Abstract
Background
Emerging evidence indicates that the tumor microenvironment (TME) influences tumor progression through the various cells it contains. Tumor‐associated neutrophils (TANs) and cancer‐associated fibroblasts (CAFs) are prominent constituents of diverse malignant solid tumors and are crucial in the TME and cancer evolution. However, the relationships and combined prognostic value of these two cell types are not known in gastric adenocarcinoma (GAC).
Materials and Methods
In total, 215 GAC patients who underwent curative surgery were enrolled. TANs were assessed by immunohistochemical staining for CD66b, and CAFs were evaluated by immunohistochemical staining for α‐smooth muscle actin (α‐SMA).
Results
The percentages of patients with high‐density TANs and CAFs in GAC tissue were 47.9% (103/215) and 43.3% (93/215), respectively. The densities of TANs and CAFs in GAC tissue samples were markedly elevated and independently correlated with GAC clinical outcomes. A strong correlation (R = .348, P < .001) was detected between TANs and CAFs in GAC. The combination of TANs and CAFs produced a more exact outcome than either factor alone. Patients with an α‐SMAlowCD66bhigh (hazard ratio [HR] = 1.791; 95% CI: 1.062‐3.021; P = .029), α‐SMAhighCD66blow (HR = 2.402; 95% CI: 1.379‐4.183; P = .002), or α‐SMAhighCD66bhigh (HR = 3.599; 95% CI: 2.330‐5.560; P < .001) phenotype were gradually correlated with poorer disease‐free survival than the subset of patients with an α‐SMAlowCD66blow phenotype. The same results were observed for disease‐specific survival in the subgroups. Noticeably, in stage II‐III patients with the α‐SMAlowCD66blow phenotype, an advantage was obtained with postoperative chemotherapeutics, and the risk of a poor prognosis was reduced compared with stage II‐III patients with the α‐SMAlowCD66bhigh, α‐SMAhighCD66blow or α‐SMAhighCD66bhigh phenotype (HR: 0.260, 95% CI: 0.124‐0.542, P < .001 for disease‐free survival; and HR: 0.258, 95% CI: 124‐0.538, P < .001 for disease‐specific survival).
Conclusion
Overall, we concluded that the combination of CD66b+ TANs and α‐SMA+ CAFs could be used as an independent factor for patient outcomes and to identify GAC patients who might benefit from the administration of postoperative chemotherapeutics.
Keywords: cancer‐associated fibroblasts, gastric adenocarcinoma, prognosis, tumor microenvironment, tumor‐associated neutrophils
We concluded that the combination of CD66b+ tumor‐associated neutrophils and α‐SMA+ cancer‐associated fibroblasts could be used as an independent factor of outcome and to identify those gastric adenocarcinoma patients who might obtain advantage from postoperative chemotherapeutics.
1. INTRODUCTION
Gastric adenocarcinoma (GAC) is the fifth most malignant tumor and the third leading cause of global cancer‐related mortality.1 Despite the development of multimodality treatment methods such as standard D2 lymphadenectomy surgery, systemic therapy, radiation therapy, and targeted treatments, the survival rate of GAC patients remains low.2, 3, 4 For patients with postoperative GAC, local recurrence and metastasis are considered major limitations, making adjuvant chemotherapy extremely critical.5 However, 85%‐90% of all gastric cancer patients respond poorly to adjuvant chemotherapy, and only a portion of patients achieve a stable condition or partial response to therapy.6 Hence, there is an urgent need to develop an exact prognostic tool that can be applied to reliably predict the risks of recurrence and metastasis and response to adjuvant chemotherapy in GAC patients. Currently, the TNM staging system is generally applied as a prognostic stratification tool by oncologists. However, the traditional TNM staging system provides only limited prognostic information and does not include information derived from the tumor microenvironment (TME). Thus, incorporating TME information with TNM staging might elevate the prognostic precision of the current model.
Gastric cancer is an inflammation‐associated tumor characterized by the invasion of multiple immune cells, including macrophagocytes, granular leukocytes, and different types of T lymphocytes.7, 8, 9 All these tumor‐related immune cells constitute a complex microenvironment that affects tumor development. Tumor‐associated neutrophils (TANs) are a predominant constituent of the inflammatory microenvironment and one of the predominant invasive immune cell populations in the tumor.10 Emerging evidence has indicated that TANs are pivotal for tumor initiation and progression.11, 12 TANs respond to signals from cancer cells or stromal cells by altering their phenotype and migratory pathways, and they also release factors that act on tumor cells.13, 14, 15 The increased frequency of TANs is associated with a poor prognosis in patients with solid tumors.12 TANs have been identified in several types of human tumors, including head and neck squamous cell carcinoma, gastric cancer, colorectal cancer, and renal cell carcinoma.16, 17, 18, 19 TANs secrete several soluble factors that cause carcinogenesis or accelerate cancer cell proliferation, cancer vasculogenesis, migration and invasion.20, 21, 22, 23 In addition, TANs also mediate tumor immune escape by inhibiting antitumor immunity.24
Cancer‐associated fibroblasts (CAFs) are spindle‐shaped fibroblast‐like interstitial cells expressing α‐smooth muscle actin (α‐SMA), constitute a primary fraction of the carcinoma stroma, and are frequently exposed to different inflammatory cells and mediators in the TME.25, 26 Thus, they may obtain new features that are not present in conventional fibroblasts, and these features tend to mediate reshaping of the TME and ultimately impact cancer evolution.27, 28, 29, 30 CAFs have an active role in mutual bidirectional interactions with tumor cells and other cell types in the TME, thereby promoting the niche that allows the tumor and promoting tumor development. Increasing studies have indicated that CAFs can be utilized as a significant prognostic marker in various tumors.31, 32, 33 Since TANs are the most common infiltrated type of immune cells in GAC, there may exist a forceful mutual effect between GAC‐derived CAFs and invasive TANs. In this research, we assessed the densities of TANs labeled with CD66b and CAFs labeled with α‐SMA in GAC by immunohistochemistry and focused on their combined effect on clinical outcomes, expecting to accurately predict patient prognosis and offer clues for stratified therapy in GAC patients.
2. MATERIAL AND METHODS
2.1. Patients
This retrospective study comprised 215 consecutive patients with GAC who underwent curative surgery with D2 lymphadenectomy at Harbin Medical University Cancer Hospital between January and December 2013. Inclusion criteria included pathologically confirmed adenocarcinoma, no preoperative chemotherapy and/or radiotherapy, accurate pathological TNM staging according to the 8th edition of the TNM classification of the American Joint Committee on Cancer staging manual, and integrated available follow‐up records. Patients who were lost to follow‐up, passed away during the perioperative period, had autoimmune diseases, with multiple cancers or previous cancers were excluded. Disease‐free survival (DFS) was calculated from the date of accepting surgery to the date of disease recurrence or metastases. Disease‐specific survival (DSS) was defined as the time between the date of accepting surgery and the date of death because of GAC. The histological subtypes were classified as well‐differentiated, moderately differentiated, and poorly differentiated GAC. Well and moderate differentiation include G1 and G2 GAC. Poor differentiation includes G3 GAC, gastric signet‐ring cell carcinoma, and mucinous GAC. The following clinicopathological parameters were collected for each patient from his/her medical records: sex, age, tumor size, tumor location, differentiation status, and pTNM stage. This research was approved by the ethics committee of Harbin Medical University Cancer Hospital.
2.2. Immunohistochemistry
Formalin‐fixed, paraffin‐embedded surgically resected tumor tissue samples were cut into 4‐μm sections. The sections were heated at 95°C for 20 minutes and dewaxed. Then, the slides were immersed in 3% H2O2 for 30 minutes to block endogenous peroxidase activity. The tissue sections were then incubated in a citrate buffer for 5 minutes on a 95‐99°C induction cooker for antigen retrieval and rinsed in phosphate‐buffered saline. Then, all sections were incubated in a humidified box at 4°C overnight with a monoclonal anti‐CD66b (555723, BD Biosciences, dilution 1:200) or primary polyclonal anti‐α‐SMA (55135‐1‐AP, Proteintech, dilution 1:200) antibody. Then, the specimens were incubated with secondary antibodies (SPN‐9001/2, anti‐rabbit/mouse IHC Kit, ZSGB‐BIO) for 1 hour at room temperature. Each slide was reacted with a 3‐3′‐diaminobenzidine reagent solution for 2 minutes and counterstained with hematoxylin for 20 seconds. Negative controls were processed in the same way without primary antibodies.
2.3. Assessment of immunostaining
Total immunohistochemical results and the CD66b‐ and α‐SMA‐positive cell densities were estimated by two independent gastroenterology pathologists (Chen K and Yan F) who were blinded to patient clinicopathological information. The number of CD66b‐positive neutrophils in each region was evaluated by applying Image‐Pro Plus 6.0 (Media Cybernetics). Uniform settings were applied to all images. Positive staining was determined in high‐power fields (HPFs, 200X). The intensity of neutrophil staining in histological sections was recorded as the average number of CD66b‐positive cells/HPF from 5 stochastic areas, and the mean was calculated. The median value was regarded as the threshold for low or high neutrophil density. α‐SMA immunoreactivity was evaluated as the percentage of positively stained cells and intensity of staining, which were scored as follows: (a) <5% colored cells as class 0, 5%‐25% as class 1, 26%‐50% as class 2, and >50% as class 3; and (b) no to weak staining intensity as class 0, moderate staining as class 1, and strong staining as class 2. The percentage and intensity scores were multiplied to form the low and high fibroblast density classes: 0‐2 indicated a low density, and higher than 2 indicated a high density.
2.4. Statistical analysis
All statistical analyses were performed using SPSS 22 (IBM Corp.). A two‐sided P < .05 was deemed significant. A paired‐sample t test was used to compare the CD66b+ TANs or α‐SMA+ CAFs in GAC specimens with those in matched paracarcinoma gastric tissue samples. The associations between quantitative variables were determined using the Pearson correlation coefficient. Chi‐square and Mann‐Whitney U tests were performed to evaluate associations between categorical variables. Kaplan‐Meier survival curves were constructed using the log‐rank test to assess DFS and DSS. Univariate and multivariate analyses using Cox proportional hazards models were applied to identify prognostic markers. Receiver operating characteristic (ROC) analysis was used to assess the value and accuracy of prognostic models.
3. RESULTS
3.1. TAN and CAF densities and their association in GAC patients
In total, 215 patients were enrolled in this research. 107 of 185 patients at Ⅱ‐Ⅲ stage or Ⅰ stage with lymph node metastasis received fluorouracil‐based postoperative adjuvant chemotherapy (at least 1 cycle), mostly including capecitabine add platinum, capecitabine alone, or S‐1. The last follow‐up date was 31 August 2018. The overall follow‐up rate was 91.16% (196/215). 134 (62.3%) patients had verified recurrence after curative resection, and 128 (59.5%) had died at last follow‐up. The median follow‐up duration was 41.4 months. We performed immunohistochemical staining for CD66b and α‐SMA in GAC tissue samples. Representative images of CD66b+ and α‐SMA+ cells in the GAC tissue samples and matched normal tissue samples are shown in Figure 1. The results demonstrated that the density of CD66b‐positive cells in 22 GAC tissue samples was markedly higher than that in noncancerous matched specimens (Figure 2A, P < .001). The neutrophil distributions in the tumor tissue samples varied greatly among the GAC specimens, ranging from 0 to 198 cells/HPF. The CD66b+ staining indicated that TANs were distributed in a diffuse manner in the tumor stroma (Figure 1A‐D). Positive staining for α‐SMA in the nontumoral gastric tissue samples was recognized to identify vascular smooth muscle cells rather than stromal fibroblasts (Figure 1E). The expression of α‐SMA was identified in fibroblasts in the tumor stroma with no positive staining in GAC cells (Figure 1F‐G). The correlation between CD66b+ and α‐SMA+ cell densities was further evaluated. There was a significant positive correlation between the CD66b+ and α‐SMA+ cell densities (R = .384, P < .001) (Figure 2B). The rate of CD66b+ cells was remarkably higher in α‐SMA‐high GAC specimens than in α‐SMA‐low GAC specimens (68.82% vs. 31.18%, respectively; P < .001) (Figure 2C). None of the clinicopathological characteristics evaluated were associated with CD66b+ TANs (all P > .05). A high density of α‐SMA+ CAFs was prominently correlated with the late pTNM stage (Table 1).
Figure 1.
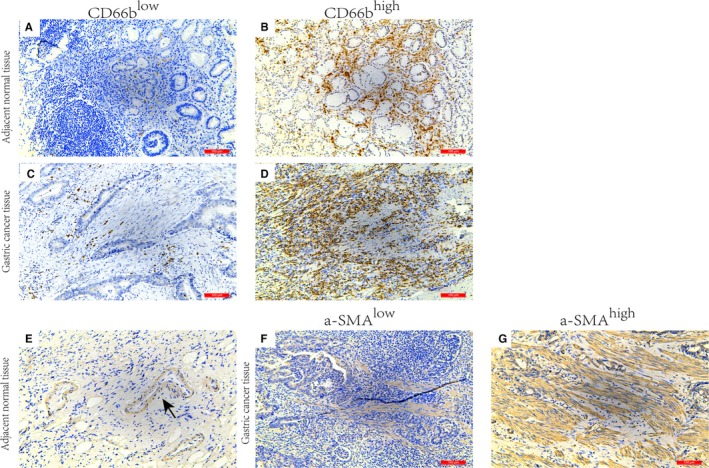
Immunohistochemical images of α‐SMA and CD66b expression in gastric adenocarcinoma tissue samples. Representative examples of low‐ and high‐density CD66b expression (A‐D) in gastric cancer tissue samples and adjacent normal tissue samples. Representative examples of low‐ and high‐density α‐SMA expression (E‐G) in gastric cancer tissue samples and adjacent normal tissue samples (arrow indicates positive α‐SMA staining of vascular smooth muscle cells). α‐SMA, α‐smooth muscle actin
Figure 2.
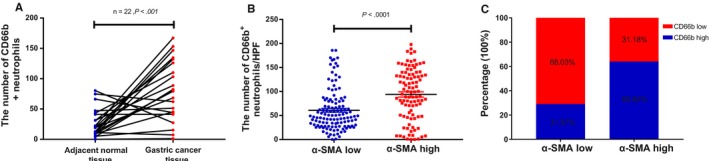
CD66b+ tumor‐associated neutrophil distribution in gastric cancer and the correlation with α‐SMA+ cancer‐associated fibroblasts. A. Assessed infiltration of CD66b+ tumor‐associated neutrophils in gastric cancer. B. Correlation between α‐SMA and CD66b expression in gastric cancer. C. CD66blow and CD66bhigh rates of patients in the α‐SMA groups. α‐SMA, α‐smooth muscle actin
Table 1.
The correlations of α‐SMA and CD66b expression with clinicopathologic characteristics in gastric cancer patients
| Characteristics | Total no, n | α‐SMA+CAFs, n (%) | P value | CD66b+TANs, n (%) | P value | ||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | ||||
| All cases | 122 (56.7) | 93 (43.3) | 112 (52.1) | 103 (47.9) | |||
| Sex | .167 | .699 | |||||
| Female | 59 | 29 (49.2) | 30 (50.8) | 32 (54.2) | 27 (45.8) | ||
| Male | 156 | 93 (59.6) | 63 (40.4) | 80 (51.3) | 76 (48.7) | ||
| Age (years) | .902 | .651 | |||||
| <60 | 103 | 58 (56.3) | 45 (43.7) | 52 (50.5) | 51 (49.5) | ||
| ≥60 | 112 | 64 (57.1) | 48 (42.9) | 60 (53.6) | 52 (46.4) | ||
| Tumor size (cm) | .086 | .102 | |||||
| <5 | 81 | 52 (64.2) | 29 (35.8) | 48 (59.3) | 33 (40.7) | ||
| ≥5 | 134 | 70 (52.2) | 64 (47.8) | 64 (47.8) | 70 (52.2) | ||
| Differentiation | .374 | .873 | |||||
| Well/moderate | 47 | 24 (51.1) | 23 (48.9) | 24 (51.1) | 23 (48.9) | ||
| Poor | 168 | 98 (58.3) | 70 (41.7) | 88 (52.4) | 80 (47.6) | ||
| Location | .161 | .561 | |||||
| Upper | 35 | 15 (42.9) | 20 (57.1) | 16 (45.7) | 19 (54.3) | ||
| Middle | 45 | 25 (55.6) | 20 (44.4) | 22 (48.9) | 23 (51.1) | ||
| Lower | 135 | 82 (60.7) | 53 (39.3) | 74 (54.8) | 61 (45.2) | ||
| pTNM stage | .008 | .321 | |||||
| I | 32 | 26 (81.3) | 6 (18.8) | 19 (59.4) | 13 (40.6) | ||
| II | 48 | 23 (47.9) | 25 (52.1) | 28 (58.3) | 20 (41.7) | ||
| III | 135 | 73 (54.1) | 62 (45.9) | 65 (48.1) | 70 (51.9) | ||
| Adjuvant chemotherapy | .411 | .151 | |||||
| Yes | 108 | 60 (54.1) | 51 (45.9) | 51 (47.2) | 57 (52.8) | ||
| No | 107 | 62 (59.6) | 42 (40.4) | 61 (57.0) | 46 (43.0) | ||
Abbreviations: α‐SMA, α‐smooth muscle actin; CAFs, cancer‐associated fibroblasts; TANs, tumor‐associated neutrophils.
P < .05 is considered statistically significant (bold).
3.2. Prognostic values of TANs and CAFs
We conducted K‐M survival analyses and log‐rank tests to identify the prognostic diversity among patients categorized by the densities of TANs and CAFs. As shown in Figure 3, concomitant high densities of TANs and CAFs were related to compromised DFS and DSS (all P < .001). Compared to clinicopathological features, including tumor size, tumor location, adjuvant chemotherapy and pTNM stage, CD66+ (hazard ratio [HR] = 1.546; 95% CI = (1.055‐2.268); P = .026) and α‐SMA+ cells (HR = 2.212; 95% CI = 1.493‐3.278; P < .001) were independent factors for DFS in GAC. Similar results were acquired for these two factors for DSS (CD66b+: HR = 1.578, 95% CI = 1.072‐2.323, P = .021; α‐SMA+: HR = 2.278, 95% CI = 1.528‐3.395, P < .001). When pathological TNM stages (I‐III) were analyzed for patient stratification, survival curve analyses showed that a high density of α‐SMA+ CAFs predicted relatively poor DFS and DSS for stage II‐III GAC but not for stage I GAC (Figure 4). However, a high density of CD66+ TANs predicted relatively poor DFS and DSS in phase III GAC only (Figure 5).
Figure 3.
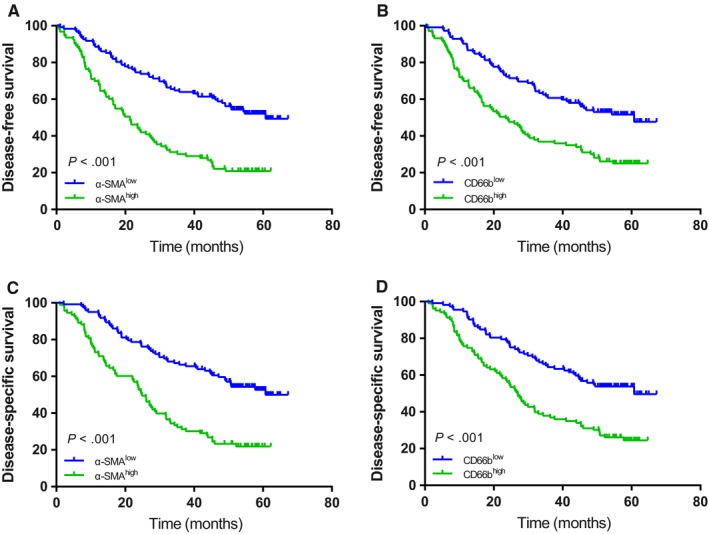
Kaplan‐Meier survival curves of patients with gastric cancer stratified according to α‐SMA and CD66b expression. DFS (A and B) and DSS (C and D) of patients with low and/or high densities of α‐SMA and CD66b in gastric cancer. α‐SMA, α‐smooth muscle actin; DFS, disease‐free survival; DSS, disease‐specific survival
Figure 4.
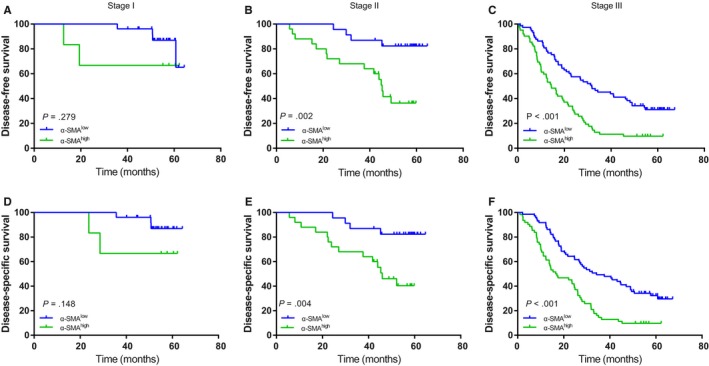
Kaplan‐Meier survival curves based on α‐SMA expression in gastric cancer patients (pTNM stage I‐III). DFS (A‐C) among subgroups stratified by α‐SMA expression. DSS (D‐F) among subgroups stratified by α‐SMA expression. α‐SMA, α‐smooth muscle actin; DFS, disease‐free survival; DSS, disease‐specific survival
Figure 5.
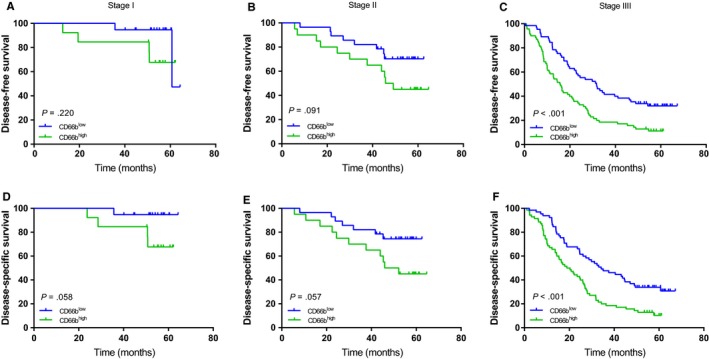
Kaplan‐Meier survival curves based on CD66b expression in gastric cancer patients (pTNM stage I‐III). DFS (A‐C) among subgroups stratified by CD66b expression. DSS (D‐F) among subgroups stratified by CD66b expression. DFS, disease‐free survival; DSS, disease‐specific survival
3.3. Prognostic value of the combination of TANs and CAFs in GAC
We divided 215 GAC patients into four subgroups according to their CD66b+ TAN and α‐SMA+ CAF densities as follows: α‐SMAlowCD66blow (n = 83, 38.6%), α‐SMAlowCD66bhigh (n = 39, 18.1%), α‐SMAhighCD66blow (n = 29, 13.5%), and α‐SMAhighCD66bhigh (n = 64, 29.8%). The patients with the α‐SMAhighCD66bhigh phenotype had the poorest DFS and DSS among all 4 subsets, while the patients with the α‐SMAlowCD66blow phenotype had the best DFS and DSS (Figure 6). pTNM stage (P = .021) was significantly correlated with patient subsets classified by the two‐marker categorizer, as shown in Table 2. Univariate Cox proportional hazard model analysis indicated that the α‐SMAlowCD66bhigh (HR = 1.791; 95% CI = 1.062‐3.021; P = .029), α‐SMAhighCD66blow (HR = 2.402; 95% CI = 1.379‐4.183; P = .002) and α‐SMAhighCD66bhigh (HR = 3.599; 95% CI = 2.330‐5.560; P < .001) patient subgroups were progressively correlated with poorer DFS compared with the α‐SMAlowCD66blow patient subgroup. In a multivariate Cox proportional hazard model, this two‐biomarker categorizer could independently predict the clinical outcome of GAC with progressively increasing hazard ratio values when the α‐SMAlowCD66blow subset was treated as a reference (Table 3).
Figure 6.
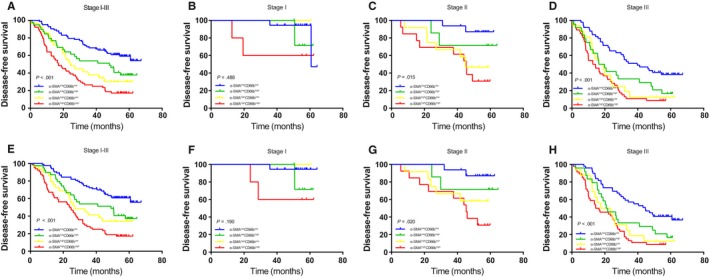
Kaplan‐Meier survival curves based on α‐SMA and CD66b coexpression in gastric cancer patients (pTNM stage I‐III). DFS (A‐D) among subgroups stratified by the combination of α‐SMA and CD66b expression. DSS (E‐H) among subgroups stratified by the combination of α‐SMA and CD66b expression. α‐SMA, α‐smooth muscle actin; DFS, disease‐free survival; DSS, disease‐specific survival
Table 2.
Associations of α‐SMA and CD66b coexpression with clinicopathologic characteristics in patients with gastric cancer
| Characteristics | α‐SMA+CAFs & CD66b+TANs, n (%) | P value | |||
|---|---|---|---|---|---|
| α‐SMAlowCD66blow | α‐SMAlowCD66bhigh | α‐SMAhighCD66blow | α‐SMAhighCD66bhigh | ||
| All cases | 83 (38.6) | 39 (18.1) | 29 (13.5) | 64 (29.8) | |
| Sex | .265 | ||||
| Female | 23 (39.0) | 6 (10.2) | 9 (15.3) | 21 (35.6) | |
| Male | 60 (38.5) | 33 (21.2) | 20 (12.8) | 43 (27.6) | |
| Age (years) | .953 | ||||
| <60 | 38 (36.9) | 20 (19.4) | 14 (13.6) | 31 (30.1) | |
| ≥60 | 45 (40.2) | 19 (17.0) | 15 (13.4) | 33 (29.5) | |
| Tumor size (cm) | .237 | ||||
| <5 | 38 (46.9) | 14 (17.3) | 10 (12.3) | 19 (23.5) | |
| ≥5 | 45 (33.6) | 25 (18.7) | 19 (14.2) | 45 (33.6) | |
| Differentiation | .540 | ||||
| Well/moderate | 15 (31.9) | 9 (19.1) | 9 (19.1) | 14 (29.8) | |
| Poor | 68 (40.5) | 30 (17.9) | 20 (11.9) | 50 (29.8) | |
| Location | .353 | ||||
| Upper | 12 (34.3) | 3 (8.6) | 4 (11.4) | 16 (45.7) | |
| Middle | 16 (35.6) | 9 (20.0) | 6 (13.3) | 14 (31.1) | |
| Lower | 55 (40.7) | 27 (20.0) | 19 (14.1) | 34 (25.2) | |
| pTNM stage | .021 | ||||
| I | 18 (56.3) | 8 (25.0) | 1 (3.1) | 5 (15.6) | |
| II | 16 (33.3) | 7 (14.6) | 12 (25.0) | 13 (27.1) | |
| III | 49 (36.3) | 24 (17.8) | 16 (11.9) | 46 (34.1) | |
| Adjuvant chemotherapy | .496 | ||||
| Yes | 39 (36.1) | 21 (19.4) | 12 (11.1) | 36 (33.3) | |
| No | 44 (41.1) | 18 (16.8) | 17 (15.9) | 28 (26.2) | |
Abbreviations: α‐SMA, α‐smooth muscle actin; CAFs, cancer‐associated fibroblasts; TANs, tumor‐associated neutrophils.
P < .05 is considered statistically significant (bold).
Table 3.
Univariate and multivariate Cox regression analyses for DFS and DSS based on α‐SMA and CD66b coexpression stratification and clinicopathologic characteristics
| Variables | Disease‐free survival | Disease‐specific survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex | .337 | .330 | ||||||
| Female | 1 | 1 | ||||||
| Male | 0.830 (0.567‐1.214) | 0.827 (0.564‐1.212) | ||||||
| Age (years) | .834 | .596 | ||||||
| <60 | 1 | 1 | ||||||
| ≥60 | 1.037 (0.736‐1.462) | 1.098 (0.777‐1.553) | ||||||
| Tumor size (cm) | .002 | .272 | <.001 | .048 | ||||
| <5 | 1 | 1 | 1 | 1 | ||||
| ≥5 | 1.830 (1.257‐2.663) | 1.245 (0.842‐1.840) | 1.986 (1.355‐2.910) | 1.492 (1.003‐2.221) | ||||
| Differentiation | .200 | .163 | ||||||
| Well/moderate | 1 | 1 | ||||||
| Poor | 1.325 (0.862‐2.036) | 1.365 (0.882‐2.113) | ||||||
| Location | .017 | .071 | .043 | .204 | ||||
| Upper | 1 | 1 | 1 | 1 | ||||
| Middle | 0.803 (0.476‐1.353) 0.409 | 0.892 (0.522‐1.525) | .677 | 0.823 (0.484‐1.401) | .473 | 0.953 (0.551‐1.648) | .863 | |
| Lower | 0.548 (0.350‐0.857) 0.008 | 0.626 (0.395‐0.993) | .047 | 0.586 (0.372‐0.923) | .021 | 0.706 (0.442‐1.128) | .145 | |
| pTNM stage | <.001 | <.001 | <.001 | <.001 | ||||
| I | 1 | 1 | 1 | 1 | ||||
| II | 2.435 (0.972‐6.098) 0.057 | .057 | 2.030 (0.793‐5.199) | .140 | 2.788 (1.035‐7.509) | .043 | 2.242 (0.817‐6.150) | .117 |
| III | 7.860 (3.444‐17.938) | <.001 | 7.368 (3.152‐17.225) | <.001 | 9.293 (3.781‐22.840) | <.001 | 8.484 (3.391‐21.228) | <.001 |
| Adjuvant chemotherapy | .004 | .001 | .003 | <.001 | ||||
| Yes | 1 | 1 | 1 | 1 | ||||
| No | 1.680 (1.183‐2.387) | 1.931 (1.342‐2.780) | 1.688 (1.191‐2.394) | 2.054 (1.423‐2.966) | ||||
| α‐SMA+CAFs & CD66b+TANs | <.001 | <.001 | <.001 | <.001 | ||||
| α‐SMAlowCD66blow | 1 | 1 | 1 | 1 | ||||
| α‐SMAlowCD66bhigh | 1.791 (1.062‐3.021) | .029 | 1.900 (1.112‐3.244) | .019 | 1.835 (1.084‐3.106) | .024 | 1.923 (1.123‐3.294) | .017 |
| α‐SMAhighCD66blow | 2.402 (1.379‐4.183) | .002 | 2.771 (1.574‐4.878) | <.001 | 2.363 (1.341‐4.163) | .003 | 2.848 (1.597‐5.082) | <.001 |
| α‐SMAhighCD66bhigh | 3.599 (2.330‐5.560) | <.001 | 3.511 (2.254‐5.469) | <.001 | 3.660 (2.361‐5.676) | <.001 | 3.689 (2.357‐5.775) | <.001 |
Abbreviations: α‐SMA, α‐smooth muscle actin; CAFs, cancer‐associated fibroblasts; CI, confidence interval; DFS, disease‐free survival; DSS, disease‐specific survival; HR, hazard ratio; TANs, tumor‐associated neutrophils.
P < .05 is considered statistically significant (bold).
3.4. Extension and accuracy of prognostic models including the two‐marker predictor
Considering this distinct prognostic value, we combined the two‐marker classifier with the pTNM staging system to investigate the actual prognostic value of the combination of TANs and CAFs. ROC analysis was applied, and area under the curve (AUC) values were compared to evaluate prognostic accuracy. The AUCs of TANs or CAFs alone were 0.647 and 0.657, respectively, while the combination of the two markers had an AUC of 0.703 (Figure 7). The AUC of pTNM staging alone was 0.757, and the AUC was elevated to 0.839 when the two‐marker predictor was added (Table 4).
Figure 7.
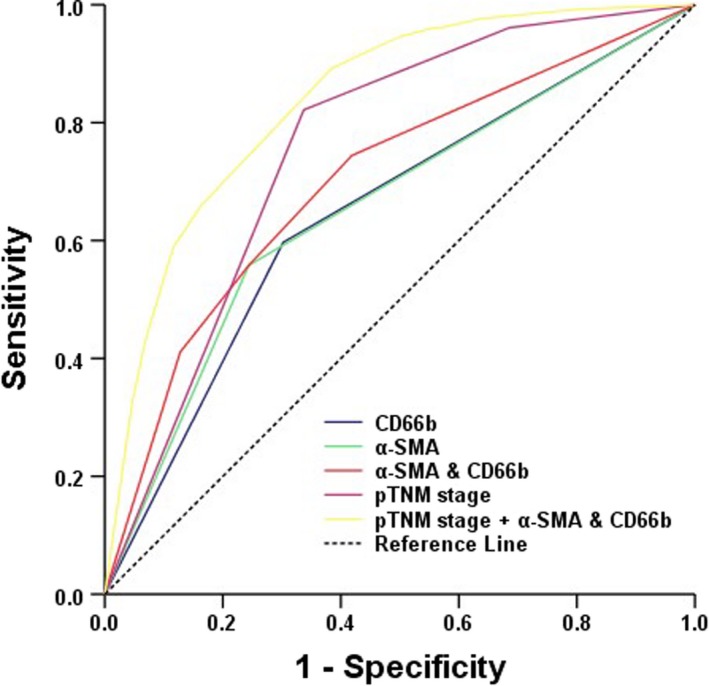
Receiver operating characteristic (ROC) curves for prognostic markers predicting survival among GAC patients. GAC, gastric adenocarcinoma
Table 4.
Areas under ROC curves for prognostic markers
| Factor | AUC (95% CI) | P‐value |
|---|---|---|
| CD66b | 0.647 (0.572‐0.722) | <.001 |
| α‐SMA | 0.657 (0.583‐0.731) | <.001 |
| α‐SMA & CD66b | 0.703 (0.633‐0.774) | <.001 |
| pTNM stage | 0.757 (0.688‐0.827) | <.001 |
| pTNM stage + α‐SMA & CD66b | 0.839 (0.784‐0.894) | <.001 |
Abbreviations: α‐SMA, α‐smooth muscle actin; AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
P < .05 is considered statistically significant (bold).
3.5. Correlations between the two‐marker predictor and adjuvant chemotherapy
In a subgroup analysis, we assessed the advantage of postoperative chemotherapeutics in TNM stage II‐III patients. There is only a weak relationship between patients with the α‐SMAlowCD66bhigh, α‐SMAhighCD66blow or α‐SMAhighCD66high phenotype and survival (DFS: P = .393, P = .500 and P = .118, respectively, Figure 8B‐D; DSS: P = .334, P = .474, P = .129, respectively, Figure 8F‐H), and these make only a small contribution to the difference between patients with surgery only and with surgery add postoperative chemotherapy adjuvant in HR. However, the patients with the α‐SMAlowCD66blow phenotype could receive a significant benefit from adjuvant chemotherapy (DFS: P < .001, Figure 8A; DSS: P < .001, Figure 8E). Treatment with adjuvant chemotherapy was associated with a decreasing risk of a poor clinical outcome in the α‐SMAlowCD66blow patient subset (HR: 0.260, 95% CI: 0.124‐0.542, P < .001; HR: 0.258, 95% CI: 0.124‐0.538, P < .001; Table 5), while such a risk decline was not found in the α‐SMAlowCD66bhigh, α‐SMAhighCD66blow or α‐SMAhighCD66high subgroup patients (Table 5).
Figure 8.
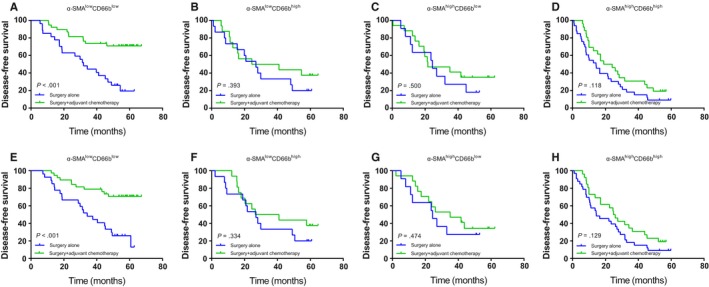
Kaplan‐Meier survival curves for gastric cancer patients who underwent surgery alone or surgery + adjuvant chemotherapy stratified according to α‐SMA and CD66b coexpression. DFS (A‐D) and DSS (E‐H) in α‐SMAlowCD66blow, α‐SMAlowCD66bhigh, α‐SMAhighCD66blow, and α‐SMAhighCD66bhigh gastric cancer patients. α‐SMA, α‐smooth muscle actin; DFS, disease‐free survival; DSS, disease‐specific survival
Table 5.
Hazard ratios for DFS and DSS in stage Ⅱ‐III GAC patients receiving adjuvant chemotherapy or not according to α‐SMA and CD66b coexpression patterns
| Factor | Patients | % | Adjuvant chemotherapeutic (yes vs no) | |||
|---|---|---|---|---|---|---|
| DFS | DSS | |||||
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |||
| α‐SMA & CD66b | 183 | 0.482 (0.337‐0.689) | <.001 | 0.476 (0.333‐0.681) | <.001 | |
| α‐SMAlowCD66blow | 65 | 35.5 | 0.260 (0.124‐0.542) | <.001 | 0.258 (0.124‐0.538) | <.001 |
| α‐SMAlowCD66bhigh | 31 | 16.9 | 0.694 (0.298‐1.613) | .396 | 0.662 (0.285‐1.538) | .337 |
| α‐SMAhighCD66blow | 28 | 15.3 | 0.736 (0.303‐1.784) | .497 | 0.694 (0.298‐1.613) | .476 |
| α‐SMAhighCD66bhigh | 59 | 32.2 | 0.643 (0.367‐1.125) | .122 | 0.650 (0.371‐1.139) | .132 |
Abbreviations: α‐SMA, α‐smooth muscle actin; CI, confidence interval; DFS, disease‐free survival; DSS, disease‐specific survival; GAC, gastric adenocarcinoma; HR, hazard ratio.
P < .05 is considered statistically significant (bold).
4. DISCUSSION
As a traditional prognostic tool for GAC patients, the TNM classification system is derived from cancer‐centered biological behaviors and overlooks the impacts of the host TME, which result in a significant decline in predictive accuracy. In fact, this is why some patients with early‐phase disease exhibit rapid disease progression, while others with high‐grade disease have stable disease for several years.19 In the current research, we detected the immunological parameters CD66b+ TANs and α‐SMA+ CAFs in 215 GAC specimens with immunohistochemical technology and analyzed the association between these two cell types and their respective and combined prognostic values.
Tumor‐associated neutrophils are a class of sensitized neutrophils in the carcinoma matrix and important components of the TME and play an essential role in tumor evolution; hence, they should be seriously evaluated.34 TANs have been considered a compromised prognostic marker in various types of tumors, including bladder cancer, hepatocellular cancer and renal cell cancer.18, 35, 36 The present research demonstrated that a high density of TANs could act as an independent poor prognostic marker in GAC. This is consistent with previous findings.17, 37 Previous studies have shown that tumor‐infiltrated neutrophils undergo polarization in different TMEs to a procancer N2 or an anticancer N1 subtype.38 Therefore, the effects of TANs on tumor cells may be quite varied. Blocking TGF‐β causes a conversion from the procancer phenotype to the anticancer subtype, suggesting that the TAN categorization paradigm resembles the M1/M2‐subtype paradigm of tumor‐associated macrophages.14, 38 In this study, a high density of TANs in a GAC specimen was associated with a poor clinical outcome and likewise demonstrated that N2‐type neutrophils might be the major cellular phenotype in GAC tissue, despite there being no particular factors that can be applied to discriminate the N1/N2 subsets. Thus, it is necessary to further investigate the precise infiltration profiles of N1 and N2 cells in GAC, their functions and potential biological mechanisms.
In recent years, increasing evidence has shown that the development of carcinoma depends on the intrinsic characteristics of carcinoma cells and the influence of the cancer matrix.39 The cancer matrix consists of fibroblasts that generate extracellular matrix (ECM) components, inflammatory cells and blood/lymphatic capillaries.40 Sensitized fibroblasts, known as CAFs, have a few similarities with myofibroblasts, including the expression of α‐SMA.41 CAFs play an important role in the origination, evolution and diffusion of epithelial carcinoma by generating soluble factors.42 They can also reshape the tumor ECM; regulate the metabolism, mobility and stem cell characteristics of carcinoma cells; and prepare disseminated niches.39 Previous studies have revealed that CAFs can be a significant prognostic marker in numerous cancers. Ju et al43 found that peritumoral CAFs were related to a compromised prognosis in hepatocellular carcinoma patients. Yamashita et al44 reported that the expression of α‐SMA was markedly higher in an invasive breast cancer dissemination subgroup than in a no dissemination subgroup and that the invasive subgroup had a worse survival rate. Similarly, Zhi et al45 demonstrated that elevated α‐SMA expression was associated with tumor invasiveness characteristics in gastric carcinoma. In the present study, a high density of α‐SMA+ CAFs was correlated with the pTNM stage and trends for poor DFS and DSS. In contrast, Valach et al46 demonstrated that the extent of α‐SMA expression was not correlated with DFS in head and neck squamous cell carcinoma. The probable cause is that α‐SMA may have diverse expression patterns and biological influences in various types of tumors.
When CD66b+ TANs and a‐SMA+ CAFs were combined for survival analysis, we discovered that patients with a low density of CD66b+ TANs combined with a low density of a‐SMA+ CAFs showed the longest DFS and DSS, followed by patients with an a‐SMAlowCD66bhigh phenotype, those with an a‐SMAhighCD66blow phenotype and finally those with an a‐SMAhighCD66bhigh phenotype. Multivariate Cox regression revealed that low‐density CD66b+ TANs combined with low‐density a‐SMA+ CAFs was a good prognostic marker in GAC patients. The combination of the densities of CD66b+ TANs and a‐SMA+ CAFs with the pathological TNM staging system could better predict the clinical outcome of GAC than the individual parameters. When patients in different pTNM stages were analyzed, the combined predictor still showed potential prognostic value. Neutropenia caused by postoperative chemotherapeutics influences the outcomes of gastric cancer, colon cancer, and breast cancer patients. A short period of neutropenia may indicate a deficient dosage and a lack of lethality.47, 48, 49 Previous studies have shown that the matrix response produces a physical barrier to defend carcinoma cells from chemotherapy in solid tumors,50 and inhibiting autophagy in CAFs contributes to the effects of chemotherapy on pancreatic cancer.51 Stage II‐III GAC patients with the SMAlowCD66blow phenotype might benefit from adjuvant chemotherapy. In other words, adjuvant chemotherapy for patients with the a‐SMAlowCD66bhigh, a‐SMAhighCD66blow or a‐SMAhighCD66bhigh phenotype should be seriously reconsidered. This discovery will help to select more suitable patients for adjuvant chemotherapy treatment and prohibit excessive toxicities and unnecessary resource waste. These conclusions require further, more predictive, multicenter studies for verification.
Molecular biology studies on the interactions between TANs and CAFs are limited. Cheng et al52 found that hepatocellular cancer‐derived CAFs affect the survival, activation, and features of neutrophils in hepatocellular cancer via the IL6‐STAT3‐PDL1 signaling pathway. In addition, Zhu et al53 suggested that gastric cancer‐derived mesenchymal stem cells protected and activated neutrophils via the IL‐6‐STAT3‐ERK1/2 signaling pathway. In turn, the gastric cancer‐derived mesenchymal stem cell‐primed neutrophils induced the differentiation of normal mesenchymal stem cells into CAFs. Therefore, the cross‐talk between TANs and CAFs is complex, and further research should be conducted on particular mechanisms to determine important targets for antineoplastic therapies.
The limitations of this study are a retrospective study and the relatively small number of patients receiving postoperative chemotherapy. In addition, the small tissues sampled may not represent the entire tumor, which may bias the results. Therefore, a prospective, a multicenter randomized trial is needed to validate these results in the future.
5. CONCLUSION
Individually, CD66b+ TANs and a‐SMA+ CAFs were valuable for predicting prognosis in GAC. However, combining the densities of CD66b+ TANs and a‐SMA+ CAFs produce a better and more precise prognostic marker that could be applied as a promising prognostic marker for clinical outcomes and indicator for adjuvant chemotherapy treatment decision‐making in GAC patients.
CONFLICT OF INTERESTS
The authors have declared that no competing interests exist.
AUTHOR CONTRIBUTIONS
Xiliang Cong contributed to conception, design, data analysis, and writing‐original draft. Yongle Zhang, Ziyu Zhu, and Sen Li contributed to provision of study materials or patients and data analysis and interpretation. Xin Yin, Yu Zhang, and Zhao Zhai contributed to collection and assembly of data; and Yingwei Xue: financial support, technical help, and fruitful discussion.
ETHICAL APPROVAL
All programs were in accordance with the ethical standards of the Human Subjects Responsibility Committee (institutions and countries) and the 1964 Helsinki Declaration and subsequent versions. This research was approved by the Ethics Committee of Harbin Medical University Cancer Hospital.
Cong X, Zhang Y, Zhu Z, et al. CD66b+ neutrophils and α‐SMA+ fibroblasts predict clinical outcomes and benefits from postoperative chemotherapy in gastric adenocarcinoma. Cancer Med. 2020;9:2761–2773. 10.1002/cam4.2939
Funding information
This work was supported by the Nn10 Program of Harbin Medical University Cancer Hospital, China (No. Nn10 PY 2017‐03).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15‐year follow‐up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439‐449. [DOI] [PubMed] [Google Scholar]
- 3. Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first‐line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24(31):4991‐4997. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet. 2010;376(9742):687‐697. [DOI] [PubMed] [Google Scholar]
- 5. GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group , Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta‐analysis. JAMA. 2010;303(17):1729‐1737. [DOI] [PubMed] [Google Scholar]
- 6. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654‐2664. [DOI] [PubMed] [Google Scholar]
- 7. Wu H, Xu JB, He YL, et al. Tumor‐associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106(4):462‐468. [DOI] [PubMed] [Google Scholar]
- 8. Rice AJ, Griffiths AP, Martin IG, Dixon MF. Gastric carcinoma with prominent neutrophil infiltration. Histopathology. 2000;37(3):289‐290. [DOI] [PubMed] [Google Scholar]
- 9. Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88(3):577‐583. [PubMed] [Google Scholar]
- 10. Wu Y, Zhao Q, Peng C, Sun L, Li XF, Kuang DM. Neutrophils promote motility of cancer cells via a hyaluronan‐mediated TLR4/PI3K activation loop. J Pathol. 2011;225(3):438‐447. [DOI] [PubMed] [Google Scholar]
- 11. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402‐1410. [DOI] [PubMed] [Google Scholar]
- 12. Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23(3):141‐148. [DOI] [PubMed] [Google Scholar]
- 13. Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90(3):222‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gregory AD, Houghton AM. Tumor‐associated neutrophils: new targets for cancer therapy. Can Res. 2011;71(7):2411‐2416. [DOI] [PubMed] [Google Scholar]
- 15. Liang W, Ferrara N. The complex role of neutrophils in tumor angiogenesis and metastasis. Cancer Immunol Res. 2016;4(2):83‐91. [DOI] [PubMed] [Google Scholar]
- 16. Trellakis S, Farjah H, Bruderek K, et al. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int J Immunopathol Pharmacol. 2011;24(3):683‐693. [DOI] [PubMed] [Google Scholar]
- 17. Li TJ, Jiang YM, Hu YF, et al. Interleukin‐17‐producing neutrophils link inflammatory stimuli to disease progression by promoting angiogenesis in gastric cancer. Clin Cancer Res. 2017;23(6):1575‐1585. [DOI] [PubMed] [Google Scholar]
- 18. Galdiero MR, Bianchi P, Grizzi F, et al. Occurrence and significance of tumor‐associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139(2):446‐456. [DOI] [PubMed] [Google Scholar]
- 19. Wang J, Bo X, Suo T, et al. Tumor‐infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Sci. 2018;109(7):2266‐2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Houghton AM, Rzymkiewicz DM, Ji H, et al. Neutrophil elastase‐mediated degradation of IRS‐1 accelerates lung tumor growth. Nat Med. 2010;16(2):219‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spicer JD, McDonald B, Cools‐Lartigue JJ, et al. Neutrophils promote liver metastasis via Mac‐1‐mediated interactions with circulating tumor cells. Can Res. 2012;72(16):3919‐3927. [DOI] [PubMed] [Google Scholar]
- 22. Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Can Res. 2010;70(14):6071‐6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP‐free MMP‐9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104(51):20262‐20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang TT, Zhao YL, Peng LS, et al. Tumour‐activated neutrophils in gastric cancer foster immune suppression and disease progression through GM‐CSF‐PD‐L1 pathway. Gut. 2017;66(11):1900‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klobukowska HJ, Munday JS. High numbers of stromal cancer‐associated fibroblasts are associated with a shorter survival time in cats with oral squamous cell carcinoma. Vet Pathol. 2016;53(6):1124‐1130. [DOI] [PubMed] [Google Scholar]
- 26. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582‐598. [DOI] [PubMed] [Google Scholar]
- 27. Jiang J, Ye F, Yang X, et al. Peri‐tumor associated fibroblasts promote intrahepatic metastasis of hepatocellular carcinoma by recruiting cancer stem cells. Cancer Lett. 2017;404:19‐28. [DOI] [PubMed] [Google Scholar]
- 28. Zhao XL, Lin Y, Jiang J, et al. High‐mobility group box 1 released by autophagic cancer‐associated fibroblasts maintains the stemness of luminal breast cancer cells. J Pathol. 2017;243(3):376‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunaydin G, Kesikli SA, Guc D. Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology. 2015;4(9):e1034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pistore C, Giannoni E, Colangelo T, et al. DNA methylation variations are required for epithelial‐to‐mesenchymal transition induced by cancer‐associated fibroblasts in prostate cancer cells. Oncogene. 2017;36(40):5551‐5566. [DOI] [PubMed] [Google Scholar]
- 31. Bello IO, Vered M, Dayan D, et al. Cancer‐associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma‐associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Yang P, Xiao Y, et al. Overexpression of α‐sma‐positive fibroblasts (CAFs) in nasopharyngeal carcinoma predicts poor prognosis. J Cancer. 2017;8(18):3897‐3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blom S, Erickson A, Östman A, et al. Fibroblast as a critical stromal cell type determining prognosis in prostate cancer. Prostate. 2019;79(13):1505‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228(7):1404‐1412. [DOI] [PubMed] [Google Scholar]
- 35. Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54(3):497‐505. [DOI] [PubMed] [Google Scholar]
- 36. Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27(28):4709‐4717. [DOI] [PubMed] [Google Scholar]
- 37. Li S, Cong X, Gao H, et al. Tumor‐associated neutrophils induce EMT by IL‐17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. 2019;38(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor‐associated neutrophil phenotype by TGF‐beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16(3):183‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cirri P, Chiarugi P. Cancer‐associated‐fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1‐2):195‐208. [DOI] [PubMed] [Google Scholar]
- 40. Polyak K, Haviv I, Campbell IG. Co‐evolution of tumor cells and their microenvironment. Trends Genet. 2009;25(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 41. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano‐regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349‐363. [DOI] [PubMed] [Google Scholar]
- 42. Galiè M, Sorrentino C, Montani M, et al. Mammary carcinoma provides highly tumourigenic and invasive reactive stromal cells. Carcinogenesis. 2005;26(11):1868‐1878. [DOI] [PubMed] [Google Scholar]
- 43. Ju M‐J, Qiu S‐J, Fan J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131(4):498‐510. [DOI] [PubMed] [Google Scholar]
- 44. Yamashita M, Ogawa T, Zhang X, et al. Role of stromal myofibroblasts in invasive breast cancer: stromal expression of alpha‐smooth muscle actin correlates with worse clinical outcome. Breast Cancer. 2012;19(2):170‐176. [DOI] [PubMed] [Google Scholar]
- 45. Zhi K, Shen X, Zhang H, Bi J. Cancer‐associated fibroblasts are positively correlated with metastatic potential of human gastric cancers. J Exp Clin Cancer Res. 2010;29:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Valach J, Fík Z, Strnad H, et al. Smooth muscle actin‐expressing stromal fibroblasts in head and neck squamous cell carcinoma: increased expression of galectin‐1 and induction of poor prognosis factors. Int J Cancer. 2012;131(11):2499‐2508. [DOI] [PubMed] [Google Scholar]
- 47. Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. Predictive value of chemotherapy‐induced neutropenia for the efficacy of oral fluoropyrimidine S‐1 in advanced gastric carcinoma. Br J Cancer. 2007;97(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shitara K, Matsuo K, Takahari D, et al. Neutropaenia as a prognostic factor in metastatic colorectal cancer patients undergoing chemotherapy with first‐line FOLFOX. Eur J Cancer. 2009;45(10):1757‐1763. [DOI] [PubMed] [Google Scholar]
- 49. Han Y, Yu Z, Wen S, Zhang B, Cao X, Wang X. Prognostic value of chemotherapy‐induced neutropenia in early‐stage breast cancer. Breast Cancer Res Treat. 2012;131(2):483‐490. [DOI] [PubMed] [Google Scholar]
- 50. Choi IK, Strauss R, Richter M, Yun CO, Lieber A. Strategies to increase drug penetration in solid tumors. Front Oncol. 2013;3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X, Schönrogge M, Eichberg J, et al. Blocking autophagy in cancer‐associated fibroblasts supports chemotherapy of pancreatic cancer cells. Front Oncol. 2018;8:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng Y, Li H, Deng Y, et al. Cancer‐associated fibroblasts induce PDL1+ neutrophils through the IL6‐STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhu Q, Zhang X, Zhang L, et al. The IL‐6‐STAT3 axis mediates a reciprocal crosstalk between cancer‐derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


