Mother-to-child-transmission of HIV-1 offers a unique setting in which maternal antibodies both within the mother and passively transferred to the infant are present at the time of viral exposure. Untreated HIV-exposed human infants are infected at a rate of 30 to 40%, meaning that some infants do not get infected despite continued exposure to virus. Since the potential of HIV-specific immune responses to provide protection against HIV is a central goal of HIV vaccine design, understanding the nature of maternal antibodies may provide insights into immune mechanisms of protection. In this study, we isolated and characterized HIV-specific antibodies from the mother of an infant whose transmitted virus has been well studied.
KEYWORDS: antibody function, human immunodeficiency virus, maternal antibodies, monoclonal antibodies, mother-to-child transmission
ABSTRACT
Infants of HIV-positive mothers can acquire HIV infection by various routes, but even in the absence of antiviral treatment, the majority of these infants do not become infected. There is evidence that maternal antibodies provide some protection from infection, but gestational maternal antibodies have not yet been characterized in detail. One of the most studied vertically infected infants is BG505, as the virus from this infant yielded an Envelope protein that was successfully developed as a stable trimer. Here, we isolated and characterized 39 HIV-specific neutralizing monoclonal antibodies (nAbs) from MG505, the mother of BG505, at a time point just prior to vertical transmission. These nAbs belonged to 21 clonal families and employed a variety of VH genes. Many were specific for the HIV-1 Env V3 loop, and this V3 specificity correlated with measurable antibody-dependent cellular cytotoxicity (ADCC) activity. The isolated nAbs did not recapitulate the full breadth of heterologous or autologous virus neutralization by contemporaneous plasma. Notably, we found that the V3-targeting nAb families neutralized one particular maternal Env variant, even though all tested variants had low V3 sequence diversity and were measurably bound by these nAbs. None of the nAbs neutralized BG505 transmitted virus. Furthermore, the MG505 nAb families were found at relatively low frequencies within the maternal B cell repertoire; all were less than 0.25% of total IgG sequences. Our findings illustrate an example of the diversity of HIV-1 nAbs within one mother, cumulatively resulting in a collection of antibody specificities that can contribute to the transmission bottleneck.
IMPORTANCE Mother-to-child-transmission of HIV-1 offers a unique setting in which maternal antibodies both within the mother and passively transferred to the infant are present at the time of viral exposure. Untreated HIV-exposed human infants are infected at a rate of 30 to 40%, meaning that some infants do not get infected despite continued exposure to virus. Since the potential of HIV-specific immune responses to provide protection against HIV is a central goal of HIV vaccine design, understanding the nature of maternal antibodies may provide insights into immune mechanisms of protection. In this study, we isolated and characterized HIV-specific antibodies from the mother of an infant whose transmitted virus has been well studied.
INTRODUCTION
Mother-to-child transmission of human immunodeficiency virus (HIV) is a unique setting for studying HIV immunity, because both the mother and her infant have circulating maternal HIV-specific neutralizing antibodies (nAbs) at the time of HIV exposure and transmission. Antibodies in the mother could neutralize the maternal virus and/or target infected cells to reduce infectiousness. In addition, during late gestation and breastfeeding, the infant has HIV-specific antibodies potentially capable of recognizing and blocking maternal viruses through similar mechanisms. However, untreated HIV-exposed infants still are infected at a rate of 30 to 40%. The specific role of maternal autologous virus-neutralizing IgG responses in driving the selection of infant transmitted founder viruses is both controversial and complex. Some studies, including the larger studies on this topic, report that viruses transmitted from mother to infant are more resistant to neutralization by maternal antibodies than the overall maternal viral population, implying maternal antibodies select against the transmission of the most neutralization sensitive variants (1–4); however, this has not been consistently observed in all studies (5, 6). Relatedly, there is also inconsistency in studies that sought to define properties of Env-specific maternal antibodies that are associated with reduced risk of mother-to-child transmission (MTCT) (7). Some studies suggest that protection is associated with antibodies that target specific epitopes, such as variable loop region 3 (V3) (8–10) or gp41 (11), while another study found no association between nAb properties and MTCT risk (12), and still others have found antibody specificities that are associated with increased infection risk (13).
Recent studies suggest that passively acquired antibodies that mediate antibody-dependent cellular cytotoxicity (ADCC) provide protection from disease in infants who acquire HIV (14). ADCC antibodies target infected cells for destruction, which have been shown to be a key correlate of MTCT via breastmilk (15). ADCC-mediating HIV-specific antibodies in breastmilk have been associated with a reduced risk of MTCT in clade A HIV-infected women (16) but not in clade C-infected women (17). Thus, a deeper understanding of the characteristics, specificities, functions, and potencies of the maternal HIV Env-specific antibody repertoire present at the time of MTCT is warranted.
Given the interactions between the virus and the maternal antibody response, the viruses transmitted to infants are of particular interest. Indeed, one of the most studied HIV-1 variants is from an infant early in infection, BG505 (1), and this infant-derived Env was used to generate the first native-like soluble Env trimer: BG505.SOSIP.664 (18). The BG505 Env has informed a range of structural and immunogen studies (19–22), including a phase I human clinical vaccine trial (ClinicalTrials registration no. NCT03699241; https://clinicaltrials.gov/ct2/show/NCT03699241). Infant BG505 was HIV negative at birth but was detected positive at 6 weeks of life, having been infected by a single transmitted variant from mother MG505 (1). MG505 was HIV seropositive at enrollment in the Nairobi Breastfeeding Trial during her third trimester of pregnancy (P31), and her infant was delivered prematurely at P32 (W0), just 1 week after enrollment. At the time of HIV transmission, MG505 had already developed a relatively broad nAb response (23). While the infant ultimately was not protected from the particular transmitted virus that seeded the infection, BG505 did not acquire a myriad of other maternal variants that coexisted at the time of MTCT, as is common in MTCT and HIV infection in general (24). Prior detailed analyses of the neutralization sensitivity of individual maternal and infant variants suggested that the selection for transmission of a particular maternal Env variant and not others was mediated, in part, by maternal HIV nAbs (1).
In this study, we characterize MG505 Env-specific nAbs that were circulating immediately prior to HIV transmission, focusing on their breadth, potencies, specificities, functions, and frequencies within the antibody repertoire. In addition to measuring nAb efficacy against relevant heterologous viruses, we defined the capacity of these monoclonal maternal nAbs to bind and neutralize seven autologous MG505 viral variants as well as the virus transmitted to infant BG505. Our results demonstrate that there was a diverse repertoire of HIV antibodies in MG505 at the time of transmission, many of which targeted the V3 epitope, bound maternal Envs, and were capable of ADCC.
RESULTS
Thirty-nine HIV-neutralizing antibodies were functionally isolated from MG505 immediately prior to MTCT.
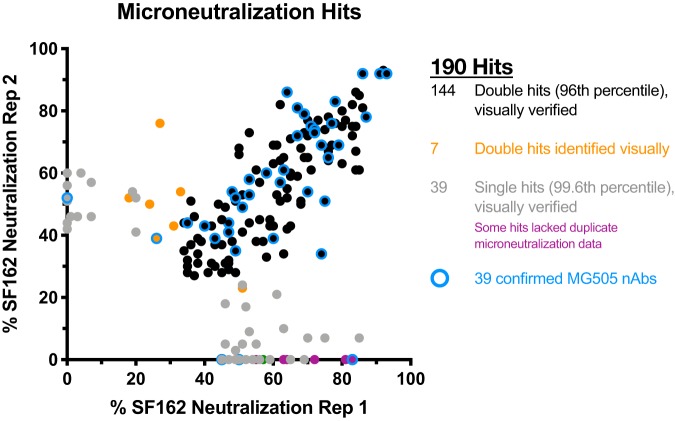
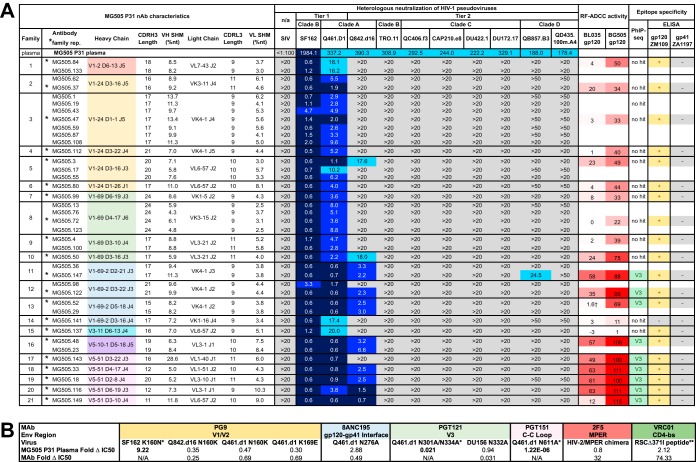
We isolated and characterized maternal nAbs present very close to the time of vertical transmission of HIV to infant BG505 by studying a maternal peripheral blood mononuclear cell (PBMC) sample from Kenyan subject MG505 collected at 31 weeks of pregnancy (P31). In this case, the P31 time point was 1 week prior to the birth of infant BG505 and 7 weeks prior to the first detection of HIV-infected cells in BG505 at 6 weeks of life (W6). After over 20 years in liquid nitrogen storage, the MG505 PBMCs were 85% viable, containing 5.2 million total live cells. Of live PBMCs, 12% were B cells (CD19+) and, of these, 32% (4% of total cells) were memory B cells (IgM− IgD−). We sorted, cultured, and screened the culture supernatant of 163,165 memory B cells for evidence of antibody activity capable of neutralizing the SF162 tier 1A HIV-1 variant. This approach identified 190 wells of interest (Fig. 1). Monoclonal Abs (MAbs) were successfully reconstructed from 97/190 wells, and 39 MAbs ultimately were confirmed to neutralize SF162 (Fig. 2). Nucleotide somatic hypermutation (SHM) ranged from 4.3 to 28.6% (heavy chain) and 2.5 to 10.3% (light chain), and CDR3 lengths ranged from 11 to 24 (CDRH3) and 9 to 11 (CDRL3) amino acids (Fig. 2). Clonal family analysis revealed that the 39 nAbs comprise 21 clonal families that collectively employ 7 VH genes, 5 VK genes, and 7 VL genes (Fig. 2A). Two nAbs contained indel events: MG505.18 (family 19) had 6 nucleotides deleted from its VH5-51 FR3 region, and MG505.72 (family 8) had 3 nucleotides inserted in its VK3-15 CDR1 region.
FIG 1.
Selection of SF162 pseudovirus-neutralizing MG505 memory B cells for immunoglobulin gene rescue. Wells demonstrating neutralization in the 96th percentile of wells across all plates for both technical replicates were selected (black), in addition to wells that were in the 99.6th percentile in only one replicate (gray and purple) and seven wells that were subjectively identified by visual inspection as possibly neutralizing (orange). Wells that were not selected are not shown. Wells that ultimately yielded a confirmed nAb are highlighted in blue.
FIG 2.
Characteristics of MG505 isolated nAbs and plasma epitopes. (A) nAb characteristics are displayed in rows, with dark lines separating clonal families. Heavy-chain rearrangements are color-coded according to VH gene usage. Neutralization of heterologous viruses by MG505 P31 plasma (top row) and nAbs is displayed as IC50 values (reciprocal dilution and μg/ml, respectively). Heterologous virus tiers, clades, and names are indicated. Simian immunodeficiency virus (SIV) was included as a negative control. Darker blue shading indicates more potent neutralization. Gray indicates that 50% neutralization was not achieved at the highest nAb concentration tested. All nAbs were initially tested at 20 μg/ml, and, only if possible low-level neutralization was observed, they were retested at 50 μg/ml. RF-ADCC activity is displayed as a percentage of target cells killed, normalized to HIVIG activity, with more potent activity shaded in darker red. PhIP-seq epitope mapping indicates V3 linear peptide specificity for nAb family representatives. ELISA results indicate binding at least 2 times above the negative control (+) or lack of binding (−) to the indicated antigen. Neutralization, PhIP-seq, and ELISA results reflect averages from at least two independent experiments, each performed in duplicate. RF-ADCC results are representative of two independent experiments. *, nAb selected to represent clonal family; †, RF-ADCC activity varies from 1 to 27% depending on PBMC donor used in the experiment. (B) MG505 plasma nAb epitopes identified through serum mapping techniques. The table shows fold change in IC50 values (WT versus mutant) for MG505 P31 plasma and control monoclonal antibodies against a panel of viruses and virus mutants that target specific epitopes. n/a, control MAb does not neutralize the WT virus. *, CD4-bs mapping fold change calculated from absorbance values measured at 450 nm via ELISA (see Materials and Methods for additional details).
Tier 1 and tier 2 heterologous HIV-1 neutralization by MG505 nAbs.
To characterize the neutralization potential of the 39 nAbs, we tested each nAb for its ability to neutralize ten heterologous HIV-1 pseudoviruses that each was neutralized by the MG505 P31 plasma (Fig. 2A). MG505 nAbs all potently neutralized tier 1A virus SF162 (clade B), which was the major selection criterion for their inclusion in this study, with 50% inhibitory concentrations (IC50s) of 4.7 μg ml−1 or lower. They also all neutralized tier 1 Q461.D1 (clade A, ∼87% nucleotide identity to autologous Envs) (Fig. 3), with variable potencies and IC50s ranging from 0.6 to 20 μg ml−1 (Fig. 2A). A third of the nAbs neutralized a tier 2 virus from the same virus clade that infected MG505 (Q842.d16, clade A, ∼91% nucleotide identity to autologous Envs) (Fig. 2A and 3). Only one nAb, MG505.147, demonstrated low-potency cross-clade neutralizing activity against tier 2 virus QB857.B3 (clade D, ∼80% nucleotide identity to autologous Envs) (Fig. 3), which is a variant that is weakly neutralized by MG505 P31 plasma (IC50 of 188 reciprocal dilution) (Fig. 2A). Neither individual nAbs nor a pool of representatives of the 21 nAb families in a polyclonal mixture was able to recapitulate the full breadth of the plasma (data not shown).
FIG 3.
Phylogenetic tree of G505 Envs. Maximum likelihood phylogenetic tree of maternal (MG505)-and infant (BG505)-derived Envelope (Env) variants. Maternal variants were isolated at the time of delivery (W0, blue); BG505 Env variants were isolated at 6 weeks of age (W6, green), when the infant was detected to be HIV positive. Heterologous Envs used in neutralization and RF-ADCC assays are included with viral clade indicated (red), as well as representative subtype reference Envs from clades A, B, C, and D (black). The tree is rooted in the clade B HXB2 reference sequence. Units for branch length estimates are nucleotide substitutions per site.
Since none of the isolated MG505 nAbs recapitulated the breadth and potency of the plasma, we performed serum mapping of MG505 P31 plasma to determine if there was a known antibody specificity that could be determined using this approach. Serum mapping of MG505 P31 nAbs using pseudotyped Env mutants targeting known epitopes revealed little in the way of dominant antibody signatures (Fig. 2B). Specifically, we observed no evidence that the disruption of the epitopes of the membrane-proximal external region (MPER), the CD4-binding site (CD4-bs), or the C2 outer domain of gp120 (N276) antibodies affected the neutralization of MG505 plasma (Fig. 2B). We also found no evidence that the disruption of the epitopes for glycan-dependent V1/V2 (N160), V3 (N301/N332), or interface PGT151-like antibodies (N611) resulted in the loss of neutralization, indicative of the presence of antibodies to those epitopes. Rather, we found that the removal of those glycans resulted in enhanced neutralization sensitivity, suggesting that removal of the glycan exposed a key epitope for the MG505 antibodies. This effect was somewhat strain dependent, as evidenced by the differences in fold change for N160, where the effect was most pronounced in the context of SF162 (Fig. 2B).
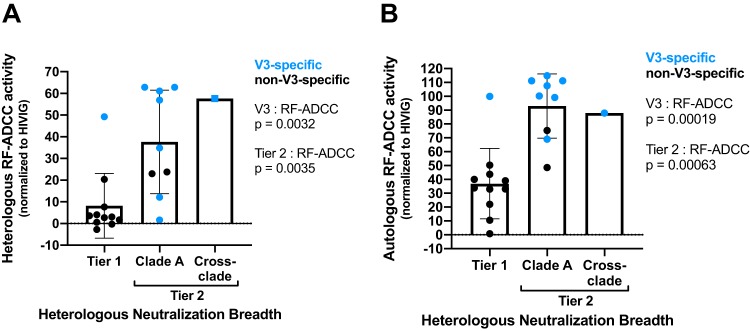
ADCC functionality is associated with V3 epitope specificity and tier 2 heterologous neutralization in MG505 nAbs.
Since we previously found that maternal plasma ADCC activity, as measured by the rapid fluorometric ADCC (RF-ADCC) assay, correlated with reduced mortality in HIV-infected infants (14), we further selected one representative nAb from each clonal family to test for RF-ADCC activity. We tested against BL035 gp120, a clade A virus isolated from the same cohort as MG505 and BG505 (∼82% nucleotide identity to autologous Envs) (Fig. 3), which we previously determined to yield the highest correlation with average RF-ADCC activities for diverse gp120 proteins across subtypes A, B, C, and D (14). In addition to tier 1 HIV neutralization, 11 of 21 families were capable of >10% RF-ADCC activity against heterologous BL035 gp120, with values ranging from 12 to 63%. Interestingly, many more of these MAbs (20 of 21) mediated ADCC against the autologous BG505 gp120; there was only one MAb that did not mediate ADCC against BG505 gp120, and it also did not mediate ADCC against BL035 gp120 (Fig. 2A).
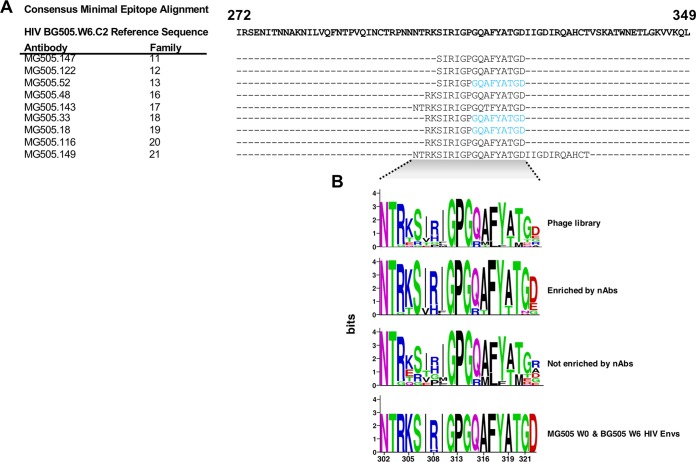
To map epitope specificities of each clonal nAb family, we used a combination of phage immunoprecipitation sequencing (PhIP-seq) (25) and enzyme-linked immunosorbent assays (ELISAs) (Fig. 2A and 4). For PhIP-seq, we utilized a previously described phage library (25) that contains multiple HIV Env sequences: consensus sequences for clades A, B, C, and D and specific sequences circulating in Kenya, including the transmitted BG505.W6.C2 virus. For 9 of the 21 nAb families, phage-displayed peptides were significantly enriched within the V3 region of HIV Envelope (spanning positions 302 to 322, based on HXB2 numbering), suggesting that this region of HIV Env comprises a key part of the epitope of these isolated nAbs (Fig. 4A). Of note, in the case of MG505.149, only a small number of peptides were significantly enriched, which likely led to the lengthening of the minimal epitope sequence defined for this nAb through to position 332. Because of this, we have less confidence in the minimal peptide target of this nAb. For three of the nAbs tested (MG505.18, MG505.33, and MG505.52), we observed weak but significant enrichments of a peptide that truncated the minimal epitope sequence, suggesting this is a core part of the epitope (Fig. 4A, blue residues).
FIG 4.
PhIP-seq analysis of nAb family representatives. (A) Sequence alignment of the minimal consensus epitopes identified by PhIP-seq for each tested nAb. See Fig. S1 for all peptides significantly enriched and not enriched for each tested nAb. Residues in blue signify where the minimal epitope was extended in cases where there was weak but significant enrichment of a peptide that truncated the minimal epitope sequence. (B) Logo plot of sequences corresponding to the minimal epitope region (HIV Env V3) present in the phage library, sequences that were significantly enriched or not by tested nAbs, and MG505 W0 and BG505 W6 Env sequences.
Since the library included sequences of several different HIV-1 variants, our PhIP-seq data also allowed us to gain some insight into which amino acids were preferred at highly variable residues within the library sequences. Interestingly, while we observed some variation in V3 peptide enrichment among different nAbs, there were cases in which peptides with residues at certain positions were consistently enriched while other peptides spanning the same sequence were not enriched. For example, while the phage library contains peptides with amino acids K, T, E, and Q at position 305, the epitope-mapped antibodies only enriched for peptides with K and T at this position (Fig. 4B; see also Fig. S1 in the supplemental material). Another example was at positions 321 to 322, where G and D were enriched, respectively (Fig. 4B and Fig. S1). Of note, in all three cases these preferentially enriched peptides were the same amino acid residues found in the autologous viruses from both MG505 and BG505 Envs (Fig. 4B). In all nine cases, the V3 peptides that bound to the nAbs were identical to the BG505.W6.C2 Env V3 sequence (Fig. S1), which is also identical to the V3 regions of six of seven MG505 W0 Env sequences (Fig. 4B) (1).
These V3-targeting families exclusively employed heavy-chain gene VH1-69-2, VH5-10-1, or VH5-51 (Fig. 2A). High heterologous ADCC activity against BL035 gp120 was associated with both V3 specificity (P = 0.0032) and tier 2 Env neutralization (P = 0.0035) (Fig. 5A). Similar associations were observed for autologous ADCC antibody activity (Fig. 5B).
FIG 5.
Relationships between nAb functionalities. (A) Heterologous RF-ADCC activity against clade A BL035 gp120 (y axis), neutralization (bins), and V3 specificity (blue). (B) Autologous RF-ADCC activity against BG505 gp120 (y axis), neutralization (bins), and V3 specificity (blue). Nonparametric two-tailed Wilcoxon tests were performed to calculate P values.
The remaining 12 nAb family representatives that were tested by PhIP-seq did not significantly enrich for any phage in the library, suggesting that they target conformational epitopes that cannot be detected in the 39-mer peptides expressed by phage in the library. To broadly map the epitope specificities of these 12 non-V3-specific nAbs, we employed ELISAs using gp120 and gp41 antigens. Eleven of twelve families bound gp120 monomer (ZM109) by ELISA; none bound the gp41 ectodomain (C.ZA.1197MB) (Fig. 2A). The single antibody representing family 14, MG505.141, did not bind either monomeric form of HIV Env.
V3-specific nAbs bound, but only rarely neutralized, autologous HIV-1 viruses.
Given the relevance of autologous virus neutralization to the prevention of vertical transmission in the context of MTCT, we next tested the nAbs for their abilities to neutralize seven nearly contemporaneous maternal autologous viruses from the time of birth (W0), which occurred 1 week after the antibody isolation time point (P31). We also tested three >99.5% identical infant viruses from the time at which infant HIV infection was first detected (W6). The MG505 P31 plasma neutralized only four autologous viruses, MG505.W0.C2, -D1, -G2, and -H3, and there was no detectable neutralization of the vertically transmitted viruses tested (Fig. 6A). We found that only the V3-specific nAb families neutralized MG505.W0.G2, which was the maternal virus most potently neutralized by the plasma (Fig. 6A). The nAbs did not neutralize any other autologous MG505 or vertically transmitted BG505 viruses.
FIG 6.
Autologous virus neutralization and binding by MG505 nAbs. (A) Neutralization IC50 values of autologous MG505 W0 Envs and vertically transmitted BG505 W6 Envs by nAb family representatives. Data are reported as averages from at least two independent experiments and displayed for the indicated pseudoviruses, as described for Fig. 2A. (B) Cell surface autologous Env binding by select nAb families. Data are displayed as the percentage of mock-subtracted Env-transfected cells bound by each nAb. Data are representative of three independent experiments. Green fill indicates V3 specificity; in gray are nAbs that do not target V3. VRC01 antibody was used as a positive binding control. SIV Env was used as a negative Env control. (C) Biolayer interferometry analysis of MG505.33 MAb (ligand, 8 μg ml−1) binding to BG505.SOSIP.664 T332N HIV Env trimer (analyte) at the indicated concentrations. The gray line shows 10E8 negative-control antibody at 1 μM. Data are representative of two independent experiments. KD, Kon, and Kdis are derived from the global best fit (purple) using a 1:1 model of ligand-analyte binding.
The V3 region of MG505.W0.G2 is identical to that of the other maternal and infant viruses, with the exception of MG505.W0.H3, which has an R310H substitution (Fig. 4B). To explore the possibility that the V3-specific nAbs bound the majority of the MG505 and BG505 variants, even though they did not neutralize them, we tested these nine families for Env binding via cell surface binding assays. Indeed, detectable binding was observed for all V3-specific nAbs to all tested maternal and infant autologous Envs expressed on the surface of cells (Fig. 6B). We also tested three gp120 nAbs that were not V3 specific, representative of the range of neutralization abilities across clonal families. These non-V3-specific nAbs also bound to cell surface-expressed Env, albeit at lower levels (Fig. 6B, shown in gray). Because cell surface-expressed Envs can include various forms of the Envelope protein in addition to the native Env trimer, we more specifically tested whether V3-specific binding, nonneutralizing antibodies could bind the trimeric form of Env. As shown in Fig. 6C, the V3-specific MG505.33, which displayed average binding to cell surface-expressed Env, bound to native-like BG505.W6.C2.T332N-SOSIP trimer by biolayer interferometry.
MG505 nAb families were at relatively low frequencies within the maternal B cell repertoire.
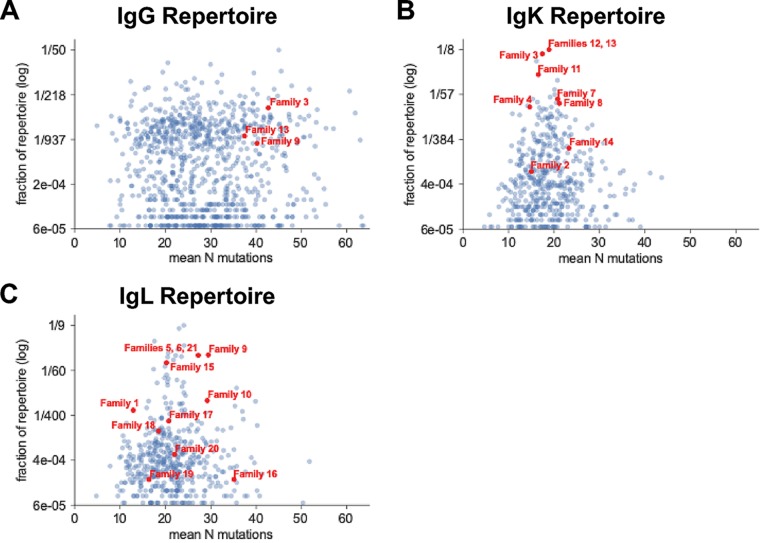
The relative frequencies of characterized HIV nAb families within a transmitting mother’s larger antibody repertoire are currently unstudied, although these statistics potentially are relevant to understanding which antibody traits permit or prevent MTCT. To better define the frequencies of our 21 nAb families within MG505’s B cell repertoire, we deeply sequenced antibody variable regions (26) from a second P31 MG505 PBMC sample (Table 1). Repertoire clonal family analysis identified IgG sequences clonally related to 3/21 functionally isolated nAb families: families 3, 9, and 13 (Fig. 7A). These nAb families ranked 65th, 400th, and 278th largest within the IgG repertoire, representing 0.24%, 0.08%, and 0.13% of the total repertoire, respectively. We did not sample any additional clonal IgG sequences stemming from the other 18 nAb families, indicating that family members of these nAbs are either absent or rare enough that we did not achieve sufficient sampling depth to detect them (Table 1).
TABLE 1.
MG505 P31 antibody-variable region deep-sequencing statisticsa
| MG505 time point | Live PBMC count (excluding nonviable) | PBMC viability(%) | Ab chain | No. of replicates | No. of raw MiSeq reads | No. of productive replicate-merged deduplicated sequences | Estimated sequencing coverage within (%): |
|
|---|---|---|---|---|---|---|---|---|
| Sampled blood PBMCs | MG505 P31 whole-body blood repertoire | |||||||
| P31 | 4.84E+06 | 97.78 | IgM | 1 | 351,376 | 168,116 | 43 | 0.09 |
| IgG | 1 | 891,354 | 595,016 | 320 | 0.71 | |||
| 2 | 795,589 | |||||||
| IgK | 1 | 222,689 | 479,405 | 83 | 0.18 | |||
| 2 | 686,922 | |||||||
| IgL | 1 | 815,155 | 661,269 | 114 | 0.25 | |||
| 2 | 529,489 | |||||||
Sequence read coverage was calculated for PBMCs using MG505 P31 B cell frequency statistics from the cell sort of the first sample aliquot from this time point, as reported in this study. Whole-body sequencing coverage was calculated assuming 10 ml of blood was sampled from a total blood volume of 4,500 ml.
FIG 7.
Clonal family analysis of the MG505 P31 B cell repertoire for IgG (A), IgK (B), and IgL (C) antibody variable regions. Each point represents a distinct clonal family, with red points indicating clonal families that contain functionally identified nAbs. Families smaller than 3 sequences were excluded from the plot.
Repertoire analysis is more nuanced for antibody light chains because of the vastly lower theoretical diversity due to the absence of D genes, shorter CDR3 lengths, and shorter nontemplated insertions. The resulting artifactual superfamilies reflect examples of preferred light-chain usage, where many families with distinct heavy chains have selected the same light chain. Regarding our nAb light chains, we identified IgK or IgL sequences apparently clonally related to all 21 nAb families, with many families using preferred gene rearrangements that appear as large superfamilies (Fig. 7B and C). One example of a superfamily that can be identified unambiguously is the largest family in our kappa analysis, representing 9.85% of the kappa repertoire, which contains members of both nAb families 12 and 13, although we know these are clonally distinct families based on their paired heavy chains. This effect can also be seen in Fig. 7, where the three nAb families found in the heavy-chain data appear as much larger fractions of the repertoire in the light-chain panels (Fig. 7B and C) than in the heavy-chain panel (Fig. 7A).
DISCUSSION
Since maternal HIV-targeting antibodies are present at the time of infant infection, MTCT provides a unique setting to explore whether antibodies play a role in determining HIV transmission and infection outcome. This study examines both the maternal B cell repertoire at the monoclonal antibody level and contemporaneous maternal autologous viruses at the time of MTCT, which, in combination, are clinically relevant to HIV transmission to infants. Here, we characterized 39 HIV-neutralizing monoclonal antibodies isolated just prior to vertical HIV transmission in a clade A-infected mother. These nAbs were diverse in their relative frequencies, specificities, and functions. Notably, V3 specificity, ADCC activity, and tier 2 heterologous HIV neutralization were associated, representing features that may be relevant to evaluating vaccine-induced HIV antibody responses in the future. Of note, while these V3-specific antibodies neutralized heterologous viruses, most did not neutralize autologous maternal or infant viruses, despite binding to the corresponding infant Env trimer.
The MG505 nAbs were isolated using a high-throughput functional screening method performed in technical replicate, which facilitated the discovery of SF162 strain HIV-neutralizing antibodies. This approach, like other commonly used approaches for isolating HIV nAbs, has strengths as well as biases (27). Part of the rationale for using a tier 1 virus for screening was that the majority of broadly neutralizing Abs (bnAbs) potently neutralize tier 1 viruses, including SF162; thus, it is a more sensitive screen for harder-to-detect activities using the functional screening approach. However, it is possible that using SF162 as the screening virus also biased the antibody discovery toward V3-targeting, ADCC-mediating antibodies. Our approach contrasts Env bait-based approaches, which tend to isolate antibodies of certain specificities and, thus, are particularly powerful when there is a dominant response to a known epitope (27). We found that in studies of polyclonal responses, many important activities can be missed with this approach (28), further highlighting that there are limitations to any given approach.
Despite the potential limitations of the screening approach, the diversity of gene usage within the resulting 39 nAbs was striking, especially considering that the screen was not saturating and that there were likely additional families that contributed to the breadth of the MG505 response. Also striking was the fact that we isolated multiple members of 10 clonal families despite their relative rarity in the repertoire, as was indicated by our failure to find additional clonal members of most of these families by deep sequencing. Despite sampling a diverse set of nAbs, including low-frequency families, we did not recapitulate the MG505 P31 plasma breadth for heterologous HIV-1 neutralization. Thus, additional nAbs, either from undiscovered families or other clones from families already identified here, likely contributed to the MG505 plasma response. It is possible that (i) the bnAb lineages responsible for the breadth are exceedingly rare populations and we missed them, (ii) the antibody-secreting cells (ASCs) and memory cells of the presumed bnAb lineages were not localized to the blood, (iii) complex germinal center dynamics resulted in functional differences between the circulating blood memory B cells that we sampled and the plasma antibody-contributing ASCs from the same bnAb lineage, or (iv) that the SF162-based functional screening approach we used failed to capture relevant antibodies. Regardless, the maternal HIV-neutralizing Ab lineages we identified represented <0.25% of the total IgG repertoire. Although relative frequencies of individual nAb lineages are not yet commonly reported, one study reported a value comparable to those we found: adult-derived Protocol C laboratory code DN HIV-1 bnAb lineage comprised up to 0.8% of the adult’s repertoire at 2 years postinfection (29). However, it is important to note that antibody lineage frequencies are known to vary greatly across time in humans (30), and that most repertoire sampling, including our own, is limited to blood samples and excludes bone marrow and other important sites of B cell residence.
We used a novel phage immunoprecipitation approach to map the epitopes of these antibodies and found that 9 of the 21 families recognized a linear epitope in V3 that includes the GPGQ sequence that is a common target for V3-specific nAbs (31, 32). These V3-specific nAbs were capable of tier 2 heterologous clade A virus neutralization, autologous ADCC activity, and heterologous clade A ADCC activity, although the tier 2 neutralization was limited to Q842.d16, which shares ∼91% nucleotide identity with autologous Envs and differs by 1 to 2 amino acids in the targeted V3 epitope. There is some evidence that V3-specific nAbs play a role in MTCT, although the results are not consistent across studies and cohorts. V3 specificity was associated with protection in the clade B Women and Infant Transmission Study (8, 9) but not in the clade C Breastfeeding and Nutrition study (12). Based on evidence suggesting that V3-targeting antibodies can select virus escape mutants and drive neutralization resistance in the autologous virus reservoir (33), it has been proposed that maternal V3-specific nAbs drive selection of neutralization-resistant transmitted viruses in MTCT (7). Here, we found that while the V3-specific nAbs bound to all autologous viruses tested, they only neutralized one out of seven variants, indicating that the autologous virus reservoir was mostly resistant to maternal V3-specific antibodies present near the time of transmission.
All but one of the nAbs were capable of mediating ADCC against gp120 from the BG505 transmitted virus, but only a subset mediated ADCC activity against a heterologous clade A envelope, BL035, which shares 82% nucleotide identity. The most potent heterologous ADCC-mediating nAbs were all V3 specific. The ability to mediate ADCC was associated with both V3 specificity and the ability to neutralize a tier 2 virus. It is possible that V3 specificity simply enables tier 2 breadth and ADCC function, especially since V3-specific antibodies can exhibit weak breadth for tier 2 viruses due to the viruses’ sampling of the open conformational states that allow for V3 binding (34). Given that ADDC activity has been correlated with infant outcomes (14), these highly potent maternal ADCC nAbs may provide clues to the mechanisms of protection of ADCC antibodies.
It was surprising that the V3-specific nAbs only neutralized one maternal variant, despite all maternal and infant viruses having identical sequences within the V3 minimal epitope. It is possible that V3 defines only part of the epitope for these nAbs and/or that factors other than minimal epitope binding affect pseudovirus neutralization, such as occlusion of V3 in the native trimer (35). Despite the lack of autologous virus neutralization, we could detect binding to the cell surface-expressed forms of Env of these same viruses. We also detected binding to the BG505 infant Env SOSIP trimer but not neutralization of the corresponding virus. The single difference between the BG505 Envs used in the binding versus neutralization studies is that the native-like SOSIP had an additional glycan at site 332, but this site is outside the minimal V3 epitope as defined by PhIP-seq. Overall, these studies support the findings of our previous studies of nAbs from infant BF520, which showed that trimer binding and neutralization are not always linked (28).
This study of maternal monoclonal antibodies provides additional context to our previous studies concerning the nature of the transmission bottleneck that existed between MG505 and BG505 at the time of vertical transmission. We previously reported that there was no detectable neutralization of three early (W6) transmitted infant Env variants, also used in this study, by maternal plasma, suggesting that the BG505 transmitted virus could have been an escape variant from maternal nAbs (1). Conversely, the seven maternal W0 Env variants isolated at the time of birth, also used in this study, varied in their susceptibility to contemporaneous maternal plasma. Interestingly, BG505 was born prematurely, which could have resulted in lower levels of passively transferred nAbs and, thus, less antibody selective pressure, although it should not have affected the specificity of the antibodies present (36). Our subsequent studies demonstrated that the transmitted BG505 Envs all were susceptible to a panel of bnAbs with known specificities, including the CD4 binding site, V3 glycan, and V2 apex, which, given the transmitted virus was a neutralization escape variant, imply that these specificities were not dominant in MG505 (37).
Consistent with BG505 transmitted virus being an escape variant from MG505 neutralization, our mapping studies of MG505 plasma indicate that the removal of some glycans in Env at N160, N301, and N611 in certain viral strains enhances neutralization susceptibility of the virus by MG505 plasma. This is similar to prior studies that have reported several of these glycan sites (N160 and N301) as global epitopes that cause an increase in neutralization sensitivity when they are removed (38, 39). These findings suggest that one of the main contributors to the MG505 plasma response is antibodies that recognize epitopes that are either proximal to or occluded by glycans present in Env. Reminiscent of the broadly neutralizing antibody N123-VRC34.01, which targets the fusion peptide and glycan N88, we observed that the removal of the glycan at site 611 resulted in an increase in neutralization sensitivity of MG505 plasma (40). However, because we did not fully recapitulate the antibodies contributing to the MG505 response, we cannot directly address the interesting possibility that responses that target a glycan-occluded epitope have shaped the antigenicity of the transmitted BG505 Env (37).
In the effort to devise an effective HIV-1 vaccine, it is important to understand and target for elicitation protective traits of polyclonal antibody responses that can prevent infection in naive individuals. Maternal antibodies may provide important insights in the context of MTCT, where only a subset of infants become infected and those that do are often infected with a virus that has escaped maternal antibody pressure. The diverse monoclonal nAb responses described here, in the setting of MTCT, may provide context for defining the features of antibodies that succeed versus fail at mediating protection against HIV infection.
MATERIALS AND METHODS
Human plasma and PBMC samples.
Plasma and peripheral blood mononuclear cell (PBMC) samples were from mother MG505, enrolled in the Nairobi Breastfeeding Clinical Trial (41), which was conducted prior to the use of antiretrovirals for the prevention of mother-to-child transmission. The infecting virus was clade A based on envelope sequence (1). Approval to conduct the Nairobi Breastfeeding Clinical Trial was provided by the ethical review committee of the Kenyatta National Hospital Institutional Review Board and the University of Washington Institutional Review Board.
B cell sorting.
A PBMC sample from MG505 from 31 weeks of pregnancy (P31) was thawed as previously described (28). Cells were stained on ice for 30 min using a cocktail of anti-CD19-BV510, anti-IgD-fluorescein isothiocyanate (FITC), anti-IgM-FITC, anti-CD3-BV711, anti-CD14-BV711, and anti-CD16-BV711. Cells then were washed once and resuspended in fluorescence-activated cell sorting (FACS) wash (1× phosphate-buffered saline [PBS], 2% fetal bovine serum [FBS]). Cells were loaded onto a BD FACS Aria II cell sorter. The gating strategy was such that memory B cells (CD3− CD14− CD16− CD19+ IgD− IgM−) were sorted into B cell medium (Iscove’s modified Dulbecco’s medium [GIBCO], 10% heat-inactivated low-IgG FBS [Life Technologies], 5 ml GlutaMAX [Life Technologies], 1 ml MycoZap plus PR [Lonza]). Immediately following the sort, memory B cells were plated at 6 B cells in 55 μl per well into 96- by 384-well plates in B cell medium supplemented with 100 U ml−1 interleukin-2 (IL-2; Roche), 50 ng ml−1 IL-21 (Invitrogen), and 1 × 105 cells ml−1 irradiated 3T3-CD40L feeder cells (ARP 12535). Cultured B cells were incubated for 12 days at 37°C in a 5% CO2 incubator based on the protocol by Huang et al. (42).
B cell culture harvest, microneutralization assay, and reconstruction of antibodies.
On day 12, B cell culture supernatants were divided into 2- by 384-well plates at 20 μl each for neutralization assays using a Tecan automated liquid handling system. B cells were frozen at −80°C in 20 μl RNA storage buffer per well. Microneutralization assays were performed as previously described (28), with one virus in technical replicate (tier 1 clade B SF162). Wells demonstrating neutralization within the 96th percentile in both replicate assays were selected for antibody gene amplification and cloning. Additional wells of interest were subjectively identified by eye, taking into account well position on the plate and surrounding background signal from negative wells. Reverse transcription-PCR amplification of IgG heavy- and light-chain variable regions was performed using previously described methods (28). Functional heavy- and light-chain variable region sequences were determined using IMGT V-QUEST (43). All nAbs were of IgG1 subclass based on 5′ constant region sequencing. Functional variable region sequences were cloned thusly into corresponding IgG1, IgK, and IgL expression vectors as previously described (28). In parallel, B cell RNA was sent to Atreca (https://www.atreca.com) for deep sequencing of the antibody heavy- and light-chain variable regions from each well of interest. In 11 cases where additional heavy- and/or light-chain sequences were amplified by the Atreca method beyond what we had already identified, the variable chains were synthesized as fragmentGENEs by Genewiz and subsequently cloned into expression vectors. The Freestyle MAX system (Invitrogen) was used to cotransfect paired heavy- and light-chain plasmids cloned from the same well, and IgG was purified as described previously (44). For each well, all possible heavy- and light-chain pairs were generated if more than one antibody was sequenced from the well.
Pseudovirus production and neutralization assays.
Methods for pseudovirus production using envelope-deficient proviral Q23Δenv backbone and TZM-bl-based neutralization assays were previously described (45). Plasma IC50 values are the reciprocal plasma dilution resulting in a 50% reduction of virus infectivity, while monoclonal antibody IC50 values represent the MAb concentration (in micrograms per milliliter) at which 50% of the virus was neutralized. Reported IC50 values are an average from at least two independent experiments performed in duplicate. If values from the two experiments disagreed by more than 3-fold, a third experiment was done and all data were averaged.
Serum mapping of MG505 plasma.
CD4-binding site mapping of MG505 plasma was performed as previously described (46, 47), using resurfaced core protein (RSC3) and a CD4-bs-defective mutant (RSC3Δ371I). These proteins were obtained via the AIDS Research and Reference Reagent Program, NIAID, NIH, from Zhi-Yong Yang, Peter Kwong, Gary Nabel, and John Mascola (numbers 12042 and 12043) (48, 49). Binding ELISAs were performed using these coat proteins at 200 ng/ml in the same manner as that detailed below. ELISA plates were incubated with 2-fold dilutions of heat-inactivated MG505 P31 plasma starting at a 1:100 dilution.
Point mutants N160K, K169E, N276A, N301A/N334A, N332A, and N611A were made previously using site-directed mutagenesis (QuikChange II; Agilent Technologies) and verified by sequencing (46, 47). At least two replicates were performed using each point mutant and the respective wild-type virus prior to calculating fold change in IC50 value (WT versus mutant), as depicted in Fig. 2B.
MPER mapping was performed using a chimera made from HIV-2 strain 7312A and the HIV-1 subtype B strain YU-2 MPER engrafted virus (7312C1) (50). Two replicates of the neutralization assay were performed with the wild-type full-length 7312A virus and 7312C1 chimera before calculating the average IC50 values and subsequent fold change (WT versus mutant).
Phylogenetic comparison of assay Envs to MG505 and BG505 Envs.
Env gp160 sequences were aligned with HIVAlign (https://www.hiv.lanl.gov) (51), using HMM-align and codon alignment. The phylogeny was generated using PhyML (52), with the GTR substitution model, BioNJ trees, a bootstrap of 100 replicates for branch support, and HXB2 (clade B reference) as the root. Units for branch length estimates are nucleotide substitutions per site. The following GenBank accession numbers were used for assay Envs: FJ866138.1 (QB857.B3), AY835445.1 (TRO.11), FJ866133.1 (QC406.f3), DQ435683.1 (CAP210.e8), DQ411854.1 (DU422.1), DQ411853.1 (DU172.17), FJ866139.1 (QD435.A4), EU123924.1 (SF162), DQ208480.1 (BL035), AF407162.1 (Q842.d16), and AF407155.1 (Q461.D1). Subtype reference alignments (https://www.hiv.lanl.gov) were used as representative HIV-1 Envs from clades A, B, C, and D.
ELISA.
Immunolon 2HB ELISA plates were coated with 1 μg ml−1 ZM109 gp120 monomer or C.ZA.1197MB gp41 ectodomain (Immune Technology Corp.) in 0.1 M sodium bicarbonate, pH 9.4, overnight at 4°C. Wells were washed four times with 300 μl wash buffer (1× PBS, 0.05% Tween 20) and blocked for 1 h at room temperature (RT) in blocking buffer (1× PBS with 10% nonfat milk and 0.05% Tween 20). MG505 nAbs and control antibodies (gp120-specific VRC01 [48] and gp41-specific QA255.006 [25]) were applied at 10 μg ml−1 in blocking buffer and incubated at 37°C for 2 h. Wells were washed and incubated with goat anti-human IgG-horseradish peroxidase (Sigma) diluted 1:2,500 in blocking buffer for 1 h at RT. After washing, TMB-ELISA solution (Pierce) was added for 10 min at RT and then stopped with equal volumes of 1N sulfuric acid. Absorption was read at 450 nm, and positive binding was assessed based on blank-subtracted duplicate average absorbance values of at least two times that of the negative control.
RF-ADCC assay.
The RF-ADCC assay was performed as described previously (25, 53). In short, CEM-NKr cells (AIDS Research and Reference Reagent Program, NIAID, NIH, from Alexandra Trkola) were double labeled with PKH-26-cell membrane dye (Sigma-Aldrich) and a cytoplasmic staining dye (Vybrant CFDA SE cell tracer kit; Life Technologies). The double-labeled cells were coated with a clade A gp120 (BL035.W6M.Env.C1; Immune Technology Corp. [1]) or autologous BG505 gp120 (BG505.W6M.Env.C2; Cambridge Biologics [1]) for 1 h at RT at a ratio of 1.5 μg protein to 1 × 105 double-stained target cells. Coated targets were washed once with complete RMPI medium (Gibco) supplemented with 10% FBS (Gibco), 4.0 mM GlutaMAX (Gibco), and 1% antibiotic-antimycotic (Life Technologies). Monoclonal antibodies were diluted in complete RPMI medium to a concentration of 500 ng/ml and mixed with 5 × 103 coated target cells for 10 min at RT. PBMCs (Bloodworks Northwest) from an HIV-negative donor then were added at a ratio of 50 effector cells per target cell. The coated target cells, antibodies, and effector cells were cocultured for 4 h at 37°C and then fixed in 1% paraformaldehyde (Affymetrix). Cells were analyzed by flow cytometry (LSR II; BD), and ADCC activity was defined as the percentage of PKH-26+ CFDA− cells with background subtracted, where background (antibody-mediated killing of uncoated cells) was between 3 to 5%, as analyzed using FlowJo software (Tree Star). All values were normalized to HIVIG (positive control) activity.
Phage display immunoprecipitation sequencing.
To precisely map the epitopes of antibodies in this study, we employed an approach that combines phage display, immunoprecipitation, and highly multiplexed sequencing, as previously described (25, 54). In brief, amplified phage (1 ml at 2 × 105-fold representation of each phage clone) that displays peptides from several Envelope and full-length HIV sequences was added to each well of a 96-deep-well plate (CoStar). Two concentrations (2 ng/ml and 20 ng/ml) of each monoclonal antibody then were added to phage in technical replicate, with the exception of MG505.52, which was tested at one concentration (20 ng). Phage subsequently were immunoprecipitated and prepared for highly multiplexed sequencing, as previously described (25).
Bioinformatics analysis of sequencing data was performed using a zero-inflated generalized Poisson significant enrichment assignment algorithm to generate a −log10(P value) for enrichment of each phage clone across all samples, as previously described (25). Of note, the −log10(P value) reproducibility threshold when testing these antibodies in PhIP-Seq was 2.3. Thus, we considered a phage-displayed peptide as significantly enriched if its −log10(P value) was ≥2.3 in both technical replicates. A phage-displayed peptide was considered part of the antibody’s epitope sequence only if it was significantly enriched under both conditions tested (2 ng and 20 ng). Fold enrichment of each phage-displayed peptide was also calculated across all monoclonal antibodies tested.
Phage that were incubated without any monoclonal antibody served as a negative control for nonspecific binding of phage and were used to identify and eliminate background hits. For each monoclonal antibody tested, enriched and unenriched peptides were aligned using Clustal Omega. The minimal epitope of an antibody was defined as the shortest amino acid sequence present in all of the enriched peptides. Logo plots were generated using WebLogo (55). For the “phage library” and “not enriched by nAbs” logo plots, only peptides that spanned the full length of the minimal epitope (at least from S308 through D322) were included.
Analysis of RF-ADCC association with heterologous neutralization tier and V3 specificity.
Nonparametric two-tailed Wilcoxon statistical tests were performed using R, version 3.5.1, via the command wilcox.test().
Cell surface Env binding assays.
Binding to cell surface-expressed Env was measured using a flow cytometry-based assay (56). 293T cells (5 × 105 cells) were transfected with the indicated 1.33 μg HIV-1 env DNA and 2.66 μg Q23Δenv using Fugene6 (Promega), harvested 48 h posttransfection, and incubated with 20 mg ml−1 MAb. Cells were washed and incubated with a 1:100 dilution of goat-anti-human IgG-phycoerythrin (PE) (Jackson ImmunoResearch), washed and fixed with 1% paraformaldehyde, and processed by flow cytometry using a BD FACS-Canto II. Data were analyzed using FlowJo software. Percent binding was calculated as the percentage of PE-positive cells with background subtracted (MAb binding to cells transfected without env typically was 0.2 to 2%). Analyses were performed in GraphPad Prism 8.
Biolayer interferometry.
MG505 monoclonal antibody binding to HIV Env SOSIP trimer was measured using biolayer interferometry on an Octet RED instrument (ForteBio). Antibodies diluted to 8 μg ml−1 in a filtered buffer solution of 1× PBS containing 1% bovine serum albumin, 0.03% Tween 20, and 0.02% sodium azide were immobilized onto anti-human IgG Fc capture biosensors (AHC). BG505.SOSIP.664 T332N was diluted to 1 μM in the same buffer as that described above, and a series of four 2-fold dilutions of Env trimer were tested as analytes in solution at a shake speed of 600 rpm at 30°C. The kinetics of MAb binding was measured with the following steps: association was monitored for 10 min, dissociation was monitored for 6 min, and regeneration was performed in 10 mM glycine HCl (pH 1.5). Binding affinity constants (KD; on rate, Kon; off rate, Kdis) were calculated using ForteBio’s Data Analysis Software 7.0. Responses (nanometer shift) were calculated using data that were background subtracted from reference wells and processed by Savitzky-Golay filtering, prior to fitting using a 1:1 model of binding kinetics.
PBMC RNA isolation for antibody sequencing.
PBMCs stored in liquid nitrogen for ∼20 years were thawed at 37°C, diluted 10-fold in prewarmed RPMI, and centrifuged for 10 min at 300 × g. Cells were washed once in phosphate-buffered saline, counted with trypan blue, and centrifuged again, and total RNA was extracted from PBMCs using the AllPrep DNA/RNA minikit (Qiagen) according to the manufacturer’s recommended protocol. RNA was stored at –80°C until library preparation. Library preparation, sequence analysis, and antibody lineage reconstruction were performed in technical duplicate, using the same RNA isolated from the MG505 P31 time point.
Antibody gene variable region sequencing.
Antibody sequencing was performed as previously described (26). Briefly, rapid amplification of cDNA ends (RACE)-ready cDNA synthesis was performed using the SMARTer RACE 5′/3′ kit (TaKaRa Bio USA) using primers with specificity to IgM, IgG, IgK, and IgL. cDNA was diluted in tricine-EDTA according to the manufacturer’s recommended protocol. First-round Ig-encoding sequence amplification (20 cycles) was performed using Q5 high-fidelity master mix (New England BioLabs) and nested gene-specific primers, as previously reported (57). Amplicons were directly used as templates for MiSeq adaption by second-round PCR amplification (20 cycles), purified, analyzed by gel electrophoresis, and indexed using Nextera XT P5 and P7 index sequences for Illumina sequencing according to the manufacturer’s instructions (10 cycles). Gel-purified, indexed libraries were quantitated using the KAPA library quantification kit (Kapa Biosystems) performed on an Applied Biosystems 7500 Fast real-time PCR machine. Libraries were denatured and loaded onto Illumina MiSeq 600-cycle V3 cartridges according to the manufacturer’s suggested workflow.
Antibody repertoire sequence analysis and clonal family clustering.
Sequences were preprocessed using FLASH, cutadapt, and FASTX-toolkit as previously described (26, 57). Sequences from both technical replicates were combined, deduplicated, and annotated with partis (https://github.com/psathyrella/partis) using default options, including per-sample germ line inference (58–60). Sequences with internal stop codons or with out-of-frame CDR3 regions were removed during this step. In an attempt to retain even very rare or undersampled sequences, we did not exclude singletons. Sequencing run statistics are detailed in Table 1. Antibody sequences were merged with the 78 functionally identified MG505 nAb heavy- and light-chain sequences to form a single comprehensive MG505 P31 antibody sequence data set. This data set then was used for clonal family analysis using both the partis unseeded and seeded clustering methods (60). For the unseeded repertoire analysis, since we were interested only in relative properties of clonal families, each data set was subsampled for computational efficiency. For each data set, three random subsamples of 50,000 sequences were analyzed, comparing results among the three to ensure that they were large enough to minimize statistical uncertainties. No subsampling was necessary for the seeded analysis.
Neutralizing antibody sequence analysis.
Heavy- and light-chain nAb sequences (i.e., seeds) were annotated, analyzed, and clustered into clonal families with the MG505 P31 NGS sequences using the partis seeded clustering method on the non-downsampled replicate-merged NGS data set described above. nAb clusters were delineated for Fig. 2A using IgH chain variable region clustering information. Percent SHM was calculated as the mutation frequency at the nucleotide level compared to the predicted naive allele, as determined by the per-subject germ line inference for MG505.
Data availability.
The MG505 P31 antibody deep-sequencing data sets generated and analyzed during the current study (Fig. 7) are publicly available under BioProject SRA accession PRJNA562912 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA562912). Maternal nAb sequences reported in this paper have GenBank accession numbers MN395490 to MN395567. The GenBank accession numbers for the maternal MG505 W0 and infant BG505 W6 HIV-1 Envs utilized in this paper are DQ208449 to DQ208455 and DQ208456 to DQ208458 (1).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Guy Cavet and Atreca, Inc., for their help with gene amplification and cloning of B cell RNA that resulted in nAb sequences. We thank Brian Oliver, Megan Stumpf, Sonja Danon, and Vrasha Chohan for technical assistance with next-generation sequencing library preparation, antibody production, and neutralization assays.
This work was supported by NIH grant R01 AI120961, by training awards T32 AI07140, F30 AI122866, and F30 AI142870, and by two new investigator awards (P30 AI027757). The research of F.A.M. was supported in part by a Faculty Scholar grant from the Howard Hughes Medical Institute and the Simons Foundation. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wu X, Parast AB, Richardson BA, Nduati R, John-Stewart G, Mbori-Ngacha D, Rainwater SM, Overbaugh J. 2006. Neutralization escape variants of human immunodeficiency virus type 1 are transmitted from mother to infant. J Virol 80:835–844. doi: 10.1128/JVI.80.2.835-844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickover R, Garratty E, Yusim K, Miller C, Korber B, Bryson Y. 2006. Role of maternal autologous neutralizing antibody in selective perinatal transmission of human immunodeficiency virus type 1 escape variants. J Virol 80:6525–6533. doi: 10.1128/JVI.02658-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliks SC, Wara DW, Landers DV, Levy JA. 1994. Features of HIV-1 that could influence maternal-child transmission. JAMA 272:467–474. doi: 10.1001/jama.1994.03520060067034. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Smith CEP, Giorgi EE, Eudailey J, Martinez DR, Yusim K, Douglas AO, Stamper L, McGuire E, LaBranche CC, Montefiori DC, Fouda GG, Gao F, Permar SR. 2018. Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma. PLoS Pathog 14:e1006944. doi: 10.1371/journal.ppat.1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouda GG, Mahlokozera T, Salazar-Gonzalez JF, Salazar MG, Learn G, Kumar SB, Dennison SM, Russell E, Rizzolo K, Jaeger F, Cai F, Vandergrift NA, Gao F, Hahn B, Shaw GM, Ochsenbauer C, Swanstrom R, Meshnick S, Mwapasa V, Kalilani L, Fiscus S, Montefiori D, Haynes B, Kwiek J, Alam SM, Permar SR. 2013. Postnatally-transmitted HIV-1 Envelope variants have similar neutralization-sensitivity and function to that of nontransmitted breast milk variants. Retrovirology 10:3. doi: 10.1186/1742-4690-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milligan C, Omenda MM, Chohan V, Odem-Davis K, Richardson BA, Nduati R, Overbaugh J. 2016. Maternal neutralization-resistant virus variants do not predict infant HIV infection risk. mBio 7:e02221-15. doi: 10.1128/mBio.02221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas AO, Martinez DR, Permar SR. 2017. The role of maternal HIV envelope-specific antibodies and mother-to-child transmission risk. Front Immunol 8:1091. doi: 10.3389/fimmu.2017.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez DR, Vandergrift N, Douglas AO, McGuire E, Bainbridge J, Nicely NI, Montefiori DC, Tomaras GD, Fouda GG, Permar SR. 2017. Maternal binding and neutralizing IgG responses targeting the C-terminal region of the V3 loop are predictive of reduced peripartum HIV-1 transmission risk. J Virol 91:e02422-16. doi: 10.1128/JVI.02422-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, Jaeger FH, Pollara J, Martelli A, Liebl BE, Lloyd K, Yates NL, Overman RG, Shen X, Whitaker K, Chen H, Pritchett J, Solomon E, Friberg E, Marshall DJ, Whitesides JF, Gurley TC, Von Holle T, Martinez DR, Cai F, Kumar A, Xia SM, Lu X, Louzao R, Wilkes S, Datta S, Sarzotti-Kelsoe M, Liao HX, Ferrari G, Alam SM, Montefiori DC, Denny TN, Moody MA, Tomaras GD, Gao F, Haynes BF. 2015. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Investig 125:2702–2706. doi: 10.1172/JCI81593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez DR, Kumar A, Tu JJ, Mangold JF, Mangan RJ, Goswami R, Giorgi EE, Chen J, Mengual M, Douglas AO, Heimsath H, Saunders K, Nicely NI, Eudailey J, Hernandez G, Morgan-Asiedu P, Wiehe K, LaBranche C, Montefiori DC, Gao F, Permar S. 3 May 2019. Maternal broadly neutralizing antibodies select for neutralization-resistant infant transmitted/founder HIV variants. Cell Rep doi: 10.2139/ssrn.3381953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diomede L, Nyoka S, Pastori C, Scotti L, Zambon A, Sherman G, Gray CM, Sarzotti-Kelsoe M, Lopalco L. 2012. Passively transmitted gp41 antibodies in babies born from HIV-1 subtype C-seropositive women: correlation between fine specificity and protection. J Virol 86:4129–4138. doi: 10.1128/JVI.06359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutucumarana CP, Eudailey J, McGuire EP, Vandergrift N, Tegha G, Chasela C, Ellington S, Horst C, Kourtis AP, Permar SR, Fouda GG, Edwards KM. 2017. Maternal humoral immune correlates of peripartum transmission of clade C HIV-1 in the setting of peripartum antiretrovirals. Clin Vaccine Immunol 24:e00062-17. doi: 10.1128/CVI.00062-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naiman NE, Slyker J, Nduati R, Overbaugh JM. 2019. Maternal envelope gp41 ectodomain-specific antibodies are associated with increased mother-to-child transmission of HIV-1. J Infect Dis 221:232–237. doi: 10.1093/infdis/jiz444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. 2015. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe 17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rousseau CM, Nduati RW, Richardson BA, John-Stewart GC, Mbori-Ngacha DA, Kreiss JK, Overbaugh J. 2004. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis 190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, Seaton KE, Deal A, Edwards RW, Tegha G, Kamwendo D, Kumwenda J, Nelson JA, Liao HX, Brinkley C, Denny TN, Ochsenbauer C, Ellington S, King CC, Jamieson DJ, van der Horst C, Kourtis AP, Tomaras GD, Ferrari G, Permar SR. 2015. Association of HIV-1 envelope-specific breast milk IgA responses with reduced risk of postnatal mother-to-child transmission of HIV-1. J Virol 89:9952–9961. doi: 10.1128/JVI.01560-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, de Val N, Kim HJ, Blattner C, de la Pena AT, Korzun J, Golabek M, de Los Reyes K, Ketas TJ, van Gils MJ, King CR, Wilson IA, Ward AB, Klasse PJ, Moore JP. 2013. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog 9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders RW, Moore JP. 2014. HIV: a stamp on the envelope. Nature 514:437–438. doi: 10.1038/nature13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward AB, Wilson IA. 2017. The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol Rev 275:21–32. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanders RW, Moore JP. 2017. Native-like Env trimers as a platform for HIV-1 vaccine design. Immunol Rev 275:161–182. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina-Ramirez M, Sanders RW, Sattentau QJ. 2017. Stabilized HIV-1 envelope glycoprotein trimers for vaccine use. Curr Opin HIV AIDS 12:241–249. doi: 10.1097/COH.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goo L, Chohan V, Nduati R, Overbaugh J. 2014. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat Med 20:655–658. doi: 10.1038/nm.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sagar M. 2010. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis 202(Suppl 2):S289–S296. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams KL, Stumpf M, Naiman NE, Ding S, Garrett M, Gobillot T, Vezina D, Dusenbury K, Ramadoss NS, Basom R, Kim PS, Finzi A, Overbaugh J. 2019. Identification of HIV gp41-specific antibodies that mediate killing of infected cells. PLoS Pathog 15:e1007572. doi: 10.1371/journal.ppat.1007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigdorovich V, Oliver BG, Carbonetti S, Dambrauskas N, Lange MD, Yacoob C, Leahy W, Callahan J, Stamatatos L, Sather DN. 2016. Repertoire comparison of the B-cell receptor-encoding loci in humans and rhesus macaques by next-generation sequencing. Clin Transl Immunol 5:e93. doi: 10.1038/cti.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCoy LE, Burton DR. 2017. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev 275:11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonich CA, Williams KL, Verkerke HP, Williams JA, Nduati R, Lee KK, Overbaugh J. 2016. HIV-1 neutralizing antibodies with limited hypermutation from an infant. Cell 166:77–87. doi: 10.1016/j.cell.2016.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLeod DT, Choi NM, Briney B, Garces F, Ver LS, Landais E, Murrell B, Wrin T, Kilembe W, Liang CH, Ramos A, Bian CB, Wickramasinghe L, Kong L, Eren K, Wu CY, Wong CH, IAVI Protocol C Investigators, IAVI African HIV Research Network, Kosakovsky Pond SL, Wilson IA, Burton DR, Poignard P. 2016. Early antibody lineage diversification and independent limb maturation lead to broad HIV-1 neutralization targeting the Env high-mannose patch. Immunity 44:1215–1226. doi: 10.1016/j.immuni.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horns F, Vollmers C, Dekker CL, Quake SR. 2019. Signatures of selection in the human antibody repertoire: selective sweeps, competing subclones, and neutral drift. Proc Natl Acad Sci U S A 116:1261–1266. doi: 10.1073/pnas.1814213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krachmarov C, Pinter A, Honnen WJ, Gorny MK, Nyambi PN, Zolla-Pazner S, Kayman SC. 2005. Antibodies that are cross-reactive for human immunodeficiency virus type 1 clade A and clade B v3 domains are common in patient sera from Cameroon, but their neutralization activity is usually restricted by epitope masking. J Virol 79:780–790. doi: 10.1128/JVI.79.2.780-790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorny MK, Williams C, Volsky B, Revesz K, Wang X-H, Burda S, Kimura T, Konings FAJ, Nádas A, Anyangwe CA, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S. 2006. Cross-clade neutralizing activity of human anti-V3 monoclonal antibodies derived from the cells of individuals infected with non-B clades of human immunodeficiency virus type 1. J Virol 80:6865–6872. doi: 10.1128/JVI.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moody MA, Gao F, Gurley TC, Amos JD, Kumar A, Hora B, Marshall DJ, Whitesides JF, Xia SM, Parks R, Lloyd KE, Hwang KK, Lu X, Bonsignori M, Finzi A, Vandergrift NA, Alam SM, Ferrari G, Shen X, Tomaras GD, Kamanga G, Cohen MS, Sam NE, Kapiga S, Gray ES, Tumba NL, Morris L, Zolla-Pazner S, Gorny MK, Mascola JR, Hahn BH, Shaw GM, Sodroski JG, Liao HX, Montefiori DC, Hraber PT, Korber BT, Haynes BF. 2015. Strain-specific V3 and CD4 binding site autologous HIV-1 neutralizing antibodies select neutralization-resistant viruses. Cell Host Microbe 18:354–362. doi: 10.1016/j.chom.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han Q, Jones JA, Nicely NI, Reed RK, Shen X, Mansouri K, Louder M, Trama AM, Alam SM, Edwards RJ, Bonsignori M, Tomaras GD, Korber B, Montefiori DC, Mascola JR, Seaman MS, Haynes BF, Saunders KO. 2019. Difficult-to-neutralize global HIV-1 isolates are neutralized by antibodies targeting open envelope conformations. Nat Commun 10:2898. doi: 10.1038/s41467-019-10899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zolla-Pazner S, Cohen SS, Boyd D, Kong XP, Seaman M, Nussenzweig M, Klein F, Overbaugh J, Totrov M. 2016. Structure/function studies involving the V3 region of the HIV-1 envelope delineate multiple factors that affect neutralization sensitivity. J Virol 90:636–649. doi: 10.1128/JVI.01645-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pou C, Nkulikiyimfura D, Henckel E, Olin A, Lakshmikanth T, Mikes J, Wang J, Chen Y, Bernhardsson AK, Gustafsson A, Bohlin K, Brodin P. 2019. The repertoire of maternal anti-viral antibodies in human newborns. Nat Med 25:591–596. doi: 10.1038/s41591-019-0392-8. [DOI] [PubMed] [Google Scholar]
- 37.Mabuka J, Goo L, Omenda MM, Nduati R, Overbaugh J. 2013. HIV-1 maternal and infant variants show similar sensitivity to broadly neutralizing antibodies, but sensitivity varies by subtype. AIDS 27:1535–1544. doi: 10.1097/QAD.0b013e32835faba5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavine CL, NIAID Center for HIV/AIDS Vaccine Immunology (CHAVI), Lao S, Montefiori DC, Haynes BF, Sodroski JG, Yang X. 2012. High-mannose glycan-dependent epitopes are frequently targeted in broad neutralizing antibody responses during human immunodeficiency virus type 1 infection. J Virol 86:2153–2164. doi: 10.1128/JVI.06201-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binley JM, Ban YE, Crooks ET, Eggink D, Osawa K, Schief WR, Sanders RW. 2010. Role of complex carbohydrates in human immunodeficiency virus type 1 infection and resistance to antibody neutralization. J Virol 84:5637–5655. doi: 10.1128/JVI.00105-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong R, Xu K, Zhou T, Acharya P, Lemmin T, Liu K, Ozorowski G, Soto C, Taft JD, Bailer RT, Cale EM, Chen L, Choi CW, Chuang G-Y, Doria-Rose NA, Druz A, Georgiev IS, Gorman J, Huang J, Joyce MG, Louder MK, Ma X, McKee K, O'Dell S, Pancera M, Yang Y, Blanchard SC, Mothes W, Burton DR, Koff WC, Connors M, Ward AB, Kwong PD, Mascola JR. 2016. Fusion peptide of HIV-1 as a site of vulnerability to neutralizing antibody. Science 352:828–833. doi: 10.1126/science.aae0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, Kreiss J. 2000. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA 283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 42.Huang J, Doria-Rose NA, Longo NS, Laub L, Lin CL, Turk E, Kang BH, Migueles SA, Bailer RT, Mascola JR, Connors M. 2013. Isolation of human monoclonal antibodies from peripheral blood B cells. Nat Protoc 8:1907–1915. doi: 10.1038/nprot.2013.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. 2009. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res 37:D1006–D1012. doi: 10.1093/nar/gkn838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams KL, Cortez V, Dingens AS, Gach JS, Rainwater S, Weis JF, Chen X, Spearman P, Forthal DN, Overbaugh J. 2015. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine 2:1464–1477. doi: 10.1016/j.ebiom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goo L, Milligan C, Simonich CA, Nduati R, Overbaugh J. 2012. Neutralizing antibody escape during HIV-1 mother-to-child transmission involves conformational masking of distal epitopes in envelope. J Virol 86:9566–9582. doi: 10.1128/JVI.00953-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goo L, Jalalian-Lechak Z, Richardson BA, Overbaugh J. 2012. A combination of broadly neutralizing HIV-1 monoclonal antibodies targeting distinct epitopes effectively neutralizes variants found in early infection. J Virol 86:10857–10861. doi: 10.1128/JVI.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortez V, Wang B, Dingens A, Chen MM, Ronen K, Georgiev IS, McClelland RS, Overbaugh J. 2015. The broad neutralizing antibody responses after HIV-1 superinfection are not dominated by antibodies directed to epitopes common in single infection. PLoS Pathog 11:e1004973. doi: 10.1371/journal.ppat.1004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch RM, CHAVI 001 Clinical Team Members, Tran L, Louder MK, Schmidt SD, Cohen M, DerSimonian R, Euler Z, Gray ES, Karim SA, Kirchherr J, Montefiori DC, Sibeko S, Soderberg K, Tomaras G, Yang Z-Y, Nabel GJ, Schuitemaker H, Morris L, Haynes BF, Mascola JR. 2012. The development of CD4 binding site antibodies during HIV-1 infection. J Virol 86:7588–7595. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decker JM, Bibollet-Ruche F, Wei XP, Wang SY, Levy DN, Wang WQ, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaschen B, Kuiken C, Korber B, Foley B. 2001. Retrieval and on-the-fly alignment of sequence fragments from the HIV database. Bioinformatics 17:415–418. doi: 10.1093/bioinformatics/17.5.415. [DOI] [PubMed] [Google Scholar]
- 52.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 53.Gomez-Roman VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, Robert-Guroff M. 2006. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods 308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Xu GJ, Kula T, Xu Q, Li MZ, Vernon SD, Ndung'u T, Ruxrungtham K, Sanchez J, Brander C, Chung RT, O'Connor KC, Walker B, Larman HB, Elledge SJ. 2015. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science 348:aaa0698. doi: 10.1126/science.aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovelace E, Xu H, Blish CA, Strong R, Overbaugh J. 2011. The role of amino acid changes in the human immunodeficiency virus type 1 transmembrane domain in antibody binding and neutralization. Virology 421:235–244. doi: 10.1016/j.virol.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simonich CA, Doepker L, Ralph D, Williams JA, Dhar A, Yaffe Z, Gentles L, Small CT, Oliver B, Vigdorovich V, Mangala Prasad V, Nduati R, Sather DN, Lee KK, Matsen FA IV, Overbaugh J. 2019. Kappa chain maturation helps drive rapid development of an infant HIV-1 broadly neutralizing antibody lineage. Nat Commun 10:2190. doi: 10.1038/s41467-019-09481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ralph DK, Matsen FA IV. 2019. Per-sample immunoglobulin germline inference from B cell receptor deep sequencing data. PLoS Comput Biol 15:e1007133. doi: 10.1371/journal.pcbi.1007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ralph DK, Matsen FA IV. 2016. Consistency of VDJ rearrangement and substitution parameters enables accurate B cell receptor sequence annotation. PLoS Comput Biol 12:e1004409. doi: 10.1371/journal.pcbi.1004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ralph DK, Matsen FA IV. 2016. Likelihood-based inference of B cell clonal families. PLoS Comput Biol 12:e1005086. doi: 10.1371/journal.pcbi.1005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MG505 P31 antibody deep-sequencing data sets generated and analyzed during the current study (Fig. 7) are publicly available under BioProject SRA accession PRJNA562912 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA562912). Maternal nAb sequences reported in this paper have GenBank accession numbers MN395490 to MN395567. The GenBank accession numbers for the maternal MG505 W0 and infant BG505 W6 HIV-1 Envs utilized in this paper are DQ208449 to DQ208455 and DQ208456 to DQ208458 (1).