Abstract
Premature ventricular contractions (PVCs) is one of the most common situations in the current cardiology practice. Although PVCs are generally benign in people without any structural heart disease, they may be associated with left ventricular dysfunction, cardiomyopathy, and, rarely, sudden death. Recently, there has been a considerable research in the pathophysiology of PVC, several clinical presentations in different situations, new proposals of successful diagnostic methods, and treatment modalities. Finally, the American College of Cardiology Electrophysiology Council has published a special report that deals with all the aspects of PVC. We reviewed the important points from this report that can be reflected in our daily practice.
Keywords: premature ventricular contraction, left ventricular, cardiomyopathy, sudden death
Introduction
Premature ventricular contractions (PVCs) are frequently detected arrhythmias in the clinical practice. Clinical presentation may vary from a benign condition in a structurally normal heart to an increased risk of sudden cardiac death (SCD) in patients with structural heart disease (SHD) (1). However, the incidence may be up to 90% in the patients with cardiomyopathy (CMP), and PVCs are more complex in such patients (2, 3). Its frequency in the general population varies according to the method used for diagnosis: 1–4% prevalence in 12-lead electrocardiography (ECG) and 40–75% in 24- to 48-h Holter monitoring (4, 5). The American College of Cardiology Electrophysiology Council has published a document that covers all the aspects of the current approaches to PVCs (1). Here, the purpose of this study is to highlight what is new in PVCs and how it can affect our daily clinical practice in approaching PVC.
All the three major electrophysiological mechanisms (enhanced automaticity, triggered activity, and re-entry) may be responsible for the development of PVC. The “triggered activity”, which is responsible from most of PVCs in normal hearts, occurs with the overload of intracellular Ca2+. The most common mechanism observed in the patients with SHD is the re-entry resulting from heterogeneous conduction due to ischemia or scarring. Parasystolic PVCs and ventricular arrhythmias observed in ischemia–reperfusion states are also associated with increased automaticity (1).
Clinical management
Many patients with PVCs may not have any complaints. Others may describe various kinds of palpitations (heart pounding, irregular, skipped, or paused heartbeats) or more general complaints (dizziness, near syncope, dyspnea, chest pain, or fatigue). Symptoms may be associated with PVCs itself, a strong beat followed by a compensatory pause, or a reduction in the cardiac output if it is frequent. Syncope or heart failure (HF) may indicate a serious condition, such as CMP, or may be associated with PVC-induced nonsustained ventricular tachycardia (VT) or ventricular fibrillation (VF). The triggering factors include stress, dehydration, poor sleep, caffeine, alcohol, medication, stimulant drugs, and hormonal cycles in women. Coronary artery disease and acute ischemia, left ventricular (LV) hypertrophy due to hypertension, mitral valve prolapse, hypertrophic CMP, cardiac sarcoidosis, HF, and PVC-induced CMP are commonly associated with PVCs. Inherited arrhythmic conditions such as arrhythmogenic right ventricular dysplasia/CMP (ARVD/C), catecholaminergic polymorphic VT, lamin A/C mutations, or hypertrophic CMP are related to PVCs and SCD (1).
Diagnostic tests
The tools used for the diagnosis of PVCs and their prognostic significance are explained in detail in subtitles (1). Here, we will briefly discuss and summarize the important points. Table 1 summarizes the main diagnostic tests used in the evaluation of PVC.
Table 1.
Main diagnostic tests for premature ventricular contraction evaluation
| Diagnostic tool | Area of use |
|---|---|
| The 12-lead ECG | • Findings showing a structural heart disease |
| • The frequency and the origin of PVCs | |
| • Unifocal or multifocal morphology | |
| Ambulatory monitoring | • The burden of PVCs |
| • Morphology (Unifocal or multifocal) | |
| • More complex ventricular arrhythmia (nonsustained or sustained VAs) | |
| • Correlation between PVCs and symptoms | |
| • The relationship between PVCs and exercise | |
| • Determination of PVCs’ origin, significant changes in QT interval or ST segment | |
| (The 12-lead ambulatory monitoring) | |
| Echocardiography | • Assessment of cardiac structure and functions |
| • Evaluation of improvement in cardiac functions after PVC treatment | |
| Cardiac MRI | • To reveal the underlying infiltrative diseases, edema, and fibrosis |
| • Discrimination between scar areas associated with ischemic and nonischemic CMP | |
| • Risk stratification of sudden cardiac death and VAs | |
| Exercise testing | • Assessment of the presence or absence of structural, coronary, or hereditary arrhythmic conditions |
| • Evaluation of decrease or increase in PVCs | |
| Coronary angiography | • Coronary anatomy in patients with ischemic symptoms or positive stress testing |
| • Coronary artery proximity during catheter ablation procedures | |
| FDG cardiac PET | • Assessment of underlying inflammation |
| • To detect and characterize SHD | |
| EPS | • Identification of PVC mechanism or origin |
| • Risk stratification for sudden cardiac death |
CMP - cardiomyopathy; ECG - electrocardiography; EPS - electrophysiologic study; FDG - fluorodeoxyglucose; MRI - magnetic resonance imaging; PET - positron emission tomography; PVC - premature ventricular contraction; SHD - structural heart disease; VA - ventricular arrhythmia
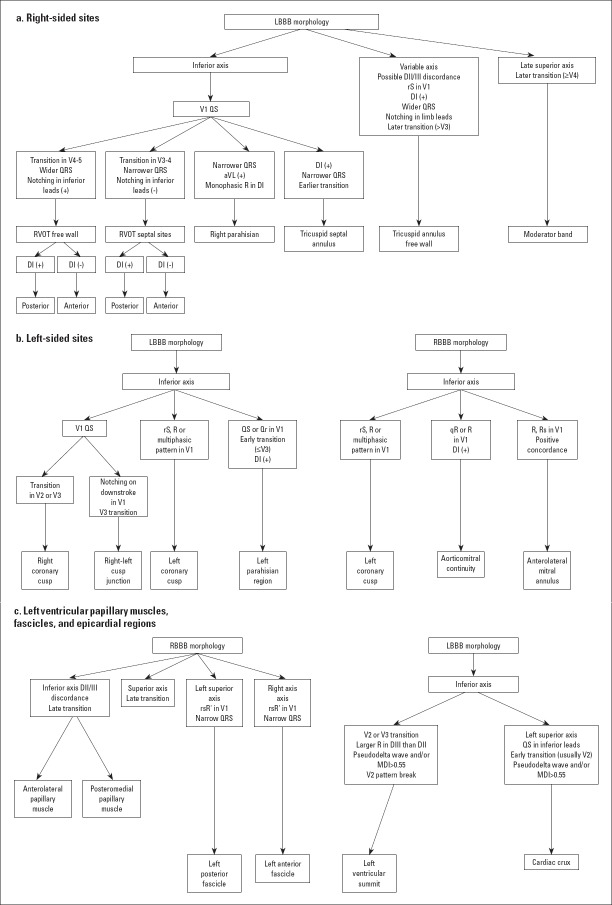
The 12-lead ECG can provide essential clues about SHD (presence of Q waves, poor R progression in ischemic scar, T-wave changes, and epsilon waves in the ARVD/C along with lateral lead QRS fragmentation along with prominent S waves in the basal lateral scar associated with nonischemic CMP. In addition, ECG has a prominent role in determining the origin of PVCs according to QRS width, QRS axis, bundle brunch block pattern, and precordial transition (Fig. 1) (6).
Figure 1.
Classic 12-lead electrocardiography findings of idiopathic premature ventricular contractions (a) For right-sided sites (b) For left-sided sites (c) For other sites
LBBB - left bundle brunch block, MDI - maximum deflection index, RBBB - right bundle brunch block, RVOT - right ventricle outflow tract
Ambulatory ECG provides vital information about the daily burden of PVCs, morphological characteristics, and variability of PVC frequency during the day. Especially, rather than a single 24-h monitoring, longer (48–72-h) or serial 24-h continuous ambulatory monitoring can provide more accurate information in terms of determining the actual burden of PVC. The 12-lead ambulatory monitoring may contribute in the determination of PVCs’ origin, significant changes in QT intervals or ST segments (1, 7). The use of a cardiac event recorder is recommended to show whether symptoms are sporadic and associated with PVC. In the presence of sporadic symptoms, implantable loop recorder is recommended if symptom–rhythm correlation cannot be demonstrated/detected by other methods (1, 7).
Echocardiography is an essential and practical diagnostic tool in the patients with PVCs in terms of determining the ventricular size and function, and excluding SHD. It can also be employed to assess the improvement in the LV systolic functions. However, it should be kept in mind that the evaluation of the LV systolic functions in patients with frequent ventricular ectopia is prone to error. The LV ejection fraction (LVEF) should be calculated from the non-PVC cycles, the second of two consecutive sinus beats, or the mean of two beats in patients with bigeminy PVCs (8, 9). In addition, echocardiography has low sensitivity in identifying the early-stage SHD and scar analysis (1).
Late gadolinium enhancement–magnetic resonance imaging (MRI) is a powerful imaging modality for identifying the infiltrative diseases, microvascular obstruction, myocardial edema, and fibrosis (10). Late gadolinium enhancement–MRI can also be employed to distinguish between scar areas associated with myocardial infarction (MI) and nonischemic CMP. Nonischemic CMP is typically presented with patchy involvement involving the mid-myocardium or epicardium. Additionally, the areas of fibrosis do not match with the distribution of the coronary arteries (11). Late gadolinium involvement may also be employed for prognostic purposes in patients with CMP. A recent review and meta-analysis showed that late gadolinium enhancement on cardiac MRI is considerably correlated with ventricular arrhythmic events in the patients with ischemic or nonischemic CMP [OR 5.62 (95% CI: 4.20–7.51)] (12). In myocarditis, MRI has a poor sensitivity at the early stages of the disease; therefore, both imaging modalities, MRI with delayed contrast enhancement and fluorodeoxyglucose positron emission tomography, are recommended for its detection in the review. An important limitation of MRI is patients with cardiac implantable electronic devices. Nowadays, with the advancement of device technology, MRI can be safely performed in most of the patients with both MR conditional and nonconditional cardiac implantable electronic devices by taking the necessary precautions (13, 14). However, artifacts resulting from such cardiac devices may adversely affect the visualization of myocardial abnormalities.
Exercise testing is beneficial for considering the structural, coronary, or hereditary arrhythmic conditions. Additionally, exercise testing may have a prognostic role in the patients with PVCs. The suppression of PVCs during a light exercise on exercise testing was associated with good prognosis in children without underlying heart disease (15). On the contrary, PVCs observed during rest and exercise phases of exercise testing have been found to be associated with increased mortality and cardiovascular events in the patients with and without SHD in the meta-analysis and cohort studies (16-18).
Coronary angiography is required in the patients with ischemic symptoms or positive stress testing. It is useful in evaluating the proximity of the coronary arteries during the procedure of catheter ablation (CA), especially in the cusp region, coronary sinus, middle cardiac vein, and on the epicardial surface via a subxiphoid approach (1).
The fluorodeoxyglucose positron emission tomography has an increasing significance in the detection of myocardial inflammation with false positivity. Localized or localized diffuse uptake of fluorodeoxyglucose with other indicators such as regional wall motion abnormalities and gadolinium enhancement is interpreted in the favor of myocarditis. It is also useful in the patients with suspected SHD for the detection and characterization of SHD (19).
Electrophysiologic study (EPS) is helpful in the identification of PVC mechanism or origin and risk stratification for SCD. If the PVC mechanism is considered, then the applied procedure during the EPS will vary. For example, the use of sympathomimetic agents will be helpful in triggered PVCs, whereas programmed stimulation and burst pacing will be needed in PVCs with a re-entrant mechanism. The coupling interval of PVC provides an idea of probable mechanism, a variable coupling interval of PVC indicates an increased automaticity or parasystole, and fixed coupling interval indicates re-entry and triggered activity (20). The reproducible induction of PVCs with ventricular programmed stimulation is consistent with a re-entrant mechanism. Rapid atrial or ventricular burst pacing, sometimes, induce PVCs due to a triggered activity (21). Adenosine-suppressed PVCs refer to the cAMP-mediated triggered activity (22). Three-dimensional mapping systems helps in visualizing the catheter, creating the activation, and pace maps to localize the PVCs, even if the PVCs are infrequent.
The characterization of PVCs
VPCs originating from the outflow tracts and LV fascicles generally do not lead to fatal arrhythmias. The underlying structural, ischemic, or electrical disease indicates a potential nonbenign character of PVCs. As compared to uniform PVCs, complex PVCs with couplets, triplets, or nonsustained forms, possibly, have a malignant character. Multifocal origins, the increasing number of PVCs with exercise, nonoutflow tract and non-LV fascicular PVCs, PVCs with short coupling intervals or wider PVCs are also carrying a malignant character (23, 24). Very rarely, the benign PVCs with short coupling interval possess the risk for VT or VF (1).
The frequency of PVCs is also clinically essential for the development of arrhythmia-induced CMP. Speckle-tracking echocardiography findings (measuring LV global longitudinal strain and mechanical dispersion) have showed that even the PVCs’ load of 8–10% may impair the myocardial systolic functions (25). Although there is no clear limit for the development of CMP, it is appropriate to periodically follow up the LV systolic functions in patients with PVCs’ burden of over 8%. In this case, wider PVC–QRS duration, retrograde atrial activation, and interpolation of PVCs are associated with LV dysfunction (26-28).
Idiopathic PVCs
Idiopathic PVCs are defined as PVCs observed in the absence of SHD. In the healthy population, PVCs have been observed up to 75% rate in 48-h Holter monitoring (29). Although idiopathic PVCs generally have a good prognosis, they can cause the development of CMP and, very rarely, induce polymorphic VT/VF. If patients are symptomatic, with class I recommendation, a treatment with a beta-blocker or nondihydropyridine calcium channel blocker is often useful in the control of symptoms and reduction of recurrent arrhythmias (19). Although the use of antiarrhythmic drugs is effective in this patient group, it is generally avoided in the patients with idiopathic PVCs due to their severe side effects. If an antiarrhythmic therapy is not effective, not tolerated, or undesired, then CA should be considered as a highly effective treatment with class I recommendation (19). In 2019 HRS/EHRA/APHRS/LAHRS Catheter Ablation Of Ventricular Arrhythmias Position Paper, CA is recommended in preference to metoprolol or propafenone in the patients with frequent and symptomatic idiopathic PVCs with class I/B level for PVCs with right ventricular outflow tract origin, with class IIa/B level for those with endocardial LV outflow tract, LV summit, or epicardial origin (6).
PVC-related cardiomyopathy
The underlying mechanisms between frequent PVCs and reversible CMP are generally considered due to electrical dyssynchrony, abnormal calcium handling from short-coupled PVCs, or abnormal filling with post-ectopic pauses (30). The PVC burden required for the development of PVC-related CMP varies between 5 and 56% depending on the studied population. Some studies have reported the threshold value of 10–26% or the number of PVCs above 10,000 beats per day as a determinant for the development of CMP. Apart from the burden of PVC, the presence of nonsustained VT, interpolated PVCs, retrograde P waves, a QRS duration longer than 150 ms, longer coupling interval dispersion, epicardial origin or RV origin as compared to LV, male gender, older ages, lack of circadian variability, lack of symptoms, and symptom duration have been suggested as the predictors of the development of LV dysfunction in the patients with frequent PVCs (1).
In patients with SHD, PVC suppression alone with antiarrhythmic drugs (AADs) did not improve survival, even increased mortality (31, 32). Only one study has showed an improved LVEF with flecainide or propafenone in the patients with nonischemic CMP and frequent PVCs refractory to prior CA (33). On the contrary, CA is an effective treatment option in the patients with and without SHD in terms of improvement of LV functions (34-36). Such patients should be monitored closely in the event of recurrence of PVCs, as LV functions may be impaired again in the recurrence of PVCs. In the 2017 ACC/AHA/ACC Ventricular Arrhythmias Guideline, if the patient’s LVEF declined from frequent PVCs (>15% burden), and AADs are ineffective, not tolerated or not desired, then CA is recommended with class IB (19). In the expert consensus statement of HRS/EHRA/APHRS/LAHRS published in 2019, the recommendation level for CA is class I/B for patients suspected to have frequent and monomorphic PVC-related CMP and for whom AADs are ineffective, not tolerated, or not preferred for long-term therapy. The recommendation level is reported as class IIa/B in the patients with SHD in whom frequent PVCs are suspected to be contributing to a CMP in the same conditions (6). Beta-blocker or amiodarone is recommended with class IIa recommendation in the patients with SHD. Calcium antagonists may also be administered; however, it should be considered that they may worsen LV function and exacerbate HF.
PVCs in other structural heart diseases
Myocarditis are responsible for 50% of unexplained cases with VAs, including PVCs. In the detection of myocarditis, combined T2-weighted CMR with early and late gadolinium enhancement plays a pivotal role in detecting myocardia and areas of fibrosis (37). In addition to acute myocarditis, some patients with VA may report chronic myocarditis findings (38). PVC treatment caused by myocarditis should be directed to the underlying etiology, and HF treatment should be provided. Steroids and immunosuppressives as an adjunctive therapy must be considered for different cases due to the wide variety of clinical outcomes. CA is appropriate and safe in drug-resistant VA cases, and requires extensive endo-epicardial mapping and often multiple procedures (39). Implantable cardioverter defibrillator decision should be made while considering the primary/secondary prevention guidelines for SCD.
In patients with ischemic heart disease, PVCs generally arise from the endocardial scar tissue. High PVC burden can worsen the LV functions in such patients, and successful CA may improve LVEF (40, 41).
As a congenital heart disease, PVCs are commonly observed in the patients with tetralogy of Fallot after repair. It is unclear whether the presence of PVC worsens the prognosis in the patients with complex congenital heart disease. The presence of PVCs did not show an increased risk in the patients with tetralogy of Fallot over a 12-year follow-up period (42).
In a systemic review of 33 mitral valve prolapse studies, the presence of complex VAs originating from the outflow tract alternating with the papillary muscle or fascicular region has been associated with an increased risk of SCD (43). In this population, SCD prevention approach is likely to be recommended to the patients with nonischemic CMP (19).
Treatments and prognosis
Lifestyle triggers are generally considered as the intake of caffeine, tobacco, or alcohol along with stress and anxiety, further resulting in electrolyte imbalances, hypoxia, or increased level of catecholamines. Avoidance or reduction of these factors may reduce the PVC burden in selected cases. Notably, PVC burden did not decrease with total abstinence from caffeine and smoking, reducing alcohol intake, and physical exercise in a six-week-long nondrug intervention study with a cohort of healthy men (44). However, if such triggers have been shown to increase the PVC burden in a patient, then reducing caffeine, alcohol, and smoking may reduce PVC. Managing stress, reducing anxiety, and administration of anti-anxiety medications may be beneficial in some patients. Hypokalemia and hypomagnesemia are the predictors of VAs, including PVCs. Intravenous replacement should be considered in the patients with severe hypokalemia and hypomagnesemia (1).
Medical treatment
PVCs can be treated with medically beta-blockers or nondihydropyridine calcium channel blockers. They should be used at the lowest effective dosage to minimize the adverse effects. The exception to this is the presence of previous MI and HF. In such patients, titration should be performed up to the recommended doses. In a randomized study, atenolol has shown effectiveness in the patients with symptomatic and high PVC burden (45).
AAD may be considered in the patients who have symptomatic PVCs without a SHD if the patients are unresponsive to beta-blockers or calcium channel blockers. (4) Most of the studies about suppression PVCs with AADs were relatively older and were conducted with a small number of patients. In these studies, flecainide has been shown to be more effective than mexiletine (46), propafenone was more effective in suppressing idiopathic PVCs than verapamil or metoprolol, and radiofrequency ablation was more effective than any other drug (47). In patients with SHD, sodium channel blockers (class IA and IC AAD) increased the mortality in the CAST trials (31, 48). Sotalol is effective in the suppression of PVCs in the patients with SHD, but QT prolongation and risks of torsades de pointes should be considered (49). Amiodarone is also effective for the suppression of PVCs in the patients with HF and reduced EF since it improves EF in the PVC-induced CMP (50). Class IC AADs also suppresses PVCs and improves EF in the patients with PVC-induced CMP (33).
Catheter ablation
The CA procedure requires combining visual information obtained from fluoroscopy and/or intracardiac echocardiography along with electrical data that indicate the site of earliest activation. Such data are often obtained with activation mapping or pace mapping. Activation mapping can be performed with point-by-point mapping by using an ablation catheter or multielectrode catheter. The earliest local activation time from the tip electrode on bipolar signaling and QS signal on unipolar recording is valuable for the origin of PVC. Activation mapping may be difficult in the cases of infrequent PVCs with multiple and different morphologies. The principle of pace mapping is the formation of the same depolarization with the pace from the earliest activation, further resulting in the same QRS morphology as the patient’s clinical PVCs. Pace mapping is a useful method when the patient’s clinical PVCs are rare, but the same QRS morphology can occur over a large area if high output pace is performed (51, 52). For re-entrant arrhythmias, the earliest region represents the exit from the critical isthmus.
Another important point to achieve successful results after finding the focus of PVCs is to create an effective and permanent lesion in the target area. Tissue contact can be evaluated by using contact force catheters or intracardiac echocardiography. Again, it is crucial to observe a decrease in impedance during ablation in terms of creating a permanent lesion (51-53). PVCs originating from the papillary muscles require circumferential ablation due to many interconnections of the distal parts of the left bundle brunch (54).
Before starting the ablation, it is extremely essential to check whether the energy to be delivered can cause damage to other structures, such as the coronary arteries and conduction system along with aortic cusps, pulmonal valve, epicardial focus, and coronary veins. Intracardiac echocardiography or coronary angiography are required for avoiding the damage of coronary artery when PVC ablation is performed in such areas. If ablation site is close to the conduction system, then cryoablation makes the procedure safe (53). When ablation extends to the deep sites of myocardium, bipolar ablation or irrigated tip catheters can be employed (55).
Conclusion
With the development of diagnostic and therapeutic methods, we have gained more information about the causes of PVCs, their localization, and clinical course. Again, with the development of catheter and three-dimensional mapping systems, the success of CA and procedure reliability in the patients with PVCs have considerably improved. The CA methods are now recommended with class I level in the guidelines and position papers. The management of the patient with VPC is discussed in detail in all the aspects in the report published by the American College of Cardiology Electrophysiology Council. Additionally, new methods such as signal averaged ECGs, noninvasive stereotactic radio-ablation methods, new agents in monoclonal antibody technology, and genetic science will provide the essential knowledge in understanding and treating arrhythmias.
Footnotes
Conflict of interest: None declared.
Peer-review: Internally peer-reviewed.
Authorship contributions: Concept – T.U., B.G.; Design – E.B., T.U.; Supervision – E.B., T.U., B.G.; Funding – None; Materials – None; Data collection and/or processing – E.B., T.U.; Analysis and/or interpretation – E.B., T.U., B.G.; Literature search – E.B., T.U.; Writing – E.B., T.U.; Critical review – E.B., T.U., B.G.
References
- 1.Gorenek B, Fisher JD, Kudaiberdieva G, Baranchuk A, Burri H, Campbell KB, et al. Premature ventricular complexes:diagnostic and therapeutic considerations in clinical practice :A state-of-the-art review by the American College of Cardiology Electrophysiology Council. J Interv Card Electrophysiol. 2019 Dec 11; doi: 10.1007/s10840-019-00655-3. doi:10.1007/s10840-019-00655-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Bigger J, Dresdale R, Heissenbuttel R, Weld FM, Wit AL. Ventricular arrhythmias in ischemic heart disease:mechanism, prevalence, significance and management. Prog Cardiovasc Dis. 1977;19:255–300. doi: 10.1016/0033-0620(77)90005-6. [DOI] [PubMed] [Google Scholar]
- 3.Moss A, Davis H, DeCamilla J, Bayer LW. Ventricular ectopic beat and their relation to sudden and non-sudden cardiac death after myocardial infarction. Circulation. 1979;60:998–1003. doi: 10.1161/01.cir.60.5.998. [DOI] [PubMed] [Google Scholar]
- 4.Ng GA. Treating patients with ventricular ectopic beats. Heart. 2006;92:1707–12. doi: 10.1136/hrt.2005.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med. 1985;312:193–7. doi: 10.1056/NEJM198501243120401. [DOI] [PubMed] [Google Scholar]
- 6.Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Arrhythm. 2019;35:323–484. doi: 10.1002/joa3.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC Scientific Document Group 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death:The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by:Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 8.Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Probl Cardiol. 2015;40:379–422. doi: 10.1016/j.cpcardiol.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Panizo JG, Barra S, Mellor G, Heck P, Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128–34. doi: 10.15420/aer.2018.23.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucciarelli-Ducci C, Baritussio A, Auricchio A. Cardiac MRI Anatomy and Function as a Substrate for Arrhythmias. Europace. 2016;18(suppl 4):iv130–5. doi: 10.1093/europace/euw357. [DOI] [PubMed] [Google Scholar]
- 11.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disertori M, Rigoni M, Pace N, Casolo G, Masè M, Gonzini L, et al. Myocardial Fibrosis Assessment by LGE Is a Powerful Predictor of Ventricular Tachyarrhythmias in Ischemic and Nonischemic LV Dysfunction:A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1046–55. doi: 10.1016/j.jcmg.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Indik JH, Gimbel JR, Abe H, Alkmim-Teixeira R, Birgersdotter-Green U, Clarke GD, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14:e97–153. doi: 10.1016/j.hrthm.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Muthalaly RG, Nerlekar N, Ge Y, Kwong RY, Nasis A. MRI in Patients with Cardiac Implantable Electronic Devices. Radiology. 2018;289:281–92. doi: 10.1148/radiol.2018180285. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen JR, Garson A, Jr, Gillette PC, McNamara DG. Premature ventricular contractions in normal children. J Pediatr. 1978;92:36–8. doi: 10.1016/s0022-3476(78)80066-3. [DOI] [PubMed] [Google Scholar]
- 16.Frolkis JP, Pothier CE, Blackstone EH, Lauer MS. Frequent ventricular ectopy after exercise as a predictor of death. N Engl J Med. 2003;348:781–90. doi: 10.1056/NEJMoa022353. [DOI] [PubMed] [Google Scholar]
- 17.Dewey FE, Kapoor JR, Williams RS, Lipinski MJ, Ashley EA, Hadley D, et al. Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med. 2008;168:225–34. doi: 10.1001/archinte.168.2.225. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kwon M, Chang J, Harris D, Gerson MC, Hwang SS, et al. Meta-Analysis of Prognostic Implications of Exercise-Induced Ventricular Premature Complexes in the General Population. Am J Cardiol. 2016;118:725–32. doi: 10.1016/j.amjcard.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death:A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91–220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 20.de Vries LJ, Martirosyan M, van Domburg RT, Wijchers SA, Géczy T, Szili-Torok T. Coupling interval variability of premature ventricular contractions in patients with different underlying pathology:an insight into the arrhythmia mechanism. J Interv Card Electrophysiol. 2018;51:25–33. doi: 10.1007/s10840-017-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantillon DJ. Evaluation and management of premature ventricular complexes. Cleve Clin J Med. 2013;80:377–87. doi: 10.3949/ccjm.80a.12168. [DOI] [PubMed] [Google Scholar]
- 22.Lerman BB, Ip JE, Shah BK, Thomas G, Liu CF, Ciaccio EJ, et al. Mechanism-specific effects of adenosine on ventricular tachycardia. J Cardiovasc Electrophysiol. 2014;25:1350–8. doi: 10.1111/jce.12510. [DOI] [PubMed] [Google Scholar]
- 23.Lee V, Perera D, Lambiase P. Prognostic significance of exercise induced premature ventricular complexes:a systematic review and meta-analysis of observational studies. Heart Asia. 2017;9:14–24. doi: 10.1136/heartasia-2016-010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli S, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–52. doi: 10.1016/s0735-1097(02)01977-0. [DOI] [PubMed] [Google Scholar]
- 25.Sadron Blaye-Felice M, Hamon D, Sacher F, Pascale P, Rollin A, Duparc A, et al. Premature ventricular contraction-induced cardiomyopathy:related clinical and electrophysiologic parameters. Heart Rhythm. 2016;13:103–10. doi: 10.1016/j.hrthm.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Yokokawa M, Kim HM, Good E, Chugh A, Pelosi F, Jr, Alguire C, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012;9:92–5. doi: 10.1016/j.hrthm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Olgun H, Yokokawa M, Baman T, Kim HM, Armstrong W, Good E, et al. The role of interpolation in PVC-induced cardiomyopathy. Heart Rhythm. 2011;8:1046–9. doi: 10.1016/j.hrthm.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Carballeira Pol L, Deyell MW, Frankel DS, Benhayon D, Squara F, Chik W, et al. Ventricular premature depolarization QRS duration as a new marker of risk for the development of ventricular premature depolarization-induced cardiomyopathy. Heart Rhythm. 2014;11:299–306. doi: 10.1016/j.hrthm.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 29.Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, Brusin MC, et al. Long-term follow-upofright ventricular monomorphic extrasystoles. J Am Coll Cardiol. 2001;38:364–70. doi: 10.1016/s0735-1097(01)01403-6. [DOI] [PubMed] [Google Scholar]
- 30.Panizo JG, Barra S, Mellor G, Heck P, Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128–34. doi: 10.15420/aer.2018.23.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 32.Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 33.Hyman MC, Mustin D, Supple G, Schaller RD, Santangeli P, Arkles J, et al. Class IC antiarrhythmic drugs for suspected premature ventricular contraction-induced cardiomyopathy. Heart Rhythm. 2018;15:159–63. doi: 10.1016/j.hrthm.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Sarrazin JF, Labounty T, Kuhne M, Crawford T, Armstrong WF, Desjardins B, et al. Impact of radiofrequency ablation of frequent post-infarction premature ventricular complexes on left ventricular ejection fraction. Heart Rhythm. 2009;6:1543–9. doi: 10.1016/j.hrthm.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountantonakis SE, Frankel DS, Gerstenfeld EP, Dixit S, Lin D, Hutchinson MD, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation:effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–14. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 36.Penela D, Van Huls Van Taxis C, Van Huls Vans Taxis C, Aguinaga L, Fernández-Armenta J, Mont L, et al. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction:a prospective multicenter study. J Am Coll Cardiol. 2013;62:1195–202. doi: 10.1016/j.jacc.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis:A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–87. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strain JE, Grose RM, Factor SM, Fisher JD. Results of endomyocardial biopsy in patients with spontaneous ventricular tachycardia but without apparent structural heart disease. Circulation. 1983;68:1171–81. doi: 10.1161/01.cir.68.6.1171. [DOI] [PubMed] [Google Scholar]
- 39.Dello Russo A, Casella M, Pieroni M, Pelargonio G, Bartoletti S, Santangeli P, et al. Drug-refractory ventricular tachycardias after myocarditis:endocardial and epicardial radiofrequency catheter ablation. Circ Arrhythmia Electrophysiol. 2012;5:492–8. doi: 10.1161/CIRCEP.111.965012. [DOI] [PubMed] [Google Scholar]
- 40.Sarrazin JF, Good E, Kuhne M, Oral H, Pelosi F, Jr, Chugh A, et al. Mapping and ablation of frequent post-infarction premature ventricular complexes. J Cardiovasc Electrophysiol. 2010;21:1002–8. doi: 10.1111/j.1540-8167.2010.01771.x. [DOI] [PubMed] [Google Scholar]
- 41.Sramko M, Hoogendoorn JC, Glashan CA, Zeppenfeld K. Advancement in cardiac imaging for treatment of ventricular arrhythmias in structural heart disease. Europace. 2019;21:383–403. doi: 10.1093/europace/euy150. [DOI] [PubMed] [Google Scholar]
- 42.Cullen S, Celermajer DS, Franklin RCG, Hallidie-Smith KA, Deanfield JE. Prognostic significance of ventricular arrhythmia after repair of tetralogy of Fallot:a 12-year prospective study. J Am Coll Cardiol. 1994;23:1151–5. doi: 10.1016/0735-1097(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 43.Spartalis M, Tzatzaki E, Spartalis E, Athanasiou A, Moris D, Damaskos C, et al. Mitral valve prolapse:an underestimated cause of sudden cardiac death-a current review of the literature. J Thorac Dis. 2017;9:5390–8. doi: 10.21037/jtd.2017.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeBacker G, Jacobs D, Prineas R, Crow R, Vilandre J, Kennedy H, et al. Ventricular premature contractions:a randomized nondrug intervention trial in normal men. Circulation. 1979;59:762–9. doi: 10.1161/01.cir.59.4.762. [DOI] [PubMed] [Google Scholar]
- 45.Krittayaphong R, Bhuripanyo K, Punlee K, Kangkagate C, Chaithiraphan S. Effect of atenolol on symptomatic ventricular arrhythmia without structural heart disease:a randomized placebo-controlled study. Am Heart J. 2002;144:e10. doi: 10.1067/mhj.2002.125516. [DOI] [PubMed] [Google Scholar]
- 46.Capucci A, Di Pasquale G, Boriani G, Carini G, Balducelli M, Frabetti L, et al. A double-blind crossover comparison of flecainide and slow-release mexiletine in the treatment of stable premature ventricular complexes. Int J Clin Pharmacol Res. 1991;11:23–33. [PubMed] [Google Scholar]
- 47.Stec S, Sikoraks A, Zaborska B, Kryński T, Szymot J, Kułakowski P. Benign symptomatic premature ventricular complexes:shortand long-term efficacy of antiarrhythmic drugs and radiofrequency ablation. Kardiol Pol. 2012;70:351–8. [PubMed] [Google Scholar]
- 48.Cardiac Arrhythmia Suppression Trial II Investigators Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227–33. doi: 10.1056/NEJM199207233270403. [DOI] [PubMed] [Google Scholar]
- 49.Hohnloser SH, Zabel M, Krause T, Just H. Short- and long-term antiarrhythmic and hemodynamic effects of d,l-sotalol in patients with symptomatic ventricular arrhythmias. Am Heart J. 1992;123:1220–4. doi: 10.1016/s0002-8703(10)80002-x. [DOI] [PubMed] [Google Scholar]
- 50.Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 51.Ling Z, Liu Z, Su L, Zipunnikov V, Wu J, Du H, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract:prospective randomized study. Circ Arrhythm Electrophysiol. 2014;7:237–43. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- 52.Baman TS, Ilg KJ, Gupta SK, Good E, Chugh A, Jongnarangsin K, et al. Mapping and ablation of epicardial idiopathic ventricular arrhythmias from within the coronary venous system. Circ Arrhythm Electrophysiol. 2010;3:274–9. doi: 10.1161/CIRCEP.109.910802. [DOI] [PubMed] [Google Scholar]
- 53.Yamada T, McElderry HT, Doppalapudi H, Okada T, Murakami Y, Yoshida Y, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit:anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol. 2010;3:616–23. doi: 10.1161/CIRCEP.110.939744. [DOI] [PubMed] [Google Scholar]
- 54.Fisher JD. Hemiblocks and the fascicular system:myths and implications. J Interv Card Electrophysiol. 2018;52:281–5. doi: 10.1007/s10840-018-0440-1. [DOI] [PubMed] [Google Scholar]
- 55.Koruth JS, Dukkipati S, Miller MA, Neuzil P, d'Avila A, Reddy VY. Bipolar irrigated radiofrequency ablation:a therapeutic option for refractory intramural atrial and ventricular tachycardia circuits. Heart Rhythm. 2012;9:1932–41. doi: 10.1016/j.hrthm.2012.08.001. [DOI] [PubMed] [Google Scholar]