Developing targeted treatment for RABV requires understanding the innate immune response to the virus because early virus clearance is essential for preventing the fatality when the infection has progressed to the CNS. Previous studies have revealed that TLR7 is involved in the immune response to RABV. Here, we establish that TLR7 recognizes RABV and facilitates the production of some interferon-stimulated genes. We also demonstrated that when RABV invades into the CNS, TLR7 enhances the production of inflammatory cytokines which contribute to immunopathology in the mouse brain. Taken together, our findings suggest that treatments for RABV must consider the balance between the beneficial and harmful effects of TLR7-triggered immune responses.
KEYWORDS: TLR7, neuroinflammation, rabies virus, recognition receptor
ABSTRACT
Rabies, caused by rabies virus (RABV), is a fatal encephalitis in humans and other mammals, which continues to present a public health threat in most parts of the world. Our previous study demonstrated that Toll-like receptor 7 (TLR7) is essential in the induction of anti-RABV antibodies via the facilitation of germinal center formation. In the present study, we investigated the role of TLR7 in the pathogenicity of RABV in a mouse model. Using isolated plasmacytoid dendritic cells (pDCs), we demonstrated that TLR7 is an innate recognition receptor for RABV. When RABV invaded from the periphery, TLR7 detected viral single-stranded RNA and triggered immune responses that limited the virus’s entry into the central nervous system (CNS). When RABV had invaded the CNS, its detection by TLR7 led to the production of cytokines and chemokines and an increase the permeability of the blood-brain barrier. Consequently, peripheral immune cells, including pDCs, macrophages, neutrophils, and B cells infiltrated the CNS. While this immune response, triggered by TLR7, helped to clear viruses, it also increased neuroinflammation and caused immunopathology in the mouse brain. Our results demonstrate that TLR7 is an innate recognition receptor for RABV, which restricts RABV invasion into the CNS in the early stage of viral infection but also contributes to immunopathology by inducing neuroinflammation.
IMPORTANCE Developing targeted treatment for RABV requires understanding the innate immune response to the virus because early virus clearance is essential for preventing the fatality when the infection has progressed to the CNS. Previous studies have revealed that TLR7 is involved in the immune response to RABV. Here, we establish that TLR7 recognizes RABV and facilitates the production of some interferon-stimulated genes. We also demonstrated that when RABV invades into the CNS, TLR7 enhances the production of inflammatory cytokines which contribute to immunopathology in the mouse brain. Taken together, our findings suggest that treatments for RABV must consider the balance between the beneficial and harmful effects of TLR7-triggered immune responses.
INTRODUCTION
Rabies is an ancient disease that can be traced back 4,000 years and is still a threat to human health today, causing over 59,000 deaths worldwide every year (1, 2). In many developing countries and rural areas with poor medical care, rabies causes heavy economic and social losses. Its causative agent, rabies virus (RABV), is a negative single-stranded RNA virus belonging to the Rhabdoviridae family. After entry into the nerve terminal of the infection site, RABV travels to the spinal cord via retrograde axonal transport and then the brain, where virus replicates rapidly. Rabies is almost always fatal after the onset of symptoms, and no effective treatment is currently available. However, RABV infection sometimes fails to cause rabies in lab or wild animals, indicating the critical importance of early virus clearance (3–6). An effective early response relies on the innate immune system, which can promote a subsequent adaptive immune response, to control early stages of RABV infection. Many questions still exist about the components and mechanisms of the innate immune response that determine RABV pathogenicity.
A number of recent studies have focused on identifying the innate recognition receptors that are responsible for the initial detection of RABV. For example, Toll-like receptor 3 (TLR3) is sensitive to the double-stranded RNA that is an occasional error output during RABV replication (7). Some groups have reported that RABV infection upregulates TLR3 expression and triggers transcription of antiviral genes that are mediated by TLR3, suggesting that TLR3 plays a role in anti-RABV defenses (8, 9). More recently, it has been found that mice deficient in TLR3 showed more resistance to rabies and had lower levels of infection in their brains (10), indicating that TLR3 does not play a critical role in limiting RABV infection. Another promising recognition receptor is RIG-I, because 5′-triphosphate single-stranded RNA and double-stranded RNA produced during viral transcription can be detected by RIG-I. Hornung et al. found that RABV-induced IFN-β production is dependent on RIG-I in both Vero cells and HEK-293 cells (11). Faul et al. found RABV-induced type I IFN production is mediated by IPS-1, which is the common adaptor for both RIG-I and MDA5 (12). However, in the same study, comparable lethality was found in IPS-1−/− mice and wild-type (WT) mice after RABV challenge, indicating that there may be other important pattern recognition receptors involved in RABV clearance.
The present study considers an alternative receptor, TLR7, which has recently been shown to be critical for inducing anti-RABV antibodies. TLR7 is expressed abundantly in plasmacytoid dendritic cells (pDCs) and plays an important role in alpha interferon (IFN-α) production during the immune response. Specifically, after binding its ligand, TLR7 recruits adaptor MyD88, which then forms a complex with TRAF6 and IRAK1 and phosphorylates IRF7. Phosphorylated IRF7 forms a homodimer or heterodimer with IRF3 and enters the nucleus to initiate type I IFN production (13–15). TLR7 recognizes many RNA viruses, including influenza A virus, West Nile virus, human immunodeficiency virus, yellow fever virus, and pneumonia virus (16–21). More closely related to RABV, vesicular stomatitis virus (VSV), which belongs to the same family as RABV, activates pDCs in a TLR7-dependent manner, suggesting that VSV-induced activation of pDCs is specific to TLR7 (22, 23). TLR7 has been implicated in responses specifically to RABV in previous research showing that TLR7−/− mice have higher mortality to RABV infection and that TLR7 augments antibody induction after RABV vaccination (24, 25). Despite suggestive evidence, it is not yet clear whether TLR7 is an innate recognition receptor for RABV, and the role of TLR7 in pathogenic RABV infection is not fully understood. In the present study, we tested the hypothesis that TLR7 is a key recognition receptor for RABV by examining how pDCs are activated by using TLR7−/− and WT mice. We then examined in detail the role of TLR7 in both early and later stages of infection in order to specify how TLR7 contributes to both immune responses and symptoms of the disease.
RESULTS
TLR7 is an innate recognition receptor for RABV.
TLR7 is abundantly expressed by pDCs (26), and the interaction of TLR7 with its ligands triggers pDC activation, reflected as upregulation of cluster of differentiation antigen 86 (CD86) on the surface and the production of IFN-α and interleukin-12 subunit p40 (IL-12p40). We therefore used measures of CD86, IFN-α, and IL-12p40 as indicators of pDC activation. In order to investigate whether TLR7 recognizes RABV, we first generated FMS-like tyrosine 3 ligand (Flt3L)-stimulated bone marrow-derived dendritic cells (FL-DCs), which contains pDCs (CD11c+ B220+ PDCA-1+). Since pDCs can also be activated by the dsDNA genome of the herpes simplex virus containing a high CpG content through the TLR9-MyD88 pathway (18), the agonist for TLR9, CpG-ODN2006, was used as a control. FL-DC cultures were stimulated with either UV-inactivated RABV, live RABV, R848 (a TLR7 agonist), or CpG-ODN 2006 for 24 h. CD86 expression on the surface of pDCs was determined by flow cytometry, and production of IFN-α and IL-12p40 in the supernatant was measured by enzyme-linked immunosorbent assay (ELISA). Expression of CD86 was significantly lower on TLR7−/− pDCs than that on WT pDCs after treatment with live RABV or R848 (Fig. 1B). However, no difference between the genotypes of pDCs was observed when stimulated with CpG-ODN (Fig. 1B). The pattern of results was similar for the production of IFN-α and IL-12p40: both IFN-α (Fig. 1C) and IL-12p40 (Fig. 1D) were lower for TLR7−/− pDCs compared to WT in response to live RABV and R848, indicating their production was dependent on TLR7. However, like measures of CD86, there was no difference between pDC genotypes in response to CpG-ODN (Fig. 1C and D). These results demonstrate that TLR7 is an innate recognition receptor for RABV and that TLR7 triggers pDCs activation following RABV infection.
FIG 1.
TLR7 can recognize RABV in pDCs. pDCs (identified as B220+ CD11C+ PDCA-1+) were prepared by stimulating the mouse bone marrow from TLR7−/− or WT mice with Flt3l for 9 days. After plating, the cells were treated with UV-inactivated RABV, live RABV at an MOI of 10, TLR7 agonist R848 (1 μg/ml), or TLR9 agonist CpG-ODN2006 (20 μg/ml) for 24 h. The cells were harvested. (A) The the CD86 expression was analyzed by flow cytometry. (B) The mean fluorescence intensity (MFI) of CD86 on B220+ CD11C+ PDCA-1+ cells was quantified. Cell supernatants were harvested for quantification of IFN-α (C) and IL-12p40 (D) production using commercial ELISA kits (n = 3). Error bars represent standard deviations (SD). Statistical differences between TLR7−/− and WT mice were determined by using Student t test and are denoted as follows: **, P < 0.01; ***, P < 0.001.
TLR7 deficiency led to impaired antiviral gene expression after intracranial infection with RABV.
After recognition of viral invasion, TLR7 can enhance antiviral genes expression by activating the downstream signaling pathway. We tested whether TLR7 affects the antiviral gene expression during RABV infection in the brain. TLR7−/− and WT mice were infected with a lab-attenuated RABV strain CVS-B2c (100 focus-forming units [FFU]/mouse) via an intracranial (i.c.) route. At 6 days postinfection (dpi), when TLR7−/− and WT mice bore comparable viral loads in the brain (Fig. 2C), the mouse brains were harvested, and we measured the activation of a number of molecules in the TLR7 downstream signaling pathway by Western blotting. The results showed that the levels of phosphorylated IRF7 and STAT1, the activated state of IRF7 and STAT1, dramatically increased in WT mice but were only slightly elevated in TLR7−/− mice after RABV infection (Fig. 2A). In addition, the production of interferon and interferon-stimulated genes (ISGs), including IFN-β, Mx1, OAS1a, IFIT2, IFIT3, IFI44, IFI203, and ISG15, in mouse brains was also impaired in TLR7−/− mice (Fig. 2B). The viral load in the brain was analyzed by qPCR at 3, 6, and 9 dpi. Viral genomic RNA in the whole brain and different parts were comparable in TLR7−/− and WT mice at 3 and 6 dpi. However, the viral load in the whole brain, cerebrum, and brainstem was higher in TLR7−/− mice than in WT mice at 9 dpi (Fig. 2C). These results demonstrate that TLR7 is responsible for inducing interferons and ISGs, which then act to restrict RABV replication in the mouse brain.
FIG 2.
TLR7 deficiency impairs the production of IFNs and ISGs in the CNS after RABV infection. TLR7−/− and WT mice in C57BL/6 background were i.c. infected with RABV CVS-B2c strain (100 FFU/mouse), and at 6 dpi the brains were removed for Western blot (A) and qPCR (B) analyses (naive mice, n = 3; infected mice, n = 7). (C) Viral genomic RNA in the whole brains, the cerebrum, the cerebellum, and the brainstem was analyzed using qPCR (day 3, n = 3; day 6, n = 5; day 9, n = 5). Error bars represent standard errors of the mean (SEM). Statistical differences between TLR7−/− and WT mice were determined using Student t test and are denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TLR7 deficiency causes attenuated pathogenicity after intracranial infection with RABV.
Next, we examined the effect of TLR7 on the pathogenicity of RABV after i.c. infection. TLR7−/− and WT mice were i.c. infected with CVS-B2c (100 FFU/mouse), and the clinical signs were observed for the next 3 weeks. Surprisingly, compared to WT mice, TLR7−/− mice had significantly less weight loss from 6 to 9 dpi (Fig. 3A). In addition, TLR7−/− mice showed significantly delayed death after RABV challenge compared to WT mice, although all infected mice died of rabies (Fig. 3B). Also, a wild-type RABV strain HuNPN01 was tested in the challenge experiment. Similarly, TLR7−/− mice showed significantly delayed death compared to WT mice (Fig. 3C). These results suggest that TLR7 deficiency causes attenuated pathogenicity during RABV infection in the central nervous system (CNS).
FIG 3.

TLR7 deficiency enhances resistance to RABV challenge via the intracranial route. TLR7−/− and WT mice were i.c. infected with RABV CVS-B2c strain (100 FFU/mouse), and clinical signs were monitored daily for 16 days. Body weight changes (A) and survival ratios (B) were calculated (WT, n = 8; TLR7−/−, n = 7). (C) Mice were i.c. infected with RABV HuNPN01 strain (20 FFU/mouse), and survival ratios were calculated (n = 9). Error bars represent the SEM. The asterisks indicate significant differences between infected TLR7−/− and WT mice at the same time points in panel A determined by two-way ANOVA and Sidak’s multiple-comparison test. The survival ratios in panels B and C were analyzed by log-rank (Mantel-Cox) test. Statistical differences are denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TLR7 deficiency downregulates inflammation-related signaling pathways during RABV infection in mouse brains.
We have shown that after i.c. inoculation with RABV, TLR7 can limit virus replication but lead to increased viral pathogenicity. In order to explain this paradox, at 6 dpi the i.c.-infected mouse brains from TLR7−/− and WT mice were collected for transcriptome sequencing (RNA-seq) analysis. By comparing the genotypes, we found that TLR7 deficiency led to downregulation of 155 transcripts and upregulation of 18 transcripts after RABV infection (Fig. 4A). The top eight differential genes analyzed by RNA-seq, including Saa3, IL-12p40, IFN-γ, etc., were confirmed by qPCR (Fig. 4B). The downregulated genes in TLR7−/− mice compared with those in WT mice were then subjected to GO and KEGG pathway analysis, respectively (Fig. 4C and D). Bioinformatics results revealed that pathways related to inflammation, including inflammatory responses, chemotaxis, cytokine-cytokine receptor interaction, and the NF-κB signaling pathway, were downregulated in TLR7−/− mice compared to those in WT mice (Fig. 4C and D).
FIG 4.
TLR7 deficiency downregulates the activation of inflammation related signaling pathways after RABV infection. TLR7−/− and WT mice were i.c. inoculated with RABV CVS-B2c strain (100 FFU/mouse), and brains were harvested and homogenized at 6 dpi. The total RNA of the brains was isolated for RNA-seq analysis. (A) Volcano plot showing quantity of differential regulated transcripts between TLR7−/− and WT mice. (B) Top eight selected upregulated expressed genes in infected WT brains compared to those in TLR7−/− brains were measured by qPCR (naive mice, n = 3; infected mice, n = 7). (C and D) Upregulated genes in infected WT brains compared to those in TLR7−/− brains were subjected to GO and KEGG pathway analysis. Inflammation-related pathways are highlighted with arrows. (E) Transcription levels of indicated inflammatory cytokines are analyzed by qPCR (naive mice, n = 3; infected mice, n = 7). (F) Expression and phosphorylation of the key molecules in inflammation related signaling pathways were confirmed by Western blotting. Error bars represent the SEM. Statistical differences between TLR7−/− and WT mice were determined using Student t test and are denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In order to confirm whether there were fewer inflammation related cytokines and chemokines transcription in the absence of TLR7 after RABV infection, we quantified several inflammatory genes, including TNF-α, IL-1β, IL-6, CCL3, and CXCL9 by qPCR. Results showed that the expression of these inflammatory molecules in TLR7−/− mice was significantly lower than that in WT mice (Fig. 4E). To further specify the role of inflammation related pathways, we used Western blot to measure the basal and phosphorylation levels of the key molecules among NF-κB and mitogen-activated protein (MAPK) signaling pathways, including p65, ERK1/2, p38, and JNK. The results showed that the phosphorylated levels of these molecules in TLR7−/− mice were lower than those in WT mice following RABV infection (Fig. 4F). These results indicate that TLR7 augments the activation of NF-κB and MAPK signaling pathways, which ultimately exacerbates neuroinflammation.
TLR7 deficiency reduces blood-brain barrier permeability during RABV infection.
According to previous research, infection of lab-attenuated RABV increases the permeability of the blood-brain barrier (BBB) (27, 28). In order to identify whether TLR7 is involved in the opening of the BBB after RABV infection, mouse brains were collected and homogenized at 6 dpi. Because inflammatory cytokines were produced in the mouse brains after RABV infection, the supernatant was collected to test its ability to cause tight conjunct protein ZO-1 degradation in bEND.3 cells, using both immunofluorescence and Western blotting. In bEND.3 cells, the immunofluorescence results showed that ZO-1 was abundantly expressed on cells when treated with the supernatant from naive mouse brains but degraded after being treated with the supernatant from RABV-infected WT mouse brains (Fig. 5A). ZO-1 degradation was also observed in bEND.3 cells treated with the supernatant from RABV-infected TLR7−/− mouse brains compared to a mock-treated brain, but the degradation was obviously weaker than for those treated with supernatants from RABV-infected WT mouse brains (Fig. 5A). Western blot results confirmed that treatment with the supernatant from RABV-infected TLR7−/− mouse brains caused less degradation of ZO-1 in bEND.3 cells than that from WT mouse brains (Fig. 5B). Finally, in a transwell model, the permeability of dextran 10000 (10 kDa) in bEND.3 cells treated with the supernatant from RABV-infected TLR7−/− mouse brains was lower than that from WT mouse brains (Fig. 5C).
FIG 5.
TLR7 deficiency decreases the permeability change of BBB after RABV infection. TLR7−/− and WT mice were i.c. infected with RABV CVS-B2c strain (100 FFU/mouse). At 6 dpi, the brains were harvested and homogenized with phenol red-free Dulbecco modified Eagle medium. After centrifugation, the supernatants were harvested and treated with UV for 30 min to eliminate live viruses. bEnd.3 cells were treated with these supernatants for 48 h and then harvested to measure the ZO-1 levels by using immunofluorescence (A) and Western blotting (B). (C) bEnd.3 cells in a transwell model were treated with the supernatants, and a transendothelial permeability assay was performed to evaluate the integrity of bEnd.3 cells (n = 3). (D) At 6 dpi, mice were injected with 100 μl of NaF (100 mg/ml) intraperitoneally, and the BBB integrity was calculated based on NaF uptake in the brains (n = 4). Error bars represent the SEM. Statistical differences between TLR7−/− and WT mice were determined using Student t test and are denoted as follows: *, P < 0.05; ***, P < 0.001. Scale bar, 50 μm.
In order to examine BBB permeability in vivo, we administered sodium fluorescein (NaF), a dye with the molecular weight of 41.99 Da, which is normally shielded by the BBB, into the mice via an intraperitoneal route at 6 dpi. There were significantly lower levels of NaF in the cerebrum, cerebellum, and brain stem, as well as the whole brain, of each TLR7−/− mouse than that in the corresponding parts of brains from WT mice (Fig. 5D). Taken together, the in vitro and in vivo results provide evidence that TLR7 promotes BBB permeability after RABV infection.
TLR7 deficiency decreases the infiltration of immune cells into the CNS after RABV infection.
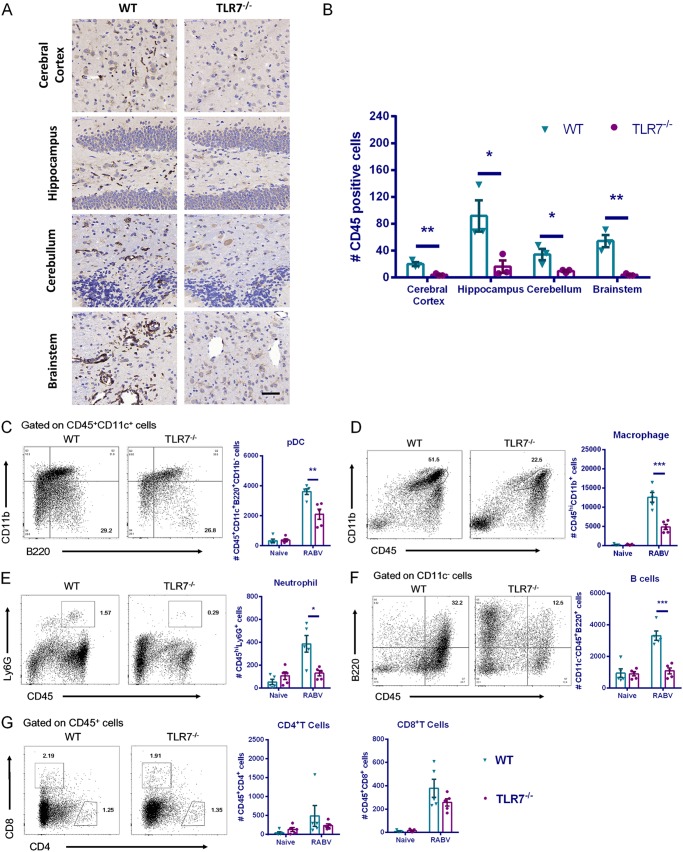
Increased BBB permeability facilitates infiltration of peripheral immune cells into the CNS. To evaluate the quantity of leukocytes in the mouse brains after RABV infection, we performed immunohistochemistry with anti-CD45 antibody at 6 dpi. Significantly fewer CD45+ cells were observed in the cerebral cortex, hippocampus, cerebellum, and brainstem from each TLR7−/− mouse brain than from WT mouse brains (Fig. 6A and B).
FIG 6.
TLR7 deficiency reduces neuroinflammation and immune cell infiltration in mouse brains after RABV infection. TLR7−/− and WT mice were i.c. infected with the RABV CVS-B2c strain (100 FFU/mouse), and the brains were harvested at 6 dpi. Mouse brains were prepared into paraffin sections, and CD45+ cells were identified by immunohistochemistry analysis. (A) Representative immunohistochemistry staining with anti-CD45 antibody. (B) Quantity of CD45 positive cells from five randomly selected fields in each indicated brain region was calculated (n = 3). (C to G) Mouse brains were homogenized, and leukocytes were isolated for analysis of pDCs (CD45+ CD11c+ B220+ CD11b−), macrophages (CD45hi CD11b+), neutrophils (CD45hi Ly6G+), B cells (CD11c− CD45+ B220+), CD4+ T cells (CD45+ CD4+), and CD8+ T cells (CD45+ CD8+) by flow cytometry. A total of 50,000 events were acquired to generate graphs (n = 5). Error bars represent the SEM. Statistical differences between TLR7−/− and WT mice were determined using Student t test and are denoted as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To specify the types of cells that had infiltrated the brain, we homogenized the mouse brains at 6 dpi, and leukocytes were isolated and analyzed by flow cytometry. We observed a number of infiltrated immune cells in RABV-infected mouse brains, including pDCs (CD45+ CD11c+ B220+ CD11b−), macrophages (CD45hi CD11b+), neutrophils (CD45hi Ly6G+), B cells (CD11c− CD45+ B220+), CD4+ T cells (CD45+ CD4+), and CD8+ T cells (CD45+ CD8+). When we compared two genotypes, significantly fewer pDCs, macrophages, neutrophils, and B cells were found in the brains from TLR7−/− mice than in those from WT mice (Fig. 6C to G), indicating that TLR7 facilitates infiltration of pDCs, macrophages, neutrophils, and B cells into the brains after RABV infection.
Excessive inflammation may cause tissue damage in the mouse brains. Hematoxylin and eosin (H&E) staining was used to detect pathological changes in the brains, and the results demonstrated the presence of neuronophagia in the cerebral cortex, gliosis in the hippocampus, and corpora quadrigemina, glia nodule in medulla, and vascular cuff and in pons and medullas of WT mice, whereas all four conditions were found significantly less in the brains of TLR7−/− mice (Fig. 7). Together, these findings support the conclusion that TLR7 enhances the infiltration of specific immune cells into the CNS and that these cells may exacerbate immunopathology.
FIG 7.
Pathological changes in the brains. TLR7−/− and WT mice were i.c. infected with RABV CVS-B2c strain (100 FFU/mouse), and the brains were removed at 6 dpi for H&E staining. Representative H&E staining images are presented, and the quantity of the pathological lesions was calculated. For the cerebral cortex, hippocampus, and corpora quadrigemina, each value represents the average amount of indicated pathological lesions from five randomly selected fields in the indicated brain regions (n = 3); for the pons and medulla, each value represents the total numbers of indicated pathological changes in indicated brain regions (n = 3). Neuronophagia, red arrows; gliosis, blue arrows; glial nodule, green arrows; vascular cuff, orange arrows. Scale bar, 50 μm.
TLR7 inhibits RABV invasion from the periphery into the CNS in the early stage.
We next examined the effect of TLR7 on the pathogenicity of RABV in peripheral infection. TLR7−/− and WT mice were intramuscularly (i.m.) infected with RABV CVS-B2c strain (5 × 105 FFU/mouse) in the hind legs, and then the virus load in the muscles of the infection sites, spinal cords, and brains was analyzed by qPCR at 2, 4, and 6 dpi. The results show that the levels of viral genomic RNA in both the muscles and the spinal cords of TLR7−/− mice were significantly higher than in WT mice at 4 dpi (Fig. 8A and B). However, no difference in virus load in the brains was found between TLR7−/− and WT mice at any time point (Fig. 8C). TLR7−/− mice showed comparable body weight loss with WT mice (Fig. 8D). Consistently, there was no significant difference in the mortality between TLR7−/− and WT mice (Fig. 8E). Similarly, for mice i.m. infected with the RABV HuNPN01 strain, TLR7 also had a dispensable effect on mortality (Fig. 8F). These results indicate that TLR7 limits the spread of RABV from the periphery into the spinal cord at the early stage of RABV infection but has no significant impact on viral entry into the mouse brain.
FIG 8.
TLR7 helps to limit RABV invasion from periphery to the CNS. TLR7−/− and WT mice were i.m. infected with RABV CVS-B2c strain (5 × 105 FFU/mouse), and clinical signs were monitored daily for 3 weeks. (A to C) Viral genomic RNA in the muscles of the infection site (A), in the spinal cords (B), and in the brains (C) was analyzed by qPCR (n = 3 to 4). The body weight changes (D) and survival ratios (E) were calculated (WT, n = 22; TLR7−/−, n = 21). (F) TLR7−/− and WT mice were i.m. infected with RABV HuNPN01 strain (500 FFU/mouse), and the survival ratio was calculated (n = 12). Error bars represent the SEM. Significant differences between infected TLR7−/− and WT mice at the same time points in in panels A to C were determined by Student t test, and those in panel D were determined by two-way ANOVA and Sidak’s multiple-comparison test. The survival ratios in panels E and F were analyzed by log-rank (Mantel-Cox) test. Statistical differences are denoted as follows: *, P < 0.05; ***, P < 0.001.
DISCUSSION
The present study demonstrates that TLR7 is a recognition receptor for RABV and provides specifics of the TLR7-triggered immune response and its relationship to RABV pathogenicity. Our focus on TLR7 was motivated by recent research that indicated the TLR7 affects RABV mortality (24) and RABV vaccine effectiveness (25). We tested the hypothesis that TLR7 is a pattern recognition receptor for RABV by investigating the role of TLR7 in triggering pDC activation and limiting viral replication during RABV infection. As expected, we found evidence that TLR7 recognizes RABV and induces the activation of downstream IFN pathway in both pDCs and mouse brains.
Given our finding that TLR7 triggers the immune response to RABV, we hypothesized that TLR7 would play a protective role during RABV infection and that the absence of TLR7 would render the animals more susceptible to viral infection. Surprisingly, we found that TLR7−/− mice were more resistant to RABV challenge, especially after intracranial inoculation. In particular, we found that compared to WT mice, TLR7−/− mice had (i) significantly higher viral load in the brain; (ii) weaker evidence of the activation of innate signaling pathways, antiviral genes, and inflammatory mediators; (iii) tighter BBB; (iv) attenuated encephalitis and brain injury; and (v) longer survival. Based on these findings and recent studies describing the role of inflammation in the pathogenesis of RABV infection, we conclude that TLR7 plays a dual role during RABV infection: on one hand, TLR7 limits RABV replication by inducing IFNs and ISGs, while on the other hand, TLR7 exacerbates neurological pathology by causing excessive inflammation in the CNS. Previous evidence supports the dual role of inflammation in response to RABV infection. It has been established that inflammation favors the clearance of WT RABV, which induces a very limited inflammatory response in the CNS during infection. Local inflammation in the CNS facilitates the interaction of infected cells and immune cells and augments the production of antibodies (29). However, while neuroinflammation may favor virus clearance, it also causes immunopathology, especially when the virus has spread throughout the brain (30, 31). For example, after mice were infected with fox-isolated RABV in the hind limb footpad, surviving animals demonstrated acute inflammation in the brainstem and spinal cord, which was accompanied by neuronal degeneration in the spinal cord and dorsal roots (32). Likewise, RABV-infected mice with less inflammation developed fewer morphological brain lesions, suggesting that inflammation contributes to neurological impairments (33). These findings have implications for treatment: when mice were infected with RABV CVS strain, treatment with U0126 (a specific inhibitor of MEK1/2) reduced inflammation and histopathological lesions (34). Similarly, therapy for RABV-infected mice that combines inhibitors of TNF-α and MAPK signaling pathways increases the survival ratio (35). In the present study, we found that TLR7 promoted the activation of NF-κB and MAPK signaling pathways after RABV infection and that, in comparison to WT mice, TLR7−/− mice showed both reduced histopathological brain lesions and extended survival. In sum, the previous and current findings indicate that the outcome of RABV infection depends on the balance between the beneficial and detrimental effects of TLR7-triggered immune responses in the CNS.
The role of inflammation in the progress of RABV infection may differ for different virus strains. For example, mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1), a scaffold protein in NF-κB, serves different roles in response to different RABV strains. After infection with the nonpathogenic RABV vaccine strain ERA, all MALT1−/− mice succumb to rabies, suggesting that MALT1 was required for the effective clearance of ERA, whereas MALT1−/− mice show decreased inflammation in the brains and extended survival postinfection with the virulent CVS-11 strain, demonstrating that MALT1 exacerbated the acute immunopathology caused by CVS-11 (36, 37). One reason for the different responses to different strains may be that strains which spread rapidly and facilitate immune cell infiltration into the CNS are more likely to present a risk of immunopathology (30). The different impact of various strains may explain why Li et al. found that TLR7−/− mice were susceptible to a nonlethal dose of RABV challenge with 60% mortality, whereas we found that TLR7−/− mice were more resistant to both CVS-B2c and WT RABV strain HuNPN01 challenges (24). A possible explanation of this discrepancy is that the present study and that of Li et al. used different virus strains, which may differ in the degree to which they trigger the innate immune responses that lead to neuroinflammation in the CNS.
Given the potentially damaging effects of inflammation, it is critical to understand how TLR7 contributes to neural inflammation. In simian immunodeficiency virus infection, increased produced miRNA-21 in extracellular vesicles could activate TLR7 and led to neuroinflammation (38). In a recent study, TLR7 has also been found to mediate brain inflammation and neurodegeneration after EV71 infection by IL-6 production (22). During the progress of RABV infection, an intact BBB impedes peripheral antibodies and immune cells from entering the CNS, limiting their ability to clear virus infection (31). On the other hand, BBB breakdown may cause excessive infiltration of immune cells into the CNS, which may cause detrimental inflammation-related damage (30). In this study, we find that TLR7 contributes to the permeability of the BBB, facilitating the infiltration of peripheral pDCs, macrophages, neutrophils and B cells. In keeping with this role, TLR7−/− mice compared to WT mice had lower levels of immune cells in their brains and consequently reduced inflammation and brain injury.
The mechanism behind the increased BBB permeability during RABV infection is not yet fully understood, although there is relevant evidence from research on some specific cells and cytokines. For example, RAG−/− mice maintained an intact BBB, while JHD−/− mice had increased permeability in their BBBs after being challenged with RABV CVS-F3 strain, implying that T cells rather than B cells mediate this process (27). In particular, Phares et al. showed that CD4+ T cells instead of CD8+ T cells play a role in RABV-induced BBB permeability (39). Another possibility is that IFN-γ production triggered by TLR7 affects the permeability of the BBB, because the level of IFN-γ is closely related to BBB leakage and virus clearance after RABV infection (39, 40). IFN-γ increases BBB permeability by promoting the production of peroxynitrite, which enhances vascular permeability (39, 41, 42). In our study, IFN-γ production is significantly downregulated in TLR7−/− mice compared to WT mice (Fig. 4B). In Japanese encephalitis virus infection, TLR7-MyD88 signaling axis induced a profound production of IL-6, which could led to disruption of endothelial barrier integrity by activate pericytes (43). We found that TLR7 increased IL-6 production after RABV infection (Fig. 4E). All of these findings indicate that the permeability change in BBB mediated by TLR7 may be due, at least in part, to increased IFN-γ and IL-6 production. It may be that CNS-resident cells that express TLR7, including astrocytes and microglia, recognize RABV upon infection and produce cytokines and chemokines that facilitate the breakdown of BBB.
In summary, our study provides evidence that TLR7 is an innate recognition receptor for RABV. More importantly, understanding that the TLR7 acts as a double-edged sword in the pathogenesis of RABV will help us to reevaluate the role of innate immune system in responding to CNS diseases and aid us in selecting potential drug targets for effective therapies in the future.
MATERIALS AND METHODS
Viruses and mice.
RABV CVS-B2c strain propagated on BSR cells was used for pDC activation and the RABV challenge experiment (for i.c. infection, 100 FFU/mouse; for i.m. infection, 5 × 105 FFU/mouse). A wild-type RABV strain HuNPN01 isolated from rabid pigs in Hunan province, China (44), was propagated in suckling ICR mouse brains and then used in the challenge experiment (for i.c. infection, 20 FFU/mouse; for i.m. infection, 500 FFU/mouse). TLR7−/− mice (C57BL/6 background; stock no. 008380; 129S1-TLR7tm1Flv/J) were obtained from Jackson Laboratories and bred in the Animal Facility at Huazhong Agricultural University. Wild-type C57BL/6 mice of the same age and gender cohoused with TLR7−/− mice were obtained from the Center for Disease Control of Hubei province, China. All animals involved in the study were housed and handled following the Guidance on the Treatment of Experimental Animals issued by the Ministry of Science and Technology of the People’s Republic of China. All animal experiments performed in the present study were approved by the Scientific Ethics Committee of Huazhong Agricultural University (permit HZAUMO-2015-023).
Preparation and stimulation of pDCs.
Bone marrow cells were harvested from the femurs and tibiae of mice. A single cell suspension was prepared and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and 100 ng/ml human Flt3 ligand (PeproTech, Rocky Hill, NJ) for 9 days, to use as pDCs. Cells stimulated with 1 μg/ml R848 (Invivogen, CA), 5 μg/ml CpG-ODN-2006 (Invivogen, San Diego, CA), and UV-inactivated RABV or live RABV at multiplicity of infection (MOI) of 10 were then harvested for the flow cytometry analysis used to quantify CD86 expression gated on pDCs (CD11c+ B220+ PDCA-1+), and supernatants were subjected to ELISA for cytokine production.
Quantitative real-time PCR.
Total RNA was extracted by TRIzol reagent (Invitrogen) and treated with DNase. Then, 1.6 μg of total RNA from a sample was converted to cDNA in a reaction volume of 20 μl by using FSQ-201 ReverTra Ace (Toyobo, Osaka, Japan). Quantitative PCR (qPCR) analysis by SYBR green (Bio-Rad, Richmond, CA) was performed on a 7300 real-time PCR system (Applied Biosystems, Foster City, CA). The primers used in this study are listed in Table 1 .
TABLE 1.
Primers used for qPCR
| Primer | Sequences (5′–3′) |
|---|---|
| Viral RNA-F | CCTCGTCGTCAGAGTTGACA |
| Viral RNA-R | GAGGAATTCTTCGGGAAAGG |
| IFN-β-F | AGATGTCCTCAACTGCTCTC |
| IFN-β-R | AGATTCACTACCAGTCCCAG |
| Mx1-F | CAACTGGAATCCTCCTGGAA |
| Mx1-R | GGCTCTCCTCAGAGGTATCA |
| OAS1a-F | CCAAGGTGGTGAAGGGTGG |
| OAS1a-R | ACCACCAGGTCAGCGTCTGA |
| IFIT2-F | GCCAAGGAACACCAAAGATTG |
| IFIT2-R | AAGTCCAAGATGAAGGTGCC |
| IFIT3-F | GCAGAAGATGAGTCCTTGGA |
| IFIT3-R | CCTCAGCTTCCCCTAAGCAT |
| IFI44-F | AACTGACTGCTCGCAATAATGT |
| IFI44-R | GTAACACAGCAATGCCTCTTGT |
| IFI203-F | TGTGAGAGAATTAAGGCACCAAGGAGA |
| IFI203-R | ACTTTCAACACCATCACTTGTTTGGGA |
| ISG15-F | GATTCACACCTACACCGTCCG |
| ISG15-R | GCATCAGCCTTTGTTGGTCC |
| Saa3-F | TTGATCCTGGGAGTTGACAG |
| Saa3-R | CACTCATTGGCAAACTGGTC |
| IL-12p40-F | TTGAAAGGCTGGGTATCGGT |
| IL-12p40-R | GAATTTCTGTGTGGCACTGG |
| Chil3-F | GGCCCACCAGGAAAGTACAC |
| Chil3-R | AGACCACGGCACCTCCTAAA |
| IFN-γ-F | GACTGTGATTGCGGGGTTGT |
| IFN-γ-R | GGCCCGGAGTGTAGACATCT |
| CXCR6-F | AGCACACTTCACTCTGGAAC |
| CXCR6-R | TTGAAGAGCCAGAAATCTCCC |
| IL-1rl1-F | GTCTCAAGAGATCGTCTGAAG |
| IL-1rl1-R | CGATTCAGGGCTTCTGATAAC |
| Igkc-F | CCACCATCCAGTGAGCAGTT |
| Igkc-R | TCGTCCTTGGTCAACGTGAG |
| Hbb-bs-F | AGGTGAACGCCGATGAAGTT |
| Hbb-bs-R | ATGCAGCTTGTCACAGTGGA |
| TNF-α-F | TCACTGGAGCCTCGAATGTC |
| TNF-α-R | GTGAGGAAGGCTGTGCATTG |
| IL-1β-F | CCTCTGATGGGCAACCACTT |
| IL-1β-R | TTCATCCCCCACACGTTGAC |
| IL-6-F | ACAGAAGGAGTGGCTAAGGA |
| IL-6-R | CGCACTAGGTTTGCCGAGTA |
| CCL3-F | TGCCCTTGCTGTTCTTCTCT |
| CCL3-R | GTGGAATCTTCCGGCTGTAG |
| CXCL9-F | CGAGGCACGATCCACTACAA |
| CXCL9-R | AGGCAGGTTTGATCTCCGTT |
Western blotting.
Tissues and cells were lysed in a radioimmunoprecipitation assay buffer, and supernatants were quantified with a BCA kit (Beyotime, Haimen, China). Samples were boiled for 5 min with SDS loading buffer, and 10 to 60 μg of total protein from a sample was resolved on SDS-PAGE gels. Proteins were transferred to polyvinylidene difluoride membranes. Membranes were incubated first with the indicated antibodies, including p-IRF7 (S437/438, catalog no. 24129), p-STAT1 (Y701, catalog no. 9167), STAT1 (catalog no. 14994), p-p65 (S536, catalog no. 3033), p65 (catalog no. 8242), p-ERK1/2 (T202/Y204, catalog no. 4370), ERK1/2 (catalog no. 4695), p-p38 (T180/Y204, catalog no. 4511), p38 (catalog no. 9212), p-JNK (T183/Y185, catalog no. 9251), JNK (catalog no. 9252) (Cell Signaling Technology, MA) and IRF7 (catalog no. ab62505; Abcam, Cambridge, MA), β-actin (catalog no. AC004), GAPDH (catalog no. AC202) (Abclonal, Wuhan, China), and then with horseradish peroxidase-conjugated secondary antibodies. Proteins were detected by an Amersham Imager 600 (GE Healthcare, PA).
RNA-seq analysis.
Total RNA was extracted from mouse brains. RNA integrity was evaluated with a Bioanalyzer 2100 system (Agilent Technologies, Carpinteria, CA). RNA samples with high integrity were chosen for RNA-Seq analyses. RNA-Seq library construction was performed using the NEB Next Ultra Directional RNA Library Prep kit for Illumina (NEB, Ipswich, MA), and four index codes were added to attribute sequences to different samples. The clustering of the index-coded samples was performed on a cBot cluster generation system using the TruSeq PE Cluster kit v3-cBot-HS (Illumina, Foster City, CA). RNA-seq libraries were sequenced on an Illumina HiSeq X-Ten platform to generate 100-bp single-end reads. Raw reads were preprocessed to remove low quality regions and adapter sequences. Read counts of each gene were summarized by the HTSeq-count30. Differentially expressed genes were identified by R package edgeR. The expression of each gene was normalized to reads per million (rpm) to compare different samples. Genes with low expression levels were removed, and genes with an expression level of at least 1 rpm in at least two samples were kept. Genes with fold change of ±1.5 and an adjusted P value of <0.05 were considered to be differentially expressed and used for GO and KEGG annotation analysis with DAVID.
BBB integrity analysis.
BBB permeability was determined by measuring sodium fluorescein (NaF) uptake as described in previous research with minor modifications (45). Briefly, mice were injected with 100 μl of NaF (100 mg/ml) intraperitoneally. After 10 min to allow circulation of NaF, the mice were anesthetized. Cardiac blood was collected, and brains were removed after mice were intracardially perfused with phosphate-buffered saline (PBS). The fluorescence in serum and brain homogenate samples was analyzed by a FLUOstar Omega microplate reader (BMG Labtech, Cary, NC) with excitation at 485 nm and emission at 530 nm. Standards (125 to 4,000 g/ml) were prepared to calculate the NaF content. NaF uptake into tissue was calculated according to the ratio (μg of fluorescence brain/mg of tissue)/(μg of fluorescence sera/ml of blood) in order to normalize values for blood levels of the dye at the time of tissue collection.
Transendothelial permeability assay.
A transendothelial permeability assay was carried out as previously described (46). bEnd.3 cells were seeded into 0.4-μm-pore-size transwell filters until reaching 100% confluence. After being treated with the indicated UV-inactivated brain supernatants, FITC-dextran 10000 (10 kDa; Sigma-Aldrich, St. Louis, MO) was added apically at 1 mg/ml for 30 min. Samples were removed from the lower chamber for fluorescence measurements (excitation, 492 nm; emission, 520 nm).
Histopathology and immunohistochemistry.
Mice were intracardially perfused with PBS. Brains were removed and placed in 4% paraformaldehyde at 4°C for more than 16 h. Samples were embedded in paraffin and sectioned (4 μm) after dehydration and wax immersion. For the histopathological analysis, sections were stained with H&E. For the immunohistochemical analysis, sections were processed using antigen retrieval and endogenous peroxidase quenching, followed by anti-CD45 antibody (catalog no. ab10558; Abcam) staining.
Isolation of leukocytes in the CNS.
Leukocytes from the brains of infected mice were isolated and analyzed as described in previous research (47). Mice were intracardially perfused with PBS. Brains were removed and digested with collagenase D and DNase I to disperse the tissue into single-cell suspensions. Viable cells were separated by discontinuous Percoll gradient (70/30%) centrifugation. Cells were stained for CD45 (30-F11, catalog no. 553079), B220 (RA3-6B2, catalog no. 561881), CD11c (N418, catalog no. 565872), CD11b (M1/70, catalog no. 557397), CD8 (53-6.7, catalog no. 553032), Ly6G (1A8, catalog no. 560602) (BD Pharmingen, San Diego, CA) and CD4 (GK1.5, catalog no. 100412; BioLegend, San Diego, CA) with directly conjugated antibodies. Data collection and analysis were performed using a BD FACSVerse flow cytometer (BD Biosciences, La Jolla, CA) and FlowJo software (TreeStar).
Statistical analysis.
Data were analyzed using Prism software (GraphPad, La Jolla, CA). Significant differences were determined by using two-way analysis of variance (ANOVA) and Sidak’s multiple-comparison test or Student t tests. The survival ratio was analyzed by a log-rank (Mantel-Cox) test. Asterisks in figures indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Data availability.
RNA-seq data are available in the NCBI Gene Expression Omnibus under GEO accession number GSE138164.
ACKNOWLEDGMENTS
This study was partially supported by the National Program for Key Research Projects of China (grant 2016YFD0500400 to L.Z.) and the National Natural Science Foundation of China (grant numbers 31522057 and 31872451 to L.Z. and grant 31720103917 to Z.F.F.).
REFERENCES
- 1.Schnell MJ, McGettigan JP, Wirblich C, Papaneri A. 2010. The cell biology of rabies virus: using stealth to reach the brain. Nat Rev Microbiol 8:51–61. doi: 10.1038/nrmicro2260. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2013. WHO expert consultation on rabies, 2nd report. World Health Organ Tech Rep Ser 2013:1–139. [PubMed] [Google Scholar]
- 3.Araujo DB, Martorelli LA, Kataoka AP, Campos AC, Rodrigues CS, Sanfilippo LF, Cunha ES, Durigon EL, Favoretto SR. 2014. Antibodies to rabies virus in terrestrial wild mammals in native rainforest on the north coast of Sao Paulo State, Brazil. J Wildl Dis 50:469–477. doi: 10.7589/2013-04-099. [DOI] [PubMed] [Google Scholar]
- 4.Machado GP, Antunes JM, Uieda W, Biondo AW, Cruvinel TM, Kataoka AP, Martorelli LF, de Jong D, Amaral JM, Hoppe EG, Guerra Neto G, Megid J. 2012. Exposure to rabies virus in a population of free-ranging capuchin monkeys (Cebus apella nigritus) in a fragmented, environmentally protected area in southeastern Brazil. Primates 53:227–231. doi: 10.1007/s10329-012-0306-6. [DOI] [PubMed] [Google Scholar]
- 5.Jorge RS, Pereira MS, Morato RG, Scheffer KC, Carnieli P Jr, Ferreira F, Furtado MM, Kashivakura CK, Silveira L, Jacomo AT, Lima ES, de Paula RC, May-Junior JA. 2010. Detection of rabies virus antibodies in Brazilian free-ranging wild carnivores. J Wildl Dis 46:1310–1315. doi: 10.7589/0090-3558-46.4.1310. [DOI] [PubMed] [Google Scholar]
- 6.Almeida MF, Massad E, Aguiar EA, Martorelli LF, Joppert AM. 2001. Neutralizing antirabies antibodies in urban terrestrial wildlife in Brazil. J Wildl Dis 37:394–398. doi: 10.7589/0090-3558-37.2.394. [DOI] [PubMed] [Google Scholar]
- 7.Ostertag D, Hoblitzell-Ostertag TM, Perrault J. 2007. Overproduction of double-stranded RNA in vesicular stomatitis virus-infected cells activates a constitutive cell-type-specific antiviral response. J Virol 81:503–513. doi: 10.1128/JVI.01218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prehaud C, Megret F, Lafage M, Lafon M. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol 79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson AC, Rossiter JP, Lafon M. 2006. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J Neurovirol 12:229–234. doi: 10.1080/13550280600848399. [DOI] [PubMed] [Google Scholar]
- 10.Menager P, Roux P, Megret F, Bourgeois JP, Le Sourd AM, Danckaert A, Lafage M, Prehaud C, Lafon M. 2009. Toll-like receptor 3 (TLR3) plays a major role in the formation of rabies virus Negri Bodies. PLoS Pathog 5:e1000315. doi: 10.1371/journal.ppat.1000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 12.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. 2010. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog 6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda K, Yanai H, Mizutani T, Negishi H, Shimada N, Suzuki N, Ohba Y, Takaoka A, Yeh WC, Taniguchi T. 2004. Role of a transductional-transcriptional processor complex involving MyD88 and IRF-7 in Toll-like receptor signaling. Proc Natl Acad Sci U S A 101:15416–15421. doi: 10.1073/pnas.0406933101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, Terai K, Matsuda M, Inoue J, Uematsu S, Takeuchi O, Akira S. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 15.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, Coban C, Ishii KJ, Kawai T, Takeuchi O, Akira S. 2005. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-α induction. J Exp Med 201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson S, Kaiko G, Loh Z, Lalwani A, Zhang V, Spann K, Foo SY, Hansbro N, Uematsu S, Akira S, Matthaei KI, Rosenberg HF, Foster PS, Phipps S. 2011. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol 186:5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 18.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandl JN, Akondy R, Lawson B, Kozyr N, Staprans SI, Ahmed R, Feinberg MB. 2011. Distinctive TLR7 signaling, type I IFN production, and attenuated innate and adaptive immune responses to yellow fever virus in a primate reservoir host. J Immunol 186:6406–6416. doi: 10.4049/jimmunol.1001191. [DOI] [PubMed] [Google Scholar]
- 20.Schlaepfer E, Audige A, Joller H, Speck RF. 2006. TLR7/8 triggering exerts opposing effects in acute versus latent HIV infection. J Immunol 176:2888–2895. doi: 10.4049/jimmunol.176.5.2888. [DOI] [PubMed] [Google Scholar]
- 21.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. 2009. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M, Brzoza KL, Hiltbold EM. 2006. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J Virol 80:2194–2205. doi: 10.1128/JVI.80.5.2194-2205.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed M, Mitchell LM, Puckett S, Brzoza-Lewis KL, Lyles DS, Hiltbold EM. 2009. Vesicular stomatitis virus M protein mutant stimulates maturation of Toll-like receptor 7 (TLR7)-positive dendritic cells through TLR-dependent and -independent mechanisms. J Virol 83:2962–2975. doi: 10.1128/JVI.02030-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Faber M, Dietzschold B, Hooper DC. 2011. The role of Toll-like receptors in the induction of immune responses during rabies virus infection. Adv Virus Res 79:115–126. doi: 10.1016/B978-0-12-387040-7.00007-X. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z, Li Y, Zhou M, Lv L, Wu Q, Chen C, Zhang Y, Sui B, Tu C, Cui M, Chen H, Fu ZF, Zhao L. 2019. Toll-Like receptor 7 enhances rabies virus-induced humoral immunity by facilitating the formation of germinal centers. Front Immunol 10:429. doi: 10.3389/fimmu.2019.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, Akira S, Reis e Sousa C. 2003. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DCs correlates with unresponsiveness to imidazoquinolines. Eur J Immunol 33:827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 27.Hooper DC, Phares TW, Fabis MJ, Roy A. 2009. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl Trop Dis 3:e535. doi: 10.1371/journal.pntd.0000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy A, Hooper DC. 2008. Immune evasion by rabies viruses through the maintenance of blood-brain barrier integrity. J Neurovirol 14:401–411. doi: 10.1080/13550280802235924. [DOI] [PubMed] [Google Scholar]
- 29.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. 1998. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol 72:3711–3719. doi: 10.1128/JVI.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooper DC, Roy A, Barkhouse DA, Li J, Kean RB. 2011. Rabies virus clearance from the central nervous system. Adv Virus Res 79:55–71. doi: 10.1016/B978-0-12-387040-7.00004-4. [DOI] [PubMed] [Google Scholar]
- 31.Laothamatas J, Hemachudha T, Mitrabhakdi E, Wannakrairot P, Tulayadaechanont S. 2003. MR imaging in human rabies. Am J Neuroradiol 24:1102–1109. [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson AC, Reimer DL. 1989. Pathogenesis of experimental rabies in mice: an immunohistochemical study. Acta Neuropathol 78:159–165. doi: 10.1007/bf00688204. [DOI] [PubMed] [Google Scholar]
- 33.Martina BEE, Smreczak M, Orlowska A, Marzec A, Trebas P, Roose JM, Zmudzinski J, Gerhauser I, Wohlsein P, Baumgartner W, Osterhaus A, Koraka P. 2018. Combination drug treatment prolongs survival of experimentally infected mice with silver-haired bat rabies virus. Vaccine doi: 10.1016/j.vaccine.2018.05.065. [DOI] [PubMed] [Google Scholar]
- 34.Manjunatha V, Singh KP, Saminathan M, Singh R, Shivasharanappa N, Umeshappa CS, Dhama K, Manjunathareddy GB. 2017. Inhibition of MEK-ERK1/2-MAP kinase signaling pathway reduces rabies virus induced pathologies in mouse model. Microb Pathog 112:38–49. doi: 10.1016/j.micpath.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 35.Marosi A, Dufkova L, Forro B, Felde O, Erdelyi K, Sirmarova J, Palus M, Honig V, Salat J, Tikos R, Gyuranecz M, Ruzek D, Martina B, Koraka P, Osterhaus A, Bakonyi T. 2018. Combination therapy of rabies-infected mice with inhibitors of proinflammatory host response, antiviral compounds and human rabies immunoglobulin. Vaccine 37:4724–4735. doi: 10.1016/j.vaccine.2018.05.066. [DOI] [PubMed] [Google Scholar]
- 36.Kip E, Staal J, Tima HG, Verstrepen L, Romano M, Lemeire K, Suin V, Hamouda A, Baens M, Libert C, Kalai M, Van Gucht S, Beyaert R. 2018. Inhibition of MALT1 decreases neuroinflammation and pathogenicity of virulent rabies virus in mice. J Virol 92:e00720-18. doi: 10.1128/JVI.00720-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kip E, Staal J, Verstrepen L, Tima HG, Terryn S, Romano M, Lemeire K, Suin V, Hamouda A, Kalai M, Beyaert R, Van Gucht S. 2018. MALT1 controls attenuated rabies virus by inducing early inflammation and T cell activation in the brain. J Virol 92:e02029-17. doi: 10.1128/JVI.02029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS. 2015. MiR-21 in extracellular vesicles leads to neurotoxicity via TLR7 signaling in SIV neurological disease. PLoS Pathog 11:e1005032. doi: 10.1371/journal.ppat.1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. 2007. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-α is neither necessary nor sufficient. J Immunol 178:7334–7343. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 40.Phares TW, Kean RB, Mikheeva T, Hooper DC. 2006. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol 176:7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 41.Hooper DC, Kean RB, Scott GS, Spitsin SV, Mikheeva T, Morimoto K, Bette M, Rohrenbeck AM, Dietzschold B, Weihe E. 2001. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J Immunol 167:3470–3477. doi: 10.4049/jimmunol.167.6.3470. [DOI] [PubMed] [Google Scholar]
- 42.Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. 2000. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol 165:6511–6518. doi: 10.4049/jimmunol.165.11.6511. [DOI] [PubMed] [Google Scholar]
- 43.Chang CY, Li JR, Ou YC, Lin SY, Wang YY, Chen WY, Hu YH, Lai CY, Chang CJ, Chen CJ. 2017. Interplay of inflammatory gene expression in pericytes following Japanese encephalitis virus infection. Brain Behav Immun 66:230–243. doi: 10.1016/j.bbi.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, Yu X, Wang L, Lu Z, Liu H, Xuan H, Hu Z, Tu C. 2008. An outbreak of pig rabies in Hunan province, China. Epidemiol Infect 136:504–508. doi: 10.1017/S0950268807008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chai Q, She R, Huang Y, Fu ZF. 2015. Expression of neuronal CXCL10 induced by rabies virus infection initiates infiltration of inflammatory cells, production of chemokines and cytokines, and enhancement of blood-brain barrier permeability. J Virol 89:870–876. doi: 10.1128/JVI.02154-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Wang Y, Yu L, Cao S, Wang K, Yuan J, Wang C, Wang K, Cui M, Fu ZF. 2015. Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol 89:5602–5614. doi: 10.1128/JVI.00143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao L, Toriumi H, Kuang Y, Chen H, Fu ZF. 2009. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol 83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-seq data are available in the NCBI Gene Expression Omnibus under GEO accession number GSE138164.