An intriguing mode of vector transmission described only for plant viruses is circulative nonpropagative transmission, whereby the virus passes through the gut and salivary glands of the insect vector without replicating. Three plant virus families are transmitted this way, but details of the molecular/cellular mechanisms of the virus-vector interaction are missing. This is striking for nanoviruses that are believed to interact with aphid vectors in ways similar to those of luteoviruses or geminiviruses but for which empirical evidence is scarce. We here confirm that nanoviruses follow a within-vector route similar to that of geminiviruses but distinct from that of luteoviruses. We show that they produce a nonstructural protein mandatory for viral entry into gut cells, a unique phenomenon for this mode of transmission. Finally, noting that nanoviruses are multipartite viruses, we demonstrate that a large number of viral particles penetrate susceptible cells of the vector, allowing distinct genome segments to remain together.
KEYWORDS: aphid, circulative, insect vector, nanovirus, vector transmission, multipartite virus, nonpropagative, plant
ABSTRACT
Vector transmission plays a primary role in the life cycle of viruses, and insects are the most common vectors. An important mode of vector transmission, reported only for plant viruses, is circulative nonpropagative transmission whereby the virus cycles within the body of its insect vector, from gut to salivary glands and saliva, without replicating. This mode of transmission has been extensively studied in the viral families Luteoviridae and Geminiviridae and is also reported for Nanoviridae. The biology of viruses within these three families is different, and whether the viruses have evolved similar molecular/cellular virus-vector interactions is unclear. In particular, nanoviruses have a multipartite genome organization, and how the distinct genome segments encapsidated individually transit through the insect body is unknown. Here, using a combination of fluorescent in situ hybridization and immunofluorescence, we monitor distinct proteins and genome segments of the nanovirus Faba bean necrotic stunt virus (FBNSV) during transcytosis through the gut and salivary gland cells of its aphid vector Acyrthosiphon pisum. FBNSV specifically transits through cells of the anterior midgut and principal salivary gland cells, a route similar to that of geminiviruses but distinct from that of luteoviruses. Our results further demonstrate that a large number of virus particles enter every single susceptible cell so that distinct genome segments always remain together. Finally, we confirm that the success of nanovirus-vector interaction depends on a nonstructural helper component, the viral protein nuclear shuttle protein (NSP), which is shown to be mandatory for viral accumulation within gut cells.
IMPORTANCE An intriguing mode of vector transmission described only for plant viruses is circulative nonpropagative transmission, whereby the virus passes through the gut and salivary glands of the insect vector without replicating. Three plant virus families are transmitted this way, but details of the molecular/cellular mechanisms of the virus-vector interaction are missing. This is striking for nanoviruses that are believed to interact with aphid vectors in ways similar to those of luteoviruses or geminiviruses but for which empirical evidence is scarce. We here confirm that nanoviruses follow a within-vector route similar to that of geminiviruses but distinct from that of luteoviruses. We show that they produce a nonstructural protein mandatory for viral entry into gut cells, a unique phenomenon for this mode of transmission. Finally, noting that nanoviruses are multipartite viruses, we demonstrate that a large number of viral particles penetrate susceptible cells of the vector, allowing distinct genome segments to remain together.
INTRODUCTION
Among hundreds of plant virus species recognized by the International Committee on Taxonomy of Viruses (ICTV), nearly 80% are transmitted from plant to plant by vectors (1). Vectors can be very diverse plant-feeding organisms or parasites (insects, mites, nematodes, and protists), but hemipteran insects (2), and particularly aphids and whiteflies, are by far the most important (1, 3). There are distinct categories of virus-vector interactions, named circulative or noncirculative depending on whether the virus penetrates and circulates within the body of its vector or more simply attaches externally to the cuticle of its mouthparts (4). In circulative transmission, the virus often replicates in the vector, as is the case for all arboviruses infecting vertebrates and for a few plant viruses in the families Tymoviridae, Rhabdoviridae, and Reoviridae or in the order Bunyavirales. An intriguing variation of circulative transmission considered to be nonpropagative has been reported solely in plant viruses (5). In this case, the virus circulates through the gut to the salivary gland (SG) cells of its insect vector but does not replicate during this process (6).
Circulative nonpropagative transmission has been reported for three economically important families of plant viruses: Luteoviridae, Geminiviridae, and Nanoviridae. In each case, the molecular/cellular interaction between virus and vector has not been not fully elucidated, and whether viruses in these three families follow similar pathways in their respective vectors is unclear. A series of pioneering electron microscopy studies have revealed the accumulation of virus particles of distinct luteovirus species in clathrin-coated vesicles of midgut or hindgut (7–9) cells of their aphid vectors. These vesicles seemingly follow the early endosomal pathway prior to the appearance of noncoated tubular vesicles within which the virions are believed to reach the basal membrane and exit gut cells into the hemolymph (10). The transcytosis process has been shown to be similar when luteovirids cross the accessory salivary gland cellular barrier (9). For geminiviruses, and particularly the best-studied genus Begomovirus, a number of reports used immunolabeling of the coat protein (CP) to demonstrate that virus particles also use a clathrin-assisted endocytosis process, following the early endosomal pathway (11). The specific receptors at the level of the gut lumen are poorly known in circulative nonpropagative transmission. Only two receptor candidates, the amino peptidase N and the ephrin receptor protein, have been identified for luteoviruses (12–14), whereas no such candidates could be identified for geminiviruses. In both families, the process by which virus particles internalized in gut/salivary gland cells successfully exit the cells and escape the endosome recycling and lysosome pathway is not understood. It has been shown that the transit of the geminivirus Tomato yellow leaf curl virus (TYLCV) across its whitefly gut cells triggers autophagy as an insect defense mechanism (15). Such a process has not been reported to date for luteoviruses. Despite important differences in their life histories, the transmission mode and the virus-vector interaction for nanoviruses are mostly inferred from knowledge acquired from geminivirus species. Very scarce empirical information is available for nanoviruses, and further investigation is needed to better comprehend circulative nonpropagative transmission and its commonalities and specificities among the three viral families.
The family Nanoviridae comprises two genera, Babuvirus and Nanovirus. Their genomes are composed of six and eight single-stranded circular DNA molecules, respectively, of approximately 1 kb (Fig. 1). Each genome segment encodes a single protein and is individually encapsidated in an icosahedral particle of 17 to 20 nm in diameter (16). All viruses of this family are transmitted by aphids (16), and one series of studies describes the route of the Babuvirus Banana bunchy top virus (BBTV) in its vector Pentalonia nigronervosa (17–19). By immunofluorescence labeling of the coat protein, the BBTV particles were localized in the aphid anterior midgut (AMG) and principal salivary glands (PSG) (17), reminiscent of the situation reported for geminiviruses in whiteflies. However, considering the specificities of nanoviruses, two prominent questions have not been addressed and are examined below.
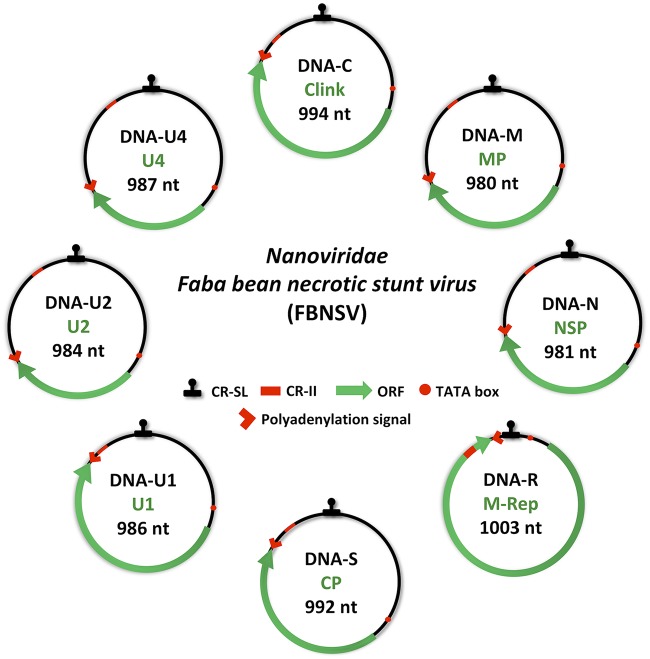
FIG 1.
Genome organization of Faba bean necrotic stunt virus (FBNSV). The eight circles represent the different genomic segments. The name and size of each genome segment and the name of the encoded protein are indicated inside circles in black and green, respectively. Clink, cell cycle-linked protein; MP, movement protein; NSP, nuclear shuttle protein; M-Rep, master replication protein; CP, coat protein; U1, U2, or U4, unknown protein 1, 2, or 4. CR-SL, common stem-loop region; CR-II, common region; ORF, open reading frame; nt, nucleotides.
One major aspect in the genome architecture of nanoviruses, contrasting with luteoviruses and (most) geminiviruses, is that they are multipartite, and so the virus population within the plant is a mixture of six to eight types of viral particles, with each type containing a distinct genome segment (20). In order to ensure successful passage of the integral genome to a new host plant, it is assumed that at least one functional particle of each type must be transmitted (21). We recently reported that the genome segments of Faba bean necrotic stunt virus (FBNSV) do not all coexist in individual plant cells and suggested that the infection proceeds within the host plant through functional complementation of the distinct genes across distinct cells (22). In this intriguing pluricellular way of life, the virus can colonize host cells with a low multiplicity of infection (MOI). In previous studies tracking the BBTV within its aphid vector (17–19), only the coat protein was monitored. The actual identity of genome segments was not documented, and so it is unknown whether nanoviruses invade individual vector cells with a small or large number of virus particles, allowing the distinct genome segments to travel all together or separately from gut to salivary glands.
Another important aspect of the transmission of nanoviruses was uncovered 2 decades ago with the species Faba bean necrotic yellows virus (FBNYV). Through a series of sequential acquisitions of highly and poorly transmissible virus isolates by aphid vectors, Franz and collaborators (23) concluded that, in addition to the virus particles, a viral factor, or helper component (HC), is required for virus transit through the vector. Recently, using agroinfectious clones in which any segment can be omitted during agroinoculation, it was demonstrated that the absence of segment N in the infected plant does not affect systemic infection but totally precludes aphid transmission (24). The authors provided strong evidence that the HC of nanoviruses is the segment N-encoded nuclear shuttle protein (NSP), whose mode of action now awaits investigation.
Here, we confirm the internalization of the distinct FBNSV genome segments within the AMG and the PSG of its aphid vector Acyrthosiphon pisum. We demonstrate that the virus particles penetrate the aphid cells in very high numbers, allowing all genome segments to travel together in all colonized aphid cells, sharply contrasting with the situation recently described in host plants (22). We further show that the NSP is mandatory for viral accumulation into aphid gut cells. Finally, we observe that both the NSP and CP colocalize with the viral genome segments, suggesting that NSP-virus particle complexes are the viral form that cycles within the aphid body.
RESULTS
FBNSV accumulates in the anterior midgut and primary salivary glands of Acyrthosiphon pisum.
We used fluorescence in situ hybridization (FISH) to localize the FBNSV DNA in aphid gut and salivary gland cells. Total viral DNA was first targeted using a whole-genome probe directed against the coding sequences of all eight segments. In the aphid gut, a specific and strong fluorescent signal could be observed in most, if not all, cells of the AMG, while downstream posterior midgut and hindgut were rarely and never labeled, respectively (Fig. 2A to C). At the intracellular level, most of the signal was observed as numerous cytoplasmic perinuclear fluorescent foci, sometimes polarized on one side of the nucleus (Fig. 2B). No viral DNA could be detected in nuclei, even in those cells with the most intense signals.
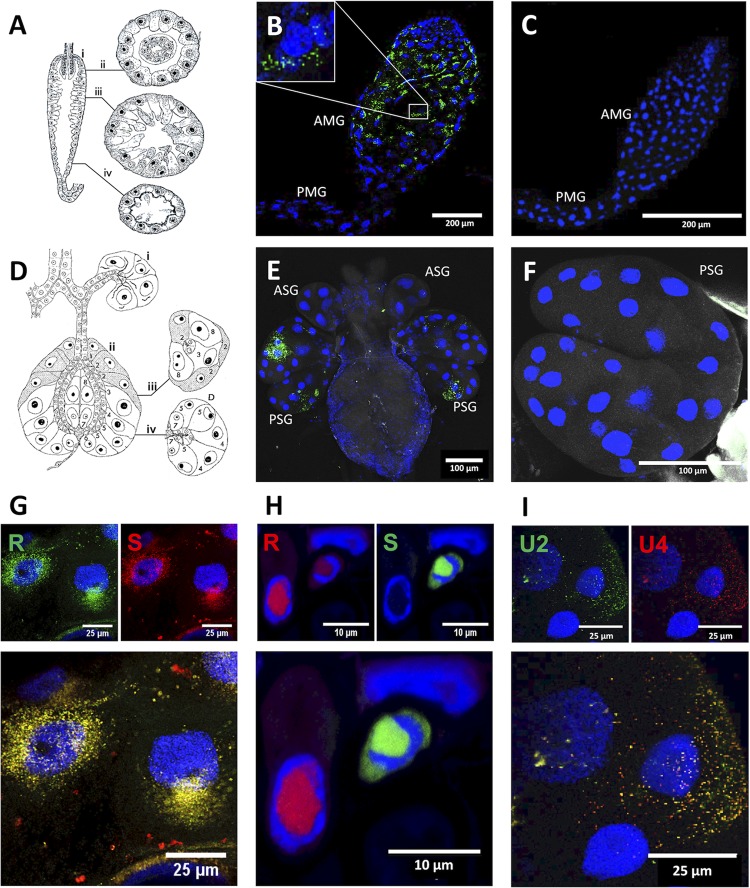
FIG 2.
Localization of the DNA segments of Faba bean necrotic stunt virus (FBNSV) in aphid versus that in plant cells. Schematic drawing of the anatomy of the AMG (A) shows longitudinal (i) and transverse sections (ii to iv). Schematic drawing of the anatomy of the salivary glands (D) shows longitudinal sections of the accessory (i) and principal glands (ii) as well as transverse sections of the principal glands (iii and iv). Both panels A and D are adapted from Ponsen (25) describing the anatomy of Myzus persicae. Ponsen’s numbering of distinct cell types (1 to 8) of the salivary glands is indicated in panel D. The accumulation of FBNSV DNA was observed in anterior midgut (AMG) of 64 viruliferous aphids from 8 experiments, and a representative image is shown in panel B. The accumulation of FBNSV DNA in a specific cell type of the principal salivary glands (PSG) was observed in 15 viruliferous aphids from 3 experiments, and a representative image is shown in panel E. In panels B and E, the viral DNA is revealed by FISH (green probe targeting all eight FBNSV segments), and nonviruliferous controls are shown in panels C and F. The respective localizations of R and S segments (probe color as indicated) are compared in AMG cells (G; representative of 24 observed aphids) and in infected faba bean phloem cells (H) (22). The respective localizations of U2 and U4 segments are compared in PSG cells (I; representative of 4 aphids observed). In panels G, H, and I, the merged-color channel image is shown at the bottom, and the corresponding split-color channel images are shown at the top. All images correspond to maximum-intensity projections. Cell nuclei are stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue). PMG, posterior midgut; ASG, accessory salivary glands.
In salivary glands, the viral DNA appeared exclusively accumulated in PSG and exhibited an intracellular pattern similar to that observed in AMG cells. Interestingly, the viral DNA appeared detectable only in specific cells of the PSG (Fig. 2D to F). Based on Ponsen’s descriptions (25) of the PSG anatomy (Fig. 2D), we propose this specific area to correspond to type 4 cells (Fig. 2E). Other cells of the PSG and accessory salivary glands were not detectably labeled, indicating that they do not or only poorly accumulate FBNSV DNA segments.
The eight FBNSV segments travel together in their insect vector.
Because FBNSV is a multipartite virus and because its eight distinct genome segments have recently been shown to accumulate in distinct cells of the host plant (21), we investigated whether they travel together or separately during their journey across the body of the aphid vector. For this purpose, we prepared segment-specific probes with distinct fluorochromes and used them in pairs. In sharp contrast to the situation earlier reported within host plants (Fig. 2H), the two segments of the pair R/S (encoding the master replication-associated protein and coat protein, respectively) appeared colocalized not only within AMG individual cells but also within each of the numerous fluorescent foci within these cells (Fig. 2G). The overall fluorescent signal was always weaker when FISH was applied to salivary glands, indicating a generally lower level of accumulation of FBNSV in this tissue. When the segment pair U2/U4 (encoding proteins 2 and 4 of unknown function, respectively) was labeled, however, the signal was sufficiently intense to similarly conclude that the two segments accumulate together in individual cells and in most, if not all, fluorescent foci within these cells (Fig. 2I).
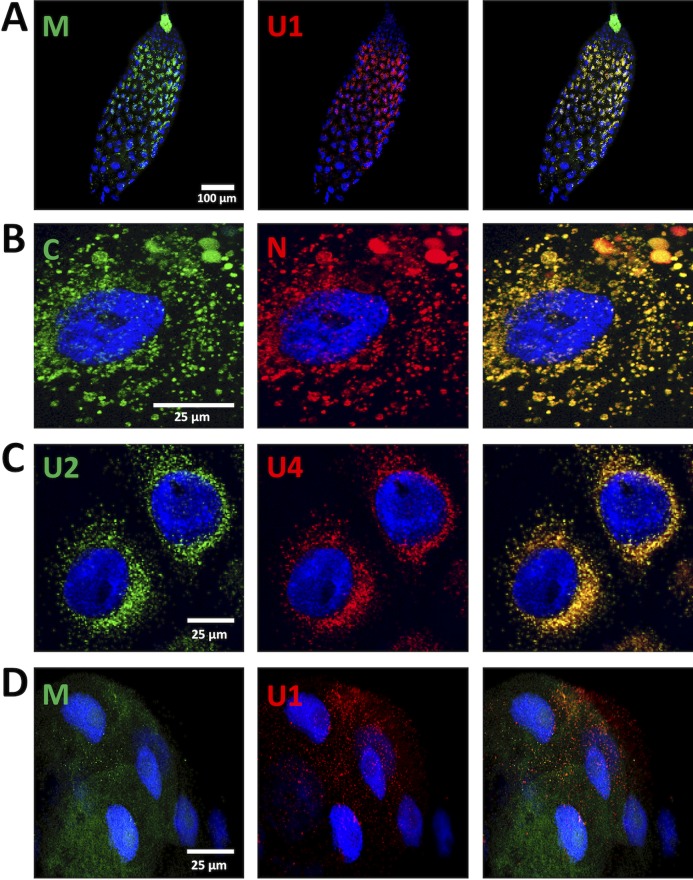
Such systematic colocalization of distinct genome segments was confirmed in the AMG with three additional segment pairs: M/U1 (encoding the movement protein and protein 1 of unknown function, respectively), C/N (encoding the cell cycle-linked protein and the nuclear shuttle protein, respectively), and U2/U4 (Fig. 3A to C). In the PSG, solely the additional pair M/U1 yielded a weak but detectable signal, and, although barely visible, the intracellular fluorescent foci also appeared to contain both segments (Fig. 3D). The different intensities of the fluorescent signal in AMG and PSG are probably due to different levels of viral accumulation in these respective organs. The average number of copies of all eight segments was 1.64 × 108 (±1e7) in the head, containing the PSG, and 8.34 × 109 (± 6e8) in the rest of the body (see Materials and Methods). All together, these observations suggest that all FBNSV segments are internalized in gut and salivary gland cells of their insect vector and undergo transcytosis as groups of virus particles large enough to contain one or more copies of each segment.
FIG 3.
Colocalization of FBNSV segments in AMG and PSG. The colors of the fluorescent probes and the targeted segment pairs are indicated. Three additional pairs of segments were tested in the AMG: 32 aphids from four experiments for the pair M/U1 and 24 aphids from three experiments for the pairs C/N and U2/U4. Illustrative images are, shown, respectively, in panels A, B, and C. In the PSG, the additional segment pair M/U1 was observed in 6 aphids from three experiments, and a representative image is shown in panel D. Split-color channels are shown in the left and middle panels whereas merged images are shown in the right panel. All images correspond to maximum-intensity projections. Cell nuclei are stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue).
Viral DNA colocalizes with CP and NSP within aphid AMG cells.
To further determine the form under which the FBNSV crosses the cellular barriers within its aphid vector, we compared the localization of the FBNSV DNA to that of the two viral proteins obviously involved in vector transmission, CP and NSP. First, we looked at the localization of the CP using immunofluorescence (IF). The CP exhibited a distribution identical to that of viral DNA with very numerous cytoplasmic fluorescent foci in AMG cells (Fig. 4A). In PSG, CP-associated fluorescent foci were visible in cells of type 4, just as viral DNA, and also in the cells with the biggest nucleus, defined by Ponsen as type 3 cells (Fig. 2C and 4E). We then used a combination of FISH and IF to more precisely colocalize viral genomic DNA and the CP. In the AMG, this DNA/protein colabeling demonstrated that all intracellular foci containing viral DNA also contained the CP (Fig. 4I and J), consistent with the assumption that nanoviruses circulate from the infected plant sap, through gut cells into the hemolymph and through salivary gland cells into the saliva, as mature virus particles (26). Noticeably, some smaller CP aggregates appeared sometimes visible in the absence of labeling of the viral DNA. In the PSG, probably due to much weaker fluorescent signals (see the Discussion), we could not observe a reliable FISH/IF double signal, and so the colocalization of viral DNA and CP could not be confirmed in this tissue.
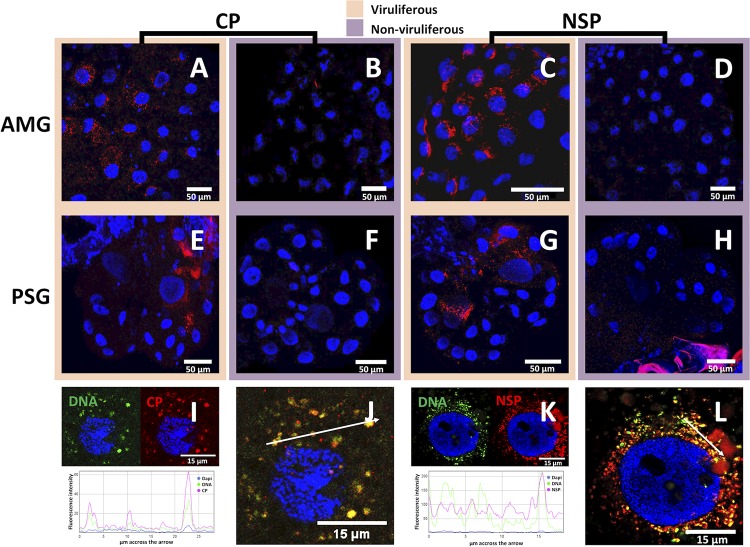
FIG 4.
Localization of FBNSV DNA, CP, and NSP in AMG and PSG of A. pisum. FBNSV CP is labeled by IF in the AMG (A and B) and PSG (E and F) of viruliferous (A and E) and nonviruliferous (B and F) aphids. NSP is labeled by IF in the AMG (C and D) and PSG (G and H) of viruliferous (C and G) and nonviruliferous (D and H) aphids. When viral DNA and either CP or NSP (FISH and IF) were colabeled in AMG, the DNA probe targeting all eight genome segments appears green whereas the specific CP or NSP antibody appears red (I to L). In each case, 30 aphids from three experiments were observed, and one representative image is shown. Split-color channel images in panels I and K correspond to the merged-color channel images in panels J and L, respectively. The graphics represent the colocalization profiles between either DNA (green curve) and CP (red curve) (I) or DNA (green curve) and NSP (red curve) (K). Fluorescence intensity was measured along the white arrows drawn in panels J and L. Images in panels A to H correspond to maximum-intensity projections, and images in panels I to L correspond to single optical sections. Cell nuclei are stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue).
The same approach was applied to the NSP, which was first labeled through IF alone. In both AMG and PSG, the distribution of the NSP-associated fluorescent foci was similar to that observed for the CP (Fig. 4C and G). Combining FISH and IF, we then colabeled NSP and viral DNA in AMG cells. As observed for the CP, the intracellular foci containing FBNSV DNA appeared to contain the NSP as well (Fig. 4K and L). However, a strong heterogeneity in the relative intensities of the signals attributable to viral DNA and NSP was observed among distinct aggregates of the same cells (Fig. 4K, graph), and this is discussed further below. This result indicates that the virus particles and the NSP likely follow the same pathway during entry and accumulation within insect cells. Unfortunately, the lack of specific antibodies that would be produced in distinct animal species precluded direct colocalization assays of the NSP and CP.
FBNSV needs NSP to accumulate in AMG cells.
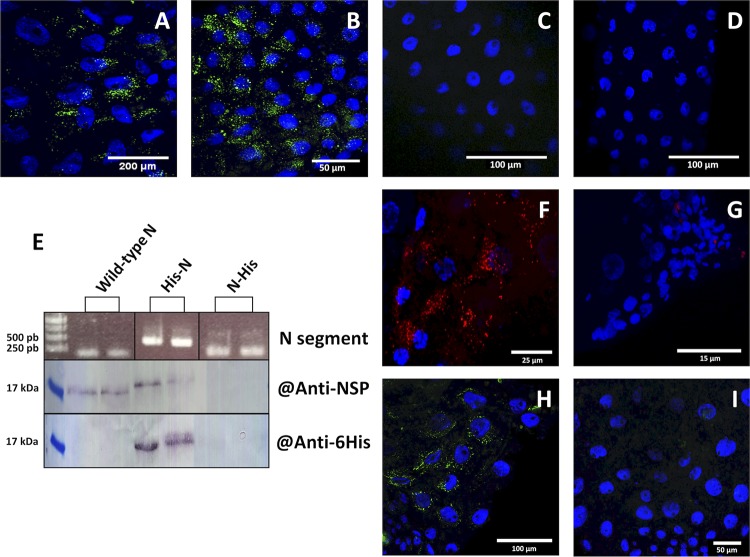
To pave the way to the future deciphering of the NSP mode of action, we questioned whether this protein is mandatory for viral accumulation in AMG cells. Earlier work investigating the dependency of FBNSV infection on the presence/absence of individual genome segments (24) demonstrated that the absence of U4 does not affect systemic infection of host plants or aphid transmission from these plants. In contrast, the absence of segment N does not affect infection but totally abolishes aphid transmission. We thus assessed whether FBNSV could accumulate within AMG cells when the virus is acquired from source plants infected with FBNSV wild type, FBNSV lacking segment U4, FBNSV lacking segment N, or an FBNSV mutant in which the start codon of the NSP in the segment N has been suppressed through mutagenesis. FISH observations showed no difference in the viral DNA accumulation patterns within AMG cells of aphids fed on plants containing or lacking segment U4 (Fig. 5A and B). In contrast, the absence of segment N in infected plants totally abolished the accumulation of the viral genome in AMG cells (Fig. 5C). Similarly, the absence of accumulation of the viral genome in aphids fed on plants infected with the ATG-mutated N segment (Fig. 5D) confirmed that it is the NSP, rather than the segment N itself, that is mandatory for FBNSV accumulation within vector gut cells.
FIG 5.
NSP-dependent accumulation of FBNSV DNA in the AMG of aphid vector. FBNSV DNA is green-labeled in gut cells of aphids fed either on infected plants containing all FBNSV segments (A; representative of 15 aphids from three experiments) or on infected plants lacking segment U4 (B; representative of 5 aphids from one experiment), or on infected plants lacking segment N (C; representative of 15 aphids from three experiments), or on infected plants with all 8 segments but where the N segment has a mutation of the ATG-start codon (D; representative of 5 aphids from one experiment). The presence of the N segment and derivatives His-N and N-His in two replicate infected plants was verified by PCR using primers specific for the coding sequence of the His tag (E, top panel). The expression of NSP, His-NSP, and NSP-His proteins in infected plant tissues was evaluated by Western blotting (E) using antiserum directed against the NSP (middle panel) or His tag (bottom panel). FBNSV NSP (F and G; red) or DNA (H and I; green) are labeled in aphids fed on infected plants expressing wild-type NSP (F and H) or its derivative His-NSP fusion (G and I). Images in panels F and H are representative of 20 aphids observed from two experiments. All confocal images are maximum-intensity projections. Cell nuclei are stained with 4′,6′-diamidino-2-phenylindole (DAPI; blue).
Modifications at the N or C terminus of NSP alter its function or stability.
Because it would be easier to further characterize NSP derivatives fused to small purification tags, we modified the sequence of segment N in order to introduce a series of six histidines at either the N (His-NSP) or C (NSP-His) terminus of the NSP. Plants were infected with either of these constructs, and PCR detection confirmed the maintenance of the modified versions of segment N during systemic plant infection (Fig. 5E). However, while the His-NSP fusion protein accumulated to a level comparable to that of wild-type NSP in infected plant tissues, NSP-His could not be detected (Fig. 5E). The failure to detect the NSP-His fusion could be due to instability of the modified protein or mRNA or to other unknown reasons.
To assess the functionality of the His-NSP fusion produced in infected plants, we tested whether aphids could acquire and transmit the virus from these plants (Fig. 5F to I). FISH and IF, respectively, showed that no viral DNA or His-NSP fusion accumulated detectably in AMG cells. Consistently, in two repeated experiments, aphids that acquired FBNSV from infected plants expressing His-NSP failed to transmit the virus (no infected plants out of 95 test plants; see Materials and Methods for details), while aphids fed on infected plants expressing wild-type NSP efficiently transmitted the virus (69 infected plants out of 105 test plants).
All together, these results indicate that modification at the N or C terminus of NSP has profound effects on accumulation and/or functionality of this protein in aphid vectors and further confirm that only a functional NSP can enter gut cells and assist the coentry of the CP and viral genome.
DISCUSSION
The route of FBNSV in its vector A. pisum.
Because very few experimental data are available concerning the cellular and molecular interactions between nanoviruses and their aphid vectors, we investigated these anew in the model species Faba bean necrotic stunt virus transmitted by Acyrthosiphon pisum. The first logical step was to precisely localize the distinct viral components that are required for successful transmission: viral genomic DNA, coat protein, and the helper component NSP. Using FISH and IF to monitor these three viral components, we could definitely confirm that FBNSV specifically accumulates in the AMG and PSG, consistent with a circulative nonpropagative mode of transmission. This observation matches the reported localization of the coat protein of BBTV in its aphid vector P. nigronervosa (17). FBNSV and BBTV, respectively, belong to the Nanovirus and Babuvirus genera, the only two genera of the family Nanoviridae. It is thus most likely that the aphid AMG and PSG are the organs specifically involved in the transmission of all nanoviruses. This within-vector route is similar to that of geminiviruses transmitted by whiteflies (27) but contrasts with that of luteoviruses, which can enter and cross cells of the hindgut (7–9) and have been reported only in accessory salivary glands of their aphid vectors (9).
In the PSG, FBNSV DNA was unambiguously detected solely in type 4 cells whereas CP and NSP were detected both in type 4 and type 3 cells. At this point, because we do not have sound biological arguments that could explain the accumulation of viral coat protein and not of DNA, we assume that the lack of detection of viral DNA in type 3 cells is due to a technical bias whereby the fluorescent signal associated with FISH is weaker than that associated with IF. In any case, it will be interesting to further investigate the specific accumulation of FBNSV in PSG cells over time, as previously reported for a begomovirus in its whitefly vector (28). At this point, we cannot exclude the possibility that FBNSV could penetrate other cell types and later accumulate preferentially in cell types 3 and 4.
Intracellular localization of FBNSV.
FBNSV is a single-stranded DNA (ssDNA) virus encoding a replication protein, M-Rep, that is not a DNA polymerase (29). On the basis of inference from data obtained from related geminiviruses, nanoviruses are thought to recruit a nonidentified cellular DNA polymerase for replication and accumulation in the nucleus of host plant cells (20). Within aphid vectors, we observed a cytoplasmic localization of FBNSV DNA and proteins, supporting the absence of replication. This result must be considered with care, however, because the question of viral replication within the vector has long been a matter of controversy in the related family Geminiviridae (30, 31). TYLCV primarily accumulates in the cytoplasm of AMG and PSG of its whitefly vector (28), but an elusive transient replication phase has nevertheless been evidenced soon after acquisition (30). In the present study, we used aphids that were all allowed a very long acquisition access period in order to maximize the detection of viral material accumulated in the cytoplasm of the AMG and PSG over time. A small transient replicative proportion of the viral DNA could eventually remain overlooked under our experimental conditions. We have earlier reported that the FBNSV genome formula (the relative amounts of each FBNSV genome segment) changes when the virus passes from the infected plant into the aphid vector (32). Among other hypotheses, replication of the virus upon entry into insect cells could explain the formula changes, and so the question of a transient replication phase of FBNSV within its aphid vector remains open.
Vesicles of the endosomal pathway have been suggested to be the entry route of luteoviruses and geminiviruses in their vectors, based on electron microscopy in viruliferous aphids (9) and colocalization of markers of cell organelles and on confocal microscopy in viruliferous whiteflies (11). An earlier attempt with markers of subcellular compartments to identify the accumulation sites of the nanovirus BBTV failed (19). The authors suggested that this failure could be due to the lack of markers specifically adapted to aphids. For this reason, we have not identified the subcellular compartment with which the FBNSV associates during transcytosis. Developing a large panel of aphid/whitefly-specific markers will be of great utility because the endocytosis/exocytosis pathways that are used downstream of the early endosome remain unknown for the three viral families.
The DNA and coat protein perfectly colocalized in the cytoplasmic fluorescent foci, supporting the general assumption that the FBNSV goes across cellular barriers of its aphid vector under the form of mature virus particles. A remarkable fact inspires caution, however. For both begomoviruses and nanoviruses, no distinctive virus particles could ever be visualized within any cell of an insect vector through electron microscopy. For luteoviruses, which are not much bigger (≈25 nm in diameter), images of virions within intracellular vesicles have long and repeatedly been published (9, 33–35). The reason precluding analogous images with begomo- and nanoviruses is intriguing. While the colocalization of DNA and coat protein indicates that the two travel together, it does not represent definitive proof that they do so as assembled virus particles.
Role of NSP in the transmission of FBNSV.
Franz and collaborators (23) demonstrated the requirement of a helper component (HC) for aphid transmission of Faba bean necrotic yellows virus (FBNYV). Nearly 2 decades later, the same research group (23) demonstrated that this HC is the viral NSP encoded by the N segment. HC molecules have been reported mostly in cases of noncirculative transmission, the best-known examples of which are caulimoviruses and potyviruses (4). In these cases, the HC creates a reversible molecular bridge between the virus and the insect mouthparts, a phenomenon called the “bridge hypothesis” (36), with one domain interacting with the viral coat protein (37, 38) and another interacting with receptor molecules of the vector (39–41). HCs had not been reported until recently in circulative transmission, whether propagative or not. With the discovery that the Rice stripe tenuivirus (RSV) (42) and FBNSV (24) have also evolved the use of an HC, the so-called “helper strategy” (36) is now found in all types of virus-vector interactions. In the case of RSV, the HC is a virus-encoded glycoprotein that binds to the CP and mediates endocytosis and entry of the virus particles inside gut cells. Here, we similarly demonstrate that the NSP of FBNSV is mandatory for viral accumulation within AMG cells and, thus, presumably for virus entry within the vector. We further show that NSP localizes, though imperfectly, in the same intracellular aggregates as viral DNA, and we can thus hypothesize that FBNSV transits within aphids as virus particle-NSP macromolecular complexes. These observations suggest that HCs have at least partly similar modes of action in noncirculative and circulative transmission: creating a molecular bridge between virus and vector. The imperfect colocalization observed for NSP and viral DNA, however, may indicate a distinct and unknown mode of action, and we believe further investigation is needed to confirm or refute the bridge hypothesis. While the NSP of the Babuvirus BBTV has been shown to bind CP (43, 44), the requirement of this interaction for successful transmission has not been demonstrated. Likewise, a direct binding of NSP to the cellular membranes in the AMG lumen and the identification of a putative specific receptor at this site await further research efforts. Unfortunately, investigation on the mode of action of NSP can be foreseen as a difficult task because our results demonstrate that modifications of the NSP N or C terminus to produce protein fusions amenable to biochemical approaches lead to very low accumulation of the recombinant protein or to a loss of its biological activity.
All FBNSV genome segments colocalize within individual cells of the aphid vector.
It is generally assumed that the multipartite lifestyle entails an important cost related to the probability of losing genome segments during transmission from cell to cell or host to host (21, 45, 46). For highly multipartite viruses, i.e., those with a genome composed of four or more segments, the multiplicity of infection (MOI) theoretically required for these viral systems to evolve has been predicted to be unrealistically high (21, 47). We have recently demonstrated that FBNSV can infect its host plant with its distinct genes (genome segments) separated in distinct cells by exchanging gene products across these cells (22). This capacity allows infection of plant cells at a very low MOI, likely alleviating the cost of the multipartite lifestyle within the host. In the present work we touch on the mechanisms through which all FBNSV segments may be transmitted between hosts. Obviously, the FBNSV massive accumulation in aphid cells is totally different from the low-MOI infection observed in plant cells (Fig. 2G and H offer a striking contrast). All susceptible cells of the AMG are packed with all genome segments. Although we did not formally quantify the segments' copy numbers, one can easily observe that each AMG cell contains hundreds of fluorescent foci. By using different pairs of segment-specific probes, we established that each of these foci systematically contains the two segments tested. This indicates that each focus contains numerous distinct segments and most probably all eight. We can thus conservatively conclude that several hundreds to thousands of virus particles enter and accumulate over time within each cell of the AMG. In the PSG, the fluorescent signal was weaker, with some foci eventually containing a dominant color (so perhaps only one segment of the tested pair), indicating a reduction of the number of viral particles accumulated in this organ. It is likely that, as described for geminiviruses (48), FBNSV is primarily stored in the AMG and slowly released into the hemolymph to reach the salivary glands and, from there, the saliva. The success of each genome segment during this transit and after inoculation into new plants is uncertain since the probability of successful transmission by individual aphids is ∼40% (47); the transmission failures could potentially be due to the lack of successful transmission of all genomic segments. It is conceivable that, on the one hand, a low viral flow from the AMG viral stock through the salivary glands allows viruliferous aphids to release viral particles and to possibly transmit virus during their whole life span. On the other hand, the much weaker virus accumulation in the salivary glands suggests that the number of viral particles released in each new visited plant is so small that single aphids often fail to transmit the virus. We cannot quantify precisely the number of virus particles in the salivary glands, but this weaker accumulation is at least qualitatively compatible with the very low numbers of copies of each segment estimated to be transmitted by aphids during host-to-host transmission (47).
MATERIALS AND METHODS
Virus isolate and clones, host plant, and aphid colony.
The FBNSV was first isolated from faba bean in Ethiopia in 1997 (49) and then characterized in 2009 (50). Each of the eight FBNSV genome segments encodes only one protein: segment C encodes the cell cycle-linked protein (clink), M encodes the movement protein (MP), N encodes the nuclear shuttle protein (NSP), S encodes the coat protein (CP), R encodes the master replication-associated protein (M-Rep), and U1, U2, and U4 encode proteins of unknown functions (Fig. 1). Each genome segment of this isolate has been inserted as a head-to-tail dimer into the binary plasmid pBin19 to create eight plasmids together constituting the FBNSV infectious clone (50).
With the aim to purify an active NSP derivative, we added a hexahistidine (His) tag at the C or N terminus of this protein using a Q5 site-directed mutagenesis kit (NEB). The plasmid encoding NSP-His was constructed by inserting the sequence 5′-CATCATCATCACCACCAC-3′ just before the stop codon of the coding sequence of the DNA-N segment (GenBank accession no. GQ150782) in the plasmid pCambia 2300-N-SL (51) to generate pCambia 2300-N-His-SL. The plasmid encoding His-NSP was constructed by inserting the same sequence immediately after the initiating ATG of DNA-N in pCambia 2300-N-SL to generate pCambia 2300-His-N-SL. The sequences of the primers used for these two constructs are listed in Table 1. We refer to these NSPs His tagged at their C or N terminus as NSP-His or His-NSP, respectively. The correct insertion of the series of six histidine codons was confirmed by Sanger sequencing. Plasmids pCambia 2300-N-His-SL and pCambia 2300-His-N-SL were finally transferred to Agrobacterium tumefaciens COR308 for subsequent agroinoculation in faba bean host plants.
TABLE 1.
List of primers
| Primer function and target DNAa | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| FISH | ||
| C | ATGGGTCTGAAATATTTCTC | TTAATTAATTACAATCTCC |
| M | GCTGCGTATCAAGACGAC | TTCTAGCATCCCAATTCCTTTC |
| N | TGGCAGATTGGTTTTCTAGT | TTCTGAGTGAATGTACAATAAACATTT |
| R | ACATTAAATAATCCTCTCTCTCCTA | CCTATCATCACTAAACATGCC |
| S | AAATGGTGAGCAATTGGAA | GCCTATGATAGTAATCATATCTTGACA |
| U1 | TTGGTCGATTATTTGTTGGTT | AATATCTCATTAGCATTAATTACATTTGAA |
| U2 | TTATGGATGCCGGCTTT | CATCAAGTATTAGAATAACGAACTTGA |
| U4 | AGCAGGTTATCGAATGTAG | ATAGATTCCCACAATCGCT |
| Mutagenesis | ||
| N-His | CACCACCACGCAGATTGGTTTTCTAGTC | ATGATGATGCATTTTTCTGCAACTTCC |
| His-N | CACCACCACTAATTAGTTGTGATGATGTAATTAATAATAATT | ATGATGATGCACTTTGATTCTGAGTGAATG |
| Control His | ||
| ATG-His | ATGCATCATCATCACCACCAC | GTTCCTGTTTCCACCATAGAAACTAC |
| His-TAA | GCATGAAAGACAAGCTCAACG | TTAGTGGTGGTGATGATGATG |
Primers were used to amplify the coding sequence of each segment for segment-specific fluorescent labeling during FISH, to generate the pCambia 2300-His-N-SL or pCambia 2300-N-His-SL (mutagenesis), and to control the presence of DNA-His-N or DNA-N-His in infected plants (control His).
Faba bean (Vica faba cv. Sevilla; Vilmorin) was used as the host plant in all agroinoculation experiments. Ten-day-old plantlets were agroinoculated with the FBNSV infectious clone as described previously (50). In some cases, the complete set of eight cloned segments was used, and in other cases either the cloned segment N or U4 was omitted. Faba beans were maintained in growth chambers under a 13/11-h day/night photoperiod at a temperature of 26/20°C day/night and 70% hygrometry. The soil of each potted plant was treated with a solution of 2 g of Trigard 75 WP (24923; Syngeta) in 5 liters of water to avoid the development of sciarid flies. All FBNSV-infected plants were analyzed by quantitative PCR (qPCR) to control for the presence/absence of each inoculated segment.
Aphid colonies of Acyrthosiphon pisum (clones 210 and LSR1) were reared on either FBNSV-infected (viruliferous aphids) or healthy (nonviruliferous aphids) plants. Every week, aphids were transferred to new plants, and the colonies were maintained under a 16/8-h day/night photoperiod at a temperature of 23/18°C day/night.
For practical space reasons, pea (Pisum sativum cv. Provencal; Vilmorin) was used as the recipient plant during transmission experiments.
Preparation of aphid midguts and salivary glands.
To facilitate the dissection and to get insects with an important virus load, we used adult aphids from the colony maintained on infected plants. To eliminate the virus present in the lumen of the gut, aphid individuals were purged by a 24-h acquisition access period on water through Parafilm membrane as described previously (52). Guts and salivary glands of aphids were dissected in 1× phosphate-buffered saline (PBS; pH 7.4) and fixed in 4% paraformaldehyde (PFA) prepared in 1× PBS for 20 min. Dissected organs were then incubated in 0.1 M glycine, pH 7.4, for at least 15 min in order to stop the fixation reaction. For posterior FISH treatments, samples were then submitted to a discoloration step of 20 min in 30% H2O2 and kept in 1× PBS at 4°C until use (maximum storage time, 3 weeks). When IF was applied to the samples, either alone or in combination with FISH, this discoloration step was omitted.
Fluorescence in situ hybridization (FISH) and immunofluorescence (IF).
Fluorescent DNA probes specific to each of the eight FBNSV segments were prepared exactly as described previously (22, 53). Briefly, the coding sequence of each FBNSV segment was first amplified by PCR. PCR products from individual segments or a mixture thereof were then used as templates for the probe synthesis by random priming and incorporation of Alexa Fluor-labeled dUTP, using a BioPrime DNA labeling system kit (Invitrogen). The primer pairs used to amplify the coding sequence of each segment were those described in Sicard et al. (22). For segment-specific labeling, amplified coding sequences of C, M, R, or U2 were labeled with Alexa Fluor 488 (green), and those of N, S, U1, or U4 were labeled with Alexa Fluor 568 (red). For detection of FBNSV DNA, organs kept in 1× PBS were rinsed three times for 5 min in hybridization buffer (20 mM Tris-HCl, pH 8, 0.9 M NaCl, 0.01% SDS, and 30% formamide) (54) and then incubated with the fluorescent probes (diluted 1/30 in hybridization buffer) overnight at 37°C. Labeling was stopped by three rinses in hybridization buffer followed by one rinse in 1× PBS. Samples were mounted on microscope slides in Vectashield antifade mounting medium (Vector Laboratories) (22) and observed with a Zeiss LSM700 confocal microscope equipped with a 10×, 20×, 40×, or 63× objective.
For localization of the coat protein, we used a mix of three previously described (50, 55) mouse monoclonal antibodies (MAbs; FBNYV-1-1F2, FBNYV-2-1A1, and FBNYV-3-4F2). This mix was named FBNSV-FBNYV anti-CP and used at a 1/200 dilution. For detection of the NSP, we used the mouse monoclonal antibody FBNSV-NSP Mab 1-3G9 (24) diluted 1/200. Dissected anterior midgut (AMG) and salivary gland (SG) samples were incubated for 10 min with 1 μg/μl of proteinase K (PK) to increase the tissue permeability (56). Then, samples were rinsed three times for 5 min in 0.1 M glycine, pH 7.4, and twice in 1× PBS to stop the PK treatment, and a second fixation was performed in 2% PFA. After this second PFA fixation, we incubated the organs in a 1× PBS and 5% bovine serum albumin (BSA) solution for 1.5 h to saturate the nonspecific fixation sites. Then, AMG and SG samples were incubated with the primary antibody in 1× PBS–5% BSA overnight at 4°C and with the secondary antibody (goat anti-mousse Alexa Fluor 594 IgG conjugate, diluted 1/250; Life Technologies) in 1× PBS–5% BSA for 1 h at 37°C. After each antibody incubation, three rinses in 1× PBS–0.2% Triton (0.2% PBST) were perform. The samples were mounted on microscope slides. All images were taken at a resolution of 512 by 512 or 1,024 by 1,024 pixels.
When FISH and IF were combined on the same samples, after initial fixation in 4% PFA, PK treatment was carried out before FISH labeling, which was always applied prior to IF. We used ImageJ software, version 1.4.3.67, to analyze images. The overall intensity of green, red, and blue signals was adjusted for each image as described in Sicard et al. (22).
Transmission tests with the mutated NSP.
Faba bean plantlets were agroinoculated with the seven wild-type plasmids of the FBNSV infectious clone, C, M, R, S, U1, U2, and U4, plus either pCambia 2300-N-His-SL or pCambia 2300-His-N-SL. At 21 days postinonculation (21 dpi), plants were tested by PCR or qPCR (see details in the next section) for the presence of the complete set of segments. Production of NSP-His or His-NSP in infected plants was controlled by Western blotting (WB). A piece of 0.6 g of an infected plant stem was finely ground in liquid nitrogen using a mortar. Then, the powdered tissue was further homogenized in 1,800 μl of extraction buffer (20 mM Tris-HCl, 0.2% Na2SO3, and 0.2% SDS) maintained on ice. The resulting crude extract was incubated under agitation at room temperature for 20 min and at 4°C for 1 h, prior to centrifugation at 8,000 × g for 15 min. Twenty microliters of supernatant was used for the WB analysis. As a primary antibody, we used either the rabbit FBNSV IgG 1511389 C-terminal anti-NSP antibody (produced from the NSP peptide sequence C-QYLKKDEDYRRKFII) or a mouse 6×His tag antibody (Invitrogen). The secondary antibody was an anti-mouse or -rabbit IgG coupled with alkaline phosphatase (produced in goat; Sigma). Finally, the membrane was revealed in a solution containing alkaline phosphatase substrate (0.15 mg/ml 5-bromo-4-chloro-3-indolylphosphate [BCIP], 0.30 mg/ml nitroblue tetrazolium [NBT], 100 mM Tris buffer, and 5 mM MgCl2 at pH 9.25 to 9.75; Sigma).
Aphids reared on plants inoculated with plasmid pCambia 2300-N-His-SL or pCambia 2300-His-N-SL were used to observe the localization of the NSP derivatives in AMG and for transmission testing. For transmission tests, L1-stage larvae were deposited on infected faba bean plants for an acquisition access period (AAP) of 3 days and transferred to healthy pea plantlets for an inoculation access period (IAP) of 3 days. Aphids were then killed with insecticide (1 g/liter Pirimor in water; Certis), and plants were observed for the appearance of symptoms 21 days after inoculation. Three experiments were run in parallel, one with plants infected with the eight wild-type segments and two others with plants infected with seven wild-type segments and either pCambia 2300-N-His-SL or pCambia 2300-His-N-SL.
DNA extraction and quantitative real-time PCR.
Extraction of total DNA from healthy or FBNSV-infected plants was performed using three leaf disks (0.6 cm each) from the two upper leaf levels squashed onto a Whatman paper. The corresponding piece of Whatman paper was then placed into a 200-μl filter tip. A total of 100 μl of modified Edwards buffer (200 mM Tris-HCl, pH 7.5, 25 mM EDTA, 250 mM NaCl, 0.5% SDS, 1% polyvinylpyrrolidone 40 [PVP40], 0.2% ascorbic acid) was added to the Whatman paper disk, and the filter tip was placed on the top of a PCR plate and centrifuged at 5,000 × g for 15 s. One hundred microliters of isopropanol was added to the liquid recovered down in the well of the PCR plate and further centrifuged for 25 min at 5,000 × g. The supernatant was discarded, and the pellet was washed with 100 μl of 70% ethanol and resuspended in 50 μl of distilled water.
Total DNA was extracted from pools of five dissected heads or bodies of viruliferous aphids, as previously described (32). Twenty pools were used to estimate by qPCR the average number of copies of the eight FBNSV genome segments accumulating in each of these body parts. qPCR was carried out on a LightCycler 480 thermocycler (Roche). A LightCycler FastStart DNA Master Plus SYBR green I kit (Roche) was used according to the manufacturer’s instructions using 5 μl of the 2× qPCR Mastermix, 2.5 to 2.7 μl of H2O, 0.3 to 0.5 μl of the primer mixes (depending of the primers; 0.3 μM final concentration for C, M, and S and 0.5 μM final concentration for the other segments), and 2 μl of DNA sample (diluted 10-fold in H2O) as the matrix. The FBNSV pair of primers used has been described in Sicard et al. (57). Forty qPCR cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s were applied to the samples. Post-PCR data analysis was as described previously (58). The plant extracts were also tested by standard PCR to verify the presence of the His tag coding sequence in the segment N, using specific primer pairs (Table 1).
ACKNOWLEDGMENTS
We are grateful to B. Gronenborn, T. Timchenko, and J. Vetten for providing antibodies against the CP and NSP of FBNSV. We acknowledge the much-valued help of S. Leblaye with all plant production and aphid maintenance, as well as with the agroinoculation of the FBNSV infectious clone.
This work was supported by ANR grants ANR-14-CE02-0014-01 and ANR-18-CE92-0028-01. J.D.M. acknowledges support from the University of Montpellier; S.B., M.Y., M.-S.V., E.P., and M.V. received support from INRA-SPE; J.-L.Z. and Y.M. received support from IRD; Y.M. received support from CNRS; and Y.G. and H.Z. received support from JKI Braunschweig Germany.
REFERENCES
- 1.Hogenhout SA, Ammar el D, Whitfield AE, Redinbaugh MG. 2008. Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327–359. doi: 10.1146/annurev.phyto.022508.092135. [DOI] [PubMed] [Google Scholar]
- 2.Ng JC, Perry KL. 2004. Transmission of plant viruses by aphid vectors. Mol Plant Pathol 5:505–511. doi: 10.1111/j.1364-3703.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 3.Nault LR. 1997. Arthropod transmission of plant viruses: a new synthesis. Ann Entomol Soc Am 90:521–541. doi: 10.1093/aesa/90.5.521. [DOI] [Google Scholar]
- 4.Blanc S, Drucker M, Uzest M. 2014. Localizing viruses in their insect vectors. Annu Rev Phytopathol 52:403–425. doi: 10.1146/annurev-phyto-102313-045920. [DOI] [PubMed] [Google Scholar]
- 5.Blanc S, Gutierrez S. 2015. The specifics of vector transmission of arboviruses of vertebrates and plants. Curr Opin Virol 15:27–33. doi: 10.1016/j.coviro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Gray S, Cilia M, Ghanim M. 2014. Circulative, “nonpropagative” virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Adv Virus Res 89:141–199. doi: 10.1016/B978-0-12-800172-1.00004-5. [DOI] [PubMed] [Google Scholar]
- 7.Gray SM, Gildow FE. 2003. Luteovirus-aphid interactions. Annu Rev Phytopathol 41:539–566. doi: 10.1146/annurev.phyto.41.012203.105815. [DOI] [PubMed] [Google Scholar]
- 8.Reinbold C, Herrbach E, Brault V. 2003. Posterior midgut and hindgut are both sites of acquisition of Cucurbit aphid-borne yellows virus in Myzus persicae and Aphis gossypii. J Gen Virol 84:3473–3484. doi: 10.1099/vir.0.19415-0. [DOI] [PubMed] [Google Scholar]
- 9.Brault V, Herrbach E, Reinbold C. 2007. Electron microscopy studies on luteovirid transmission by aphids. Micron 38:302–312. doi: 10.1016/j.micron.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ali M, Anwar S, Shuja MN, Tripathi RK, Singh J. 2018. The genus luteovirus from infection to disease. Eur J Plant Pathol 151:841–860. doi: 10.1007/s10658-018-1425-8. [DOI] [Google Scholar]
- 11.Xia WQ, Liang Y, Chi Y, Pan LL, Zhao J, Liu SS, Wang XW. 2018. Intracellular trafficking of begomoviruses in the midgut cells of their insect vector. PLoS Pathog 14:e1006866. doi: 10.1371/journal.ppat.1006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linz LB, Liu S, Chougule NP, Bonning BC. 2015. In vitro evidence supports membrane alanyl aminopeptidase N as a receptor for a plant virus in the pea aphid vector. J Virol 89:11203–11212. doi: 10.1128/JVI.01479-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang SL, Linz LB, Bonning BC, Pohl NL. 2015. Automated solution-phase synthesis of insect glycans to probe the binding affinity of pea enation mosaic virus. J Org Chem 80:10482–10489. doi: 10.1021/acs.joc.5b01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulot M, Monsion B, Boissinot S, Rastegar M, Meyer S, Bochet N, Brault V. 2018. Transmission of turnip yellows virus by Myzus persicae is reduced by feeding aphids on double-stranded RNA targeting the ephrin receptor protein. Front Microbiol 9:457. doi: 10.3389/fmicb.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LL, Wang XR, Wei XM, Huang H, Wu JX, Chen XX, Liu SS, Wang XW. 2016. The autophagy pathway participates in resistance to tomato yellow leaf curl virus infection in whiteflies. Autophagy 12:1560–1574. doi: 10.1080/15548627.2016.1192749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vetten HJ, Dale JL, Grigoras I, Gronenborn B, Harding R, Randles JW, Thomas JE, Timchenko T, Yeh HH. 2011. Nanoviridae, p 395–404. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom. [Google Scholar]
- 17.Bressan A, Watanabe S. 2011. Immunofluorescence localisation of Banana bunchy top virus (family Nanoviridae) within the aphid vector, Pentalonia nigronervosa, suggests a virus tropism distinct from aphid-transmitted luteoviruses. Virus Res 155:520–525. doi: 10.1016/j.virusres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Bressan A. 2013. Tropism, compartmentalization and retention of banana bunchy top virus (Nanoviridae) in the aphid vector Pentalonia nigronervosa. J Gen Virol 94:209–219. doi: 10.1099/vir.0.047308-0. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe S, Borthakur D, Bressan A. 2016. Localization of Banana bunchy top virus and cellular compartments in gut and salivary gland tissues of the aphid vector Pentalonia nigronervosa. Insect Sci 23:591–602. doi: 10.1111/1744-7917.12211. [DOI] [PubMed] [Google Scholar]
- 20.Gronenborn B. 2004. Nanoviruses: genome organisation and protein function. Vet Microbiol 98:103–109. doi: 10.1016/j.vetmic.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Iranzo J, Manrubia SC. 2012. Evolutionary dynamics of genome segmentation in multipartite viruses. Proc Biol Sci 279:3812–3819. doi: 10.1098/rspb.2012.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicard A, Pirolles E, Gallet R, Vernerey MS, Yvon M, Urbino C, Peterschmitt M, Gutierrez S, Michalakis Y, Blanc S. 2019. A multicellular way of life for a multipartite virus. Elife 8:e43599. doi: 10.7554/eLife.43599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz AW, van der Wilk F, Verbeek M, Dullemans AM, van den Heuvel JF. 1999. Faba bean necrotic yellows virus (genus Nanovirus) requires a helper factor for its aphid transmission. Virology 262:210–219. doi: 10.1006/viro.1999.9904. [DOI] [PubMed] [Google Scholar]
- 24.Grigoras I, Vetten HJ, Commandeur U, Ziebell H, Gronenborn B, Timchenko T. 2018. Nanovirus DNA-N encodes a protein mandatory for aphid transmission. Virology 522:281–291. doi: 10.1016/j.virol.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Ponsen MB. 1972. The site of potato leafroll virus multiplication in its vector, Myzus persicae: an anatomical study. H. Veenman, Wageningen, The Netherlands. [Google Scholar]
- 26.Whitfield AE, Falk BW, Rotenberg D. 2015. Insect vector-mediated transmission of plant viruses. Virology 479–480:278–289. doi: 10.1016/j.virol.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Czosnek H, Hariton-Shalev A, Sobol I, Gorovits R, Ghanim M. 2017. The incredible journey of begomoviruses in their whitefly vector. Viruses 9:e273. doi: 10.3390/v9100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J, Zhao JJ, Zhang T, Li FF, Ghanim M, Zhou XP, Ye GY, Liu SS, Wang XW. 2014. Specific cells in the primary salivary glands of the whitefly Bemisia tabaci control retention and transmission of begomoviruses. J Virol 88:13460–13468. doi: 10.1128/JVI.02179-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timchenko T, de Kouchkovsky F, Katul L, David C, Vetten HJ, Gronenborn B. 1999. A single rep protein initiates replication of multiple genome components of faba bean necrotic yellows virus, a single-stranded DNA virus of plants. J Virol 73:10173–10182. doi: 10.1128/JVI.73.12.10173-10182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pakkianathan BC, Kontsedalov S, Lebedev G, Mahadav A, Zeidan M, Czosnek H, Ghanim M. 2015. Replication of tomato yellow leaf curl virus in its whitefly vector, Bemisia tabaci. J Virol 89:9791–9803. doi: 10.1128/JVI.00779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez-Campos S, Rodríguez-Negrete EA, Cruzado L, Grande-Pérez A, Bejarano ER, Navas-Castillo J, Moriones E. 2016. Tomato yellow leaf curl virus: no evidence for replication in the insect vector Bemisia tabaci. Sci Rep 6:30942. doi: 10.1038/srep30942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicard A, Zeddam JL, Yvon M, Michalakis Y, Gutierrez S, Blanc S. 2015. Circulative nonpropagative aphid transmission of nanoviruses: an oversimplified view. J Virol 89:9719–9726. doi: 10.1128/JVI.00780-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris KF, Bath JE. 1972. The fate of pea enation mosaic virus in its pea aphid vector, Acyrthosiphon pisum (Harris). Virology 50:778–790. doi: 10.1016/0042-6822(72)90432-1. [DOI] [PubMed] [Google Scholar]
- 34.Garret A, Kerlan C, Thomas D. 1993. The intestine is a site of passage for potato leafroll virus from the gut lumen into the haemocoel in the aphid vector, Myzus persicae Sulz. Arch Virol 131:377–392. doi: 10.1007/bf01378639. [DOI] [PubMed] [Google Scholar]
- 35.Gildow F. 1993. Evidence for receptor-mediated endocytosis regulating luteovirus acquisition by aphids. Phytopathology 83:270–277. doi: 10.1094/Phyto-83-270. [DOI] [Google Scholar]
- 36.Pirone TP, Blanc S. 1996. Helper-dependent vector transmission of plant viruses. Annu Rev Phytopathol 34:227–247. doi: 10.1146/annurev.phyto.34.1.227. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt I, Blanc S, Esperandieu P, Kuhl G, Devauchelle G, Louis C, Cerutti M. 1994. Interaction between the aphid transmission factor and virus particles is a part of the molecular mechanism of cauliflower mosaic virus aphid transmission. Proc Natl Acad Sci U S A 91:8885–8889. doi: 10.1073/pnas.91.19.8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanc S, Lopez-Moya JJ, Wang R, Garcia-Lampasona S, Thornbury DW, Pirone TP. 1997. A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus. Virology 231:141–147. doi: 10.1006/viro.1997.8521. [DOI] [PubMed] [Google Scholar]
- 39.Blanc S, Ammar ED, Garcia-Lampasona S, Dolja VV, Llave C, Baker J, Pirone TP. 1998. Mutations in the potyvirus helper component protein: effects on interactions with virions and aphid stylets. J Gen Virol 79:3119–3122. doi: 10.1099/0022-1317-79-12-3119. [DOI] [PubMed] [Google Scholar]
- 40.Moreno A, Hebrard E, Uzest M, Blanc S, Fereres A. 2005. A single amino acid position in the helper component of cauliflower mosaic virus can change the spectrum of transmitting vector species. J Virol 79:13587–13593. doi: 10.1128/JVI.79.21.13587-13593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzest M, Gargani D, Drucker M, Hebrard E, Garzo E, Candresse T, Fereres A, Blanc S. 2007. A protein key to plant virus transmission at the tip of the insect vector stylet. Proc Natl Acad Sci U S A 104:17959–17964. doi: 10.1073/pnas.0706608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu G, Li S, Zhou C, Qian X, Xiang Q, Yang T, Wu J, Zhou X, Zhou Y, Ding XS, Tao X. 2019. Tenuivirus utilizes its glycoprotein as a helper component to overcome insect midgut barriers for its circulative and propagative transmission. PLoS Pathog 15:e1007655. doi: 10.1371/journal.ppat.1007655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji XL, Yu NT, Qu L, Li BB, Liu ZX. 2019. Banana bunchy top virus (BBTV) nuclear shuttle protein interacts and re-distributes BBTV coat protein in Nicotiana benthamiana. 3 Biotech 9:121. doi: 10.1007/s13205-019-1656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu N, Wang J, Yu N, Zheng X, Zhou Q, Liu Z. 2019. Bioinformatics analysis of the interaction between coat protein and nuclear shuttle protein in babuvirus. Am J Plant Sci 10:622–630. doi: 10.4236/ajps.2019.104045. [DOI] [Google Scholar]
- 45.Sicard A, Michalakis Y, Gutierrez S, Blanc S. 2016. The strange lifestyle of multipartite viruses. PLoS Pathog 12:e1005819. doi: 10.1371/journal.ppat.1005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucia-Sanz A, Manrubia S. 2017. Multipartite viruses: adaptive trick or evolutionary treat? NPJ Syst Biol Appl 3:34. doi: 10.1038/s41540-017-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallet R, Fabre F, Thebaud G, Sofonea MT, Sicard A, Blanc S, Michalakis Y. 2018. Small bottleneck size in a highly multipartite virus during a complete infection cycle. J Virol 92:e00139-18. doi: 10.1128/JVI.00139-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Czosnek H, Ghanim M, Ghanim M. 2002. The circulative pathway of begomoviruses in the whitefly vector Bemisia tabaci—insights from studies with tomato yellow leaf curl virus. Ann Appl Biol 140:215–231. doi: 10.1111/j.1744-7348.2002.tb00175.x. [DOI] [Google Scholar]
- 49.Franz A, Makkouk KM, Vetten HJ. 1997. Host range of faba bean necrotic yellows virus and potential yield loss in infected faba bean. Phytopathol Mediterr 36:94–103. [Google Scholar]
- 50.Grigoras I, Timchenko T, Katul L, Grande-Perez A, Vetten HJ, Gronenborn B. 2009. Reconstitution of authentic nanovirus from multiple cloned DNAs. J Virol 83:10778–10787. doi: 10.1128/JVI.01212-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicard A. 2014. Fonctionnement des populations de virus multipartites de plantes au cours des différentes étapes de leur cycle de vie. PhD thesis INRA CIRAD SupAgro, Montpellier, France. [Google Scholar]
- 52.Akey DH, Beck SD. 1971. Continuous rearing of the pea aphid, Acyrthosophon pisum, on a holidic diet. Ann Entomol Soc Am 64:353–356. doi: 10.1093/aesa/64.2.353. [DOI] [Google Scholar]
- 53.Vernerey M, Pirolles P, Sicard A. 2019. Localizing genome segments and protein products of a multipartite virus in host plant cells. Bio Protoc 9:e3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghanim M, Brumin M, Popovski S. 2009. A simple, rapid and inexpensive method for localization of Tomato yellow leaf curl virus and Potato leafroll virus in plant and insect vectors. J Virol Methods 159:311–314. doi: 10.1016/j.jviromet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Franz A, Makkouk KM, Katul L, Vetten HJ. 1996. Monoclonal antibodies for the detection and differentiation of Faba bean necrotic yellows virus isolates. Ann Appl Biol 128:255–268. doi: 10.1111/j.1744-7348.1996.tb07321.x. [DOI] [Google Scholar]
- 56.Lin GW, Chang CC. 2016. Identification of critical conditions for immunostaining in the pea aphid embryos: increasing tissue permeability and decreasing background staining. J Vis Exp 108:e53883. doi: 10.3791/53883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sicard A, Yvon M, Timchenko T, Gronenborn B, Michalakis Y, Gutierrez S, Blanc S. 2013. Gene copy number is differentially regulated in a multipartite virus. Nat Commun 4:2248. doi: 10.1038/ncomms3248. [DOI] [PubMed] [Google Scholar]
- 58.Gallet R, Fabre F, Michalakis Y, Blanc S. 2017. The number of target molecules of the amplification step limits accuracy and sensitivity in ultradeep-sequencing viral population studies. J Virol 91:e00561-17. doi: 10.1128/JVI.00561-17. [DOI] [PMC free article] [PubMed] [Google Scholar]