Abstract
Background
This is an update of a previous review (McGuinness 2006).
Hypertension and cognitive impairment are prevalent in older people. Hypertension is a direct risk factor for vascular dementia (VaD) and recent studies have suggested hypertension impacts upon prevalence of Alzheimer's disease (AD). Therefore does treatment of hypertension prevent cognitive decline?
Objectives
To assess the effects of blood pressure lowering treatments for the prevention of dementia and cognitive decline in patients with hypertension but no history of cerebrovascular disease.
Search methods
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group, The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS as well as many trials databases and grey literature sources were searched on 13 February 2008 using the terms: hypertens$ OR anti‐hypertens$.
Selection criteria
Randomized, double‐blind, placebo controlled trials in which pharmacological or non‐pharmacological interventions to lower blood pressure were given for at least six months.
Data collection and analysis
Two independent reviewers assessed trial quality and extracted data. The following outcomes were assessed: incidence of dementia, cognitive change from baseline, blood pressure level, incidence and severity of side effects and quality of life.
Main results
Four trials including 15,936 hypertensive subjects were identified. Average age was 75.4 years. Mean blood pressure at entry across the studies was 171/86 mmHg. The combined result of the four trials reporting incidence of dementia indicated no significant difference between treatment and placebo (236/7767 versus 259/7660, Odds Ratio (OR) = 0.89, 95% CI 0.74, 1.07) and there was considerable heterogeneity between the trials. The combined results from the three trials reporting change in Mini Mental State Examination (MMSE) did not indicate a benefit from treatment (Weighted Mean Difference (WMD) = 0.42, 95% CI 0.30, 0.53). Both systolic and diastolic blood pressure levels were reduced significantly in the three trials assessing this outcome (WMD = ‐10.22, 95% CI ‐10.78, ‐9.66 for systolic blood pressure, WMD = ‐4.28, 95% CI ‐4.58, ‐3.98 for diastolic blood pressure). Three trials reported adverse effects requiring discontinuation of treatment and the combined results indicated no significant difference (OR = 1.01, 95% CI 0.92, 1.11). When analysed separately, however, more patients on placebo in Syst Eur 1997 were likely to discontinue treatment due to side effects; the converse was true in SHEP 1991. Quality of life data could not be analysed in the four studies. Analysis of the included studies in this review was problematic as many of the control subjects received antihypertensive treatment because their blood pressures exceeded pre‐set values. In most cases the study became a comparison between the study drug against a usual antihypertensive regimen.
Authors' conclusions
There is no convincing evidence from the trials identified that blood pressure lowering in late‐life prevents the development of dementia or cognitive impairment in hypertensive patients with no apparent prior cerebrovascular disease. There were significant problems identified with analysing the data, however, due to the number of patients lost to follow‐up and the number of placebo patients who received active treatment. This introduced bias. More robust results may be obtained by conducting a meta‐analysis using individual patient data.
Plain language summary
There is no convincing evidence from randomized controlled trials that blood pressure lowering in late life prevents the development of cognitive impairment and dementia in patients without apparent prior cerebrovascular disease.
Hypertension and cognitive impairment are prevalent in older people. We found four trials suitable for analysing the effectiveness of blood pressure lowering for preventing development of cognitive impairment and dementia. However, for several reasons, including the differing methodologies of the trials, the number of drop‐outs from the trials, and active treatment of subjects in the control groups, we were unable to assess definitively the effectiveness of antihypertensive treatments for preventing cognitive impairment and dementia in people with no evidence of previous cerebrovascular disease.
Background
This is an update of a previous Cochrane review (McGuinness 2006) which found no convincing evidence that blood pressure lowering prevents the development of dementia or cognitive impairment in hypertensive patients with no apparent prior cerebrovascular disease.
Description of the condition
The relationship between blood pressure, cognition and dementia is complex and not fully established. Analysis of the epidemiological data must take account of the following methodological issues. Firstly, longitudinal studies can suggest whether a causal relationship exists between blood pressure and cognition or dementia, whereas cross‐sectional studies can not determine causality. Secondly, the definitions of high or low blood pressure and cognitive impairment are heterogeneous across the studies and may limit comparability. The methods used to screen for and to examine in more detail cognitive impairment are also heterogeneous. The diagnosis of dementia, and its aetiology, does follow established, standardised criteria, however. Thirdly, studies may vary in controlling for the effect of other vascular risk factors or antihypertensive treatment, for example.
Nevertheless, analysis of the available epidemiological data does allow some conclusions to be drawn. The reader is referred to recent authoritative reviews including detailed analysis of the relevant literature which is beyond the scope of this updated review (Qui 2005; Kennelly 2009).
Several longitudinal studies have reported a consistent relationship between elevated blood pressure in mid‐life (age 40‐64 years) and cognitive impairment in late‐life (age >65 years) (Elias 1993; Launer 1995; Kilander 1998; Swan 1998; Kilander 2000; Elias 2004). This effect was more marked if the high blood pressure was not treated (Elias 1993; Kilander 2000).
Several of the same data sets also indicated a relationship between mid‐life hypertension and the incidence of dementia and AD in late‐life (Launer 2000; Kivipelto 2001; Wu 2003; Whitmer 2005). In the Honolulu‐Asia Aging Study (Launer 2000), this association was only present in those participants not treated with antihypertensive agents. One Japanese study reported a relationship of mid‐life high blood pressure to late‐life VaD but not to AD (Yamada 2003).
The available data regarding late‐life blood pressure and cognition or dementia are inconsistent. Some studies showed no relationship between high blood pressure and cognitive impairment (Hebert 2004; Tervo 2004) or dementia (Yoshitake 1995; Brayne 1998). Other studies did find a relationship between late‐life high blood pressure and cognitive decline (Elias 1993) or dementia (Skoog 1996). Low blood pressure in late‐life has been more consistently associated with cognitive impairment and dementia (Guo 1996; Ruitenberg 2001; Morris 2001; Verghese 2003; Qiu 2003; Qiu 2004).
Description of the intervention
Treatment of high blood pressure has been reported in several observational studies to be protective against cognitive impairment and dementia (Guo 1999; in't Veld 2001; Qiu 2003a; Yasar 2005; Khachaturian 2006). Effective agents have included calcium channel blockers (Yasar 2005) and diuretics (Qiu 2003a; Khachaturian 2006). Other studies have reported no benefit of treatment (Morris 2001; Lindsay 2002). These studies did not find a relationship between high blood pressure and incident dementia.
Evidence of the true effectiveness of treating high blood pressure on preventing cognitive impairment and dementia is needed from randomised, controlled clinical trials (RCTs).
Several RCTs which are ineligible for this review have investigated the effect on cognition of blood pressure lowering therapy. The reasons for exclusion of these studies from the current meta‐analysis, according to the prespecified criteria in the Methods, may be found in the Characteristics of excluded studies section. The Hypertensive Old People in Edinburgh (HOPE) study showed that treatment of hypertension is not hazardous to cognitive function in older people with pre‐existing cognitive impairment and that long‐term adequate control of blood pressure may even reverse cognitive impairment associated with pre‐existing hypertension (Starr 1996). The Medical Research Council trial (Prince 1996) comparing the treatment of moderate hypertension with either hydrochlorothiazide plus amiloride or atenolol found no effect on cognition over 54 months (Prince 1996a). A brief trial comparing the angiotension receptor blocker valsartan and the ACE inhibitor enalapril showed that valsartan was more effective in lowering blood pressure and improving memory function (Fogari 2004). In people with established cerebrovascular disease (and therefore excluded from the current meta‐analysis), treatment with an ACE inhibitor based regime reduced the risk of dementia with recurrent stroke, the overall risk of cognitive decline and the risk of cognitive decline with recurrent stroke but not the overall risk of dementia in secondary outcomes (PROGRESS 2003).
There is scant evidence in the literature on the effects on cognition of non‐pharmacological interventions which lower blood pressure. Such interventions might include salt restriction, weight reduction, exercise, and reduction in alcohol intake.
Recent hypertension treatment trials (ALLHAT 2003; ASCOT 2005; VALUE 2004) did not report data on cognition or dementia.
How the intervention might work
The pathological processes through which hypertension might influence cognitive function are numerous. Cerebral ischaemia caused by atherosclerosis of large and small vessels, vascular smooth muscle hyperplasia, infarction, and altered cerebral blood flow are important factors and interact with other factors such as age, genetic predisposition, and smoking. Changes in cerebral white matter, or leukoaraiosis, can also occur as a result of sustained high blood pressure (Starr 1992).
Why it is important to do this review
The aim of this systematic review is to determine the effects of blood pressure lowering on development of cognitive decline and dementia in patients with hypertension but no history of stroke or transient ischaemic attacks (TIAs). The effects of antihypertensive interventions for people with established cerebrovascular disease are the subject of a separate Cochrane review (Ratnasabapathy 2003). Given the burden of both hypertension and dementia, identification of treatments that reduce the rate of cognitive decline or incidence of dementia would be of considerable clinical, public health, and societal benefit.
This update is important to ensure that relevant data published since the first review are included in the meta‐analysis, allowing the best possible estimate of the effect of blood pressure lowering on cognitive decline to be obtained.
Objectives
Primary objective In hypertensive patients with no history of cerebrovascular disease, to assess the effects of blood pressure lowering treatments for the prevention of: (a) dementia (b) cognitive decline.
Secondary objective To assess whether: (a) there is an optimal blood pressure level for prevention of dementia or cognitive decline (b) there is an optimal antihypertensive agent, or class of antihypertensive agent, for the prevention of dementia or cognitive decline (c) there are different effects of treatment according to aspects of baseline risk including sex, age, blood pressure level, pulse pressure, associated cardiovascular disease, smoking and diabetes.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, double‐blind, placebo‐controlled trials in which pharmacological or non‐pharmacological interventions to lower blood pressure were administered for more than six months. Six months was chosen as this was felt to be the minimum length of time required to be on treatment before any benefit could be attained.
Types of participants
Patients included had a diagnosis of hypertension made according to the established criteria for the time. Blood pressure readings were at least 160/90 mmHg for entry into essential hypertension studies. Systolic blood pressure was 160‐219 mmHg and diastolic blood pressure was <90 mmHg for entry into an isolated systolic hypertension study. The patients had no clinical history or signs suggestive of previous cerebrovascular disease. Patients with a diagnosis of hypertension, who were cognitively impaired, but did not fulfil the accepted criteria for the classification of dementia, were included, but examined separately. Studies involving people with dementia were analysed separately. Participants were not excluded on the basis of level of blood pressure, age, or prior use of antihypertensive therapy.
For the diagnosis of dementia, standard criteria based on American Psychiatric Association DSM (APA 1987), ICD 10 (WHO 1992), NINCDS‐ADRDA (National Institute of Neurological and Communicative Disorders and Stroke ‐ Alzheimer's Disease and Related Disorders Association) (McKhann 1984), or acceptable equivalents were used.
Cognitive function was assessed using the MMSE (Mini‐Mental State Examination), GDS (Global Deterioration Scale), CDR (Clinical Dementia Rating scale) or acceptable alternative.
Types of interventions
Pharmaceutical agents, or classes of therapy, for this review include:
ACE inhibitors
Angiotensin II receptor antagonists
Beta adrenergic blockers
Combined alpha and beta blockers
Calcium channel blockers
Diuretics
Alpha adrenergic blockers
Central sympatholytics
Direct vasodilators
Peripheral adrenergic antagonists
Sympathomimetics
All dosages of drugs were considered. Only long term treatment (six months or longer) were considered.
Non‐Pharmacological Interventions include:
Salt restriction
Weight reduction
Exercise
Alcohol restriction
Smoking cessation
Any other dietary or lifestyle modification aimed at reducing blood pressure
Types of outcome measures
Primary outcomes
Incidence of dementia, diagnosed according to standard diagnostic criteria or those appropriate at the time
Cognitive change from baseline
Secondary outcomes
Blood pressure level
Incidence and severity of adverse effects
Quality of life
Search methods for identification of studies
The Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) was searched on 13 February 2008 for all years up to December 2005. This register contains records from the following major healthcare databases The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing trial databases and other grey literature sources. The following search term was used: hypertens$. For this update, CDCIG was searched on 24 September 2008 for January 2005 to present.
Trials were also identified through a search of the following databases on 13 February 2008:
CENTRAL: issue 1/2008 #1=(cognit* or memor* or "quality of life") and (hypertens* or antihypertens* or anti‐hypertens* or "blood pressure") #2=(placebo* or "control group*" or controls) in AB, TI, MeSH #3=random* #4=#2 or #3 #5=#1 and #4 #6=((TG=animal) not (TG=human)) and (TG=animal) #7=#5 not #6
MEDLINE: 1966‐2008/06 #1=(cognit* or memor* or "quality of life") and (hypertens* or antihypertens* or anti‐hypertens* or "blood pressure") #2=(placebo* or "control group*" or controls) in AB, TI, MeSH #3=random* #4=#2 or #3 #5=#1 and #4 #6=((TG=animal) not (TG=human)) and (TG=animal) #7=#5 not #6
EMBASE: 1980‐2008/06 #1=(cognit* or memor* or "quality of life") and (hypertens* or antihypertens* or anti‐hypertens* or "blood pressure") #2="randomized‐controlled‐trial"/ all subheadings #3=random* #4=(placebo* or "control group*" or controls) in TI, AB, DER, DRR #5=#2 or #3 or #4 #6=#1 and #5 #7=animal* in DER #8=#6 not #7
PsycINFO: 1872‐2008/01 #1=(random* or placebo* or "control group*" or controls) in TI, AB, KC, DE #2=(cognit* or memor* or "quality of life") and ((hypertens* or antihypertens* or anti‐hypertens* or "blood pressure") in TI, AB, KC, DE) #3=#1 and #2 #4=animal* in PO #5=#3 not #4
CINAHL: 1982‐2008/02 #1=(cognit* or memor* or "quality of life") and ((hypertens* or antihypertens* or anti‐hypertens* or "blood pressure") in TI, AB, DE) #2=(random* or placebo* or "control group*" or controls) in TI, AB, DE #3=#1 and #2 #4=animal* in DE #5=#3 not #4
On 13 Febraury 2008, the Specialized Register consisted of records from the following databases:
Healthcare databases:
The Cochrane Library: (2006, Issue 1);
MEDLINE (1966 to 2006/07, week 5);
EMBASE (1980 to 2006/07);
PsycINFO (1887 to 2006/08, week 1);
CINAHL (1982 to 2006/06);
SIGLE (Grey Literature in Europe) (1980 to 2005/03);
LILACS: Latin American and Caribbean Health Science Literature (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&base=LILACS&lang=i&form=F) (last searched 29 August 2006).
Conference proceedings:
ISTP (http://portal.isiknowledge.com/portal.cgi) (Index to Scientific and Technical Proceedings) (to 29 August 2006);
INSIDE (BL database of Conference Proceedings and Journals) (to June 2000);.
Theses:
Index to Theses (formerly ASLIB) (http://www.theses.com/) (UK and Ireland theses) (1716 to 11 August 2006);
Australian Digital Theses Program (http://adt.caul.edu.au/): (last update 24 March 2006);
Canadian Theses and Dissertations (http://www.collectionscanada.ca/thesescanada/index‐e.html): 1989 to 28 August 2006);
DATAD ‐ Database of African Theses and Dissertations (http://www.aau.org/datad/backgrd.htm);
Dissertation Abstract Online (USA) (http://wwwlib.umi.com/dissertations/gateway) (1861 to 28 August 2006).
Ongoing trials: UK
National Research Register (http://www.update‐software.com/projects/nrr/) (last searched issue 3/2006);
ReFeR (http://www.refer.nhs.uk/ViewWebPage.asp?Page=Home) (last searched 30 August 2006);
Current Controlled trials: Meta Register of Controlled trials (mRCT) (http://www.controlled‐trials.com/) (last searched 30 August 2006) :
ISRCTN Register ‐ trials registered with a unique identifier
Action medical research
Kings College London
Laxdale Ltd
Medical Research Council (UK)
NHS Trusts Clinical Trials Register
National Health Service Research and Development Health Technology Assessment Programme (HTA)
National Health Service Research and Development Programme 'Time‐Limited' National Programmes
National Health Service Research and Development Regional Programmes
The Wellcome Trust
Stroke Trials Registry (http://www.strokecenter.org/trials/index.aspx) (last searched 31 August 2006);
Netherlands
Nederlands Trial Register (http://www.trialregister.nl/trialreg/index.asp) (last searched 31 August 2006);
USA/International
ClinicalTrials.gov (http://www.ClinicalTrials.gov) (last searched 31 August 2006) (contains all records from http://clinicalstudies.info.nih.gov/);
IPFMA Clinical trials Register: www.ifpma.org/clinicaltrials.html. The Ongoing Trials database within this Register searches http://www.controlled‐trials.com/isrctn, http://www.ClinicalTrials.gov and http://www.centerwatch.com/. The ISRCTN register and Clinicaltrials.gov are searched separately. Centerwatch is very difficult to search for our purposes and no update searches have been done since 2003.
The IFPMA Trial Results databases searches a wide variety of sources among which are:
http://www.astrazenecaclinicaltrials.com (Seroquel, statins)
http://www.centerwatch.com
http://www.clinicalstudyresults.org
http://clinicaltrials.gov
http://www.controlled‐trials.com
http://ctr.gsk.co.uk
http://www.lillytrials.com (Zyprexa)
http://www.roche‐trials.com (anti‐a beta antibody)
http://www.organon.com
http://www.novartisclinicaltrials.com (rivastigmine)
http://www.bayerhealthcare.com
http://trials.boehringer‐ingelheim.com
http://www.cmrinteract.com
http://www.esteve.es
http://www.clinicaltrials.jp
This part of the IPFMA database is searched and was last updated on 4 September 2006;
Lundbeck Clinical Trial Registry (http://www.lundbecktrials.com) (last searched 15 August 2006);
Forest Clinical trial Registry (http://www.forestclinicaltrials.com/) (last searched 15 August 2006).
The search strategies used to identify relevant records in MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS can be found in the Group's module on The Cochrane Library.
Blood pressure, hypertension, cardiovascular, dementia, neurology, psychiatry, and geriatric journals and conference proceedings were hand searched.
We contacted relevant drug companies, colleagues and researchers to identify further published and unpublished studies.
The reference lists of all trials identified were screened.
Data collection and analysis
IDENTIFICATION OF STUDIES The search and screening of publications was undertaken by two authors (BMcG, supported by ST). For this update, the search and screening of publications was undertaken by two authors (ST, supported by BMcG). The MeSH terms and search strategy used were agreed upon and tested by both reviewers. The other authors (PP and RB) acted as adjudicators and reviewed the process.
Authors independently selected trials for relevance against the defined inclusion criteria. Those trials that did not fulfil the criteria were excluded from further analysis. Excluded studies may be referred to in the discussion section.
QUALITY ASSESSMENT The methodological quality of the included trials was assessed with particular emphasis on the concealment of treatment allocation. Trials were ranked using the Cochrane approach (Mulrow 1997):
Grade A: Adequate concealment This is where the report describes allocation of treatment by: (i) some form of centralized randomized scheme, such as having to provide details of an enrolled participant to an office, by phone to receive the treatment group allocation; (ii) some form of randomization scheme controlled by a pharmacy; (iii) numbered or coded containers, such as in a pharmaceutical trial in which capsules from identical‐looking numbered bottles are administered sequentially to enrolled participants; (iv) an on‐site or coded computer system, given that the allocations were in a locked, unreadable file that could be accessed only after inputting the characteristics of an enrolled participant; or (v) if assignment envelopes were used, the report should at least specify that they were sequentially numbered, sealed, opaque envelopes; (vi) other combinations of described elements of the process that provides assurance of adequate concealment.
Grade B: Uncertain This is where the report describes allocation of treatment by: (i) use of a 'list' or 'table' to allocate assignments; (ii) use of 'envelopes' or 'sealed envelopes'; (iii) stating the study as 'randomized' without further detail.
Grade C: Inadequate concealment This is where the report describes allocation of treatment by: (i) alternation; (ii) reference to case record numbers, dates of birth, day of the week, or any other approach; (iii) any allocation procedure that is entirely transparent before assignment, such as an open list of random numbers or assignments.
Empirical research has shown that lack of adequate allocation concealment is associated with bias. Trials with unclear concealment measures have been shown to yield more pronounced estimates of treatment effects than trials that have taken adequate measures to conceal allocation schedules, but less pronounced than inadequately concealed trials (Chalmers 1983; Schulz 1995). Thus trials were included if they conformed to category A and those falling into categories B or C were excluded.
Other aspects of trial quality were not assessed by a scoring system although details were noted of blinding, whether intention‐to‐treat analyses were extractable from the published data, and the number of patients lost to follow up.
INCLUSION CRITERIA Identified trials with the above quality assessment were included. Any disagreement in the independent selection was resolved with discussion.
DATA EXTRACTION Data were extracted from the published reports. The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at each time point was extracted.
For binary data the numbers in each treatment group and the numbers experiencing the outcome of interest were sought. The baseline assessment was defined as the latest available assessment prior to randomization, but no longer than two months prior.
For each outcome measure, data was sought on every patient assessed. To allow an intention‐to‐treat analysis, the data was sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible, or otherwise excluded from treatment or follow‐up. If intention‐to‐treat data were not available in the publications, "on‐treatment" or the data of those who complete the trial was sought and indicated as such.
DATA ANALYSIS The analysis would assess the effect of:
All blood pressure lowering interventions
Combined effect of all antihypertensive medications
The outcomes measured in clinical trials of dementia and cognitive impairment often arise from ordinal rating scales. Where the rating scales used in the trials had a reasonably large number of categories (more than 10) the data were treated as continuous outcomes arising from a normal distribution.
Summary statistics (n, mean and standard deviation (SD)) were required for each rating scale at each assessment time for each treatment group in each trial for change from baseline.
When change from baseline results was not reported, the required summary statistics were calculated from the baseline and assessment time treatment group means and standard deviations.
The meta‐analysis requires the combination of data from the trials that may not use the same rating scale to assess an outcome. The measure of the treatment difference for any outcome was the weighted mean difference where the pooled trials use the same rating scale or test, and the standardised mean difference, which is the absolute mean difference divided by the standard deviation, where they used different rating scales or tests.
For binary outcomes, such as dead or alive, development of dementia or no dementia, the odds ratio was used to measure treatment effect. A weighted estimate of the treatment effect across trials was calculated. Overall estimates of the treatment difference were presented. In all cases the overall estimate from a fixed effects model was presented and a test for heterogeneity using a standard chi‐square statistic was performed. If there was significant heterogeneity a random effects model was presented.
Sensitivity analyses were undertaken to assess the robustness of the results to fixed effect versus random effect models and on the inclusion or exclusion of studies of poor quality. If the treatment effect in the sensitivity analysis were of the similar magnitude and precision as that of the main analysis, a definite conclusion about the treatment effectiveness will be made; otherwise no definite conclusion will be made on the effectiveness of the treatment.
Results
Description of studies
This update identified one additional randomized, placebo‐controlled trial (HYVET 2008) for inclusion in the review. The total number of hypertensive subjects included is 15,936. Ages ranged from 60‐89 years, average age was 75.4 years. SCOPE 2003 included only elderly patients aged 70‐89 years. SHEP 1991 and Syst Eur 1997 included patients 60 years and older. HYVET 2008 included only people aged 80 years or older, with an average age of 83.5 years.
Participants were recruited mainly from industrialised countries: Europe 63%, North America 27%. 8% of participants were Chinese. 62% of subjects were female. SHEP 1991, the only trial based wholly in the USA, reported ethnicity: 30% Hispanic, 14% African‐American. Most subjects in HYVET 2008 were recruited from eastern Europe (56%) and China (40%); only a small proportion of participants were recruited from western Europe, Australasia and Tunisia. Ethnicity was not reported. Syst Eur 1997 and SCOPE 2003 did not report ethnicity.
Study populations predominantly consisted of ambulatory patients recruited from the community or primary care facilities. No studies included patients with dementia. Patients in Syst Eur 1997 had a median MMSE of 29, those in SCOPE 2003 had MMSE ≥ 24, and median MMSE was 26 in HYVET 2008. Syst Eur 1997 and SCOPE 2003 reported years of education, with means of 12.3 and 11.7 years respectively. In HYVET 2008, 27% of subjects had no education, 28% primary, 29% secondary, 12% higher and 3% further.
Mean blood pressure at entry across studies was 171/86 mmHg. Syst Eur 1997 and SHEP 1991 included only persons with isolated systolic hypertension (ISH). Patients in both these studies had systolic BP 160‐219 mmHg and diastolic BP < 90 mmHg. SCOPE 2003 included patients with essential hypertension. In HYVET 2008, entry blood pressure criteria was sitting systolic 160‐199mmHg with standing systolic 140 mmHg or greater and diastolic less than 110mmHg. Please refer to the "Participants" heading in the Characteristics of included studies table for a complete description of each study's blood pressure inclusion criteria.
All trials instituted a stepped care approach to hypertension treatment. A variety of drugs were used as first line treatment in the treatment groups. Syst Eur 1997 started treatment with a calcium‐channel blocker. Patients in SHEP 1991 and HYVET 2008 were started on a thiazide diuretic while those in SCOPE 2003 began treatment with an angiotensin II type 1 receptor blocker. Second and third line drugs included diuretics, beta blockers, centrally acting agents, and ACE inhibitors. Please refer to the "Interventions" heading in Characteristics of included studies table for a complete description of each study's drug treatment protocol.
Planned mean length of study follow‐up was 5 years in each of the trials. All trials were multisite.
In SHEP 1991, dementia was diagnosed by an expert and confirmed by the central coding panel according to the DSM‐III‐R criteria (APA 1987) after referral triggered by score on the short‐Comprehensive Assessment and Referral Evaluation (short‐CARE) at follow up. Syst Eur 1997, SCOPE 2003 and HYVET 2008 administered the MMSE at follow up visits. Triggers for (a) further diagnostic evaluation and (b) the criteria for diagnosis of dementia were:
Syst Eur 1997 ‐ (a) MMSE≤23 or symptoms or signs reported by subject or carer or found by doctor (b) DSM‐III‐R, with all cases validated by a treatment allocation blinded review board
SCOPE 2003 ‐ (a) significant cognitive decline was a reduction by 4 or more points in the MMSE in two consecutive visits in comparison to baseline (b) ICD10 criteria, adjudicated by Independent Clinical Event Commiittee based on information supplied by the local investigator
HYVET 2008 ‐ (a) decline in MMSE to <24 or fall of >3 points in one year (b) DSM‐IV (APA 1994), with consensus from a treatment allocation blinded central committee.
Level of blood pressure change was provided by all trials.
Quality of life (QOL) data was provided by two trials, (SHEP 1991; Syst Eur 1997). Syst Eur 1997 used three QOL assessments: Sickness Impact Profile, Brief Assessment Index and a checklist of 32 symptoms associated with hypertension and the side effects of anti‐hypertensive treatment, while SHEP 1991 used the short‐CARE, Centre for Epidemiologic Studies‐Depression Scale (CES‐D), Activities of Daily Living (ADL) and Social Network Questionnaires. A SCOPE 2003 substudy (Degl'Innocenti 2004) reported QOL data using three validated assessments: Psychological General Well‐being index (PGWB), Subjective Symptoms Assessment Profile (SSA‐P), and EuroQoL Health Utility Index (EuroQoL).
Adverse events were recorded by all included studies.
Risk of bias in included studies
Only randomized, double‐blind, placebo‐controlled trials were included. All trials were ranked grade A in terms of treatment allocation using the Cochrane approach (Mulrow 1997). All subjects, providers of therapy, and outcome assessors were blinded to therapy.
SCOPE 2003 reported loss to follow‐up of < 1%. SHEP 1991 and Syst Eur 1997 reported loss to follow‐up of < 5%.
A proportion of the subjects assigned to the control group may have received antihypertensive treatment because their blood pressure exceeded pre‐set 'escape criteria'. Also, a proportion of subjects assigned to the treatment group may have stopped taking their medications due to side effects or because they achieved normal blood pressure off medication. The degree to which subjects cross over from one group to another reduces the strength of the results of the study. The percentage of patients assigned to the control group who were receiving antihypertensive medication by the end of the trial were: SCOPE 2003 84%, Syst Eur 1997 27%, and SHEP 1991 44%. The percentage of patients assigned to the treatment group who had ceased taking antihypertensive treatment by the end of the trial were: SCOPE 2003 0%, Syst Eur 1997 18%, and SHEP 1991 10%. The percentage of patients taking initially assigned medication alone were: SCOPE 2003 25%, Syst Eur 1997 30%, and SHEP 1991 30%.
In this update, risk of bias tables have been completed for all included studies and may be found in the Characteristics of included studies section. In HYVET 2008, at the 2 year follow‐up, 0.8% of the active treatment group were not taking one of the three treatment steps specified in the protocol. 0.6% of the control group were not taking one of the matching placebo steps specified in the protocol.
Effects of interventions
Analyses were performed on the combined results of all four trials.
Incidence of dementia (Analysis 1.1): Incidence of dementia was a secondary outcome in the included trials (SHEP 1991; Syst Eur 1997; SCOPE 2003; HYVET 2008). The combined results of the four trials showed no significant difference between treatment and placebo (OR = 0.89; 95% CI 0.74, 1.07) (Figure 1). Blood pressure reduction resulted in 11% relative risk reduction of dementia in patients with no prior cerebrovascular disease but this effect was not statistically significant (p = 0.21) and there was no noticeable heterogeneity between the trials.
1.1. Analysis.
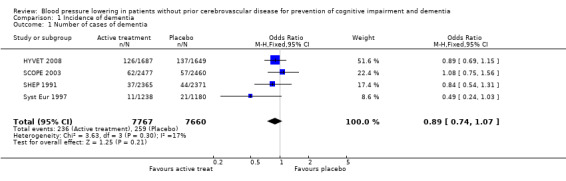
Comparison 1 Incidence of dementia, Outcome 1 Number of cases of dementia.
1.
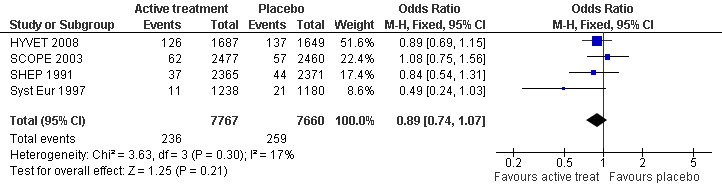
Forest plot of comparison: 1 Incidence of dementia, outcome: 1.1 Number of cases of dementia.
Of the individual studies, only Syst Eur 1997 reported significance with results indicating a benefit. There were 21 cases of incident dementia in the placebo group and 11 in the active treatment group. The number of patient years was 2737 and 2885 respectively. Active treatment reduced the rate of dementia by 50% (95% CI 0, 76) from 7.7 to 3.8 cases per 1000 patient‐years (p=0.05). However, when the numbers of subjects in the active treatment (1238) and control groups (1180) are considered, consistent with analysis of the other included studies, the benefit with active treatment is no longer significant (OR 0.49; 95% CI 0.24, 1.03) (Figure 1). In SHEP 1991 from 2365 patients in the active treatment group 1.6% (37) had a positive diagnosis of dementia and from 2371 patients in the control group 1.9% (44) had a positive diagnosis of dementia. There was no evidence that active treatment reduced the incidence of dementia (OR 0.84; 95% CI 0.51, 1.31). In SCOPE 2003 there was no significant difference in the proportion of patients who developed dementia; 62 from 2477 patients in the candesartan arm and 57 from 2460 patients in the control arm (OR 1.08; 95% CI 0.75, 1.56). In HYVET 2008 no significant difference in the rate of incident dementia was found, with 126 cases in the treatment group (1687 subjects) and 137 in the control group (1649 subjects) (OR 0.89; 95% CI 0.69, 1.15).
A sensitivity analysis was carried out with SCOPE 2003 removed (due to problems with the placebo group) and there was no difference in the incidence of dementia outcome.
To control for the possible effect of differential drop‐out, an analysis was carried out to determine the incidence of dementia in the first year of study treatment (Analysis 1.2). No significant effect was noted in the combined results of the three trials which reported or provided this data (SHEP 1991; Syst Eur 1997; HYVET 2008) (Figure 2).
1.2. Analysis.
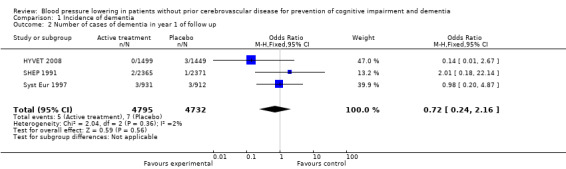
Comparison 1 Incidence of dementia, Outcome 2 Number of cases of demen tia in year 1 of follow up .
2.
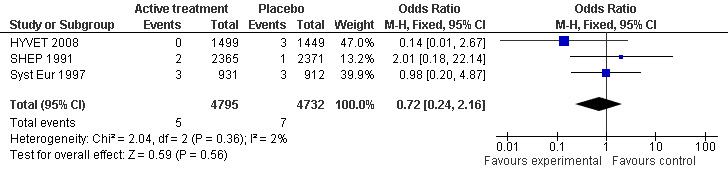
Forest plot of comparison: 1 Incidence of dementia, outcome: 1.2 Number of cases of dementia in year 1 of follow up.
Cognitive change from baseline (Analysis 2.1): Syst Eur 1997, SCOPE 2003 and HYVET 2008 also provided figures for cognitive change from baseline. The combined results from the three trials (SCOPE 2003; Syst Eur 1997; HYVET 2008) reporting change in MMSE indicate a significant benefit from treatment (WMD = 0.42; 95% CI 0.30, 0.53) (Figure 3). Of the individual studies, only HYVET 2008 indicated a benefit of treatment.
2.1. Analysis.
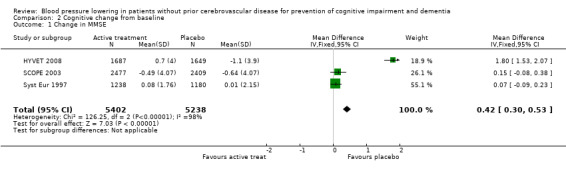
Comparison 2 Cognitive change from baseline, Outcome 1 Change in MMSE.
3.
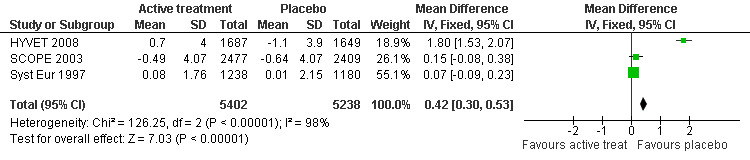
Forest plot of comparison: 2 Cognitive change from baseline, outcome: 2.1 Change in MMSE.
Change in systolic blood pressure level (Analysis 3.1): The combined results from the included trials (SCOPE 2003; SHEP 1991; Syst Eur 1997; HYVET 2008) reporting change in systolic blood pressure indicated a significant benefit of treatment (WMD = ‐10.22; 95% CI ‐10.78, ‐9.66) in reducing systolic blood pressure (Figure 4). All included studies indicated a significant benefit of treatment.
3.1. Analysis.
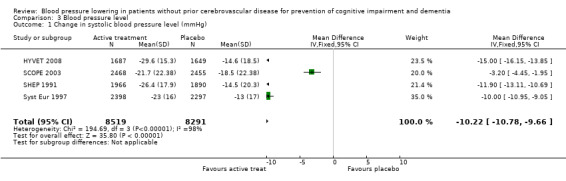
Comparison 3 Blood pressure level, Outcome 1 Change in systolic blood pressure level (mmHg).
4.
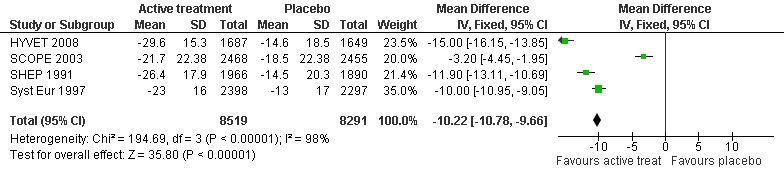
Forest plot of comparison: 3 Blood pressure level, outcome: 3.1 Change in systolic blood pressure level (mmHg).
Change in diastolic blood pressure level (Analysis 3.2): The combined results from the included trials (SCOPE 2003; SHEP 1991; Syst Eur 1997; HYVET 2008) reporting change in diastolic blood pressure level indicated a significant benefit from treatment (WMD = ‐4.28; 95% CI ‐4.58, ‐3.98) in reducing diastolic blood pressure (Figure 5). All included studies indicated a significant benefit of treatment although Syst Eur 1997 included patients with isolated systolic hypertension only.
3.2. Analysis.
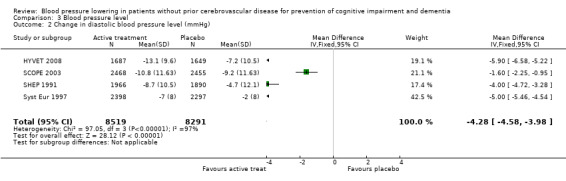
Comparison 3 Blood pressure level, Outcome 2 Change in diastolic blood pressure level (mmHg).
5.
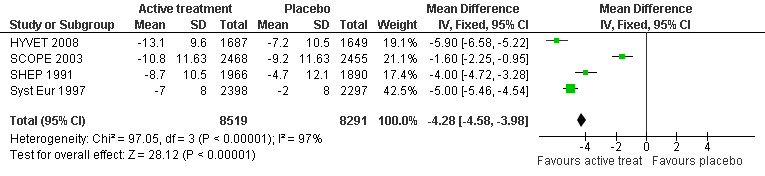
Forest plot of comparison: 3 Blood pressure level, outcome: 3.2 Change in diastolic blood pressure level (mmHg).
Incidence and severity of adverse effects (Analysis 4.1): The combined results from three trials (SHEP 1991; Syst Eur 1997 and SCOPE 2003) reporting adverse effects requiring discontinuation of treatment indicated that there was no significant difference between the active treatment and placebo groups (OR = 1.01; 95% CI 0.92, 1.11) (Figure 6). Of the individual studies, Syst Eur 1997 reached significance with patients on active treatment less likely to discontinue treatment due to side effects (OR = 0.42; 95% CI 0.33, 0.54). Conversly, in SHEP 1991 significantly more patients on active treatment withdrew due to adverse events (OR = 1.49; 95%CI 1.30, 1.70). No significant difference between the active treatment and placebo groups in adverse events requiring discontinuation of treatment was found in SCOPE 2003, possibly because of the large proportion of subjects in the placebo group commenced on active treatment during the study.
4.1. Analysis.
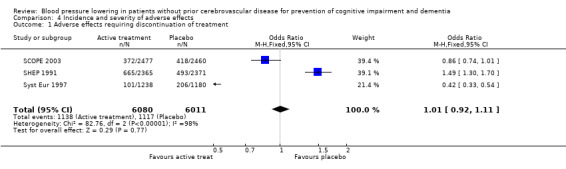
Comparison 4 Incidence and severity of adverse effects, Outcome 1 Adverse effects requiring discontinuation of treatment.
6.
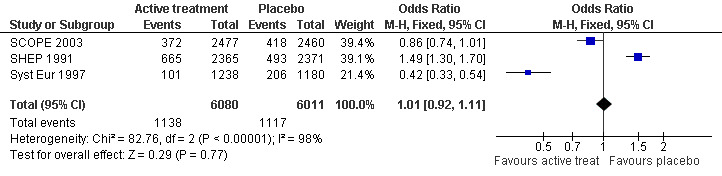
Forest plot of comparison: 4 Incidence and severity of adverse effects, outcome: 4.1 Adverse effects requiring discontinuation of treatment.
HYVET 2008 reported fewer 'serious adverse events' in the active treatment group than in the placebo group (358 vs 448, p=0.001). Data was not recorded for adverse effects requiring discontinuation of treatment so HYVET 2008 could not be included in the present analysis.
Quality of life: Quality of life data could not be analysed on the included studies.
Control Rates: Control rates provide insight regarding baseline risk of study populations and explain the differences in outcomes between individual trials. Incidence of dementia ranged from 1.8% ‐ 8.3% in the control groups. Syst Eur 1997 had a rate of dementia of 1.8% in the control group, SCOPE 2003 had a rate of 2.3%, SHEP 1991 had a rate of 1.9%, and HYVET 2008 had a rate of 8.3%. MMSE score in the control group changed by 0.01 (SD 2.15) in Syst Eur 1997, by ‐0.64 (SD 4.07) in SCOPE 2003, and by ‐1.1 (SD 3.9) in HYVET 2008. Data were not provided in SHEP 1991. The problem with the control group in this review is that many of the subjects in the control group received antihypertensive treatment as their blood pressure exceeded pre‐set values. For ethical reasons these patients had to be treated with an antihypertensive agent and in most cases the study became a comparison of the study drug against a usual antihypertensive regimen.
Substudies: Follow‐up substudies were carried out on three trials (SHEP 1991; Syst Eur 1997; SCOPE 2003) addressing cognitive decline. These substudies were open label follow‐up or retrospective and therefore did not meet inclusion criteria for the current analysis. The results will be assessed in the discussion section and in more detail in Appendix 1.
Discussion
The aim of this systematic review was to determine the effects of blood pressure lowering on development of cognitive decline and dementia on patients with hypertension but no history of stroke or TIAs. There have been numerous RCTs assessing blood pressure lowering treatment but most did not assess cognitive function. There have also been numerous cross‐sectional, longitudinal, and observational studies assessing relation of hypertension to subsequent cognitive decline and dementia. These were not included in the review as randomized, double‐blind, placebo‐controlled studies are considered the gold standard.
A systematic review that included all population based studies relating to blood pressure and cognitive decline or dementia concluded that the studies provided substantial evidence of a risk effect of mid‐life high blood pressure on the development of late‐life cognitive decline and dementia, particularly if left untreated (Qui 2005). There is no strong evidence from the longitudinal studies, however, to indicate that late‐life hypertension is a risk factor for cognitive decline and dementia. The authors summarized that the observational studies showed some beneficial effects of antihypertensive treatment against cognitive decline and dementia in elderly people but that RCTs were necessary to verify these effects to take into account different antihypertensive agents and the extent of blood pressure reduction (Qui 2005).
From the four RCTs identified which met the inclusion criteria, there is no convincing evidence that blood pressure lowering in late‐life prevents the development of dementia or cognitive impairment in hypertensive patients without apparent prior cerebrovascular disease. Syst Eur 1997 reported a benefit from treatment in prevention of dementia but neither SCOPE 2003, SHEP 1991 nor HYVET 2008 showed a benefit and, when the trials were entered in a meta‐analysis, no statistically significant effect was seen.
The secondary objectives of the review were difficult to meet. From the studies identified the optimal blood pressure level for prevention of dementia or cognitive decline could not be determined. Nitrendipine, a calcium channel antagonist, was found to be effective in reducing incidence of dementia in Syst Eur 1997. The trialists felt it may have had a neuroprotective effect outside blood pressure lowering but further studies would be required to confirm this hypothesis. Without individual patient data (IPD), it is difficult to assess if there are different effects of treatment according to aspects of baseline risk such as sex, age, or blood pressure level.
Analysis of this data was problematic due to the number of patients lost to follow‐up, with differential loss between the assigned groups, and the number of placebo patients who received active medication as their blood pressure exceeded certain pre‐set values, thus introducing bias. The bias may be reduced by analysing one year data but true cognitive effects may not be seen over such a short time period. IPD would be required to be certain of outcomes in each study and there may be an advantage in extending length of follow‐up to be certain of true incidence of cognitive decline. More detailed discussion addressing quality of the included studies is found in Appendix 1 and Characteristics of included studies.
However, examining the literature from cross‐sectional, longitudinal, and observational studies along with the RCTs, there is moderately strong evidence to support the view that hypertension in mid‐life, especially if not treated effectively, negatively affects cognition and contributes to the development of dementia and AD in later life (Qui 2005). It is proposed that high blood pressure in middle age can cause a long term cumulative effect, which leads to increased severity of atherosclerosis and more vascular comorbidities in later life. There is less evidence from these studies that the same negative effect on cognition is present for hypertension in late‐life.
A number of other meta‐analyses have been published since our initial review (Wang 2003; Feigin 2005; Birns 2006). Wang 2003 reported a meta‐analysis of placebo‐controlled and active controlled trials of the risks of stroke, stroke recurrence and dementia with blood pressure lowering therapy in participants both with and without evidence of prior cerebrovascular disease. Four trials were included in the prevention of dementia outcome (SHEP 1991; Syst Eur 1997; PROGRESS 2003; SCOPE 2003). Across the four trials, the pooled odds ratio was not significantly lower in the active treatment group (OR 0.89; 95% CI 0.75, 1.04; p=0.15). A sensitivity analysis found a significant difference if only those studies initiating treatment with a dihydropyridine calcium channel blocker or diuretic were considered (SHEP 1991; Syst Eur 1997; PROGRESS 2003 combination therapy subgroup) (OR 0.75; 95% CI 0.60, 0.94; p=0.01) whereas those studies initiating an inhibitor of the renin‐angiotensin‐aldosterone system (SCOPE 2003; PROGRESS 2003 perindopril only subgroup) did not have an effect on dementia (OR 1.08; 95%CI 0.84, 1.38; p=0.54). RCTs to determine if specific blood pressure lowering treatments have differing effects on the prevention of dementia were advised (Wang 2003).
Feigin 2005 included data from PROGRESS 2003 as well as SHEP 1991, Syst Eur 1997 and SCOPE 2003 in their aggregated data meta‐analysis of the effects of blood pressure lowering treatment on dementia and/or cognitive decline in patients with cardiovascular and/or cerebrovascular disease. In Syst Eur 1997 'cardiovascular complications' were present in 29.9% of patients at randomisation. However, only 4.1% and 11.6%, respectively, had a history of stroke and myocardial infarction. SCOPE 2003 reported that at baseline 4.5% in the candesartan group and 4.6% in the control group had previous (longer than 6 months) myocardial infarction; the corresponding figures for previous stroke were 3.9% and 3.9%. In SHEP 1991, 4.9% of participants in both active treatment and control groups had history of myocardial infarction. History of stroke was present in 1.5% of the active treatment group and 1.3% of the control group. Thus, the majority of the participants in these three trials did not have cardiovascular or cerebrovascular disease. PROGRESS 2003 was a trial of blood pressure lowering therapy following stroke. Feigin and colleagues combined data on significant cognitive decline and dementia from SCOPE 2003. Counting the majority of this trial's participants twice in their meta‐analysis introduces bias. Feigin et al report a non‐significant reduction in risk of dementia or cognitive decline with blood pressure lowering treatment (RR 0.80; 95% CI 0.63, 1.02) (Feigin 2005).
Birns and colleagues included RCTs measuring the effect of blood pressure reduction on any measure of cognitive performance (Birns 2006). Trials were included in their meta‐analysis that compared different antihypertensive treatments as well as placebo controlled trials. Additionally, the presence of cerebrovascular disease was not an exclusion criteria, in contrast to this Cochrane review. Analysis of incident dementia was not carried out. Outcomes were reported on measures of: global cognitive function (MMSE); memory (immediate and delayed logical memory); perceptual processing and executive function (digit span, trail‐making, digit symbol substitution; and learning capacity (paired associate learning). In an analysis including Syst Eur 1997, SCOPE 2003 and PROGRESS 2003, decrease in the MMSE score was 0.19 (95% CI 0.19, 0.19) points less in the active treatment group. PROGRESS 2003 had a weight of 99.88% in that analysis. SHEP 1991 could not be included in any analysis, as the authors note resulting in bias. It was concluded that antihypertensive treatment decreases decline in global cortical function and memory but may not have a similar effect on executive function or learning capacity (Birns 2006).
Peters 2008 performed a meta‐analysis including their trial (HYVET 2008) along with three other placebo‐controlled trials of antihypertensive treatment that assessed dementia incidence (SHEP 1991; Syst Eur 1997; PROGRESS 2003). The pooled relative risk was of borderline significance (RR 0.87; 95% CI 0.76,1.00; p=0.045). The results are similar to those of this updated Cochrane review (Analysis 1.1). As previously noted, PROGRESS 2003 was excluded from our review as it enrolled subjects with cerebrovascular disease. PROGRESS 2003 provided the majority of the weight for the meta‐analysis by Peters et al. which used a random effects model. As no significant heterogeneity was found in our analysis (Figure 1), a fixed effects model was presented.
HYVET 2008, with 40% Chinese participants, had more non‐Caucasian participation than is reported or may be inferred from the geographic locations of the other included studies. The consistent results across the discussed meta‐analyses suggests a common effect across populations.
Based on data provided by authors of included studies (SHEP 1991; Syst Eur 1997; HYVET 2008), an analysis was performed of incident dementia in the first year of follow up, assuming lower degree of differential dropout between the active treatment and control groups across that time period (Analysis 1.2). No significant effect of active treatment was found (OR 0.72; 95% CI 0.24, 2.16; p=0.56) (Figure 2).
In this update the values for Syst Eur 1997 were amended to the number of subjects in each group rather than patient‐years of follow up which previously had been used. This kept the denominator consistent across the included studies. This did not alter the point estimate of the effect of blood pressure lowering therapy on incidence of dementia.
It is also important to note that cardiovascular outcomes were the primary end‐points in all of the included studies in this review. Dementia and cognitive function were secondary outcomes. The trials had to be terminated once the benefits for the primary end‐points were shown. It is therefore possible that beneficial effects on cognition are not observed before the cardiovascular benefits become apparent. Following patients in placebo‐controlled trials from mid‐life would be very informative in terms of cognition but would not be ethical.
In Syst Eur 1997 it was speculated that dementia prevention was facilitated by a neuroprotective role of the calcium‐channel blocker, nitrendipine. The meta‐analysis by Wang and colleagues suggests differential effects between dihydropyridine calcium channel blockers and diuretics on the one hand and inhibitors of the renin‐angiotensin system on the other (Wang 2003). It would be informative to address this class effect further but again there are ethical considerations.
Authors' conclusions
Implications for practice.
The addition of the HYVET 2008 trial to the updated meta‐analysis does not alter the conclusions from the initial review, which remain that blood pressure lowering in late‐life is not indicated with the aim of prevention of cognitive decline or dementia alone.
The benefits of blood pressure lowering treatments on cardiovascular and cerebrovascular outcomes are now firmly established. As further placebo‐controlled trials investigating cognitive outcomes would not be ethical, it is unlikely that additional trials would be included in future updates of this review.
Treatment of elevated blood pressure in late‐life, even into the ninth decade, is not associated with significant deleterious effects on cognitive performance.
Implications for research.
Recommendations arising from this review are:
1. Further analysis of the studies using IPD, although this would be time consuming and expensive. This would allow analysis of the data taking into account differential drop‐out and may lead to more robust results.
2. Further trials on different classes of drugs. In Syst Eur 1997 it was speculated that dementia prevention was facilitated by a neuroprotective role of the calcium channel blocker, nitrendipine. The meta‐analysis by Wang and colleagues suggests differential effects between dihydropyridine calcium channel blockers and diuretics on the one hand and inhibitors of the renin‐angiotensin system on the other (Wang 2003). For ethical reasons further trials must be head to head, however, as placebo controlled trials could no longer be justified. Based on the epidemiological data (Qui 2005), trials of blood pressure lowering treatment regimens should be designed to include subjects with hypertension in middle age with prolonged follow up and assessment of cognition into older age which are adequately powered to determine, as a primary outcome, whether cognitive impairment and dementia can be prevented.
Feedback
Peer review and editorial comment, 3 July 2009
Summary
Peer reviewers and editor suggested rewriting parts of background section and discussion from initial review to facilitate ease of reading as parts of these sections were also repeated in other sections of the review, for example characteristics of included studies.
A few inconsistencies in presentation through the various sections of the review were highlighted.
Comments throughout initial review and this update have returned to the included and excluded studies.
Reply
Rewriting of the background and discussion sections in the updated review done as suggested.
Consistent use of terms and phrasing now used throughout the updated review.
The brief given to the reviewers by the Review Group was to analyse the effect of blood pressure lowering on cognitive impairment in subjects with no evidence of prior cerebrovascular disease. The reasons for exclusion of certain studies and the problems with analysis of the included studies have been dealt with in detail in the initial review and in this update.
Contributors
ST and BMcG; reviewed and commented on by PP.
What's new
| Date | Event | Description |
|---|---|---|
| 27 July 2009 | Feedback has been incorporated | Further feedback from editor incorporated into updated review. |
| 3 July 2009 | Feedback has been incorporated | Feedback from editor incorporated into updated review. |
| 11 May 2009 | Feedback has been incorporated | Feedback from external peer reviewers incorporated into updated review. |
| 21 January 2009 | Feedback has been incorporated | Feedback from contact editor and editorial base incorporated into updated review. |
| 26 November 2008 | New citation required and conclusions have changed | The authors have recommended that no further trials are conducted assessing the effect of antihypertensive interventions for cognition. |
| 26 November 2008 | New search has been performed | Update of search of February 2008 retrieved several studies for consideration by the authors: upon assessment, one new study has been included (HYVET 2008); there are now four studies included in the review, with a total of 15,936 participants. In addition, two new studies have been excluded. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 8 February 2006 | New citation required and conclusions have changed | Substantive amendment |
| 30 September 2005 | New search has been performed | Minor update |
Acknowledgements
We are grateful for help from Gerard McVeigh, consumer editor. We would also like to express gratitude for support from the Cochrane Dementia and Cognitive Improvement Group, particularly advice from Sarah Sampler, Katherine Hicks, Jacqueline Birks and Dymphna Hermans.
For this update, we appreciate advice and support from Helen Collins and Reem Malouf at the Cochrane Dementia and Cognitive Impairment Group. Furthermore, we gratefully acknowledge provision of additional information from authors of the following included studies: Drs Lutgarde Thijs, Francoise Forette, and Jan Staessen for the Syst Eur research group, Dr Ruth Peters for the HYVET study group, and Ms Sara L. Pressel for the SHEP study group.
Appendices
Appendix 1. Discussion of t he individual included studies and associated follow up studies
SHEP 1991 Large multicentre trial which mostly fits the inclusion criteria (1.4% reported history of stroke). It Included only patients with ISH (systolic BP > 160 mmHg, diastolic BP < 90 mmHg) and age older than 60 years. There was no evidence that active treatment reduced the incidence of dementia. One problem identified was open label treatment was given if SBP > 240 mmHg or DBP > 115 mmHg at a single visit. As more people in the placebo group than in the treatment group needed open label treatment the imbalance may have biased the results. Analysing one year data may be more useful.
A follow‐up study carried out by Di Bari 2001 aimed to evaluate whether assessment of cognitive and functional outcomes was biased by differential dropout. Characteristics of subjects who did or did not participate in follow‐up cognitive assessments were compared. The relative risk of incident cognitive impairment was assessed in the two groups, with the use of reported findings and under the assumption that the proportion of cognitive impairments among dropouts increased. Assignment to placebo group and the occurrence of cardiovascular events independently predicted missed assessments. When 20‐30% of the subjects who missed the assessment were assumed to be cognitively impaired, assignment to active treatment reduced the risk of cognitive impairment. The authors concluded that in SHEP 1991 the cognitive evaluations were biased toward the null effect by differential dropout. This might have obscured the appraisal of a protective effect of treatment on the incidence of cognitive decline in older hypertensive adults.
Syst Eur 1997 Large multicentre trial which mostly fulfilled the inclusion criteria (7.3% had prior cerebrovascular disease and 4.1% had a history of stroke). The trial was stopped early as the primary endpoint of a significant benefit for stroke was reached after the second interim analysis. The vascular dementia project study by intention‐to‐treat analysis found the incidence of dementia was reduced by 50%. There was difficulty analysing the trial as it was difficult to account for the progression of all people through the trial. It was not clear how many people reached endpoint in each year of follow up nor was it clear how the patients were followed up. More than 50% of the placebo group received active treatment i.e. an antihypertensive. It was difficult to interpret the results of the intention to treat analysis as it was difficult to ascertain who exactly was in each group; this includes results for change in BP, incidence of dementia and cognitive change. The results above should therefore be interpreted with caution because of the element of bias. Individual patient data (IPD) would be required to be certain of outcomes.
Substudy of Syst Eur 1997 (Forette 2002): this was an open‐label, active treatment follow‐up study in the same population based on the original active study medication. The median follow‐up was 3.9 years. 80.5% of control patients ended up on active treatment due to blood pressure level. Throughout follow‐up, systolic/diastolic blood pressure was 7.0/3.2 mmHg higher in the 1417 control patients than in the 1485 subjects randomized to active treatment. Compared with the controls, long‐term antihypertensive therapy reduced the risk of dementia by 55% from 7.4 to 3.3 cases per 1000 patient‐years (43 versus 21 cases, p < 0.001). There was no significant change in MMSE between control and active treatment groups. Again this follow‐up study needs to be interpreted with caution as most of the control patients received antihypertensive medication and it was open label.
SCOPE 2003 Large multicentre trial. The problem with interpreting the results of this trial is that only 16% of the patients in the control group received placebo alone; 84% received active antihypertensive treatment. The SCOPE trial was initiated as a placebo‐controlled study. However, because of changes in treatment guidelines and for ethical reasons, it became necessary during the recruitment period to recommend open‐label active antihypertensive therapy in both treatment groups for patients whose blood pressure remained high. As a result, the trial actually compared a candesartan‐based regimen with a usual treatment regimen not containing candesartan. There was no significant difference found between the groups in terms of significant cognitive decline (reduction in MMSE of 4 points or more at two consecutive visits in comparison to baseline) or incidence of dementia but one would have to assume the control group had been compromised. It was also difficult to interpret values for mean reduction in BP and change in MMSE as it was not clear whether all subjects on open label treatment were included. One year data may be more useful.
A sensitivity analysis was carried out with SCOPE 2003 removed (due to problems with the placebo group) and there was no difference in the incidence of dementia outcome (OR 0.84, 95% CI 0.68,1.03).
One substudy of SCOPE 2003 assessed outcomes in patients not receiving add‐on therapy after randomization (Lithell 2004). This was a post hoc analysis and so did not meet inclusion criteria for the review. It is, however, worthy of comment as it analysed patients who most closely reflected the original intention of the placebo controlled trial. Out of 4937 patients in SCOPE 2003, 2098 did not receive add‐on therapy. Of these 1253 received candesartan 8‐16 mg daily, and 845 received placebo. When these two groups were directly compared, there was no significant difference between the candesartan and placebo groups with respect to change in MMSE score or development of cognitive impairment or dementia. The study investigators felt the lack of beneficial effect of candesartan on cognitive outcomes may have been explained by the relatively short follow‐up (median 3.6 years) and the rather small blood pressure differences between the groups (mean SBP at baseline for candesartan group 164.7 mmHg; placebo group 164.6 mmHg; mean DBP at baseline for candesartan group 90.5 mmHg; placebo group 90.4 mmHg). It was also felt patients in SCOPE had very good cognitive function at baseline and were therefore at low risk for marked impairment. This was also thought to limit the possibility of demonstrating a preventive effect. It may be useful, therefore, to follow patients in this trial over a longer period of time to assess whether there is any significant difference in cognitive impairment over time.
Another substudy of SCOPE 2003 (Papademetriou 2004) assessed elderly patients with isolated systolic hypertension (ISH) only. The original study looked at patients with essential hypertension. This means results can be compared with SHEP 1991 and Syst Eur 1997 as both these trials only included patients with ISH. A subgroup analysis of outcome results in SCOPE patients with ISH was carried out; this was retrospective so did not fit inclusion criteria for the meta analysis. From 4964 patients, 1518 met criteria for ISH (systolic BP > 160 mmHg diastolic BP < 90 mmHg). Average duration of follow‐up was 3.6 years. 754 patients received candesartan and 764 received control medications, although again 82% of control patients received active therapy due to blood pressure level. In both groups SBP and DBP reductions were significant but there was no significant change in MMSE between the candesartan and control groups and no significant difference in incidence of dementia between the two groups. This substudy therefore showed candesartan to be effective in treatment of ISH in elderly patients like SHEP 1991 and Syst Eur 1997, but showed no benefit in terms of protecting against cognitive decline. Again the results need to be interpreted with caution due to the number of control group patients on active treatment and short follow‐up.
A further substudy (Degl'Innocenti 2004) looked at Health Related Quality of Life (HRQL) of patients in the SCOPE trial. This showed that HRQL in SCOPE patients was generally good at baseline and well preserved during follow‐up in the presence of substantial blood pressure reductions in both groups. The conclusions were that there should be no reason to withhold modern antihypertensive therapy in elderly patients due to concerns for a negative effect on HRQL.
This update identified one further study (HYVET 2008) which fulfilled the inclusion criteria.
HYVET 2008 was a well‐conducted, international, multicentre trial which mostly fulfilled the inclusion criteria (6.7% of the active treatment group and 6.9% of the placebo group had history of stroke at baseline). The lack of definitive data advising on the treatment of hypertension in the included age group (80 years or older) probably reflects the adherence to assigned study treatment, with less than 1% of participants not taking one of the three steps of active treatment or matching placebo. The trial was stopped early, after a median follow up of 1.8 years, due to the finding of a significant reduction in the primary end‐point, the rate of any stroke, in the active treatment group at a prespecified interim analysis. A secondary end‐point, death from any cause, was also significantly lower in the active treatment group. The majority of subjects (>95%) were recruited from eastern Europe and China.
The HYVET‐COG substudy (Peters 2008) reported on the 3336 subjects from the total trial population of 3845 who attended for at least one follow‐up assessment (1 year). The 509 participants who did not meet inclusion criteria for this substudy were comparable in baseline data to those included. Prevalent dementia or cognitive decline at baseline was not reported, although MMSE scores ranged from 15 to 30. There were 263 incident cases of dementia, which was much greater than for the other included studies and reflective of the older mean age of participants in this trial compared to the other included studies. No significant difference in the rate of incident dementia or incident cognitive decline was found.
Data and analyses
Comparison 1. Incidence of dementia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of cases of dementia | 4 | 15427 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.74, 1.07] |
| 2 Number of cases of demen tia in year 1 of follow up | 3 | 9527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.24, 2.16] |
Comparison 2. Cognitive change from baseline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in MMSE | 3 | 10640 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.30, 0.53] |
Comparison 3. Blood pressure level.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in systolic blood pressure level (mmHg) | 4 | 16810 | Mean Difference (IV, Fixed, 95% CI) | ‐10.22 [‐10.78, ‐9.66] |
| 2 Change in diastolic blood pressure level (mmHg) | 4 | 16810 | Mean Difference (IV, Fixed, 95% CI) | ‐4.28 [‐4.58, ‐3.98] |
Comparison 4. Incidence and severity of adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects requiring discontinuation of treatment | 3 | 12091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.92, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
HYVET 2008.
| Methods | Multisite study. Randomization: stratified by age, sex, and centre. Participants randomly allocated through interactive voice response system setup by coordinating centre. Patients blinded; investigators blinded; outcome assessors blinded. Planned duration: 5 years. Lost to follow‐up: 6 in active treatment; 11 in control group. % not on assigned treatment at 2 years: 0.8% active treatment; 0.6% control group. | |
| Participants | Geographic region: Europe, China, Australasia, Tunisia. 195 centres in 13 countries. Study setting: community. n=3845 (60.5% female). Age: ≥80 years, mean 83.6 years. Ethnicity: not reported; 86 western Europe, 2144 eastern Europe, 1526 China, 19 Australasia, 70 Tunisia. Mean sitting blood pressure (BP) at entry: 173.0/90.8mmHg. BP entry criteria: sustained systolic BP 160‐199mmHg (1 month apart); standing systolic BP >140mmHg; sitting diastolic BP 90‐109mmHg (protocol amendment 2003 <110mmHg). | |
| Interventions | Minimum 2 month single daily placebo tablet run‐in phase with all other antihypertensive treatment stopped. Treatment: step 1 ‐ indapamide SR 1.5mg/day; step 2 ‐ indapamide SR 1.5mg/day and perindopril 2mg/day; step 3 ‐ indapamide SR 1.5mg/day and perindopril 4mg/day. Control: matching placebos. Target sitting systolic BP<150mmHg and diastolic<80mmHg (sitting systolic BP ≥150mmHg accepted if standing systolic BP <120mmHg). Average follow‐up: median 1.8 years (mean, 2.1; range, 0‐6.5). Difference in BP at end of study (treatment ‐ control) systolic/diastolic: ‐15.0/‐6.1 mmHg (sitting). | |
| Outcomes | Analysed in the review: blood pressure level; incidence of dementia; change in cognitive function (measured by MMSE); incidence of side‐effects. Not analysed in the review. Primary: fatal and non‐fatal stroke. Secondary: total mortality; cardiovascular mortality; cardiac mortality; stroke mortality; skeletal fracture rate. Other outcomes: incidence of retinal lesions; overt heart failure; renal failure; dissecting aortic aneurysm; acute myocardial infarction (MI). Cognitive function outcomes not specified in original trial protocol. |
|
| Notes | Exclusion criteria: known accelerated hypertension; overt clinical congestive heart failure, requiring treatment with diuretic or ACE inhibitor; renal failure (serum creatinine >150μmol/l); documented cerebral or subarachnoid haemorrhage in previous 6 months; condition expected to severely limit survival; known secondary hypertension; gout; clinical diagnosis of dementia; nursing home residence; contraindication to trial medication (serum potassium <3.5mmol/l or >5.5mmol/l); inability to stand or walk. Funding: British Heart Foundation and the Institut de Recherches Internationales Servier. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'patients underwent randomization, provided all inclusion and exclusion criteria were met. Randomization stratified according to age and sex; permuted blocks of 4 and 6 of any 10 patients used to ensure roughly equal assignment to each group within large centres' |
| Allocation concealment? | Low risk | 'when coordinating centre has received entry form and checked they are eligible, they inform interactive voice response system (IVRS) to permit randomization according to schedule. IVRS automatically faxes, in real‐time, centre to inform that patient is eligible and that local investigator can call system to receive number of treatment pack patient is to receive' |
| Blinding? All outcomes | Low risk | Active treatment and matching placebo allocation concealed from investigator and participant. End‐Points Committee blinded to treatment allocation. |
| Incomplete outcome data addressed? All outcomes | Low risk | 'data analyzed for the groups to which patients were assigned, regardless of which study drugs (or doses) patient actually received and regardless of other protocol irregularities. Patients from closed centres were included in intention‐to ‐treat population and contributed (data) up to date of closure' Comment: Similar numbers for each event for which data were censored between treatment and placebo groups. |
| Free of selective reporting? | Low risk | Outcomes specified in the protocols reported in the main results and cognitive substudy. |
| Free of other bias? | High risk | Study was stopped early based on the significant reduction in the primary outcome at prespecified interim analysis by independent data monitoring committee. Comment: The shorter than planned follow‐up may bias ability to detect effect of treatment on cognitive change and dementia. |
SCOPE 2003.
| Methods | Multisite study. Randomization: patients allocated by a central, computer‐generated randomization schedule in a 1:1 ratio. Patients blinded; providers blinded; outcome assessors blinded. Eight patients lost to follow‐up. % not on assigned therapy at study end: 84% control group, 75% treatment group. Duration of trial: 5 years. | |
| Participants | Geographic region: Europe, Canada, USA and Israel. Study setting: community. n = 4964. 64% female. Age range: 70‐89 years, average 76.4 years. Mean blood pressure at entry 166/90 mmHg. Blood pressure entry criteria: SBP 160‐179 mmHg or DBP 90‐99 mmHg, or both. Mini Mental State Examination score of 24 or above. | |
| Interventions | Control: matching placebo. Treatment: step 1 ‐ candesartan 8 mg daily; step 2 ‐ candesartan 16 mg daily; step 3 ‐ hydrochlorothiazide 12.5 mg daily. Other drugs, except angiotensin‐converting enzyme inhibitors and AT1‐receptor blockers, could be added later. Mean duration of trial: 44.6 months. Difference in blood pressure at study end (Treatment‐Control) systolic/diastolic: ‐3.2/‐1.6 mmHg | |
| Outcomes | Analysed in the review: blood pressure level, incidence of dementia, cognitive change measured by MMSE, incidence of side effects. Not analysed in the review: major cardiovascular events (cardiovascular deaths, non‐fatal MI, non‐fatal stroke), total mortality, fatal and non‐fatal stroke, new onset diabetes mellitus. Dropouts due to side effects (no significant difference between groups): placebo: 17%; treatment: 15%. Quality of life or functional status outcomes:Both treatment regimens were well tolerated. |
|
| Notes | Exclusions: Related to hypertension ‐ secondary hypertension, SBP > 180 mmHg, orthostatic hypotension, need for antihypertensive treatment other than hydrochlorothiazide during run‐in; stroke or MI within 6 months; decompensated heart failure; serum AST or ALT > 3 times upper limit of normal (ULN); serum creatinine >180 micromol/l (men) or >140 micromol/l (women); contraindication to study drug or hydrochlorothiazide; serious concomitant diseases affecting survival; alcoholism or drug abuse. Related to dementia: dementia; treatment with drugs for dementia; conditions which preclude MMSE; vitamin B12 deficiency treated < 12 months; hypothyroidism treated < 12 months; neurosyphilis or AIDS; severe brain disorder which may interfere with cognitive function; certain mental disorders; psychopharmacological treatment started within 6 months. Study funding: Astra‐Zeneca. 'Executive and Steering committees had full access to all data and were free to suggest analyses, interpret results and write ... independently of study sponsor'. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Patients allocated by central, computer‐generated randomization schedule, in a 1:1 ratio'. |
| Allocation concealment? | Low risk | 'Investigators sent fax with patient data for central randomization and received treatment allocation (patient study number) by return fax'. |
| Blinding? All outcomes | Low risk | 'Patients randomized in double‐blind fashion to receive (active treatment) or matching placebo tablet.' 'Suspected clinical events reported to coordinating centre. Adjudicated by Independent Clinical event Committee. All clinical events strictly and prospectively defined. Every person involved in adjudication ... blinded to ... allocation' |
| Incomplete outcome data addressed? All outcomes | Low risk | 6 in treatment group and 2 in control group lost to follow‐up at study end; included in intention‐to ‐treat analysis. 27 excluded of 4964 randomized (all 13 at 1 centre due to concerns on individual patient data; 14 ‐ no study drug dispensed). |
| Free of selective reporting? | Low risk | Outcomes specified in protocol reported. |
| Free of other bias? | High risk | 84% of placebo group ended study on antihypertensive agent (not ACE or ARB) to ensure ethical treatment of hypertension. Comment: Fewer than anticipated events and lower between group difference in BP reduced power of study to detect outcome differences. |
SHEP 1991.
| Methods | Multisite study. Double blind, placebo controlled stepped‐care treatment programme. Study duration: 5 years. Randomization: stratified by site and by antihypertensive medication status at initial contact; patients randomly allocated by coordinating centre. Patients blinded; providers blinded; morbidity and mortality assessors blinded. Type of trial: randomized, placebo controlled. % lost to follow ‐up: < 1%. % not on assigned therapy at study end: placebo group: 44%; treatment group: 10% | |
| Participants | Geographic region: United States of America. Study setting: community. n = 4736 (55.8% female). Age range 60 to > 80 years, mean 71.6 (SD 6.7). Race: White non‐Hispanic (79.2%), Black (13.8%), Hispanic (1.8%), Asian (4.3%), other (0.9%). Education level: mean 11.7 years (SD 3.5). Diagnosis: systolic hypertension. Mean blood pressure at entry: 170/77 mmHg. Blood pressure (BP) entry criteria: systolic BP 160‐219 mmHg and diastolic BP < 90 mmHg. | |
| Interventions | Control: matching placebo. Treatment: step 1 ‐ chlorthalidone 12.5 or 25 mg daily; step 2 ‐ atenolol 25 or 50 mg or reserpine 0.05 or 0.10 mg daily. Average length of follow up: 4.5 years. Difference in blood pressure at study end (Treatment ‐ Control) systolic/diastolic: ‐11.1/‐3.4 mmHg. | |
| Outcomes | Analysed in the review: incidence of dementia; quality of life; incidence of side effects including depression; blood pressure level. Not analysed in the review: total mortality; total stroke; sudden cardiac death; rapid cardiac death; non‐fatal MI; fatal MI; left ventricular failure; other cardiovascular death; transient ischaemic attack (TIA); coronary artery bypass grafting or angioplasty; renal dysfunction. Dropouts due to side effects: Control group: 7%; Treatment group: 13%. Quality of life or functional outcomes: no perceptible negative effect of treatment compared to control on measures of cognitive, physical and emotional function. |
|
| Notes | Exclusions: history and/or signs of major cardiovascular diseases likely to require pharmacologic and other treatment (e.g., previous MI, coronary artery surgery, major arrhythmias, conduction defect, recent stroke, carotid artery disease, history of TIA with bruit matched with TIA localisation, two or more TIAs and signs or symptoms in a single neurological distribution); other major diseases (e.g. cancer, alcoholic liver disease, established renal dysfunction) with competing risk factors for the primary endpoint ‐ stroke; presence of medical management problems (e.g. insulin dependent diabetes, history of dementia, evidence of alcohol abuse); bradycardia; people maintained on beta‐blockers, diuretics, other antihypertensive drugs, anticoagulants, or experimental drugs on recommendation of their physicians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'After verification of eligibility ... randomly allocated by coordinating centre to one of two treatment groups. Randomization stratified by centre and antihypertensive medication use at initial contact.' Comment: Probably adequate |
| Allocation concealment? | Low risk | 'Random assignment ... made by coordinating centre and transmitted to local centre by telephone after verification of eligibility.' Comment: probably adequately concealed. |
| Blinding? All outcomes | Low risk | 'Participants randomized in a double‐blind manner to once‐daily dose of active drug treatment or matching placebo.' Data on study end points collected by investigators and confirmed by a panel of three physicians blind to allocation. |
| Incomplete outcome data addressed? All outcomes | Low risk | 'All analyses were by treatment assignment at randomization.' Comment: Subsequent publication (Di Bari 2001) addressed effect of differential drop‐out between treatment and control groups. |
| Free of selective reporting? | Low risk | Prespecified outcomes reported. |
| Free of other bias? | High risk | About 35% of placebo group took antihypertensive medication during study for predefined BP levels. |
Syst Eur 1997.
| Methods | Multisite study. Randomization: stratified by centre, sex and previous cardiovascular complications. Patients randomly allocated to placebo or active treatment by a centralised system or local computer system. Patients blinded; providers blinded; outcome assessors blinded. Study duration: 5 years. Lost to follow‐up: 2% at 2 years. % not on assigned therapy at study end (2 years) including open follow‐up and lost to follow‐up: placebo group: 27%; treatment group: 18% | |
| Participants | Geographic region: 23 countries across western and eastern Europe, mainly from Finland, Bulgaria, the Russian Federation, Belgium, Italy, Israel, UK, France, Estonia, Lithuania, Spain, Poland and Romania. Study setting: community based and referral clinic. n = 4695 (66.8% female) Age range: ≥60 years; mean 70.3 years. Race: not reported. Mean BP at entry: 174/86 mmHg. BP entry criteria: sitting systolic BP 160‐219 mmHg and sitting diastolic BP< 95 mmHg. Standing systolic BP at least 140 mmHg. | |
| Interventions | Treatment: Step 1 ‐ nitrendipine 10‐40 mg/day; Step 2 ‐ enalapril 5‐20 mg/day and/or hydrochlorothiazide 12.5‐25 mg/day. Control: matching placebos with stepped therapy schedule similar to treatment groups. Mean daily doses: nitrendipine 28.2 mg; enalapril 13.5 mg; hydrochlorothiazide 21.2 mg. Average follow‐up: 2 years (median). Difference in blood pressure at end of study (Treatment ‐ Control) systolic/diastolic: ‐10.1/‐4.5 mmHg at 2 years. | |
| Outcomes | Analysed in the review: Blood pressure level. Not analysed in the review: Total mortality; coronary heart disease (CHD) mortality; CHD morbidity and mortality (M&M); cerebrovascular mortality; cerebrovascular M&M; cardiovascular mortality; cardiovascular M&M. Dropouts due to side effects (no significant difference between groups): placebo: < 7.3%; treatment: < 7.8%. Quality of life or functional status outcomes: Across 4 years of follow‐up, patients receiving active treatment were more likely to report problems on the Social Interaction scale than placebo treated patients (OR 1.32, 95% CI 1.02, 1.69). There were no significant differences between active and placebo treatment in the other Sickness Impact Profile dimensions or in the measure of depression. |
|
| Notes | Exclusions: hypertension secondary to a disorder that needed specific medical or surgical treatment; retinal haemorrhage or papilloedema; congestive heart failure; dissecting aortic aneurysm; serum creatinine concentration at presentation of 180 micromols/l or more; history of severe nose bleeds, stroke or MI in the year before the study; dementia; substance abuse; any disorder prohibiting a sitting or standing position; any severe concomitant or non‐cardiovascular disease. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'After stratification by centre, sex, and previous cardiovascular complications, ... randomly assigned eligible patients ... by means of a computerised random function.' |
| Allocation concealment? | Low risk | 'Randomization by coordinating centre of patients satisfying all entry criteria and in whom none of the exclusion criteria are present' |
| Blinding? All outcomes | Low risk | 'Placebo tablets were identical to study drugs, with similar schedule'. 'Endpoint committee was unaware of patients treatment status' |
| Incomplete outcome data addressed? All outcomes | Low risk | Open follow‐up of patients who withdrew from treatment; if this was not possible, data collected annually on vital status, endpoints and antihypertensive medication use. Comment: Similar numbers of non‐supervised open and lost‐to follow‐ups in each group; higher number of supervised open follow‐ups in placebo group. |
| Free of selective reporting? | Low risk | Prespecified outcomes reported |
| Free of other bias? | High risk | Study stopped early after second prespecified interim analysis found significant decrease in primary outcome (stroke) in active treatment group. 144 subjects in placebo group and 72 subjects in active treatment group taking open‐label antihypertensives at final visit. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ALLHAT 2003 | No data on cognition. Not placebo controlled. |
| ASCOT 2005 | No data on cognition. Not placebo controlled. |
| Black 2001 | No data on cognition |
| Framingham 1993 | Prospective study. Not double blind, randomised, placebo controlled |
| Hofman 1997 | Prospective population based follow‐up study |
| HOT 1998 | Aspirin versus placebo in patients treated with felodipine as baseline therapy |
| Hsieh 2003 | No data on cognition |
| Kilander 1998 | Prospective population‐based study |
| Lasser 1989 | Age of participants 25‐55 years, mean age 51.3 years, not at risk of dementia. |
| Launer 1995 | Prospective population based study |
| LOMIR‐MCT‐IL | No data on cognition |
| MRC 1996 | Single blind study |
| Murray 2002 | Longitudinal analysis |
| PROGRESS 2003 | Patients had prior stroke or transient ischaemic attack |
| Richards 2000 | Community based survey |
| Skoog 1996 | Longitudinal population study |
| Starr 1996 | Not placebo controlled. Patients received either captopril or bendrofluazide |
| STOP 1991 | No end points as specified for this review |
| Syst China 1998 | Not double blind or adequately randomised |
| VALUE 2004 | No data on cognition |
Contributions of authors
BMcG: drafting of versions, search for trials, obtaining hard copy of trials, selection of trials for inclusion/exclusion, extraction of data, entry of data, interpretation of data‐analysis
ST: drafting of versions, search for trials, obtaining hard copy of trials, selection of trials for inclusion/exclusion, extraction of data, entry of data, interpretation of data analysis
PP: adjudicator and reviewer of process
RB: adjudicator and reviewer of process
Contact editors: Richard Harvey of CDCIG, Jim Wright and Francois Gueyffier of Cochrane Hypertension Group Consumer editor: Gerard McVeigh
For this update:
ST: drafting of versions, search for trials, obtaining hard copy of trials, selection of trials for inclusion/exclusion, extraction of data, entry of data, interpretation of data analysis
BMG: drafting of versions, search for trials, obtaining hard copy of trials, selection of trials for inclusion/exclusion, extraction of data, interpretation of data analysis
PP: adjudicator and reviewer of process
RB: adjudicator and reviewer of process
Contact editor: Leon Flicker
Sources of support
Internal sources
-
No source of support received for the completion of this update , Not specified.
None
External sources
-
No source of support was received for the completion of this update , Not specified.
None
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
HYVET 2008 {published and unpublished data}
- Bulpitt C, Fletcher A, Beckett N, Coope J, Gil‐Extremera B, Fore tte F, et al. Hypertension in the Very Elderly Trial (HYVET): protocol for the main trial . Drugs & Aging 2001 ; 18 :151‐164. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Nunes M, Fletcher A, Forette F, Bulpitt C. A substudy protocol of the Hypertension in the Very Elderly Trial assessing cognitive decline and dementia incidence (HYVET‐COG): an ongoing randomised, double‐blind, placebo‐controlled trial . Drugs Aging 2006 ; 23 :83‐92. [DOI] [PubMed] [Google Scholar]
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumiterscu D, et al. for the HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. NEJM 2008;358:1887‐1898. [DOI] [PubMed] [Google Scholar]
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. for the HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurology 2008;7:683‐689. [DOI] [PubMed] [Google Scholar]
SCOPE 2003 {published data only}
- Degl'Innocenti A, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A, Wiklund I. Health‐related quality of life during treatment of elderly patients with hypertension: results from the Study on COgnition and Prognosis in the Elderly (SCOPE). Journal of Human Hypertension 2004;18(4):239‐45. [DOI] [PubMed] [Google Scholar]
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A for the SCOPE Study Group. The Study of COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add‐on therapy after randomization. Journal of Hypertension 2004;22(8):1605‐12. [DOI] [PubMed] [Google Scholar]
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A for the SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomised double‐blind intervention trial. Journal of Hypertension 2003;21:875‐886. [DOI] [PubMed] [Google Scholar]
- Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A for the SCOPE Study Group. Stroke Prevention With the Angiotension II Type‐1 Receptor Blocker Candesartan in Elderly Patients With Isolated Systolic Hypertension. The Study on Cognition and Prognosis in the Elderly (SCOPE). Journal of the American College of Cardiology 2004;44(6):1175‐80. [DOI] [PubMed] [Google Scholar]
- Skoog I, Lithell H, Hansson L, Elmfeldt D, Hofman A, Olofsson B, et al. for the SCOPE Study Group. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). American Journal of Hypertension 2005; Vol. 18:1052‐1059. [DOI] [PubMed]
SHEP 1991 {published data only}
- Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, et al. Impact of the Treatment of Isolated Systolic Hypertension on Behavioral Variables. Results From the Systolic Hypertension in the Elderly Program. Arch Intern Med 1994;154:2154‐2160. [PubMed] [Google Scholar]
- Bari M, Pahor M, Franse LV, Shorr RI, Wan JY, Ferrucci L, et al. Dementia and Disability Outcomes in Large Hypertension Trials: Lessons Learned from the Systolic Hypertension in the Elderly Program (SHEP) Trial. American Journal of Epidemiology 2001;153:72‐8. [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group. Prevention of Stroke by Antihypertensive Drug Treatment in Older Persons With Isolated Systolic Hypertension. Final Results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA 1991;265:3255‐64. [PubMed] [Google Scholar]
- The Systolic Hypertension in the Elderly Program (SHEP) Cooperative Research Group. Rationale and Design of a Randomized Clinical Trial on Prevention of Stroke in Isolated Systolic Hypertension. Journal of Clinical Epidemiology 1988;41:1197‐1208. [DOI] [PubMed] [Google Scholar]
Syst Eur 1997 {published and unpublished data}
- Amery A, Birkenhager W, Bulpitt CJ, Clement D, Leeuw P, Dollery CT, et al. Syst‐Eur. A multicentre trial on the treatment of isolated systolic hypertension in the elderly: Objectives, protocol, and organization. Aging Clin Exp Res 1991;3:287‐302. [DOI] [PubMed] [Google Scholar]
- Fletcher AE, Bulpitt CJ, Thijs L, Toumilehto J, Antikainen R, Bossini A, et al. Quality of life on randomised treatment for isolated hypertension: results from the Syst‐Eur Trial. Journal of Hypertension 2002;20:2069‐2079. [DOI] [PubMed] [Google Scholar]
- Fletcher AE, Bulpitt CJ, Tuomilehto J, Browne J, Bossini A, Kawecka‐Jaszcz K, et al. Quality of life of elderly patients with isolated systolic hypertension: baseline data from the Syst‐Eur Trial. Journal of Hypertension 1988;16:1117‐1124. [DOI] [PubMed] [Google Scholar]
- Forette F, Seux M‐L, Staessen JA, Thijs L, Babarskiene M‐R, Babeanu S, et al. The Prevention of Dementia With Antihypertensive Treatment. New Evidence From the Systolic Hypertension in Europe (Syst‐Eur) Study. Archives of Internal Medicine 2002;162:2046‐2052. [DOI] [PubMed] [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH. Reduction of dementia by calcium‐antagonist‐based antihypertensive treatment. European Heart Journal Supplements 2000;2:D17‐D19. [Google Scholar]
- Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, et al. Prevention of dementia in randomised double‐blind placebo‐controlled Systolic Hypertension in Europe (Syst‐Eur) Trial. Lancet 1998;352:1347‐51. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Lancet 1997;350:757‐764. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ALLHAT 2003 {published data only}
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Diuretic Versus Alpha‐Blocker as First‐Step Antihypertensive Therapy. Final Results From the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension 2003;42:239‐246. [DOI] [PubMed] [Google Scholar]
ASCOT 2005 {published data only}
- Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomized controlled trial. Lancet 2005;366:895‐906. [DOI] [PubMed] [Google Scholar]
Black 2001 {published data only}
- Black HR, Elliott WJ, Weber MA, Frishman WH, Strom JA, Liebson PR, et al. One‐Year Study of Felodipine or Placebo for Stage 1 Isolated Systolic Hypertension. Hypertension 2001;38:1118‐1130. [DOI] [PubMed] [Google Scholar]
Framingham 1993 {published data only}
- Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated Blood Pressure Level is Inversely Related to Cognitive Functioning: The Framingham Study. American Journal of Epidemiology 1993;138:353‐364. [DOI] [PubMed] [Google Scholar]
- Farmer ME, White LR, Abbott RD, Kittner SJ, Kaplan E, Wolz MM, et al. Blood Pressure and Cognitive Performance. The Framingham Study. American Journal of Epidemiology 1987;126:1103‐1114. [DOI] [PubMed] [Google Scholar]
Hofman 1997 {published data only}
- Hofman A, Ott A, Breteler MMB, Bots ML, Slooter AJC, Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. The Lancet 1997;349:151‐54. [DOI] [PubMed] [Google Scholar]
HOT 1998 {published data only}
- Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. The Lancet 1998;351:1755‐62. [DOI] [PubMed] [Google Scholar]
Hsieh 2003 {published data only}
- Hsieh M‐H, Chan P, Sue Y‐M, Liu J‐C, Liang TH, Huang T‐Y, et al. Efficacy and Tolerability of Oral Stevioside in Patients with Mild Essential Hypertension: A Two‐Year, Randomised, Placebo‐Controlled Study. Clinical Therapeutics 2003;25:2797‐2808. [DOI] [PubMed] [Google Scholar]
Kilander 1998 {published data only}
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is Related to Cognitive Impairment. A 20‐Year Follow‐up of 999 men. Hypertension 1998;31:780‐786. [DOI] [PubMed] [Google Scholar]
Lasser 1989 {published data only}
- Lasser NL, Nash J, Lasser VI, Hamill SJ, Batey DM. Effects of antihypertensive therapy on blood pressure control, cognition and reactivity. A placebo‐controlled comparison of prazosin, propranolol, and hydrochlorothiazide. Am J Med 1989;86:98‐103. [DOI] [PubMed] [Google Scholar]
Launer 1995 {published data only}
- Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The Association Between Midlife Blood Pressure Levels and Late‐Life Cognitive Function. The Honolulu‐Asia Aging Study. JAMA 1995;274:1846‐1851. [PubMed] [Google Scholar]
- Syme SL, Marmot MG, Kagan A, Kato H, Rhoads G. Epidemiologic Studies of Coronary Heart Disease and Stroke in Japanese Men Living in Japan, Hawaii and California: Introduction. American Journal of Epidemiology 1975;102:477‐480. [DOI] [PubMed] [Google Scholar]
LOMIR‐MCT‐IL {published data only}
- Amir M, Cristal N, Bar‐On D, Loidl A. Does the Combination of ACE Inhibitor and Calcium Antagonist Control Hypertension and Improve Quality of Life? The LOMIR‐MCT‐IL Study Experience. Blood Pressure 1994;3(Suppl 1):40‐42. [PubMed] [Google Scholar]
- Bar‐On D, Amir M. Reexamining the Quality of Life of Hypertensive Patients. A New Self‐Structured Measure. American Journal of Hypertension 1993;6:62S‐66S. [DOI] [PubMed] [Google Scholar]
- Yodfat Y, Cristal N. A Multicenter, Double‐Blind, Randomized, Placebo‐Controlled Study of Isradipine and Methyldopa as Monotherapy or in Combination With Captopril in the Treatment of Hypertension. American Journal of Hypertension 1993;6:57S‐61S. [DOI] [PubMed] [Google Scholar]
MRC 1996 {published data only}
- MRC W orking Party. Medical Research Council trial of treatment of hypertension in older adults: principal results . BMJ 1992 ; 304 :405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart S. Results of MRC (UK) trial of drug therapy for mild hypertension. Clin Invest Med 1987;10:616‐20. [PubMed] [Google Scholar]
- Prince M, Lewis G, Bird A, Blizard R, Mann A. A longitudinal study of factors predicting change in cognitive test scores over time, in an older hypertensive population. Psychological Medicine 1996;26:555‐568. [DOI] [PubMed] [Google Scholar]
- Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council's treatment trial of hypertension in older adults. BMJ 1996;312:801‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2002 {published data only}
- Murray MD, Lane KA, Sujuan G, Evans RM, Unverzagt FM, Hall KS, Hendrie H. Preservation of Cognitive Function With Antihypertensive Medications: A Longitudinal Analysis of a Community‐Based Sample of African Americans Preservation of Cognitive Function With Antihypertensive Medications: A Longitudinal Analysis of a Community‐Based Sample of African Americans. Archives of Internal Medicine 2002;162(18):2090‐2096. [DOI] [PubMed] [Google Scholar]
PROGRESS 2003 {published data only}
- The PROGRESS Collaborative Group. Effects of Blood Pressure Lowering With Perindopril and Indapamide Therapy on Dementia and Cognitive Decline in Patients With Cerebrovascular Disease. Arch Intern Med 2003;163:1069‐1075. [DOI] [PubMed] [Google Scholar]
Richards 2000 {published data only}
- Richards SS, Emsley CL, Roberts J, Murray MD, Hall K, Sujuan G, Hendrie HC. The Association Between Vascular Risk Factor‐Mediating Medications and Cognition and Dementia Diagnosis in a Community‐Based Sample of African‐Americans. Journal of the American Geriatrics Society 2000;48:1035‐1041. [DOI] [PubMed] [Google Scholar]
Skoog 1996 {published data only}
- Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15‐year longitudinal study of blood pressure and dementia. The Lancet 1996;347:1141‐45. [DOI] [PubMed] [Google Scholar]
Starr 1996 {published data only}
- Starr JM, Whalley LJ, Deary IJ. The Effects of Antihypertensive Treatment on Cognitive Function: Results from the HOPE Study. Journal of the American Geriatric Society 1996;44:411‐415. [DOI] [PubMed] [Google Scholar]
STOP 1991 {published data only}
- Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester P‐O. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP Hypertension). Lancet 1991;338:1281‐85. [DOI] [PubMed] [Google Scholar]
Syst China 1998 {published data only}
- Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Journal of Hypertension 1998;16:1823‐1829. [DOI] [PubMed] [Google Scholar]
VALUE 2004 {published data only}
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022‐31. [DOI] [PubMed] [Google Scholar]
Additional references
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington DC: APA, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders . 4th Edition. Washington DC : APA , 1994 . [Google Scholar]
Birns 2006
- Birns J, Morris R, Donaldson N, Kalra L. The effects of blood pressure reduction on cognitive function: a review of effects based on pooled data from clinic al trials . Journal of Hypertension 2006 ; 24 :1907‐1914. [DOI] [PubMed] [Google Scholar]
Brayne 1998
- Brayne C, Gill C, Huppert FA, et al. Vascular risk s and incident dementia :results from a cohort study of the very old . Dementia and Geriatric Cognitive Disorders 1998 ; 9 :175‐180. [DOI] [PubMed] [Google Scholar]
Chalmers 1983
- Chalmers TC, Celano P, Sacks HS, Smith H Junior. Bias in Treatment Assignment in Controlled Clinical Trials. N Eng J Med 1983;309:1358‐61. [DOI] [PubMed] [Google Scholar]
Degl'Innocenti 2004
- Degl'Innocenti A, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A, Wiklund I. Health‐related quality of life during treatment of elderly patients with hypertension: results from the Study on COgnition and Prognosis in the Elderly (SCOPE). Journal of Human Hypertension 2004;18(4):239‐45. [DOI] [PubMed] [Google Scholar]
Di Bari 2001
- Bari M, Pahor M, Franse LV, Shorr RI, Wan JY, Ferrucci L, Somes GW, Applegate WB. Dementia and disability outcomes in large hypertension trials: lessons learned from the systolic hypertension in the elderly program (SHEP) trial. American Journal of Epidemiology 2001;153(1):72‐8. [DOI] [PubMed] [Google Scholar]
Elias 1993
- Elias MF, Wolf PA, Agostino RBD, Cobb J, White LR. Untreated blood pressure is inversely related to cognitive functioning: the Framingham Study. Journal of Epidemiology 1993;138(6):352‐64. [DOI] [PubMed] [Google Scholar]
Elias 2004
- Elias PK, Elias M F, Robbins MA, Budge MM. Blood pressure related cognitive decline: does age make a difference? . Hypertension 2004 ; 44 :631‐636. [DOI] [PubMed] [Google Scholar]
Feigin 2005
- Feigin V, Ratnasabapathy Y, Anderson C. Does blood pressure lowering treatment prevent deme ntia or cognitive decline in patients with cardiovascular and cerebrovascular disease? . Journal of the N eurological Sciences 2005 ; 229 :151‐155. [DOI] [PubMed] [Google Scholar]
Fogari 2004
- Fogari R, Mu gellini A, Zoppi A, et al. Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension . European Journal of Clinical Pharmacology 2004 ; 59 :863‐868. [DOI] [PubMed] [Google Scholar]
Forette 2002
- Forette F, Seux M‐L, Staessen JA, Thijs L, Babarskiene M‐R, Babeanu S, et al. The Prevention of Dementia With Antihypertensive Treatment. New Evidence From the Systolic Hypertension in Europe (Syst‐Eur) Study. Archives of Internal Medicine 2002;162:2046‐2052. [DOI] [PubMed] [Google Scholar]
Guo 1996
- Guo Z, Viitan en M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people : the Kungsholmen project . BMJ 1996 ; 312 :805‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 1999
- Guo Z, Fratiglioni L, Zhu L, Fastborn J, Wi nblad B, Viitanen M. Occurence and progression of dementia in a community population aged 75 years and older: relationship of antihypertensive medication use . Archives of Neurology 1999 ; 56 :991‐996. [DOI] [PubMed] [Google Scholar]
Hebert 2004
- Hebert LE, Scherr PA, Bennet DA, et al. Blood pressure and late‐life cognitive function change: a biracial longitudinal population study . Neurology 2004 ; 62 :2021‐202 4. [DOI] [PubMed] [Google Scholar]
in't Veld 2001
- in't Veld BA, Ruitenberg A, Hofman A, Stricker BHC, Breteler MMB. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiology of Aging 2001;22:407‐412. [DOI] [PubMed] [Google Scholar]
Kennelly 2009
- Kennelly SP, Lawlor BA, Kenny RA. Blood pressure and the risk for dementia ‐ a d ouble edged sword . Ageing Research Reviews 2009 ; 8 :61‐70. [DOI] [PubMed] [Google Scholar]
Khachaturian 2006
- Khachaturian AS, Zandi PP, Lyketsos CG, Hayden KM, Skoog I, Norton MC, Tschanz, Mayer LS, Welsh‐Bohmer KA, Breitner JCS. Antihypertensive medic ation use and incident Alzheimer disease: the Cache County Study . Archives of Neurology 2006 ; 63 :686‐692. [DOI] [PubMed] [Google Scholar]
Kilander 2000
- Kilander L, Nyman H, Boberg M, Lithell H. The association b etween low diastolic blood pressure in middle age and cognitive function in old age: a population based study . Age Ageing 2000 ; 29 :243‐248. [DOI] [PubMed] [Google Scholar]
Kivipelto 2001
- Kivipelto M, Helkala E‐L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissien A. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study . BMJ 2001 ; 322 :1447‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Launer 2000
- Launer LJ, Ross GW, Petrovich H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu‐Asia aging study . N eurobiology of Aging 2000 ; 21 :49‐55. [DOI] [PubMed] [Google Scholar]
Lindsay 2002
- Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospe ctive analysis from the Canadian Study of Health and Aging . American Journal of Epidemiology 2002 ; 156 :445‐453. [DOI] [PubMed] [Google Scholar]
Lithell 2004
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P and Zanchetti A for the SCOPE Study Group. The Study of COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add‐on therapy after randomization. Journal of Hypertension 2004;22(8):1605‐12. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;4:939‐44. [DOI] [PubMed] [Google Scholar]
Morris 2001
- Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study . Archives of Neurology 2001 ; 58 :1640‐1646. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD (editors). Cochrane Collaboration Handbook, The Cochrane Library [database on disc and CDROM ]. Vol. Issue 1, Oxford: Update Software, 1997. [Google Scholar]
Papademetriou 2004
- Papademetriou V, Farsang C, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I, Trenkwalder P, Zanchetti A for the SCOPE Study Group. Stroke Prevention With the Angiotension II Type‐1 Receptor Blocker Candesartan in Elderly Patients With Isolated Systolic Hypertension. The Study on Cognition and Prognosis in the Elderly (SCOPE). Journal of the American College of Cardiology 2004;44(6):1175‐80. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. for the HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurology 2008;7:683‐689. [DOI] [PubMed] [Google Scholar]
Prince 1996
- Prince M, Lewis G, Bird A, Blizard R, Mann A. A longitudinal study of factors predicting change in cognitive test scores over time, in an older hypertensive population. Psychological Medicine 1996;26(3):555‐68. [DOI] [PubMed] [Google Scholar]
Prince 1996a
- Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council's treatment trial of hypertension in older adults. BMJ 1996;312:801‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qiu 2003
- Qiu C, Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project : a 6‐year follow‐up study . Archives of Neurology 2003 ; 60 :223‐228. [DOI] [PubMed] [Google Scholar]
Qiu 2003a
- Qiu C, Winblad B, Fastbom J, Fratiglioni L. Combined effects of APOE genotype, blood pressure and antihypertensive drug use on incident AD . Neurology 2003 ; 61 :655‐660. [DOI] [PubMed] [Google Scholar]
Qiu 2004
- Qiu C, Strauss E, W inblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: a longitudi nal study from the Kungsholmen Project . Stroke 2004 ;35 :1810‐1815. [DOI] [PubMed] [Google Scholar]
Qui 2005
- Qui C, Winblad B, Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology 2005;4:487‐99. [DOI] [PubMed] [Google Scholar]
Ratnasabapathy 2003
- Ratnasabapathy Y, Chi‐Lun Lee A, Feigin V, Anderson C. Blood pressure lowering interventions for preventing dementia in patients with cerebrovascular disease (Protocol). Cochrane Database of Systematic Reviews 2003, Issue 2 Art. No.: CD004130.. [DOI: 10.1002/14651858] [DOI] [Google Scholar]
Ruitenberg 2001
- Ruitenberg A, Skoog I, Ott A, et al. Blood pressure and risk of dementia: results from the Rotterdam Study and the Gothenburg H‐70 S tudy . Dementia and Geriatric Cognitive Disorders 2001 ; 12 :33‐39. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Starr 1992
- Starr JM, Whalley LJ. Senile hypertension and cognitive impairment: an overview. Journal of Hypertension 1992;10(2):S31‐42. [PubMed] [Google Scholar]
Swan 1998
- Swan GE, DeCarli C, Miller BL, et al. Associa tion of midlife blood pressure to late‐life cognitive decline and brain morphology . Neurology 1998 ; 51 :986‐993. [DOI] [PubMed] [Google Scholar]
Tervo 2004
- Tervo S, Kivipelto M, Hänninen T, et al. Incidence and risk factors for mild cognitive impairment: a population based three‐year follow‐up study of cognitively healthy elderly subjects . Dementia and Geriatric Cognitive Dis orders 2004 ; 17 :196‐203. [DOI] [PubMed] [Google Scholar]
Verghese 2003
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Matz MJ. Low blood pressure and the risk of dementia in very old individuals . Neurology 2003 ; 61 :1667‐1672. [DOI] [PubMed] [Google Scholar]
Wang 2003
- Wang JG, Staessen JA, Birkenhä ger, WH. Antihypertensive treatment and prevention of stroke and dementia . Seminars in Cerebrovascular Diseases and Stroke 2003 ; 3 :155‐164. [Google Scholar]
Whitmer 2005
- Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life . Neurology 2005 ; 64 :277‐281. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organisation. The ICD‐10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. Geneva: WHO, 1992. [Google Scholar]
Wu 2003
- Wu C, Zhou D, Wen C, Zhang I, Como P, Qiao Y. Relationship between blood pressure and Alzheimer's disease in Linxian county . Life Science 2003 ; 72 :1125‐1133. [DOI] [PubMed] [Google Scholar]
Yamada 2003
- Yamada M, Kasagi F, Sasaki H, Maunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the radiation effects research foundation adult health study . Journal of the American Geriatrics Society 2003 ; 51 :410‐414. [DOI] [PubMed] [Google Scholar]
Yasar 2005
- Yasar S, Cor rada M, Brookmeyer R, Kawas C. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging . Neurobiology of Aging 2005 ; 26 :157‐163. [DOI] [PubMed] [Google Scholar]
Yoshitake 1995
- Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese study: the Hisayama Study . Neurology 1995 ; 45 :1161‐1168. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
McGuinness 2006
- McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database of Systematic Reviews 2006, Issue 2. [CD004034. DOI: 10.1002/14651858.CD004034.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


