Introduction
Although pericardial effusion is a common finding, in most of the cases, its etiology remains unclear (1). Sometimes, however, by using a multidisciplinary approach, a treatable condition can be diagnosed.
Case Report
A 68-years-old woman with a 1-year-long history of dyspnea occurring at minimal exertion and complaining of dizziness was admitted to our hospital. She reported a weight loss of 66 lbs (30 kg) in a year. Diffuse articular complaints were intermittently present. No fever, nocturnal sweating, headache, or any other symptoms were present. Her physical examination was unremarkable, and no tenderness or enlargement of the temporal artery could be detected.
The year before, due to difficulty at breathing, an emergency chest computed tomography (CT) was performed in this patient. An intramural hematoma of the ascending aorta was described, which was treated conservatively. During the same year, the hoarseness occurred. Left-sided recurrent nerve paralysis was discovered, and endoscopic arytenoid lateropexy was performed. The patient’s medical history was remarkable for diabetes mellitus, hypertension, and cataract on both eyes. Despite the cataract extraction, her left eye’s visual acuity remained restricted for 4 years.
These symptoms could point toward several different diseases, such as cardiac failure, coronary disease, aortic dissection, renal disease, malignancy, autoimmune disease, and panic syndrome.
Upon admission, the patient’s blood pressure was in the normal range, and the electrocardiography (ECG) and the chest X-ray did not indicate any anomalies. The blood test showed only an elevated erythrocyte sedimentation rate (52 mm/h). The blood count, C-reactive protein, and renal and thyroid functions were within normal limits. During the 6-minute walk test, the patient could barely walk for 120 meters, and her oxygen saturation dropped from 97% to 82%. TTE revealed a dilated ascending aorta (35 mm) and pericardial effusion near the right ventricle and the apex with a thickness of 10 mm, as well as Grade I aortic, mitral, and tricuspid insufficiency. It was possible to rule out constrictive pericarditis with Doppler measurements. Thoracic CT angiography depicted a thickened aortic wall, but no intramural hematoma could be seen. By performing transesophageal echocardiography, we found that the ascending aortic wall was 9 mm, and the aortic arch was 5 mm thick, while the descending aorta was not affected. By then, the results of the autoimmune serology had arrived, which confirmed the ANCA positivity with elevated proteinase 3 (>200 U/mL) and elevated anti-RF IgM (149 U/mL) and anti-RF IgG (93 U/mL) levels. The ANA, SS-A, SS-B, anti-Scl-70, anti-Jo-1, anti-RNP-70, anti-RNP, anti-Sm, anti-centromere, and anti-MPO antibody levels were within normal limits. No signs of intraabdominal inflammation could be seen on the abdominal Doppler ultrasound. Cardiac CT showed that the thickened aortic wall caused a 40% stenosis in the proximal part of the right coronary artery and that the walls of the pulmonary trunk and pulmonary arteries were also affected. In addition, a stenosis of 53% could be observed on the left pulmonary artery (Fig. 1). The carotid Doppler ultrasound revealed a well-defined thickening of the wall of the right common carotid artery. Regarding the left-side amaurosis, despite the patient’s previous cataract operation, an ophthalmologist consultant was involved. The physical examination of our patient’s eyes revealed the discoloration of the optic disc. The visually evoked potential on the left side was subnormal, while on the right side, it was physiological. During the pattern electroretinography, normal response was depicted on the right side, but on the left side, the potential was hardly elevated from the noise level, which was highly suggestive of anterior ischemic optic neuropathy.
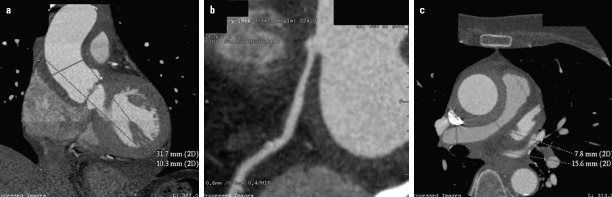
Figure 1.
Cardiac computed tomography after admission: the thickened aortic wall (a), the stenosis in the right coronary artery (b), and the thickening of the wall of the pulmonary trunk and the pulmonary arteries with stenosis on the left pulmonary artery (c)
These results supported the diagnosis of arteritic ischemic optic neuropathy. Having considered the involvement of the great arteries, the ophthalmological complication (2), and the lack of characteristic symptoms of Wegener’s granulomatosis, giant cell arteritis was diagnosed. The patient was put on methylprednisolon and methotrexate therapy, and her symptoms and pericardial effusion gradually decreased.
After 3 years of therapy, the aorta was 5–8 mm thick on CT (Fig. 2), with a minimal pericardial effusion that reoccurred, but the patient was still free of symptoms, and her erythrocyte sedimentation rate also became normal.
Figure 2.
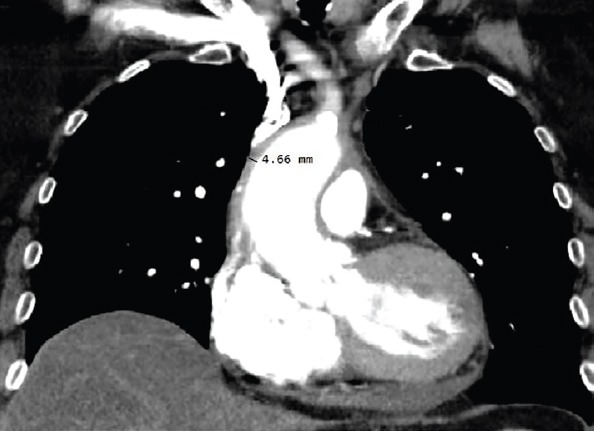
Thoracic computed tomography after 3 years of therapy: The ascending aorta and the aortic arch were 5–8 mm thick
Discussion
Giant cell arteritis is a chronic, often granulomatous vasculitis of large- and medium-sized arteries, involving mainly the cranial branches of the aorta, which occurs in patients aged >50 years (3). The most common symptoms are new-onset localized headache, visual disturbances, polymyalgia rheumatica, jaw claudication, and an elevated erythrocyte sedimentation rate and/or C-reactive protein. Four to five percent of patients have normal erythrocyte sedimentation rate and C-reactive protein at diagnosis (4).
The enlargement of either the pulmonary artery or the aorta can result in a left-sided recurrent nerve palsy, although this has rarely been reported as a complication of giant cell arteritis (5). Cardiac involvement is also rare in giant cell arteritis, and it usually appears as coronary arteritis and myocardial infarction (6). Pulmonary involvement is a rare complication and can be the cause of respiratory symptoms. Its diagnosis is challenging, requiring often the use of positron emission tomography/CT to assess the exact extension of arteritis (7). Unfortunately, this method was unavailable at that time in our center.
The gold standard of the diagnosis is temporal artery biopsy, although this can give a false-negative result in 15%–40% of the cases (8). Because of a great possibility that the temporal artery was not affected, temporal artery biopsy was considered unnecessary.
In the present case, the diagnostic difficulties were caused by the atypical appearance of giant cell arteritis. The vascular involvement was specific to both Takayasu- and giant cell arteritis, although our patient was much older than 50 years at the onset of the disease, which would have been very uncommon in Takayasu arteritis. In addition, some of the main manifestations of temporal arteritis were also missing. Although the autoimmune serology suggested the presence of granulomatosis with polyangiitis, the clinical appearance was against small vessel vasculitis. The ophthalmologic examination with electrophysiological examination led to the final proof: According to the literature, arteritic anterior ischemic optic neuropathy is associated with giant cell arteritis in most of the cases (2).
Conclusion
Pericardial effusion is still an extraordinary manifestation of temporal arteritis, but the exact prevalence is unknown. Although it is usually asymptomatic, it can lead to the final diagnosis, and the development of aortic aneurysm can be prevented.
Video 1
Thickened aortic wall on transesophageal echocardiography: The ascending aortic wall was 9 mm and the aortic arch was 5 mm thick.
Acknowledgments:
The corresponding author gratefully acknowledges the teaching efforts of the co-authors and the assistance of the nurses in the 2nd Department of Internal Medicine of University of Szeged.
Footnotes
Informed consent: Written informed consent was obtained from the patient.
References
- 1.Khandaker MH, Espinosa RE, Nishimura RA, Sinak LJ, Hayes SN, Melduni RM, et al. Pericardial disease:diagnosis and management. Mayo Clin Proc. 2010;85:572–93. doi: 10.4065/mcp.2010.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biousse V, Newman NJ. Ischemic Optic Neuropathies. N Engl J Med. 2015;372:2428–36. doi: 10.1056/NEJMra1413352. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 4.Solans-Laque R, Fonseca E, Escalante B, Martinez-Zapico A, Fraile G, Perez-Conesa M, et al. Giant cell arteritis with normal inflammatory markers at diagnosis. Ann Rheum Dis. 2018;77(Suppl 2):1490. [Google Scholar]
- 5.Edrees A. Ortner's syndrome as a presenting feature of giant cell arteritis. Rheumatol Int. 2012;32:4035–6. doi: 10.1007/s00296-010-1533-z. [DOI] [PubMed] [Google Scholar]
- 6.Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]
- 7.Shu X, Xu X, Peng Q, Lu X, Ma L, Mi N, et al. Diagnostic value of PET/CT for giant cell arteritis combined with pulmonary embolism presenting:Case report and literature review. Medicine (Baltimore) 2017;96:e7651. doi: 10.1097/MD.0000000000007651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong EW, Robertson AJ. Is temporal artery biopsy a worthwhile Procedure? ANZ J Surg. 2005;75:388–91. doi: 10.1111/j.1445-2197.2005.03399.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.