Abstract
The endothelial glycocalyx is a vital regulator of vascular permeability. Damage to this delicate layer can result in increased protein and water transit. The clinical importance of albuminuria as a predictor of kidney disease progression and vascular disease has driven research in this area. This review outlines how research to date has attempted to measure the contribution of the endothelial glycocalyx to vessel wall permeability. We discuss the evidence for the role of the endothelial glycocalyx in regulating permeability in discrete areas of the vasculature and highlight the inherent limitations of the data that have been produced to date. In particular, this review emphasizes the difficulties in interpreting urinary albumin levels in early disease models. In addition, the research that supports the view that glycocalyx damage is a key pathologic step in a diverse array of clinical conditions, including diabetic complications, sepsis, preeclampsia, and atherosclerosis, is summarized. Finally, novel methods are discussed, including an ex vivo glomerular permeability assay that enhances the understanding of permeability changes in disease.
Water and solute exchange across the walls of the microcirculation are dynamic processes that are fundamental to tissue homeostasis. The net rates of exchange are regulated by alterations in systemic blood pressure (hydrostatic pressure), vessel density and size (surface area), flow rate, concentration gradients, and the intrinsic permeability properties of the vessel wall. The basic structure of the capillary wall is conserved throughout the body, consisting of an endothelial cell monolayer, basement membrane, and supporting cells. However, a high level of specialization occurs within discrete areas of the vasculature, optimizing the structure for the individual demands placed on it. The endothelial glycocalyx layer found on the luminal surface of all endothelial cells contributes to the permeability barrier formed by the vessel wall.1,2 Glycocalyx literally translates from the Greek for sugar coat (glykys means sweet and kalyx means husk). This adherent structure includes proteoglycans, glycoproteins, and glycolipids (Figure 1).1,3, 4, 5 Proteoglycans consist of core proteins (eg, syndecans and glypicans) with covalently bound glycosaminoglycan side chains [eg, heparan sulfate (HS) and chondroitin sulfate]. The glycocalyx is not uniform across its depth.6 The 2-layer fiber matrix model suggests a dense 200- to 300-nm meshlike inner layer rich in proteoglycans covalently bound to the endothelial cell membrane and adherent glycosaminoglycans, including long chains of hyaluronan (HA), and an outer, more porous, gellike layer up to 1-μm thick, including adsorbed plasma proteins.6,7 This review discusses the evidence for the importance of the endothelial glycocalyx as a regulator of endothelial and vascular permeability, while noting the inherent difficulties in studying it. The human diseases in which glycocalyx damage and associated permeability alterations appear to be key pathogenic steps are also reviewed.
Figure 1.
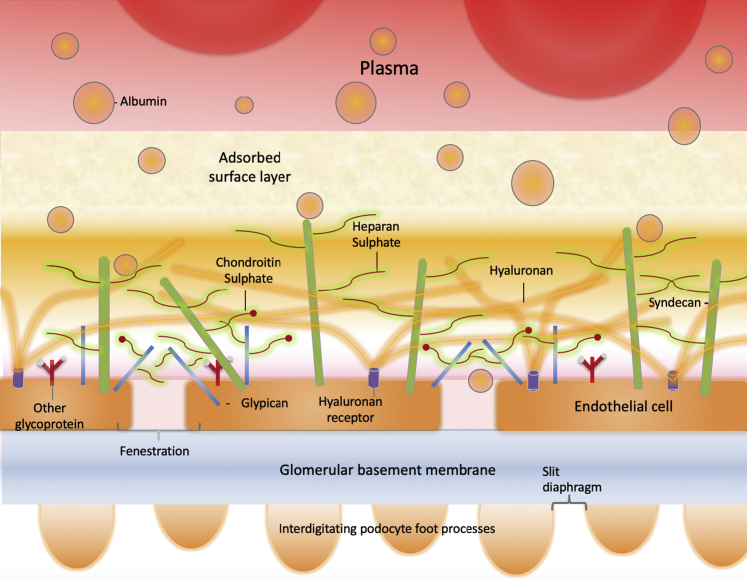
The glomerular filtration barrier consists of glomerular endothelial cells (GEnCs), the glomerular basement membrane, and podocytes. GEnCs possess numerous transcellular fenestrations, which permit the high hydraulic permeability necessary for filtration, and a glycocalyx, which covers the luminal surface, extending over the fenestrations. Podocytes form a second cellular layer by interdigitating their foot processes, which connect at the slit diaphragms. The glycocalyx is a complex structure that contains core proteoglycans, such as syndecans and glypicans, holding glycosaminoglycans, heparan sulfate, chondroitin sulfate, and hyaluronan to the cell surface. The glycocalyx contributes to the filtration barrier by depleting the filtrated protein concentration before it reaches the fenestrated glomerular endothelial cell surface. The generation of a zone of protein-depleted filtrate adjacent to the luminal membrane of endothelial cells (shown in pink) limits loss of macromolecules from the plasma and reduces the effective oncotic pressure across the endothelial cell body.
The Complexities of Studying the Endothelial Glycocalyx as a Permeability Barrier
Endothelial cells in vitro produce a surface glycocalyx that provides an accessible model to study glycocalyx functions, including shear stress sensing2 and permeability regulation.4 However, it is much thinner than the glycocalyx seen in vivo, limiting the applicability of in vitro research.4,8,9 As a result, most studies on the permeability of the glycocalyx have been conducted in vivo.
Much research to date has focused on the glomerular endothelial glycocalyx, which may be driven by the physiologic importance of permeability in glomerular function and the clinical importance of albuminuria. Furthermore, urinary albumin creatinine ratios (uACRs) can be easily quantified to provide a measure of albumin permeability across the glomerular filtration barrier (GFB) (Figure 1). Early models of the GFB overestimated the contribution of the slit diaphragm to the restriction of albumin filtration.10 Recent models of the GFB, however, suggest that the glycocalyx represents a significant protein barrier.11 This finding concurs with accumulating experimental evidence,12 indicating that the remaining components of the GFB, including the endothelial glycocalyx, provide the major barrier to protein permeability (Figure 1).4,13, 14, 15 These estimates, combined with the increased understanding of cellular crosstalk within the GFB, should make us question assumptions about the pathogenesis of albuminuria in multiple historical models.16,17
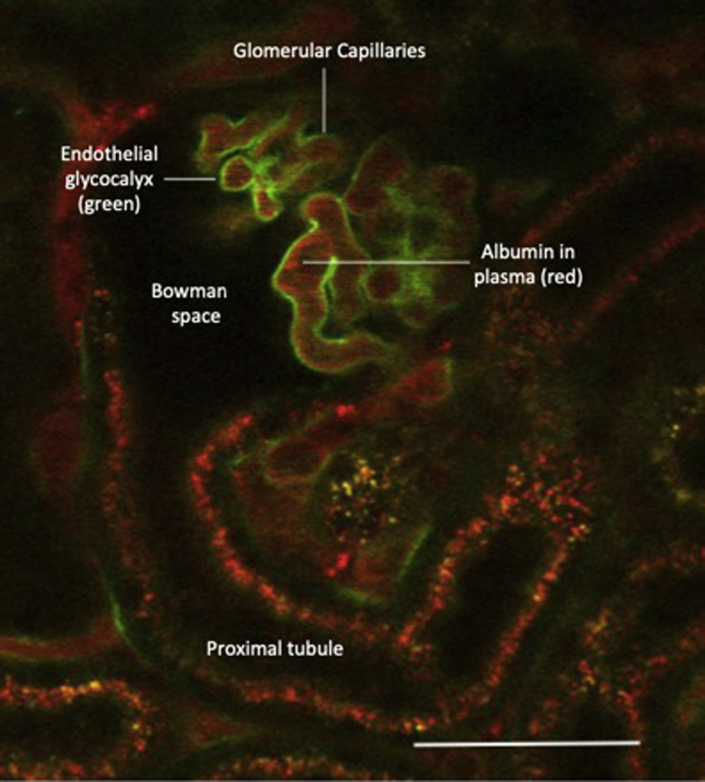
Albuminuria is the net result of albumin passage across the GFB (influenced by permeability) and albumin reabsorption and metabolism within the renal tubules. However, despite the widespread use of uACR, there is increasing evidence in rodent models that uACR is not a sensitive test for changes in GFB permeability and hence not an ideal index of glycocalyx integrity. Using in vivo multiphoton microscopy in mice (Figure 2) (image provided by M.J.B.), we found that glomerular albumin leakage can be significantly increased before detectable levels of albumin appear in the urine.2 Other groups have reported similar data, demonstrating increased glomerular albumin leakage but no significant change in uACR.18 Evidence suggests that that this discrepancy is explained by tubular reabsorption of filtered albumin, resulting in a threshold effect whereby increased glomerular albumin permeability will not result in uACR increases until mechanisms of uptake and metabolism are overwhelmed.19 The role of tubular albumin uptake in humans is debated, but diseases resulting in tubular dysfunction, such as Fanconi syndrome and Dent disease result in significant albuminuria.20 Historical studies in patients with diabetes using lysine to inhibit tubular albumin reabsorption similarly indicate that tubular albumin uptake may influence uACR results.21 In summary, the clinical importance of albuminuria is now well established, but the absence of albuminuria in early rodent models of disease does not exclude increased albumin passage across the GFB.
Figure 2.
A live perfused mouse glomerulus under anesthesia was imaged using multiphoton microscopy. The image was taken 10 minutes after an intravenous bolus of fluorescein isothiocyanate–wheat germ agglutinin. This lectin binds to sialic acid residues within the glycocalyx (labeled green). The plasma within the capillary loops was labeled red with Alexa Flur–conjugated albumin. The dark areas within the capillary loops represent circulating blood cells. The glomerular endothelial glycocalyx is a continuous layer within the glomerular capillaries that contribute to the restriction of macromolecule and water leakage from the glomerulus into the Bowman space. Scale bar = 50 μm.
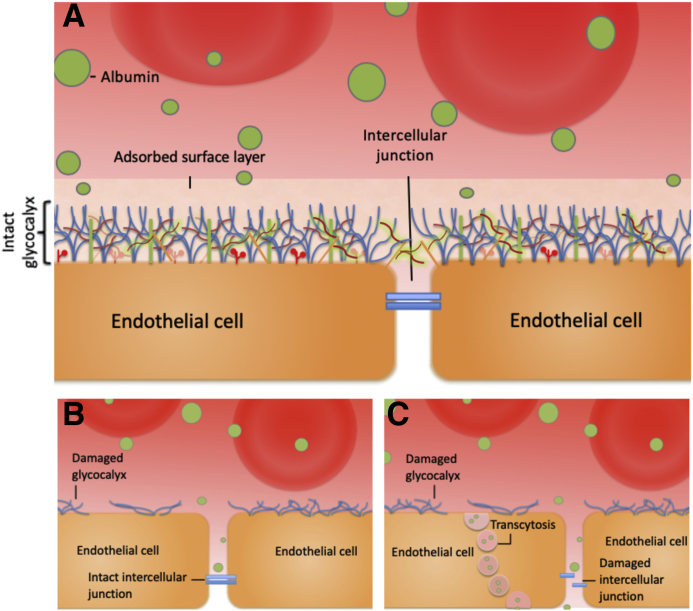
Relatively little research has been conducted studying glycocalyx-dependent permeability changes in the systemic vasculature. In part, this lack of research is attributable to the complex methods needed to study glycocalyx-specific changes. Previous studies in mesenteric microvessels by intravital confocal microscopy have demonstrated that neuraminidase, which disrupts sialic acid residues and reduces glycocalyx depth, increases microvessel permeability.1 Work that compares the depth of the endothelial glycocalyx within the continuous capillaries of the systemic and pulmonary vasculature highlighted significant variability in glycocalyx depth.22 To date, little is known about whether the composition of the glycocalyx varies among these sites. It seems likely that the glycocalyx within discrete areas of the vasculature is specialized, adapting to perform the combination of tasks needed at each tissue site optimally. Variability in the glycocalyx structure means that pathologic insults may not affect all areas of the glycocalyx equally. Specialization is also seen in the endothelial monolayer itself. The double barrier concept was first introduced by Rehm et al23 and is illustrated in Figure 3. They found in guinea pig hearts that simultaneous disruption of both the cellular barrier (using ischemia or histamine) and glycocalyx damage (using heparinase) was needed to increase coronary vessel leakage.23 This work led to the hypothesis that glycocalyx damage overlying a tight cellular barrier will have minimal (direct) influence on monolayer or vessel wall permeability. In contrast, identical glycocalyx damage overlying a leaky cellular monolayer will result in rapid measurable increases in vessel permeability. When glycocalyx-dependent permeability changes within the systemic vasculature are studied, it is therefore important to consider how both glycocalyx structural adaptations and the underlying endothelial cell phenotype will influence detectable permeability changes.
Figure 3.
The double barrier concept. A: In health, both the intact glycocalyx and a tight endothelial monolayer can limit vascular wall permeability to macromolecules (including albumin). B: When damage to the endothelial barrier is limited to the glycocalyx, in areas of the vasculature where a tight cellular monolayer exists, an intact second barrier remains. This tight endothelial monolayer continues to limit macromolecule transit. In such areas, it is currently not possible to directly measure the contribution of the endothelial glycocalyx to the vessel wall permeability. C: When damage affects both the glycocalyx and the permeability of the underlying monolayer, marked increases in the vascular wall permeability will result, but again calculating the relative contribution of the glycocalyx to the vessel wall permeability is not possible. In summary, to directly measure the contribution of the glycocalyx to vessel permeability, vessels that lack a tight endothelial monolayer must be selected until new techniques are developed that can measure macromolecule concentrations in the subglycocalyx space.
To measure the contribution of the endothelial glycocalyx to vessel permeability, comparisons have generally been made after a glycocalyx insult. Enzymatic removal or genetic knockdown of a specific glycocalyx component is commonly used for this purpose. However, the method used can significantly alter the results of such studies. Rapid removal of HS, using human heparanase or bacterial heparinase III, increased albumin passage across endothelial monolayers.4 However, knockout of the HS proteoglycan syndecan 1 did not result in albuminuria in mice.24 In addition, mice that lacked endothelial N-deacetylase and N-sulfotransferase, a key enzyme in modifying HS chains, did not become albuminuric.25
A similar pattern of results was seen in experiments that target HA. Hyaluronidase, an enzyme that degrades HA, has a plasma half-life of approximately 3 minutes before being taken up in the liver via a mannose-dependent mechanism.26 The short plasma half-life and the relatively high molecular weight (55 to 61 kDa) of hyaluronidase result in a high level of glycocalyx-degrading activity with limited off-target effects. Several studies found that removing HA from the endothelial glycocalyx, using hyaluronidase, increased glomerular albumin transit 5.6-fold, a figure consistent with findings.27, 28, 29 Tamoxifen-induced endothelial-specific knockdown of HA synthase 2 (a membrane-bound HA synthesis enzyme) also resulted in significant albuminuria from 4 weeks after induction, which persisted to 12 weeks (experimental end point).30 In contrast, Dane et al18 found that 4 weeks of hyaluronidase infusion did not result in measurable albuminuria, although they found increased glomerular albumin leakage in 90% of glomeruli.
Although this work, again, highlights the fact that albuminuria is a poor measure of low-level glomerular albumin leakage, it also suggests that the differences in time scale, as well as the precise component targeted, may influence glycocalyx permeability changes. Knockdown in genetic models may have resulted in compensatory adaptations (eg, up-regulation of other glycocalyx components). In contrast, the rapid enzymatic removal of glycocalyx components may prevent adaptations from occurring before functional assessments are made.25,27, 28, 29,31 In addition, the rapid removal of a single structural component of the glycocalyx may leave the structure as a whole vulnerable to further nonspecific destruction by the shear forces applied by the circulation. Another contrast between enzymatic removal and genetic knockdown of a glycocalyx component is the generation of circulating glycocalyx fragments. The enzymatic release of fragments is also nonspecific. Heparanase increased syndecan 1 and 4 loss from the glycocalyx structure, possibly by exposing cleavage sites for the actions of other circulating enzymes.32 The influence of active signaling fragments released after glycocalyx enzymatic degradation on vessel permeability has not been directly investigated, but they represent a potentially important pharmacologic target.33,34
In summary, when the glycocalyx is studied, careful consideration needs to be given to the chosen method and tissue. Although the glomerular endothelial glycocalyx lends itself to studying permeability, the uACR should be interpreted with caution. Measures of systemic permeability need to be considered in context. A double barrier can exist, and in the absence of specific manipulations, the relative contributions of the components are difficult to establish. With the development of more sensitive and specific methods to assess permeability, the role of individual glycocalyx components are expected to be better understood in the next decade.35
The Relevance of Glycocalyx Permeability Changes in Disease
Diabetes
There is evidence that the diabetic milieu affects the vasculature globally. Nieuwdorp et al36 measured the total glycocalyx volume by comparing the volume of distribution of erythrocytes and dextran 40 in healthy volunteers. They found that 6 hours of hyperglycemia reduced the glycocalyx volume to 50% of the baseline value. More recently, an intervention trial found that intensification of glycemic control in poorly controlled type 2 diabetic patients during 12 months (glycated hemoglobin reduction from 74 to 54 mol/mol) was associated with a significant increase in glycocalyx depth compared with baseline vaulues.37 Glycocalyx depth in this study was measured using side-stream dark field imaging to assess the perfused boundary region depth (a measure inversely proportional to glycocalyx thickness) on sublingual vessels, which suggests that systemic glycocalyx damage in early diabetes is at least partially reversible.37 Changes to the endothelial glycocalyx occur early in the disease course, suggesting that they could represent a valuable direct therapeutic target.35 Electron microscopy on glomerular capillaries showed that the percentage surface covered by the glycocalyx is one of the earliest detectable vascular changes in diabetes.35 Consistent with this, Targosz-Korecka et al38 used atomic force microscopy to map the glycocalyx on diabetic db/db mouse aortas. They found that endothelial glycocalyx coverage was significantly reduced by week 11.38 At this time point, significant reductions in the glycocalyx depth were not detected.38 Although glycocalyx damage occurs early in diabetic disease, the clinical manifestations of this damage may be significantly delayed and remain dependent on the function of the vascular bed studied.
Diabetic Nephropathy
The hallmark of diabetic nephropathy (DN) is microalbuminuria. In diabetes, uACR is a key screening test, predicting patients at the highest risk of progressive renal and vascular disease.39 Electron microscopy to study glomerular structural changes in patients with diabetes found that loss of endothelial fenestration area correlated with the uACR more strongly than podocyte detachment.40 These data suggest that endothelial damage is likely to be one of the key steps that results in albuminuria in diabetes.40 The authors of this study did not examine the glycocalyx, but it seems likely that the endothelial cell damage, resulting in fenestration loss, was the result of altered vascular endothelial growth factor (VEGF) signaling.41,42 Altered VEGF signaling results in glycocalyx loss.15,29,43 Given the evidence that supports the importance of the glycocalyx in glomerular filtration, it seems likely that glycocalyx damage contributed to the microalbuminuria seen in these patients.
Experimental evidence supports this view. On cultured monolayers of conditionally immortalized glomerular endothelial cells, high concentrations of glucose result in a marked reduction in the biosynthesis of the glycocalyx component HS, with an associated increase in albumin permeability.44 Jeansson et al45 confirmed that increased glomerular albumin leakage contributed to the albuminuria detected in animal models of diabetes by cooling isolated kidneys to 8°C to limit tubular effects. Loss of HA and HS from the glomerular capillary wall has been confirmed in streptozocin-induced diabetic rats and Zucker fatty rats, suggesting that glycocalyx damage is likely to contribute to the increase in albumin filtration seen in these disease models.46,47 More recently, it is reported that diabetic glomerular glycocalyx damage can be prevented in the mouse streptozocin model of diabetes by administering a matrix metalloprotease (MMP) 2/9 inhibitor.48 A daily injection of MMP2/9 inhibitor reduced syndecan 4 loss from the glycocalyx and significantly reduced albuminuria by reducing glomerular albumin permeability.48 As previously reported, high-power electron microscopy images of the GFB and glomerular endothelial glycocalyx suggested that glycocalyx changes had occurred before other significant changes to the GFB.29,35,48 An isolated glomerular albumin permeability assay used early in the diabetic disease course confirmed that glomerular glycocalyx loss is associated with increased glomerular albumin permeability in both the mouse and rat streptozocin diabetic models.29,48 This unique assay has the advantage of studying glomerular permeability in the absence of hemodynamic influence, ensuring that alterations in the glomerular perfusion pressure will not influence permeability measurements. In addition, this assay allows us to study changes in glomerular permeability in isolation from the renal tubules and hence confounding tubular albumin reuptake. This assay found that glomerular albumin leakage may be significantly increased before an increase in uACR is detectable. In summary, the current evidence suggests that glycocalyx injury is likely to contribute to the early pathogenesis of DN.
Diabetic Retinopathy
Diabetic retinopathy (DR), like DN, is a manifestation of diabetic microvascular damage. The retina is a continuation of the central nervous system with a blood retina barrier. The pathogenesis of DR has been reviewed in detail elsewhere,49,50 but the role of the retinal endothelial glycocalyx and its impairment in diabetes is an area of ongoing research. DR is characterized by microaneurysms, leukocyte-endothelial adhesion, hemorrhage, capillary occlusion, neovascularization, and increased permeability.51 Diabetic rats have a significantly thinner retinal endothelial glycocalyx compared with control rats (28.3 versus 60.2 nmol/L, P < 0.01), measured using transmission electron microscopy.52 In addition, in the Akita mouse model of type 1 diabetes, anesthetized diabetic mice have a significantly thinner glycocalyx in retinal arterioles.51 In human volunteers, combined fluorescein and indocyanine green angiography also demonstrated a significantly thinner glycocalyx in patients with type 2 diabetes compared with healthy controls.53 In the same study, the authors measured the rate at which 125I-labeled albumin left the plasma, confirming an increased rate of loss in patients with diabetes, which is consistent with an increase in systemic albumin permeability.53 In streptozocin-induced diabetic rats, retinal permeability was also increased (measured by fluorescein isothiocyanate–dextran accumulation in retinal tissue).54 In the same study, overexpression of endomucin prevented damage to the endothelial glycocalyx, reduced leukocyte adhesion, and reduced retinal vessel permeability.54 A reduction in tight junctions within the interendothelial cleft of retinal vessels has also been reported early in diabetic disease, making the direct contribution of glycocalyx injury impossible to assess.54,55 However, it seems likely that glycocalyx injury is contributing to the pathogenesis of DR through direct increases in permeability and by facilitating leukocyte adhesion; glycocalyx preservation therefore remains a promising therapeutic option.54
Sepsis
During sepsis, the endothelial glycocalyx becomes thinner and the cover sparser, contributing to tissue edema.56,57 In mice, intravenous lipopolysaccharide injection results in activation of the innate immune system, shock, lactic acidosis, myocardial impairment, and increased levels of circulating tumor necrosis factor-α and IL-6.58 After lipopolysaccharide administration, mice have significantly thinner aortic glycocalyx compared with controls.59 Elevated levels of tumor necrosis factor-α are likely to contribute to glycocalyx damage in this model via increased MMP activity and syndecan loss.60 In human volunteers, a low-dose endotoxin model resulted in a significant reduction in the depth of the sublingual vessel glycocalyx (measured using side stream imaging) and a concurrent elevation in plasma HA, suggesting glycocalyx shedding.61 Multiple circulating biomarkers of glycocalyx shedding have been studied in humans as markers of sepsis, including syndecan 1, HS, and HA.56,62,63 It is hoped that in the future these targets may provide practitioners with additional diagnostic and prognostic information. In sepsis, circulating sheddases, including A disintegrin and metalloproteinase 15, heparanase, and MMP2/9, have been implicated in the degradation of the glycocalyx.63, 64, 65, 66 It seems likely therefore that the albuminuria observed in sepsis, at least in part, results from glycocalyx injury and increased glomerular albumin permeability.14,67, 68, 69
Increased pulmonary vascular permeability in sepsis manifests as acute lung injury and acute respiratory distress syndrome, which can occur early in sepsis.70 However, the evidence to date suggests that evidence should not be extrapolated, supporting peripheral glycocalyx damage mediating increased vasculature permeability, to the pulmonary vasculature in sepsis. Mouse pulmonary endothelial glycocalyx appears to be substantially thicker than that seen in the mouse systemic vessels (cremaster muscle).71 Conflicting evidence exists supporting the role of the pulmonary vessel glycocalyx as a permeability barrier. Although a study using bovine lung microvasculature endothelial cells in vitro suggested that HS within the glycocalyx contributed to cell barrier function, a study using ex vivo rat lungs did not.72,73 In addition, in isolated mouse lungs perfused with 4% Evans blue–labeled albumin, neither water nor albumin permeability was measurably altered by glycocalyx degradation. In vivo heparinase III enzyme infusion did not result in detectable pulmonary edema in mice.74 These findings could be explained by the double barrier hypothesis (Figure 3). Glycocalyx injury in isolation may not result in measurable increases in pulmonary permeability to macromolecules in the presence of an intact second barrier (the endothelial monolayer). However, by altering the level of immune cell extravazation and inflammation, the pulmonary glycocalyx may still have a key role in maintaining pulmonary homeostasis.74
The endothelial cells within the cerebral circulation form part of the blood brain barrier, limiting transcytosis and solute diffusion.75 The glycocalyx forms the most luminal layer of the blood brain barrier. Although cytokines smaller than 40 kDa are likely to freely cross the glycocalyx, the passage of larger molecules and interactions between circulating cells and the endothelium are restricted by an intact glycocalyx.75,76 Groups are currently working to investigate the function and structure of the cerebral glycocalyx.76,77 In vivo imaging in mice suggests that the cerebral arteries and capillaries have an intact glycocalyx, whereas veins and venules do not.76 As yet, it is not known if the same distribution will be seen in the human cerebral circulation. During sepsis, patients commonly develop cognitive impairment (acute delirium). The pathogenesis of this condition is complex, with evidence to date suggesting a role for cytokines (IL-1, IL-6, and tumor necrosis factor-α) penetrating the blood brain barrier and activating astrocytes.78 Studies now suggest that during sepsis HS fragments penetrate the hippocampal blood brain barrier to inhibit long-term potentiation—the process responsible for memory formation.34,79 The blood brain barrier of the hippocampal region appears to be susceptible to damage, with an apparent predisposition to age-related vascular dysfunction.80 It seems possible in sepsis, therefore, that an increase in the permeability of the blood brain barrier of the hippocampus to HS fragments, possibly as a direct result of sepsis-induced glycocalyx shedding, contributes to the hippocampal HS fragment accumilation.34 Interestingly, a limited clinical study using side-stream dark field imaging found that the glycocalyx depth varies between cortical and hippocampal microvessels.77 The hippocampal endothelial glycocalyx was thicker than the cortical endothelial glycocalyx, suggesting that structural differences in the glycocalyx at the two sites may exit.77 Further work is needed to determine whether the hippocampal microvascular glycocalyx is predisposed to damage during sepsis because this could represent an exciting therapeutic target in the future to limit the comorbidity associated with sepsis.
Preeclampsia
Preeclampsia is a complication that affects 3% to 5% of pregnancies. The hallmark of the condition is endothelial cell damage, leading to altered microvascular permeability.81,82 Endothelial glycocalyx dysfunction may contribute to this. Activation and dysfunction of the maternal endothelium in preeclampsia is mediated (in part) through the release of placentally derived factors.83 Hypoxic trophoblasts release antiangiogenic cytokines, including soluble fms-like tyrosine kinase 1 into the maternal circulation. In addition, there is a reduction in the proangiogenic placental growth factor, a member of the VEGF family.84 The resulting imbalance contributes to the altered vascular permeability seen in preeclampsia.85 Historically, VEGF-A, VEGF-C, and VEGF-A165b have altered the endothelial glycocalyx.15,43 Interestingly, a failure in first trimester up-regulation of VEGF-A165b is predictive of the development of preeclampsia.86 The important link among VEGF, permeability, and the endothelial glycocalyx is an increasing focus in PE research.
The net effect of PE is glycocalyx degradation, illustrated by an increased perfused boundary region in sublingual capillaries, assessed by side stream imaging in patients with early-onset preeclampsia.87 However the mechanism of damage remains unclear because of conflicting data to date. Both HS and HA are elevated in early-onset preeclampsia (onset before 34 weeks), severe preeclampsia, and the related syndrome of hemolysis, elevated liver enzyme levels, and low platelet counts.87, 88, 89 However, although soluble syndecan-1 increases with advancing gestation in normal pregnancy and preeclampsia, its utility as a marker of preeclampsia remains unclear.87,90,91 The sample size, diagnostic criteria, and different preeclampsia subtypes may explain the discrepancies observed. Recently developed electron microscopy–based methods have reliably measured the glycocalyx depth in the fetal and maternal circulation of human placental tissue to aid future work in this area.92
Atherosclerosis
The major focus of this review has been on the function of the endothelial glycocalyx within the microvasculature. However, there is now an emerging field of research on the role of the glycocalyx in the prevention of atherosclerosis in larger blood vessels. Atherosclerosis is a chronic arterial vascular disease that results from lipid-filled plaque accumulation.93 The subsequent erosion or rupture of plaques can result in acute arterial occlusion, myocardial infarction, or cerebrovascular accident. Damage to the endothelial glycocalyx has been linked to the pathogenesis of atheroma through multiple pathways, which have recently been reviewed by Mitra et al,93 but alterations in transendothelial permeability appear to be a key step. Atheroma tends to form in areas of nonlaminar blood flow, adjacent to bends or branch points.93,94 In vitro, nonuniform fluid flow rapidly results in reduced glycocalyx expression of HS and sialic acids, with an associated reduction in glycocalyx thickness and coverage.93 Areas of glycocalyx damage correlated with oxidized low-density lipoprotein cholesterol uptake.93 In vivo, lipid accumulation in apolipoprotein E–deficient (ApoE−/−) mice increases in areas of endothelium with incomplete glycocalyx coverage (71% glycocalyx coverage in regions of plaque versus 97% coverage in plaque-free regions).95 Low-density lipoprotein cholesterol uptake is considered important because it triggers CD40/CD40 ligand signaling pathways, and subsequent macrophage uptake and degradation of oxidized low-density lipoprotein results in their transformation into foam cells, a key finding on histologic examination of plaques.96
Conclusions
Further research into the structural variability of the glycocalyx and the contribution of the endothelial glycocalyx to vessel wall permeability is needed. This research needs to be targeted to both the organ and vessel of interest. The glycocalyx is a highly specialized structure, and pathologic states should not be expected to affect remote vascular beds or the different vessels within them in the same way. Highly sensitive tools, such as the ex vivo glomerular permeability assay, are providing new evidence that glycocalyx injury in early disease models has functional consequences and should not be ignored. Glycocalyx damage is associated with increased vessel wall permeability in multiple clinical conditions and has massive potential medical relevance. Restoring or maintaining the glycocalyx represents an inviting therapeutic strategy, but for the full potential of this strategy to be realized, an increased understanding of how the glycocalyx structure responds to disease and the functional consequences of glycocalyx damage is needed.
Footnotes
Glycocalyx in Human Disease Theme Issue
Supported by NIH grant S10 OD021833 (USC Multi-Photon Microscopy Core), and Medical Research Council grant MR/M018237/1 (M.J.B.), and the National Institute for Health Research (M.J.B.).
Disclosures: M.J.B. is a National Institute for Health Research–funded clinical lecturer in renal medicine.
This article is part of a review series on glycocalyx in human disease.
References
- 1.Betteridge K.B., Arkill K.P., Neal C.R., Harper S.J., Foster R.R., Satchell S.C., Bates D.O., Salmon A.H.J. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol. 2017;595:5015–5035. doi: 10.1113/JP274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler M.J., Ramnath R., Kadoya H., Desposito D., Riquier-Brison A., Ferguson J.K., Onions K.L., Ogier A.S., ElHegni H., Coward R.J., Welsh G.I., Foster R.R., Peti-Peterdi J., Satchell S.C. Aldosterone induces albuminuria via matrix metalloproteinase-dependent damage of the endothelial glycocalyx. Kidney Int. 2019;95:94–107. doi: 10.1016/j.kint.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dane M.J., van den Berg B.M., Lee D.H., Boels M.G., Tiemeier G.L., Avramut M.C., van Zonneveld A.J., van der Vlag J., Vink H., Rabelink T.J. A microscopic view on the renal endothelial glycocalyx. Am J Physiol Renal Physiol. 2015;308:F956–F966. doi: 10.1152/ajprenal.00532.2014. [DOI] [PubMed] [Google Scholar]
- 4.Singh A., Satchell S.C., Neal C.R., McKenzie E.A., Tooke J.E., Mathieson P.W. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 5.Curry F.R. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 6.Curry F.E. Layer upon layer: the functional consequences of disrupting the glycocalyx-endothelial barrier in vivo and in vitro. Cardiovasc Res. 2017;113:559–561. doi: 10.1093/cvr/cvx044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curry F.E., Michel C.C. The endothelial glycocalyx: barrier functions versus red cell hemodynamics: a model of steady state ultrafiltration through a bi-layer formed by a porous outer layer and more selective membrane-associated inner layer. Biorheology. 2019;56:113–130. doi: 10.3233/BIR-180198. [DOI] [PubMed] [Google Scholar]
- 8.Potter D.R., Damiano E.R. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- 9.Chappell D., Jacob M., Paul O., Rehm M., Welsch U., Stoeckelhuber M., Conzen P., Becker B.F. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ Res. 2009;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- 10.Edwards A., Daniels B.S., Deen W.M. Ultrastructural model for size selectivity in glomerular filtration. Am J Physiol. 1999;276:F892–F902. doi: 10.1152/ajprenal.1999.276.6.F892. [DOI] [PubMed] [Google Scholar]
- 11.Punyaratabandhu N., Kongoup P., Dechadilok P., Katavetin P., Triampo W. Transport of spherical particles through fibrous media and a row of parallel cylinders: applications to glomerular filtration. J Biomech Eng. 2017;139:121005. doi: 10.1115/1.4037550. [DOI] [PubMed] [Google Scholar]
- 12.Satchell S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol. 2013;9:717–725. doi: 10.1038/nrneph.2013.197. [DOI] [PubMed] [Google Scholar]
- 13.Weinbaum S., Tarbell J.M., Damiano E.R. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 14.Salmon A.H., Satchell S.C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226:562–574. doi: 10.1002/path.3964. [DOI] [PubMed] [Google Scholar]
- 15.Oltean S., Qiu Y., Ferguson J.K., Stevens M., Neal C., Russell A., Kaura A., Arkill K.P., Harris K., Symonds C., Lacey K., Wijeyaratne L., Gammons M., Wylie E., Hulse R.P., Alsop C., Cope G., Damodaran G., Betteridge K.B., Ramnath R., Satchell S.C., Foster R.R., Ballmer-Hofer K., Donaldson L.F., Barratt J., Baelde H.J., Harper S.J., Bates D.O., Salmon A.H. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J Am Soc Nephrol. 2015;26:1889–1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eremina V., Jefferson J.A., Kowalewska J., Hochster H., Haas M., Weisstuch J., Richardson C., Kopp J.B., Kabir M.G., Backx P.H., Gerber H.P., Ferrara N., Barisoni L., Alpers C.E., Quaggin S.E. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimke H., Maezawa Y., Quaggin S.E. Crosstalk in glomerular injury and repair. Curr Opin Nephrol Hypertens. 2015;24:231–238. doi: 10.1097/MNH.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dane M.J., van den Berg B.M., Avramut M.C., Faas F.G., van der Vlag J., Rops A.L., Ravelli R.B., Koster B.J., van Zonneveld A.J., Vink H., Rabelink T.J. Glomerular endothelial surface layer acts as a barrier against albumin filtration. Am J Pathol. 2013;182:1532–1540. doi: 10.1016/j.ajpath.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 19.Lazzara M.J., Deen W.M. Model of albumin reabsorption in the proximal tubule. Am J Physiol Renal Physiol. 2007;292:F430–F439. doi: 10.1152/ajprenal.00010.2006. [DOI] [PubMed] [Google Scholar]
- 20.Norden A.G., Scheinman S.J., Deschodt-Lanckman M.M., Lapsley M., Nortier J.L., Thakker R.V., Unwin R.J., Wrong O. Tubular proteinuria defined by a study of Dent's (CLCN5 mutation) and other tubular diseases. Kidney Int. 2000;57:240–249. doi: 10.1046/j.1523-1755.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 21.Gambaro G., Baggio B., Cicerello E., Mastrosimone S., Marzaro G., Borsatti A., Crepaldi G. Abnormal erythrocyte charge in diabetes mellitus: link with microalbuminuria. Diabetes. 1988;37:745–748. doi: 10.2337/diab.37.6.745. [DOI] [PubMed] [Google Scholar]
- 22.Ando Y., Okada H., Takemura G., Suzuki K., Takada C., Tomita H., Zaikokuji R., Hotta Y., Miyazaki N., Yano H., Muraki I., Kuroda A., Fukuda H., Kawasaki Y., Okamoto H., Kawaguchi T., Watanabe T., Doi T., Yoshida T., Ushikoshi H., Yoshida S., Ogura S. Brain-specific ultrastructure of capillary endothelial glycocalyx and its possible contribution for blood brain barrier. Sci Rep. 2018;8:17523. doi: 10.1038/s41598-018-35976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehm M., Zahler S., Lotsch M., Welsch U., Conzen P., Jacob M., Becker B.F. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Rops A.L., Gotte M., Baselmans M.H., van den Hoven M.J., Steenbergen E.J., Lensen J.F., Wijnhoven T.J., Cevikbas F., van den Heuvel L.P., van Kuppevelt T.H., Berden J.H., van der Vlag J. Syndecan-1 deficiency aggravates anti-glomerular basement membrane nephritis. Kidney Int. 2007;72:1204–1215. doi: 10.1038/sj.ki.5002514. [DOI] [PubMed] [Google Scholar]
- 25.Garsen M., Rops A.L., Rabelink T.J., Berden J.H., van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol Dial Transplant. 2014;29:49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 26.Earnshaw J.S., Curtis C.G., Powell G.M., Dodgson K.S., Olavesen A.H., Gacesa P. The fate of intravenously administered highly purified bovine testicular hyaluronidase (Hyalosidase) in the rat. Biochem Pharmacol. 1985;34:2199–2203. doi: 10.1016/0006-2952(85)90418-6. [DOI] [PubMed] [Google Scholar]
- 27.Landsverk S.A., Tsai A.G., Cabrales P., Intaglietta M. Impact of enzymatic degradation of the endothelial glycocalyx on vascular permeability in an awake hamster model. Crit Care Res Pract. 2012;2012:842545. doi: 10.1155/2012/842545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeansson M., Haraldsson B. Glomerular size and charge selectivity in the mouse after exposure to glucosaminoglycan-degrading enzymes. J Am Soc Nephrol. 2003;14:1756–1765. doi: 10.1097/01.asn.0000072742.02714.6e. [DOI] [PubMed] [Google Scholar]
- 29.Onions K.L., Gamez M., Buckner N.R., Baker S.L., Betteridge K.B., Desideri S., Dallyn B.P., Ramnath R.D., Neal C.R., Farmer L.K., Mathieson P.W., Gnudi L., Alitalo K., Bates D.O., Salmon A.H.J., Welsh G.I., Satchell S.C., Foster R.R. VEGFC reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes. 2019;68:172–187. doi: 10.2337/db18-0045. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg B.M., Wang G., Boels M.G.S., Avramut M.C., Jansen E., Sol W., Lebrin F., Jan van Zonneveld A., de Koning E.J.P., Vink H., Grone H.J., Carmeliet P., van der Vlag J., Rabelink T.J. Glomerular function and structural integrity depend on hyaluronan synthesis by glomerular endothelium. J Am Soc Nephrol. 2019;30:1886–1897. doi: 10.1681/ASN.2019020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil N., Goldberg R., Neuman T., Garsen M., Zcharia E., Rubinstein A.M., van Kuppevelt T., Meirovitz A., Pisano C., Li J.P., van der Vlag J., Vlodavsky I., Elkin M. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208–216. doi: 10.2337/db11-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung O., Trapp-Stamborski V., Purushothaman A., Jin H., Wang H., Sanderson R.D., Rapraeger A.C. Heparanase-induced shedding of syndecan-1/CD138 in myeloma and endothelial cells activates VEGFR2 and an invasive phenotype: prevention by novel synstatins. Oncogenesis. 2016;5:e202. doi: 10.1038/oncsis.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim E.Y., Roshanravan H., Dryer S.E. Syndecan-4 ectodomain evokes mobilization of podocyte TRPC6 channels and their associated pathways: an essential role for integrin signaling. Biochim Biophys Acta. 2015;1853:2610–2620. doi: 10.1016/j.bbamcr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X., Han X., Xia K., Xu Y., Yang Y., Oshima K., Haeger S.M., Perez M.J., McMurtry S.A., Hippensteel J.A., Ford J.A., Herson P.S., Liu J., Schmidt E.P., Linhardt R.J. Circulating heparin oligosaccharides rapidly target the hippocampus in sepsis, potentially impacting cognitive functions. Proc Natl Acad Sci U S A. 2019;116:9208–9213. doi: 10.1073/pnas.1902227116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desideri S., Onions K.L., Qiu Y., Ramnath R.D., Butler M.J., Neal C.R., King M.L.R., Salmon A.E., Saleem M.A., Welsh G.I., Michel C.C., Satchell S.C., Salmon A.H.J., Foster R.R. A novel assay provides sensitive measurement of physiologically relevant changes in albumin permeability in isolated human and rodent glomeruli. Kidney Int. 2018;93:1086–1097. doi: 10.1016/j.kint.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nieuwdorp M., van Haeften T.W., Gouverneur M.C., Mooij H.L., van Lieshout M.H., Levi M., Meijers J.C., Holleman F., Hoekstra J.B., Vink H., Kastelein J.J., Stroes E.S. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 37.Lambadiari V., Pavlidis G., Kousathana F., Maratou E., Georgiou D., Andreadou I., Kountouri A., Varoudi M., Balampanis K., Parissis J., Triantafyllidi H., Katogiannis K., Birba D., Lekakis J., Dimitriadis G., Ikonomidis I. Effects of different antidiabetic medications on endothelial glycocalyx, myocardial function, and vascular function in type 2 diabetic patients: one year follow-up study. J Clin Med. 2019;8 doi: 10.3390/jcm8070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Targosz-Korecka M., Jaglarz M., Malek-Zietek K.E., Gregorius A., Zakrzewska A., Sitek B., Rajfur Z., Chlopicki S., Szymonski M. AFM-based detection of glycocalyx degradation and endothelial stiffening in the db/db mouse model of diabetes. Sci Rep. 2017;7:15951. doi: 10.1038/s41598-017-16179-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satchell S.C., Tooke J.E. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714–725. doi: 10.1007/s00125-008-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyoda M., Najafian B., Kim Y., Caramori M.L., Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes. 2007;56:2155–2160. doi: 10.2337/db07-0019. [DOI] [PubMed] [Google Scholar]
- 41.Tschulakow A., Christner S., Julien S., Ludinsky M., van der Giet M., Schraermeyer U. Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS One. 2014;9:e113701. doi: 10.1371/journal.pone.0113701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eleftheriadis T., Antoniadi G., Pissas G., Liakopoulos V., Stefanidis I. The renal endothelium in diabetic nephropathy. Ren Fail. 2013;35:592–599. doi: 10.3109/0886022X.2013.773836. [DOI] [PubMed] [Google Scholar]
- 43.Foster R.R., Armstrong L., Baker S., Wong D.W., Wylie E.C., Ramnath R., Jenkins R., Singh A., Steadman R., Welsh G.I., Mathieson P.W., Satchell S.C. Glycosaminoglycan regulation by VEGFA and VEGFC of the glomerular microvascular endothelial cell glycocalyx in vitro. Am J Pathol. 2013;183:604–616. doi: 10.1016/j.ajpath.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh A., Friden V., Dasgupta I., Foster R.R., Welsh G.I., Tooke J.E., Haraldsson B., Mathieson P.W., Satchell S.C. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300:F40–F48. doi: 10.1152/ajprenal.00103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeansson M., Granqvist A.B., Nystrom J.S., Haraldsson B. Functional and molecular alterations of the glomerular barrier in long-term diabetes in mice. Diabetologia. 2006;49:2200–2209. doi: 10.1007/s00125-006-0319-z. [DOI] [PubMed] [Google Scholar]
- 46.Satoh M., Kobayashi S., Kuwabara A., Tomita N., Sasaki T., Kashihara N. In vivo visualization of glomerular microcirculation and hyperfiltration in streptozotocin-induced diabetic rats. Microcirculation. 2010;17:103–112. doi: 10.1111/j.1549-8719.2009.00010.x. [DOI] [PubMed] [Google Scholar]
- 47.Kuwabara A., Satoh M., Tomita N., Sasaki T., Kashihara N. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats. Diabetologia. 2010;53:2056–2065. doi: 10.1007/s00125-010-1810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramnath R.D., Butler M.J., Newman G., Desideri S., Russell A., Lay A.C., Neal C.R., Qiu Y., Fawaz S., Onions K., Gamez M., Crompton M., Michie C., Finch N., Coward R.J., Welsh G.I., Foster R.R., Satchell S.C. Blocking matrix metalloproteinase-mediated syndecan-4 shedding restores the endothelial glycocalyx and glomerular filtration barrier function in early diabetic kidney disease. Kidney Int. 2019 doi: 10.1016/j.kint.2019.09.035. [Epub ahead of print] doi: 10.1016/j.kint.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowluru R.A., Mishra M. Regulation of matrix metalloproteinase in the pathogenesis of diabetic retinopathy. Prog Mol Biol Transl Sci. 2017;148:67–85. doi: 10.1016/bs.pmbts.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Wong T.Y., Cheung C.M., Larsen M., Sharma S., Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 51.Leskova W., Pickett H., Eshaq R.S., Shrestha B., Pattillo C.B., Harris N.R. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp Eye Res. 2019;179:125–131. doi: 10.1016/j.exer.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumase F., Morizane Y., Mohri S., Takasu I., Ohtsuka A., Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama. 2010;64:277–283. doi: 10.18926/AMO/40502. [DOI] [PubMed] [Google Scholar]
- 53.Broekhuizen L.N., Lemkes B.A., Mooij H.L., Meuwese M.C., Verberne H., Holleman F., Schlingemann R.O., Nieuwdorp M., Stroes E.S., Vink H. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:2646–2655. doi: 10.1007/s00125-010-1910-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu T., Zhao M., Jiang Y., Xing X., Shi X., Cheng L., Jin H., Liu K. Endomucin restores depleted endothelial glycocalyx in the retinas of streptozotocin-induced diabetic rats. FASEB J. 2019;33:13346–13357. doi: 10.1096/fj.201901161R. [DOI] [PubMed] [Google Scholar]
- 55.Wallow I.H., Engerman R.L. Permeability and patency of retinal blood vessels in experimental diabetes. Invest Ophthalmol Vis Sci. 1977;16:447–461. [PubMed] [Google Scholar]
- 56.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. 2019;23:16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chelazzi C., Villa G., Mancinelli P., De Gaudio A.R., Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care. 2015;19:26. doi: 10.1186/s13054-015-0741-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fink M.P. Animal models of sepsis. Virulence. 2014;5:143–153. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiesinger A., Peters W., Chappell D., Kentrup D., Reuter S., Pavenstadt H., Oberleithner H., Kumpers P. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8:e80905. doi: 10.1371/journal.pone.0080905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramnath R., Foster R.R., Qiu Y., Cope G., Butler M.J., Salmon A.H., Mathieson P.W., Coward R.J., Welsh G.I., Satchell S.C. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J. 2014;28:4686–4699. doi: 10.1096/fj.14-252221. [DOI] [PubMed] [Google Scholar]
- 61.Nieuwdorp M., Meuwese M.C., Mooij H.L., van Lieshout M.H., Hayden A., Levi M., Meijers J.C., Ince C., Kastelein J.J., Vink H., Stroes E.S. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 62.Steppan J., Hofer S., Funke B., Brenner T., Henrich M., Martin E., Weitz J., Hofmann U., Weigand M.A. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165:136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 63.Becker B.F., Jacob M., Leipert S., Salmon A.H., Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80:389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang X., Meegan J.E., Jannaway M., Coleman D.C., Yuan S.Y. A disintegrin and metalloproteinase 15-mediated glycocalyx shedding contributes to vascular leakage during inflammation. Cardiovasc Res. 2018;114:1752–1763. doi: 10.1093/cvr/cvy167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lygizos M.I., Yang Y., Altmann C.J., Okamura K., Hernando A.A., Perez M.J., Smith L.P., Koyanagi D.E., Gandjeva A., Bhargava R., Tuder R.M., Faubel S., Schmidt E.P. Heparanase mediates renal dysfunction during early sepsis in mice. Physiol Rep. 2013;1:e00153. doi: 10.1002/phy2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui N., Wang H., Long Y., Su L., Liu D. Dexamethasone suppressed LPS-induced matrix metalloproteinase and its effect on endothelial glycocalyx shedding. Mediators Inflamm. 2015;2015:912726. doi: 10.1155/2015/912726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adembri C., Sgambati E., Vitali L., Selmi V., Margheri M., Tani A., Bonaccini L., Nosi D., Caldini A.L., Formigli L., De Gaudio A.R. Sepsis induces albuminuria and alterations in the glomerular filtration barrier: a morphofunctional study in the rat. Crit Care. 2011;15:R277. doi: 10.1186/cc10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koike K., Aiboshi J., Shinozawa Y., Sekine K., Endo T., Yamamoto Y. Correlation of glomerular permeability, endothelial injury, and postoperative multiple organ dysfunction. Surg Today. 2004;34:811–816. doi: 10.1007/s00595-004-2834-1. [DOI] [PubMed] [Google Scholar]
- 69.De Gaudio A.R., Adembri C., Grechi S., Novelli G.P. Microalbuminuria as an early index of impairment of glomerular permeability in postoperative septic patients. Intensive Care Med. 2000;26:1364–1368. doi: 10.1007/s001340000593. [DOI] [PubMed] [Google Scholar]
- 70.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., Smith L.P., Cheng S.S., Overdier K.H., Thompson K.R., Geraci M.W., Douglas I.S., Pearse D.B., Tuder R.M. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dull R.O., Mecham I., McJames S. Heparan sulfates mediate pressure-induced increase in lung endothelial hydraulic conductivity via nitric oxide/reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1452–L1458. doi: 10.1152/ajplung.00376.2006. [DOI] [PubMed] [Google Scholar]
- 73.Dull R.O., Cluff M., Kingston J., Hill D., Chen H., Hoehne S., Malleske D.T., Kaur R. Lung heparan sulfates modulate K(fc) during increased vascular pressure: evidence for glycocalyx-mediated mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2012;302:L816–L828. doi: 10.1152/ajplung.00080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y., Schmidt E.P. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1 doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galea I., Perry V.H. The blood-brain interface: a culture change. Brain Behav Immun. 2018;68:11–16. doi: 10.1016/j.bbi.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 76.Yoon J.H., Lee E.S., Jeong Y. In vivo imaging of the cerebral endothelial glycocalyx in mice. J Vasc Res. 2017;54:59–67. doi: 10.1159/000457799. [DOI] [PubMed] [Google Scholar]
- 77.Haeren R.H.L., Rijkers K., Schijns O., Dings J., Hoogland G., van Zandvoort M., Vink H., van Overbeeke J.J. In vivo assessment of the human cerebral microcirculation and its glycocalyx: a technical report. J Neurosci Methods. 2018;303:114–125. doi: 10.1016/j.jneumeth.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Piva S., McCreadie V.A., Latronico N. Neuroinflammation in sepsis: sepsis associated delirium. Cardiovasc Hematol Disord Drug Targets. 2015;15:10–18. doi: 10.2174/1871529x15666150108112452. [DOI] [PubMed] [Google Scholar]
- 79.Hippensteel J.A., Anderson B.J., Orfila J.E., McMurtry S.A., Dietz R.M., Su G., Ford J.A., Oshima K., Yang Y., Zhang F., Han X., Yu Y., Liu J., Linhardt R.J., Meyer N.J., Herson P.S., Schmidt E.P. Circulating heparan sulfate fragments mediate septic cognitive dysfunction. J Clin Invest. 2019;129:1779–1784. doi: 10.1172/JCI124485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montagne A., Barnes S.R., Sweeney M.D., Halliday M.R., Sagare A.P., Zhao Z., Toga A.W., Jacobs R.E., Liu C.Y., Amezcua L., Harrington M.G., Chui H.C., Law M., Zlokovic B.V. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boeldt D.S., Bird I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27–R44. doi: 10.1530/JOE-16-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tranquilli A.L., Dekker G., Magee L., Roberts J., Sibai B.M., Steyn W., Zeeman G.G., Brown M.A. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 83.O'Brien M., Baczyk D., Kingdom J.C. Endothelial dysfunction in severe preeclampsia is mediated by soluble factors, rather than extracellular vesicles. Sci Rep. 2017;7:5887. doi: 10.1038/s41598-017-06178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levine R.J., Maynard S.E., Qian C., Lim K.H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., Sibai B.M., Sukhatme V.P., Karumanchi S.A. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 85.Maynard S.E., Min J.-Y., Merchan J., Lim K.-H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., Karumanchi S.A. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bills V.L., Varet J., Millar A., Harper S.J., Soothill P.W., Bates D.O. Failure to up-regulate VEGF(165)b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin Sci (Lond) 2009;116:265–272. doi: 10.1042/CS20080270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weissgerber T.L., Garcia-Valencia O., Milic N.M., Codsi E., Cubro H., Nath M.C., White W.M., Nath K.A., Garovic V.D. Early onset preeclampsia is associated with glycocalyx degradation and reduced microvascular perfusion. J Am Heart Assoc. 2019;8:e010647. doi: 10.1161/JAHA.118.010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Osmers R.G., Schutz E., Diedrich F., Wehry B., Krauss T., Oellerich M., Kuhn W. Increased serum levels of hyaluronic acid in pregnancies complicated by preeclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Obstet Gynecol. 1998;178:341–345. doi: 10.1016/s0002-9378(98)80023-9. [DOI] [PubMed] [Google Scholar]
- 89.Berg S., Engman A., Holmgren S., Lundahl T., Laurent T.C. Increased plasma hyaluronan in severe pre-eclampsia and eclampsia. Scand J Clin Lab Invest. 2001;61:131–137. doi: 10.1080/00365510151097647. [DOI] [PubMed] [Google Scholar]
- 90.Hofmann-Kiefer K.F., Knabl J., Martinoff N., Schiessl B., Conzen P., Rehm M., Becker B.F., Chappell D. Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: an observational study. Reprod Sci. 2013;20:318–325. doi: 10.1177/1933719112453508. [DOI] [PubMed] [Google Scholar]
- 91.Gandley R.E., Althouse A., Jeyabalan A., Bregand-White J.M., McGonigal S., Myerski A.C., Gallaher M., Powers R.W., Hubel C.A. Low soluble syndecan-1 precedes preeclampsia. PLoS One. 2016;11:e0157608. doi: 10.1371/journal.pone.0157608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabre-Gray A.C.M., Down C.J., Neal C.R., Foster R.R., Satchell S.C., Bills V.L. Imaging the placental glycocalyx with transmission electron microscopy. Placenta. 2018;74:59–61. doi: 10.1016/j.placenta.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 93.Mitra R., O'Neil G.L., Harding I.C., Cheng M.J., Mensah S.A., Ebong E.E. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep. 2017;19:63. doi: 10.1007/s11883-017-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zand T., Majno G., Nunnari J.J., Hoffman A.H., Savilonis B.J., MacWilliams B., Joris I. Lipid deposition and intimal stress and strain: a study in rats with aortic stenosis. Am J Pathol. 1991;139:101–113. [PMC free article] [PubMed] [Google Scholar]
- 95.Cancel L.M., Ebong E.E., Mensah S., Hirschberg C., Tarbell J.M. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016;252:136–146. doi: 10.1016/j.atherosclerosis.2016.07.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parthasarathy S., Raghavamenon A., Garelnabi M.O., Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]