulrichsen SP, Rorth MR, Sorensen HT. Survival after bone metastasis by primary cancer type: a Danish population‐based cohort study. BMJ Open. 2017;7(9):e016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suva LJ, Washam C, Nicholas RW, Griffin RJ. Bone metastasis: mechanisms and therapeutic opportunities. Nat Rev Endocrinol. 2011;7(4):208‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF‐beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182(5):2795‐2807. [DOI] [PubMed] [Google Scholar]
- 41. Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck‐Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606‐612. [PubMed] [Google Scholar]
- 42. Fournier PG, Chirgwin JM, Guise TA. New insights into the role of T cells in the vicious cycle of bone metastases. Curr Opin Rheumatol. 2006;18(4):396‐404. [DOI] [PubMed] [Google Scholar]
- 43. Capietto AH, Faccio R. Immune regulation of bone metastasis. Bonekey Rep. 2014;3:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhattacharya AA, Grüne T, Curry S. Crystallographic analysis reveals common modes of binding of medium and long‐chain fatty acids to human serum albumin 1 1Edited by R. Huber. J Mol Biol. 2000;303(5):721‐732. [DOI] [PubMed] [Google Scholar]
- 45. Bal W, Sokołowska M, Kurowska E, Faller P. Binding of transition metal ions to albumin: sites, affinities and rates. Biochimica et Biophysica. Acta (BBA) ‐ General Subjects. 2013;1830(12):5444‐5455. [DOI] [PubMed] [Google Scholar]
- 46. Arroyo V, García‐Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61(2):396‐407. [DOI] [PubMed] [Google Scholar]
- 47. Yamasaki K, Chuang VTG, Maruyama T, Otagiri M. Albumin–drug interaction and its clinical implication. Biochimica et Biophysica. Acta (BBA) – General Subjects. 2013;1830(12):5435‐5443. [DOI] [PubMed] [Google Scholar]
- 48. Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104(8):1258‐1264. [DOI] [PubMed] [Google Scholar]
- 49. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Djaladat H, Bruins HM, Miranda G, Cai J, Skinner EC, Daneshmand S. The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. 2014;113(6):887‐893. [DOI] [PubMed] [Google Scholar]
- 51. Jiang H, Li H, Li A, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7(44):72076‐72083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu J, Dai Y, Zhou F, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol Oncol. 2016;34(11):484.e481‐484.e488. [DOI] [PubMed] [Google Scholar]
- 53. Li J, Cheng Y, Liu G, Ji Z. The association of pretreatment serum albumin with outcomes in bladder cancer: a meta‐analysis. Onco Targets Ther. 2018;11:3449‐3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Colino J, Duke L, Snapper CM. Autologous albumin enhances the humoral immune response to capsular polysaccharide covalently coattached to bacteria‐sized latex beads. Eur J Immunol. 2014;44(5):1433‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giles S, Czuprynski C. Novel role for albumin in innate immunity: serum albumin inhibits the growth of Blastomyces dermatitidis yeast form in vitro. Infect Immun. 2003;71(11):6648‐6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32a(7):1135‐1141. [DOI] [PubMed] [Google Scholar]
- 57. Jang RW, Caraiscos VB, Swami N, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Practice. 2014;10(5):e335‐341. [DOI] [PubMed] [Google Scholar]
- 58. Cona M, Lecchi M, Cresta S, et al. Combination of baseline LDH, performance status and age as integrated algorithm to identify solid tumor patients with higher probability of response to anti PD‐1 and PD‐L1 monoclonal antibodies. Cancers. 2019;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Matar P, Alaniz L, Rozados V, et al. Immunotherapy for liver tumors: present status and future prospects. J Biomed Sci. 2009;16(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mazzolini GD, Malvicini M. Immunostimulatory monoclonal antibodies for hepatocellular carcinoma therapy. Trends Perspect. 2018;78(1):29‐32. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


