Identifying the acquired structural traits in virus capsids is important for elucidating what functions are essential among viruses that infect different hosts. The Picornavirales viruses infect a broad spectrum of hosts, ranging from unicellular algae to insects and mammals and include many human pathogens. Those viruses that infect unicellular protists, such as algae, are likely to have undergone fewer structural changes during the course of evolution compared to those viruses that infect multicellular eukaryotes and thus still share some characteristics with the Picornavirales ancestor. This article describes the first atomic capsid structure of an alga Marnavirus, CtenRNAV-II. A comparison to capsid structures of the related invertebrate and vertebrate viruses identified a number of structural traits that have been functionally acquired or lost during the course of evolution. These observations provide new insights on past theories on the viability and evolution of Picornavirales viruses.
KEYWORDS: Chaetenuissarnavirus II, Marnaviridae, Picornavirales, alga virus, cryo-EM, diatom virus, evolution, viron structure
ABSTRACT
The order Picornavirales includes viruses that infect different kinds of eukaryotes and that share similar properties. The capsid proteins (CPs) of viruses in the order that infect unicellular organisms, such as algae, presumably possess certain characteristics that have changed little over the course of evolution, and thus these viruses may resemble the Picornavirales ancestor in some respects. Herein, we present the capsid structure of Chaetoceros tenuissimus RNA virus type II (CtenRNAV-II) determined using cryo-electron microscopy at a resolution of 3.1 Å, the first alga virus belonging to the family Marnaviridae of the order Picornavirales. A structural comparison to related invertebrate and vertebrate viruses revealed a unique surface loop of the major CP VP1 that had not been observed previously, and further, revealed that another VP1 loop obscures the so-called canyon, which is a host-receptor binding site for many of the mammalian Picornavirales viruses. VP2 has an N-terminal tail, which has previously been reported as a primordial feature of Picornavirales viruses. The above-mentioned and other critical structural features provide new insights on three long-standing theories about Picornavirales: (i) the canyon hypothesis, (ii) the primordial VP2 domain swap, and (iii) the hypothesis that alga Picornavirales viruses could share characteristics with the Picornavirales ancestor.
IMPORTANCE Identifying the acquired structural traits in virus capsids is important for elucidating what functions are essential among viruses that infect different hosts. The Picornavirales viruses infect a broad spectrum of hosts, ranging from unicellular algae to insects and mammals and include many human pathogens. Those viruses that infect unicellular protists, such as algae, are likely to have undergone fewer structural changes during the course of evolution compared to those viruses that infect multicellular eukaryotes and thus still share some characteristics with the Picornavirales ancestor. This article describes the first atomic capsid structure of an alga Marnavirus, CtenRNAV-II. A comparison to capsid structures of the related invertebrate and vertebrate viruses identified a number of structural traits that have been functionally acquired or lost during the course of evolution. These observations provide new insights on past theories on the viability and evolution of Picornavirales viruses.
INTRODUCTION
As the most abundant biological entity in oceans, viruses are major players in the marine ecosystem by influencing biogeochemical and ecological processes, such as nutrient and energy cycling, and microbial diversity and distribution, subjects which have been extensively reviewed and discussed (1–6). Diatoms are unicellular algae, so-called microalgae, that also play important roles in the marine ecosystem as producers of considerable oxygen and as the base of the aquatic food web. However, some diatoms cause harmful algal blooms that can have devastating effects on people and aquatic life. An algal bloom is a massive increase of an alga population that suddenly appears and disappears, a growth pattern that repeats itself another year. Environmental conditions, such as nutrient level, sunlight, and temperature, are important factors for the algal bloom growth dynamics, but several studies also demonstrate the importance of algal viruses for terminating the blooms and for influencing the clonal composition (7–10).
Chaetoceros tenuissimus Meunier is a marine diatom with a cell size of up to 11 μm in diameter and is common in the brackish water layer of fiords and inlets. The cell walls of Chaetoceros spp. are heavily silicified, which in combination with their ability to form long chains, makes them harmful, as they can physically damage and clog fish gills (11). The positive-sense single-stranded RNA [(+)ssRNA] virus from C. tenuissimus, CtenRNAV-II, was previously isolated from the Hiroshima Bay, Japan (12). CtenRNAV-II (species Chaetenuissarnavirus II) is a Sogarnavirus of the family Marnaviridae, which belongs to the order Picornavirales (13). There are currently 20 members of the Marnavirus family (https://talk.ictvonline.org/taxonomy/), but large-scale genomic studies indicate that a great number of uncharacterized picorna-like viruses exist in the ocean (14, 15). Apart from the protist Marnaviridae family, the order Picornavirales (13) includes viruses that infect vertebrates (Picornaviridae), plants (Secoviridae), invertebrates (Dicistroviridae and Iflaviridae), and arthropods (Polycipiviridae) and includes members that are of great public health and economic concern as being responsible for diseases in animals (e.g., the common cold) (16), plants (e.g., sharka) (17), and insects (e.g., sacbrood) (18). Based on structural and functional characteristics, some members of the Picornaviridae family have previously been described as “primordial,” and thus, the term primordial Picornaviridae will be used here to group these viruses (19, 20). The current understanding of unicellular protists as the earliest eukaryotes implies that they were hosts of the most ancient groups of viruses. Large-scale metagenomic studies suggest that present-day marine unicellular organisms are exclusively infected by (+)ssRNA viruses and that the so-called picorna-like viruses are one of the groups with the most ancient origins (21–23). Recent advancements in metagenomics have had a great impact on research regarding RNA virus diversity and evolution (21, 23–25). Structural information on viruses can, despite being obtained at a comparably slower pace, provide additional valuable information that can advance the field further. Moreover, for clarifying the host-specific tropism and the infection mechanisms of alga viruses, currently poorly understood on a molecular level, more virus structures need to be determined.
Viruses from the order Picornavirales have a nonenveloped icosahedral capsid of about 30 nm in diameter that encloses a (+)ssRNA genome. The CtenRNAV-II genome has two open reading frames (ORFs), which encode the replication proteins and structural proteins in ORF1 and ORF2, respectively (12). The order in which the structural proteins are encoded is VP2, VP4, VP3, and VP1, which is the same as for members of the invertebrate virus families Dicistroviridae and Iflaviridae and differs from the Picornaviridae viruses, which have the order VP4, VP2, VP3, and VP1. The maturation of the capsids of many viruses of the Picornavirales is dependent on cleavage of capsid protein (CP) VP4 from the N terminus of a precursor subunit, called VP0. In the invertebrate viruses (families Dicistroviridae and Iflaviridae) (26, 27) and CtenRNAV-II, the cleavage of the precursor VP0 generates VP4 and VP3, whereas in Picornaviridae viruses, it generates VP4 and VP2 (28). The cleavage of VP0 is probably autocatalytic, since there is no evidence of a proteolytic enzyme inside the particle. Among the invertebrate viruses, a conserved Asp-Asp-Phe (DDF) motif in VP1 has been suggested to be responsible for the VP0 cleavage (26, 27), whereas in the Picornaviridae viruses, a conserved histidine residue in VP2 has been identified as important and suggested to activate local water molecules that make a nucleophilic attack on the scissile bond (29), commonly at cleavage site N/S or A/D (29, 30).
Apart from some members of the family Secoviridae, the capsid adopts a pseudo T = 3 geometry and is built up by 60 units composed of the three major CPs—VP1, VP2, and VP3—where the VP1 proteins are located around the five-fold axes, and VP2 and VP3 alternate around the three-fold axes. Each major CP has one jelly-roll domain (31). Some species included in the families Picornaviridae, Dicistroviridae, Iflaviridae, and Marnaviridae encode an additional minor VP4 protein located underneath the five-fold axes (26, 32, 33). Some members of the family Secoviridae are composed of three CPs, equivalent to other families of the Picornavirales order, whereas others have either one (folded in three jelly-rolls) or two CPs (one large CP with two jelly-rolls and one small CP with one jelly-roll) (34). A number of Picornavirales structures have been determined from the vertebrate, plant, and invertebrate virus families Picornaviridae, Secoviridae, Dicistroviridae, and Iflaviridae, but no previous structure exists of a virus from the protist Marnaviridae family.
RESULTS
Summary of the structure determination.
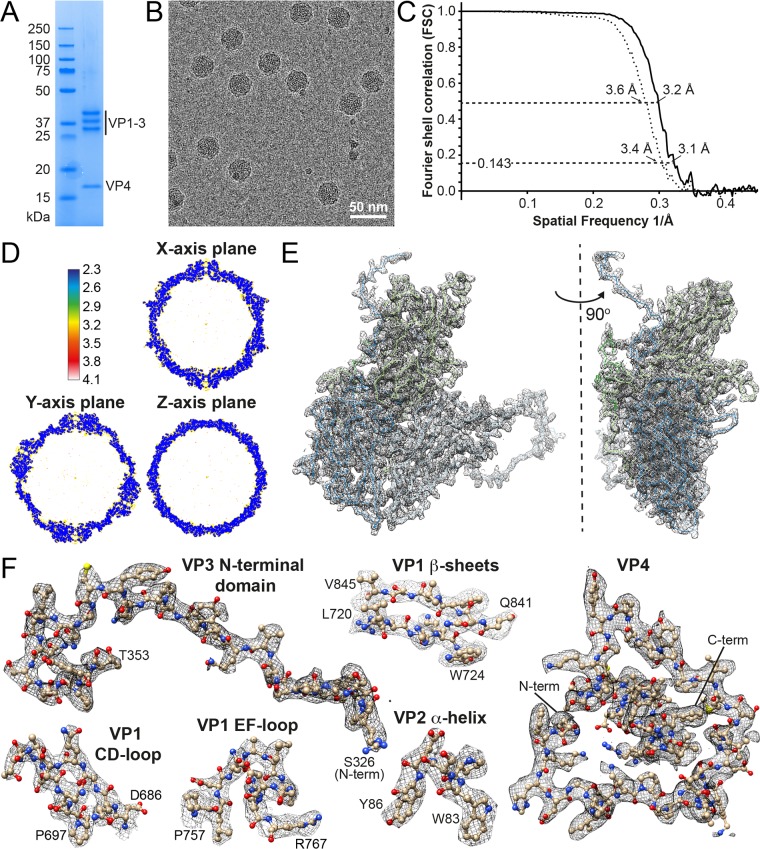
CtenRNAV-II was purified by sucrose gradient centrifugation and characterized by SDS-polyacrylamide gel electrophoresis, which showed the presence of four CPs (Fig. 1A). The capsid structure of the CtenRNAV-II viron was determined using cryo-electron microscopy (cryo-EM). A Titan Krios microscope (Thermo Fisher Scientific) equipped with a K2 Summit direct electron detector (Gatan) (see Materials and Methods) was used to record micrographs (Fig. 1B) and confirmed mature virons containing an RNA genome. The particles had a diameter of approximately 30 nm and appeared to be morphologically reproducible. The three-dimensional (3D) reconstruction was performed with RELION (35) by imposing icosahedral symmetry and using 8,315 particles, which generated a final map with an overall resolution of 3.1 Å (PDB 6SHL and EMD-10200) using the “gold standard” Fourier shell correlation (FSC) = 0.143 criterion (36, 37) (Fig. 1C). The local resolution was distributed between 2.3 and 4.1 Å and estimated using ResMap (38) (Fig. 1D). Data acquisition and processing are summarized in Table 1. The polypeptide main chain and side chains were well resolved (Fig. 1E and F, see Movie S1 in the supplemental material), allowing an atomic model to be manually built (39). The model was refined (40) using standard X-ray crystallographic metrics to validate the refined structure (Table 1).
FIG 1.
Characterization and structure determination of CtenRNAV-II. (A) SDS-PAGE for viral protein composition analysis. (B) Cryo-EM raw image of CtenRNAV-II. (C) The gold standard FSC resolution curves of masked (solid line) and unmasked (dotted line) reconstructions of the CtenRNAV-II particle. The resolution at FSC = 0.143 (gold standard threshold [36, 37] is 3.1 Å) and at FSC = 0.5 is 3.2 Å. (D) A local resolution map of the final reconstruction generated by ResMap, showing a resolution distribution from 2.3 to 4.1 Å. (E) Cα backbone trace of a single icosahedral protomer and the corresponding electron density map. The individual proteins are colored according to the following code: VP1, light green; VP2, light blue; VP3, dark blue; VP4, dark green. Left: view from the outside of the capsid showing VP1-3 located on the surface. Right: view when rotated 90° to the right, visualizing VP4 beneath the surface. A video showing the electron density map and fit of the model is provided in Movie S1. (F) Refined side chains of representatives of secondary structural elements and the entire VP4.
TABLE 1.
Cryo-EM data collection, refinement, and validation statistics
| Parameter or statistic | CtenRNAV-II VPs (EMD-10200 and PDB 6SHL) |
|---|---|
| Data collection and processing | |
| Magnification | 140,000 |
| Voltage (kV) | 300 |
| Electron exposure (e–/Å2) | 29 |
| Defocus range (μm) | –1.00 to –3.00 |
| Pixel size (Å) | 1.06 |
| Symmetry imposed | I |
| No. of initial particle images | 9,920 |
| No. of final particle images | 8,315 |
| Map resolution (Å) | 3.1 |
| FSC threshold | 0.143 |
| Map resolution range (Å) | 2.3–4.1 |
| Refinement | |
| Map sharpening B factor (Å2) | –129.95 |
| Model composition | |
| Nonhydrogen atoms | 378,240 |
| Protein residues | 48,600 |
| B factors (Å2) | |
| Protein (min/max/mean) | 12.53/51.60/21.05 |
| RMSDa | |
| Bond lengths (Å) | 0.009 |
| Bond angles (°) | 1.386 |
| Validation | |
| MolProbity score | 1.78 |
| Clashscore | 4.54 |
| Poor rotamers (%) | 0.88 |
| Ramachandran plot | |
| Favored (%) | 89.90 |
| Allowed (%) | 9.85 |
| Disallowed (%) | 0.25 |
| CC (main chain/side chain) | 0.87/0.85 |
| EMRinger score | 4.51 |
| d 99 (masked/unmasked) | 3.2/3.1 |
RMSD, root mean square deviation.
Overall structure of the CtenRNAV-II viron and CPs.
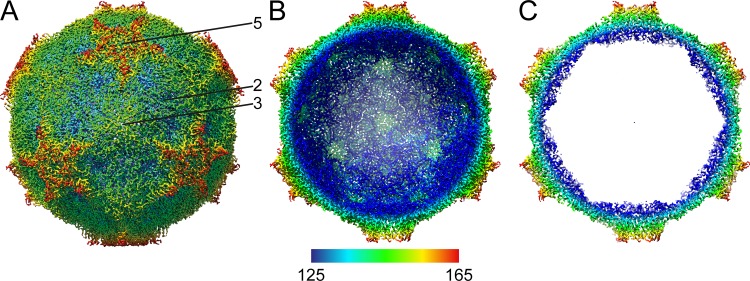
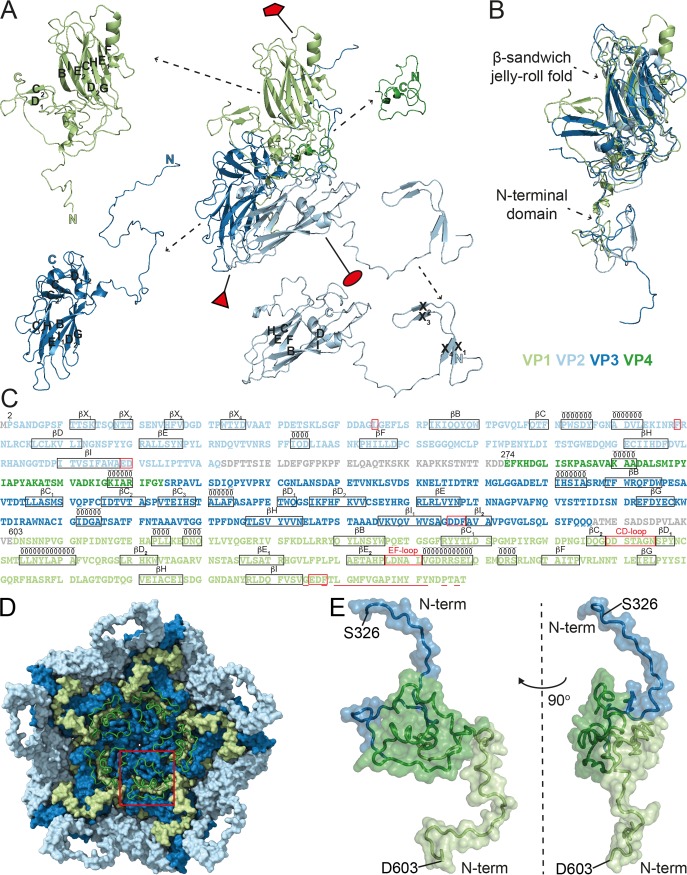
The surface of the CtenRNAV-II viron is characterized by the presence of star-shaped plateaus at the five-fold axes and depressions at the two-fold axes (Fig. 2A) and resembles what has been observed among cardioviruses, such as Theiler’s virus (41). All regions of the cryo-EM map were modeled as CPs, and thus, the internal features of the particle seen in Fig. 2B and C originate from them. The CtenRNAV-II viron is built from the three major subunits, VP1, VP2, and VP3 (Fig. 3A), arranged in a capsid with pseudo T = 3 icosahedral symmetry, where VP1 surrounds the five-fold axes, and VP2 and VP3 alternate around the two- and three-fold axes. An annulus is formed by five VP3 N termini below each five-fold vertex and possibly provides intrapentamer stability, a characteristic also found in human parechovirus 1 and 3 (42, 43). The three major CPs adopt a β-sandwich jelly-roll fold with an N-terminal extension (Fig. 3B). The β-strands are named B to I in accordance with the jelly-roll fold convention forming the two antiparallel β-sheets containing strands BIDG and CHEF (28, 44) (Fig. 3A and C). The N terminus of VP2 has four additional strands not included in the β-sandwich, named X1 to X4 (Fig. 3A and C). The smaller VP4 proteins are located around the five-fold axes on the interior of the particle (Fig. 3D) and interact with the N termini of VP1 and VP3 (Fig. 3D and E). VP4 adopts a compact conformation similar to the invertebrate Picornavirales viruses (26, 45, 46) and in contrast to the Picornaviridae viruses (47–49). The C termini of the VPs are exposed on the capsid surface. The N termini of VP1 and VP2 are located on the inside of the capsid in close proximity around the three-fold axes, while the VP3 N termini are buried around the five-fold axes. The CtenRNAV-II model includes 811 out of the 868 residues that were expected from the genome sequence. The missing regions, colored gray in Fig. 3C, are located between VP2 and VP4 and between VP3 and VP1.
FIG 2.
Cryo-EM 3D reconstruction of the CtenRNAV-II capsid. The capsid is viewed down the three-fold axis and radially colored from blue to red corresponding to 125 Å and 165 Å from the center. (A) The complete CtenRNAV-II particle with the icosahedral five-fold, three-fold, and two-fold axes labeled as 5, 3, and 2, respectively. (B) The front half of the CtenRNAV-II particle removed to visualize the inside of the capsid. (C) A thin slice of the central section of the CtenRNAV-II particle.
FIG 3.
Structures and the amino acid sequences of the CtenRNAV-II CPs. The individual proteins are colored according to the following code: VP1, light green; VP2, light blue; VP3, dark blue; VP4, dark green. (A) The secondary structure for one single icosahedral protomer. The positions of the five-, three-, and two-fold axes are shown by a red pentamer, triangle, and ellipse, respectively. The β-strands within the β-sheets are named alphabetically according to the conventional jelly-roll fold nomenclature (B to I). (B) Superimposition of the three major CPs, VP1-3, exhibits the β-sandwich jelly-roll folds and N-terminal extensions in all three CPs. (C) The amino acid sequences of the CtenRNAV-II CPs. The proteins are listed in the order in which they appear in the genome, and the residue numbering follows this order, which is the same as in PDB entry 6SHL. The number of the first residue that was modeled in each protein is written out above those residues. Each line has 100 residues and is further subdivided into blocks of 10 residues by spaces within the sequence. Unobserved regions of the proteins are shown in gray text. The assigned secondary structure is shown schematically above the sequence. Areas discussed in this paper are highlighted in red: L54 and F99 of VP2 that might be of importance for the N-terminal domain swap; the EDF and DDF proteolytic motifs following strand βI in VP1 and VP3, respectively, as well as the ED residues at the corresponding position in VP2; the CD- and EF-loop in VP1; and the residues considered hydrophobic in the N terminus of VP1 and discussed in regard to cell entry are underlined. (D) A pentamer of CP protomers as seen from the inside looking down the five-fold axis. The VP1-3 proteins are rendered with a surface representation, and the VP4 protein is rendered with a ribbon representation. The red square highlights the position of one VP4 molecule. The N terminus of VP4 is located toward the five-fold axis. (E) The position of VP4 in relation to VP1 and VP3. The figure shows the N-terminal domains of VP3 (S326 to D367) and VP1 (D603 to R648) in the top and bottom of the picture, respectively. Left: same view as in (D), i.e., looking at the inside of the capsid; right: rotated 90° to the left.
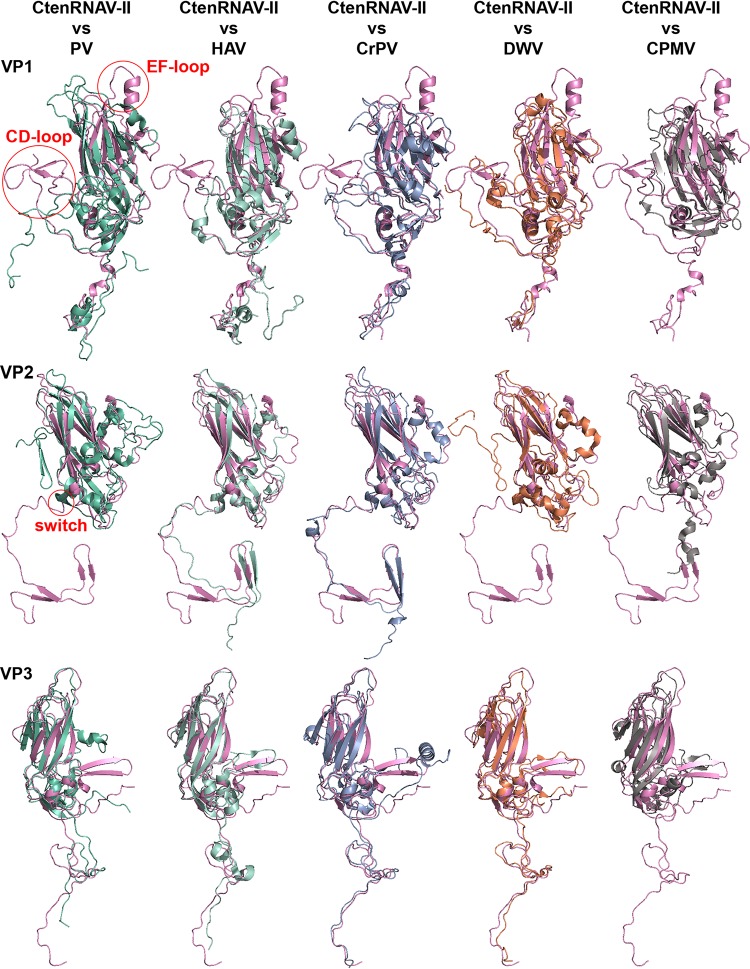
The CtenRNAV-II capsid structure was compared with members of the families Picornaviridae, Dicistroviridae, Iflaviridae, and Secoviridae, as no structure has been determined yet of any member of the Polycipiviridae family. To our knowledge, the Secoviridae structures determined to date are composed of either one or two CPs, and thus, the comparisons of VP1-3 in CtenRNAV-II were performed against the jelly-roll domains in the Secoviridae viruses that are located at the corresponding position in the capsid.
The VPs of CtenRNAV-II exhibit noncanonical jelly-roll folds. Instead of the conventional eight β-strands, VP2 and VP3 are composed of seven (missing βG and βF, respectively). VP1 contains an additional ninth strand, referred to as βE2 in Fig. 3A and C, that inserts in front of βC, thus forming a sheet according to ECHEF. Among the capsid structures determined of members of the Picornavirales order, the noncanonical jelly-roll folds are mostly found within the Dicistroviridae and Secoviridae families. Nine-stranded jelly-roll folds are observed in the Cricket paralysis virus (CrPV) and Triatoma virus (TrV) at the corresponding position of βE2 in CtenRNAV-II, although they are shorter (26, 50). The Israeli Acute bee paralysis virus (IAPV) also has seven-stranded jelly-roll folds, but in VP1 and VP3 (45). VP1 and VP3 of the mud crab dicistrovirus (MCDV) each contain six strands (46). Among members of the Secoviridae family, noncanonical jelly-rolls of either nine or ten strands are primarily found in the domain that surrounds the fivefold axes, i.e., the jelly-roll corresponding to VP1 in CtenRNAV-II (51, 52).
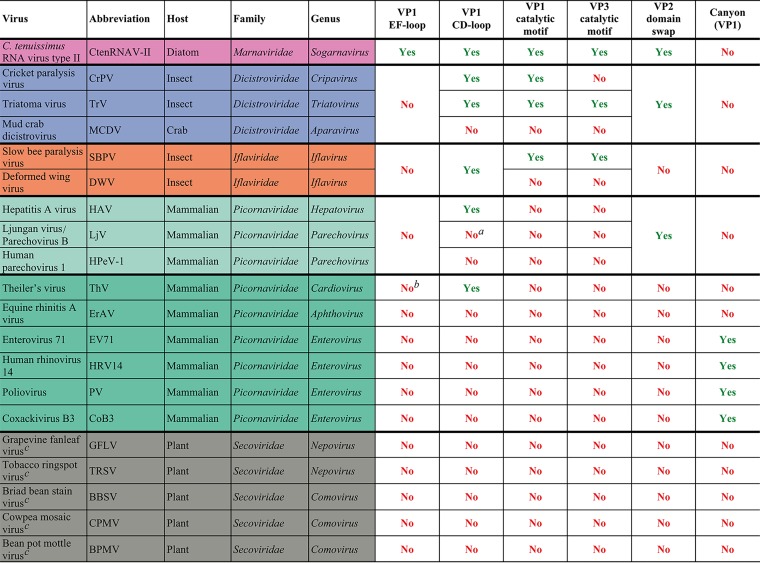
Four structural traits were observed in the CtenRNAV-II capsid—a unique conformation of the VP1 EF-loop, the location of the VP1 CD-loop, a domain swap of VP2, and the position of putative autoproteolytic motifs in VP1 and VP3. The details and the functional and evolutionary implications of these are described and discussed in the following sections and summarized in Table 2.
TABLE 2.
Summary of the most important structural traits discussed in this paper and their existence in the CtenRNAV-II virus and other viruses of the order Picornaviralesd
However, an unassigned surface protrusion at the corresponding position was observed in the structure.
A smaller EF-loop is observed but is less assessable on the capsid surface.
Members of the family Secoviridae do not contain VP1-3; however, the comparisons were performed against the corresponding jelly-roll domains.
The listed viruses are the same representatives as used to reconstruct the phylogenetic tree (Fig. 9).
VP1 has a protruding EF-loop.
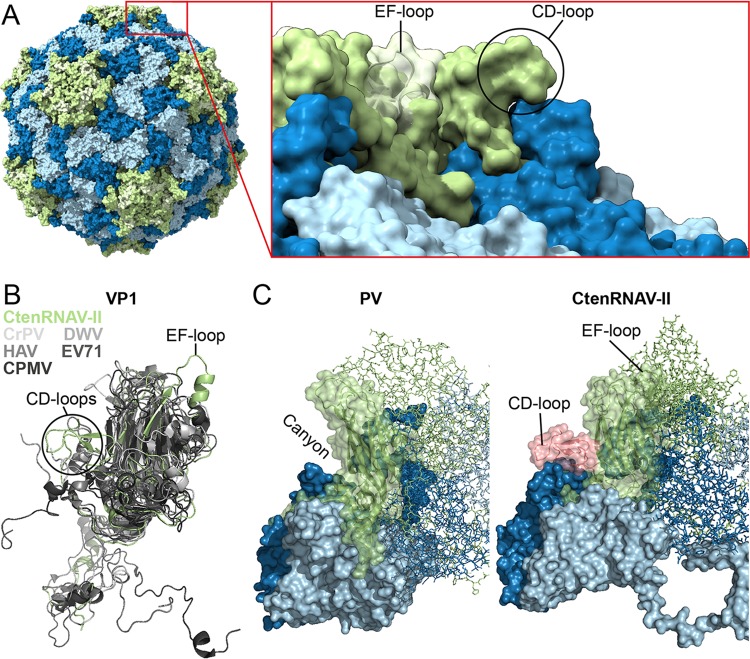
Unique to CtenRNAV-II, compared to other members of the order Picornavirales, is a protruding EF-loop in VP1, which is connected to the extra β-strand (βE2) in the CHEF-sheet previously mentioned (Fig. 4A and B, 5). As described, nine-stranded jelly-roll folds are present in the insect viruses CrPV and TrV at the corresponding position, although much shorter (26, 50), but in neither of them does the strand extend out in a protruding surface loop, as for CtenRNAV-II (Fig. 4B, Fig. 5). The EF-loop continues into a long helix leading the polypeptide chain back into the jelly-roll and the F-strand. The EF-loop is highly accessible on the capsid surface and positioned around the five-fold axis (Fig. 4A). The modeled EF-loop and corresponding cryo-EM map are displayed in Fig. 1F. Cardioviruses (family Picornaviridae) possess smaller EF-loops at a similar position (41); nevertheless, they do not carry the additional ninth E2-strand, and the loops are not as assessable on the capsid surface.
FIG 4.
Surface properties of the CtenRNAV-II capsid. The major CPs are colored according to the following code: VP1, light green; VP2, light blue; VP3, dark blue. (A) Unique EF-loop of VP1 in CtenRNAV-II. The ninth strand (βE2), EF-loop, and the following helix of VP1 in CtenRNAV-II are highlighted in pale green. The EF-loop is exposed on the surface of the capsid around the five-fold axes. (B) Superimposition of VP1 from CtenRNAV-II (light green) with VP1 from representatives of the invertebrate, vertebrate, and plant virus families of the order Picornavirales: Cricket paralysis virus (CrPV) (1B35), Deformed wing virus (DWV) (5L8Q), hepatitis A virus (HAV) (4QPI), enterovirus 71 (EV71) (3VBF), and Cowpea mosaic virus (CPMV) (1NY7) in different shades of gray. (C) Structural comparison between poliovirus (PV) (1HXS) and CtenRNAV-II with a closer look at the surfaces of the biological protomers. For CtenRNAV-II, the C-terminal residues and the CD-loop of VP1, which obscure the canyon indicated for PV, are shown in pink.
FIG 5.
Superimpositions of major capsid proteins. CtenRNAV-II (pink) is compared with the following representatives from the Picornavirales order: poliovirus (PV) (1HXS), hepatitis A virus (HAV), (4QPI), Cricket paralysis virus (CrPV) (1B35), Deformed wing virus (DWV) (5L8Q), and Cowpea mosaic virus (CPMV) (1NY7). The CPMV is composed of two CPs, one small with a single jelly-roll and one large with two jelly-rolls. VP1 from CtenRNAV-II was superimposed on the small CP, whereas VP2 and VP3 were superimposed on the N- and C-terminal domains, respectively, of the large CP. The structural traits, CD- and EF-loop in VP1 and the N-terminal switch in VP2, discussed in this paper are highlighted in red in the left-most superimposition. The P-protein in VP3 of DWV is excluded.
The VP1 CD-loop obscures the canyon.
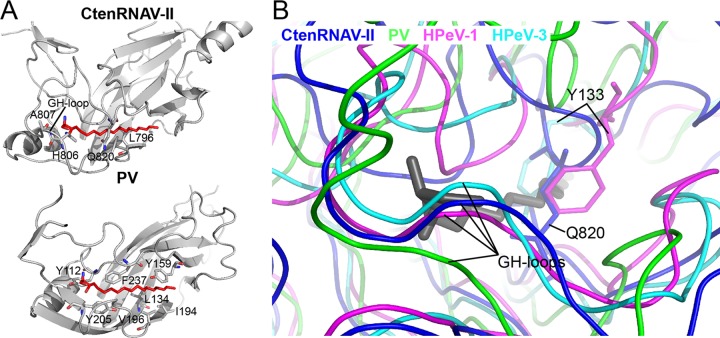
A common surface feature of enterovirus virons is a depression around the five-fold axes called the “canyon” (28), which serves as a host-receptor binding site. A hydrophobic “pocket factor” is located below the canyon in a pocket of VP1 and is released upon receptor binding, which in turn, initiates uncoating (53). In place of a continuous canyon, CtenRNAV-II has five “pits” around each five-fold axis (Fig. 2A). The CD-loop of VP1 fills the volume that corresponds to the canyon in enteroviruses (Fig. 4C) and is located close to the above-described EF-loop of an adjacent VP1 protein (Fig. 4A). The modeled CD-loop and corresponding cryo-EM map are displayed in Fig. 1F. Protruding CD-loops of various sizes in VP1 are a common feature among the invertebrate picornavirads (26, 27, 33, 50, 54) (Fig. 5). Secoviridae viruses do not possess CD-loops at the corresponding position (52) (Fig. 4B and 5) and, likewise, most Picornaviridae viruses lack large CD-loops in VP1. One exception is primordial Picornaviridae viruses, where hepatitis A virus (HAV) has a short CD-loop (19) (Fig. 4B and 5) and where Ljungan virus (LjV) (20) has unassigned protrusions at the corresponding position. Another exception is cardioviruses, which do have large CD-loops at the corresponding position in VP1, which contributes to forming the star-shaped plateaus at the five folds, similar to CtenRNAV-II (41). No canyon has been observed in any of the above-described viruses. Perhaps correlated with the absence of the canyon, no pocket factor was observed within the VP1 β-barrel volume of CtenRNAV-II, and the side chains of amino acids Q820 and L796 as well as the main chain of residues T819-G821 fill the equivalent volume of the β-barrel cavity (Fig. 6A). Possibly, the GH-loop with residues A807 and H806 occlude the mouth of the pocket (Fig. 6A and B). Examples of medically relevant viruses that do not form a pocket include human parechovirus 1 and 3, in which several bulky residues fill the volume of the β-barrel cavities. The common obstructing features in these viruses are the side chain of a conserved Y133 and the GH-loop that occludes the mouth of the pocket (43, 55), the latter of which is similar to CtenRNAV-II (Fig. 6B).
FIG 6.
VP1 of CtenRNAV-II does not contain a hydrophobic pocket. (A) The cryo-EM structure of the CtenRNAV-II capsid does not display any pocket factor. The VP1 proteins of CtenRNAV-II and poliovirus (PV) (1ASJ) are shown as cartoon representations. The pocket factor is shown as a stick model in red. The side chains of residues in poliovirus that interact with the pocket factor are also shown as sticks. The poliovirus pocket factor is represented in CtenRNAV-II and was positioned in the structure by superimposing the two VP1 proteins. The pocket is not formed in CtenRNAV-II, and the side chains of several residues clash with the pocket factor. (B) Superimpositions of CtenRNAV-II and human parechovirus 1 (HPeV-1) (4Z92) and 3 (HPeV-3) (5APM) to PV (1ASJ) to compare how the hydrophobic pocket is blocked in medically relevant picornaviruses. Here, the pocket factor is colored gray.
VP2 has an N-terminal domain swap.
VP2 of CtenRNAV-II has an N-terminal tail (Fig. 3A and B) that exchanges with its two-fold symmetry-related neighbor, resulting in a domain swap relative to the arrangement in, e.g., poliovirus (Fig. 7A). This organization leads to adjacent protomers in one pentamer being sewn together, which theoretically could lead to additional stability of the capsid. The domain swap has previously been observed in all structures determined to date of viruses from the invertebrate Dicistroviridae family (26, 45, 46, 50, 56), the primordial Picornaviridae viruses HAV, LjV, and human parechovirus 1 (HPeV-1) (19, 20, 55) but is, to date, unperceived in viruses from the other invertebrate family, Iflaviridae (27, 33, 54). Compared to other members of the Picornavirales order, Secoviridae viruses have fewer amino acids in the N terminus before the first strand (βB). Additionally, the secondary structure of the tail and its arrangement relative to the jelly-roll domain are different (Fig. 5). As a consequence, they do not possess a domain swap relative to, e.g., poliovirus (52).
FIG 7.
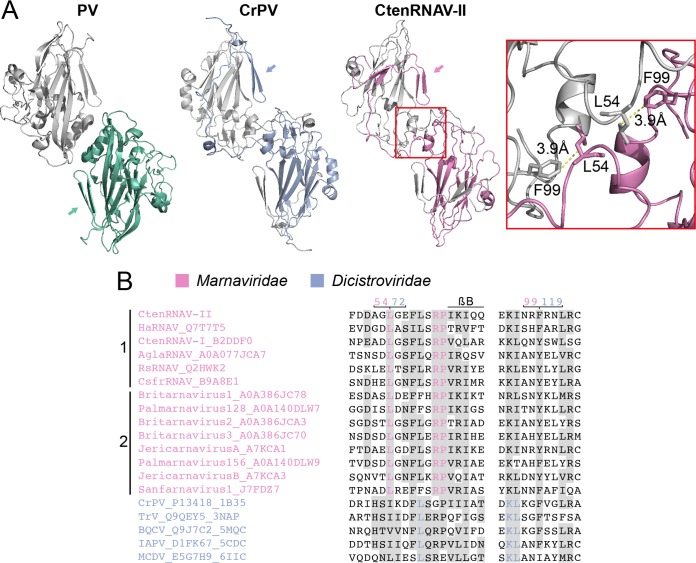
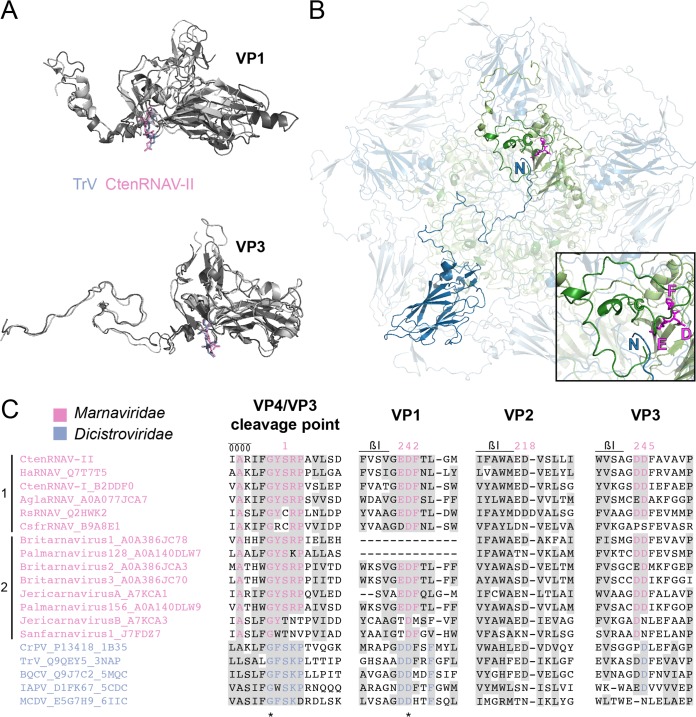
VP2 N-terminal domain swap. (A) Comparison of two-fold symmetry-related VP2 proteins from poliovirus (PV) (1HXS), Cricket paralysis virus (CrPV) (1B35), and CtenRNAV-II (6SHL) as seen from the inside of the viruses. The arrows indicate the location of the N termini of the bottom VP2 proteins of each virus, color-coded green, blue, and pink, respectively. PV does not contain the domain swap, but CrPV and CtenRNAV-II do. The two residues, L54 and F99, of CtenRNAV-II that might be of importance for the domain swap are represented as sticks in the right-most image. The distance between L54 from one of the VP2 proteins to F99 of the other VP2 protein is 3.9 Å. (B) Selected areas from a sequence alignment (76) of VPs from representatives of viruses that are currently classified by ICTV to the Marnaviridae family and viruses of the Dicistroviridae family that have structures available in the PDB. The indication of a secondary structure above the alignments follows CtenRNAV-II and is the same as in PDB entry 6SHL. Six of the Marnaviridae viruses, including CtenRNAV-II, are identified as diatom viruses (1), whereas the other eight were discovered through metagenomic analysis (2). At least 75% identities, either within each virus family or within the whole alignment, are colored in pink and blue, respectively, and similarity (80) across each family or across the entire alignment is shaded gray. The residue numbering follows CtenRNAV-II (pink) and CrPV (blue).
Based on the CrPV structure (26) and a sequence alignment of several insect viruses, it was previously suggested by Liljas et al. that the domain swap probably would be a common feature among “cricket paralysis-like” viruses, since the amino acids involved in the domain swap are partly conserved. Residues 70 to 72 (HSI) in CrPV were found to be relatively conserved, and the isoleucine was found to interact with a fourth conserved residue, F119, in the neighboring two-fold related VP2 protein (57). A sequence alignment (Fig. 7B) including the CtenRNAV-II and representatives of other members of the Marnaviridae family, as well as viruses of the Dicistroviridae family with capsid structures determined, shows that there is a strictly conserved leucine (L54 for CtenRNAV-II) within the Marnaviridae family at the corresponding position of I72 in CrPV and that the interacting residue F119 of CrPV corresponds to either of the two aromatic residues phenylalanine or tyrosine (F99 in CtenRNAV-II) among the Marnaviridae viruses. The conservation of these residues suggests that the domain swap is a common feature among the Marnaviridae viruses as well. The side chains of L54 and F99 in CtenRNAV-II are separated by a distance of 3.9 Å (red box in Fig. 7A).
CtenRNAV-II has two putative autoproteolytic motifs.
As previously described, a conserved Asp-Asp-Phe (DDF) motif in VP1 in the invertebrate viruses has been suggested to be involved in the cleavage of the precursor protein VP0. In dicistroviruses, the cleavage point has the sequence GF/SKP (26, 27). The proteolytic motif is located in a loop immediately following strand βI of VP1 and is found in members of the Dicistroviridae family, although not entirely conserved (Fig. 8A, top, and Fig. 8C) (26, 45, 46, 50, 56). CtenRNAV-II has a similar motif (residues 847 to 849) located in the corresponding loop that follows strand βI, but instead of DDF, CtenRNAV-II has EDF (Fig. 8C). The cleavage point between VP4 and VP3 in CtenRNAV-II is GY/SRP (Fig. 3C, 8C). The glutamic acid in EDF of CtenRNAV-II and the first aspartic acid in the DDF motif of the dicistroviruses are located at the same position in the structure (Fig. 8A, top). The glutamic acid in CtenRNAV-II points toward a gap between the VP4 C terminus and the N terminus of VP3 from an adjacent five-fold related protomer, suggesting that the cleavage takes place after at least a penton has been formed during viron maturation (Fig. 8B), an observation also reported for Dicistroviridae viruses (45). The EDF motif and the cleavage sequence GY/SRP are rather conserved among viruses of the Marnaviridae family (Fig. 8C).
FIG 8.
Putative autoproteolytic motifs in VP1 and VP3. (A) Superimposition of VP1 and VP3 proteins of CtenRNAV-II (dark gray) and Triatoma virus (TrV) (3NAP) (light gray). The catalytic motifs are shown in stick representation in pink and blue, respectively. (B) A CtenRNAV-II pentamer viewed from the inside of the capsid. The C terminus of a VP4 protein (dark green) and N terminus of a VP3 protein (dark blue), located in an adjacent five-fold related protomer, are marked. The position of the catalytic motif EDF in VP1 (light green) is highlighted in magenta, where the glutamic acid points toward the gap between VP4 and VP3. The inset shows a closeup of VP4, the N terminus of VP3, and the EDF motif in VP1. (C) Selected areas from a sequence alignment (76) of VPs from representatives of viruses that are currently classified by ICTV to the Marnaviridae family and viruses of the Dicistroviridae family that have structures available in the PDB. The indication of a secondary structure above the alignments follows CtenRNAV-II and is the same as in PDB entry 6SHL. Six of the Marnaviridae viruses, including CtenRNAV-II, are identified as diatom viruses (1), whereas the other eight were discovered through metagenomic analysis (2). At least 75% identity, either within each virus family or within the whole alignment, is colored in pink and blue, respectively, and similarity (80) across each family or across the entire alignment is shaded gray. Identical residues among all sequences are marked with an asterisk. The residue numbering follows CtenRNAV-II.
The dicistroviruses TrV and black queen cell virus (BQCV) (50, 56) have an additional DDF sequence at the corresponding position in VP3 (in a loop following strand βI) (Fig. 8A, bottom). CtenRNAV-II (residues 566 to 568) and many members of the Marnaviridae family also contain this second motif (Fig. 8A, bottom, and Fig. 8C). It has previously been suggested that the second aspartic acid has a structural role, while the first aspartic acid that points toward a region in the N-terminus tail of VP1 possesses the catalytic function. It was speculated that an RNA rearrangement might activate cleavage at this position prior to genome exit (50). Of the three iflavirus structures determined to date, the two DDF motifs in VP1 and VP3 are also present in the Slow bee paralysis virus (SBPV); however, the cleavage point is NC/DNP (27).
No VP4 protein has been reported for Secoviridae viruses, and thus, the VP1 motif is not expected. Interestingly, though, the DDF sequence was found in the Torradovirus lettuce necrotic leaf curl virus (LNLCV) (GenBank accession number KC855267) and was aligned with the EDF/DDF motifs in VP1 of Marnaviridae and Dicistroviridae viruses in a multiple sequence alignment. Other viruses in the generas Torradovirus and Cheravirus had similar amino acids at the same position but no full EDF or DDF motif. Both torradoviruses and cheraviruses encode three CPs, but since no structure has been determined to date, the positions of these residues in the jelly-roll are not known.
No complete EDF or DDF motif is found at the corresponding position in VP2 of CtenRNAV-II. However, a glutamic acid followed by an aspartic acid (i.e., ED) is found at a similar position (residues 219 to 221). The glutamic acid is pointing here toward a segment in the adjacent two-fold related VP2 protein. These two residues are also present among many other Marnaviridae and Dicistroviridae viruses but are much less conserved than the catalytic motifs in VP1 and VP3 (Fig. 8C).
Evolutionary relationship to other viruses of the order Picornavirales.
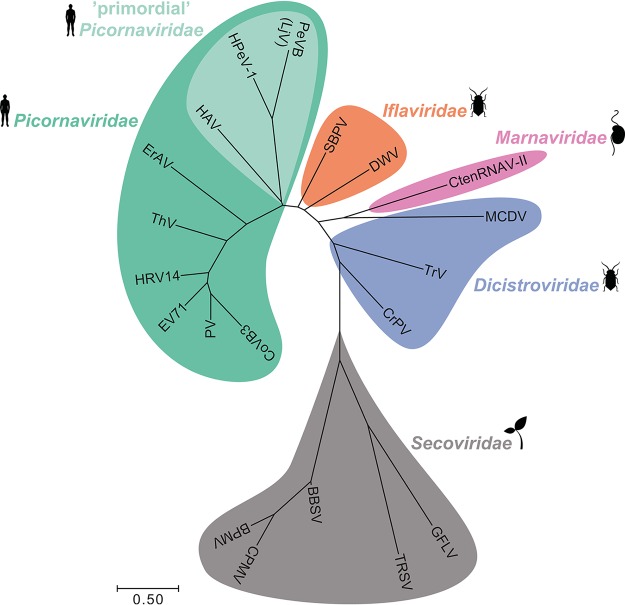
The evolutionary relationship between CtenRNAV-II and viruses of the Dicistroviridae, Iflaviridae, Picornaviridae, and Secoviridae families was investigated based on structural alignment of the major CPs (Fig. 9). The tree is largely similar to those described by others; the four above-mentioned virus families are clearly separated, and the primordial Picornaviridae viruses are placed as an evolutionary link between the invertebrate and vertebrate virus families (54, 56). The CtenRNAV-II is positioned in the same clade as one of the Dicistroviridae viruses—the mud crab dicistrovirus.
FIG 9.
Phylogeny. The tree is constructed based on structural similarity between CtenRNAV-II and other viruses in the order Picornavirales. For CtenRNAV-II and members of the families Picornaviridae, Iflaviridae, and Dicistroviridae, the comparison is performed between VP1-3, whereas for members of the Secoviridae family, the comparison is performed on either the single CP (TRSV and GFLV) or CP1-2 (BPMV, BBSV, and CPMV). For details on the construction of the tree and its limitations, see Materials and Methods. Abbreviations in alphabetical order: Bean pot mottle virus (BPMV), Broad bean stain virus (BBSV), Cowpea mosaic virus (CPMV), coxsackievirus B3 (CoB3), Cricket paralysis virus (CrPV), C. tenuissimus RNA virus type II (CtenRNAV-II), Deformed wing virus (DWV), Equine rhinitis A virus (ErAV), enterovirus 71 (EV71), Grapevine fanleaf virus (GFLV), hepatitis A virus (HAV), human parechovirus 1 (HPeV-1), human rhinovirus 14 (HRV14), mud crab dicistrovirus (MCDV), parechovirus B (PeVB)/Ljungan virus (LjV), poliovirus (PV), Slow bee paralysis virus (SBPV), Theiler’s virus (ThV), Tobacco ringspot virus (TRSV), and Triatoma virus (TrV). Superimpositions of VP1-3 between CtenRNAV-II and representatives from each family included in the tree are displayed in Fig. 5. The structural traits discussed in this paper and their existence in the CtenRNAV-II virus and other viruses of the order Picornavirales are summarized in Table 2.
DISCUSSION
Infection mechanisms of picornavirads.
The observation of the canyon in enteroviruses gave rise to the canyon hypothesis, which suggests that the canyon, inaccessible to immunoglobulins, serves to hide its host receptor binding site and thus prevent an attack by the host’s immune system (28, 58). The canyon is obstructed by surface loops in the alga CtenRNAV-II (Fig. 4C) and in almost all invertebrate viruses (26, 27, 33, 50, 54) whose hosts do not possess a humoral immune system. The primordial viruses also carry short loops or unassigned protrusions at the corresponding position (19, 20). These facts imply that the loops have been shortened as an adaptation to the host immune system in vertebrate hosts, thereby reinforcing the canyon hypothesis. However, exceptions of invertebrate viruses that do not possess large surface loops (46) and vertebrate viruses that do not have a canyon (48) do exist, and hence, the canyon hypothesis is not a complete explanation. The absence of a canyon in CtenRNAV-II implies that a different and more primitive way of recognizing its alga host exists. VP1 appears to be the most important protein for receptor binding in picornaviruses (53, 59–62). Adjacent to the corresponding position of the canyon, VP1 of the algae CtenRNAV-II has an additional ninth strand (βE2) that continues out to form a unique surface EF-loop (Fig. 4A and B). The fact that this loop has not been observed in any Picornavirales structure determined to date suggests its importance for marnaviruses or alga marnaviruses in particular. The observation of higher sequence divergence in the VP1 domain, compared to other parts of the genome in a recent pan-genomic analysis of marine picorna-like viruses, also suggests that VP1 is important for receptor binding, since sequence variation is a response to host evolution (63). In picornaviruses, the N terminus of VP1 is highly hydrophobic and is responsible for anchoring the viron to the endosomal membrane during cell entry (64). Similar to picornaviruses, the N terminus of VP1 in CtenRNAV-II is highly hydrophobic (Fig. 3C), and it is intriguing to speculate that the N terminus of CtenRNAV-II has a similar role in the infection process as in Picornaviridae viruses.
Because of their strong clinical impact, Picornaviridae viruses have been extensively studied compared to other families of the Picornavirales order. These viruses utilize a variety of receptors, such as immunoglobulins, integrins and sialic acids, to facilitate binding to cell surfaces, which results in endocytosis. Receptor binding and/or pH changes in the endosomal system lead to genome release, which was recently shown to be facilitated by the loss of capsid-protein pentamers (65). The hydrophobic N terminus of VP1 anchors the viron to the endosomal membrane, and the minor VP4 protein forms hexameric membrane pores (64, 66). Of the invertebrate virus families, the viral entry and replication mechanisms are best described for the dicistroviruses. From the few studies conducted, it is evident that many processes are similar to those of Picornaviridae viruses; viruses can enter via a clathrin-mediated endocytosis pathway (67), alkaline pH can trigger genome release, which is likely taking place through pentameric disassembly (68, 69), and VP4 is responsible for forming membrane pores (70). Which cell surface receptors are responsible for the initial interaction with the host is yet to be determined. Knowledge about the infection mechanisms of alga viruses is sparse. Strategies such as hijacking autophagy pathways that were previously thought to be exclusive to mammalian viruses have been suggested to be exploited also by some alga viruses (71). Recently, Wagstaff et al. demonstrated that Prymnesium parvum is capable of de novo synthesis of sialic acids and that sialic acid biosynthesis is probably widespread among microalgae (72). Sialic acids could therefore be receptor candidates for the alga CtenRNAV-II, a theory already demonstrated in some mammalian Picornaviridae viruses, such as the coxsackievirus A24 variant (59).
As described above, several steps of the infection process appear to be shared between the invertebrate and vertebrate Picornavirales families, and likewise, the alga virus CtenRNAV-II possesses features such as characteristics of VP1 that are similar to the former. Perhaps the overall strategy and function of the individual CPs between different members of the order Picornavirales are similar, and only details, such as host receptor recognition, had to be adapted during the eukaryotic evolution.
Evolution of capsids of the Picornavirales from a structural perspective.
When the HAV and LjV capsid structures were determined (19, 20), the VP2 domain swap had previously been observed only in insect dicistroviruses and was therefore regarded as a primordial feature. However, the VP2 protein of viruses from the family Iflaviridae, structures which were released later (27, 33, 54), does not possess an N-terminal tail, and thus, does not possess the corresponding domain swap. It became unclear whether the VP2 domain swap is a primordial feature or not. The fact that the domain swap is present in CtenRNAV-II, and based on the sequence alignment, is probably also widespread within the family Marnaviridae (Fig. 7B), suggests that it is indeed a primordial trait.
Based on the CtenRNAV-II capsid structure, it is evident that the ancestral structural traits previously observed in the invertebrate and the primordial vertebrate picornavirads are also features of the protist Marnaviridae family (summarized in Table 2). These include the VP2 domain swap and the VP1 CD-loop. Further, VP1-3 of CtenRNAV-II has noncanonical jelly-roll folds, and VP4 adopts a compact conformation (Fig. 3), features that appear to be more prevalent among the invertebrate viruses. In contrast, the conformation of the VP1 EF-loop is absent in other picornavirad structures determined to date. From a sequence-based point of view, CtenRNAV-II has the same order of the CPs in the genome as the invertebrate viruses. Moreover, the two possible autoproteolytic motifs in VP1 and VP3 were shown to have the same location in the two VPs, which also corresponds to the same in the invertebrate viruses (Fig. 8A). A third motif or a remnant of an ancestral motif might exist in VP2 as well (Fig. 8C). The sequence identity between VP1, VP2, and VP3 within CtenRNAV-II is between 10 and 17%. Considering the low sequence identity, the conservation of the catalytic motifs in VP1 and VP3, as well as the two residues at a similar position in VP2, is interesting from an evolutionary perspective, as it has been postulated that the pseudo T = 3 viruses arose from an ancestor that only encoded a single jelly-roll CP (21, 57), as discussed by Squires et al. (50). Likewise, it would also explain why the VP2 N-terminal tail found in CtenRNAV-II is more prevalent in viruses infecting invertebrates and its presence in the primordial Picornaviridae viruses. The above-described similarities between CtenRNAV-II and the invertebrate viruses are also reflected in the structure-based phylogenetic tree (Fig. 9).
CONCLUSIONS AND FUTURE OUTLOOK
This work presents the first capsid structure of a virus from the Marnaviridae family of the order Picornavirales. The CtenRNAV-II structure was compared with previously determined capsid structures of other viruses belonging to the order that infects invertebrates (families Iflaviridae and Dicistroviridae), vertebrates (family Picornaviridae), and plants (family Secoviridae). Four ancestral structural traits (summarized in Table 2) were observed—the conformation of the VP1 EF-loop, the conformation of the VP1 CD-loop, the VP2 N-terminal domain swap, and the putative autoproteolytic motifs in VP1 and VP3. Future investigations should elucidate the role of the EF-loop in terms of the virus-host interaction. To our knowledge, only one alga virus prior to the CtenRNAV-II structure has been determined to near-atomic resolution (73). More virus structures need to be determined for clarifying the host-specific tropism and infection mechanisms of alga viruses. The observed ancestral structural traits and the phylogenetic analysis strengthen the three previous theories—the canyon hypothesis, the hypothesis that the VP2 domain swap is a primordial trait of picornavirads, and the hypothesis that algae picorna-like viruses share characteristics with the Picornavirales ancestor (21, 22). With more marnavirus structures determined in the future, it will be uncovered whether the VP1 EF-loop is a common feature and on what level—within the Marnaviridae family, among alga viruses within the family, or perhaps within the genera. Questions that arose for the authors during the course of this study and that remain to be elucidated in future studies include the following: What is the function of the EF-loop? What (if any) are the cleavage products of the VP3 DDF motif? Does VP4 play a similar role as in the related invertebrate and vertebrate viruses? Is the VP1 hydrophobic tail capable of anchoring the membrane? What host receptors are involved, and how do they interact with the virus? We anticipate that the CtenRNAV-II structure can guide future molecular work designed to target these questions and unravel molecular details about alga virus infection mechanisms that are currently sparsely described in the literature.
MATERIALS AND METHODS
Virus production and purification.
CtenRNAV-II was produced as previously described (12). Briefly, exponentially growing cultures of C. tenuissimus strain NIES-3715 were inoculated with CtenRNAV-II suspensions (1% vol/vol) and allowed to grow for 2 weeks at 20°C. The resultant lysate was passed through a 0.4-μm pore-size polycarbonate membrane filter (Nuclepore; Merck, Darmstadt, Germany) to remove cellular debris. Polyethylene glycol 6,000 (Wako Pure Chemical Industries Ltd., Osaka, Japan) was added to the filtrate to achieve a final concentration of 10% (wt/vol), and the suspension was stored at 4°C in the dark overnight. After centrifugation at 2,600 × g at 4°C for 2 h, the pellet was washed with ultrapure water and added to an equal volume of chloroform. After vigorous vortex mixing, the suspensions were centrifuged at 2,600 × g for 20 min at room temperature to remove the chloroform. Each water phase was collected and ultracentrifuged at 217,000 × g for 4 h at 4°C to collect the virus particles. The virus particles were resuspended in 300 μl of ultrapure water (i.e., virus suspension). The suspension was loaded onto 15 to 50% (wt/vol) sucrose density gradients and centrifuged at 40,000 × g for 18 h at 4°C. The lower band was pooled and subjected to centrifugation at 202,000 × g for 3 h at 4°C. The pellet was resuspended in 50 mM Tris (pH 7.4), 100 mM NaCl, and 0.1 mM EDTA.
Cryo-EM and data collection.
An aliquot (3 μl) of purified CtenRNAV-II virons (∼10 mg ml−1) was deposited onto freshly glow-discharged holey carbon-coated copper grids (Quantifoil R 2/2, 300 mesh, copper) followed by 2 s of blotting in 100% relative humidity for plunge-freezing (Vitrobot Mark IV) in liquid ethane. Images were acquired using a Titan Krios microscope (Thermo Fisher Scientific) operated at 300 kV and equipped with a K2 Summit direct electron detector (Gatan) and an energy filter. Micrographs were collected at a defocus between –1.0 and –3.0 μm as movies (32 frames, 8 s each, giving a total dose of 29 eV Å−2) at a magnification of ×140,000, resulting in a pixel size of 1.06 Å. The data collection parameters are summarized in Table 1.
Image processing and 3D reconstruction.
The micrographs were corrected for beam-induced drift using MotionCor2 (74), and contrast transfer function (CTF) parameters were estimated using CTFFIND4 (75). The RELION 2.1 package (35) was used for automatic particle picking, 2D and 3D classifications, de novo 3D model generation, and refinement. A total of 9,920 particles were automatically picked from 2,592 micrographs but further reduced after manual selection of particles and several rounds of 2D and 3D classifications. Final reconstruction was performed using 8,315 particles, and the resolution was estimated using the gold standard Fourier shell correlation (threshold, 0.143 criterion) (36, 37). Icosahedral symmetry was imposed on the volumes during the refinement process. The local resolution was estimated using ResMap (38). The data set and image processing are summarized in Table S1.
Model building and refinement.
The atomic models of CtenRNAV-II CPs VP1, VP2, VP3, and VP4 were manually built into the density map using Coot (39) and further refined using PHENIX (40). Refinement statistics are summarized in Table S1.
Structure and sequence analysis.
Multiple sequence alignments were carried out using Clustal Omega (76). Figures were generated using the programs UCSF Chimera (77), UCSF Chimera X (78), and PyMOL (PyMOL Molecular Graphics System version 1.8.4.0; Schrödinger, LLC). Structure-based pairwise alignments of biological protomers of various picornavirads were prepared using the “super” or “cealign” command in PyMOL, and the root mean square deviation (RMSD) values provided were used as an evolutionary distance to construct a matrix file, which was converted into a phylogenetic tree, using the neighbor-joining method in MEGA software version 7.0.26 (79). Structural data are powerful to reconstruct deeper evolutionary branches. Nevertheless, it should be noted that the RMSD-based neighbor-joining method has some limitations compared to performing a multiple sequence alignment and maximum-likelihood analysis; for instance, multiple substitutions of amino acid residues at sequence positions are not allowed, and branch support cannot be provided. The alignment was performed using the following Protein Data Bank (PDB) entries: 1PGW (Bean pod mottle virus), 6QCC (Broad bean stain virus), 1NY7 (Cowpea mosaic virus), 1COV (coxsackievirus B3), 1B35 (Cricket paralysis virus), 6SHL (CtenRNAV-II), 5L8Q (Deformed wing virus), 2WFF (Equine rhinitis A virus), 3VBF (enterovirus 71), 2Y26 (Grapevine fanleaf virus), 4QPI (hepatitis A virus), 4Z92 (human parechovirus 1), 4RHV (human rhinovirus 14), 6IIC (mud crab dicistrovirus), 3JB4 (Ljungan virus/parechovirus B), 1HXS (poliovirus), 5J96 (Slow bee paralysis virus), 1TME (Theiler’s virus), 1A6C (Tobacco ringspot virus), and (3NAP (Triatoma virus).
Data availability.
The atomic coordinates of CtenRNAV-II have been submitted to the Protein Data Bank under accession no. 6SHL. The cryo-EM density map of CtenRNAV-II has been deposited at the Electron Microscopy Data Bank under no. EMD-10200 (https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10200).
Supplementary Material
ACKNOWLEDGMENTS
The data were collected at the Cryo-EM Swedish National Facility funded by the Knut and Alice Wallenberg, Erling Persson Family, and Kempe Foundations, SciLifeLab, Stockholm University and Umeå University. We thank Marta Carroni and Julian Conrad for help with data collection.
Funding was provided by the following agencies: Vetenskapsrådet (VR)/The Swedish Research Council (to K.O., grant no. 2018-03387), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) (to Janos Hajdu and K.O., grant no. JA2014-5721), FORMAS research grant from the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (to K.O., grant no. 2018-00421), the Royal Swedish Academy of Sciences (to K.O., grant no. BS2018-0053), and the Japan Society for the Promotion of Science KAKENHI (to Keizo Nagasaki, K.K., and Y.T., grant no. 16H06429, 16K21723, 16H06437, and 19H00956).
A.M., K.K., Y.T., and K.O. prepared the cryo-EM samples. A.M. and K.O. designed the experiments. A.M. and K.O. collected the cryo-EM data and analyzed the data. A.M. and K.O. wrote the manuscript. All of the authors discussed the results and proofread the manuscript.
We declare no competing financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Suttle CA. 2007. Marine viruses—major players in the global ecosystem. Nat Rev Microbiol 5:801–812. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 2.Rohwer F, Thurber RV. 2009. Viruses manipulate the marine environment. Nature 459:207–212####212. doi: 10.1038/nature08060. [DOI] [PubMed] [Google Scholar]
- 3.Suttle CA. 2005. Viruses in the sea. Nature 437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 4.Fuhrman JA. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 5.Danovaro R, Corinaldesi C, Dell’Anno A, Fuhrman JA, Middelburg JJ, Noble RT, Suttle CA. 2011. Marine viruses and global climate change. FEMS Microbiol Rev 35:993–1034. doi: 10.1111/j.1574-6976.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 6.Short SM. 2012. The ecology of viruses that infect eukaryotic algae. Environ Microbiol 14:2253–2271. doi: 10.1111/j.1462-2920.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 7.Bratbak G, Egge JK, Heldal M. 1993. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar Ecol Prog Ser 93:39–48. doi: 10.3354/meps093039. [DOI] [Google Scholar]
- 8.Nagasaki K, Ando M, Imai I, Itakura S, Ishida Y. 1994. Virus-like particles in Heterosigma akashiwo (Raphidophyceae): a possible red tide disintegration mechanism. Mar Biol 119:307–312. doi: 10.1007/BF00349570. [DOI] [Google Scholar]
- 9.Nagasaki K, Tomaru Y, Nakanishi K, Hata N, Katanozaka N, Yamaguchi M. 2004. Dynamics of Heterocapsa circularisquama (Dinophyceae) and its viruses in Ago Bay, Japan. Aquat Microb Ecol 34:219–226. doi: 10.3354/ame034219. [DOI] [Google Scholar]
- 10.Tomaru Y, Tarutani K, Yamaguchi M, Nagasaki K. 2004. Quantitative and qualitative impacts of viral infection on a Heterosigma akashiwo (Raphidophyceae) bloom in Hiroshima Bay, Japan. Aquat Microb Ecol 34:227–238. doi: 10.3354/ame034227. [DOI] [Google Scholar]
- 11.Landsberg JH. 2002. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci 10:113–390. doi: 10.1080/20026491051695. [DOI] [Google Scholar]
- 12.Kimura K, Tomaru Y. 2015. Discovery of two novel viruses expands the diversity of single-stranded DNA and single-stranded RNA viruses infecting a cosmopolitan marine diatom. Appl Environ Microbiol 81:1120–1131. doi: 10.1128/AEM.02380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Gall O, Christian P, Fauquet CM, King AMQ, Knowles NJ, Nakashima N, Stanway G, Gorbalenya AE. 2008. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch Virol 153:715–727. doi: 10.1007/s00705-008-0041-x. [DOI] [PubMed] [Google Scholar]
- 14.Culley AI, Lang AS, Suttle CA. 2006. Metagenomic analysis of coastal RNA virus communities. Science 312:1795–1798. doi: 10.1126/science.1127404. [DOI] [PubMed] [Google Scholar]
- 15.Culley AI, Lang AS, Suttle CA. 2003. High diversity of unknown picorna-like viruses in the sea. Nature 424:1054–1057. doi: 10.1038/nature01886. [DOI] [PubMed] [Google Scholar]
- 16.Whitton JL, Cornell CT, Feuer R. 2005. Host and virus determinants of picornavirus pathogenesis and tropism. Nat Rev Microbiol 3:765–776. doi: 10.1038/nrmicro1284. [DOI] [PubMed] [Google Scholar]
- 17.Sochor J, Babula P, Adam V, Krska B, Kizek R. 2012. Sharka: the past, the present and the future. Viruses 4:2853–2901. doi: 10.3390/v4112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisder S, Genersch E. 2017. Viruses of commercialized insect pollinators. J Invertebr Pathol 147:51–59. doi: 10.1016/j.jip.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Ren J, Gao Q, Hu Z, Sun Y, Li X, Rowlands DJ, Yin W, Wang J, Stuart DI, Rao Z, Fry EE. 2015. Hepatitis A virus and the origins of picornaviruses. Nature 517:85–88. doi: 10.1038/nature13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu L, Wang X, Ren J, Porta C, Wenham H, Ekström J-O, Panjwani A, Knowles NJ, Kotecha A, Siebert CA, Lindberg AM, Fry EE, Rao Z, Tuthill TJ, Stuart DI. 2015. Structure of Ljungan virus provides insight into genome packaging of this picornavirus. Nat Commun 6. doi: 10.1038/ncomms9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf YI, Kazlauskas D, Iranzo J, Lucía-Sanz A, Kuhn JH, Krupovic M, Dolja VV, Koonin EV. 2018. Origins and evolution of the global RNA virome. mBio 9:e02329-18. doi: 10.1128/mBio.02329-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koonin EV, Wolf YI, Nagasaki K, Dolja VV. 2008. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Microbiol 6:925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- 23.Dolja VV, Koonin EV. 2018. Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Res 244:36–52. doi: 10.1016/j.virusres.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi M, Lin X-D, Chen X, Tian J-H, Chen L-J, Li K, Wang W, Eden J-S, Shen J-J, Liu L, Holmes EC, Zhang Y-Z. 2018. The evolutionary history of vertebrate RNA viruses. Nature 556:197–202. doi: 10.1038/s41586-018-0012-7. [DOI] [PubMed] [Google Scholar]
- 25.Shi M, Lin X-D, Tian J-H, Chen L-J, Chen X, Li C-X, Qin X-C, Li J, Cao J-P, Eden J-S, Buchmann J, Wang W, Xu J, Holmes EC, Zhang Y-Z. 2016. Redefining the invertebrate RNA virosphere. Nature 540:539–543. doi: 10.1038/nature20167. [DOI] [PubMed] [Google Scholar]
- 26.Tate J, Liljas L, Scotti P, Christian P, Lin T, Johnson JE. 1999. The crystal structure of cricket paralysis virus: the first view of a new virus family. Nat Struct Biol 6:765–774. doi: 10.1038/11543. [DOI] [PubMed] [Google Scholar]
- 27.Kalynych S, Přidal A, Pálková L, Levdansky Y, de Miranda JR, Plevka P. 2016. Virion structure of Iflavirus slow bee paralysis virus at 2.6-Angstrom resolution. J Virol 90:7444–7455. doi: 10.1128/JVI.00680-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht H-J, Johnson JE, Kamer G, Luo M, Mosser AG, Rueckert RR, Sherry B, Vriend G. 1985. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 29.Hindiyeh M, Li Q-H, Basavappa R, Hogle JM, Chow M. 1999. Poliovirus mutants at histidine 195 of VP2 do not cleave VP0 into VP2 and VP4. J Virol 73:9072–9079. doi: 10.1128/JVI.73.11.9072-9079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curry S, Fry E, Blakemore W, Abu-Ghazaleh R, Jackson T, King A, Lea S, Newman J, Stuart D. 1997. Dissecting the roles of VP0 cleavage and RNA packaging in picornavirus capsid stabilization: the structure of empty capsids of foot-and-mouth disease virus. J Virol 71:9743–9752. doi: 10.1128/JVI.71.12.9743-9752.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Racaniello V. 2013. Picornaviridae: the viruses and their replication, p 453–489. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 32.Hogle JM, Chow M, Filman DJ. 1985. Three-dimensional structure of poliovirus at 2.9 A resolution. Science 229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 33.Procházková M, Füzik T, Škubník K, Moravcová J, Ubiparip Z, Přidal A, Plevka P. 2018. Virion structure and genome delivery mechanism of sacbrood honeybee virus. Proc Natl Acad Sci U S A 115:7759–7764. doi: 10.1073/pnas.1722018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson JR, Dasgupta I, Fuchs M, Iwanami T, Karasev AV, Petrzik K, Sanfaçon H, Tzanetakis I, van der Vlugt R, Wetzel T, Yoshikawa N, ICTV Report Consortium. 2017. ICTV Virus Taxonomy Profile: Secoviridae. J Gen Virol 98:529–531. doi: 10.1099/jgv.0.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheres SHW. 2012. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J Struct Biol 180:519–530. doi: 10.1016/j.jsb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson R, Sali A, Baker ML, Carragher B, Devkota B, Downing KH, Egelman EH, Feng Z, Frank J, Grigorieff N, Jiang W, Ludtke SJ, Medalia O, Penczek PA, Rosenthal PB, Rossmann MG, Schmid MF, Schröder GF, Steven AC, Stokes DL, Westbrook JD, Wriggers W, Yang H, Young J, Berman HM, Chiu W, Kleywegt GJ, Lawson CL. 2012. Outcome of the first electron microscopy validation task force meeting. Structure 20:205–214. doi: 10.1016/j.str.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheres SHW, Chen S. 2012. Prevention of overfitting in cryo-EM structure determination. Nat Methods 9:853–854. doi: 10.1038/nmeth.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucukelbir A, Sigworth FJ, Tagare HD. 2014. Quantifying the local resolution of cryo-EM density maps. Nat Methods 11:63–65. doi: 10.1038/nmeth.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 40.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant RA, Filman DJ, Fujinami RS, Icenogle JP, Hogle JM. 1992. Three-dimensional structure of Theiler virus. Proc Natl Acad Sci U S A 89:2061–2065. doi: 10.1073/pnas.89.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shakeel S, Evans JD, Hazelbaker M, Kao CC, Vaughan RC, Butcher SJ. 2018. Intrinsically-disordered N-termini in human parechovirus 1 capsid proteins bind encapsidated RNA. Sci Rep 8:5820. doi: 10.1038/s41598-018-23552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shakeel S, Westerhuis BM, Domanska A, Koning RI, Matadeen R, Koster AJ, Bakker AQ, Beaumont T, Wolthers KC, Butcher SJ. 2016. Multiple capsid-stabilizing interactions revealed in a high-resolution structure of an emerging picornavirus causing neonatal sepsis. Nat Commun 7:11387. doi: 10.1038/ncomms11387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison SC, Olson AJ, Schutt CE, Winkler FK, Bricogne G. 1978. Tomato bushy stunt virus at 2.9 Å resolution. Nature 276:368–373. doi: 10.1038/276368a0. [DOI] [PubMed] [Google Scholar]
- 45.Mullapudi E, Přidal A, Pálková L, de Miranda JR, Plevka P. 2016. Virion structure of Israeli acute bee paralysis virus. J Virol 90:8150–8159. doi: 10.1128/JVI.00854-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao Y, Liu S, Huang J, Wang Q, Li K, He J, He J, Weng S, Zhang Q. 2019. Cryo-electron microscopy structures of novel viruses from mud crab Scylla paramamosain with multiple infections. J Virol 93. doi: 10.1128/JVI.02255-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, Walter TS, Evans G, Axford D, Owen R, Rowlands DJ, Wang J, Stuart DI, Fry EE, Rao Z. 2012. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 19:424–429. doi: 10.1038/nsmb.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fry EE, Newman JWI, Curry S, Najjam S, Jackson T, Blakemore W, Lea SM, Miller L, Burman A, King AMQ, Stuart DI. 2005. Structure of Foot-and-mouth disease virus serotype A1061 alone and complexed with oligosaccharide receptor: receptor conservation in the face of antigenic variation. J Gen Virol 86:1909–1920. doi: 10.1099/vir.0.80730-0. [DOI] [PubMed] [Google Scholar]
- 49.Venkataraman S, Reddy SP, Loo J, Idamakanti N, Hallenbeck PL, Reddy VS. 2008. Structure of Seneca Valley Virus-001: an oncolytic picornavirus representing a new genus. Structure 16:1555–1561. doi: 10.1016/j.str.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Squires G, Pous J, Agirre J, Rozas-Dennis GS, Costabel MD, Marti GA, Navaza J, Bressanelli S, Guérin DMA, Rey FA. 2013. Structure of the Triatoma virus capsid. Acta Crystallogr D Biol Crystallogr 69:1026–1037. doi: 10.1107/S0907444913004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasekar V, Johnson JE. 1998. The structure of tobacco ringspot virus: a link in the evolution of icosahedral capsids in the picornavirus superfamily. Struct Lond Engl 6:157–171. doi: 10.1016/S0969-2126(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 52.Lin T, Chen Z, Usha R, Stauffacher CV, Dai J-B, Schmidt T, Johnson JE. 1999. The refined crystal structure of cowpea mosaic virus at 2.8 Å resolution. Virology 265:20–34. doi: 10.1006/viro.1999.0038. [DOI] [PubMed] [Google Scholar]
- 53.Rossmann MG, He Y, Kuhn RJ. 2002. Picornavirus-receptor interactions. Trends Microbiol 10:324–331. doi: 10.1016/s0966-842x(02)02383-1. [DOI] [PubMed] [Google Scholar]
- 54.Škubník K, Nováček J, Füzik T, Přidal A, Paxton RJ, Plevka P. 2017. Structure of deformed wing virus, a major honey bee pathogen. Proc Natl Acad Sci U S A 114:3210–3215. doi: 10.1073/pnas.1615695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalynych S, Pálková L, Plevka P. 2016. The structure of human parechovirus 1 reveals an association of the RNA genome with the capsid. J Virol 90:1377–1386. doi: 10.1128/JVI.02346-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spurny R, Přidal A, Pálková L, Kiem HKT, de Miranda JR, Plevka P. 2017. Virion structure of black queen cell virus, a common honeybee pathogen. J Virol 91:e02100-16. doi: 10.1128/JVI.02100-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liljas L, Tate J, Lin T, Christian P, Johnson JE. 2002. Evolutionary and taxonomic implications of conserved structural motifs between picornaviruses and insect picorna-like viruses. Arch Virol 147:59–84. doi: 10.1007/s705-002-8303-1. [DOI] [PubMed] [Google Scholar]
- 58.Rossmann MG. 1989. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem 264:14587–14590. [PubMed] [Google Scholar]
- 59.Zocher G, Mistry N, Frank M, Hähnlein-Schick I, Ekström J-O, Arnberg N, Stehle T. 2014. A sialic acid binding site in a human picornavirus. PLoS Pathog 10:e1004401. doi: 10.1371/journal.ppat.1004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao C, Bator-Kelly CM, Rieder E, Chipman PR, Craig A, Kuhn RJ, Wimmer E, Rossmann MG. 2005. The crystal structure of coxsackievirus A21 and its interaction with ICAM-1. Structure 13:1019–1033. doi: 10.1016/j.str.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Verdaguer N, Fita I, Reithmayer M, Moser R, Blaas D. 2004. X-ray structure of a minor group human rhinovirus bound to a fragment of its cellular receptor protein. Nat Struct Mol Biol 11:429–434. doi: 10.1038/nsmb753. [DOI] [PubMed] [Google Scholar]
- 62.He Y, Chipman PR, Howitt J, Bator CM, Whitt MA, Baker TS, Kuhn RJ, Anderson CW, Freimuth P, Rossmann MG. 2001. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat Struct Biol 8:874–878. doi: 10.1038/nsb1001-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vlok M, Lang AS, Suttle CA. 2019. Marine RNA virus quasispecies are distributed throughout the oceans. mSphere 4:e00157-19. doi: 10.1128/mSphereDirect.00157-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fricks CE, Hogle JM. 1990. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol 64:1934–1945. doi: 10.1128/JVI.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buchta D, FüZik T, Hrebík D, Levdansky Y, Sukeník L, Mukhamedova L, Moravcová J, Vácha R, Plevka P. 2019. Enterovirus particles expel capsid pentamers to enable genome release. Nat Commun 10. doi: 10.1038/s41467-019-09132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Danthi P, Tosteson M, Li Q.-h, Chow M. 2003. Genome delivery and ion channel properties are altered in VP4 mutants of poliovirus. J Virol 77:5266–5274. doi: 10.1128/jvi.77.9.5266-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang S, Yu J, Fan Z, Gong S, Tang H, Pan L. 2018. Bub1 facilitates virus entry through endocytosis in a model of Drosophila pathogenesis. J Virol 92:00254-18. doi: 10.1128/JVI.00254-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sánchez-Eugenia R, Durana A, López-Marijuan I, Marti GA, Guérin D. 2016. X-ray structure of Triatoma virus empty capsid: insights into the mechanism of uncoating and RNA release in dicistroviruses. J Gen Virol 97:2769–2779. doi: 10.1099/jgv.0.000580. [DOI] [PubMed] [Google Scholar]
- 69.Snijder J, Uetrecht C, Rose RJ, Sanchez-Eugenia R, Marti GA, Agirre J, Guérin DMA, Wuite GJL, Heck AJR, Roos WH. 2013. Probing the biophysical interplay between a viral genome and its capsid. Nat Chem 5:502–509. doi: 10.1038/nchem.1627. [DOI] [PubMed] [Google Scholar]
- 70.Sánchez-Eugenia R, Goikolea J, Gil-Cartón D, Sánchez-Magraner L, Guérin DMA. 2015. Triatoma virus recombinant VP4 protein induces membrane permeability through dynamic pores. J Virol 89:4645–4654. doi: 10.1128/JVI.00011-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schatz D, Shemi A, Rosenwasser S, Sabanay H, Wolf SG, Ben-Dor S, Vardi A. 2014. Hijacking of an autophagy-like process is critical for the life cycle of a DNA virus infecting oceanic algal blooms. New Phytol 204:854–863. doi: 10.1111/nph.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wagstaff BA, Rejzek M, Field RA. 2018. Identification of a Kdn biosynthesis pathway in the haptophyte Prymnesium parvum suggests widespread sialic acid biosynthesis among microalgae. J Biol Chem 293:16277–16290. doi: 10.1074/jbc.RA118.004921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fang Q, Zhu D, Agarkova I, Adhikari J, Klose T, Liu Y, Chen Z, Sun Y, Gross ML, Van Etten JL, Zhang X, Rossmann MG. 2019. Near-atomic structure of a giant virus. Nat Commun 10:388. doi: 10.1038/s41467-019-08319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng SQ, Palovcak E, Armache J-P, Verba KA, Cheng Y, Agard DA. 2017. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rohou A, Grigorieff N. 2015. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol 192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Madeira F, Park YM, Lee J, Buso N, Gur T, Madhusoodanan N, Basutkar P, Tivey ARN, Potter SC, Finn RD, Lopez R. 2019. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res 47:W636–W641. doi: 10.1093/nar/gkz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera: a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 78.Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, Ferrin TE. 2018. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci 27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates of CtenRNAV-II have been submitted to the Protein Data Bank under accession no. 6SHL. The cryo-EM density map of CtenRNAV-II has been deposited at the Electron Microscopy Data Bank under no. EMD-10200 (https://www.ebi.ac.uk/pdbe/entry/emdb/EMD-10200).