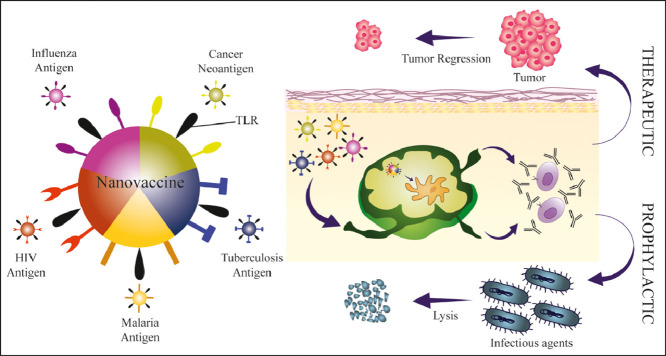
Abstract
Vaccines activate suitable immune responses to fight against diseases but can possess limitations such as compromised efficacy and immunogenic responses, poor stability, and requirement of adherence to multiple doses. ‘Nanovaccines’ have been explored to elicit a strong immune response with the advantages of nano-sized range, high antigen loading, enhanced immunogenicity, controlled antigen presentation, more retention in lymph nodes and promote patient compliance by a lower frequency of dosing. Various types of nanoparticles with diverse pathogenic or foreign antigens can help to overcome immunotolerance and alleviate the need of booster doses as required with conventional vaccines. Nanovaccines have the potential to induce both cell-mediated and antibody-mediated immunity and can render long-lasting immunogenic memory. With such properties, nanovaccines have shown high potential for the prevention of infectious diseases such as acquired immunodeficiency syndrome (AIDS), malaria, tuberculosis, influenza, and cancer. Their therapeutic potential has also been explored in the treatment of cancer. The various kinds of nanomaterials used for vaccine development and their effects on immune system activation have been discussed with special relevance to their implications in various pathological conditions.
Statement of Significance
Interaction of nanoparticles with the immune system has opened multiple avenues to combat a variety of infectious and non-infectious pathological conditions. Limitations of conventional vaccines have paved the path for nanomedicine associated benefits with a hope of producing effective nanovaccines. This review highlights the role of different types of nanovaccines and the role of nanoparticles in modulating the immune response of vaccines. The applications of nanovaccines in infectious and non-infectious diseases like malaria, tuberculosis, AIDS, influenza, and cancers have been discussed. It will help the readers develop an understanding of mechanisms of immune activation by nanovaccines and design appropriate strategies for novel nanovaccines.
Keywords: Nanovaccine, Prophylactic, Therapeutic, Biomimetic, Antibody-mediated immunity, Cell-mediated immunity, Immune activation, Memory responses
Graphical abstract
1. Introduction
Since the advent of the first vaccine centuries ago, researchers have been trying to find the answer of most common pandemic diseases through immunotherapy. Vaccination has successfully managed to eradicate a lot of prevalent diseases, but associated off-target responses, lack of prolonged protection across variable pathogenic strains and allergy, limits its horizon for the prevention or treatment of other globally prevalent diseases like tuberculosis, AIDS, malaria, influenza and cancer. Though prophylactic vaccines use live-attenuated or inactivated pathogens, lack of control over immune response to certain pathogenic portions or co-administered adjuvants and pathogen reversion possibility pose an area of concern over their safety and efficacy. Similarly, these limitations are specific to any therapy involving target-specific immune activation or tolerance [1].
1.1. Nanovaccines outperform free antigen-based immunization
With the advancement in the field of nanotechnology, ‘Nanovaccines’ have been explored by combining pathogen-specific antigens with synthetic or natural nanomaterials in an effort to elicit controlled immune response. This requires the use of necessary components of the pathogen known as subunits to make vaccines more tunable and safer. Such subunits can be peptides, proteins, membranes, polysaccharides, capsules and toxins. Vaccines comprising of these subunits possess more safety, controlled immune response and protection against diverse pathogenic strains [2]. In addition, nanoparticles have the ability to impart more stability with high antigen loading and less proteasomal degradation of antigenic subunits. Small and more specific subunits often tend to be less immunogenic, which can be overcome by the use of adjuvants that produce co-immunostimulatory or immunomodulatory signals. Side effects associated with such conventional adjuvants with individual-specific response, immunotolerance to the target antigen and unwanted reactions towards self-antigens limit their use [3]. Therefore, biocompatible nanoparticles can also be directly used as adjuvants and may mitigate the need of strong conventional adjuvants (e.g., alum). Another reason associated with low subunit immunogenicity is their low cellular internalization and rapid clearance from the body. Nanoparticles with tunable physicochemical properties can overcome this limitation by enhancing circulation time, bio-accumulation in lymphoid organs and efficient targeting of immune cells. They can also increase cross-presentation by antigen-presenting cells (APCs) and activate the immune system at much lower doses. For instance, poly (lactic-co-glycolic acid) (PLGA)-based nanoparticles enhanced the cross presentation of ovalbumin through major histocompatibility complex-I (MHC-I) in bone marrow derived primary dendritic cells due to which T cells were stimulated at 1000-fold lower concentrations as compared to soluble antigen [4].
Different kinds of nanoparticles (lipid-based, polymeric, inorganic and protein/peptide-based) have been extensively used as adjuvants, immunogens and antigen delivery vehicles for immune activation [5]. Most of the synthetic nanoparticles interact with APCs structurally to increase internalization rather than functionally as they lack specific binding locations for cell receptors. On the contrary, protein-based nanoparticles can interact both structurally and functionally as they can carry antigens as well as interact with the pattern associated receptors on APCs [6]. Nanovaccines have the ability to induce both the components of human adaptive immunity i.e. cell-mediated as well as antibody-mediated immunity with induction of memory response. Prolonged release of antigens from nanoparticle depots can cause enhanced stimulation for a long period of time, alleviating the need for frequent booster doses and therefore, nanovaccines can be used as both prophylactic or therapeutic which involves its administration before or after the occurrence of the disease respectively. However, the safety, biodistribution and residence of the nanoparticles must be optimized to obtain preferred immune responses towards nanoparticle-based vaccines.
In this review, the potential and pros and cons of nanoparticle-based vaccines are explored. The role of different nanovaccines in activating various arms of immunity with an intent to abate the use of frequent booster doses as vaccines for tuberculosis, malaria, HIV (human immunodeficiency virus), influenza, and cancer are discussed. The applications and advantages of nanovaccines against various infectious and non- infectious diseases are also further delineated in this article.
2. Immunological mechanism of nanovaccines
The ultimate goal for vaccination is to generate safe and efficient primary as well as secondary immune responses in the body. In a simplified way, primary immune responses protect the body from potential damages that might occur on first exposure of the pathogen or antigen. On the other hand, secondary immune responses, which are comparatively rapid and stronger, are elicited depending on the immunological memory generated during first exposure and protect the body from future encounters of the same epitope [7]. Nanoparticle-based vaccine delivery emerged as an appealing platform for boosting both primary as well as secondary immune responses in the body [8,9]. There are multiple mechanisms by which nanovaccines help enhancing immune responses. Nanovaccines facilitate uptake of antigens in dendritic cells (DCs), which can further be enhanced by decorating the particle surface with ligands that selectively recognize receptors on DCs (refer Fig. 1 ). For example, ligands including Fc fragment, mannose and anti-DEC-205 (mAb) have been widely explored which selectively binds Fc receptors, mannose receptors and DEC-205 receptors respectively on DCs [10,11]. In addition to efficient internalization, ability to co-deliver multiple-antigens and adjuvants, inherent adjuvant property, rapid lymph node drainage and efficient antigen presentation on DC’s surface are other predominant mechanisms by which nanovaccines help increase the duration as well as the magnitude of the immune responses in immunized animals [12,13]. Interestingly, these properties of nanoparticles have been substantially engineered to achieve desirable immune response for developing either prophylactic or therapeutic vaccines [9]. For designing a safe and effective nanovaccine, it is imperative to understand the mechanism by which the nanovaccine activates both innate and adaptive immunity in the body [14].
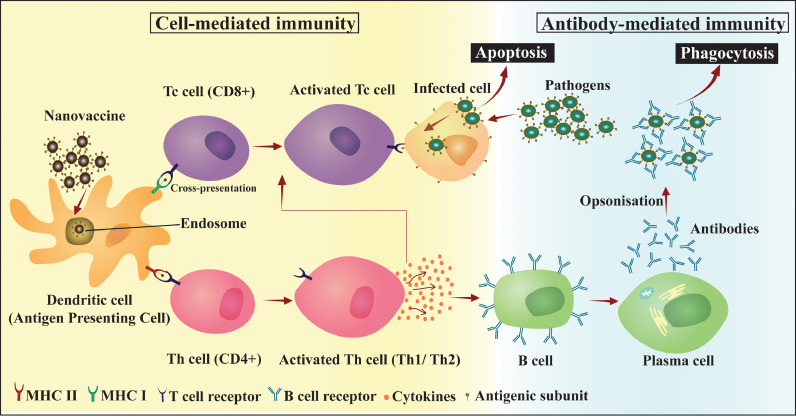
Fig. 1.
Activation of adaptive immunity by nanovaccines: uptake and presentation of antigenic subunit by APCs elicits cell-mediated and antibody-mediated immune response leading to apoptosis of infected cells and phagocytosis of antibody-pathogen complex.
2.1. Activation of innate immunity by nanovaccines
Prophylactic nanovaccines are generally administered subcutaneously, intramuscularly, or intranasally whereas therapeutic vaccines (e.g. for cancer treatment) are injected intravenously. Depending on the route of immunization, nanovaccines first encounter immune cells including neutrophils, macrophages, DCs and natural killer (NK) cells which quickly recognize the nanovaccine (foreign particle) based on the pathogen associated molecular patterns (PAMPs) associated with them [15]. PAMPs (e.g. bacterial LPS, teichoic acid, lectins, oligonucleotides or material from the carrier nanoparticle etc.) serve as the ligands for PRRs [e.g. toll-like receptors (TLRs), C-type lectin receptors (CLRs), retinoic acid-inducible gene-I-like receptors (RLRs) etc.] abundantly present on these cells [16,17]. Thus, interaction of PAMPs with PRR trigger endocytosis of larger particles (usually >500 nm) preferentially by macrophages and smaller particles by DCs. Engulfment of nanovaccines by macrophages results in gradual degradation of nanovaccine particles and the encapsulated antigens. To prevent this, nanovaccine particles have been engineered to survive the attack by macrophages and facilitate direct delivery to APCs [18,19]. Additionally, depending on the type of PAMPs, neutrophils and macrophage secrete a variety of cytokines and chemokines which further activate APCs. Besides PAMPs, there are several unknown mechanisms which can activate innate immunity for example, induction of immune responses (potential adjuvant effect) by glycolic acid component of PLGA co-polymer [20] or by cationic liposomes [21]. Eventually, the secreted cytokines and chemokines stimulate maturation and activation of APCs (mainly DCs) which initiate strong adaptive immune response (refer Fig. 1). Therapeutic nanovaccines developed for treatment of metastatic cancer directly target DCs for strengthening adaptive immune responses in body [22]. Activated DCs serve as the link between innate and adaptive immunity.
2.2. Activation of adaptive immunity by nanovaccines
Adaptive immunity generally has two arms; cell-mediated and antibody-mediated immunity. Adaptive immune responses are generated within few days to several weeks and last longer. Essentially, both types of immunity are necessary for a protective and prolonged immune response. However, their requirement is different in both prophylactic and therapeutic nanovaccines. For instance, strong cell-mediated cytotoxic responses are more desirable in case of therapeutic nanovaccine in order to effectively kill the metastatic cancer cells [23].
2.2.1. Activation of cell-mediated immunity
Vaccination provokes cell-mediated immunity generated by B cells and T cells which serve to neutralize the pathogen/antigen and simultaneously stimulating the body for developing immunological memory [24]. The CMI responses are triggered by migration of activated DCs (following uptake of nanovaccine particle) to lymphatic organs (spleen and lymph noted). Activated DCs present their antigens via MHC class I receptor to CD8+ cytotoxic T lymphocytes (CTLs) (refer Fig. 1). Activated CTLs elicit strong CMI and selectively kill target cells (infected altered cells or cancerous cell) by inducing apoptosis in them [20]. Killing target cells is a complex immunological process that attracts several compliment systems (CS) proteins and co-stimulatory molecules. Therapeutic cancer nanovaccines are preferentially designed to strengthen CTLs responses by ex vivo educating DCs via adoptive transfer of antigen (for example, Sipuleucel-T, a therapeutic vaccine approved for treatment of metastatic contraction resistant prostate cancer) [25].
On other hand, activated DCs also present antigens to CD4+ T-helper cells (Th cells) via MHC-II receptor. Subsequently, effector Th cells undergo activation and maturation leading to secretion of variety of cytokines signals (refer Fig. 1). Depending on the type of cytokine secreted, Th populations are divided into Th1 cells and Th2 cells subsets [7]. Th1 subset majorly secretes pro-inflammatory cytokines (e.g. INF-γ, TNF-α, IL-1) which stimulates proliferation of CTLs and strengthen the function of CMI. Th2 subset secrete class of cytokines (e.g. IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13) which stimulate B cell proliferation during antibody-mediated immune responses [7,26]. A subtle balance between Th1/Th2 responses determines overall therapeutic or prophylactic vaccine potential of candidate nanovaccine [27]. In some cases, nanovaccines also work by suppressing T-regulatory (Treg) cells, which naturally suppress activation and proliferation of effector T cells in the body [28].
2.2.2. Activation of antibody-mediated immunity
Depending on the potential of nanovaccines to generate strong Th2 cytokine response, naïve B cells located in spleen and lymph nodes get stimulated and bind to soluble antigen via B cell receptors (BCRs). Activated B cell population undergoes proliferation in the germinal center. Further, by somatic hyper mutation, B cells becomes specific to particular epitope of an antigen and then only epitope-specific B cells are selectively (clonal selection) proliferated. At this stage, activated B cells either differentiate into antibody-secreting plasma cells and secrete soluble antibodies against target antigen or remain dormant as memory cells for a future encounter of the same antigen (refer Fig. 1) [29].
2.3. Nanovaccines boost immunological memory
Plasma cells are short-lived and their number gradually declines over time, as do the corresponding antibody titers [30]. In that case, memory cells (B cells and T cells) which were generated and stored in the lymphatic organs or bone marrow for several months, come into play and protect the body from reinfection of the same antigen. In such situations, memory B cells quickly proliferate and turn to antibody-secreting (mainly IgG) plasma cells to neutralize the antigen. On the other hand, memory T cells (both types CD4+ and CD8+) serve to produce more cytokine and chemokine signals to stimulate enhanced CMI and antibody-mediated responses. However, in case the structure of the antigen (epitope) has undergone significant changes due to cleavage, aggregation or re-folding, even memory cells cannot provide the required protection [31]. Although, in some cases, primary immunization can offer up to 90% protection, however, as even the rest 10% can be disastrous, therefore booster doses are scheduled to increase protection up to 100% [32]. Booster doses promote generating of more population and a variety of memory cells. Generally, booster doses are scheduled after the antibody titer from plasma cells has dramatically declined to allow more competitive binding of memory cells to the injected antigen [33,34]. In a related context, the use of nanoparticles help recalling more robust immunological memory even at a low dose [35]. Nanoparticles (e.g. PLGA based nanovaccine) facilitate prolonged availability of intact antigen in blood due to sustained release that leads to a higher proliferation of B cells and hence more memory cells [36,37]. Also due to small size, nanoparticles can travel through the narrow lymphatic system to lymph nodes inducing differentiation of more memory cells [38]. For example, Demento et al., reported that over alum-based (free antigen) or liposome-based (burst release) carriers, mice immunized with OVA-expressing Listeria monocytogenes (LM-OVA) encapsulated in PLGA particles (sustained-release) showed the highest protection against a lethal dose of L. monocytogenes even after 13 weeks post immunization. They observed that mice immunized with antigen in PLGA nanoparticles had a robust population of CD8+ T memory cells [36]. Similarly, Kanchan et al., showed that encapsulation of tetanus toxoid (TT) inside poly(lactic acid) (PLA) particles generated long lasting primary antibody response in mice and interestingly even after 18 months re-exposing the animal using very low antigen dose induced heightened and robust B cell responses [39].
3. Approaches for designing of nanovaccines
Nanomaterials can be efficiently used for designing nanovaccines for the enhancement of immune-modulation. Based on their potential use, they can be employed as adjuvants, immunogens or nanocarriers for enhanced and prolonged antigenic delivery. These properties of a nanoparticle are strongly governed by its size, shape, composition, surface chemistry and route of administration. It plays an important role in deciding its capability to induce inflammatory response or expression of specific defense-related genes. Different types of nanoparticles like protein, lipid, polymer, or inorganic particles (such as gold, iron, carbon and silica nanoparticles) can induce immune stimulation and have been discussed below [40].
3.1. Traditional adjuvants
Adjuvants are used with vaccines to enhance their potency by combination of mechanisms. Adjuvants activate innate immune responses that shape and trigger the adaptive immune responses to vaccines [41]. Traditionally used adjuvants such as alum, complete Freund's adjuvant and AS04 (combination of alum and monophosphoryl lipid A) are known to form local depots that induce secretion of cytokines, chemokines and recruitment of immune cells. Absorption of antigen on surface of adjuvants enhances antigen uptake and presentation, thus promoting antigen transport to draining lymph nodes [42]. Activation of TLRs has been potentially used for designing vaccine adjuvants. TLR ligands or agonists have served as potential immunostimulatory molecules as TLRs activate innate immune responses by identifying PAMPs and down signaling the transcription factors responsible for immune responses such as NF-κB, IRFs and lymphocyte activation [43]. The co-delivery of antigen along with TLR ligands promotes antigen presentation to CD4+ T cells via MHC-II signaling and cross-presentation to activate CD8+ T cells [44]. TLR agonist such as CpG up-regulates expression of CD40, CD54, CD80, CD86, and MHC class II molecules thus enhancing antigen processing and presentation in plasmacytoid DCs [45].
3.2. Nanoparticles as adjuvants, carriers and immunogens
Nanovaccines generate amplified, specific and robust responses as compared to traditional vaccines despite having similar mechanism of immune activation. The exaggerated immune response generated by nanoparticle associated antigen is due to their ability to specifically target dendritic cells with enhanced cellular uptake and antigen presentation. Moreover, nanoparticles can be used as adjuvants with specific antigens or as immunogen themselves.
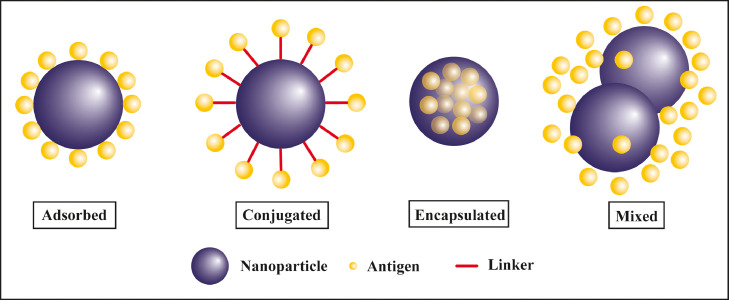
Vaccine antigens are either encapsulated inside or surface decorated over nanoparticles to get delivered efficiently (refer Fig. 2 ). There are chances that free antigens may degrade and elicit local immune responses at the site of administration, therefore, encapsulating free antigen inside nanoparticles prevents their degradation and provides prolonged and controlled release at the target site. The controlled release of antigen prevents burst responses and eliminates the need of booster doses. Surface decoration of nanoparticles with antigen also confers the advantage of presenting the antigen to cells in a similar way as presented by pathogens [46]. Polyanhydride-based nanoparticles encapsulating F1-V antigen when administered intranasally induced an immune response that persisted for 23 weeks and elicited a high anti-F1-V IgG1 antibody response post-vaccination and conferred long-lived protective immunity against Yersinia pestis infections compared to recombinant F1-V antigen [47]. Similar nanoparticles were functionalized with di-mannose targeted macrophage mannose receptor (MMR). These carbohydrate functionalized nanoparticles enabled sustained release of immunogen for 30 days and enhanced the uptake of antigen by dendritic cells simultaneously upregulating the co-stimulatory molecule CLR, CD206, and CD40 in cells as compared to non-functionalized nanoparticles [48]. Apart from encapsulation, several other strategies have been exploited for the co-delivery of antigens and nanoparticle such as surface absorption, conjugation, and mixture (refer Fig. 2). Nevagi et al., observed enhanced immune activation against GAS (Group A Streptococcus) antigen using tri-methyl chitosan (TMC) nanoparticle conjugated with B cell and T cell epitope as compared to antigen mixed with TMC [49].
Fig. 2.
Design of nanovaccines: nanovaccines are formulated either by physical adsorption, chemical conjugation, encapsulation or physical mixing of antigen with nanoparticles.
Nanoparticles have also shown potential to be used as adjuvants because of their immunomodulating activities such as inflammasome activation, complement system activation and recruitment of immune cells [50]. Due to above-mentioned effects, nanoparticles act as a better adjuvant in comparison to complete Freund's adjuvant and conventional aluminum containing salts by inducing higher immunogenic responses at low antigen doses [51]. Also, their small size facilitates internalization by dendritic cells and induces adaptive immune responses. Studies by Swaminathan et al., have proved enhanced immune responses by lipid nanoparticle (LNP) adjuvant formulations against ovalbumin (OVA) and hepatitis B virus surface antigen (HBsAg) in BALB/c and C57BL/6 mice injected with HBsAg along with LNPs as compared to HBsAg alone [52]. Co-administration of quarternized chitosan nanoparticles as adjuvants along with ovalbumin intranasally in female BALB/c mice induced activation of APCs followed by enhanced lymphocyte proliferation and differentiation as compared to OVA mixed with aluminum hydroxide gel [53]. Asgary et al., also reported that silver nanoparticles showed increased antibody-mediated responses against CVS-11 rabies and reduced toxicity as compared to conventionally used adjuvants such as alum [54]. Although nanoparticles possess great adjuvant properties, their co-delivery along with generally used adjuvants such as TLR agonists can provide very robust immune protection. The use of TLR agonists is restricted because of their instability, particularly while using RNA as TLR agonists. Nugyen et al., have designed lipidoids to effectively deliver immunostimulatory RNA to activate innate and adaptive immune responses. These lipid-RNA nanoparticles enhanced IFN-γ responses and anti-OVA cell-mediated and antibody-mediated responses. These lipidoids were superior to the conventionally used N- [1-(2,3-dioleoyloxy) propyl]-N,N,N-tri-methyl ammonium methyl sulfate–RNA delivery system [55]. Similarly, Siefert et al., have synthesized PLGA based bacterial biomimetic nanoparticles co-presenting monophosphoryl Lipid A (MPLA) and unmethylated CpG-rich oligodeoxynucleotides (CpG), a TLR agonist. These nanoparticles induced pro inflammatory responses and antigen-specific antibody and cell-mediated immunity. Co-localization of MPLA and CpG also induced functional memory CD8+ cells. These findings clearly demonstrated the ability of bacterial elements and TLR agonists towards developing a more effective vaccine design [56]. In addition, TLR agonists have been used in designing disease-specific nanovaccines, which are discussed later in this review.
Layer by layer protein nanoclusters synthesized from recombinant trimeric hemagglutinin were immunogenic by themselves and induced protective antibody-mediated responses without the use of adjuvants. Such high immunogenic potential of peptide nanoclusters is because of the high antigen density available on the surface of nanoparticles [57].
Various types of nanoparticles have been used for nanovaccine formulation and each type serves a different function, which has been elaborated in the next section.
3.3. Nanovaccines employ a broad range of materials
3.3.1. Protein/peptide-based nanovaccines
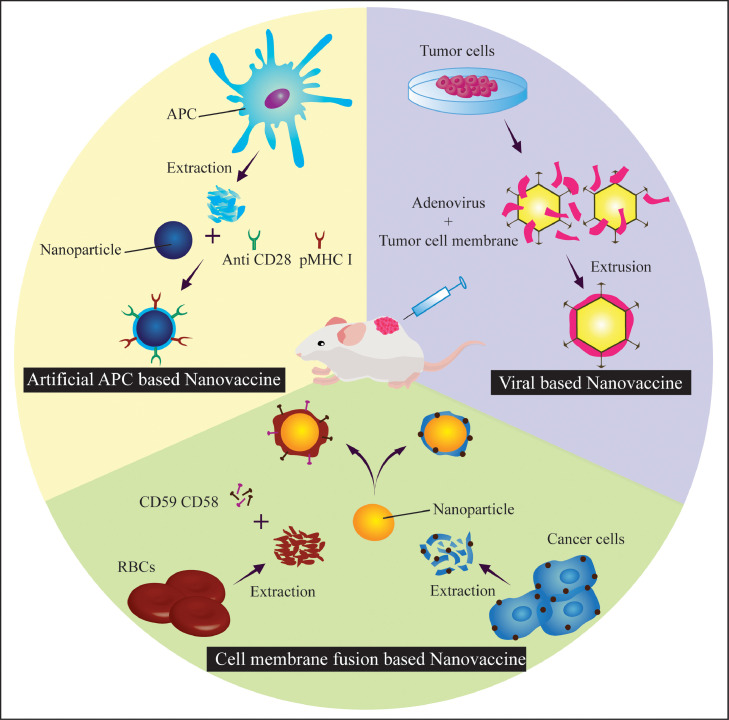
Protein-based nanovaccine can serve both structural as well as functional purpose by carrying antigens and engaging pattern recognition receptors of APCs. The protein-based nanovaccine can be categorized as biomimetic (virus-like particles, cages, vaults), rationally designed (nanofibrils, self- assembled protein nanoparticles, nanoclusters) and micelles.
Biomimetic protein-based nanovaccines: Biomimetic protein-based nanovaccines are of viral origin with self-assembled viral capsid proteins devoid of genetic material and are categorized as virus-like particles (VLPs) (refer Fig. 3(E)). On the other hand, those of prokaryotic or eukaryotic origin are called protein cages and vaults. Cages and Vaults are generally used for biocatalysis or molecular transport. They share common properties with VLPs like nanoscale, symmetry and container like geometries. All these structures can be modified chemically or biologically with antigens for immune activation [6]. VLPs (60–80 nm) of Salmonella typhimurium bacteriophage P22 were engineered to encapsulate two respiratory syncytial virus (RSV) protein antigens, matrix (M) and matrix 2 (M2). Mice vaccination and intranasal boosting showed antigen-specific CD4+ and CD8+ T cell responses. Upon subsequent RSV challenge, 1000-fold reduction in viral load and increased antigen-specific CD8+ T cells were observed in the lungs of vaccinated mice as compared to empty P22 vaccinated and unvaccinated animals [58].
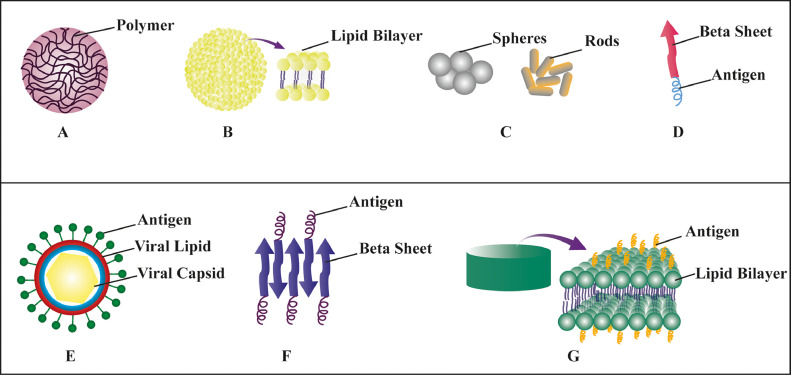
Fig. 3.
Types of nanovaccines: various nanoparticles have been used for the development of nanovaccines. (A) Polymeric nanoparticle, (B) Liposome, (C) Inorganic nanoparticles, (D)–(G): Protein-based nanoparticles.
Cages like 25 nm E2 cage derived from the pyruvate dehydrogenase complex of Bacillus stearothermophilus [59], 13 nm cage of human heavy chain ferritin (an iron storage protein) [60], ~24 nm encapsulins (class of icosahedral cage structures) of bacteria and archaea are used for the presentation of different antigens to elicit immune response after immunization [61]. A recent study on non-viral E2 protein-based biomimetic nanoparticle-containing acid-labile CpG (DC activating molecule) and SIINFEKL peptide epitope conjugate has shown the potential to enhance the CpG mediated DC activation by 25-fold in vitro as compared to unbound CpG. Interestingly, co-delivery of SIINFEKL peptide conjugated with CpG has shown elevated cross-presentation and CD8+ T cell activation by inducing a 3-fold greater display of SIINFEKL on MHC-I by DCs as compared to unbound peptide or peptide bound directly to E2 protein. This shows the ability of biomimetic protein-based nanoparticles to facilitate enhanced immune response by DC activation and cross-presentation [62].
Vaults are eukaryotic ribonucleoproteins assembly of a cage-like barrel-shaped structure (approximately 70 nm × 40 nm × 40 nm) made from the major vault protein (MVP) [63]. It has been shown that increased antigen-specific CD4+ T cell response with a reduction in genital bacterial load and inflammation was observed after Chlamydia muridarum polymorphic membrane protein G-1(PmpG) encapsulating vaults were administered intranasally [64].
Rationally designed protein-based nanovaccines: Nanofibrils are generally made of engineered β-sheets or α-helical coiled coils. β-sheet nanofibrils can be formed with peptide sequences like Q11 (QQKFQFQFEQQ) and KFE8 (FKFEFKFE) appended to C terminus of a peptide antigen with a short linker sequence (refer Fig. 3(D)). Similarly, α-helical coiled-coil can also be formed into fibrils from α-helical peptide-like coil29 [65]. Such peptide sequences undergo assembly into fibrils with the antigenic epitope sticking outward from the fibril surface [6]. These fibrils have been assembled with various epitopes of Plasmodium falciparum [66], Mycobacterium tuberculosis [67], Streptococcus aureus [68], T-helper cells epitope PADRE [68] and OVA epitopes [69]. Conjugation of E2EP3 immunogenic epitope of Chikungunya virus E2 glycoprotein with β-sheet self-adjuvanted amyloid assemblies showed cytocompatibility with enhanced macrophage uptake and robust IgG response against E2EP3 epitope [70].
Self-assembling protein nanoparticles (SAPNs) are 20–30 nm icosahedrons containing pentameric and trimeric helical coiled-coils conjugated with (glycine)2 linker (refer Fig. 3(F)). Antigens and adjuvants can be inserted depending on the expected structure from in silico modeling [71]. Subunit vaccine SAPNs have been reported to enhance immune response in numerous diseases like malaria [72], HIV [73], toxoplasma [74] and severe acute respiratory syndrome [75]. Wahome et al., encapsulated two HIV protein epitopes (4E10 and 2F5) onto SAPN surface and observed enhanced production of epitope-specific neutralizing antibodies. This indicates the potential of SAPN as nanovaccine to activate immune response against HIV [73].
Nanoclusters have also emerged as a prominent vaccine platform prepared from desolvation of proteins and peptides. They are generally made up of antigens and crosslinkers with an intent to increase antigen loading and eliminate off-target sequences. Intranasal vaccination of influenza matrix protein 2 (M2e) containing self-assembled protein nanoclusters elicited enhanced immune response and complete protection after lethal challenge with hetero and homo-subtypic virus [76]. Since the preparation of nanoclusters requires organic solvents, it may alter the secondary or quaternary structure of full protein antigens [57].
Micelles: Antigenic molecules can be conjugated to micelles in two different ways. Either they can be covalently linked onto the micellar surface or can be conjugated to the variety of alkyl tails to form amphiphilic peptide micelles (PAMs). Micelles of different sizes and shapes affect their immune activation potential [77]. Studies have shown that cylindrical or spherical PAMs are efficiently able to reach lymph nodes and carry the required amount of antigen and adjuvant [78]. Studies have reported PAM mediated increased immunogenicity of T cells [79] and B cells epitopes [80]. Such peptide lipid conjugates also form nano-disc vaccines (refer Fig. 3(G)). Subcutaneous vaccination with neo-antigen ADPGK nanodiscs showed enhanced CD8+ cellular response, APC uptake and increase survival after B16F10 melanoma challenge as compared to intramuscular vaccination [81]. Peptide-polymeric micelles have also been explored as a vaccine platform by conjugating hydrophilic peptide antigen with dendrimer polymers for immunotherapy [24].
3.3.2. Lipid-based nanovaccines
The most common lipid-based nanovaccines getting explored from the past few decades are liposomal nanovaccines (refer Fig. 3(B)). They offer a distinct advantage over other nanovaccine systems in terms of being biodegradable, tolerable, less immunogenic and having tunable physicochemical properties. They provide a wide repertoire to load antigens of different philicity inside and outside of liposomes with easy surface conjugation [82]. Different physicochemical properties (like size [83], surface charge [84], surface modifications (PEG) [85], bilayer composition and fluidity [86]) affect the immunological outcome of liposomal nanovaccines.
Size-dependent differential activation of immune response indicated the activation of Th2 response by 100 nm sized liposomes as compared to Th1 response by liposomes ≥400 nm [83]. Transdermal immunization of mice with dissolving microneedles loaded with opposite charged transferosomes indicated the enhancement of Th1 immune response by cationic transferosomes as compared to anionic counterparts by exhibiting strong endocytic escape property with fecilitating antigen processing via MHC-I pathway and larger accumulation in lymph nodes [87].
Kaur et al., showed the effect of bilayer fluidity on immune activation by varying cholesterol concentrations. Relatively lesser in vivo IgG production and IFN-γ was observed by liposomal formulations containing high cholesterol [85]. Similarly, the effect of another important factor affecting bilayer fluidity on liposomal mediated immune activation was observed by preparing liposomes with phospholipids having different transition temperatures (Tm). High Tm phospholipids containing liposomes stimulated Th1 immune response as compared to low Tm phospholipids that induced Th2 response in mice immunized with leishmaniasis rgp63 antigen [86].
Numerous clinical studies showed the use of liposomes as adjuvants in therapeutic vaccines. Liposome encapsulated D. pteronyssinus vaccination reduced allergen bronchial provocation induced inflammatory changes and improved condition of asthma patient after sustained mite exposure [89]. A phase III study of tecetomide vaccine, containing BLP lipopeptide antigen incorporated into DMPG:DPPC:Chol:MPL-liposomes was performed for stage III non-small cell lung cancer after subcutaneous immunization (NCT01015443) [90]. However, the study was terminated due to no effect on survival and primary or secondary endpoint in a phase II study [91]. A phase I trial (NCT00922363) showed safety and immunogenicity of CAF01 (liposomal adjuvant system) given with a subunit vaccine against M. tuberculosis antigen H1. This adjuvanted vaccine showed long-lasting T cell response with no antibody response observed in healthy adults [92].
Viral liposomes called “virosomes” have also been studied in the literature. Virosome exhibits viral capsid proteins onto the liposomal surface for effective fusing with the target cell membrane. Recently, virosomes and liposomes mediated immune response was investigated on human respiratory tract triple culture model. Both virosomes and liposomes were prepared from the same neutral phospholipids, DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) and POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine), but virosomes were also fused with influenza membrane protein. Results showed enhanced internalization of virosomes in epithelial cells of triple culture as compared to liposomes of similar sizes [93].
A recent study has shown the use of lipid-based 40–60 nm particles (ISCOMATRIX particles) as adjuvant with chimera peptide vaccine containing gp21, Tax, gp46 and gag epitopes of human T cell lymphotropic virus type 1 when given subcutaneously in mice. Enhanced production of mucosal IgA and IgG2a antibody titers along with IFN-γ and IL-10 cytokines were observed as compared to vaccine formulation [94].
Another class of lipid-based nanocarrier includes ‘hexosomes’ and ‘cubosomes’ that can provide a high membrane surface area for antigen and membrane protein loading. Hexosomes are generally made up of lipids known to form non-lamellar structure in hydrated systems with inverse hexagonal liquid crystalline phase. Comparative adjuvant activity of mono mycoloyl glycerol-1 (MMG-1) immunopotentiator containing hexosomes and cationic liposomes (CAF04) loaded with Chlamydia trachomatis major outer membrane protein (MOMP) antigen in CB6/F1 mice after vaccination was studied by Rodrigues et al. MMG-1 hexosomes made up of lipid phytantriol induced a strong MOMP-specific antibody-mediated immune response whereas MMG-1 cationic liposomes elicited much stronger MOMP-specific cell-mediated response. It indicated the immune activation by two lipid-based adjuvants via different mechanisms [95]. In addition, cubosomes are generally made up of lipid bilayers forming continuous periodic cubic lattice structures with interwoven water channels and a stabilizing polymeric outer corona [96]. Phytantriol, pluronic F127 and propylene glycol-based cubosome formulation containing (imiquimod and monophosphoryl lipid A (MPL)) adjuvants and model OVA antigen elicited robust IFN-γ production and CD4+ and CD8+ T cell proliferation as compared to similar adjuvant containing liposomal formulation and alum [97]. Such lipid-based self-assembled structures can provide high antigen and adjuvant loading with enhanced immunoactivation in comparison to conventional lipid-based carriers like liposomes.
3.3.3. Synthetic or natural polymer-based nanovaccines
Polymeric particles showed a great possibility to be used as nanovaccines due to their ease of preparation, biocompatibility and biodegradability, fine-tuning of surface properties and controlled release kinetics (refer Fig. 3(A)) [98]. The most commonly used polymeric nanoparticle for antigen delivery is poly (lactic-co-glycolic acid) PLGA or poly (lactic acid) PLA that has been used to deliver a broad range of antigens like hepatitis B virus antigen [99], tetanus toxoid [100], Bacillus anthracis antigen [101], Mycobacterium tuberculosis antigen [102] and ovalbumin [36]. Other natural polymers like chitosan [103], alginate [104], pullulans [105], and inulin [106] have also been used as adjuvants and antigen carriers. Chitosan alginate-based nanovaccines for hepatitis B virus [103] and DNA-based chitosan vaccines for mycobacterium tuberculosis (Mtb H37Rv) showed a marked increase in immunologic and protective efficacy in immunized mice [107].
Hyperbranched polyglycerol (HbPG) globular polymer can be a potential candidate for inert dendrimer based multi-valent vaccine coupled with B cell epitope for tumor-associated antigen (MUC1) and T cell epitope for tetanus toxin P2. It showed enhanced immune response and IgG antibodies against breast cancer cells. Hence polymeric nanoparticles can be used for potential multi-valent vaccines [108].
3.3.4. Inorganic-based nanovaccines
Several inorganic nanoparticles (Gold, Iron, Carbon, Silica nanoparticles) are being explored for vaccine delivery in different disease models (refer Fig. 3(C)). Ease of antigen functionalization onto easily accessible surface groups, robust properties with reproducibility outweighs their low biodegradability and gives them an edge to be used as nanovaccines. They also increase antigen stability by preventing premature proteolytic degradation. However, there are reports showing dose and size-dependent clinical toxicities of these nanoparticles [109]. Amongst all the inorganic nanoparticles, gold nanoparticles have gained maximum attention in vaccine delivery. These particles have been used for the epitope delivery against HIV [110], influenza [111], malaria [112] and tumor [113]. Effect of shape of gold nanovaccine on antibody production against WNV envelope E protein showed that rods induced only 50% of the antibody response as compared to spherical nanoparticles [114]. Multicopy multivalent nano-glycoconjugates of gold nanoparticles decorated with Tn-antigen glycan generated strong long-lasting antibodies against breast cancer expressing aberrant mucin glycan [115].
Inorganic carbon spherical nanoparticles and nanotubes were used as adjuvants to increase immunogenicity and act as delivery vehicles for peptides and protein against various viral infections [116,117]. Oral immunization of bovine serum albumin (BSA) loaded carbon nanoparticles showed effective stimulation of mucosal IgA antibodies in intestinal, vaginal and salivary mucosa along with Th1 and Th2 responses [116]. Abundance of silanol groups over silica nanoparticle surface provides an advantage for easy conjugation of targeting moiety for various viral infections [118]. Silica vesicles adsorbing E2 glycoprotein of bovine viral diarrhea virus (BVDV) showed 6-month antibody-mediated and cell-mediated immune response with no histopathological changes at the site of infection [119]. The use of magnetic iron oxide nanoparticles for tumor-associated carbohydrate antigen (TACA) through phospholipid functionalization of TACA glycopeptide showed enhanced antibody titers against both human and mouse tumor cells expressing that glycopeptide [120]. Calcium nanoparticles have also been reported for their superior adjuvant property with DNA vaccines and to confer mucosal immunity. They can also induce enhanced antibody production against viral antigens as compared to aluminum adjuvants [121].
Since different types of nanoparticles impart different properties to the developed nanovaccine, their vast use in immune activation against a variety of antigens have been observed. Though clear demarcation or direct comparison across nanocarriers has not been studied but there are unique properties and advantages associated with each nanocarrier that gives them an edge over each other. Biomimetic carriers like VLPs are expected to possess inherent immunogenicity that can elicit antibody reaction against carrier antigens itself. This could lead to the competition with the target antigen and alter antibody repertoire or memory response against it. Reactive toxicities or neutralization upon repeated dosing can also limit their multiple use in a patient [122]. Such problems can be avoided by the use of nanoclusters that are solely made up of single type of antigen. These protein-based biomimetic and rationally designed nanocarriers are most suitable for the incorporation of a highly hydrophobic antigens of varied sizes with an intent to preserve its native structure, whereas hydrophilic peptides can be well delivered using amphiphilic micelles [6]. Carrier specific immune response has also been observed for certain polymeric and lipid-based carriers but it can be avoided using natural or biomimetic components (e.g., cellular lipid isolates or extracellular matrix polymers) [6]. Control over spatiotemporal antigen release, biodegradability, biocompatibility and ease of modification makes them two of the most widely explored nanocarriers for vaccine development. Precise control over size with high reproducibility and ease of antigen functionalization gives inorganic nanoparticles an edge over other nanocarriers but reports suggesting their dose-dependent clinical toxicity because of prolonged exposure limits their potential use. Different examples of nanoparticle-based vaccines and their current clinical status has been summarized in Table 1 .
Table 1.
Examples and clinical status of nanocarriers-based prophylactic vaccines.
| Infectious/Non- infectious | Targeted disease | Nanocarrier | Exploited antigen | Clinical status | Ref. |
|---|---|---|---|---|---|
| Infectious | Influenza | T7 phage VLP | HA, M2e | Preclinical | [167] |
| Influenza | Human ferritin cage | M2e | Preclinical | [60] | |
| Influenza | α-helix self- assembling peptide nanoparticles | M2e/CFA+IFA | Preclinical | [168] | |
| Influenza | α-helix self- assembling peptide nanoparticles | Helix C, M2e/flagellin, PADRE | Preclinical | [169] | |
| Influenza | Liposomes | H1N1 Split virus | Phase I | [170] | |
| Influenza | Gold nanoparticles | Extracellular portion of M2 protein (influenza virus) | Preclinical | [171] | |
| Influenza | Thermotoga maritima encapsulin | M2e, GFP/CFA | Preclinical | [172] | |
| Influenza | Self-assembled peptide nanofibers (Q11 self-assembly domain) | Influenza acid polymerase (PA) | Preclinical | [69] | |
| Malaria | Self-assembling protein nanoparticle (SAPN) | FMP014 | Preclinical | [173] | |
| Malaria | β-sheet fibers | (NANP)3 | Preclinical | [66] | |
| Malaria | α-helix fibers | PbCSP | Preclinical | [72] | |
| Malaria | Liposomes | RTS,S | Phase I/II | [174], [175], [176] | |
| Malaria | Iron oxide nanoparticles | Merozoite surface protein | Preclinical | [141] | |
| Malaria | Gold nanoparticles | Pf CSP (P. falciparum) | Preclinical | [177] | |
| HIV | α-helix self- assembling peptide nanoparticles | 2F5, 4E10/IFA | Preclinical | [131] | |
| HIV | Gold nanoparticles | HIV-1 Env plasmid | Preclinical | [178] | |
| HIV | Gold nanoparticles | HIV Gag p17 and CMV pp65 | Preclinical | [161] | |
| HIV | Chitosan and Hyaluronic acid nanoparticles | PCS peptide | Preclinical | [159] | |
| HIV | Silver nanorods | HIV Gag | Preclinical | [160] | |
| Tuberculosis | β-sheet fibers | ESAT6, TB10.4, Ag85B/Pam2Cys | Preclinical | [67] | |
| Tuberculosis | Self-assembling peptide nanofibers | Mtb-specific CD8+ or CD4+ T cell epitopes | Preclinical | [67] | |
| Tuberculosis | Liposomes | M72, H1 protein | Phase I | [179] | |
| Tuberculosis | Chitosan nanoparticle | DNA encoding T cell epitopes of Esat-6 and FL | Preclinical | [107] | |
| Tuberculosis | Chitosan nanoparticle | Mycobacterium lipids | Preclinical | [136] | |
| Pneumonia | Qβ phage | Tetra saccharide/NKT cell adjuvant | Preclinical | [180] | |
| Pneumonia | Polyanhydride nanoparticle | Pneumococcal surface protein A (PspA) | Preclinical | [123] | |
| Respiratory syncytial virus | P22 phage | M, M2 | Preclinical | [58] | |
| Respiratory syncytial virus | Liposomes | Envelope (E) protein | Preclinical | [181] | |
| MCMV respiratory infection | PLGA micro/nano | EP67 conjugated to MCMV CTL epitope | Preclinical | [182] | |
| Hepatitis B | Chitosan nanoparticles | Recombinant Hepatitis B surface antigen (rHBsAg) | Preclinical | [183] | |
| Hepatitis B | Poly (D,L-lactic-co-glycolic acid) nanospheres | Hepatitis B surface antigen | Preclinical | [99] | |
| Chlamydia | Vault | PmpG | Preclinical | [64] | |
| Toxoplasmosis | α-helix self- assembling peptide nanoparticles | 5 CD8+ T cell epitopes/ PADRE, flagellin, GLA-SE | Preclinical | [74] | |
| GAS infection | Lipopeptide based nano carrier systems (LCP) | B cell epitopes of the M protein (J14) and the SfbI protein (FNBR-B) | Preclinical | [184] | |
| Group A Streptococcus (GAS) infections | PLGA | Lipopeptide-based vaccine candidate (LCP-1) | Preclinical | [185] | |
| human T cell lymphotropic virus type 1 (HTLV-1) | ISCOMATRIX | Tax, gp21, gp46, and gag | Preclinical | [94] | |
| Dengue | Liposomes | Envelope (E) protein | Preclinical | [181] | |
| Meningitidis | Liposomes | Outer membrane proteins and deacetylated lipo-oligosaccharide | Phase I | [186] | |
| Anthrax | Poly (D,L-lactic-co-glycolic acid) nanospheres | PAD4 | Preclinical | [101] | |
| Newcastle disease | Chitosan nanoparticles | Live virus vaccine | Preclinical | [187] | |
| Tetanus | Gold nanoparticles | Tetanus toxoid bulk from Clostridium tetani | Preclinical | [188] | |
| Foot and mouth disease | Gold nanoparticles | FMDV peptide | Preclinical | [189] | |
| Enterohemorrhagic Infection | Gold nanoparticles | LomW and EscC | Preclinical | [129] | |
| Non-infectious | Melanoma | Hepatitis B VLP | Melanoma peptides | Preclinical | [190] |
| Melanoma | E2 cage | gp100 | Preclinical | [59] | |
| Cancer | Metal organic framework nanoparticle | Cytomembrane of fused DCs and Cancer Cells | Preclinical | [77] | |
| Cancer | Fe3O4 magnetic nanoclusters | Cancer cell membrane | Preclinical | [191] | |
| Cancer (EGFRvIII) | α-helix fibers | PEPvIII, SIINFEKL/ PADRE | Preclinical | [65] | |
| Cystic fibrosis | Nanoemulsion | Proteobacterial outer membrane protein (OMPs) | Preclinical | [192] |
4. Prophylactic and therapeutic properties of nanovaccines
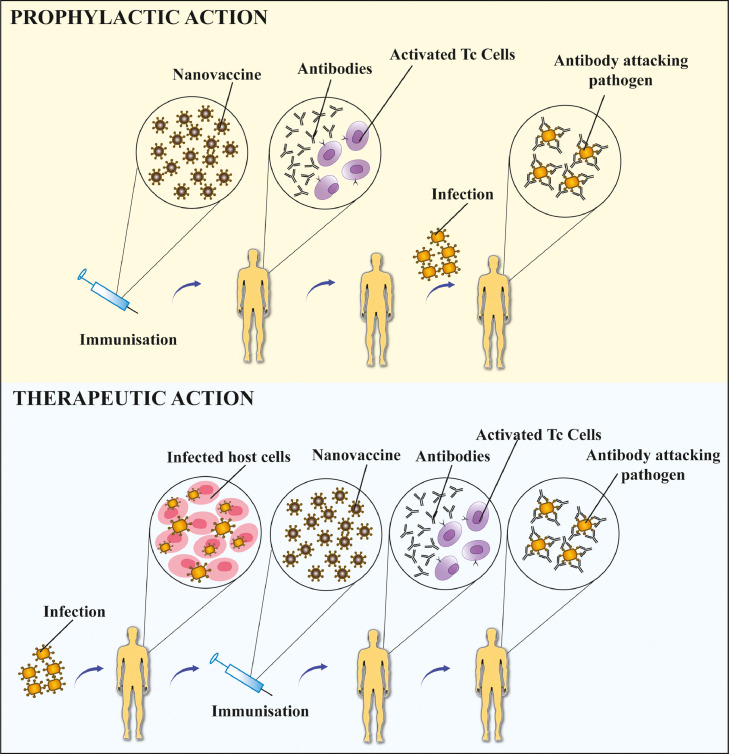
Different type of nanovaccines can escalate the immunogenic potential of antigens by boosting the immunogenic responses and memory generated against antigens at low doses. Immunogenic responses generated against certain antigens can be used in two different scenarios by varying the use of vaccines as prophylactic or therapeutic (refer Fig. 4 ).
Fig. 4.
Prophylactic and therapeutic action of nanovaccines: strategy for using nanovaccines in prevention and treatment of an infection.
Prophylactic vaccines aim to develop protective immunity and are administered before the onset of disease. The goal of prophylactic vaccines is to develop immunogenic memory against certain infections. Major challenge of prophylaxis is obtaining sufficiently long-lasting immunogenic memory. For instance, single-dose immunization of mice with polyanhydride nanoparticle containing pneumococcal surface protein A (PspA), a virulence factor of S. pneumonia activated protective immunity and enhanced the survival of animals upon challenge even at 25-fold reduction in dose. PspA based nanovaccine formulation performed comparable to protein adjuvanted with alum, with much less tissue reactivity at the site of immunization [123]. Prophylactic nanovaccines confer protective immunity mostly against infectious conditions at low doses of immunostimulating antigen and reduced need for adjuvants, thus mitigating associated toxicities.
Therapeutic vaccines are administered after the onset of diseases to alter the course of disease by encouraging the immune system to fight harder against the prevailing conditions. Unique immune responses are generated against disease-specific antigens. Generally, therapeutic vaccines are used for cancer treatment, but there are compelling evidences suggesting their potential use in autoimmune disorders also. The advantage of using therapeutic vaccines instead of conventional pharmaceutical treatment lies in their specificity [124]. Mono-specific, disease-relevant pMHC complexes when coated on nanoparticle surfaces triggered the selective expansion of memory-like autoregulatory CD8+ T cells that arise only in affected individuals. Such engineered nanoparticles have the potential to become suitable vaccines with the capacity of resolving organ and disease-specific autoimmune responses. Systemic delivery of Type-I diabetes relevant peptide-MHC-nanoparticle complex triggered expansion of memory autoregulatory T cells and suppressed the autoimmune attack against insulin-producing beta cells, thus restoring glucose balance [125].
In certain cases, nanovaccines can be formulated to achieve both prophylactic and therapeutic responses. Poly (γ-glutamic acid) based synthetic vaccine nanoparticles (SVNPs) loaded with ovalbumin and toll-like receptor 3 agonist (poly (I:C)) were synthesized by Kim et al., both of them enhanced the uptake of antigens by APCs and enhanced the secretion of inflammatory cytokines (TNF-α, IL-6) and type I interferon (IFN-α, IFN-β). Simultaneous injection of both SVNP-OVA and SVNP-IC conferred both the protective as well as therapeutic immunities by inhibiting tumor growth in EG7-OVA tumor-bearing mice [126].
It is clear from the above-mentioned examples that nanovaccines modulate the immune system either for providing prophylaxis or therapeutic responses against pathological conditions. Majorly prophylactic vaccines are used to prevent infections (Table 1) and therapeutic vaccines have been used for cancer (Table 2 ) treatment.
Table 2.
Examples and clinical status of nanocarriers-based therapeutic vaccines.
| Infectious/Non-infectious | Targeted disease | Nanocarrier | Exploited antigen | Clinical status | Ref. |
|---|---|---|---|---|---|
| Infectious | HIV | Liposomes | Cocktail of peptides | Phase I | [200] |
| HPV16-related cancer | Self-assembling protein nanoparticle (SAPN) | Tat-E7/pGM-CSF | Preclinical | [201] | |
| Non-infectious | Melanoma | Filamentous phage fd | α-galactosylceramide | Preclinical | [202] |
| Melanoma | VLPs | Toll-like receptors ligands | Preclinical | [203] | |
| Melanoma | Nanodiscs | Neoantigen (Adpgk) | Preclinical | [81] | |
| Melanoma | Liposomes | HSP-70 | Phase I | [204] | |
| Melanoma | Gold glyconanoparticles | Listeriolysin O peptide | Preclinical | [205] | |
| Cancer | Qβ phage | MUC-1 | Preclinical | [206] | |
| Cancer | Poly(d,l-lactide-co-glycolide) (PLGA) | imiquimod and monophosphoryl Lipid A | Preclinical | [207] | |
| Prostate cancer | Liposomes | LHRH peptide and tetanus toxoid T-helper epitope | In vitro | [208] | |
| Prostate cancer | PLGA nanoparticle | STEAP peptide | Preclinical | [209] | |
| Gastric carcinoma | Liposomes | Heat Shock Protein (HSP) | Phase I | [204] | |
| Hodgkin lymphoma | Liposomes | HSP | Phase I | [204] | |
| Glioblastoma | Liposomes | HSPPC- 96 | Phase I–II | [210] | |
| Lung cancer | Liposomes | BLP25 | Phase III | [90] | |
| Breast cancer | Liposomes | dHER2 protein | Phase I | [211] | |
| Neuroblastoma | PLA-PEG | SN38 | Preclinical | [212] | |
| Persistent allergic asthma | VLP | QbG10 | Phase II | [170] |
4.1. Prophylactic nanovaccine examples
Infections that can be prevented by vaccination still contribute to approximately half of the child morbidity each year [127].Treatment and immunization of such infectious conditions is a greater challenge due to the rapid emergence of new pathogenic variants. In spite of advancements in vaccine development over the years, many vaccines are associated with the risk of regaining their pathogenicity under certain immuno-compromised conditions. So, there is an imperative need for the development of risk-free and effective vaccines that confers desired antibody-mediated and cell-mediated immunity against infectious diseases [128].
Subunit nanovaccine against enterohemorrhagic Escherichia coli (EHEC) was developed using gold nanoparticle conjugated with EHEC antigens and induced high titers of serum IgG upon subcutaneous administration in mice. It has also prevented colonization of EHEC in both the cecum and large intestine at 3 days post-immunization [129]. Mice immunized against Anthrax with a single dose of PAD4-NP (PLGA-based protective antigen domain, 4 nanoparticles) elicited robust antibody-mediated and cell-mediated responses with mixed IgG1/IgG2a subtypes and Th1/Th2 response. The survival rate of mice immunized with PAD4-NP was more than for PAD4 immunized mice after a lethal challenge with Bacillus anthracis spores [101].
Nanovaccines have also shown great prospects in mitigating parasitic invasion. Chimeric peptides containing Leishmania infantum epitopes (histone H1, kinetoplastid membrane protein 11 and cysteine peptidase A) were encapsulated in PLGA nanoparticles. Stimulation with the peptide-based nanovaccine induced maturation of DCs and IL-12 production subsequently promoting allogeneic T cell proliferation and production of IFN-γ by CD4+ and CD8+ T cell. On immunization of transgenic mice with this peptide-based nanovaccine induced peptide-specific IFN-γ producing CD8+ T cells and conferred protection against L. infantum infection [130]. Multiple self-assembling peptide-based nanovaccines containing epitopes of Toxoplasma gondii specific antigens have been designed. Immunization with self-assembled protein nanoparticles activated CD8+ T cells to produce IFN-γ and protected transgenic mice against subsequent challenge with Type II parasites [131,132]. Some more example of nanovaccines for highly prevalent infectious diseases have been discussed below in detail.
4.1.1. Nanovaccines for tuberculosis
Tuberculosis being the deadliest infectious disease, is the greatest cause of mortality worldwide. 10% of infected individuals develop the disease within one or two years of infection and the rest develop the disease in later stages of life when immune functions are compromised. Co-infection of HIV patients with Mtb (Mycobacterium tuberculosis), further complicates the situation with 33% mortality each year [133]. BCG (Bacille Calmette-Guerin) is a widely accepted vaccine against TB (Tuberculosis), but its protective efficacy is limited by age due to the absence of consensus genomic loci that are present in most of the virulent strains of Mtb. It induces short term and variable immune responses from 0% to 80% [134,135].
There is an urgent requirement of booster vaccines that augment T cell immunity in the lungs of BCG-vaccinated individuals. To address this, Ansari et al., have synthesized archeosomes encapsulating a T cell antigen Rv3619c, that upon immunization elicited type-1 cytokine response in mice. Also, it increased the production of IgG2a antibodies, antigen-specific T lymphocytes and enhanced expression of co-stimulatory molecules on the surface of APCs. By the virtue of all these properties, vaccination with archeosomes containing antigen reduced mycobacterial burden in the lungs and spleen of animals upon challenging with Mtb [135]. Similarly, self-assembled peptide nanofibers, when loaded with T cell epitope, also showed similar responses by inducing corresponding effector memory T cells and increasing cytotoxic T cell population in the lungs upon intranasal immunization. Mice vaccinated with co-assembly of nanofibers containing CD8+ T cell epitopes and TLR2 agonists in the lungs increased the expansion of multi-functional cytotoxic T cell population. On further challenge with Mtb, significant reduction in lung bacterial load was observed in comparison to BCG primed mice [67]. In another study, Mtb lipid-based nanovaccines were used wherein chitosan nanoparticles bound with Mtb lipids induced cell-mediated and antibody-mediated immunity by secretion of immunoglobulins (IgG, IgM) and prominent Th1 and Th2 cytokines in lymph node and spleen cells [136].
The higher potency of nanoparticle encapsulated antigen preparation is because of their ability to form depots with a slow and steady release in the surrounding milieu and enhanced uptake of antigen by antigen-presenting cells [135], [136], [137]. As shown in a study by Diogo et al., liposome-encapsulated antigens elicited more profound antibody-mediated and cell-mediated responses than free antigens. Mice were immunized with free antigens and liposome-encapsulated antigen, further challenging mice 16 weeks post immunization with Mtb, it was observed that Mtb burden in the lungs and spleen was significantly less in mice immunized with liposome-encapsulated antigen as compared to free BCG vaccine [137]. The potential of nanoparticles in augmenting the prophylactic activity of antigens against TB was well understood and human trials were initiated. First-in-man trial on novel liposome CAF01 as an adjuvant along with the tuberculosis vaccine Ag85B-ESAT-6 (H1) is reported in 2014 [46]. Human volunteers were vaccinated with escalating doses of CAF01at 0 and 8 weeks. After immunization strong antigen-specific T cell responses persisted for 150 weeks and did not cause local or systemic adverse effects [92].
Above discussed preclinical and clinical assessment of various antigen-loaded nanoparticles indicates the potential of nanovaccines in inducing robust and long-lasting cellular immunity against Mtb and shows hope for the development of a different and more effective prophylactic regime against TB. It is clear that with the advent of nanotechnology, adjuvant and delivery vehicle associated problems have been reduced, but the effect of the host environment and lack of consensus genetic loci still remains an inevitable challenge while developing efficient vaccines against TB.
4.1.2. Nanovaccines for malaria
Malaria is known to affect almost 200 million people annually, with half a million deaths worldwide [138]. Fighting malaria is challenging because of its multistage life cycle. Various efforts have been made in designing vaccines against the pre-erythrocytic and blood stage of malaria [139,140]. Similarly, nanovaccines have been used to target multiple stages of malaria. Iron oxide (IO) nanoparticles have been used for the delivery of merozoite surface proteins (rMSP1). Immunization of mice with rMSP-1 loaded nanoparticles induced parasite inhibitory antibodies with high titers. Macrophages and dendritic cells showed more than 90% internalization of IO nanoparticles and enhanced the expression of cytokines and chemokines [141].
Immune responses generated against sexual stage antigens of malaria impairs its transmission and reduces the disease burden. Pfs25 is one such malaria transmission-blocking vaccine antigen, but its immunogenicity is limited in humans. Hence, Pfs25 has been used along with nanoformulations to achieve desired immune responses. For example, codon harmonized Pfs25 (CHrPfs25) has been used with gold nanoparticles as adjuvants for the induction of immunity against transmission. Strong transmission-blocking antibodies elicited by simultaneous delivery of CHrPfs25 with gold nanoparticles could be due to the co-ingestion of nanoparticles and antigen by immune cells [112]. Similarly, recombinant polyhistidine-tagged (his-tagged) Pfs25 when mixed with preformed co-porphyrin containing liposomes at the time of immunization resulted in spontaneous nanoliposome antigen particularization (SNAP). Immunization of mice and rabbits with SNAP resulted in higher functional antibody generation as compared with other ‘mix-and-inject’ adjuvants. They have also been used for developing multi-antigen vaccines (apical membrane antigen-1, Pfg27, Pfs230 and the NANP peptide), targeting multiple stages of the plasmodium life cycle using liposomes [142].
Ovalbumin loaded carboxylated polystyrene nanoparticles acted as an adjuvant and induced IL-10 and granulocyte colony-stimulating factor, that affects the migratory and homing ability of dendritic cells. Mice challenged with malaria two weeks after immunization with nanoparticles produced anti-malaria antibodies and create the state of immune readiness to a subsequent infectious challenge [143]. Stable nanomimic, which are polymersomes with receptors for parasite attachment were synthesized. They bind to host cells and block re-invasion of the parasite after their release from host cells in vitro. This strategy can be used in the future to modulate immune responses against malaria and in the efficient designing of the vaccine [144]. Thus, the increased role of nanovaccines in targeting multiple life cycle stages of malaria shows new avenues in building a robust vaccination regime for prevention against malaria infection.
4.1.3. Nanovaccines for influenza
Three types of influenza viruses A, B and C have different viral nucleoproteins and matrix proteins giving rise to variable antigenic differences among them. Influenza virus A and B cause epidemics attacking both adults and children, causing around 5 million infections annually [145]. Presently, two types of vaccines are used against influenza based on strain A and strain B that target and produce antibody-mediated protective responses against hemagglutinin and neuraminidase. These antigens are highly prone to antigenic shift and drift due to error-prone RNA dependent RNA replication in influenza [146,147]. Due to environmental selection and antigenic variations, chances of influenza epidemic still persists [148]. Although animal influenza virus is distinct from the human virus, zoonotic animal viruses can still occasionally infect humans through direct/indirect contact. Avian and swine influenza viruses have been known to infect humans in some countries. Thus, there is an unmet need for producing more efficient vaccines that can provide both antibody-mediated and cell-mediated immunity to confront homologous and heterologous variants [149]. Association of various influenza antigens with nanoparticles have shown promising preliminary results in providing enhanced immune responses against variable influenza antigens.
As the antigenic variability limits the use of a single vaccine against influenza A virus (IAV), there are efforts to develop nanovaccines conferring protection against more than one serotype by using most conserved ectodomain of influenza matrix protein 2 (M2e). Nanoparticles containing self-assembling recombinant cage of human heavy chain ferritin presented with 3-sequential repeats of M2e protein was observed to elicit elevated levels of M2e-specific IgG and mucosal secretory IgA antibodies with enhanced T cell response against H1N1 and H5N1 lethal infection after intranasal administration in mice [60]. Nanovaccines against the conserved ectodomains can confer protection against inter-species viral infection. Self-assembled virus-like particles (VLPs) containing multiple copies of M2e protein inserted into capsid (Cap) protein of porcine circovirus type 2 (PCV2) have shown dual protection against IAV and PCV2 lethal challenge in mice and pigs after generating M2e specific and PCV2 neutralizing antibodies [150].
Hemagglutinin trimer has also been exploited as a potential antigen for inducing protective immunity against influenza virus. Pham et al., immunized BALB/C mice with nanodiamond conjugated trimeric H7 and observed a significant higher H7 specific IgG production as compared to immunization with H7 trimeric antigen alone [151]. Similarly, Ross et al., have shown that intranasal or subcutaneous immunization of BALB/c mice with polyanhydride nanoparticles encapsulated H53 induced high titer neutralizing antibodies. Also, significantly higher CD4+ T cell expansion was observed in vaccinated mice as compared to non-vaccinated mice, thus providing cell-mediated and antibody-mediated protective immunity to mice. Mice were further challenged intranasally with A/H5N1 VNH5N1-PR8CDC-RG influenza virus at 63 days post immunization and viral load was significantly reduced as compared to saline-administered mice [152]. It has also been demonstrated that the polyanhydride nanoparticles give equivalent responses at 64-fold lower doses as compared to free antigen [153]. Also, encapsulation of antigen in polyanhydride nanovaccines exhibited stability of antigen for one year at room temperature, thus providing a major benefit for maintaining stocks of pandemic vaccines [154].
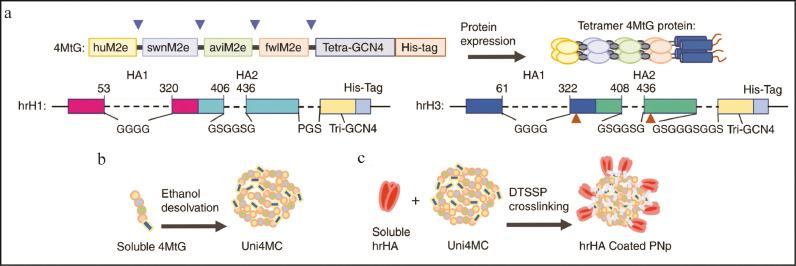
As discussed above, the nanovaccines have been designed by various research groups as combinations of nanomaterials and different antigens, but they provide protection from a particular strain of the virus. Due to antigenic variations, multiple strains of influenza virus have emerged over the years posing serious public threats, indicating the need for a universal vaccine, which includes broad cross-presentation against multiple strains of influenza to reduce community threats. Insights into the structural properties of antigens or peptides can be further explored for the rational designing of broad-spectrum nanovaccines using multiple antigens. On similar lines, to increase the breadth and potency of nanovaccines, Deng et al., have designed double-layered protein nanoparticles. The core of the protein nanoparticles (4MtG) contains four types of M2e from human (huM2e), swine (swnM2e), avian (aviM2e), and domestic fowl (fwlM2e) viral consensus sequences coated with HA variants hrH1 and hrH3 (refer Fig. 5 ). Mice were immunized intramuscularly with Uni4MC (PNp), Uni4C1 (hrH1-coated double-layer PNp) and Uni4C3 (hrH3-coated double-layer PNp) and Uni4C13 (Cocktail of Uni4C1 and Uni4C3). Robust antibody-mediated responses were generated and induced M2e antibodies were strongly cross-reactive to diverse antigens. Also, Uni4C13 group produced broad cellular responses by significantly inducing more IFN-γ secreting splenocytes after stimulation with diverse antigen peptides. Naïve mice were vaccinated with sera from pre-immunized mice with DPBS, Uni4MC, or Uni4C13 and then challenged intranasally with PR8 or Aic after 24 hours. Sera from Uni4C13 immunized mice prevented the mice from a viral infection, indicating the role of serum antibodies. Also, antibodies specific to human M2e, PR8, and Aic were viable for four months after the immunization in mice and implied long-lasting protection [155].
Fig. 5.
Recombinant protein construction and PNp generation and characterization: (A) Cartoon models of the construction and expression of recombinant proteins 4MtG, hrH1, and hrH3. The numbering of hrH1 and hrH3 are based on the amino acid sequences of PR8 and Aic HA, respectively. Above arrows indicate the location of flexible linkers in 4MtG. Dashed lines indicate the sequences replaced with linkers shown below. Below arrows indicate the site-mutations, V325C and S438C in hrH3. (B) Schematic diagram of Uni4MC (desolvated 4MtG PNp) fabrication. Recombinant 4MtG protein was self-assembled into PNps by desolvation as described in the Materials and Methods. (C) Schematic diagram of double-layered nanoparticle generation. An additional layer of trimeric hrHA proteins was crosslinked onto the desolvated Uni4MC PNp surface via DTSSP crosslinking. Adapted from Deng, Lei, et al. "Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses." Nature communications 9.1 (2018): 1–12.http://creativecommons.org/licenses/by/4.0/.
From the examples discussed above, it is evident that with the help of structurally important antigens, smartly designed protein nanomaterials will have the potential of inducing long-lasting immunity against broad spectrum of antigens, thus providing platforms for producing efficient nanovaccines to combat pandemic viruses.
4.1.4. Nanovaccines for HIV
HIV is the sixth leading cause of death worldwide. HIV infection causes systemic depletion of CD4+ T cells, hence compromising the activity of the immune system and the development of AIDS. Immune functions can be preserved in the early stages of HIV by using antiretroviral therapy (ART). It targets the HIV life cycle, but the major challenge is strict adherence to complex ART regimens. This necessitates the urgent need for the development of a prophylactic vaccine against HIV. The most recent and promising HIV vaccine RV144 is in the phase III trial [156]. IgG antibodies raised against V1V2 loop were inversely correlated with the risk of infection in the clinical trial of RV144 [157]. SAPN-based vaccine reduces the need for essential glycosylation of the HIV-1 Env V1V2 loop to form a native-like structure to act as potential immunogen [157]. Although self-assembling VLPs as vectors have shown great potential in the development of immunity against HIV, anti-vector immunity is a significant concern. Here, synthetic nanoparticles offer versatile platform technologies that can induce strong adaptive immune responses while avoiding anti vector immunity and toxicity issues [156].
PLGA based nanoparticles have been used for co-utilization of TLR agonist and the antigen. Intradermal immunization of mice with HIV-1P24-Nef/FLiC/PLGA (antigen/agonist/nanoparticle) enhanced IgG production even at 4-fold lesser dose of antigen. Nanovaccines shifted the immune response towards Th1 polarization and enhanced the Th1 cytokine pattern [158]. Recently, protease cleavage sites (PCS) has been utilized as a strategy to hamper the maturation and infectivity of HIV. Due to low immunogenicity of peptide antigens, they have been crosslinked with chitosan and hyaluronic based nano-formulation. Nanoparticles containing PCS showed enhanced activation of APCs and the production of anti-PCS antibody. Also, these nanoparticles generated memory T cells at longer time points after the last booster dose, indicating their capacity to elicit a good immune response upon infection [159]. Silver nanoparticles coated with amantadine triggered the production of HIV specific CTLs in the spleen of mice with 8-fold stronger TNF-α production in vivo. The co-culture of these HIV specific CTLs with HIV infected cells in vitro enhanced the death of infected cells and reduced the production of HIV [160]. Similarly, HIV-1 peptide and oligosaccharide loaded AuNPs enhanced antigen presentation to isolated T cells from HIV-1 patients. Treatment with AuNP formulated vaccine increased proliferation of HIV-specific CD4+ and CD8+ T cells with increased secretion of pro-Th1 cytokines and pro-inflammatory cytokines in vitro [161].
Malik et al., have shown the prophylactic potential of intravaginally delivered nanogold loaded formulation. Poloxomer and hyaluronic based thermogels were loaded with gold niosomes and mannosylated gold niosomes along with efavirenz (EFV). EFV and GNPs inhibited viral dissemination in PBMCs (peripheral blood mononuclear cells) host cells, but their individual potential in inhibiting pre-interaction viral dissemination was lower than that of the combination. A substantial 42.6% increase in activity was seen when EFV was given with GNPs. Both the moieties had a dual-faced attack wherein GNPs inhibited HIV entry into the cell by causing denaturation of gp120 glycoprotein and EFV inhibited transcriptase enzyme. The mannosylated EFV-GNPs (man(EGNz)) showed further potential activity in inhibiting viral dissemination when the host was pre-exposed to them before viral infection. Thermogels containing EFV-GNP and man(EGNz) showed 87.6 ± 2.4% and 98.1 ± 0.2% inhibition respectively in p24 antigen during anti-HIV prophylactic challenged study [162]. Various such formulations have been developed over the years that enhanced the prophylactic action of raltegravir and efavir [163], phthalate and efavirenz [164] or efavirenz and saquinavir [165].
Bayon et al., have also shown the capacity of nano-lipid complexes (NLC) to induce p24 specific immune responses against HIV in non-human primates (NHP). Four intradermal immunization over a period of 7 months showed that NLC-loaded p-24 antigen elicited significantly higher p24-specific antibodies as compared to the combination of free p24 antigen and CpG adjuvant [166].
There have been advancements in understanding the role of nanovaccines in fighting HIV and development of nanovaccine loaded formulations for prophylaxis of HIV. With the emerging use of nanotechnology development of an efficient vaccine system for HIV prevention is not that far.
4.1.5. Nanovaccine for the prevention of cancer
Despite constant efforts to develop effective cancer vaccines, their efficacy in clinics has always been limited. Nonetheless, two prophylactic vaccines for hepatitis B virus-associated liver cancer and human papillomavirus virus-associated cervical cancer have been approved by US FDA till now [50]. The prophylactic vaccine development for cancer eradication is a challenge due to inefficient identification of tumor-specific antigens (neoantigens), rapid clearance with less accumulation in the lymphoid organs and generation of weak memory immune response. Therefore, the use of nanoparticles to overcome these limitations would be an advantageous strategy.
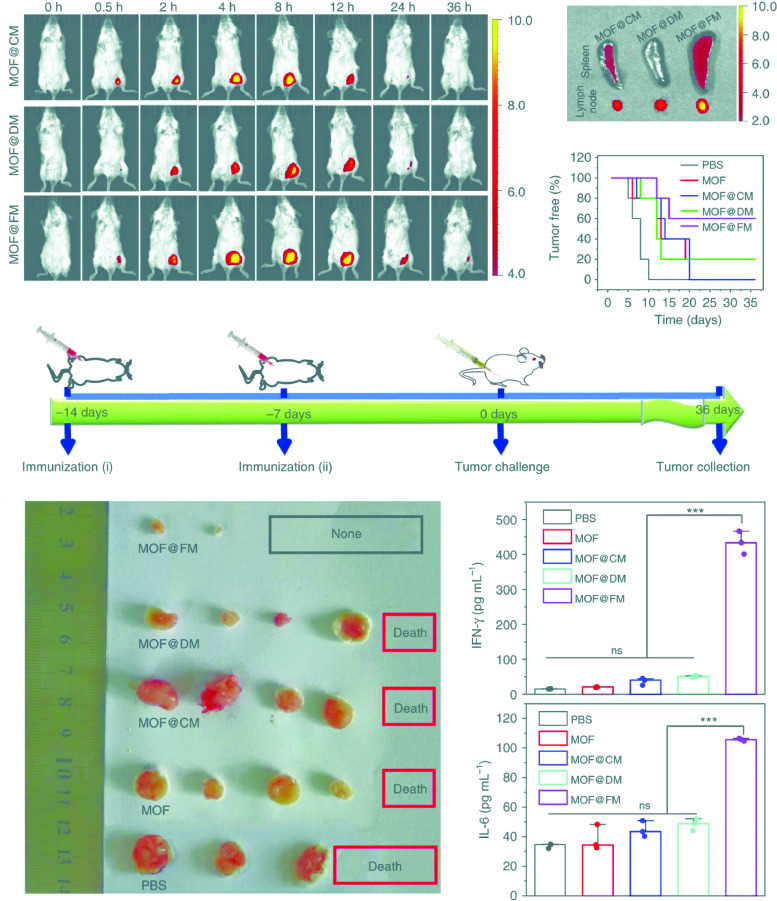
Prolonged and sustained delivery of antigen would be extremely important for the generation of immunologic memory and booster dose reduction. Immunization of mice with protein nanoparticles conjugated to melanoma-associated epitope gp100 and CpG adjuvant enhanced the production of melanoma-specific CD8+ T cells. Pre-immunization with nanoparticles delayed onset of tumor growth and increased the survival rate to 40%, showing prophylactic potential of protein nanoparticles [59]. Luo et al., showed strong cytotoxic T cell response by a physical mixture of PC7A polymeric nanoparticle and OVA antigen. This was achieved by enhanced cytosolic delivery and antigen presentation in APCs of draining lymph node by PC7A NPs and activation of type-I interferon stimulating genes. Nanovaccines showed tumor growth inhibition in melanoma, human papilloma virus E6/E7 tumor and colon cancer models. The combination of anti-PD1 with PC7A NPs showed synergism with 100% survival in a TC-1 model over 60 days. Further, re-challenging of tumor-free mice with TC-1 cells after immunization with PC7A nanoparticles and anti-PD1 showed complete tumor growth inhibition. This indicates the prophylactic response of nanovaccine by generating long-term anti-tumor memory [46]. Liu et al., developed a unique prophylactic nanovaccines using metallo-organic nanoparticles (MOF) coated with fused membrane (FM) of dendritic cells and cancer cells. Presence of whole tumor antigens and immunological co-stimulatory molecules on its cytomembranes impart them APC like properties for cancer specific T cell immuno-activation. Since, fusion of DC and cancer membrane is accompanied by DC maturation, MOF@FM nanovaccines possessed lymph node homing molecules. Therefore, enhanced retention and homing of MOF@FM in lymph nodes and spleen as compared to only cancer membrane fused (MOF@CM) and dendritic cell membrane fused (MOF@DM) nanovaccines was observed. Pre-immunization of mice with MOF@FM has significantly prevented the development of 4T1 tumors by promoting differentiation of CD8+ T cell precursors into cytotoxic T cells with enhanced generation of IFN-ɣ and IL-6 immuno-stimulatory cytokines (refer Fig. 6 ) [77].
Fig. 6.
MOF@FM as a vaccine for tumor prevention. (A) In vivo fluorescence imaging at the indicated time points after the subcutaneous injection of samples. (B) Ex vivo fluorescent images of lymph node and spleen at 36 h after subcutaneous injection. (C) Illustration of the experiment design. Healthy mice were immunized twice in every week by subcutaneous injection and tumor challenge at 7 d after the last immunization. (D) Percentage of tumor-free mice after tumor challenge. (E) Photos of harvested tumors at 36 d after tumor challenge. (F) Levels of secreted IFN-γ in mice serum measured by ELISA kit. The mean values and s.d. were presented and measurements were taken from distinct samples (one-way ANOVA; ns not significant, ***p < 0.001, n = 3). (G) Levels of secreted IL-6 in mice serum measured by ELISA kit. The mean values and s.d. were presented and measurements were taken from distinct samples (oneway ANOVA; ns: not significant, ***p < 0.001, n = 3). Adapted from Liu, Wen-Long, et al. "Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells." Nature communications 10.1 (2019): 1–12.http://creativecommons.org/licenses/by/4.0/.
Tumor heterogeneity with differential expression of antigens makes it challenging to develop a prophylactic treatment strategy. Therefore, most of the nanovaccines being developed for cancer are therapeutic. The detailed description of the same is given in the next section.
4.2. Therapeutic nanovaccines examples
Unlike conventional therapeutics, therapeutic nanovaccines offer the prospect of eliciting antigen-specific responses without any additional non-specific responses. Therapeutic vaccines have been used against autoimmune disorders by antigen-specific targeting of T-regulatory cells [193]. Clinical studies revealed the therapeutic efficacy of VLP in allergen-induced asthma. Bacteriophage derived VLPs were packed with CpG and TLR 9 and induced Th1 response in immune system. A total of 63 asthmatic patients were treated with 7 injections of VLPs as a part of double-blind randomized trial and it was observed that the asthma was well-controlled in patients treated with VLPs as compared to the placebo group [170].
Unlike cancer, nanovaccines showed promising results in pathogenic infections because of the presence of extremely potent foreign non-self antigens. Therefore, vaccine-based therapy is still limited in cancer treatment but can provide an alternative to, or complement conventional cancer treatments.
4.2.1. Therapeutic nanovaccines for cancer
Till now, cancer vaccines have failed to make a mark clinically as compared to other immunotherapies like T cell therapy and checkpoint blockade. The only US FDA approved therapeutic cancer vaccine, Provenge (Sipuleucel-T), has shown a moderate increase in clinical efficacy in prostate cancer [25]. The major problem in successfully developing a cancer vaccine is the selection of tumor-specific antigens called “neoantigens”. Conventionally, cancer vaccines were developed by targeting tumor-associated antigens (TAAs), which are overexpressed specifically in a particular tumor type across patients. However, TAAs are also present in normal tissues; therefore vaccines specific for them can induce peripheral or central immunotolerance leading to autoimmunity or low vaccination efficiency [88].
Neoantigens are tumor-specific antigens derived from random somatic mutations but absent in normal cells. Hence, vaccines targeting such neoantigens using nanoparticles may enhance the outcome of cancer immunotherapy. Cancer nanovaccines can present unique and highly immunogenic tumor-specific antigens with increased antigen loading and efficient delivery, controlled antigen presentation, and retention in lymphoid organs [194].
A recent study showed the targeting of bone marrow and splenic DCs with peptide TY using mesoporous silica nanoparticles conjugated with OVA/CpG for immune activation (MSN-TY/OVA/CpG). Therapeutic application of (MSN-TY/OVA/CpG) in naïve C57BL/6J B16-OVA mice enhanced DC activation and tumor elimination by eliciting tumor-specific CD8+ T cell response, with prolonged survival and less systemic toxicity [195]. To overcome inadequate loading efficiency, weak immune response, and complex preparation process, Dong et al., prepared antigen nanoparticles (ovalbumin NPs) containing CpG adjuvant (ONPs-CpG) with high antigen and adjuvant loading for cancer immunotherapy. The in vitro and in vivo results showed enhanced immune response including DC maturation, T cell activation and IFN- γ production. ONPs-CpG induced remarkable anti-tumor efficacy in mouse lymphoma model [121].
Biomimetic-approach for developing cancer nanovaccines: Overcoming cancer heterogeneity by using patient-derived cell membranes for the development of smart nanovaccines can be a promising biomimetic approach for personalized therapy. Various strategies have been explored in the form of developing artificial antigen-presenting cells (aAPCs) based nanovaccines, use of different cell membrane fused nanovaccines and membrane coated viruses for enhanced cross-presentation and immune stimulation (refer Fig. 7 ).
Fig. 7.
Schematic representation of strategies to design biomimetic nanovaccines for personalized cancer therapy.
Recently, Kuai et al., have shown the anti-tumor efficacy of high-density lipoprotein (HDL) nanodiscs loaded with neo-antigenic peptide and CpG T cell receptor agonist in B16F10 and MC-38 colon carcinoma model. Nanodiscs have delivered the neoantigen to draining lymph nodes and enhanced antigen presentation by APCs. It causes enhanced stimulation of cytotoxic T cell response that potentially inhibited tumor growth. Delivery of nanodiscs with checkpoint blockade (anti-PD1 and anti-CTLA-4) therapy also eradicated established B16F10 and MC-38 colon carcinomas. This strategy showed a powerful therapy to generate personalized cancer medicine [196]. Zhang et al., developed a magnetic artificial antigen-presenting cell (aAPC) for enhancing CD8+ T cell-mediated tumorigenic response. They developed Fe3O4 magnetic nanoclusters coated with leucocyte membrane antigens decorated with MHC-I loaded peptide (SIINFEKL) and anti-CD28 co-stimulatory ligand for EG-7 tumors. These biomimetic aAPCs stimulated OT-1 CD8+ T cells and visually guided them to tumor magnetically, causing reduced tumor burden [197]. This offers a great platform for T cell-based immunotherapy.
The fusion of biological membranes with nanovaccines has emerged as an interesting concept towards biomimetic nanovaccines. A recent study showed biomimetic cancer-derived magnetosome with enhanced retention in lymph nodes after subcutaneous administration. Toll-like receptor (TLR) agonist (CpG) loaded Fe3O4 magnetic nanoclusters camouflaged with cancer membrane and was decorated with anti-CD205 for preferential recognition by CD8+ DC population for cross-presentation to CD8+ T cells via MHC-I. Enhanced stimulation of tumor-specific CD8+ T cells due to prolonged retention of magnetically guided nanocluster in lymph nodes showed anti-tumor efficacy with improved survival in different mice models [191]. Similarly, Guo et al., developed an erythrocyte membrane coated PLGA nanoparticle-containing hgp10025-33 antigenic peptide and monophosphoryl lipid-A (MPL-A) TLR-4 agonist for enhanced anti-tumor immunity. Intradermal injection in C57BL/6 had shown reduced tumor burden along with metastasis and prolonged tumor occurring time after re-challenge in metastatic models [198]. Studies exploiting cancer cell membranes as an envelope to impart a wide repertoire of cancer-specific antigens can offer an advantage to develop various personalized vaccines towards multiple tumor types.
Another interesting strategy for developing personalized biomimetic cancer nanovaccines is the use of cancer cell membrane coated virus for increased adjuvanticity, infectivity and oncolytic activities to generate a strong anti-tumor immune response. A recent study showed the prophylactic and therapeutic potential of a 117.7±0.8 nm biohybrid artificially enveloped virus ExtraCRAd (Extra Conditionally Replicating Adenovirus). Oncolytic adenovirus serotype 5 containing a CpG island with a 24 base pair deletion was encapsulated into B16.OVA cancer cell line membrane. Coating of the cancer membrane was found to enhance the in vitro cytotoxicity of ExtraCRAd as compared to the naked virus indicating the enhanced infectivity of viral vaccine as compared to the naked virus in multiple cell lines. Intra-tumoral injection of ExtraCRAd in C57BL/6 mice containing B16.Ova, B16F10 and LL/2 induced models showed in vivo therapeutic vaccine efficacy in facilitating reduced tumor proliferation as compared to the naked virus, individual vaccine component controls and virus membrane mixture because of enhanced cross-presentation and T cell activation. The prophylactic potential was explored by vaccinating mice 3 times with ExtraCRAd.CMT64.OVA and ExtraCRAd.B16F10 vaccine before CMT64.OVA and B16F10 cells re-challenge, respectively. Prolonged overall survival and enhanced inhibition of tumor proliferation in homologous membrane enveloped viral vaccinated groups as compared to the naked virus and heterologous membrane enveloped group indicated the strong potential of viral-based vaccine imparting wide repertoire of cancer-specific antigens as preventive vaccines [199]. Another viral protein-coated dendrimer nanovaccine containing E6 and E7 peptide epitope of HPV showed enhanced cytotoxic T cell stimulation followed by HPV infected cells eradication. Tumor elimination was observed in 50% of treated mice with no recurrence up to 3 months post initial challenge [24].
Therefore, to address the problems associated with cancer vaccine development, special emphasis has been given on biomimetic personalized nanovaccines. The use of an APCs and nanoparticles coated with the patient's own cancer cell membranes can provide effective antigenic diversity to overcome tumor heterogeneity and tumor-specific immune response. This provides a key strategy to train one's own immune system with appropriate antigens. Research using such a biomimetic personalized approach can significantly improve the outcome of current treatment modalities. Nanovaccines have shown potential in augmenting the therapeutic and prophylactic efficacy of cancer vaccines.
5. Summary
In the past decade, research on vaccines has taken a big leap forward, exploring their vast potential to combat numerous diseases. Specifically, strong interactions of nanoparticles with the immune system and the associated benefits have attracted attention with the hope of producing less toxic and effective nanovaccines. Physicochemical variations in nanoparticles are one of the prime factors affecting their fate as “nanovaccines” inside the body in terms of their clearance, specific bioaccumulation, adjuvanticity, antigenicity and toxicity. Potent antigenicity with prolonged retention and release can cause nanovaccines to elicit both cell-mediated and antibody-mediated antibody along with memory effector response, thus alleviating the use of frequent booster doses. Although nanovaccines are still in their infancy because of its limited clinical trials but new generation nanovaccines possess enormous potential both as prophylactic or therapeutic. Hence, exploring new avenues to increase nanovaccine safety and efficacy should be the driving force for further research in this domain of bio-nanotechnology.
6. Challenges and future prospects
Nanomedicine toxicity, scaling-up processes and lack of regulatory guidelines can be considered as the limiting factors in the production of nanovaccines. The nano-scale size can work as a double edge sword as pre-clinical and clinical reports showed their dose-dependent acute and chronic toxicities with preferential bioaccumulation depending on the route of administration. Few classes of nanoparticles possess inherent toxicity after prolonged exposure like inorganic nanoparticles (e.g., metallic nanoparticles). Moreover, ISCOMs are being used in a number of animal vaccines, but toxicity associated with saponin-based adjuvants prohibited their use in humans. Therefore, concerns over nanoparticle usage for vaccines are still in existence. Scaling up is another major problem that has been minimized up to an extent because of technological advancements, but scale-up in a sterile environment is still a significant challenge [213].
Though, the conventional treatments in this era of modern medicine is saving countless lives. The steady rise in antibiotic resistance and lack of curative cancer therapy because of continuous causative strain variations and cancer heterogeneity respectively drives the focus towards personalized therapeutics. Hence, on similar lines, vaccines against bacterial infections and tumors could benefit significantly, but the current vaccine's antigenic breadth and lack of potency are the limiting factors. Therefore, researchers have recently shifted towards biomimetic nanotechnology to develop biomimetic nanovaccines as personalized medicines. Such nanovaccines can be inherently immuno-stimulatory and multi-antigenic. Coating of bacterial outer membrane vesicles (OMVs) onto nanoparticles for anti-virulence vaccination to deliver and neutralize bacterial toxins outplays the pathogen by utilizing its own survival mechanism. This can prevent bacterial colonization effectively and reduce direct selective pressure to develop antibiotic resistance. A large number of such nano-toxoid formulations can be developed by varying the outer membrane coating of these biomimetic nanovaccines [213]. Despite the advancements in the field of cancer nano-based immunotherapy, the major challenge is the toxicological assessments of these nanovaccines. Prior evaluation of autoimmune side effects generated from nanovaccine induced immune activation needs to be done. Over-activation of antigen presenting cells by nanovaccine can prove to be detrimental as it may cause the death of dendritic cells [47]. Last but not least, increased complexity, cost of manufacturing, and commercialization hurdle related to nanovaccine therapy requires strategies to minimize these limitations for the successful translation of nano-based immunotherapy.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors would like to thank IIT Bombay, Ministry of Human Resource and Development, Government of India, for their research fellowships.
References
- 1.Nandy A., Basak S.C. A brief review of computer-assisted approaches to rational design of peptide vaccines. Int. J. Mol. Sci. 2016;17:390. doi: 10.3390/ijms17050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: progress and challenges. Vaccines. 2014;2:515–536. doi: 10.3390/vaccines2030515. 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zepp F. Principles of vaccine design-lessons from nature. Vaccine. 2010;28(Suppl 3):C14–C24. doi: 10.1016/j.vaccine.2010.07.020. 392. [DOI] [PubMed] [Google Scholar]
- 4.Shen H., Ackerman A.L., Cody V., Giodini A., Hinson E.R., Cresswell P., Edelson R.L., Saltzman W.M., Hanlon D.J. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L.C., Chattopadhyay S., Lin J.C., Hu C.J. Advances and opportunities in nanoparticle- and nanomaterial-based vaccines against bacterial infections. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701395. 393. [DOI] [PubMed] [Google Scholar]
- 6.Tsoras A.N., Champion J.A. Protein and peptide biomaterials for engineered subunit vaccines and immunotherapeutic applications. Annu. Rev. Chem. Biomol. Eng. 2019;10:337–359. doi: 10.1146/annurev-chembioeng-060718-030347. 134. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B., Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leleux J., Roy K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv. Healthc. Mater. 2013;2:72–94. doi: 10.1002/adhm.201200268. 184. [DOI] [PubMed] [Google Scholar]
- 9.Amorij J.P., Kersten G.F., Saluja V., Tonnis W.F., Hinrichs W.L., Slutter B., Bal S.M., Bouwstra J.A., Huckriede A., Jiskoot W. Towards tailored vaccine delivery: needs, challenges and perspectives. J. Controll. Release: Offic. J. Controll. Release Soc. 2012;161:363–376. doi: 10.1016/j.jconrel.2011.12.039. 185. [DOI] [PubMed] [Google Scholar]
- 10.Rosalia R.A., Cruz L.J., van Duikeren S., Tromp A.T., Silva A.L., Jiskoot W., de Gruijl T., Lowik C., Oostendorp J., van der Burg S.H., Ossendorp F. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. 186. [DOI] [PubMed] [Google Scholar]
- 11.Tacken P.J., de Vries I.J., Torensma R., Figdor C.G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 2007;7:790–802. doi: 10.1038/nri2173. 187. [DOI] [PubMed] [Google Scholar]
- 12.Song W., Musetti S.N., Huang L. Nanomaterials for cancer immunotherapy. Biomaterials. 2017;148:16–30. doi: 10.1016/j.biomaterials.2017.09.017. 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z., Tongchusak S., Mizukami Y., Kang Y.J., Ioji T., Touma M., Reinhold B., Keskin D.B., Reinherz E.L., Sasada T. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. 189. [DOI] [PubMed] [Google Scholar]
- 14.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blok B.A., Arts R.J., van Crevel R., Benn C.S., Netea M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J. Leukocyte Biol. 2015;98:347–356. doi: 10.1189/jlb.5RI0315-096R. 191. [DOI] [PubMed] [Google Scholar]
- 16.Demento S.L., Siefert A.L., Bandyopadhyay A., Sharp F.A., Fahmy T.M. Pathogen-associated molecular patterns on biomaterials: a paradigm for engineering new vaccines. Trends Biotechnol. 2011;29:294–306. doi: 10.1016/j.tibtech.2011.02.004. 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010;10:787–796. doi: 10.1038/nri2868. 193. [DOI] [PubMed] [Google Scholar]
- 18.Toy R., Roy K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016;1:47–62. doi: 10.1002/btm2.10005. 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalluru R., Fenaroli F., Westmoreland D., Ulanova L., Maleki A., Roos N., Paulsen Madsen M., Koster G., Egge-Jacobsen W., Wilson S., Roberg-Larsen H., Khuller G.K., Singh A., Nystrom B., Griffiths G. Poly(lactide-co-glycolide)-rifampicin nanoparticles efficiently clear Mycobacterium bovis BCG infection in macrophages and remain membrane-bound in phago-lysosomes. J. Cell Sci. 2013;126:3043–3054. doi: 10.1242/jcs.121814. 195. [DOI] [PubMed] [Google Scholar]
- 20.Gross B.P., Wongrakpanich A., Francis M.B., Salem A.K., Norian L.A. A therapeutic microparticle-based tumor lysate vaccine reduces spontaneous metastases in murine breast cancer. AAPS J. 2014;16:1194–1203. doi: 10.1208/s12248-014-9662-z. 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasievich E.A., Chen W., Huang L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol. Immunother. CII. 2011;60:629–638. doi: 10.1007/s00262-011-0970-1. 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulis L.E., Mandal S., Kreutz M., Figdor C.G. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr. Opin. Immunol. 2013;25:389–395. doi: 10.1016/j.coi.2013.03.001. 198. [DOI] [PubMed] [Google Scholar]
- 23.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussein W.M., Liu T.Y., Jia Z., McMillan N.A.J., Monteiro M.J., Toth I., Skwarczynski M. Multiantigenic peptide-polymer conjugates as therapeutic vaccines against cervical cancer. Bioorganic Med. Chem. 2016;24:4372–4380. doi: 10.1016/j.bmc.2016.07.036. 463. [DOI] [PubMed] [Google Scholar]
- 25.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. 170. [DOI] [PubMed] [Google Scholar]
- 26.Walsh K.P., Mills K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. 201. [DOI] [PubMed] [Google Scholar]
- 27.Tran E., Turcotte S., Gros A., Robbins P.F., Lu Y.C., Dudley M.E., Wunderlich J.R., Somerville R.P., Hogan K., Hinrichs C.S., Parkhurst M.R., Yang J.C., Rosenberg S.A. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vignali D.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkowska M.A., Driessen G.J., Bikos V., Grosserichter-Wagener C., Stamatopoulos K., Cerutti A., He B., Biermann K., Lange J.F., van der Burg M., van Dongen J.J., van Zelm M.C. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–2158. doi: 10.1182/blood-2011-04-345579. 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang H., Benoist C., Mathis D. Rituximab specifically depletes short-lived autoreactive plasma cells in a mouse model of inflammatory arthritis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:4658–4663. doi: 10.1073/pnas.1001074107. 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine T.P., Chain B.M. The cell biology of antigen processing. Crit. Rev. Biochem. Mol. Biol. 1991;26:439–473. doi: 10.3109/10409239109086790. 206. [DOI] [PubMed] [Google Scholar]
- 32.Seidman J.C., Richard S.A., Viboud C., Miller M.A. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir. Viruses. 2012;6:52–62. doi: 10.1111/j.1750-2659.2011.00268.x. 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHugh K.J., Guarecuco R., Langer R., Jaklenec A. Single-injection vaccines: progress, challenges, and opportunities. J. Controll. Release: Off. J. Controll. Release Soc. 2015;219:596–609. doi: 10.1016/j.jconrel.2015.07.029. 208. [DOI] [PubMed] [Google Scholar]
- 34.Rosado M.M., Scarsella M., Pandolfi E., Cascioli S., Giorda E., Chionne P., Madonne E., Gesualdo F., Romano M., Ausiello C.M., Rapicetta M., Zanetti A.R., Tozzi A., Carsetti R. Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example. Eur. J. Immunol. 2011;41:1800–1808. doi: 10.1002/eji.201041187. 209. [DOI] [PubMed] [Google Scholar]
- 35.de Titta A., Ballester M., Julier Z., Nembrini C., Jeanbart L., van der Vlies A.J., Swartz M.A., Hubbell J.A. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl. Acad. Sci. U.S.A. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demento S.L., Cui W., Criscione J.M., Stern E., Tulipan J., Kaech S.M., Fahmy T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials. 2012;33:4957–4964. doi: 10.1016/j.biomaterials.2012.03.041. 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice-Ficht A.C., Arenas-Gamboa A.M., Kahl-McDonagh M.M., Ficht T.A. Polymeric particles in vaccine delivery. Curr. Opin. Microbiol. 2010;13:106–112. doi: 10.1016/j.mib.2009.12.001. 212. [DOI] [PubMed] [Google Scholar]
- 38.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P., Lee L.K., Swartz M.A., Hubbell J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. 213. [DOI] [PubMed] [Google Scholar]
- 39.Kanchan V., Katare Y.K., Panda A.K. Memory antibody response from antigen loaded polymer particles and the effect of antigen release kinetics. Biomaterials. 2009;30:4763–4776. doi: 10.1016/j.biomaterials.2009.05.075. 214. [DOI] [PubMed] [Google Scholar]
- 40.Smith D.M., Simon J.K., Baker J.R., Jr. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013;13:592–605. doi: 10.1038/nri3488. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Gregorio E., Caproni E., Ulmer J.B. Vaccine adjuvants: mode of action. Front. Immunol. 2013;4:214. doi: 10.3389/fimmu.2013.00214. 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Awate S., Babiuk L.A., Mutwiri G. Mechanisms of action of adjuvants. Front. Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maisonneuve C., Bertholet S., Philpott D.J., De Gregorio E. Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12294–12299. doi: 10.1073/pnas.1400478111. 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerkmann M., Rothenfusser S., Hornung V., Towarowski A., Wagner M., Sarris A., Giese T., Endres S., Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. 525. [DOI] [PubMed] [Google Scholar]
- 46.Gregory A.E., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3:13. doi: 10.3389/fcimb.2013.00013. 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulery B.D., Kumar D., Ramer-Tait A.E., Metzger D.W., Wannemuehler M.J., Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PloS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vela Ramirez J.E., Roychoudhury R., Habte H.H., Cho M.W., Pohl N.L., Narasimhan B. Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells. J. Biomater. Sci. Polym. Ed. 2014;25:1387–1406. doi: 10.1080/09205063.2014.940243. 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nevagi R.J., Khalil Z.G., Hussein W.M., Powell J., Batzloff M.R., Capon R.J., Good M.F., Skwarczynski M., Toth I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018;80:278–287. doi: 10.1016/j.actbio.2018.09.037. 325. [DOI] [PubMed] [Google Scholar]
- 50.Zhu M., Wang R., Nie G. Applications of nanomaterials as vaccine adjuvants. Hum. Vaccines Immunother. 2014;10:2761–2774. doi: 10.4161/hv.29589. 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaman M., Good M.F., Toth I. Nanovaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. 465. [DOI] [PubMed] [Google Scholar]
- 52.Swaminathan G., Thoryk E.A., Cox K.S., Meschino S., Dubey S.A., Vora K.A., Celano R., Gindy M., Casimiro D.R., Bett A.J. A novel lipid nanoparticle adjuvant significantly enhances B cell and T cell responses to sub-unit vaccine antigens. Vaccine. 2016;34:110–119. doi: 10.1016/j.vaccine.2015.10.132. 387. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S., Huang S., Lu L., Song X., Li P., Wang F. Curdlan sulfate-O-linked quaternized chitosan nanoparticles: potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int. J. Nanomed. 2018;13:2377–2394. doi: 10.2147/IJN.S158536. 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asgary V., Shoari A., Baghbani-Arani F., Sadat Shandiz S.A., Khosravy M.S., Janani A., Bigdeli R., Bashar R., Cohan R.A. Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. Int. J. Nanomed. 2016;11:3597–3605. doi: 10.2147/IJN.S109098. 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen D.N., Mahon K.P., Chikh G., Kim P., Chung H., Vicari A.P., Love K.T., Goldberg M., Chen S., Krieg A.M., Chen J., Langer R., Anderson D.G. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc. Natl. Acad. Sci. U.S.A. 2012;109:E797–E803. doi: 10.1073/pnas.1121423109. 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siefert A.L., Caplan M.J., Fahmy T.M. Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials. 2016;97:85–96. doi: 10.1016/j.biomaterials.2016.03.039. 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L., Chang T.Z., He Y., Kim J.R., Wang S., Mohan T., Berman Z., Tompkins S.M., Tripp R.A., Compans R.W., Champion J.A., Wang B.Z. Coated protein nanoclusters from influenza H7N9 HA are highly immunogenic and induce robust protective immunity. Nanomedicine. 2017;13:253–262. doi: 10.1016/j.nano.2016.09.001. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz B., Morabito K.M., Ruckwardt T.J., Patterson D.P., Avera J., Miettinen H.M., Graham B.S., Douglas T. Viruslike Particles Encapsidating Respiratory Syncytial Virus M and M2 Proteins Induce Robust T Cell Responses, ACS Biomater. Sci. Eng. 2016;2:2324–2332. doi: 10.1021/acsbiomaterials.6b00532. 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molino N.M., Neek M., Tucker J.A., Nelson E.L., Wang S.W. Viral-mimicking protein nanoparticle vaccine for eliciting anti-tumor responses. Biomaterials. 2016;86:83–91. doi: 10.1016/j.biomaterials.2016.01.056. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi M., Zhang X.E., Sun X., Zhang X., Yao Y., Liu S., Chen Z., Li W., Zhang Z., Chen J., Cui Z. Intranasal nanovaccine confers homo- and hetero-subtypic influenza protection. Small. 2018;14 doi: 10.1002/smll.201703207. 479. [DOI] [PubMed] [Google Scholar]
- 61.Giessen T.W. Encapsulins: microbial nanocompartments with applications in biomedicine, nanobiotechnology and materials science. Curr. Opin. Chem. Biol. 2016;34:1–10. doi: 10.1016/j.cbpa.2016.05.013. 139. [DOI] [PubMed] [Google Scholar]
- 62.Molino N.M., Anderson A.K., Nelson E.L., Wang S.W. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7:9743–9752. doi: 10.1021/nn403085w. 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casanas A., Guerra P., Fita I., Verdaguer N. Vault particles: a new generation of delivery nanodevices. Curr. Opin. Biotechnol. 2012;23:972–977. doi: 10.1016/j.copbio.2012.05.004. 140. [DOI] [PubMed] [Google Scholar]
- 64.Jiang J., Liu G., Kickhoefer V.A., Rome L.H., Li L.X., McSorley S.J., Kelly K.A. A protective vaccine against chlamydia genital infection using vault nanoparticles without an added adjuvant. Vaccines. 2017;5:141. doi: 10.3390/vaccines5010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y., Norberg P.K., Reap E.A., Congdon K.L., Fries C.N., Kelly S.H., Sampson J.H., Conticello V.P., Collier J.H. A supramolecular vaccine platform based on alpha-helical peptide nanofibers. ACS Biomater. Sci. Eng. 2017;3:3128–3132. doi: 10.1021/acsbiomaterials.7b00561. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudra J.S., Mishra S., Chong A.S., Mitchell R.A., Nardin E.H., Nussenzweig V., Collier J.H. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chesson C.B., Huante M., Nusbaum R.J., Walker A.G., Clover T.M., Chinnaswamy J., Endsley J.J., Rudra J.S. Nanoscale peptide self-assemblies boost BCG-primed cellular immunity against mycobacterium tuberculosis. Sci. Rep. 2018;8:12519. doi: 10.1038/s41598-018-31089-y. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pompano R.R., Chen J., Verbus E.A., Han H., Fridman A., McNeely T., Collier J.H., Chong A.S. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthc. Mater. 2014;3:1898–1908. doi: 10.1002/adhm.201400137. 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Si Y., Wen Y., Kelly S.H., Chong A.S., Collier J.H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8(+) T cell responses. J. Controll. Release: Off. J. Controll. Release Soc. 2018;282:120–130. doi: 10.1016/j.jconrel.2018.04.031. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo M., Liu Z., Zhang X., Han C., Samandi L.Z., Dong C., Sumer B.D., Lea J., Fu Y.X., Gao J. Synergistic STING activation by PC7A nanovaccine and ionizing radiation improves cancer immunotherapy. J. Controll. Release: Off. J. Controll. Release Soc. 2019;300:154–160. doi: 10.1016/j.jconrel.2019.02.036. 376. [DOI] [PubMed] [Google Scholar]
- 71.Doll T.A., Dey R., Burkhard P. Design and optimization of peptide nanoparticles. J. Nanobiotechnol. 2015;13:73. doi: 10.1186/s12951-015-0119-z. 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaba S.A., McCoy M.E., Doll T.A., Brando C., Guo Q., Dasgupta D., Yang Y., Mittelholzer C., Spaccapelo R., Crisanti A., Burkhard P., Lanar D.E. Protective antibody and CD8+ T-cell responses to the Plasmodium falciparum circumsporozoite protein induced by a nanoparticle vaccine. PloS One. 2012;7:e48304. doi: 10.1371/journal.pone.0048304. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wahome N., Pfeiffer T., Ambiel I., Yang Y., Keppler O.T., Bosch V., Burkhard P. Conformation-specific display of 4E10 and 2F5 epitopes on self-assembling protein nanoparticles as a potential HIV vaccine. Chem. Biol. Drug Des. 2012;80:349–357. doi: 10.1111/j.1747-0285.2012.01423.x. 159. [DOI] [PubMed] [Google Scholar]
- 74.El Bissati K., Zhou Y., Paulillo S.M., Raman S.K., Karch C.P., Roberts C.W., Lanar D.E., Reed S., Fox C., Carter D., Alexander J., Sette A., Sidney J., Lorenzi H., Begeman I.J., Burkhard P., McLeod R. Protein nanovaccine confers robust immunity against Toxoplasma. NPJ Vaccines. 2017;2:24. doi: 10.1038/s41541-017-0024-6. 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pimentel T.A., Yan Z., Jeffers S.A., Holmes K.V., Hodges R.S., Burkhard P. Peptide nanoparticles as novel immunogens: design and analysis of a prototypic severe acute respiratory syndrome vaccine. Chem. Biol. Drug Des. 2009;73:53–61. doi: 10.1111/j.1747-0285.2008.00746.x. 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L., Hess A., Chang T.Z., Wang Y.C., Champion J.A., Compans R.W., Wang B.Z. Nanoclusters self-assembled from conformation-stabilized influenza M2e as broadly cross-protective influenza vaccines. Nanomedicine. 2014;10:473–482. doi: 10.1016/j.nano.2013.08.005. 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu W.L., Zou M.Z., Liu T., Zeng J.Y., Li X., Yu W.Y., Li C.X., Ye J.J., Song W., Feng J., Zhang X.Z. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat. Commun. 2019;10:3199. doi: 10.1038/s41467-019-11157-1. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y., Du M., Huang G., Wang C., Chen X., Porembka M.R., Lea J., Frankel A.E., Fu Y.X., Chen Z.J., Gao J. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Black M., Trent A., Kostenko Y., Lee J.S., Olive C., Tirrell M. Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv. Mater. 2012;24:3845–3849. doi: 10.1002/adma.201200209. 152. [DOI] [PubMed] [Google Scholar]
- 80.Trent A., Ulery B.D., Black M.J., Barrett J.C., Liang S., Kostenko Y., David N.A., Tirrell M.V. Peptide amphiphile micelles self-adjuvant group A streptococcal vaccination. AAPS J. 2015;17:380–388. doi: 10.1208/s12248-014-9707-3. 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuai R., Sun X., Yuan W., Xu Y., Schwendeman A., Moon J.J. Subcutaneous nanodisc vaccination with neoantigens for combination cancer immunotherapy. Bioconjugate Chem. 2018;29:771–775. doi: 10.1021/acs.bioconjchem.7b00761. 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan L., Weissig V., Gregoriadis G. Comparison of the immune response against polio peptides covalently-surface-linked to and internally-entrapped in liposomes. Asian Pac. J. Allergy Immunol. 1991;9:25–30. 332. [PubMed] [Google Scholar]
- 83.Badiee A., Khamesipour A., Samiei A., Soroush D., Shargh V.H., Kheiri M.T., Barkhordari F., Robert Mc Master W., Mahboudi F., Jaafari M.R. The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp. Parasitol. 2012;132:403–409. doi: 10.1016/j.exppara.2012.09.001. 125. [DOI] [PubMed] [Google Scholar]
- 84.Henriksen-Lacey M., Christensen D., Bramwell V.W., Lindenstrom T., Agger E.M., Andersen P., Perrie Y. Liposomal cationic charge and antigen adsorption are important properties for the efficient deposition of antigen at the injection site and ability of the vaccine to induce a CMI response. J. Controll. Release: Off. J. Controll. Release Soc. 2010;145:102–108. doi: 10.1016/j.jconrel.2010.03.027. 335. [DOI] [PubMed] [Google Scholar]
- 85.Kaur R., Bramwell V.W., Kirby D.J., Perrie Y. Manipulation of the surface pegylation in combination with reduced vesicle size of cationic liposomal adjuvants modifies their clearance kinetics from the injection site, and the rate and type of T cell response. J. Controll. Release: Off. J. Controll. Release Soc. 2012;164:331–337. doi: 10.1016/j.jconrel.2012.07.012. 336. [DOI] [PubMed] [Google Scholar]
- 86.Badiee A., Jaafari M.R., Khamesipour A., Samiei A., Soroush D., Kheiri M.T., Barkhordari F., McMaster W.R., Mahboudi F. Enhancement of immune response and protection in BALB/c mice immunized with liposomal recombinant major surface glycoprotein of Leishmania (rgp63): the role of bilayer composition, Colloids and surfaces B. Biointerfaces. 2009;74:37–44. doi: 10.1016/j.colsurfb.2009.06.025. 337. [DOI] [PubMed] [Google Scholar]
- 87.Wu X., Li Y., Chen X., Zhou Z., Pang J., Luo X., Kong M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunisation. J. Mater. Chem. B. 2019;7:4854–4866. doi: 10.1039/c9tb00448c. 333. [DOI] [PubMed] [Google Scholar]
- 88.Tagliamonte M., Petrizzo A., Tornesello M.L., Buonaguro F.M., Buonaguro L. Antigen-specific vaccines for cancer treatment. Hum. Vaccines Immunother. 2014;10:3332–3346. doi: 10.4161/21645515.2014.973317. 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvarez M.J., Echechipia S., Garcia B., Tabar A.I., Martin S., Rico P., Olaguibel J.M., Liposome-entrapped D. pteronyssinus vaccination in mild asthma patients: effect of 1-year double-blind, placebo-controlled trial on inflammation, bronchial hyperresponsiveness and immediate and late bronchial responses to the allergen. Clin. Exp. Allergy: J. Br. Soc. Allergy Clin. Immunol. 2002;32:1574–1582. doi: 10.1046/j.1365-2222.2002.01514.x. 340. [DOI] [PubMed] [Google Scholar]
- 90.Wu Y.L., Park K., Soo R.A., Sun Y., Tyroller K., Wages D., Ely G., Yang J.C., Mok T. INSPIRE: A phase III study of the BLP25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer. 2011;11:430. doi: 10.1186/1471-2407-11-430. 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tandrup Schmidt S., Foged C., Korsholm K.S., Rades T., Christensen D. Liposome-based adjuvants for subunit vaccines: formulation strategies for subunit antigens and immunostimulators. Pharmaceutics. 2016;8:341. doi: 10.3390/pharmaceutics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Dissel J.T., Joosten S.A., Hoff S.T., Soonawala D., Prins C., Hokey D.A., O'Dee D.M., Graves A., Thierry-Carstensen B., Andreasen L.V., Ruhwald M., de Visser A.W., Agger E.M., Ottenhoff T.H., Kromann I., Andersen P. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine. 2014;32:7098–7107. doi: 10.1016/j.vaccine.2014.10.036. 133. [DOI] [PubMed] [Google Scholar]
- 93.Blom R.A., Erni S.T., Krempaska K., Schaerer O., van Dijk R.M., Amacker M., Moser C., Hall S.R., von Garnier C., Blank F. A triple co-culture model of the human respiratory tract to study immune-modulatory effects of liposomes and virosomes. PloS One. 2016;11 doi: 10.1371/journal.pone.0163539. 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kabiri M., Sankian M., Hosseinpour M., Tafaghodi M. The novel immunogenic chimeric peptide vaccine to elicit potent cellular and mucosal immune responses against HTLV-1. Int. J. Pharmaceut. 2018;549:404–414. doi: 10.1016/j.ijpharm.2018.07.069. 129. [DOI] [PubMed] [Google Scholar]
- 95.Rodrigues L., Raftopoulos K.N., Tandrup Schmidt S., Schneider F., Dietz H., Rades T., Franzyk H., Pedersen A.E., Papadakis C.M., Christensen D., Winter G., Foged C., Hubert M. Immune responses induced by nano-self-assembled lipid adjuvants based on a monomycoloyl glycerol analogue after vaccination with the Chlamydia trachomatis major outer membrane protein. J. Controll. Release: Off. J. Controll. Release Soc. 2018;285:12–22. doi: 10.1016/j.jconrel.2018.06.028. 180. [DOI] [PubMed] [Google Scholar]
- 96.Barriga H.M.G., Holme M.N., Stevens M.M. Cubosomes: the next generation of smart lipid nanoparticles? Angew. Chem. 2019;58:2958–2978. doi: 10.1002/anie.201804067. 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rizwan S.B., McBurney W.T., Young K., Hanley T., Boyd B.J., Rades T., Hook S. Cubosomes containing the adjuvants imiquimod and monophosphoryl lipid A stimulate robust cellular and humoral immune responses. J. Controll. Release: Off. J. Controll. Release Soc. 2013;165:16–21. doi: 10.1016/j.jconrel.2012.10.020. 182. [DOI] [PubMed] [Google Scholar]
- 98.Li X., Deng X., Huang Z. In vitro protein release and degradation of poly-dl-lactide-poly(ethylene glycol) microspheres with entrapped human serum albumin: quantitative evaluation of the factors involved in protein release phases. Pharmaceut. Res. 2001;18:117–124. doi: 10.1023/a:1011043230573. 103. [DOI] [PubMed] [Google Scholar]
- 99.Thomas C., Rawat A., Hope-Weeks L., Ahsan F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharmaceut. 2011;8:405–415. doi: 10.1021/mp100255c. 316. [DOI] [PubMed] [Google Scholar]
- 100.Diwan M., Tafaghodi M., Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J. Controll. Release: Off. J. Controll. Release Soc. 2002;85:247–262. doi: 10.1016/s0168-3659(02)00275-4. 105. [DOI] [PubMed] [Google Scholar]
- 101.Manish M., Rahi A., Kaur M., Bhatnagar R., Singh S. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PloS One. 2013;8:e61885. doi: 10.1371/journal.pone.0061885. 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lima V.M., Bonato V.L., Lima K.M., Dos Santos S.A., Dos Santos R.R., Goncalves E.D., Faccioli L.H., Brandao I.T., Rodrigues-Junior J.M., Silva C.L. Role of trehalose dimycolate in recruitment of cells and modulation of production of cytokines and NO in tuberculosis. Infect. Immun. 2001;69:5305–5312. doi: 10.1128/IAI.69.9.5305-5312.2001. 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borges O., Cordeiro-da-Silva A., Tavares J., Santarem N., de Sousa A., Borchard G., Junginger H.E. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur. J. Pharmaceut. Biopharmaceut.: Off. J. Arbeitsgemeinschaft fur Pharm. Verfahr. 2008;69:405–416. doi: 10.1016/j.ejpb.2008.01.019. 112. [DOI] [PubMed] [Google Scholar]
- 104.Li P., Luo Z., Liu P., Gao N., Zhang Y., Pan H., Liu L., Wang C., Cai L., Ma Y. Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. J. Controll. Release: Off. J. Controll. Release Soc. 2013;168:271–279. doi: 10.1016/j.jconrel.2013.03.025. 320. [DOI] [PubMed] [Google Scholar]
- 105.Hasegawa K., Noguchi Y., Koizumi F., Uenaka A., Tanaka M., Shimono M., Nakamura H., Shiku H., Gnjatic S., Murphy R., Hiramatsu Y., Old L.J., Nakayama E. In vitro stimulation of CD8 and CD4 T cells by dendritic cells loaded with a complex of cholesterol-bearing hydrophobized pullulan and NY-ESO-1 protein: Identification of a new HLA-DR15-binding CD4 T-cell epitope. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2006;12:1921–1927. doi: 10.1158/1078-0432.CCR-05-1900. 318. [DOI] [PubMed] [Google Scholar]
- 106.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feng G., Jiang Q., Xia M., Lu Y., Qiu W., Zhao D., Lu L., Peng G., Wang Y. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of Esat-6 and FL against Mycobacterium tuberculosis infection. PloS One. 2013;8:e61135. doi: 10.1371/journal.pone.0061135. 113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108.Glaffig M., Palitzsch B., Hartmann S., Schull C., Nuhn L., Gerlitzki B., Schmitt E., Frey H., Kunz H. A fully synthetic glycopeptide antitumor vaccine based on multiple antigen presentation on a hyperbranched polymer. Chemistry. 2014;20:4232–4236. doi: 10.1002/chem.201400256. 324. [DOI] [PubMed] [Google Scholar]
- 109.Zhang X.D., Wu H.Y., Wu D., Wang Y.Y., Chang J.H., Zhai Z.B., Meng A.M., Liu P.X., Zhang L.A., Fan F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010;5:771–781. doi: 10.2147/IJN.S8428. 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiodo F., Enriquez-Navas P.M., Angulo J., Marradi M., Penades S. Assembling different antennas of the gp120 high mannose-type glycans on gold nanoparticles provides superior binding to the anti-HIV antibody 2G12 than the individual antennas. Carbohydr. Res. 2015;405:102–109. doi: 10.1016/j.carres.2014.07.012. 326. [DOI] [PubMed] [Google Scholar]
- 111.Tao W., Gill H.S. M2e-immobilized gold nanoparticles as influenza A vaccine: role of soluble M2e and longevity of protection. Vaccine. 2015;33:2307–2315. doi: 10.1016/j.vaccine.2015.03.063. 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kumar R., Ray P.C., Datta D., Bansal G.P., Angov E., Kumar N. Nanovaccines for malaria using Plasmodium falciparum antigen Pfs25 attached gold nanoparticles. Vaccine. 2015;33:5064–5071. doi: 10.1016/j.vaccine.2015.08.025. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Almeida J.P.M., Lin A.Y., Figueroa E.R., Foster A.E., Drezek R.A. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small. 2015;11:1453–1459. doi: 10.1002/smll.201402179. 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y., Kawaguchi A., Hasegawa H., Kajino K., Ninomiya T., Ijiro K., Sawa H. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. 310. [DOI] [PubMed] [Google Scholar]
- 115.Parry A.L., Clemson N.A., Ellis J., Bernhard S.S., Davis B.G., Cameron N.R. Multicopy multivalent' glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013;135:9362–9365. doi: 10.1021/ja4046857. 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang T., Zou M., Jiang H., Ji Z., Gao P., Cheng G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharmaceut. Sci.: Off. J. Eur. Fed. Pharmaceut. Sci. 2011;44:653–659. doi: 10.1016/j.ejps.2011.10.012. 311. [DOI] [PubMed] [Google Scholar]
- 117.Villa C.H., Dao T., Ahearn I., Fehrenbacher N., Casey E., Rey D.A., Korontsvit T., Zakhaleva V., Batt C.A., Philips M.R., Scheinberg D.A. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano. 2011;5:5300–5311. doi: 10.1021/nn200182x. 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo H.C., Feng X.M., Sun S.Q., Wei Y.Q., Sun D.H., Liu X.T., Liu Z.X., Luo J.X., Yin H. Immunisation of mice by hollow mesoporous silica nanoparticles as carriers of porcine circovirus type 2 ORF2 protein. Virol. J. 2012;9:108. doi: 10.1186/1743-422X-9-108. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mody K.T., Mahony D., Cavallaro A.S., Zhang J., Zhang B., Mahony T.J., Yu C., Mitter N. Silica vesicle nanovaccine formulations stimulate long-term immune responses to the bovine viral diarrhoea virus E2 protein. PloS One. 2015;10 doi: 10.1371/journal.pone.0143507. 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sungsuwan S., Yin Z., Huang X. Lipopeptide-coated iron oxide nanoparticles as potential glycoconjugate-based synthetic anticancer vaccines. ACS Appl. Mater. Interfaces. 2015;7:17535–17544. doi: 10.1021/acsami.5b05497. 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dong X., Liang J., Yang A., Qian Z., Kong D., Lv F. A visible codelivery nanovaccine of antigen and adjuvant with self-carrier for cancer immunotherapy. ACS Appl. Mater. Interfaces. 2019;11:4876–4888. doi: 10.1021/acsami.8b20364. 374. [DOI] [PubMed] [Google Scholar]
- 122.Da Silva D.M., Pastrana D.V., Schiller J.T., Kast W.M. Effect of preexisting neutralizing antibodies on the anti-tumor immune response induced by chimeric human papillomavirus virus-like particle vaccines. Virology. 2001;290:350–360. doi: 10.1006/viro.2001.1179. 179. [DOI] [PubMed] [Google Scholar]
- 123.Wagner-Muniz D.A., Haughney S.L., Kelly S.M., Wannemuehler M.J., Narasimhan B. Room temperature stable PSPA-based nanovaccine induces protective immunity. Front. Immunol. 2018;9:325. doi: 10.3389/fimmu.2018.00325. 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsai S., Shameli A., Yamanouchi J., Clemente-Casares X., Wang J., Serra P., Yang Y., Medarova Z., Moore A., Santamaria P. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. 397. [DOI] [PubMed] [Google Scholar]
- 125.Clemente-Casares X., Tsai S., Yang Y., Santamaria P. Peptide-MHC-based nanovaccines for the treatment of autoimmunity: a "one size fits all" approach? J. Mol. Med. 2011;89:733–742. doi: 10.1007/s00109-011-0757-z. 398. [DOI] [PubMed] [Google Scholar]
- 126.Kim S.Y., Noh Y.W., Kang T.H., Kim J.E., Kim S., Um S.H., Oh D.B., Park Y.M., Lim Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56–66. doi: 10.1016/j.biomaterials.2017.03.034. 399. [DOI] [PubMed] [Google Scholar]
- 127.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0433. 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pati R., Shevtsov M., Sonawane A. Nanoparticle vaccines against infectious diseases. Front. Immunol. 2018;9:2224. doi: 10.3389/fimmu.2018.02224. 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanchez-Villamil J.I., Tapia D., Torres A.G. Development of a gold nanoparticle vaccine against enterohemorrhagic escherichia coli O157:H7. mBio. 2019;10:78. doi: 10.1128/mBio.01869-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Athanasiou E., Agallou M., Tastsoglou S., Kammona O., Hatzigeorgiou A., Kiparissides C., Karagouni E. A poly(lactic-co-glycolic) acid nanovaccine based on chimeric peptides from different leishmania infantum proteins induces dendritic cells maturation and promotes peptide-specific IFNgamma-producing CD8(+) T cells essential for the protection against experimental visceral leishmaniasis. Front. Immunol. 2017;8:684. doi: 10.3389/fimmu.2017.00684. 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El Bissati K., Zhou Y., Dasgupta D., Cobb D., Dubey J.P., Burkhard P., Lanar D.E., McLeod R. Effectiveness of a novel immunogenic nanoparticle platform for Toxoplasma peptide vaccine in HLA transgenic mice. Vaccine. 2014;32:3243–3248. doi: 10.1016/j.vaccine.2014.03.092. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.El Bissati K., Chentoufi A.A., Krishack P.A., Zhou Y., Woods S., Dubey J.P., Vang L., Lykins J., Broderick K.E., Mui E., Suzuki Y., Sa Q., Bi S., Cardona N., Verma S.K., Frazeck L., Reardon C.A., Sidney J., Alexander J., Sette A., Vedvick T., Fox C., Guderian J.A., Reed S., Roberts C.W., McLeod R. Adjuvanted multi-epitope vaccines protect HLA-A*11:01 transgenic mice against Toxoplasma gondii. JCI Insight. 2016;1:e85955. doi: 10.1172/jci.insight.85955. 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Multidrug and extensively drug-resistant TB (M/XDR-TB) WHO; 2010. 2010 Global Report Surveillance and Response; p. 466. [Google Scholar]
- 134.Ansari M.A., Zubair S., Mahmood A., Gupta P., Khan A.A., Gupta U.D., Arora A., Owais M. RD antigen based nanovaccine imparts long term protection by inducing memory response against experimental murine tuberculosis. PloS One. 2011;6:e22889. doi: 10.1371/journal.pone.0022889. 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dhanasooraj D., Kumar R.A., Mundayoor S. Vaccine delivery system for tuberculosis based on nano-sized hepatitis B virus core protein particles. Int. J. Nanomed. 2013;8:835–843. doi: 10.2147/IJN.S40238. 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Das I., Padhi A., Mukherjee S., Dash D.P., Kar S., Sonawane A. Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of gammadelta T cells in mice. Nanotechnology. 2017;28 doi: 10.1088/1361-6528/aa60fd. 323. [DOI] [PubMed] [Google Scholar]
- 137.Diogo G.R., Hart P., Copland A., Kim M.Y., Tran A.C., Poerio N., Singh M., Paul M.J., Fraziano M., Reljic R. Immunisation with mycobacterium tuberculosis antigens encapsulated in phosphatidylserine liposomes improves protection afforded by BCG. Front. Immunol. 2019;10:1349. doi: 10.3389/fimmu.2019.01349. 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.WHO . World Health Organization. 2017. World Malaria Report, 2017; p. 196. [Google Scholar]
- 139.Duffy P.E., Sahu T., Akue A., Milman N., Anderson C. Pre-erythrocytic malaria vaccines: identifying the targets. Exp. Rev. Vaccines. 2012;11:1261–1280. doi: 10.1586/erv.12.92. 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Miura K. Progress and prospects for blood-stage malaria vaccines. Exp. Rev. Vaccines. 2016;15:765–781. doi: 10.1586/14760584.2016.1141680. 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pusic K., Aguilar Z., McLoughlin J., Kobuch S., Xu H., Tsang M., Wang A., Hui G. Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol. 2013;27:1153–1166. doi: 10.1096/fj.12-218362. 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Huang W.C., Deng B., Lin C., Carter K.A., Geng J., Razi A., He X., Chitgupi U., Federizon J., Sun B., Long C.A., Ortega J., Dutta S., King C.R., Miura K., Lee S.M., Lovell J.F. A malaria vaccine adjuvant based on recombinant antigen binding to liposomes. Nat. Nanotechnol. 2018;13:1174–1181. doi: 10.1038/s41565-018-0271-3. 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiang S.D., Kong Y.Y., Hanley J., Fuchsberger M., Crimeen-Irwin B., Plebanski M. Nanoparticles modify dendritic cell homeostasis and induce non-specific effects on immunity to malaria. Trans. R. Soc. Trop. Med. Hygiene. 2015;109:70–76. doi: 10.1093/trstmh/tru182. 403. [DOI] [PubMed] [Google Scholar]
- 144.Najer A., Wu D., Bieri A., Brand F., Palivan C.G., Beck H.P., Meier W. Nanomimics of host cell membranes block invasion and expose invasive malaria parasites. ACS Nano. 2014;8:12560–12571. doi: 10.1021/nn5054206. 471. [DOI] [PubMed] [Google Scholar]
- 145.WHO; 2014. WHO Influenza (Seasonal) p. 473. [Google Scholar]
- 146.Houser K., Subbarao K. Influenza vaccines: challenges and solutions. Cell Host Microbe. 2015;17:295–300. doi: 10.1016/j.chom.2015.02.012. 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen J., Deng Y.M. Influenza virus antigenic variation, host antibody production and new approach to control epidemics. Virol. J. 2009;6:30. doi: 10.1186/1743-422X-6-30. 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Blackburne B.P., Hay A.J., Goldstein R.A. Changing selective pressure during antigenic changes in human influenza H3. PLoS Pathogens. 2008;4 doi: 10.1371/journal.ppat.1000058. 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Woodland D.L., Hogan R.J., Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol. Res. 2001;24:53–67. doi: 10.1385/IR:24:1:53. 478. [DOI] [PubMed] [Google Scholar]
- 150.Ding P., Jin Q., Chen X., Yang S., Guo J., Xing G., Deng R., Wang A., Zhang G. Nanovaccine confers dual protection against Influenza A virus and porcine circovirus Type 2. Int. J. Nanomed. 2019;14:7533–7548. doi: 10.2147/IJN.S218057. 480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151.Pham N.B., Ho T.T., Nguyen G.T., Le T.T., Le N.T., Chang H.C., Pham M.D., Conrad U., Chu H.H. Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J. Nanobiotechnol. 2017;15:69. doi: 10.1186/s12951-017-0305-2. 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ross K.A., Loyd H., Wu W., Huntimer L., Ahmed S., Sambol A., Broderick S., Flickinger Z., Rajan K., Bronich T., Mallapragada S., Wannemuehler M.J., Carpenter S., Narasimhan B. Hemagglutinin-based polyanhydride nanovaccines against H5N1 influenza elicit protective virus neutralizing titers and cell-mediated immunity. Int. J. Nanomed. 2015;10:229–243. doi: 10.2147/IJN.S72264. 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Huntimer L., Wilson Welder J.H., Ross K., Carrillo-Conde B., Pruisner L., Wang C., Narasimhan B., Wannemuehler M.J., Ramer-Tait A.E. Single immunisation with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. J. Biomed. Mater. Res. Part B, Appl. Biomater. 2013;101:91–98. doi: 10.1002/jbm.b.32820. 483. [DOI] [PubMed] [Google Scholar]
- 154.Petersen L.K., Phanse Y., Ramer-Tait A.E., Wannemuehler M.J., Narasimhan B. Amphiphilic polyanhydride nanoparticles stabilize Bacillus anthracis protective antigen. Mol. Pharmaceut. 2012;9:874–882. doi: 10.1021/mp2004059. 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Deng L., Mohan T., Chang T.Z., Gonzalez G.X., Wang Y., Kwon Y.M., Kang S.M., Compans R.W., Champion J.A., Wang B.Z. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 2018;9:359. doi: 10.1038/s41467-017-02725-4. 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Aikins M.E., Bazzill J., Moon J.J. Vaccine nanoparticles for protection against HIV infection. Nanomedicine. 2017;12:673–682. doi: 10.2217/nnm-2016-0381. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Karch C.P., Matyas G.R., Burkhard P., Beck Z. Glycosylation of the HIV-1 Env V1V2 loop to form a native-like structure may not be essential with a nanoparticle vaccine. Fut. Virol. 2019;14:51–54. doi: 10.2217/fvl-2018-0174. 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rostami H., Ebtekar M., Ardestani M.S., Yazdi M.H., Mahdavi M. Co-utilization of a TLR5 agonist and nano-formulation of HIV-1 vaccine candidate leads to increased vaccine immunogenicity and decreased immunogenic dose: a preliminary study. Immunol. Lett. 2017;187:19–26. doi: 10.1016/j.imlet.2017.05.002. 406. [DOI] [PubMed] [Google Scholar]
- 159.Dacoba T.G., Omange R.W., Li H., Crecente-Campo J., Luo M., Alonso M.J. Polysaccharide nanoparticles can efficiently modulate the immune response against an HIV peptide antigen. ACS Nano. 2019;13:4947–4959. doi: 10.1021/acsnano.8b07662. 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li W., Balachandran Y.L., Hao Y., Hao X., Li R., Nan Z., Zhang H., Shao Y., Liu Y. Amantadine surface-modified silver nanorods improves immunotherapy of HIV vaccine against HIV-infected cells. ACS Appl. Mater. Interfaces. 2018;10:28494–28501. doi: 10.1021/acsami.8b10948. 408. [DOI] [PubMed] [Google Scholar]
- 161.Climent N., Garcia I., Marradi M., Chiodo F., Miralles L., Maleno M.J., Gatell J.M., Garcia F., Penades S., Plana M. Loading dendritic cells with gold nanoparticles (GNPs) bearing HIV-peptides and mannosides enhance HIV-specific T cell responses. Nanomedicine. 2018;14:339–351. doi: 10.1016/j.nano.2017.11.009. 409. [DOI] [PubMed] [Google Scholar]
- 162.T. Malik, G. Chauhan, G. Rath, R.N. Kesarkar, A.S. Chowdhary, A.K. Goyal, Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system, Artif. Cells Nanomed. Biotechnol.. 46 (2018) 79-90.415 [DOI] [PubMed]
- 163.Date A.A., Shibata A., Goede M., Sanford B., La Bruzzo K., Belshan M., Destache C.J. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antivir. Res. 2012;96:430–436. doi: 10.1016/j.antiviral.2012.09.015. 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Date A.A., Shibata A., McMullen E., La Bruzzo K., Bruck P., Belshan M., Zhou Y., Destache C.J. Thermosensitive gel containing cellulose acetate phthalate-efavirenz combination nanoparticles for prevention of HIV-1 infection. J. Biomed. Nanotechnol. 2015;11:416–427. doi: 10.1166/jbn.2015.1942. 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chaowanachan T., Krogstad E., Ball C., Woodrow K.A. Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis. PloS One. 2013;8:e61416. doi: 10.1371/journal.pone.0061416. 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bayon E., Morlieras J., Dereuddre-Bosquet N., Gonon A., Gosse L., Courant T., Le Grand R., Marche P.N., Navarro F.P. Overcoming immunogenicity issues of HIV p24 antigen by the use of innovative nanostructured lipid carriers as delivery systems: evidences in mice and non-human primates. NPJ Vaccines. 2018;3:46. doi: 10.1038/s41541-018-0086-0. 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ramirez A., Morris S., Maucourant S., D'Ascanio I., Crescente V., Lu I.N., Farinelle S., Muller C.P., Whelan M., Rosenberg W. A virus-like particle vaccine candidate for influenza A virus based on multiple conserved antigens presented on hepatitis B tandem core particles. Vaccine. 2018;36:873–880. doi: 10.1016/j.vaccine.2017.12.053. 488. [DOI] [PubMed] [Google Scholar]
- 168.Babapoor S., Neef T., Mittelholzer C., Girshick T., Garmendia A., Shang H., Khan M.I., Burkhard P. A novel vaccine using nanoparticle platform to present immunogenic M2e against avian influenza infection. Influenza Res. Treat. 2011;2011 doi: 10.1155/2011/126794. 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Karch C.P., Li J., Kulangara C., Paulillo S.M., Raman S.K., Emadi S., Tan A., Helal Z.H., Fan Q., Khan M.I., Burkhard P. Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge. Nanomedicine. 2017;13:241–251. doi: 10.1016/j.nano.2016.08.030. 490. [DOI] [PubMed] [Google Scholar]
- 170.Beeh K.M., Kanniess F., Wagner F., Schilder C., Naudts I., Hammann-Haenni A., Willers J., Stocker H., Mueller P., Bachmann M.F., Renner W.A. The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J. Allergy Clin. Immunol. 2013;131:866–874. doi: 10.1016/j.jaci.2012.12.1561. 418. [DOI] [PubMed] [Google Scholar]
- 171.Tao W., Ziemer K.S., Gill H.S. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine. 2014;9:237–251. doi: 10.2217/nnm.13.58. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lagoutte P., Mignon C., Stadthagen G., Potisopon S., Donnat S., Mast J., Lugari A., Werle B. Simultaneous surface display and cargo loading of encapsulin nanocompartments and their use for rational vaccine design. Vaccine. 2018;36:3622–3628. doi: 10.1016/j.vaccine.2018.05.034. 509. [DOI] [PubMed] [Google Scholar]
- 173.Seth L., Bingham Ferlez K.M., Kaba S.A., Musser D.M., Emadi S., Matyas G.R., Beck Z., Alving C.R., Burkhard P., Lanar D.E. Development of a self-assembling protein nanoparticle vaccine targeting plasmodium falciparum circumsporozoite protein delivered in three Army Liposome Formulation adjuvants. Vaccine. 2017;35:5448–5454. doi: 10.1016/j.vaccine.2017.02.040. 491. [DOI] [PubMed] [Google Scholar]
- 174.Lell B., Agnandji S., von Glasenapp I., Haertle S., Oyakhiromen S., Issifou S., Vekemans J., Leach A., Lievens M., Dubois M.C., Demoitie M.A., Carter T., Villafana T., Ballou W.R., Cohen J., Kremsner P.G. A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon. PloS One. 2009;4:e7611. doi: 10.1371/journal.pone.0007611. 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kester K.E., Cummings J.F., Ofori-Anyinam O., Ockenhouse C.F., Krzych U., Moris P., Schwenk R., Nielsen R.A., Debebe Z., Pinelis E., Juompan L., Williams J., Dowler M., Stewart V.A., Wirtz R.A., Dubois M.C., Lievens M., Cohen J., Ballou W.R., Heppner D.G., Jr., Rts S.V.E.G. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J. Infect. Dis. 2009;200:337–346. doi: 10.1086/600120. 493. [DOI] [PubMed] [Google Scholar]
- 176.Polhemus M.E., Remich S.A., Ogutu B.R., Waitumbi J.N., Otieno L., Apollo S., Cummings J.F., Kester K.E., Ockenhouse C.F., Stewart A., Ofori-Anyinam O., Ramboer I., Cahill C.P., Lievens M., Dubois M.C., Demoitie M.A., Leach A., Cohen J., Ballou W.R., Heppner D.G., Jr. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PloS One. 2009;4:e6465. doi: 10.1371/journal.pone.0006465. 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McCoy M.E., Golden H.E., Doll T.A., Yang Y., Kaba S.A., Zou X., Gerbasi V.R., Burkhard P., Lanar D.E. Mechanisms of protective immune responses induced by the Plasmodium falciparum circumsporozoite protein-based, self-assembling protein nanoparticle vaccine. Malar. J. 2013;12:136. doi: 10.1186/1475-2875-12-136. 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu L., Liu Y., Chen Z., Li W., Liu Y., Wang L., Liu Y., Wu X., Ji Y., Zhao Y., Ma L., Shao Y., Chen C. Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012;12:2003–2012. doi: 10.1021/nl300027p. 496. [DOI] [PubMed] [Google Scholar]
- 179.Montoya J., Solon J.A., Cunanan S.R., Acosta L., Bollaerts A., Moris P., Janssens M., Jongert E., Demoitie M.A., Mettens P., Gatchalian S., Vinals C., Cohen J., Ofori-Anyinam O. A randomized, controlled dose-finding Phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J. Clin. Immunol. 2013;33:1360–1375. doi: 10.1007/s10875-013-9949-3. 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Polonskaya Z., Deng S., Sarkar A., Kain L., Comellas-Aragones M., McKay C.S., Kaczanowska K., Holt M., McBride R., Palomo V., Self K.M., Taylor S., Irimia A., Mehta S.R., Dan J.M., Brigger M., Crotty S., Schoenberger S.P., Paulson J.C., Wilson I.A., Savage P.B., Finn M.G., Teyton L. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Investig. 2017;127:1491–1504. doi: 10.1172/JCI91192. 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu G., Song L., Beasley D.W., Putnak R., Parent J., Misczak J., Li H., Reiserova L., Liu X., Tian H., Liu W., Labonte D., Duan L., Kim Y., Travalent L., Wigington D., Weaver B., Tussey L. Immunogenicity and efficacy of flagellin-envelope fusion dengue vaccines in mice and monkeys. Clin. Vaccine Immunol.: CVI. 2015;22:516–525. doi: 10.1128/CVI.00770-14. 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Karuturi B.V.K., Tallapaka S.B., Yeapuri P., Curran S.M., Sanderson S.D., Vetro J.A. Encapsulation of an EP67-conjugated CTL peptide vaccine in nanoscale biodegradable particles increases the efficacy of respiratory immunisation and affects the magnitude and memory subsets of vaccine-generated mucosal and systemic CD8(+) T Cells in a diameter-dependent manner. Mol. Pharmaceut. 2017;14:1469–1481. doi: 10.1021/acs.molpharmaceut.6b01088. 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Prego C., Paolicelli P., Diaz B., Vicente S., Sanchez A., Gonzalez-Fernandez A., Alonso M.J. Chitosan-based nanoparticles for improving immunisation against hepatitis B infection. Vaccine. 2010;28:2607–2614. doi: 10.1016/j.vaccine.2010.01.011. 501. [DOI] [PubMed] [Google Scholar]
- 184.Schulze K., Ebensen T., Chandrudu S., Skwarczynski M., Toth I., Olive C., Guzman C.A. Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomedicine. 2017;13:2463–2474. doi: 10.1016/j.nano.2017.08.015. 502. [DOI] [PubMed] [Google Scholar]
- 185.Marasini N., Khalil Z.G., Giddam A.K., Ghaffar K.A., Hussein W.M., Capon R.J., Batzloff M.R., Good M.F., Skwarczynski M., Toth I. Lipid core peptide/poly(lactic-co-glycolic acid) as a highly potent intranasal vaccine delivery system against Group A streptococcus. Int. J. Pharmaceut. 2016;513:410–420. doi: 10.1016/j.ijpharm.2016.09.057. 510. [DOI] [PubMed] [Google Scholar]
- 186.Zollinger W.D., Babcock J.G., Moran E.E., Brandt B.L., Matyas G.R., Wassef N.M., Alving C.R. Phase I study of a Neisseria meningitidis liposomal vaccine containing purified outer membrane proteins and detoxified lipooligosaccharide. Vaccine. 2012;30:712–721. doi: 10.1016/j.vaccine.2011.11.084. 503. [DOI] [PubMed] [Google Scholar]
- 187.Zhao K., Chen G., Shi X.M., Gao T.T., Li W., Zhao Y., Zhang F.Q., Wu J., Cui X., Wang Y.F. Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PloS One. 2012;7:e53314. doi: 10.1371/journal.pone.0053314. 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Barhate G., Gautam M., Gairola S., Jadhav S., Pokharkar V. Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J. Pharmaceut. Sci. 2014;103:3448–3456. doi: 10.1002/jps.24161. 505. [DOI] [PubMed] [Google Scholar]
- 189.Chen Y.S., Hung Y.C., Lin W.H., Huang G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21 doi: 10.1088/0957-4484/21/19/195101. 93. [DOI] [PubMed] [Google Scholar]
- 190.Shukla G.S., Sun Y.J., Pero S.C., Sholler G.S., Krag D.N. Immunisation with tumor neoantigens displayed on T7 phage nanoparticles elicits plasma antibody and vaccine-draining lymph node B cell responses. J. Immunol. Methods. 2018;460:51–62. doi: 10.1016/j.jim.2018.06.009. 506. [DOI] [PubMed] [Google Scholar]
- 191.Li F., Nie W., Zhang F., Lu G., Lv C., Lv Y., Bao W., Zhang L., Wang S., Gao X., Wei W., Xie H.Y. Engineering magnetosomes for high-performance cancer vaccination. ACS Central Sci. 2019;5:796–807. doi: 10.1021/acscentsci.9b00060. 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Makidon P.E., Knowlton J., Groom J.V., 2nd, Blanco L.P., LiPuma J.J., Bielinska A.U., Baker J.R., Jr. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med. Microbiol. Immunol. 2010;199:81–92. doi: 10.1007/s00430-009-0137-2. 508. [DOI] [PubMed] [Google Scholar]
- 193.Keijzer C., van der Zee R., van Eden W., Broere F. Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front. Immunol. 2013;4:245. doi: 10.3389/fimmu.2013.00245. 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Blattman J.N., Greenberg P.D. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. 377. [DOI] [PubMed] [Google Scholar]
- 195.Yating L., Lintong Y. Dendritic Cell targeting peptide-based nanovaccines for enhanced cancer immunotherapy. ACS Appl. Bio. Mater. 2019;2:1241–1254. doi: 10.1021/acsabm.8b00811. 373. [DOI] [PubMed] [Google Scholar]
- 196.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater. 2017;16:489–496. doi: 10.1038/nmat4822. 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Q., Wei W., Wang P., Zuo L., Li F., Xu J., Xi X., Gao X., Ma G., Xie H.Y. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-Cell-based anticancer therapy. ACS Nano. 2017;11:10724–10732. doi: 10.1021/acsnano.7b04955. 486. [DOI] [PubMed] [Google Scholar]
- 198.Guo Y., Wang D., Song Q., Wu T., Zhuang X., Bao Y., Kong M., Qi Y., Tan S., Zhang Z. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9:6918–6933. doi: 10.1021/acsnano.5b01042. 71. [DOI] [PubMed] [Google Scholar]
- 199.Fusciello M., Fontana F., Tahtinen S., Capasso C., Feola S., Martins B., Chiaro J., Peltonen K., Ylosmaki L., Ylosmaki E., Hamdan F., Kari O.K., Ndika J., Alenius H., Urtti A., Hirvonen J.T., Santos H.A., Cerullo V. Artificially cloaked viral nanovaccine for cancer immunotherapy. Nat. Commun. 2019;10:5747. doi: 10.1038/s41467-019-13744-8. 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roman V.R., Jensen K.J., Jensen S.S., Leo-Hansen C., Jespersen S., da Silva Te D., Rodrigues C.M., Janitzek C.M., Vinner L., Katzenstein T.L., Andersen P., Kromann I., Andreasen L.V., Karlsson I., Fomsgaard A. Therapeutic vaccination using cationic liposome-adjuvanted HIV type 1 peptides representing HLA-supertype-restricted subdominant T cell epitopes: safety, immunogenicity, and feasibility in Guinea-Bissau. AIDS Res. Hum. Retrovir. 2013;29:1504–1512. doi: 10.1089/AID.2013.0076. 511. [DOI] [PubMed] [Google Scholar]
- 201.Tang J., Yin R., Tian Y., Huang Z., Shi J., Fu X., Wang L., Wu Y., Hao F., Ni B. A novel self-assembled nanoparticle vaccine with HIV-1 Tat(4)(9)(-)(5)(7)/HPV16 E7(4)(9)(-)(5)(7) fusion peptide and GM-CSF DNA elicits potent and prolonged CD8(+) T cell-dependent anti-tumor immunity in mice. Vaccine. 2012;30:1071–1082. doi: 10.1016/j.vaccine.2011.12.029. 512. [DOI] [PubMed] [Google Scholar]
- 202.Sartorius R., D'Apice L., Barba P., Cipria D., Grauso L., Cutignano A., De Berardinis P. Vectorized delivery of alpha-galactosylceramide and tumor antigen on filamentous bacteriophage fd induces protective immunity by enhancing tumor-specific T Cell response. Front. Immunol. 2018;9:1496. doi: 10.3389/fimmu.2018.01496. 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mohsen M.O., Vogel M., Riether C., Muller J., Salatino S., Ternette N., Gomes A.C., Cabral-Miranda G., El-Turabi A., Ruedl C., Kundig T.M., Dermime S., Knuth A., Speiser D.E., Bachmann M.F. Targeting mutated plus germline epitopes confers pre-clinical efficacy of an instantly formulated cancer nano-vaccine. Front. Immunol. 2019;10:1015. doi: 10.3389/fimmu.2019.01015. 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shevtsov M., Multhoff G. Heat shock protein-peptide and HSP-based immunotherapies for the treatment of cancer. Front. Immunol. 2016;7:171. doi: 10.3389/fimmu.2016.00171. 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Teran-Navarro H., Calderon-Gonzalez R., Salcines-Cuevas D., Garcia I., Marradi M., Freire J., Salmon E., Portillo-Gonzalez M., Frande-Cabanes E., Garcia-Castano A., Martinez-Callejo V., Gomez-Roman J., Tobes R., Rivera F., Yanez-Diaz S., Alvarez-Dominguez C. Pre-clinical development of Listeria-based nanovaccines as immunotherapies for solid tumours: insights from melanoma. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2018.1541534. 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yin Z., Wu X., Kaczanowska K., Sungsuwan S., Comellas Aragones M., Pett C., Yu J., Baniel C., Westerlind U., Finn M.G., Huang X. Antitumor humoral and T Cell responses by Mucin-1 conjugates of bacteriophage Qbeta in wild-type mice. ACS Chem. Biol. 2018;13:1668–1676. doi: 10.1021/acschembio.8b00313. 516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Niu L., Chu L.Y., Burton S.A., Hansen K.J., Panyam J. Intradermal delivery of vaccine nanoparticles using hollow microneedle array generates enhanced and balanced immune response. J. Controll. Release: Off. J. Controll. Release Soc. 2019;294:268–278. doi: 10.1016/j.jconrel.2018.12.026. 517. [DOI] [PubMed] [Google Scholar]
- 208.Rueda F., Eich C., Cordobilla B., Domingo P., Acosta G., Albericio F., Cruz L.J., Domingo J.C. Effect of TLR ligands co-encapsulated with multiepitopic antigen in nanoliposomes targeted to human DCs via Fc receptor for cancer vaccines. Immunobiology. 2017;222:989–997. doi: 10.1016/j.imbio.2017.06.002. 518. [DOI] [PubMed] [Google Scholar]
- 209.Chen Q., Bao Y., Burner D., Kaushal S., Zhang Y., Mendoza T., Bouvet M., Ozkan C., Minev B., Ma W. Tumor growth inhibition by mSTEAP peptide nanovaccine inducing augmented CD8(+) T cell immune responses. Drug Deliv. Transl. Res. 2019;9:1095–1105. doi: 10.1007/s13346-019-00652-z. 519. [DOI] [PubMed] [Google Scholar]
- 210.Bloch O., Crane C.A., Fuks Y., Kaur R., Aghi M.K., Berger M.S., Butowski N.A., Chang S.M., Clarke J.L., McDermott M.W., Prados M.D., Sloan A.E., Bruce J.N., Parsa A.T. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro-oncology. 2014;16:274–279. doi: 10.1093/neuonc/not203. 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hamilton E., Blackwell K., Hobeika A.C., Clay T.M., Broadwater G., Ren X.R., Chen W., Castro H., Lehmann F., Spector N., Wei J., Osada T., Lyerly H.K., Morse M.A. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected] J. Transl. Med. 2012;10:28. doi: 10.1186/1479-5876-10-28. 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nguyen F., Alferiev I., Guan P., Guerrero D.T., Kolla V., Moorthy G.S., Chorny M., Brodeur G.M. Enhanced intratumoral delivery of SN38 as a tocopherol oxyacetate prodrug using nanoparticles in a neuroblastoma Xenograft model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018;24:2585–2593. doi: 10.1158/1078-0432.CCR-17-3811. 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou J., Kroll A.V., Holay M., Fang R.H., Zhang L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2019 doi: 10.1002/adma.201901255. 421. [DOI] [PMC free article] [PubMed] [Google Scholar]