Abstract
Background
Radiotherapy has been proposed as a treatment to prevent new vessel growth in people with neovascular age‐related macular degeneration (AMD).
Objectives
The aim of this review was to examine the effects of radiotherapy on neovascular AMD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) in The Cochrane Library Issue 3, 2010, MEDLINE (January 1950 to March 2010), EMBASE (January 1980 to March 2010), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2010), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (March 2010) and ClinicalTrials.gov (http://clinicaltrials.gov) (March 2010). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 23 March 2010. We also wrote to investigators of trials included in the review to ask if they were aware of any other studies.
Selection criteria
We included all randomised controlled trials in which radiotherapy was compared to another treatment, sham treatment, low dosage irradiation or no treatment in people with choroidal neovascularisation secondary to AMD.
Data collection and analysis
Two review authors independently extracted the data. We combined relative risks using a random‐effects model. We estimated the percentage of the variability in effect estimates that was due to heterogeneity, rather than sampling error, using I2.
Main results
Thirteen trials (n=1154) investigated external beam radiotherapy with dosages ranging from 7.5 to 24 Gy; one additional trial (n=88) used plaque brachytherapy (15Gy at 1.75mm for 54 minutes/12.6 Gy at 4mm for 11 minutes). Most studies found effects (not always significant) that favoured treatment. Overall there was a small statistically significant reduction in risk of visual acuity loss in the treatment group. There was considerable inconsistency between trials and the trials were considered to be at risk of bias, in particular because of the lack of masking of treatment group. Subgroup analyses did not reveal any significant interactions, however, there were small numbers of trials in each subgroup (range three to five). There was some indication that trials with no sham irradiation in the control group reported a greater effect of treatment. The incidence of adverse events was low in all trials; there were no reported cases of radiation retinopathy, optic neuropathy or malignancy. Three trials found non‐significant higher rates of cataract progression in the treatment group.
Authors' conclusions
This review currently does not provide convincing evidence that radiotherapy is an effective treatment for neovascular AMD. If further trials are to be considered to evaluate radiotherapy in AMD then adequate masking of the control group must be considered.
Plain language summary
Radiotherapy for neovascular age‐related macular degeneration
Radiotherapy (as commonly used in the treatment of cancer) has been proposed as a treatment for wet AMD as it may prevent the growth of new vessels in the retina. This review identified 14 randomised controlled trials of radiotherapy for wet AMD. Most of these trials showed effects (not always significant) that favoured treatment with radiotherapy to prevent vision loss. However, overall this review does not provide convincing evidence that radiotherapy is an effective treatment for wet AMD, in part because the results of different trials were inconsistent, but also because it is possible that the treatment effects could be explained by the fact that it was not possible to mask the participants, and people measuring outcome, to the treatment group. The incidence of adverse effects reported in these trials was low ‐ nobody developed any radiation‐specific side effects although in three trials higher rates of cataract were reported in the radiotherapy group.
Summary of findings
Summary of findings for the main comparison. Radiotherapy versus control for neovascular age‐related macular degeneration.
| Radiotherapy versus control for neovascular age‐related macular degeneration | ||||||
| Patient or population: patients with neovascular age‐related macular degeneration Settings: Intervention: RADIATION THERAPY VERSUS CONTROL | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | RADIATION THERAPY VERSUS CONTROL | |||||
| Three or more lines visual acuity lost Follow‐up: 12 months | Medium risk population1 | RR 0.90 (0.74 to 1.1) | 759 (8 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 544 per 1000 | 490 per 1000 (403 to 598) | |||||
| Three or more lines visual acuity lost Follow‐up: 24 months | Medium risk population1 | RR 0.81 (0.63 to 1.03) | 428 (4 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 757 per 1000 | 613 per 1000 (477 to 780) | |||||
| Six or more lines visual acuity lost Follow‐up: 12 months | Medium risk population1 | RR 0.62 (0.44 to 0.87) | 576 (7 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 342 per 1000 | 212 per 1000 (150 to 298) | |||||
| Six or more lines visual acuity lost Follow‐up: 24 months | Medium risk population1 | RR 0.81 (0.64 to 1.03) | 428 (4 studies) | ⊕⊕⊕⊝ moderate3 | ||
| 444 per 1000 | 360 per 1000 (284 to 457) | |||||
| difference in visual acuity logMAR acuity. Scale from: ‐0.2 to 2. Follow‐up: 12 months | The mean difference in visual acuity in the intervention groups was 0.08 lower (0.14 to 0.01 lower) | 799 (8 studies) | ⊕⊕⊝⊝ low6,7 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Median control group risk in included studies 2 Serious limitations in design: only 3 of 8 trials adequately reported sequence generation and allocation concealment; in only 3 of 8 trials were participants and outcome assessors properly masked to treatment group; in none of the trials was incomplete outcome data properly assessed. 3 Serious limitations in design: 2 of the 4 trials adequately reported sequence generation and allocation concealment; in only 1 of the 4 trials were participants and outcome assessors properly masked to treatment group; in 1 of the 4 trials incomplete outcome data was properly assessed. 4 Serious inconsistency: chi‐sq for heterogeneity=0.04, I2=63%. Risk ratios ranged from 0.58 to 1.03. The confidence intervals for the trials showing most extreme effects overlapped to only a small extent. Too few trials to explore this heterogeneity. 5 Serious limitations in design: only 2 of 7 trials adequately reported sequence generation and allocation concealment; in only 2 of 7 trials were participants and outcome assessors properly masked to treatment group; in none of the trials was incomplete outcome data properly assessed. 6 Serious limitations in design: only 4 of 9 trials adequately reported sequence generation and allocation concealment; in only 4 of 9 trials were participants and outcome assessors properly masked to treatment group; in none of the trials was incomplete outcome data properly assessed. 7 Selective outcome bias a possibility for these analyses as only some trials reported mean final visual acuity and only some trials reported mean change in visual acuity since baseline.
Background
Description of the condition
The macula, the central area of the retina, is used for detailed vision such as reading, recognising faces and driving. Age‐related macular degeneration (AMD) is the leading cause of blindness in the developed world. It is difficult to get a clear definition of AMD. The term 'age‐related' is used partly due to its unknown pathogenesis. It is believed that both genetic and environmental factors play a significant role in the development of the disease. From a clinical perspective, AMD primarily affects the macular region. The term 'degeneration' is used to distinguish AMD from other genetic macular dystrophies which run in families and those where there is a clear environmental cause such as an infection or trauma.
There are several signs appearing in the retina that are associated with increasing age and increased risk of developing AMD. These signs, known as age‐related maculopathy (ARM), include the presence of drusen (yellow spots beneath the retina), pigmentary disturbance and small focal areas of atrophy. In general, ARM is not associated with significant visual loss. Some people with ARM will go on to develop AMD.
There are two types of AMD: geographic atrophy (large area of atrophy centred in the macula) and choroidal neovascularisation (CNV) also known as wet AMD. This review is concerned with treatment for neovascular AMD.
In neovascular AMD, CNV develops beneath the retina. In the initial phase the CNV might cause visual distortion due to leakage of fluid into the surrounding retina. At this stage the retinal function is only mildly affected and the CNV is potentially reversible. However, the CNV may leak serum lipid and protein leading to exudation and significant swelling of the retina. The CNV may bleed and the haemorrhages may be toxic. Both exudation and haemorrhages induce a scarring response. These are associated with extensive damage to the architecture of the retina‐retinal pigment epithelium‐choroid complex, leading to significant visual loss.
Choroidal neovascularisation is defined as classic or occult according to its appearance on fluorescein angiography, where fluorescent dye is injected intravenously and imaged as it passes through the blood vessels of the eye. Classic membranes are clearly delineated and can be seen at the early frames of the angiogram. Occult membranes present as either late leakage, which cannot be seen in the early frames, or fibrovascular pigment epithelial detachment. Most lesions have both classic and occult components.
Description of the intervention
Radiotherapy is commonly used in oncology and its use is increasing in the treatment of non‐neoplastic diseases. It is believed that it can preferentially damage dividing and fast growing cells more than normal supporting cells. In rats, photoreceptor cell death is not seen at doses less than 10 Gy and the retinal pigment epithelial cell loss does not occur under 20 Gy in single‐fraction. There is also evidence to suggest that fractionation of irradiation greatly reduces the toxicity but preserves the DNA‐damaging effects in rapidly dividing cells.
How the intervention might work
Clinical experience suggests that cumulative doses of up to 25 Gy cause no damage to the retina or optic nerve. As the endothelial cells in CNV are dividing it is possible that radiotherapy can stop the growth of CNV without significant damage to the retina.
Why it is important to do this review
There are several RCTs of radiotherapy for neovascular AMD using different dosage and fractionation schemes. The aim of this review was to assess systematically the results of these studies with a view to providing an overall estimate of treatment effect.
Objectives
The aim of this review was to examine the effects of radiotherapy on neovascular AMD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
We included trials in which participants were people with CNV secondary to AMD as defined by the study investigators.
Types of interventions
We included studies in which radiotherapy, no matter how it was delivered, was compared to another treatment, low dosage irradiation, sham treatment or no treatment.
Types of outcome measures
Primary outcomes
The primary outcome for this review was loss of visual acuity. We considered two measures of loss of visual acuity ‐ 3 or more lines lost on a logMAR chart (equivalent to doubling of visual angle or worse) and 6 or more lines lost (equivalent to quadrupling of visual angle or worse). We also considered mean visual acuity and change in visual acuity as a continuous score.
Secondary outcomes
The secondary outcomes for this review were:
measures of contrast sensitivity;
new vessel growth;
quality of life measures ‐ any validated measurement scale which aims to measure the impact of visual function loss on quality of life of participants;
any adverse outcomes as reported in trials.
Follow up We measured outcomes at six, 12 and 24 months after radiation treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Group Trials Register) in The Cochrane Library Issue 3, 2010, MEDLINE (January 1950 to March 2010), EMBASE (January 1980 to March 2010), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to March 2010), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (March 2010) and ClinicalTrials.gov (http://clinicaltrials.gov) (March 2010). There were no language or date restrictions in the search for trials. The electronic databases were last searched on 23 March 2010.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), mRCT (Appendix 5) and ClinicalTrials.gov (Appendix 6).
Searching other resources
We contacted the investigators of the trials included in this review for information about further trials. We searched the reference lists of relevant studies for further trial reports. We did not perform manual searches of conference proceedings or journals.
Data collection and analysis
Selection of studies
Two review authors independently scanned the titles and abstracts resulting from the searches. We obtained full copies of all potentially or definitely relevant articles. Two review authors assessed the full copies according to the 'Criteria for considering studies for this review'. We resolved disagreements by discussion.
Data extraction and management
Two review authors independently extracted data using a form developed by the Cochrane Eyes and Vision Group. We resolved discrepancies by discussion. In the original review, one author entered data into RevMan 4.2 using the double data‐entry facility to check for errors. For the updates in RevMan 5, data were entered onto a spreadsheet and cut and pasted into RevMan.
Assessment of risk of bias in included studies
Two review authors independently assessed study quality according to methods set out in Section 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). The review authors were not masked to any trial details during the assessment. We considered four parameters of quality when grading the articles: allocation concealment and method of allocation to treatment; masking of providers and recipients of care; masking of outcome assessment; and completeness of follow up. We graded each parameter of trial quality: A ‐ adequate; B ‐ unclear; or C ‐ inadequate. We resolved disagreement between the review authors on assessments by discussion. We contacted the trial authors for clarification on any parameter graded B ‐ unclear. We excluded any trial scoring C ‐ inadequate on allocation concealment and method of allocation to treatment.
For the update in 2009, we used the Cochrane Collaboration's tool for assessing the risk of bias (Higgins 2009). We assessed the extent to which bias could have been introduced in the following aspects of study design and execution: sequence generation, allocation concealment, blinding (masking), incomplete outcome data and selective outcome reporting.
Measures of treatment effect
The primary outcome of visual acuity loss was assessed at six, 12 and 24 months. We used two outcomes, loss of 3 or more lines on a logMAR chart and loss of 6 or more lines. As the proportion of people experiencing these outcomes was high in the control group (more than 10%) we used the relative risk as our effect measure. Not all trials reported visual acuity outcomes in this dichotomous format. We contacted investigators for data but these requests were not successful. We, therefore, also included mean visual acuity and change in visual acuity as a continuous score.
Unit of analysis issues
Most studies randomised participants and then studied one eye per person. One trial (Jaakkola 2005) reported data from 88 eyes in 86 participants. As the numbers of people with both eyes erroneously included in the analysis was small in this study, and it was not possible to extract data for people, this error was ignored and data on eyes used in the analysis. For one trial (Kacperek 2001) it was not clear how the analysis was done but data could not be extracted for the review in any case.
Dealing with missing data
Our main analyses assume that missing data is missing at random. However, to see how reasonable this assumption might be we also did sensitivity analyses with different assumptions about the missing data using methods as set out by White et al (White 2008). The "informative missingness odds ratio" (IMOR) refers to the ratio of the odds of the outcome among participants for whom data were missing and the odds of the outcome among participants who were observed. These IMORs can be assumed to be equal or different in the two trial arms. We did four sensitivity analyses. Firstly we assumed the IMOR was 2 in treatment and control groups i.e. that people who were not seen were twice as likely to have the outcome. Secondly, we assumed that the IMOR was ½ in both treatment and control groups i.e. that people who were not seen were half as likely to have the outcome. For the third and fourth sensitivity analyses, we assumed that the IMOR was opposite in treatment and control groups ‐ i.e. 2 or ½.
All analyses were done using the metamiss command in Stata (version 10.1, StataCorp LP, 4905 Lakeway Drive, College Station, TX 77845 USA).
Assessment of heterogeneity
We assessed heterogeneity by looking at the forest plots to see whether the confidence intervals for the estimates of effect overlapped and by looking at the χ2 and I2 value.
Assessment of reporting biases
We planned to investigate publication bias by doing a scatter plot of the effect estimates from the individual studies against their standard error. An asymmetric graph may indicate that smaller studies that are not statistically significant have not been published although it also may indicate that the effects of treatment are different in small studies. Currently not enough trials are included in the analyses to assess publication bias.
We investigated selective outcome reporting by doing an "outcome matrix" and classifying missing outcomes according to the ORBIT classification (Kirkham 2010).
A: States outcome analysed but only reported that the treatment differences were not statistically significant B: States outcome analysed but only reported that treatment differences were significant C: Clear that outcome was analysed but insufficient data presented to be included in meta‐analysis or full tabulation D: Clear that outcome was analysed but no results reported E: Clear that outcome was measured (for example, includes structurally related outcomes) but not necessarily analysed F: States that outcome was not measured G: Not mentioned but clinical judgement says likely to have been measured H: Not mentioned but clinical judgement says unlikely to have been measured I: Other give details
Data synthesis
We used a random‐effects model to combine results.
There was considerable statistical heterogeneity between studies. However, the amount of heterogeneity varied with the outcome. We have included the pooled analyses and I2 estimates on the graphs for information but have not reported the pooled results in the abstract.
There were not enough data reported for other potential outcome measures (growth of new vessels, contrast sensitivity and quality of life) to enable a statistical analysis but these are discussed in the results section.
Subgroup analysis and investigation of heterogeneity
Not all of the trials reported data for all outcomes. This meant that our options for exploring the sources of heterogeneity were limited. In our protocol we specified three factors of interest for subgroup analyses (method of delivery, dosage and type of CNV). All but one trial used the same method of delivery. Table 3 shows the details of dosage in these trials. Table 4 shows the details of CNV.
1. External beam radiotherapy dosage.
| Study | Total dose (Gy) | Number of fractions | Fraction size (Gy) | Control |
| Bergink 1998 | 24 | 4 | 6 | Observation |
| AMDRT 2004 | 20 | 5 | 4 | Observation and sham radiotherapy |
| Eter 2002 | 20 | 10 | 2 | Observation |
| Kobayashi 2000 | 20 | 10 | 2 | Observation |
| AMDLRTSG 2003 | 20 | 10 | 2 | Observation |
| Kacperek 2001 | 18 | 4 | 4.5 | Observation |
| Ciulla 2002 | 16 | 2 | 8 | Sham irradiation |
| RAD 1999 | 16 | 8 | 2 | Sham irradiation (0 Gy) |
| Marcus 2001 | 14 | 7 | 2 | Sham irradiation |
| SFRADS 2002 | 12 | 6 | 2 | Observation |
| Anders 1998 | 12 | 6 | 2 | Observation |
| Valmaggia 2002 | 8 | 4 | 2 | Low dose irradiation (1 Gy) |
| Char 1999 | 7.5 | 1 | 7.5 | Observation |
Only one trial ‐ Jaakkola 2005 ‐ used plaque brachytherapy. One plaque delivered a dose of 15 Gy at a depth of 1.75 mm for 54 minutes but as this took too long another plaque was used which delivered a dose of 12.6 Gy at 4 mm depth for 11 minutes.
2. Type of choroidal neovascularisation.
| Study | % classic | % occult | % mixed |
| AMDLRTSG | No information | ||
| AMDRT 2004 | 17.5 (predominantly classic) | 21.3 (occult only) | 61.3 (minimally classic) |
| Anders 1998 | No information | ||
| Bergink 1998 | 51.5 | 23.5 | 25 |
| Char 1999 | 48.1 | 51.9 | |
| Ciulla 2002 | 46.4 | 14.3 | 39.3 |
| Eter 2002 | 37.0 | Mixed/occult = | 63.0 |
| Jaakkola 2005 | 40 ("a classic component" | 52 ("occult no classic") | |
| Kacperek 2001 | No information | ||
| Kobayashi 2000 | 50.5 | 12.9 | 20.8 |
| Marcus 2001 | 12.0 | 42.2 | 43.4 |
| RAD 1999 | 37.7 | 62.3 | |
| SFRADS 2002 | 52.3 | 1.5 | 43.2 |
| Valmaggia 2002 | 57.1 | 42.9 |
During the course of doing the review we identified one additional aspect of study design as of interest for subgroup analysis. This was whether or not sham irradiation was carried out in the control group.
Using these factors we performed stratified analyses, the purpose of which was to determine whether the outcome varied significantly with type of explanatory variable. We used data from the 12 month follow‐up and divided the trials into two groups for each factor: high dose (more than 14 Gy) versus low dose (less than or equal to 14 Gy); 50% or more of participants with classic CNV versus less than 50% with classic CNV; and trials with no sham irradiation versus those with sham irradiation. As the numbers of trials were small and the purpose of this analysis was to compare treatment effects only, we used odds ratios pooled using a fixed‐effect model. We calculated an 'interaction effect' (Altman 2003) i.e. compared the pooled odds ratio in the two subgroups.
Sensitivity analysis
Our main sensitivity analyses were regarding missing data (see "Dealing with missing data" above).
Results
Description of studies
Results of the search
The searches identified 149 reports. A further two potentially relevant reports were identified by subsequent electronic searching carried out for another project. We obtained full copies of 28 reports which referred to 23 potentially relevant studies. We excluded 12 of these trials largely because the treatment groups were not randomly allocated (see 'Characteristics of excluded studies' table). A total of 11 trials were considered suitable for inclusion in the review (see 'Characteristics of included studies' table). The included studies all stated that they were RCTs but did not always specify how they performed the randomisation (see below).
An updated search done in March 2010 identified 487 reports of trials. After initial assessment by the Trials Search Co‐ordinator, 477 references were excluded as they were deemed not relevant to the scope of the review and the review authors subsequently assessed ten reports. Of these ten reports, three were relevant trials (AMDLRTSG 2003; AMDRT 2004; Jaakkola 2005), six were ineligible trials (Avila 2009; Barak 2005; Churei 2004; Heier 2008; Marcus 2004; Zambarakji 2006) and one was a report on quality of life outcomes in SFRADS 2002.
Included studies
For additional information see the 'Characteristics of included studies' table.
Types of participants
The 14 trials randomised a total of 1242 people. The studies took place in Germany (Anders 1998; Eter 2002; RAD 1999), the Netherlands (Bergink 1998), Finland (Jaakkola 2005), USA (AMDRT 2004; Char 1999; Ciulla 2002; Marcus 2001), Japan (AMDLRTSG 2003; Kobayashi 2000), UK (Kacperek 2001; SFRADS 2002) and Switzerland (Valmaggia 2002). In all studies the mean age of participants was around 75 years; in most studies the majority of participants were women, however, the percentage female ranged from 30% to 64%.
All studies recruited participants with subfoveal CNV associated with AMD. Most studies, with the exception of AMDLRTSG 2003, Anders 1998 and Kacperek 2001, classified the CNV lesion as classic, occult or mixed. In most trials the percentage of participants with classic or predominantly classic CNV ranged between 37% and 57% (Table 4). In Marcus 2001 a lower percentage of participants with classic CNV was recruited (12%).
Two studies did not specify visual acuity criteria for entry to the trial (Eter 2002; Valmaggia 2002). Most studies specified that eligible participants should have a worst visual acuity in the study eye, usually between 6/60 and 6/120 (AMDLRTSG 2003; AMDRT 2004; Anders 1998; Bergink 1998; Ciulla 2002; Jaakkola 2005; Kacperek 2001; Marcus 2001; RAD 1999; SFRADS 2002); two studies did not specify a worst acuity (Char 1999; Kobayashi 2000). Four studies specified that there should be some visual loss, usually to 6/12 or less (Anders 1998; Char 1999; Ciulla 2002; Kobayashi 2000).
Types of intervention
Table 3 shows the dosage of radiotherapy applied in the different studies. Thirteen studies used external beam radiotherapy. The dosages ranged from 24 Gy (four fractions of 6 Gy) (Bergink 1998) to 7.5 Gy (one fraction) (Char 1999). Only one study used plaque brachytherapy with a dose of 12.6 Gy delivered over 11 minutes (Jaakkola 2005).
Nine of the studies gave no treatment to the control group (AMDLRTSG 2003; Anders 1998; Bergink 1998; Char 1999; Eter 2002; Jaakkola 2005; Kacperek 2001; Kobayashi 2000; SFRADS 2002); three studies used sham irradiation (Ciulla 2002; Marcus 2001; RAD 1999) and one study used very low‐dose irradiation (1 Gy) (Valmaggia 2002). In AMDRT 2004 some participants in the control group received sham irradiation and others received no treatment.
Types of outcome measures
In all studies the primary outcome was visual acuity. In most cases this was measured using the ETDRS chart or equivalent logMAR chart. The exception to this was Bergink 1998 where Snellen acuity was measured. Most studies considered some aspect of the clinical progression of CNV such as area of CNV (AMDLRTSG 2003; AMDRT 2004; Kobayashi 2000; Valmaggia 2002) and appearance of the fundus on fluorescein angiography (Jaakkola 2005; Marcus 2001; RAD 1999). Near vision (SFRADS 2002) and reading ability (Valmaggia 2002) were also considered. Three studies specifically considered safety (AMDRT 2004; Kobayashi 2000; SFRADS 2002).
Excluded studies
See 'Characteristics of excluded studies' table.
Risk of bias in included studies
Figure 1 and Figure 2 summa rise the assessment of the risk of bias in included studies.
1.
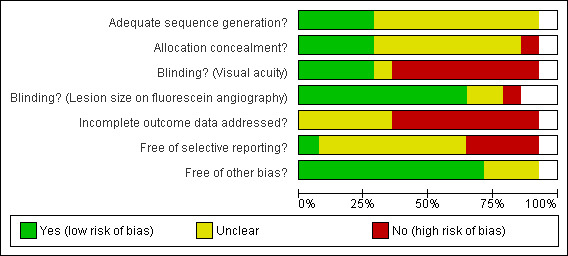
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
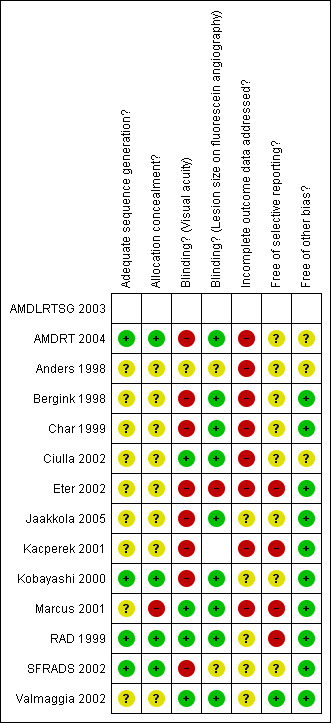
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
In four studies (Kobayashi 2000; Marcus 2001; RAD 1999; SFRADS 2002) trial reports indicated that randomisation had been executed properly, that is, an unpredictable sequence of treatment allocation was concealed properly from people recruiting participants into the trial.
Blinding
Studies that did not perform sham irradiation (Anders 1998; Bergink 1998; Char 1999; Eter 2002; Kacperek 2001; Kobayashi 2000; SFRADS 2002) were at greater risk of performance bias with participants and providers in general being aware of the treatment group. However, in three of these studies efforts were made to mask the outcome assessor to treatment group (detection bias) (Char 1999; Kobayashi 2000; SFRADS 2002).
Incomplete outcome data
Table 5; Table 6 and Table 7 summa rise the follow‐up in the included studies at six, 12 and 24 months. Follow‐up rates were not described clearly in four studies (AMDLRTSG 2003; Bergink 1998; Char 1999; Kacperek 2001). In two studies, not enough information was given on people excluded after randomisation (Ciulla 2002; Eter 2002) so estimates of follow‐up for these studies may underestimate loss to follow‐up. In one study (SFRADS 2002) a strictly intention‐to‐treat analysis was not performed as one patient randomised to the control group received treatment and was analysed in the treatment group. However, this was unlikely to have had a major impact on the results of the study. None of the authors included participants lost to follow up in the analyses.
3. Follow‐up at 6 months.
| Study | Radiotherapy group | Control group | ||||
| Randomised | Number seen at six months | % seen at six months | Randomised | Number seen at six months | % seen at six months | |
| AMDLRTSG 2003* | 38 | 37 | 97% | 31 | 28 | 90% |
| AMDRT 2004 | 41 | 35 | 85% | 47 | 35 | 74% |
| Jaakkola 2005 | 43 | 42 | 98% | 45 | 41 | 91% |
| Marcus 2001 | 41 | 39 | 95% | 42 | 34 | 81% |
| SFRADS 2002 | 99 | 93 | 94% | 100 | 87 | 87% |
| Valmaggia 2002 | 52 | 49 | 94% | 52 | 48 | 92% |
* Number of patients randomised unclear ‐ study reports mentions 100, 70 and 69.
4. Follow‐up at 12 months.
| Study | Radiotherapy group | Control group | ||||
| Randomised | Number seen at 12 months | % seen at 12 months | Randomised | Number seen at 12 months | % seen at 12 months | |
| AMDLRTSG 2003 | 38 | 35 | 92% | 31 | 26 | 84% |
| AMDRT 2004 | 41 | 31 | 76% | 47 | 31 | 66% |
| Bergink 1998 | 37 | 34 | 92% | 37 | 29 | 78% |
| Char 1999 | 14 | 14 | 100% | 13 | 13 | 100% |
| Jaakkola 2005 | 43 | 43 | 100% | 45 | 41 | 91% |
| Marcus 2001 | 41 | 37 | 90% | 42 | 33 | 79% |
| RAD 1999 | 101 | 88 | 87% | 104 | 95 | 91% |
| SFRADS 2002 | 99 | 93 | 94% | 100 | 90 | 90% |
| Valmaggia 2002 | 52 | 43 | 83% | 52 | 44 | 85% |
5. Follow‐up at 24 months.
| Study | Radiotherapy group | Control group | ||||
| Randomised | Number seen at 12 months | % seen at 12 months | Randomised | Number seen at 12 months | % seen at 12 months | |
| AMDLRTSG 2003 | 38 | 30 | 79% | 31 | 21 | 68% |
| Jaakkola 2005 | 43 | 41 | 95% | 45 | 41 | 91% |
| Kobayashi 2000 | 51 | 45 | 88% | 50 | 40 | 80% |
| SFRADS 2002 | 99 | 87 | 88% | 100 | 88 | 88% |
| Valmaggia 2002 | 52 | 43 | 83% | 52 | 43 | 83% |
Appendix 7 and Appendix 8 show the sensitivity analyses making different assumptions as to risk of outcome in people not seen. Five different assumptions are shown:
Missing at random (available case analysis)
Odds of outcome in not observed twice odds of outcome in observed in treatment and control groups
Odds of outcome in not observed half odds of outcome in observed in treatment and control groups
Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group
Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group
The pooled estimates did not appear to be at substantial risk of bias due to missing data in the included studies (Appendix 7). The pooled risk ratio, under various assumptions about the risk of outcome in people who were not observed, varied on average by less than 10% from the available case analysis. The exception to this was loss of 6+ lines at six months where making more extreme assumptions about outcome in people who were not seen resulted in approximately 15% change in the pooled risk ratio. If we assume that the odds of the outcome in people in the treatment group who were not seen was twice that of the people who were seen, and that the odds of the outcome in people in the control group who were not seen was only half that of people who were seen, the observed risk ratio showing a beneficial effect becomes non‐statistically significant.
Looking at the effect of missing data on individual studies (Appendix 8) AMDRT 2004, Bergink 1998; Kobayashi 2000Marcus 2001; and Valmaggia 2002 all had some outcomes affected by assumptions about missing data ‐ in particular the assumption that the outcome was different in non‐observed participants in treatment and control (twice the odds in treatment and half in control). This assumption, for some outcomes, leads to a change in risk ratio of greater than 10%.
Selective reporting
Table 8 shows the outcome reporting grid for the primary outcome: visual acuity at six, 12 or 24 months. Visual acuity can be presented in several different ways: loss of 3+ or 6+ lines of visual acuity, mean visual acuity or change in visual acuity. Decisions about which method of analysis to use can be influenced by the statistical significance of the results and therefore this can lead to bias. No study reported all visual acuity measures.
6. Outcome reporting grid: primary outcome.
| 6 months: Loss of 3+ lines | 6 months: Loss of 6+ lines | 6 months: Mean VA | 6 months: Change in VA | 12 months: Loss of 3+ lines | 12 months: Loss of 6+ lines | 12 months: Mean VA | 12 months: Change in VA | 24 months: Loss of 3+ lines | 24 months: Loss of 6+ lines | 24 months: Mean VA | 24 months: Change in VA | |
| AMDLRTSG 2003 | E | E | ✓ | E | E | E | ✓ | E | E | E | ✓ | E |
| AMDRT 2004 | ✓ | ✓ | E | E | ✓ | ✓ | E | E | H | H | H | H |
| Anders 1998 | E | E | ✓ | E | E | E | ✓ | E | E | E | ✓ | E |
| Bergink 1998 | E | E | E | E | ✓ | ✓ | E | E | H | H | H | H |
| *Char 1999 | ||||||||||||
| Ciulla 2002 | E | E | ✓ | E | E | E | ✓ | E | E | E | ✓ | E |
| Eter 2002 | E | E | A | E | H | H | H | H | H | H | H | H |
| Jaakkola 2005 | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ |
| Kacperek 2001 | E | E | C | E | E | E | C | E | H | H | H | H |
| Kobayashi 2000 | E | E | ✓ | ✓ | E | E | ✓ | ✓ | ✓(2 lines) | ✓ | ✓ | ✓ |
| Marcus 2001 | ✓ | ✓ | A(median) | A(median) | ✓ | ✓ | A(median) | A(median) | H | H | H | H |
| RAD 1999 | E | E | E | E | ✓ | E | E | ✓ | E | E | A | A |
| SFRADS 2002 | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ |
| Valmaggia 2002 | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ | ✓ | ✓ | E | ✓ |
*Char 1991: Small study of 27 patients. Individual visual acuity data at baseline and last follow‐up only reported. Average follow‐up 14 months, range 0 to 32 months. Data extracted for the review on mean VA and assumed related approximately to 12 month follow‐up. Other analyses e.g., of loss of 3+ lines etc theoretically possible but probably meaningless.
A: States outcome analysed but only reported the P‐value > 0.05 i.e.. NS. E: Clear that outcome was measured (for example, includes structurally related outcomes) but not necessarily analysed. H: Not mentioned but clinical judgement says unlikely to have been measured (adapted from list provided by Paula Williamson at Cochrane training workshop on selective outcome reporting bias, Edinburgh March 2009).
Effects of interventions
See: Table 1
Primary outcomes
Data on visual acuity were not available in a form suitable for inclusion in the review for two studies (Eter 2002; Kacperek 2001). In Eter 2002 45 eyes of 45 participants were assigned in a ratio of 2:1 to either radiation treatment (20 Gy in 10 fractions) or observation. There were no statistically significant differences between treatment and control groups six months after treatment. In Kacperek 2001 38 people were treated with radiotherapy (18 Gy in 4 fractions) and compared to 28 people who were not treated. At 12 months visual acuity was measured on 28 participants in the treatment group and 20 in the control group. Participants in the control group had lost more vision than the treatment group (Mann Whitney test P = 0.028).
Follow up at six months Five trials provided data on the primary outcome (3 or more lines visual acuity lost) at six months (AMDRT 2004; Jaakkola 2005; Marcus 2001; SFRADS 2002; Valmaggia 2002) (Analysis 1.1). There was some inconsistency in trial results. The I2 value (percentage of total variation across studies that was due to heterogeneity rather than chance) (Higgins 2003) was 41%. The relative risk of losing 3 or more lines six months after treatment varied from 0.40 (95% CI 0.18 to 0.88) (Valmaggia 2002) to 1.06 (95% CI 0.71 to 1.57) (Marcus 2001). There was similar inconsistency in the outcome 6 or more lines visual acuity lost (I2 = 47%) however all the risk ratios were in the direction of benefit varying from 0.07 (95% CI 0.0 to 1.11) (Valmaggia 2002) to 0.83 (95% CI 0.47 to 1.46) (SFRADS 2002) (Analysis 1.4).
1.1. Analysis.
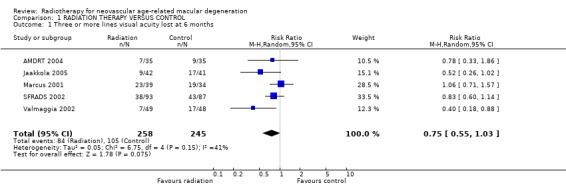
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 1 Three or more lines visual acuity lost at 6 months.
1.4. Analysis.
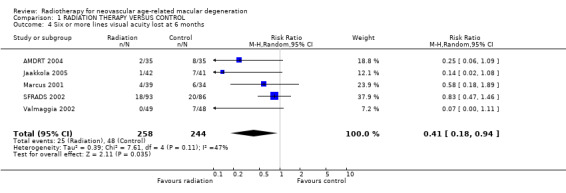
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 4 Six or more lines visual acuity lost at 6 months.
Follow up at 12 months Eight trials provided data on visual acuity outcomes at 12 months (AMDRT 2004; Bergink 1998; Char 1999; Jaakkola 2005; Marcus 2001; RAD 1999; SFRADS 2002; Valmaggia 2002). Again there was inconsistency in trial results for the outcome of 3 or more lines visual acuity lost (I2 = 42%) with the relative risk varying from 0.37 (95% CI 0.15 to 0.90) (Char 1999) to 1.22 (95% CI 0.91 to 1.62) (Marcus 2001) (Analysis 1.2). There was less inconsistency for the outcome of 6 or more lines visual acuity lost (I2 = 17%) (Analysis 1.5). Most trials provided results in the direction of benefit with the exception of Marcus 2001 1.23 (95% CI 0.56 to 2.68). The pooled risk ratio (random‐effects model) was 0.62 (95% CI 0.44 to 0.87).
1.2. Analysis.
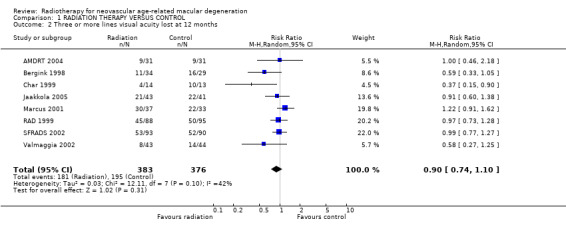
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 2 Three or more lines visual acuity lost at 12 months.
1.5. Analysis.
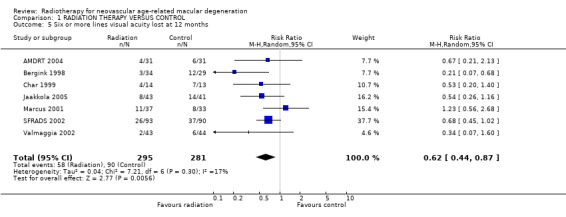
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 5 Six or more lines visual acuity lost at 12 months.
Follow up at 24 months Four trials provided data on visual acuity outcomes at 24 months (Jaakkola 2005; Kobayashi 2000; SFRADS 2002; Valmaggia 2002). There was considerable inconsistency in trial results for the outcome of 3 or more lines lost (I2 = 63%) (Analysis 1.3). There was no inconsistency in trial results for the outcome of 6 or more lines lost (I2 = 0%) (Analysis 1.6). The random‐effects pooled relative risk was 0.81 (95% CI 0.64 to 1.03). Using a fixed‐effect model the relative risk was 0.79 (95% CI 0.62 to 1.01).
1.3. Analysis.
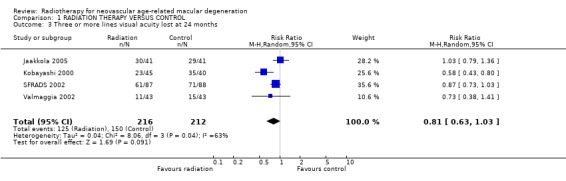
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 3 Three or more lines visual acuity lost at 24 months.
1.6. Analysis.
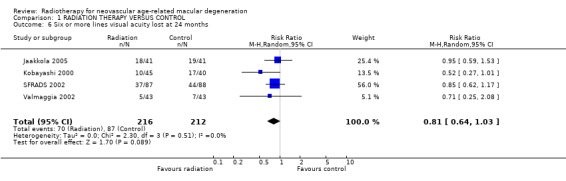
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 6 Six or more lines visual acuity lost at 24 months.
Effects of missing data
Table 5; Table 6 and Table 7 show follow‐up in the included studies. The analyses presented so far assume data were missing at random.
See Appendix 7 and Appendix 8 for sensitivity analyses and "Incomplete outcome data" above for discussion on the effects of missing data. With regard to the pooled analyses, we are interested in whether our conclusions would change as a result of different assumptions about reasons for data being missing. Overall, the size and statistical significance of the effect was similar in the available case analyses (data missing at random) and assuming that there was a different risk of outcome in non‐observed people (see table below). There were a few exceptions to this, however the differences were still relatively small and the fact that the statistical significance changed probably reflects the fact these were borderline cases anyway and the upper confidence interval was close to 1 (no effect).
| Outcome | Available case analysis risk ratio (95% CI) | Assumption about missing data | Risk ratio (95% CI) under this assumption |
| 3+ lines at 6 months | 0.755 (0.556, 1.025) | IMOR ½ 2 | 0.7 (0.516, 0.949) |
| 3+ lines at 24 months | 0.81 (0.636, 1.033) | IMOR ½ 2 | 0.768 (0.593, 0.994) |
| 6+ lines at 6 months | 0.423 (0.191, 0.934) | IMOR 2 ½ | 0.488 (0.225, 1.055) |
| 6+ lines at 24 months | 0.811 (0.638, 1.032) | IMOR ½ 2 | 0.741 (0.58, 0.947) |
IMOR 2 ½: Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group.
IMOR ½ 2: Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group.
Visual acuity as a continuous outcome
Not all trials reported visual acuity outcomes in a dichotomous format. In order to include data from the trials that did not, we also collected data on logMAR visual acuity as a continuous variable. These data were available for most trials at 12 months, either as mean visual acuity at follow‐up or change in visual acuity since the start of the trial (Analysis 1.7). There was less heterogeneity in these outcomes. For example, for the trials reporting change in visual acuity, the I2 value was 15%. The pooled weighted mean difference was ‐0.10 (95% CI ‐0.16 to ‐0.04). These results were consistent with a mean change in visual acuity of 1.5 lines of visual acuity in favour of the treated group to approximately one third of a line of visual acuity in favour of the treatment group.
1.7. Analysis.
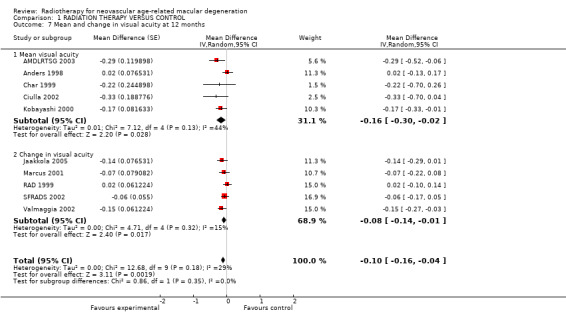
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 7 Mean and change in visual acuity at 12 months.
These analyses may be at risk of selective outcome bias because continuous data may be analysed two ways ‐ as final visual acuity or change in visual acuity from baseline. It is possible that the choice of which outcome to present was influenced by the results.
Investigation of heterogeneity
With only 14 trials included in the review, and only some of these trials providing data for some outcomes, our ability to determine the causes of the heterogeneity or inconsistency between trials was limited. Using the factors prespecified in the protocol (dosage and type of CNV) and one factor not prespecified in the protocol (sham irradiation in the control group) we performed stratified analyses for the visual acuity outcome (3 or more lines lost) at 12 months (because this was the time period for which most data were available) (see 'Table 9'). There were no statistically significant interactions. There was some indication that trials with no sham irradiation reported a greater effect of treatment as did trials with a greater percentage of participants with classic CNV. There was little evidence for any effect of dosage. Analysis 1.9 shows the forest plot for the subgroup analysis by dosage with trials ordered according to dosage (highest dosage at top and lowest dosage at bottom of plot). There was little evidence for any trend in effect of radiotherapy according to dosage.
7. Stratified analyses (3 or more lines lost at 12 months).
| Subgroup | Subgroup | Number of trials | Pooled OR | 95% CI | *Ratio of the subgroup odds ratios | **95% CI |
| 1 | Classic < 50% | 5 | 0.91 | 0.62, 1.34 | ||
| 2 | Classic 50%+ | 3 | 0.70 | 0.45, 1.10 | 0.77 | 0.43, 1.39 |
| 1 | > 14 Gy | 3 | 0.80 | 0.50, 1.25 | ||
| 2 | <= 14 Gy | 5 | 0.83 | 0.56, 1.21 | 1.04 | 0.57, 1.89 |
| 1 | No sham irradiation | 5 | 0.73 | 0.49, 1.07 | ||
| 2 | Sham irradiation | 3 | 0.95 | 0.60, 1.48 | 1.30 | 0.36, 1.34 |
*The log odds ratio of subgroup 1 was subtracted from the log odds ratio of subgroup 2 and the resulting figure transformed back to the odds ratio scale.
**Calculated using the following formula for the standard error: √(variance (subgroup 1 log OR) + variance (subgroup 2 log OR)) where variance is the square of the standard error (Altman 2003).
1.9. Analysis.

Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 9 Investigating heterogeneity: dosage.
Secondary outcomes
Our secondary outcome measures included change in membrane size and contrast sensitivity. Of the trials that specifically studied change in lesion size a beneficial outcome for treatment was found by one (Kobayashi 2000). No difference in the growth rate between treatment and controls were reported by four trials (Bergink 1998; Char 1999; Marcus 2001; Valmaggia 2002). Of the trials that specifically studied changes in contrast sensitivity, SFRADS 2002 reported a statistically significant difference in the loss of 0.3 log units of contrast sensitivity in favour of treatment at 24 months but not three months. No statistically significant difference in contrast sensitivity between treated and control groups was reported by Marcus 2001.
Quality of life outcomes were reported in SFRADS 2002. Visual functioning was assessed by the Daily Living Tasks Dependent on Vision (DLTV) questionnaire (Hart 1999). There were no differences between treatment and control groups on any dimension of the DLTV 12 or 24 months after treatment.
Adverse effects
The incidence of adverse events was low in all the trials reviewed.
Three trials found slightly higher rates of cataract progression in the treatment groups but this was not statistically significant (Kobayashi 2000; Marcus 2001; RAD 1999).
There were no reported cases of radiation retinopathy, optic neuropathy or the development of malignancy. However, the duration of follow‐up was likely to be too short to detect this. Given the mean age of participants this may not be a major concern.
Although there was an overall beneficial effect for treatment with regard to vision, Bergink 1998 reported a drop in central vision with a loss of 3 or more lines in a substantial proportion of patients in the treatment group. This was not reported by trials using standard fractions (2 Gy) in the treatment protocol.
Other complications reported in the treatment group included one case of rhegmatogenous retinal detachment and one case of a large non‐clearing vitreous haemorrhage (Marcus 2001); transient conjunctival injection in two participants (Kobayashi 2000); and transient disturbance of the precorneal tear film, found to be significant (SFRADS 2002).
Discussion
Summary of main results
We identified 14 trials of the effect of radiotherapy on neovascular AMD, which randomised 1242 participants. One of these trials studied plaque brachytherapy, the rest external beam radiotherapy. Not all of these trials could be included in each of our planned analyses because of differences in the way outcomes were presented and follow‐up times. Table 1 summarises the effects of radiotherapy on visual loss at 12 months follow‐up. Overall the quality of the evidence ranged from low to moderate. There was some evidence for an effect of radiotherapy on severe visual acuity loss (loss of 6+ lines) over 12 months with a statistically significant 40% relative risk reduction. However, this effect was not seen for more moderate visual loss (loss of 3+ lines) and was not maintained at 24 months. However, it must be noted that different trials contribute to these analyses. However, when repeating the analyses for 6+ lines using only three trials that had data for 12 and 24 months a similar pattern was observed.
There was considerable clinical and statistical inconsistency between trials. Most trials found effects that favoured treatment, but these were not always significant. The exception was Marcus 2001 which consistently found non‐significant effects that favoured the control group. It is difficult to ascertain why this trial should be different but it had sham irradiation in the control group and a very low percentage of participants with classic CNV (12%).
With only 14 trials in the review and differences between trials in terms of outcome reporting it was difficult to explore the sources of heterogeneity. Subgroup analyses comparing groups of trials with different attributes (i.e. low versus high dosage; low versus high percentage with classic CNV; and sham irradiation versus observation of the control group) did not reveal any statistically significant interactions. With small numbers of trials in each subgroup (range three to five) this was not surprising.
It is encouraging that there were no significant adverse effects noted with up to 20 Gy of radiotherapy deployed in 2 Gy fractions. The occurrence of severe visual loss in some treated patients receiving 24 Gy in larger fractions questions the safety of higher doses. Higher doses of radiation are associated with greater morbidity such as radiation retinopathy and optic neuropathy. Given the lack of a clear benefit of higher doses it cannot be assumed that these may be used safely in clinical practice. The long‐term risk to the fellow eye from collateral radiation exposure also needs to be determined. Neovascular AMD is a heterogenous disease with variation in CNV composition and disease presentation. Differences in lesion composition, size and time in the natural history at presentation may be a source of variability when assessing treatment outcome among the different trials. Evidence from the TAP (TAP Study 1999) and VIP (Bressler 2002) trials showed that many people with minimally classic (less than 50% classic) and occult with no classic lesions had relatively good natural history. Despite presenting as large lesions, they maintained reasonably good visual acuity throughout 24 months follow up without treatment. In contrast, the majority of predominantly classic (more than 50% classic) lesions were four disc areas or less and were more likely to present with lower visual acuity.
Kobayashi 2000 found a significant treatment benefit in participants with smaller CNV (less than 1.5 mm2) with regard to smaller increase in lesion size and significantly smaller decrease in LogMAR visual acuity for over two years. They also found that there was no significant difference in visual outcome in participants with larger CNV (more than 1.5 mm2). In contrast, Marcus 2001 did not find lesion size (less than one to more than six disc areas) determined treatment outcome. When the composition of the lesion was considered, Bergink 1998 and Kobayashi 2000 found a better treatment outcome for occult lesions. SFRADS 2002 suggested that one possible reason for the negative outcome in their trial was the predominance of wholly classic and predominantly classic subgroups. This finding was not supported by the other trials included in this review.
Overall completeness and applicability of evidence
Although there are 14 trials published, because of the different dosages used, and different outcome measures and follow‐up times reported, the overall completeness of the evidence is less than might be expected from the number of trials. It is possible that there is an optimum treatment regime that has not yet been identified.
Quality of the evidence
The evidence was moderate to low quality depending on the outcome (Table 1).
Authors' conclusions
Implications for practice.
It is possible that a moderate treatment benefit from radiotherapy exists in terms of prevention of severe visual loss. However, considerable clinical and statistical heterogeneity between published trials makes it difficult to draw firm conclusions. It is also possible that the moderate treatment effects seen could be explained by biases in the way that the studies were conducted. Overall, we can say that the results of this review do not currently support the use of radiotherapy in people with neovascular AMD.
Implications for research.
Future trials should have a sufficient sample size to detect moderate effects and should report data on visual acuity outcomes so as to enable their inclusion in systematic overviews. Consistent reporting of data on factors such as lesion size and composition would also facilitate synthesis. Adequate masking of the treatment groups should be considered a priority. It is possible that radiotherapy may have a role as adjunctive treatment in conjunction with pharmacological treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2010 | New search has been performed | Issue 5 2010: Updated searches yielded 3 new trials. |
| 31 March 2010 | New citation required but conclusions have not changed | Review substantially updated including new assessment of risk of bias and preparation of summary of findings tables. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 17 March 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Eyes and Vision Group editorial team prepared and executed the electronic searches. We are grateful to Rebecca Wong, Catey Bunce and Roberta Scherer for peer review. We thank Zoe Ockrim for her contribution to the original published version of the review. We would also like to thank Takehiro Yamashita and Ryo Asaoka for translating a Japanese article for the 2010 update.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Macular Degeneration #2 MeSH descriptor Retinal Degeneration #3 MeSH descriptor Neovascularization, Pathologic #4 (macula* near degenerat*) #5 (macula* near neovasc*) #6 (retina* near degener*) #7 (retina* near neovasc*) #8 (choroid* near degener*) #9 (choroid* near neovasc*) #10 (maculopath*) #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Radiotherapy #13 (radiotherap* or radiat* or irradiat*) #14 (teletherap* or tele‐therap* or proton* or plaque) #15 (external near beam) #16 (external‐beam) #17 (#12 OR #13 OR #14 OR #15 OR #16) #18 (#11 AND #17)
Appendix 2. MEDLINE search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 exp macular degeneration/ 14 exp retinal degeneration/ 15 exp retinal neovascularization/ 16 exp choroidal neovascularization/ 17 exp macula lutea/ 18 (macula$ adj2 lutea).tw. 19 maculopath$.tw. 20 ((macul$ or retina$ or choroid$) adj3 degener$).tw. 21 ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 22 or/13‐21 23 exp radiotherapy/ 24 (radiotherap$ or radiat$ or irradiat$ or teletherap$ or proton$ or plaque).tw. 25 (external adj3 beam).tw. 26 or/23‐25 27 22 and 26 28 12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. EMBASE search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. (397882) 6 or/1‐5 (453431) 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or propspectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 31 30 not (11 or 23) 32 11 or 24 or 31 33 exp retina macula age related degeneration/ 34 exp retina degeneration/ 35 exp neovascularization pathology/ 36 ((macul$ or retina$ or choroid$) adj3 degener$).tw. 37 ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 38 maculopath$.tw. 39 or/33‐38 40 exp radiotherapy/ 41 (radiotherap$ or radiat$ or irradiat$ or teletherap$ or proton$ or plaque).tw. 42 (external adj3 beam).tw. 43 or/40‐42 44 39 and 43 45 32 and 44
Appendix 4. LILACS search strategy
macula$ or retina$ or choroid$ and degenerat$ or neovasc$ and radiotherap$ or radiat$ or irradiat$ or teletherap$ or proton$ or plaque
Appendix 5. metaRegister of Controlled Trials search strategy
macular degeneration AND radiotherapy
Appendix 6. ClinicalTrials.gov search strategy
macular degeneration AND radiotherapy
Appendix 7. Sensitivity analyses: effect of different assumptions regarding missing data on pooled estimates
| Outcome | Assumption | Risk ratio | Lower 95% CI | Upper 95% CI | % change from available case analysis |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | 0.755 | 0.556 | 1.025 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.742 | 0.555 | 0.994 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.77 | 0.559 | 1.061 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.815 | 0.596 | 1.114 | ‐8% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.7 | 0.516 | 0.949 | 7% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | 0.905 | 0.745 | 1.1 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.899 | 0.745 | 1.084 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.915 | 0.748 | 1.118 | ‐1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.975 | 0.804 | 1.183 | ‐8% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.837 | 0.683 | 1.024 | 8% | |
| Loss of 3+ lines visual acuity at 24 months | Missing at random (available case analysis) | 0.81 | 0.636 | 1.033 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.817 | 0.649 | 1.028 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.807 | 0.627 | 1.038 | 0% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.856 | 0.683 | 1.074 | ‐6% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.768 | 0.593 | 0.994 | 5% | |
| Loss of 6+ lines at 6 months | Missing at random (available case analysis) | 0.423 | 0.191 | 0.934 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.406 | 0.186 | 0.888 | 4% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.44 | 0.199 | 0.973 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.488 | 0.225 | 1.055 | ‐15% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.365 | 0.163 | 0.82 | 14% | |
| Loss of 6+ lines at 12 months | Missing at random (available case analysis) | 0.62 | 0.443 | 0.868 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.61 | 0.441 | 0.845 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.633 | 0.45 | 0.891 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.683 | 0.481 | 0.97 | ‐10% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.561 | 0.401 | 0.785 | 10% | |
| Loss of 6+ lines at 24 months | Missing at random (available case analysis) | 0.811 | 0.638 | 1.032 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | 0.812 | 0.644 | 1.023 | 0% | |
| Odds of outcome in not observed half odds of outcome in observed | 0.815 | 0.637 | 1.042 | 0% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | 0.89 | 0.701 | 1.13 | ‐10% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | 0.741 | 0.58 | 0.947 | 9% |
Appendix 8. Sensitivity analyses: effect of different assumptions regarding missing data on effect estimates from individual studies
| Outcome | Assumption | Study | Risk ratio | Lower 95% CI | Upper 95% CI | % change from available case analysis |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | AMDRT 2004 | 0.778 | 0.326 | 1.856 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | AMDRT 2004 | 0.742 | 0.324 | 1.701 | 5% | |
| Odds of outcome in not observed half odds of outcome in observed | AMDRT 2004 | 0.816 | 0.336 | 1.981 | ‐5% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | AMDRT 2004 | 0.958 | 0.403 | 2.274 | ‐23% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | AMDRT 2004 | 0.632 | 0.269 | 1.482 | 19% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | AMDRT 2004 | 1 | 0.459 | 2.178 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | AMDRT 2004 | 0.955 | 0.467 | 1.955 | 5% | |
| Odds of outcome in not observed half odds of outcome in observed | AMDRT 2004 | 1.047 | 0.466 | 2.351 | ‐5% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | AMDRT 2004 | 1.321 | 0.611 | 2.856 | ‐32% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | AMDRT 2004 | 0.757 | 0.355 | 1.613 | 24% | |
| Loss of 6+ lines visual acuity at 6 months | Missing at random (available case analysis) | AMDRT 2004 | 0.25 | 0.057 | 1.095 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | AMDRT 2004 | 0.244 | 0.057 | 1.037 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | AMDRT 2004 | 0.261 | 0.059 | 1.157 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | AMDRT 2004 | 0.318 | 0.073 | 1.383 | ‐27% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | AMDRT 2004 | 0.2 | 0.046 | 0.867 | 20% | |
| Loss of 6+ lines visual acuity at 12 months | Missing at random (available case analysis) | AMDRT 2004 | 0.667 | 0.208 | 2.133 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | AMDRT 2004 | 0.644 | 0.213 | 1.946 | 3% | |
| Odds of outcome in not observed half odds of outcome in observed | AMDRT 2004 | 0.697 | 0.213 | 2.28 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | AMDRT 2004 | 0.934 | 0.296 | 2.949 | ‐40% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | AMDRT 2004 | 0.48 | 0.153 | 1.506 | 28% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | Bergink 1998 | 0.586 | 0.326 | 1.054 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Bergink 1998 | 0.575 | 0.328 | 1.007 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | Bergink 1998 | 0.608 | 0.332 | 1.112 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Bergink 1998 | 0.655 | 0.363 | 1.18 | ‐12% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Bergink 1998 | 0.534 | 0.3 | 0.95 | 9% | |
| Loss of 6+ lines visual acuity at 12 months | Missing at random (available case analysis) | Bergink 1998 | 0.213 | 0.067 | 0.683 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Bergink 1998 | 0.209 | 0.067 | 0.655 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | Bergink 1998 | 0.223 | 0.069 | 0.721 | ‐5% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Bergink 1998 | 0.247 | 0.077 | 0.791 | ‐16% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Bergink 1998 | 0.188 | 0.059 | 0.597 | 12% | |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | Jaakkola 2005 | 0.517 | 0.261 | 1.024 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 0.506 | 0.258 | 0.994 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 0.529 | 0.266 | 1.053 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 0.542 | 0.273 | 1.076 | ‐5% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 0.493 | 0.25 | 0.973 | 5% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | Jaakkola 2005 | 0.91 | 0.599 | 1.382 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 0.886 | 0.588 | 1.337 | 3% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 0.937 | 0.613 | 1.43 | ‐3% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 0.937 | 0.613 | 1.43 | ‐3% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 0.886 | 0.588 | 1.337 | 3% | |
| Loss of 3+ lines visual acuity at 24 months | Missing at random (available case analysis) | Jaakkola 2005 | 1.034 | 0.789 | 1.356 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 1.026 | 0.79 | 1.334 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 1.045 | 0.79 | 1.383 | ‐1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 1.063 | 0.808 | 1.399 | ‐3% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 1.009 | 0.772 | 1.319 | 2% | |
| Loss of 6+ lines visual acuity at 6 months | Missing at random (available case analysis) | Jaakkola 2005 | 0.139 | 0.018 | 1.084 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 0.134 | 0.017 | 1.037 | 4% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 0.144 | 0.018 | 1.119 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 0.149 | 0.019 | 1.154 | ‐7% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 0.13 | 0.017 | 1.005 | 6% | |
| Loss of 6+ lines visual acuity at 12 months | Missing at random (available case analysis) | Jaakkola 2005 | 0.545 | 0.256 | 1.16 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 0.522 | 0.247 | 1.105 | 4% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 0.565 | 0.264 | 1.207 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 0.565 | 0.264 | 1.207 | ‐4% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 0.522 | 0.247 | 1.105 | 4% | |
| Loss of 6+ lines visual acuity at 24 months | Missing at random (available case analysis) | Jaakkola 2005 | 0.947 | 0.588 | 1.528 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Jaakkola 2005 | 0.934 | 0.586 | 1.489 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | Jaakkola 2005 | 0.961 | 0.592 | 1.562 | ‐1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Jaakkola 2005 | 0.995 | 0.617 | 1.606 | ‐5% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Jaakkola 2005 | 0.902 | 0.562 | 1.448 | 5% | |
| Loss of 3+ lines visual acuity at 24 months | Missing at random (available case analysis) | Kobayashi 2000 | 0.584 | 0.429 | 0.795 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Kobayashi 2000 | 0.598 | 0.447 | 0.802 | ‐2% | |
| Odds of outcome in not observed half odds of outcome in observed | Kobayashi 2000 | 0.574 | 0.415 | 0.794 | 2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Kobayashi 2000 | 0.62 | 0.458 | 0.84 | ‐6% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Kobayashi 2000 | 0.554 | 0.405 | 0.758 | 5% | |
| Loss of 6+ lines visual acuity at 24 months | Missing at random (available case analysis) | Kobayashi 2000 | 0.523 | 0.272 | 1.006 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Kobayashi 2000 | 0.52 | 0.277 | 0.975 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | Kobayashi 2000 | 0.535 | 0.274 | 1.044 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Kobayashi 2000 | 0.606 | 0.316 | 1.163 | ‐16% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Kobayashi 2000 | 0.459 | 0.24 | 0.876 | 12% | |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | Marcus 2001 | 1.055 | 0.709 | 1.57 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Marcus 2001 | 1.014 | 0.696 | 1.478 | 4% | |
| Odds of outcome in not observed half odds of outcome in observed | Marcus 2001 | 1.105 | 0.73 | 1.672 | ‐5% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Marcus 2001 | 1.135 | 0.755 | 1.706 | ‐8% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Marcus 2001 | 0.987 | 0.673 | 1.448 | 6% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | Marcus 2001 | 1.216 | 0.913 | 1.621 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Marcus 2001 | 1.178 | 0.904 | 1.535 | 3% | |
| Odds of outcome in not observed half odds of outcome in observed | Marcus 2001 | 1.265 | 0.927 | 1.727 | ‐4% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Marcus 2001 | 1.298 | 0.959 | 1.757 | ‐7% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Marcus 2001 | 1.148 | 0.873 | 1.51 | 6% | |
| Loss of 6+ lines visual acuity at 6 months | Missing at random (available case analysis) | Marcus 2001 | 0.581 | 0.179 | 1.889 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Marcus 2001 | 0.533 | 0.168 | 1.696 | 8% | |
| Odds of outcome in not observed half odds of outcome in observed | Marcus 2001 | 0.621 | 0.19 | 2.035 | ‐7% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Marcus 2001 | 0.661 | 0.203 | 2.152 | ‐14% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Marcus 2001 | 0.501 | 0.157 | 1.604 | 14% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | Marcus 2001 | 1.226 | 0.562 | 2.677 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Marcus 2001 | 1.142 | 0.541 | 2.41 | 7% | |
| Odds of outcome in not observed half odds of outcome in observed | Marcus 2001 | 1.297 | 0.585 | 2.873 | ‐6% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Marcus 2001 | 1.423 | 0.65 | 3.112 | ‐16% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Marcus 2001 | 1.041 | 0.487 | 2.227 | 15% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | RAD 1999 | 0.972 | 0.735 | 1.285 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | RAD 1999 | 0.986 | 0.754 | 1.288 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | RAD 1999 | 0.957 | 0.717 | 1.277 | 2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | RAD 1999 | 1.041 | 0.79 | 1.371 | ‐7% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | RAD 1999 | 0.906 | 0.684 | 1.2 | 7% | |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | SFRADS 2002 | 0.827 | 0.598 | 1.143 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.812 | 0.594 | 1.111 | 2% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.845 | 0.607 | 1.177 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 0.886 | 0.64 | 1.227 | ‐7% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.774 | 0.562 | 1.065 | 6% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | SFRADS 2002 | 0.986 | 0.768 | 1.266 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.977 | 0.767 | 1.243 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.998 | 0.772 | 1.29 | ‐1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 1.033 | 0.804 | 1.328 | ‐5% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.943 | 0.737 | 1.208 | 4% | |
| Loss of 3+ lines visual acuity at 24 months | Missing at random (available case analysis) | SFRADS 2002 | 0.869 | 0.732 | 1.031 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.876 | 0.745 | 1.03 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.862 | 0.718 | 1.033 | 1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 0.905 | 0.764 | 1.073 | ‐4% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.834 | 0.701 | 0.993 | 4% | |
| Loss of 6+ lines visual acuity at 6 months | Missing at random (available case analysis) | SFRADS 2002 | 0.842 | 0.478 | 1.482 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.81 | 0.466 | 1.41 | 4% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.868 | 0.491 | 1.536 | ‐3% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 0.929 | 0.528 | 1.635 | ‐10% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.757 | 0.433 | 1.325 | 10% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | SFRADS 2002 | 0.68 | 0.452 | 1.024 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.675 | 0.453 | 1.007 | 1% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.688 | 0.455 | 1.042 | ‐1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 0.73 | 0.486 | 1.098 | ‐7% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.636 | 0.424 | 0.955 | 6% | |
| Loss of 6+ lines visual acuity at 24 months | Missing at random (available case analysis) | SFRADS 2002 | 0.851 | 0.617 | 1.173 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | SFRADS 2002 | 0.858 | 0.631 | 1.166 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | SFRADS 2002 | 0.847 | 0.608 | 1.179 | 0% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | SFRADS 2002 | 0.929 | 0.676 | 1.277 | ‐9% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | SFRADS 2002 | 0.782 | 0.567 | 1.077 | 8% | |
| Loss of 3+ lines visual acuity at 6 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.403 | 0.184 | 0.884 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.406 | 0.187 | 0.881 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.405 | 0.184 | 0.892 | 0% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.434 | 0.199 | 0.948 | ‐8% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.379 | 0.173 | 0.828 | 6% | |
| Loss of 3+ lines visual acuity at 12 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.585 | 0.273 | 1.251 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.606 | 0.292 | 1.258 | ‐4% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.575 | 0.266 | 1.246 | 2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.698 | 0.33 | 1.474 | ‐19% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.5 | 0.234 | 1.064 | 15% | |
| Loss of 3+ lines visual acuity at 24 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.733 | 0.382 | 1.409 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.746 | 0.401 | 1.389 | ‐2% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.729 | 0.374 | 1.423 | 1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.868 | 0.456 | 1.651 | ‐18% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.627 | 0.328 | 1.198 | 14% | |
| Loss of 6+ lines visual acuity at 6 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.065 | 0.004 | 1.113 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.065 | 0.004 | 1.108 | 0% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.066 | 0.004 | 1.122 | ‐2% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.071 | 0.004 | 1.215 | ‐9% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.06 | 0.004 | 1.023 | 8% | |
| Loss of 6+ lines visual acuity at 12 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.341 | 0.073 | 1.598 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.354 | 0.077 | 1.62 | ‐4% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.336 | 0.071 | 1.586 | 1% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.425 | 0.092 | 1.972 | ‐25% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.28 | 0.06 | 1.303 | 18% | |
| Loss of 6+ lines visual acuity at 24 months | Missing at random (available case analysis) | Valmaggia 2002 | 0.714 | 0.246 | 2.076 | 0% |
| Odds of outcome in not observed twice odds of outcome in observed | Valmaggia 2002 | 0.722 | 0.256 | 2.038 | ‐1% | |
| Odds of outcome in not observed half odds of outcome in observed | Valmaggia 2002 | 0.712 | 0.243 | 2.092 | 0% | |
| Odds of outcome in not observed twice odds of outcome in observed in treatment group and odds of outcome in not observed half odds of outcome in observed in control group | Valmaggia 2002 | 0.882 | 0.307 | 2.536 | ‐24% | |
| Odds of outcome in not observed half odds of outcome in observed in treatment group and odds of outcome in not observed twice odds of outcome in observed in control group | Valmaggia 2002 | 0.584 | 0.202 | 1.682 | 18% |
Data and analyses
Comparison 1. RADIATION THERAPY VERSUS CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Three or more lines visual acuity lost at 6 months | 5 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 2 Three or more lines visual acuity lost at 12 months | 8 | 759 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.74, 1.10] |
| 3 Three or more lines visual acuity lost at 24 months | 4 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.03] |
| 4 Six or more lines visual acuity lost at 6 months | 5 | 502 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.94] |
| 5 Six or more lines visual acuity lost at 12 months | 7 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.44, 0.87] |
| 6 Six or more lines visual acuity lost at 24 months | 4 | 428 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.03] |
| 7 Mean and change in visual acuity at 12 months | 10 | Mean Difference (Random, 95% CI) | ‐0.10 [‐0.16, ‐0.04] | |
| 7.1 Mean visual acuity | 5 | Mean Difference (Random, 95% CI) | ‐0.16 [‐0.30, ‐0.02] | |
| 7.2 Change in visual acuity | 5 | Mean Difference (Random, 95% CI) | ‐0.08 [‐0.14, ‐0.01] | |
| 8 Investigating heterogeneity: type of CNV | 8 | 759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.09] |
| 8.1 Classic < 50% | 5 | 426 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.62, 1.34] |
| 8.2 Classic 50%+ | 3 | 333 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.10] |
| 9 Investigating heterogeneity: dosage | 8 | 759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.09] |
| 9.1 > 14 Gy | 3 | 308 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.50, 1.25] |
| 9.2 <= 14 Gy | 5 | 451 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.56, 1.21] |
| 10 Investigating heterogeneity: sham irradiation in control group | 8 | 759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.09] |
| 10.1 Control group observation only | 5 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.07] |
| 10.2 Control group sham irradiation | 3 | 340 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.60, 1.48] |
1.8. Analysis.

Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 8 Investigating heterogeneity: type of CNV.
1.10. Analysis.
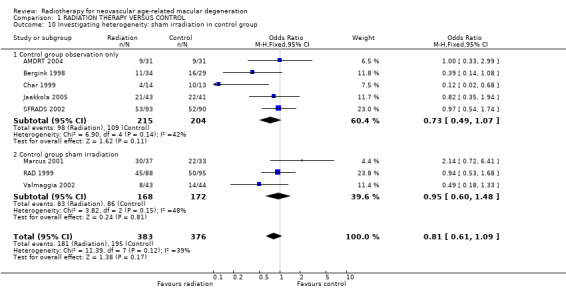
Comparison 1 RADIATION THERAPY VERSUS CONTROL, Outcome 10 Investigating heterogeneity: sham irradiation in control group.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMDLRTSG 2003.
| Methods | 2‐year prospective randomised controlled study at 18 sites in Japan. | |
| Participants | People with CNV due to AMD. | |
| Interventions | 10 fractions of 2 Gy external beam radiotherapy versus observation. | |
| Outcomes | Visual acuity, size of CNV. | |
| Notes | Information from trial from summary translation. | |
AMDRT 2004.
| Methods | Multicentre study: 10 sites. Randomisation stratified by lesion type (new or recurrent CNV following thermal laser photocoagulation) and blood (< 50% or >= 50%). |
|
| Participants | Country: USA.
Number randomised: 88. New CNV arm: mean age 77 years (range 63 to 92). Recurrent CNV arm: mean age 80 years (range 73 to 78). 58% women. Inclusion: visual acuity of at least 20/320 and subfoveal CNV (occult CNV, minimally classic CNV or predominantly classic CNV) with fibrosis if present comprising < 50% of the lesion not amenable to treatment. AMD confirmed by drusen > 63 μm or focal hyperpigmentation in either eye or evidence of CNV, geographic atrophy or serous detachment of the pigment epithelium in the non study eye. |
|
| Interventions | Treatment (n=41):External beam radiotherapy 20 Gy (5 x 4 Gy) 6 mv. Control : observation (n=25) or sham radiotherapy (n=22) depending on centre. | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Age‐related macular degeneration radiotherapy trial (AMDRT). Funded by the National Eye Institute and each participating institution. Sample size 100 patients; stopped early because of a low rate of recruitment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomised treatment assignment schedules, stratified by lesion type (new or recurrent) and status of blood (<50% or >=50% of the lesion) were generated for each clinical site" Page 819, methods, enrolment and randomisation procedures, 2nd paragraph. |
| Allocation concealment? | Low risk | “After required examinations and photography were completed, an eligibility checklist was faxed to the Coordinating Center. The enrolling ophthalmologist and clinic coordinator verbally confirmed eligibility of the patient by telephone with a Coordinating Center staff member. For centres performing sham radiotherapy, sealed, black‐lined security envelopes containing a randomized assignment were provided to the ophthalmology clinical staff. At enrollment, the clinic co‐ordinator confirmed with the Co‐ordinating center the assignment of the patient to the next sequentially numbered envelope for the appropriate strata. The sealed envelope was sent to the Radiation Oncology Department and opened by the radiation oncologist and radiation physicist immediately before treatment. For centers not performing sham radiotherapy, the coordinator called the Co‐ordinating center to obtain the treatment assignment" Page 819, methods, enrolment and randomisation procedures, 1st and 2nd paragraphs. |
| Blinding? Visual acuity | High risk | “At the outset, each center had the option to choose sham radiotherapy or observation only as the control treatment for active radiotherapy. Three centers chose sham radiotherapy.” Page 819, methods, 1st paragraph. “During follow‐up, examiners were masked to the patient’s treatment assignment” Page 820, 1st paragraph. It was obvious which group received radiotherapy. Only 3 out of 10 centers chose to perform sham radiotherapy. Only some of the control group (22/47) received sham radiotherapy. Visual acuity assessment was masked to treatment group, however, it is possible that an individual’s performance on the visual acuity test could be influenced by their perceptions as to which treatment they received. |
| Blinding? Lesion size on fluorescein angiography | Low risk | “Certified photographers performed all fundus photography and fluorescein angiography following SST protocols. Initial visit photography was required within 42 days of enrollment. Expert readers at the FPRC, masked to treatment assignment, reviewed all baseline photographs and angiograms for eligibility.” Page 820, photography and fluorescein angiography, 1st and 2nd paragraphs. Although the report does not explicitly state that photograph graders were masked to treatment assignment when considering follow‐up photographs and angiograms it is highly likely that they were and it is unlikely that a participant’s knowledge of treatment group would influence the appearance of photographs or fluorescein angiograms. |
| Incomplete outcome data addressed? All outcomes | High risk | 31/41 (76%) in treatment group seen at 12 months; 31/47 (66%) of the control group seen at 12 months. 12 enrolled patients were subsequently considered ineligible; all these patients included in the analysis. 5 patients did not get the treatment they were assigned but were analysed in the original group to which they were assigned. “Among all missed visits, the most common reason for not completing the visit was patient refusal; other reasons were illness and transportation problems” The follow‐up in the control group was rather low which is why this is marked “no”. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Unclear risk | "Patient enrollment began in January 2000 with a goal of 100 patients. One center had been conducting a single center clinical trial with the same protocol and consent procedures and had enrolled 23 patients before their multi‐center certification; these patients are included in the analysis. In September 2001, the Data and Safety Monitoring Committee (DSMC) recommended that recruitment be halted because of a low rate of enrollment." Page 819, methods, second paragraph. |
Anders 1998.
| Methods | Single centre. Allocation: not stated. Masking: participant ‐ no; provider ‐ no; outcome ‐ no. Exclusions after randomisation: not stated | |
| Participants | Country: Germany. Number randomised: 76. Mean age: 77.7. Sex: 67% women. Inclusion Criteria: 50+ years; visual acuity decrease (0.05 and 0.5); angiographically proven CNV. Exclusion criteria: previous laser photocoagulation to macula; previous radiation; other eye disease. | |
| Interventions | Treatment: 12 Gy (6 x 2 Gy). Control: observation. Duration: 8 days | |
| Outcomes | Visual acuity, near and distance; FFA. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | Unclear risk | Not reported. |
| Blinding? Lesion size on fluorescein angiography | Unclear risk | Not reported. |
| Incomplete outcome data addressed? All outcomes | High risk | 19/39 radiation group and 18/37 control group seen at 12 months. No information as to the reason for loss to follow‐up given. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Unclear risk | Not enough information. |
Bergink 1998.
| Methods | Single centre. Allocation: not stated. Masking: participant ‐ no; provider ‐ no; outcome ‐ no. Exclusions after randomisation: 3. | |
| Participants | Country: Netherlands. Number randomised: 74. Mean age: 74. Sex: 56% women. Inclusion criteria: 55+ years; visual acuity 20/200 or better; angiographically proven CNV; clinical signs of ARM; informed consent. Exclusion criteria: previous laser photocoagulation to macula; radiation for ear nose and throat or brain disease; diabetes. | |
| Interventions | Treatment: 24 Gy (4 x 6 Gy). Control: observation. Duration: 21 days. | |
| Outcomes | Visual acuity (Snellen); Doubling of CNV size (FFA). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "...patients were assigned randomly to either radiation treatment or observation." Page 322, materials and methods. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | High risk | "The patients in the control group did not receive a sham radiation treatment" Page 322, materials and methods. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "The readers were blinded for treatment status." Page 322, materials and methods. |
| Incomplete outcome data addressed? All outcomes | High risk | "Initially, 74 patients were included in the study. Of these, one died and two stopped before the first control, one because of fear of malignancies due to the treatment. In addition, one was excluded because of previously unnoted diabetes mellitus and two patients showed insufficient evidence for CNV on the angiogram later on. As a result, 68 patients, 36 in the treatment group and 32 in the observation group completed at least 3 months/ follow‐up. Twelve months follow‐up was obtained in 63 patients." Page 322, results. No information on the numbers originally randomised to treatment and control. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Low risk | |
Char 1999.
| Methods | Single centre. Allocation: not stated. Masking: participant ‐ no; provider ‐ no; outcome ‐ unclear (yes for FFA). Exclusions after randomisation: not stated. | |
| Participants | Country: USA. Number randomised: 27. Mean age: 76. Sex: 52% women. Inclusion criteria: Subfoveal CNV secondary to AMD with visual acuity less than 20/40. Exclusion criteria: | |
| Interventions | Treatment: 7.5 Gy. Control: observation. Duration: one day | |
| Outcomes | Visual acuity (ETDRS chart). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients were randomly assigned to either no treatment or to treatment with...." Page 575, methods. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | High risk | "... visual acuity examination with refraction by a trained ophthalmic technician, who was masked to the patients' status in the trial" Page 575, methods. However, patients were not masked which may influence visual acuity assessment. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "Initial and serial fluorescein angiograms were read in a masked manner by two observers...." Page 575, methods. Lack of masking of patients is unlikely to influence this outcome. |
| Incomplete outcome data addressed? All outcomes | High risk | 27 patients were entered in the trial with a mean follow‐up of 15 months (range of 7 to 32 months). In the radiation group mean follow‐up was 17 months. In the group assigned to observation the mean follow‐up was 16 months. In the methods it states that patients "were followed on a 3‐month basis" however it was not clear from the report why different patients had different lengths of follow‐up. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Low risk | |
Ciulla 2002.
| Methods | Single centre. Allocation: not stated. Masking: participant ‐ yes; provider ‐ yes; outcome ‐ yes. Exclusions after randomisation: not stated. | |
| Participants | Country: USA. Number randomised: 37. Median age: 71. Sex: 38% women. Inclusion criteria: Subfoveal CNV due to AMD; visual impairment of affected eye less than 6 months duration; best‐corrected VA of affected eye < = 20/40 and > = 20/400. Exclusion criteria: Unable to maintain steady fixation; preexisting retinal eye disease or media opacity; no informed consent. | |
| Interventions | Treatment: 16 Gy (2 x 8 Gy). Control: sham irradiation (not described). Duration: 2 days | |
| Outcomes | Visual acuity (ETDRS chart). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | Low risk | "Masked assessment of angiography and analysis of visual acuity between groups were performed" Page 905. Although this statement is not very clear as to whether the measurement of visual acuity was masked as the control group had sham irradiation we have assumed that measurement of visual acuity was masked. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "Masked assessment of angiography and analysis of visual acuity between groups were performed" Page 905. |
| Incomplete outcome data addressed? All outcomes | High risk | "Of the 37 subjects enrolled in this investigation [...] no data were recovered from seven subjects owing to four baseline discrepancies, one off‐protocol treatment due to equipment failure, and two discontinuations before the first treatment." Page 906. However, no information given as to which treatment group these exclusions belonged to and only data for 30 patients analysed.. At 12 months, 16/20 and 7/10 patients in treatment and control group respectively seen. Page 906, table 1. No reason given for loss to follow‐up. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Unclear risk | "Recruitment was halted at 37 subjects for ethical reasons regarding randomization to sham treatment when Foot and Drug Administration approval of Visudyne [...] was anticipated." Page 905. |
Eter 2002.
| Methods | Multicentre: 3 centres. Allocation: central telephone; blocked by centre. Masking: participant: no; provider: no; outcome: no. Exclusions after randomisation: 3 treatment, 1 control. | |
| Participants | Country: Germany. Number randomised: 45. Median age: 74. Sex: 53% women. Inclusion criteria: age 45+ years; classic/occult CNV; informed consent; no prior radiation treatment to head; no vascular eye disease; no prior treatment of AMD. Exclusion criteria: | |
| Interventions | Treatment: 20 Gy (10 x 2 Gy). Control: observation. Duration: one week. | |
| Outcomes | Visual acuity (logarithmic chart). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Forty‐five eyes of 45 patients [...] were assigned randomly in a ratio of 2:1 to either radiation treatment or observation." Page 14, patients and methods. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | High risk | Not reported. As control group was observation only assumed visual acuity assessment not masked. |
| Blinding? Lesion size on fluorescein angiography | High risk | Not reported. As control group was observation only assumed CNV assessment not masked. |
| Incomplete outcome data addressed? All outcomes | High risk | "Although 45 patients were randomized to either treatment or follow‐up, 27 patients in the radiation group and 15 patients in the control group could be enrolled in the study. Three patients were lost to follow‐up because motivation for further examinations was low and because they needed to be accompanied by relatives due to their age and visual acuity." Page 14, patients and methods. However, no information given as to which group the excluded patients belonged. No information given as to numbers examined at six month follow‐up. |
| Free of selective reporting? | High risk | See additional table 3. |
| Free of other bias? | Low risk | |
Jaakkola 2005.
| Methods | Single centre, masked. | |
| Participants | Country : Finland.
Number randomised: 86. Mean age: 75.5. 43 (40%) men; 52 (60%) women. |
|
| Interventions | Episceral brachytherapy. 8mm diameter, 16 Gy for 54 min vs 4 mm diameter ,12.6 Gy for 11 min | |
| Outcomes | Visual acuity (ETDRS chart). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported. |
| Allocation concealment? | Unclear risk | "Treatment allocation was performed by envelope randomization within CNV categories, as described below." Page 568, materials and methods, study design. Not really enough information to judge whether this was done properly. |
| Blinding? Visual acuity | High risk | "Visual acuity was measured [...] by an examiner masked against the treatment given to the patient." Page 569, evaluations and patient follow‐up. However patients were not masked. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "The angiograms were evaluated in a masked manner...." Page 569, angiographic and clinical evaluation. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 43/43 patients in radiotherapy group seen at 12 months however it was also reported that two patients had died in the interim. 39/43 patients in the control group (91%) seen at 12 months. Flow chart was confusing because at 6 months it was reported that four patients refused and at 12 months it was reported one patient refused. However same numbers 39/43 seen at both time points. Page 569, figure 1. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Low risk | |
Kacperek 2001.
| Methods | Single centre. Allocation: unclear. Masking: participant ‐ no; provider ‐ no; outcome ‐ no. Exclusions after randomisation: not stated. | |
| Participants | Country: UK. Number randomised: 66. Mean age: 76 years. Sex: Inclusion criteria: Aged 50+ with subfoveal CNV (classic) and evidence of AMD e.g. drusen, VA > 6/60. Exclusion criteria: diabetes, severe hypertension and retinal vascular disease, myopia. | |
| Interventions | Treatment: 18 Gy (4 x 4.5 Gy). Control: observation. Duration:4 days. | |
| Outcomes | Visual acuity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Patients [...] were randomised to between treatment and control". Page 7, introduction. |
| Allocation concealment? | Unclear risk | Not reported. |
| Blinding? Visual acuity | High risk | No masking reported. No sham intervention in the control group. |
| Incomplete outcome data addressed? All outcomes | High risk | 38 patients in the treatment arm, 28 for the control arm. 28/38 and 20/28 seen at 12 months. No information on people not seen. |
| Free of selective reporting? | High risk | See additional table 3. |
| Free of other bias? | Low risk | |
Kobayashi 2000.
| Methods | Single centre. Allocation: computer generated. Masking: participant ‐ no; provider ‐ yes; outcome ‐ unclear (yes for FFA). Exclusions after randomisation: not stated. | |
| Participants | Country: Japan. Number randomised: 101. Mean age: 72. Sex: 64% female. Inclusion criteria: 60+ years; unsuitability for laser under macular photocoagulation criteria; three or less months of new or progressive CNV; visual acuity 20/50 or worse. Exclusion criteria: pre‐existing ocular disease (glaucoma, severe myopia, chronic inflammation, neoplasia); diabetes; uncontrolled hypertension; known life‐threatening disease. | |
| Interventions | Treatment: 20 Gy (10 x 2 Gy). Control: observation. Duration: 14 days. | |
| Outcomes | Visual acuity (ETDRS); area of CNV (FFA); safety. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "One eye of each of the 101 patients was prospectively randomized to receive radiotherapy or no treatment." and "Within 24 hours after enrollment, the patients were randomized by means of computer‐generated numbers; patients assigned 0 received low‐dose radiotherapy and those assigned 1 received no treatment. Page 618, patients and methods. |
| Allocation concealment? | Low risk | "The treating physician (HK) was unaware of the patients' randomization state". Page 618, patients and methods. |
| Blinding? Visual acuity | High risk | "Assessment of outcomes, including visual acuity, angiographic interpretation, and assessment of complications and adverse events, was performed in a masked fashion." Page 618, patients and methods. However, patients not masked. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "Assessment of outcomes, including visual acuity, angiographic interpretation, and assessment of complications and adverse events, was performed in a masked fashion." Page 618, patients and methods. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | "The overall complete follow‐up rate was 84.1% (85/101) (Table 1 and Figure 1). there was no significant difference between the two groups; the complete follow‐up rate was 88.2% (45/51) and 80.0% (40/50) in the treatment group and control group, respectively. Six treated patients and 10 untreated patients were not evaluated, because five patiens died with intercurrent disease, six patients were to ill or frail to attend, and it was not possible to contact five patients. Page 619, results. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Low risk | |
Marcus 2001.
| Methods | Single centre. Allocation: computer generated; blocked. Masking: participant ‐ yes; provider ‐ yes; outcome ‐ yes. Exclusions after randomisation: not stated | |
| Participants | Country: USA. Number randomised: 83. Mean age: 76. Sex: 61% female. Inclusion criteria: active subfoveal CNV secondary AMD; >48 years of age; visual acuity > / = 20/400; clinical and angiographic evidence of a choroidal neovascular membrane, which is itself or its contiguous blood involving the centre of the foveal avascular zone. Exclusion criteria: previous laser treatment; choroidal neovascularisation due to other causes; retinal vascular diseases e.g. diabetes; previous ocular, orbital or periorbital radiation; likely candidates for chemotherapeutic agents. | |
| Interventions | Treatment: 14 Gy (7 x 2 Gy). Control: 1 sham treatment. Duration: 7 working days. | |
| Outcomes | Visual acuity (ETDRS); contrast sensitivity; appearance of fundus (FFA and photography). | |
| Notes | Patients with subfoveal choroidal neovascular membranes who were eligible for subfoveal laser according to macular photocoagulation study guidelines were offered laser versus radiation or observation versus radiation (this study). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The randomization incorporated blocking, which is recommended any time patient recruitment extends for a long period of time. Blocks of size 2 or 4 were assigned randomly, and a separate random permutation was used to assign the 2 treatments to the blocks. Page 172, patient selection, entry, and follow‐up. |
| Allocation concealment? | High risk | "A randomization schedule was printed and sent to the radiology team, how then sequentially allocated the patients to the sham or actual radiation treatments. Page 172, patient selection, entry, and follow‐up. |
| Blinding? Visual acuity | Low risk | "The patient, examining ophthalmologist, and ophthalmic technician were unaware of the assignment to observation or radiation treatment groups." Page 172, patient selection, entry, and follow‐up. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "The patient, examining ophthalmologist, and ophthalmic technician were unaware of the assignment to observation or radiation treatment groups." Page 172, patient selection, entry, and follow‐up. |
| Incomplete outcome data addressed? All outcomes | High risk | Radiation group n=41. 37 (90%) seen at one year, 4 with missing data. Control n=42. 33 (79%) seen at one year, 6 with missing data, 3 withdrawn. Page 175, table 2. |
| Free of selective reporting? | High risk | See additional table 3. |
| Free of other bias? | Low risk | |
RAD 1999.
| Methods | Multicentre: 9 centres. Allocation: computer generated. Masking: participant ‐ yes; provider ‐ yes; outcome ‐ yes. Exclusions after randomisation: | |
| Participants | Country: Germany. Number randomised: 205. Mean age: 74. Sex: 60% female. Inclusion criteria: 50+ years old; written informed consent; exudative AMD with subfoveal involvement and signs of ARM in the fellow eye; CNV 6+ disc diameters in size; visual acuity 20/320 or better in study eye; symptoms for six months or less. Exclusion criteria: ocular disease that could compromise the visual acuity in the study eye; haemorrhage; previous macular photocoagulation or PDT; history of antiangiogenic drugs. | |
| Interventions | Treatment: 16 Gy (8 x 2 Gy). Control: 8 x 0 Gy. Duration: 10 days. | |
| Outcomes | Visual acuity (ETDRS); FFA and fundus photography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "The randomization list was compiled generating random numbers using the statistical analysis systems SWAS, version 6.12. " Page 2240, Method of radiation and sham treatment, randomization procedure and masking |
| Allocation concealment? | Low risk | "To ensure concealment, external randomization by telephone was performed by the Biostatistics and Data Centre, Heidelberg, Germany." Page 2240, Method of radiation and sham treatment, randomization procedure and masking. |
| Blinding? Visual acuity | Low risk | "Patients in the placebo group were similarly planed and placed at the linear accelerator for 8 fractions with a dose of 8 x 0Gy. The machine noise during irradiation was simulated, and the technicians were instructed not to inform the patient about the mode of treatment. The sham treatment method was spread out over an identical time course as the radiation treatment." Page 2240, method of radiation therapy and sham treatment. "To ensure masking of patients and ophthalmologists, only the respective departments of radiation therapy were informed about treatment allocation. "Page 2240, Method of radiation and sham treatment, randomization procedure and masking. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "All angiograms were read by reviewers masked to treatment assignments." Page 2240, angiographic evaluation. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Radiation group 88/101 (87.1%) completed study 7 of these protocol deviations. Sham therapy group 95/104 (91.3%) completed study. Detailed information given on loss to follow‐up. Page 2241, figure 1. |
| Free of selective reporting? | High risk | See additional table 3. |
| Free of other bias? | Low risk | |
SFRADS 2002.
| Methods | Multicentre: 3 centres. Allocation: central telephone; blocked by centre. Masking: participant: no; provider: no; outcome: yes. Exclusions after randomisation: 3 treatment, 1 control. | |
| Participants | Country: UK. Number randomised: 203. Mean age: 75. Sex: 57% female. Inclusion criteria: Aged 60+; subfoveal CNV; 20/200 or better in study eye. Exclusion criteria: Inability to give informed consent; late leakage of indeterminate origin; blood under geometric centre of the fovea; other ocular disease; diabetes; other trials; prior radiotherapy. | |
| Interventions | Treatment: 12 Gy (6 X 2 Gy). Control: observation. Duration: | |
| Outcomes | Visual acuity (ETDRS chart); near vision (Bailey‐Lovie chart); radiation‐associated problems. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "To ensure balance within each of the 3 centers, the randomization was blocked." Hart et al, top of page 1031. |
| Allocation concealment? | Low risk | "The randomization code was kept at the coordinating center (Belfast) and released by telephone on receipt of patient details."Hart et al, bottom of page 1030 and top of page 1031. |
| Blinding? Visual acuity | High risk | "The optometrists who undertook visual assessments were unaware of the treatment status of the patients; however, neither the treating physicians nor the patients were masked". Hart et al, page 1030, patients and methods, 2nd paragraph. Although visual acuity assessment was masked to treatment group, physicians and patients were not. It is possible that an individual’s performance on the visual acuity test could be influenced by their perceptions as to which treatment they received. |
| Blinding? Lesion size on fluorescein angiography | Unclear risk | Outcome not reported so far. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | 101 allocated to treatment 102 to observation. 93/101 and 91/100 seen at 12 months. Not very good documentation for reasons for no follow‐up. |
| Free of selective reporting? | Unclear risk | See additional table 3. |
| Free of other bias? | Low risk | |
Valmaggia 2002.
| Methods | Single centre. Allocation: not stated. Masking: participant ‐ yes; provider ‐ yes; outcome ‐ yes. Exclusions after randomisation: not stated. | |
| Participants | Country: Switzerland. Number randomised: 161. Mean age ‐ 75. Sex: 58% female. Inclusion criteria: Symptoms of reduced vision, central scotoma or metamorphopsia. Exclusion criteria: foveal haemorrhage; severe haemorrhage impeding measurement of CNV; PED; other ocular disease (glaucoma, severe myopia, diabetic retinopathy). | |
| Interventions | Treatment: 8 Gy (4 X 2 Gy) or 16 Gy (4 X 4 Gy). Control: 1 Gy (4 X 0.25 Gy). Duration: 4 days. | |
| Outcomes | Visual acuity (logMAR chart); reading ability; CNV size (FFA/indocyanine green); radiation‐associated side effects (ocular irritation, conjunctivitis, cataract, radiation retinopathy, radiation optic neuropathy). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "The patients were stratified in four different subgroups according to the CNV type, size and duration of the symptoms" "According to the stratification, patients were randomized and treated in the Department of Radiation‐Oncology." Page 522, stratification. |
| Allocation concealment? | Unclear risk | "The collaborators in the Department of Ophthalmology and patients were not aware of the applied radiation dose. Colleagues in the Department of Radiation‐Oncology were only informed about the eye to be treated and the stratification code. " Page 522, stratification. |
| Blinding? Visual acuity | Low risk | "The collaborators in the Department of Ophthalmology and patients were not aware of the applied radiation dose." Page 522, stratification. |
| Blinding? Lesion size on fluorescein angiography | Low risk | "The collaborators in the Department of Ophthalmology and patients were not aware of the applied radiation dose." Page 522, stratification. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Control group 44/52 (85%) seen at 12 months; 8Gy group 52/57 (91%) seen at 12 months; 16Gy group 43 (83%) seen at 12 months. Page 524, table 2. |
| Free of selective reporting? | Low risk | See additional table 3. |
| Free of other bias? | Low risk | |
AMD: age‐related macular degeneration ARM: age‐related maculopathy CNV: choroidal neovascularisation ETDRS: Early Treatment of Diabetic Retinopathy Study FFA: fundus fluorescein angiography Gy: gray PDT: photodynamic therapy PED: pigment epithelial detachment
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avila 2009 | Not a randomised controlled trial. |
| Barak 2005 | No control group. |
| Bergink 1995 | Treatment groups probably not randomly allocated. |
| Brown 1997 | Treatment groups allocated sequentially. |
| Churei 2004 | Treatment groups not randomly allocated. |
| Eter 2001 | One eye treated and fellow eye served as a control. Unclear whether first eye treated randomly. |
| Heier 2008 | Avastin but not radiotherapy allocated randomly. |
| Honjo 1997 | Treatment groups probably not randomly allocated. |
| Mandai 1998 | Treatment groups probably not randomly allocated. |
| Mandai 2000 | Retrospective study ‐ groups not allocated randomly. |
| Marcus 2004 | Non‐randomised dose escalation study. |
| Matsuhashi 1996 | Treatment groups not allocated randomly. |
| Matsuhashi 2000 | Treatment groups not allocated randomly. Control group consisted of people who had refused radiation or laser treatment. |
| Postgens 1997 | Retrospective study ‐ groups not allocated randomly. |
| Saric 2001 | Control group consisted of patients who had refused treatment. |
| Taniguchi 1996 | Treatment and control groups probably not randomly allocated. |
| Tholen 2000 | This study initially began as an RCT but the trial was stopped because of radiogenic complications in the high dose group (36 Gy). The study was continued as a non‐randomised study and the reports did not distinguish randomised and non‐randomised comparisons. |
| Zambarakji 2006 | No untreated control group. |
Differences between protocol and review
The review has been substantially updated since the original protocol was written and new methods, such assessment of risk of bias, assessing the impact of missing data incorporated.
Contributions of authors
Conceiving the review: NHVC Designing the review, writing the protocol: NHVC Co‐ordinating the review: NHVC, JE Data collection for the review: ZO, VS, JE Screening search results: ZO, VS, JE Organising retrieval of papers: ZO, VS Screening retrieved papers against inclusion criteria: ZO, VS, JE Appraising quality of papers: ZO, VS, JE Abstracting data from papers: ZO, VS, JE Writing to authors of papers for additional information: NHVC, JE Obtaining and screening data on unpublished studies: ZO, VS Data management for the review: ZO, VS, JE Entering data into RevMan: ZO, VS, JE Analysis of data: JE Interpretation of data: JE, VS, NHVC Providing a clinical perspective: NHVC Writing the review: JE, VS, NHVC Updating the review: JE
Guarantor for the review: JE
Sources of support
Internal sources
No sources of support supplied
External sources
Guide Dogs for the Blind Association, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AMDLRTSG 2003 {published data only}
- Treatment of Age‐related Macular Degeneration with Low‐dose Radiation Therapy Study Group. The results of randomized controlled trial of low‐dose radiation for wet‐type age‐related macular degeneration on a 1‐year term basis. Nippon Ganka Gakkai Zasshi 2003;107(6):326‐30. [PubMed] [Google Scholar]
AMDRT 2004 {published data only}
- Marcus DM, Peskin E, Maguire M, Weissgold D, Alexander J, Fine S, et al. The AMDRT Research Group. The age‐related macular degeneration radiotherapy trials (AMDRT): one year results from a pilot study. American Journal of Ophthalmology 2004;138(5):818‐28. [DOI] [PubMed] [Google Scholar]
Anders 1998 {published data only}
- Anders N, Stahl H, Dorn A, Walkow T, Hosten N, Wust P, et al. Radiotherapy of exudative senile macular degeneration. A prospective controlled study [Strahlentherapie der exsudativen altersabhangigen Makuladegeneration. Eine prospektive kontrollierte Studie]. Ophthalmologe 1998;95(11):760‐4. [DOI] [PubMed] [Google Scholar]
Bergink 1998 {published data only}
- Bergink GJ, Hoyng CB, Maazen RW, Vingerling JR, Daal WA, Deutman AF. A randomised controlled trial of efficacy of radiation therapy in the control of subfoveal neovascularisation in age‐related macular degeneration: radiation verses observation. Graefe's Archive for Clinical and Experimental Ophthalmology 1998;236(5):321‐5. [DOI] [PubMed] [Google Scholar]
- Hoyng CB, Tromp AI, Meulendijks CFM, Leys A, Maazen RWM, Deutman AF, et al. Side effects after radiotherapy of age‐related macular degeneration with the Nijmegen technique. Graefe's Archive for Clinical and Experimental Ophthalmology 2002;240(5):337‐41. [DOI] [PubMed] [Google Scholar]
Char 1999 {published data only}
- Char DH, Irvine AI, Posner MD, Quivey J, Phillips TL, Kroll S. Randomised trial of radiation for age‐related macular degeneration. American Journal of Ophthalmology 1999;127(5):574‐8. [DOI] [PubMed] [Google Scholar]
Ciulla 2002 {published data only}
- Ciulla TA, Danis RP, Klein SB, Malinovsky VE, Soni PS, Pratt LM, et al. Proton therapy for exudative age‐related macular degeneration: a randomized, sham‐controlled clinical trial. American Journal of Ophthalmology 2002;134(6):905‐6. [DOI] [PubMed] [Google Scholar]
Eter 2002 {published data only}
- Eter N, Schuller H, Spitznas M. Radiotherapy for age‐related macular degeneration: is there a benefit for classic CNV?. International Ophthalmology 2002;24(1):13‐9. [DOI] [PubMed] [Google Scholar]
Jaakkola 2005 {published data only}
- Jaakkola A, Heikkonen J, Tommila P, Laatikainen L, Immonen I. Strontium plaque brachytherapy for exudative age‐related macular degeneration: three‐year results of a randomized study. Ophthalmology 2005;112(4):567‐73. [DOI] [PubMed] [Google Scholar]
Kacperek 2001 {published data only}
- Kacperek A, Briggs M, Sheen M, Damato BE, Errington RD, Harding S. Macular degeneration treatment at Clatterbridge Centre for oncology: treatment and preliminary results. Physica Medica 2001;17(Suppl 3):7‐9. [Google Scholar]
Kobayashi 2000 {published data only}
- Kobayashi H, Kobayashi K. Age‐related macular degeneration: long‐term results of radiotherapy for subfoveal neovascular membranes. American Journal of Ophthalmology 2000;130(5):617‐35. [DOI] [PubMed] [Google Scholar]
Marcus 2001 {published data only}
- Marcus DM, Camp MW, Sheils WC, McIntosh SB, Leibach DB, Johnson MH, et al. Sham radiation in clinical trials assessing radiotherapy for exudative age‐related macular degeneration. Retina 1999;19(6):525‐30. [DOI] [PubMed] [Google Scholar]
- Marcus DM, Sheils C, Johnson MH, McIntosh SB, Leibach DB, Maguire A, et al. External beam irradiation of subfoveal choroidal neovascularisation complicating age‐related macular degeneration. Archives of Ophthalmology 2001;1(19):171‐80. [PubMed] [Google Scholar]
RAD 1999 {published data only}
- The Radiation Therapy for Age‐Related Macular Degeneration (RAD) Group. A prospective, randomised, double‐masked trial on radiation therapy for neovascular age‐related macular degeneration. Ophthalmology 1999;106(12):2239‐47. [DOI] [PubMed] [Google Scholar]
- Vacha P, Debus J, Wiegel T, Schuchardt U, Schaefer U, Engenhart‐Cabillic R. A prospective randomized, double‐blind multicenter‐trial on radiation therapy for neovascular age‐related macular degeneration. European Journal of Cancer 2001;37:275 (A1015). [Google Scholar]
SFRADS 2002 {published data only}
- Hart PM, Chakravarthy U, Mackenzie G, Chisholm IH, Bird AC, Stevenson MR, et al. Visual outcomes in the subfoveal radiotherapy study: a randomized controlled trial of teletherapy for age‐related macular degeneration. Archives of Ophthalmology 2002;120(8):1029‐38. [DOI] [PubMed] [Google Scholar]
- Stevenson MR, Hart PM, Chakravarthy U, MacKenzie G, Bird AC, Owens SL, Chisholm IH, Hall V, Houston RF, McCulloch DW, Plowman N. Visual functioning and quality of life in the SubFoveal Radiotherapy Study (SFRADS): SFRADS report 2. British Journal of Ophthalmology 2005;89(8):1045‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valmaggia 2002 {published data only}
- Valmaggia C, Ries G, Ballinari P. A 5‐year follow‐up study for distance visual acuity after low dose radiation on subfoveal choroidal neovascularization in age‐related macular degeneration. Documenta Ophthalmologica 2001;103(3):201‐9. [DOI] [PubMed] [Google Scholar]
- Valmaggia C, Ries G, Ballinari P. Radiotherapy for subfoveal choroidal neovascularization in age‐related macular degeneration: a randomized clinical trial. American Journal of Ophthalmology 2002;133(4):521‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avila 2009 {published data only}
- Avila MP, Farah ME, Santos A, Duprat JP, Woodward BW, Nau J. Twelve‐month short‐term safety and visual‐acuity results from a multicentre prospective study of epiretinal strontium‐90 brachytherapy with bevacizumab for the treatment of subfoveal choroidal neovascularisation secondary to age‐related macular degeneration. British Journal of Ophthalmology 2009;93(3):305‐9. [DOI] [PubMed] [Google Scholar]
Barak 2005 {published data only}
- Barak A, Hauser D, Yipp P, Morse L, Leigh B, Kubo D, et al. A phase I trial of stereotactic external beam radiation for subfoveal choroidal neovascular membranes in age‐related macular degeneration. British Journal of Radiology 2005;78(933):827‐31. [DOI] [PubMed] [Google Scholar]
Bergink 1995 {published data only}
- Bergink GJ, Deutman AF, Broek JECM, Daal WAJ, Maazen RMW. Radiation therapy for age‐related subfoveal choroidal neovascular membranes. Documenta Ophthalmologica 1995;90(1):67‐74. [DOI] [PubMed] [Google Scholar]
Brown 1997 {published data only}
- Brown GC, Freire JE, Vander J, Brady LW, Shields CL, Shields JA, et al. Strontium‐90 brachytherapy for exudative, age related macular degeneration: a pilot study. International Journal of Radiation Oncology, Biology, Physics 1997;39(2, Suppl 1):332 (A2184). [Google Scholar]
Churei 2004 {published data only}
- Churei H, Ohkubo K, Nakajo M, Hokotate H, Baba Y, Ideue J, et al. External‐beam radiation therapy for age‐related macular degeneration: Two years' follow‐up results at a total dose of 20 Gy in 10 fractions. Radiation Medicine 2004;22(6):398‐404. [PubMed] [Google Scholar]
Eter 2001 {published data only}
- Eter N, Schuller H. External beam radiotherapy for age‐related macular degeneration causes transient objective changes in tear‐film function. Graefe's Archive for Clinical and Experimental Ophthalmology 2001;239(12):923‐6. [DOI] [PubMed] [Google Scholar]
Heier 2008 {published data only}
- Heier JS, Farah M, Duprat JP, Avila M, Fujii GY, Rossi J, et al. A study to evaluate the safety and feasibility of radiotherapy and bevacizumab (Avastin) for the treatment of subfoveal choroidal neovascularization (CNV) secondary to age‐related macular degeneration (AMD). The Macular Society 2008;186. [Google Scholar]
Honjo 1997 {published data only}
- Honjo M, Mandai M, Hiroshiba N, Miyamoto H, Takahashi M, Ogura Y, et al. Evaluation of the early‐phase response of neovascular membrane in age‐related macular degeneration to low‐dose radiation. Japanese Journal of Clinical Ophthalmology 1997;51(9):1563‐9. [Google Scholar]
Mandai 1998 {published data only}
- Mandai M, Takahashi M, Miyamoto H, Hiroshiba N, Kimura H, Ogura Y, et al. Longterm effects of radiation treatment for age‐related macular degeneration. Japanese Journal of Clinical Ophthalmology 1998;52:567‐71. [DOI] [PubMed] [Google Scholar]
Mandai 2000 {published data only}
- Mandai M, Takahashi M, Miyamoto H, Hiroshiba N, Kimura H, Ogura Y, et al. Long‐term outcome after radiation therapy for subfoveal choroidal neovascularization associated with age‐related macular degeneration. Japanese Journal of Ophthalmology 2000;44(5):530‐7. [DOI] [PubMed] [Google Scholar]
Marcus 2004 {published data only}
- Marcus DM, Sheils WC, Young JO, McIntosh SB, Johnson MH, Alexander J, et al. Radiotherapy for recurrent choroidal neovascularisation complicating age related macular degeneration. British Journal of Ophthalmology 2004;88(1):114‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuhashi 1996 {published data only}
- Matsuhashi H, Takahashi D, Noda Y, Mariya Y, Tarusawa N, Yoshimoto H, et al. Low‐dose radiation, therapy for choroidal neovascularization in age‐related macular degeneration. Nippon Ganka Gakkai Zasshi 1996;100:803‐9. [PubMed] [Google Scholar]
Matsuhashi 2000 {published data only}
- Matsuhashi H, Noda Y, Takahashi D, Mariya Y. Radiation therapy for small choroidal neovascularization in age‐related macular degeneration. Japanese Journal of Ophthalmology 2000;44(6):653‐60. [DOI] [PubMed] [Google Scholar]
Postgens 1997 {published data only}
- Postgens H, Bodanowitz S, Kroll P. Low‐dose radiation therapy for age‐related macular degeneration. Graefe's Archive for Clinical and Experimental Ophthalmology 1997;235(10):656‐61. [DOI] [PubMed] [Google Scholar]
Saric 2001 {published data only}
- Saric B, Sikic J, Katusic D, Vukojevic N. Brachytherapy ‐ optional treatment for choroidal neovascularization secondary to age‐related macular degeneration. Collegium Antropologicum 2001;25 Suppl:89‐96. [PubMed] [Google Scholar]
Taniguchi 1996 {published data only}
- Taniguchi T, Mandai M, Honjo M, Matsuda N, Miyamoto H, Takahashi M, et al. Radiation treatment for age‐related macular degeneration. Japanese Journal of Clinical Ophthalmology 1996;50:1821‐6. [Google Scholar]
Tholen 2000 {published data only}
- Tholen AM, Meister A, Bernasconi PP, Messmer EP. Radiotherapy for choroidal neovascularization (CNV) in age‐related macular degeneration (AMD) [Radiotherapie von choroidalen Neovaskularisationen (CNV) bei altersabhangiger Makuladegeneration (AMD)]. Klinische Monatsblatter fur Augenheilkunde 2000;216(2):112‐5. [DOI] [PubMed] [Google Scholar]
- Tholen AM, Meister A, Bernasconi PP, Messmer EP. Radiotherapy for choroidal neovascularization in age‐related macular degeneration. A pilot study using low‐ versus high‐dose photon beam radiation [Radiotherapie von subretinalen Neovaskularisationsmembranen bei altersabhangiger Makuladegeneration (AMD). Niedrig‐ versus hochdosierte Photonenbestrahlung]. Ophthalmologe 1998;95(10):691‐8. [DOI] [PubMed] [Google Scholar]
Zambarakji 2006 {published data only}
- Zambarakji HJ, Lane AM, Ezra E, Gauthier D, Goitein M, Adams JA, Munzenrider JE, Miller JW, Gragoudas ES. Proton beam irradiation for neovascular age‐related macular degeneration. Ophthalmology 2006;113(11):2012‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bressler 2002
- Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age‐related macular degeneration: two‐year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization. Verteporfin in photodynamic therapy report 2. Ophthalmology 2002;133(1):168‐9. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Hart 1999
- Hart PM, Chakravarthy U, Stevenson MR, Jamison JQ. A vision specific functional index for use in patients with age related macular degeneration. British Journal of Ophthalmology 1999;83(10):1115‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Section 6. In:The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2009
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane‐handbook.org.
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, Williamson PR. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
TAP Study 1999
- TAP Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age‐related macular degeneration with verteporfin: one‐year results of 2 randomized clinical trials ‐ TAP report. Archives of Ophthalmology 1999;117(10):1329‐45. [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JPT, Wood AM. Allowing for uncertainty due to missing data in meta‐analysis‐Part 1: two‐stage methods. Statistics in Medicine 2008;27(5):711‐27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sivagnanavel 2004
- Sivagnanavel V, Evans JR, Ockrim Z, Chong V. Radiotherapy for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004004.pub2] [DOI] [PubMed] [Google Scholar]


