Abstract
Background
Patients suffering from inoperable chronic critical leg ischaemia (NR‐CCLI) face amputation of the leg. Spinal cord stimulation (SCS) has been proposed as a helpful treatment in addition to standard conservative treatment.
Objectives
To find evidence for an improvement on limb salvage, pain relief, and the clinical situation using SCS compared to conservative treatment alone.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched January 2013) and CENTRAL (2012, Issue 12).
Selection criteria
Controlled studies comparing the addition of SCS with any form of conservative treatment to conservative treatment alone in patients with NR‐CCLI.
Data collection and analysis
Both authors independently assessed the quality of the studies and extracted data.
Main results
Six studies comprising nearly 450 patients were included. In general the quality of the studies was good. No study was blinded due to the type of intervention.
Limb salvage after 12 months was significantly higher in the SCS group (risk ratio (RR) 0.71, 95% confidence interval (CI) 0.56 to 0.90; risk difference (RD) ‐0.11, 95% CI ‐0.20 to ‐0.02). Significant pain relief occurred in both treatment groups, but was more prominent in the SCS group where the patients required significantly less analgesics. In the SCS group, significantly more patients reached Fontaine stage II than in the conservative group (RR 4.9, 95% CI 2.0 to 11.9; RD 0.33, 95% CI 0.19 to 0.47). Overall, no significantly different effect on ulcer healing was observed with the two treatments.
Complications of SCS treatment consisted of implantation problems (9%, 95% CI 4 to 15%) and changes in stimulation requiring re‐intervention (15%, 95% CI 10 to 20%). Infections of the lead or pulse generator pocket occurred less frequently (3%, 95% CI 0 to 6%). Overall risk of complications with additional SCS treatment was 17% (95% CI 12 to 22%), indicating a number needed to harm of 6 (95% CI 5 to 8).
Average overall costs (one study) at two years were EUR 36,500 (SCS group) and EUR 28,600 (conservative group). The difference (EUR 7900) was significant (P < 0.009).
Authors' conclusions
There is evidence to favour SCS over standard conservative treatment alone to improve limb salvage and clinical situations in patients with NR‐CCLI. The benefits of SCS must be considered against the possible harm of relatively mild complications and the costs.
Keywords: Humans; Amputation, Surgical; Chronic Disease; Controlled Clinical Trials as Topic; Ischemia; Ischemia/therapy; Leg; Leg/blood supply; Limb Salvage; Limb Salvage/methods; Randomized Controlled Trials as Topic; Spinal Cord Stimulation; Spinal Cord Stimulation/adverse effects; Spinal Cord Stimulation/methods
Plain language summary
Spinal cord stimulation for patients with chronic critical leg ischaemia who cannot have blood vessel surgery
Peripheral arterial disease is relatively common, particularly in late middle age. Blockages in the leg arteries can reduce blood flow in the legs enough to cause fatigue and pain in the muscles when walking (called intermittent claudication). This can become severe and cause critical limb ischaemia which can result in rest pain, leg ulceration, and gangrene that requires amputation. Surgery may improve blood flow but is not possible for everybody. Drugs may be used to relieve pain, improve leg circulation, and treat infection. Another option for patients who cannot have surgery is spinal cord stimulation (SCS). This involves stimulating nerves in the spine to help reduce pain and increase healing of ulcers by improving the local blood circulation in the affected leg. The review authors included five randomised and one controlled clinical trial involving a total of nearly 450 patients. In general the quality of the studies was good. Amputation after 12 months was required less often when SCS was added to standard care. Significant pain relief occurred with and without SCS but patients in the SCS group required fewer pain killers. Overall there was no difference on ulcer healing rates between the two treatment groups. Complications of SCS treatment consisted of problems with initially implanting the electrodes, in 8% of patients, and the need for repeat surgery because of electrode or lead failures in 12% of patients; infections occurred less frequently (3%). The average overall costs at two years were calculated in one study and found to be EUR 36,500 in the SCS group and EUR 28,600 in the conservative treatment alone group.
Background
The treatment of chronic critical limb ischaemia (CCLI) is a challenge in vascular medicine since comorbidity (the presence of two or more coexisting medical conditions) and mortality are high due to the presence of systemic atherosclerosis. The natural history of CCLI is difficult to describe because of differences in definitions used and the proportion of patients who are revascularised. It is estimated that 10% to 30% of patients with CCLI will die within six months and another 25% to 35% will undergo a major amputation (TASC 2000a). Revascularisation (surgical restoration of blood flow to an organ) is the therapy of choice in CCLI patients, used to relieve ischaemic rest pain, heal ischaemic ulcers or gangrene, and to avoid major (foot or higher) amputation (TASC 2000b). Nevertheless, for some patients vascular surgery has no realistic chance of success despite technical progress, an aggressive surgical approach, and repetitive reconstructions. Their pain is often disabling, adversely affects their quality of life and severely limiting their activity levels.
The only option for these patients with non‐reconstructable chronic critical leg ischaemia (NR‐CCLI), so far, is conservative treatment consisting of analgesics, vasodilators, and anticoagulants; before amputation ultimately becomes necessary. Alternative or additional therapies have been sought to improve limb salvage and quality of life. These include surgical interruption of the sympathetic nerves to promote vasodilation and relieve pain (sympathectomy), or spinal cord stimulation (SCS). SCS has been proposed as an aid in the management of chronic, intractable pain of the trunk or limbs. Several pre‐clinical and clinical studies using SCS have been performed to investigate potential beneficial effects such as reduction in amputation rate, pain relief, and healing of ulcers. The most desired effect is limb salvage.
Cook et al were the first investigators to use SCS in patients with peripheral vascular disease (Cook 1976). They reported a striking relief of pain and increased healing of ulcers. In some publications SCS appeared to provide good‐to‐excellent pain control in 60% to 82% of patients (Bunt 1991; Claeys 1998; Fiume 1989; Horsch 1994; Jacobs 1990; Kumar 1997; Mingoli 1993; Rickman 1994; Tesfaye 1996). A reduction in use of oral pain medications was found (Mingoli 1993; Spiegelmann 1991; Tesfaye 1996) whilst others described an improvement in claudication distance (Tallis 1983). Increases in skin temperature have been registered by thermographic recordings and measurements of skin temperature (Augustinsson 1985; Broseta 1986). Improvements in activities of daily living were also shown (Fiume 1989; Linderoth 1992; Rickman 1994; Spiegelmann 1991; Tesfaye 1996). These results have been confirmed by other studies (Augustinsson 1985; Broseta 1986; Jacobs 1990).
Postulated beneficial effects of SCS on the ischaemic leg could be attributed to an improvement in the microcirculatory status of the limb. The nutrition of the skin is determined by the peripheral microcirculatory blood flow. Several authors saw an increase in direct and indirect parameters of skin microcirculation, such as capillary flow and density of capillaries perfused (Jacobs 1990), as well as skin temperature of the foot (Augustinsson 1985; Broseta 1986). Local transcutaneous oxygen tension (TcpO2) was suggested as a predictive factor of success (Galley 1994; Gersbach 1997; Kumar 1997; Spincemaille 2001; Ubbink 1999). Doppler ultrasound recordings showed a tendency toward normalisation of the pulse wave morphology (Broseta 1986). However, few of the above‐mentioned studies were randomised or controlled.
Objectives
To identify and summarise the evidence for the effectiveness of SCS in the treatment of patients suffering from non‐reconstructable chronic critical leg ischaemia (NR‐CCLI) as compared with conservative treatment alone. This usually consists of analgesic, vasodilatory or anticoagulant medications and local wound care.
Primary objective
To assess the effect of SCS on limb salvage.
Secondary objective
To assess the effect of SCS on pain relief, wound healing, quality of life, costs and complications caused by SCS.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) evaluating the effectiveness of SCS in patients with NR‐CCLI. Controlled clinical trials (CCTs) were also included as it was expected that the number of RCTs would be limited.
To be in agreement with the reporting standards definition of CCLI (TASC 2000c), these studies had to present an objective measure of peripheral arterial disease, such as a systolic ankle blood pressure < 50 mm Hg, a toe blood pressure < 30 mm Hg, a TcpO2 < 30 mm Hg, to ensure that the clinical manifestations of CCLI were indeed caused by peripheral arterial disease.
Studies with patients suffering from severe peripheral arterial disease according to their peripheral pressures but without symptoms of CCLI (subcritical ischaemia) were excluded.
Types of participants
Inclusion criteria
Men and women aged over 18 years with atherosclerotic non‐reconstructable chronic critical leg ischaemia (NR‐CCLI).
Chronic critical leg ischaemia was defined according to the TransAtlantic inter‐Society Consensus document (TASC 2000c), which is based on the clinical symptoms predominantly caused by peripheral arterial disease (that is ischaemic rest pain and ulceration).
Non‐reconstructability was defined as the situation in which the treating physician saw no surgical options for the patient to treat the critically ischaemic leg at the time of trial inclusion. This can occur when no crural arteries can be found on the angiogram, as potential sites for a distal bypass procedure; no suitable veins are available as bypass material; or when the patient's condition precludes an operation.
Exclusion criteria
The term 'atherosclerotic' excluded patients suffering from critical leg ischaemia solely due to non‐atherosclerotic vascular diseases, like Raynaud's disease or Buerger's disease. Inclusion of these patients would cause an undesired heterogeneity of the study population.
Milder forms of leg ischaemia, in particular intermittent claudication, are not considered suitable for SCS treatment because this condition does not generally require vascular reconstruction.
Types of interventions
SCS treatment was compared with other forms of non‐surgical treatment, such as analgesics, vasodilatory or anticoagulant medications (including prostaglandins) and local wound care.
Types of outcome measures
Primary outcomes
Limb salvage, which ended the moment a major (foot or higher) amputation took place.
Secondary outcomes
Pain relief, wound healing, SCS complications, quality of life, and costs; according to the scales used in the various articles.
Search methods for identification of studies
Searches were not limited by language or publication status.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched January 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 12, part of The Cochrane Library, (www.thecochranelibrary.com). See (Appendix 1) for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the (Specialised Register) section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
For the original review, the authors searched MEDLINE, EMBASE, and CINAHL using a search strategy devised from the key words listed in (Appendix 2).
Searching other resources
We contacted authors of included RCTs or CCTs for additional data or unpublished studies, and searched the bibliographies of the trials identified through the search process. We also approached the research centre of the major manufacturer of neurostimulators (Bakken Research Centre, Maastricht, The Netherlands). They were not aware of any recently finished or ongoing studies at the time of updating the review.
Data collection and analysis
Selection of studies
The titles and abstracts of references identified by the search were screened independently by two authors (DU, HV) for their potential relevance and design. Full versions of articles were obtained and checked independently to identify those that met the inclusion criteria.
Assessment of methodological quality
The methodological quality of each trial was systematically assessed by the same two authors (DU, HV). A third independent referee (ASG) from the Department of Clinical Epidemiology and Biostatistic assessed the two trials co‐authored by DU. The assessment was performed according to the Dutch Cochrane Centre list of factors (Dutch CC) relating to internal and external validity. This list includes the following.
1. Was allocation of intervention randomised? 2. Was allocation concealed from the researcher who included the patients? 3. Was the proportion of participants with completed follow up > 80%? 4. Was an intention‐to‐treat analysis used? 5. Were all participants blinded to the intervention? 6. Were all healthcare workers blinded for the intervention? 7. Were all outcome assessors blinded for the intervention? 8. Were both groups comparable at baseline? 9. Were both groups, apart from the investigated intervention, treated the same? 10. Are the results of this trial valid and applicable? 11. Was the sample size based on a proper a priori calculation?
Data collection and extraction
Details of the studies selected were extracted and summarised independently by two authors (DU, HV) using a data extraction sheet from the Dutch Cochrane Centre (Dutch CC). If data were missing from reports of trials, attempts were made to contact the authors to obtain the missing information. Trials published in duplicate were included only once. Using the Dutch Cochrane Centre data form, the following information was captured:
(1) characteristics of the trial (design, method of randomisation, withdrawals and dropouts, funding source); (2) trial participants (gender, age, wound characteristics, comorbidity, co‐interventions); (3) intervention (SCS); (4) comparison intervention (non‐surgical therapies); (5) primary outcome measure (limb salvage); (6) secondary outcome measures (pain relief, wound healing, quality of life, complications, costs, TcpO2); (7) results.
Data analysis
Quantitative data were entered into the Cochrane RevMan 4.1 software and analysed using MetaView. For each trial and outcome, summary estimates of treatment effect (with 95% confidence intervals (CI)) were calculated for each comparison. For dichotomous outcomes the risk ratio (RR) and risk reduction (RD) were calculated (Deeks 1998). For continuous outcomes the mean difference (MD), or standardised mean difference (SMD), was used, where appropriate.
Analyses were performed for the studies, separately and combined. Stratification for the presence of diabetes mellitus could not be performed because too little information was given as to the endpoints in diabetic patients. Results of clinically homogeneous trials (trials for which the participants, interventions, outcome measures, and timing of the follow‐up measurements were considered to be similar enough) were combined using a fixed‐effect model. A random‐effects model was used in the case of statistical heterogeneity (assessed by visual inspection of the forest plots) and the availability of at least five studies. No pooling could be performed when an endpoint was addressed by only one study or when the study did not mention the standard deviations.
Results
Description of studies
Nineteen reports were found on the basis of the inclusion criteria, nine of which were excluded. Of the nine excluded studies: four were excluded because they were not RCTs or CCTs (Claeys 1997b; Palombo 1995; Petrakis 2000; Tallis 1983). One reference was only a description of the study design (Klomp 1995), and one included reconstructable patients (Guarnera 1994). Three references were duplicate publications (Claeys 1997a; Claeys 1998b; Claeys 1999).
The resulting 10 papers, all published since 1994, described six trials. There were five national studies including: one Belgian (Suy 1994), one Swedish (Jivegard 1995), one German (Claeys 1996), and two Dutch studies (ESES; Spincemaille 2000a); and one international study, SCS‐EPOS, (Amann 2003). Data were obtained for inclusion in the review. The study sizes varied from 37 to 120 patients, adding up to a total of 444 participating patients. Five out of the 10 papers (Klomp 1999; Klomp 2006; Spincemaille 2000b; Spincemaille 2000c; Ubbink 1999) focused on the Dutch ESES study (ESES), each addressing a different endpoint or aspect. In particular, Klomp (Klomp 1999), described overall limb salvage, ulcer healing, and costs; Klomp 2006 compared costs and effectiveness; Spincemaille (Spincemaille 2000b), focused on the effects on pain and quality of life; Spincemaille (Spincemaille 2000c) addressed the complications of SCS; and Ubbink (Ubbink 1999) described the effects on TcpO2.
All studies included patients suffering from non‐reconstructable critical leg ischaemia, that is having either ischaemic rest pain or ulcers smaller than 3 cm in diameter and on the basis of their clinical symptoms, peripheral blood pressure parameters and angiographic findings. The percentage of patients having only ischaemic rest pain ranged from 24% to 49% among the studies. In the German study (Claeys 1996) only patients with ischaemic ulcers were included; while in the Belgian study (Suy 1994) 11 patients with Buerger's disease were also enrolled. These 11 patients were excluded from the limb salvage meta‐analysis.
In all studies, patients received control treatment with or without SCS. In the German study (Claeys 1996) and SCS‐EPOS study (Amann 2003), a period of test stimulation was applied before definitive implantation of the SCS pulse generator. Control treatment consisted of optimum conservative treatment (local wound care, analgesics, anticoagulants, and antibiotics, if deemed necessary), which was comparable among the studies. In the German study (Claeys 1996) additional prostaglandin therapy was given to both groups.
In two studies, the transcutaneous oxygen (TcpO2) measurements were used for patient classification. In the ESES study (ESES) a subgroup analysis was performed after classification of the patients on the basis of their baseline TcpO2 values. The SCS‐EPOS study (Amann 2003) compared: patients treated with SCS who matched certain TcpO2 and stimulation criteria; patients receiving SCS but not matching these criteria; and patients who received control treatment as SCS was regarded not to be a useful option, either on the basis of the initial TcpO2 or an insufficient response to a period of test stimulation.
All studies used limb salvage (major amputation rate after 12 months) as the primary endpoint. Furthermore, in most studies pain relief and Fontaine stage (Fontaine 1954) were scored, and in some studies complications of SCS and quality of life were also noted.
Risk of bias in included studies
All studies were randomised trials except for the SCS‐EPOS study (Amann 2003), which was a controlled clinical trial. This study refrained from randomisation in order to obtain sufficient patients for evaluation as it was anticipated from the experience of the previous studies that the number of eligible, non‐reconstructable patients with chronic limb ischaemia would be limited. To pursue a randomised design would require a very long study duration, which was considered not to be feasible. Nevertheless, this study was included because it comprised the same target population, and the therapeutic decision making reflected the actual clinical situation.
Randomisation in the ESES study (ESES) was performed by means of a random numbers table via an independent randomisation institute. The other studies provided no specific allocation information. Sample size calculation was performed only in the Klomp (ESES) and SCS‐EPOS (Amann 2003) studies.
Blinding of the treating physicians and patients was impossible due to the nature of the treatment, which is based on the correct location of the electrode so that the paraesthesiae (tingling sensation) induced by the electrical stimulation reaches the painful area. This prerequisite requires communication between physician and patient. The effect authors were, therefore, also not blinded.
In every study the groups were comparable at baseline as to the patients' demographic and risk factors. In most studies a considerable number of patients died during follow up. This was due to the fact that the studies included elderly patients who suffered from generalised atherosclerosis, which reduces life expectancy. Drop outs due to death during follow up were accounted for in all studies. The follow up on the remaining patients was (nearly) complete in all studies. In all RCTs an intention‐to‐treat analysis was performed. In the ESES study (ESES) an additional per protocol analysis was done because 13% of the patients received suboptimal stimulation.
Effects of interventions
Limb salvage
The Belgian (Suy 1994), Swedish (Jivegard 1995), German (Claeys 1996), ESES (ESES), and SCS‐EPOS (Amann 2003) studies did not show a significant difference between groups in amputation frequency after 12, 18, or 24 months of follow up; although all studies showed a trend towards a better amputation‐free salvage in the SCS group (for example Spincemaille 2000a: P = 0.08). This trend was stronger in a subgroup of patients selected by their initial TcpO2 as compared to the overall result (P = 0.17 versus P = 0.47, respectively) (Ubbink 1999). In the SCS‐EPOS study (Amann 2003), the difference in cumulative limb salvage was significantly better in the patients treated with SCS (P = 0.003), and in particular in a subgroup selected based on their initial TcpO2 and response to test stimulation (P = 0.002). In a subgroup of normotensive patients, the amputation rate after 18 months was lower in the SCS group (P = 0.045) (Jivegard 1995).
The baseline risk of amputation was about 50% in all studies (ranging from 46% to 64%), except in the Claeys study (Claeys 1996) in which it was 20%.
Pooling of the results yielded a significant effect of SCS after 12 months (RR 0.74, 95% CI 0.57 to 0.94; RD ‐0.11, 95% CI ‐0.20 to ‐0.02). This effect did not change substantially after exclusion of the single non‐randomised SCS‐EPOS study (Amann 2003) (RR 0.78, 95% CI 0.58 to 1.04; RD ‐0.09, 95% CI ‐0.19 to 0.01), or the Claeys study (Claeys 1996) which included only patients in Fontaine stage IV (RR 0.73, 95% CI 0.56 to 0.94; RD 0.13, 95% CI 0.02 to 0.23). This result means that nine patients need to be treated to prevent one more major amputation (NNT 9, 95% CI 5 to 50).
Pain relief
When judged with the visual analogue scale (VAS) (Carlsson 1983), pain relief was found to be significantly better in the SCS group after three months (P = 0.0004) (Spincemaille 2000a) and 12 months (P < 0.01) (Jivegard 1995). However, in the ESES study (Spincemaille 2000b) a highly significant reduction in pain scores (from a mean of 50 to 25) during follow up was found but with no difference between the two treatment groups.
The pain rating index (PRI), as part of the McGill pain questionnaire (Melzack 1975), also showed a significant decrease during follow up in both groups (Spincemaille 2000b). During follow up, patients receiving SCS used significantly less non‐narcotic and narcotic analgesics than the patients treated conservatively (ESES; Spincemaille 2000b), as measured with the medication quantification scale (Masters‐Steed 1992).
In patients who underwent a major amputation during the follow‐up period, the VAS score declined significantly (P < 0.001) in both treatment groups. This held also for the PRI (P = 0.001). Thus, pain relief in amputated patients was substantially better than in non‐amputated patients, irrespective of the treatment (Spincemaille 2000b).
Pooling could not be performed due to missing standard deviations.
Clinical improvement
In the studies of Claeys (Claeys 1996) and Suy (Suy 1994), the number of patients whose clinical stage improved from critical leg ischaemia to claudication was significantly higher in the SCS than in the conservative group (P = 0.0014) (Claeys 1996). When pooled, the RR was 4.9 (95% CI 2.0 to 11.9) and the RD 0.33 (95% CI 0.19 to 0.47). This means three patients have to be treated with SCS for one patient to reach Fontaine stage II (NNT 3, 95% CI 2 to 5).
Two studies reported on the healing of ischaemic ulcers (Claeys 1996; ESES). Claeys found SCS had a significantly better effect on wound healing than conservative treatment (P = 0.013), whereas Klomp (ESES) found no significant difference. Pooling resulted in no significant difference between the two treatment modalities.
When subgroups of patients with and without hypertension were compared for ulcer healing, Claeys (Claeys 1996) found a significantly better effect of SCS than conservative treatment in normotensives, whereas Suy (Suy 1994) found a better SCS effect in hypertensives.
No significant differences in treatment effects were found between diabetic and non‐diabetic patients (Claeys 1996; ESES).
Ankle brachial pressure index (ABPI)
The study of Claeys (Claeys 1996) showed a significant difference in ABPI change during follow up: 0.03, an increase of 10% compared to baseline in the SCS group; and ‐0.058, a decrease of 17% compared to baseline in the conservative treatment group (P < 0.02). Only SCS patients who achieved complete ulcer healing showed a significant ABPI increase of 0.09 (P < 0.01). However, Jivegard (Jivegard 1995) found no change in ABPI in either groups during treatment. Pooling could not be performed due to missing standard deviations.
Quality of life
In the SCS‐EPOS study (Amann 2003) an SF‐12 questionnaire was used during follow up only for the SCS‐treated patients. This showed no worsening of the overall quality of life. The ESES study (ESES), described by Spincemaille (Spincemaille 2000b), applied the Nottingham health profile (NHP) and the Euroqol in both treatment groups. The overall score of the NHP decreased (that is quality of life improved) during follow up in both treatment groups. The mobility score of the NHP was significantly better in the patients treated with SCS (P < 0.01). In case of an amputation, mobility was reduced and not influenced by rehabilitation programmes. The Euroqol also showed an improvement after 12 months, in the SCS and conservative groups (SCS: from 54 to 11; conservative: from 51 to 10). After amputation these scores worsened to 66 and 61, respectively, but recovered over a period of several months to reach similar values to those of non‐amputated patients.
Local transcutaneous oxygen tension (TcpO2)
In the Claeys study (Claeys 1996) the TcpO2 (measured with a Hellige device), which was comparable at baseline (SCS: 10 mm Hg, conservative: 12 mm Hg), was significantly higher in the SCS group after 12 months of treatment (21 mm Hg versus 11 mm Hg; P < 0.001).
In the ESES study (Ubbink 1999) TcpO2 (using a radiometer device) was similar at baseline (10 mm Hg in both groups) and increased significantly (P < 0.05) during follow up, but was not significantly different between groups. This was found carrying forward the results before amputation.
Only the mean TcpO2 results after 12 months could be pooled. Using a random‐effects model, no significant difference could be detected.
Complications
No differences in mortality were observed. Usually only the complications of SCS treatment were mentioned. In the ESES study (ESES) side effects of medical treatment in the conservative group were upper gastrointestinal bleeding (three), nausea (seven), and dizziness (two); while in the SCS group duodenal perforation (one), nausea (two), and pruritus (itching) (one) were recorded.
Initial implantation problems (technical or anatomical problems causing failure of positioning the electrode in the epidural space) were recorded in two trials. In the SCS‐EPOS study (Amann 2003) two out of a total of 75 patients could not have electrodes implanted. In the ESES study (Spincemaille 2000c) this was nine out of 60 patients. After pooling, the risk of implantation problems was 8% (random‐effects model: 95% CI ‐6 to 22%), implying that in every 12 patients an implantation problem could be expected.
All trials reported the number of changes in stimulation requiring a surgical re‐intervention, which could be because of dislocation of the electrode or fracture of the lead. Multicentre trials showed the highest complication incidence. Pooling resulted in a risk of 12% (random‐effects model: 95% CI 5 to 20%). Infections of the lead or subcutaneous pulse generator pocket occurred less frequently. The pooled risk from the six studies was 3% (95% CI 0 to 6%). Depletion of the battery within 18 months of follow up also occurred: in five patients in the SCS‐EPOS study (Amann 2003), and in three patients in the ESES study (Spincemaille 2000c).
The overall number of complications of SCS treatment (comprising infection of lead or impulse generator pocket, dislocation or breakage of the lead, and early depletion of the battery) were pooled over the six trials. The overall complication risk was 0.18 (random‐effects model: 95% CI 0.03 to 0.32), indicating a number needed to harm of 6 (95% CI 3 to 33).
Costs
Only the ESES study (ESES) included a cost comparison (seeTable 1). The average overall costs of hospitalisation, rehabilitation, operative procedures, stimulator, outpatient care, professional home care, medication and non‐medical costs at two years were EUR 36,500 in the SCS group and EUR 28,600 in the conservative group. This was a significant difference (P < 0.009). After adjustment for mortality these figures were EUR 31,340 (SCS) and EUR 23,780 (conservative) (P < 0.002).
1. Costs.
| Costs (Euros) | SCS (Euros) | Conservative (Euros) |
| Hospitalisation | 11,779 | 12,321 |
| Rehabilitation | 6748 | 7471 |
| Operative procedures | 8362 | 417 |
| Stimulator implant | 7215 | 0 |
| Professional home care | Equal | Equal |
| Outpatient costs | Negligible | Negligible |
| Medication | Negligible | Negligible |
| Non‐medical | Negligible | Negligible |
| TOTAL | 36,502 | 28,617 |
Discussion
As compared with the large total number of publications on SCS for non‐reconstructable critical leg ischaemia (CLI), only a few randomised studies have been performed. One of the reasons may be the limited number of patients with CLI regarded as non‐reconstructable, and thus considered eligible candidates for such a trial, a fact illustrated by the relatively small study sizes of randomised studies. The chance of publication bias is regarded as small, considering that the manufacturers of nearly all SCS devices are keeping track of all studies that are or have been performed on SCS in this patient group.
Due to the non‐blinded character of the studies, limb salvage results could be biased if amputations were postponed in SCS‐treated patients. However, progressive necrosis (localised tissue death) with imminent sepsis (infection) and intractable pain despite maximum analgesic therapy (which was allowed in both treatment groups) were the absolute indications for an amputation. Hence, it is not likely this has had a significant influence on eventual limb salvage.
The ongoing discussion about the definition of critical leg ischaemia and non‐reconstructability further obscures the indication for such a therapy. Furthermore, this patient group has a limited life expectancy due to their increased age and the presence of generalised atherosclerosis, which limits the benefit of any treatment. On the other hand, this patient group faces the ultimate prospect of amputation. Hence, any alternative treatment option with a limb saving and pain relieving potential deserves proper evaluation.
The included trials all focused on limb salvage, clinical improvement, and complications, comparing SCS with optimum non‐surgical therapy. Only a few studies addressed quality of life and cost. Although blinding was impossible, the overall quality of the studies was good. The results of some (secondary) endpoints (for example ABPI, TcpO2) are difficult to interpret as they are influenced by the loss from the trial of patients undergoing an amputation during follow up, which leaves only the 'better' patients. This positively biases the results.
None of the individual RCTs revealed a significant beneficial effect on limb salvage. Probably the individual studies, which took approximately five years for each to be completed, tended to be underpowered. To include a larger number of patients that matched the strict inclusion criteria would have taken too much time. However, after pooling a positive effect on limb salvage was found at the cost of a significantly higher number of (correctable) complications and (probably) higher costs. Additionally, there is some evidence of a beneficial effect on ulcer healing and pain relief. The finding that TcpO2, but not ABPI, seems to increase during follow up suggests SCS treatment mainly acts on the local microcirculation. Pain relief and ulcer healing may also improve walking ability, which may further enhance local circulation.
The general quality of life seemed to remain unchanged. Apparently this is influenced by other major factors in life, or the questionnaires used are too insensitive to measure specific changes due to the treatment of CLI. To date, we are still in want of specific questionnaires to appreciate quality of life in CLI. A major amputation can still be considered as an alternative treatment because it leads to good pain relief, albeit at the cost of a (temporary) reduction in mobility.
Authors' conclusions
Implications for practice.
There is evidence that SCS is better than conservative treatment alone in order to achieve amputation risk reduction, pain relief, and improvement of the clinical situation in patients with non‐reconstructable chronic stable critical limb ischaemia. The effect of SCS appears to be stronger after further patient selection on the basis of a beneficial local transcutaneous oxygen tension (TcpO2) and test stimulation period. This beneficial effect should be weighed against the harm SCS may cause (implantation failures and relatively mild complications). In addition, the impact of SCS treatment on healthcare budgets needs to be considered.
If pain relief is the primary goal, a major amputation may be the treatment of choice in patients with an already limited mobility.
The higher complication rate, as found in multicentre studies enrolling few patients per centre, indicates that this treatment should be reserved to specialised centres which have the facilities and expertise to provide SCS.
Implications for research.
Given the present evidence, SCS treatment appears beneficial to a small subgroup of CLI patients. Despite the expected low inclusion rates, randomised controlled trials with clear inclusion criteria may further elucidate which patients may benefit from SCS therapy. Quality of life, cost‐effectiveness, and pain assessment in patients after an amputation should be among the endpoints. To assess the quality of life, the SF36 (Ware 1998) is recommended as the best validated general questionnaire while still in want of a specific questionnaire for CLI.
Inclusion criteria should aim at including patients who are most likely to benefit from SCS treatment. A period of test stimulation and TcpO2 monitoring to assess the local circulation appear useful for this purpose. Further research is required in this area. Usually effect parameters such as the TcpO2 are calculated during follow up without carrying forward the data obtained from those who previously underwent an amputation. This leaves only the participants who retained their leg and probably had better oxygenation. This bias should be avoided.
Given the evidence that limb salvage obtained with SCS is comparable to (distal) bypass surgery, other indications for experimental SCS treatment may also be considered (for example reconstructable CLI) and compared with less promising surgical interventions such as very distal or infrainguinal prosthetic bypass procedures.
Feedback
Klomp, October 2004
Summary
22 October 2004 This review [1] was recently published as well in the British Journal of Surgery [2], but contains major problems. The review describes 6 studies, with patient numbers varying from 27 to 120 patients. One (randomised) study was never reviewed (Suy), one study was NOT randomised (Amann), one study was stopped prematurely (Spincemaille), and for at least three studies concealment of treatment allocation was dubious. The selected data for the "amputation rate after 12 months" are incorrect for our study [3], and the use of data from a subgroup in the smallest study (Suy) is unjustifiable.
At least the choices made in the process should be adequately described. Presently, the debatable choices of data input (non‐randomised data, subgroup‐data) are not properly addressed in the methodological or discussion sections.
The same meta‐analysis, performed with the, in our view proper amputation data input of 5 randomised studies, generates a risk difference of ‐0.07 (95% CI: ‐0.17 to +0.03) instead of ‐0.13 (95% CI: ‐0.22 to ‐0.04). The main conclusion, that spinal cord stimulation is better than conservative treatment alone in achieving a reduction in amputation risk, is not justified. If SCS is beneficial, the magnitude of the effect is very small.
We think that the issues are of such relevance that they should at least be available to users of the Cochrane library who read the Ubbink review of SCS treatment. So modifications are required in the present Cochrane review [4,5].
Houke Klomp, Amsterdam Ewout Steyerberg, Rotterdam
References 1.Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical leg ischaemia. Cochrane Database Syst Rev. 2003:CD004001.
2.Ubbink DT, Vermeulen H, Spincemaille GH, Gersbach PA, Berg P, Amann W. Systematic review and meta‐analysis of controlled trials assessing spinal cord stimulation for inoperable critical leg ischaemia. Br J Surg. 2004;91:948‐55.
3.Klomp HM, Spincemaille GH, Steyerberg EW, Habbema JD, van Urk H. Spinal‐cord stimulation in critical limb ischaemia: a randomised trial. ESES Study Group. Lancet. 1999;353:1040‐4.
4.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Jama. 1995;273:408‐12.
5.Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses? Lancet. 1998;352:609‐13.
Reply
4 November 2004 We do not agree with the comment that our review would contain major problems and improper descriptions of our methodology for the following reasons:
1.The methodological quality and validity of every trial was judged according to standards supplied by the Dutch Cochrane Centre (while DU was replaced as author when judging trials of which he was co‐author). This implies that even the non‐peer‐reviewed paper by Suy et al. was methodologically checked by independent authors. This has also been mentioned in the 'Methods of the review' section.
2.The trials we included in our study were defined beforehand, as stated in the 'Criteria For Considering Studies For This Review', under 'Types of studies', which comprised controlled clinical trials, including the motivation for this. Because we are aware of the possible effects of including non‐randomised data, we have conducted a sensitivity analysis, as mentioned in the Results section under 'Limb salvage': Excluding this trial (Amann study) did not influence the results substantially. The Spincemaille study stopped prematurely, probably because the Dutch multicenter study was about to start, which they joined. This is no reason for excluding the study from our meta‐analysis.
3.We have checked with the commenter the data regarding the ESES trial she disagreed with. Instead of the 27 versus 32 amputations at 12 months we present, she mentioned 24 versus 29 amputations, which was not apparent from the Lancet paper. We repeated the meta‐analysis with these data and found that this does not alter the results, but we will correct these data in the next update of our review.
4.The smallest trial by Suy et al. included patients with non‐reconstructable critical leg ischaemia as well as Buerger's disease. In order not to compromise homogeneity of the patients in this meta‐analysis, only CLI patients were included, which has been addressed in the 'Description of studies' section. Again, we re‐analysed the data after excluding these (few) data from the meta‐analysis. This did not influence limb salvage risk difference significantly.
5.The same meta‐analysis, performed with the adapted amputation data from six controlled trials generates a risk difference of ‐0.11 (95% CI: ‐0.20 to ‐0.02).
6.In general, systematic reviews offer the highest level of evidence available, even if not all available studies confirm concealment of allocation. This evidence supports the beneficial effect of SCS. We agree that the magnitude of this effect may appear small, but to us, a risk difference of around 10% as to major amputations within one year seems clinically relevant. Even if the confidence interval would cross zero, one should be careful to conclude there is no effect (Alderson P, Chalmers I. Survey of claims of no effect in abstracts of Cochrane reviews. BMJ. 2003; 326(7387): 475).
Dirk Ubbink and Hester Vermeulen, November 2004.
Contributors
Comments by: Houke Klomp, Amsterdam and Ewout Steyerberg, Rotterdam
Response to comments by: Dirk Ubbink and Hester Vermeulen, Amsterdam
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2013 | Review declared as stable | This Cochrane review has been marked stable and will only be updated when new studies are identified. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 17 January 2013 | New citation required but conclusions have not changed | Searches re‐run but no new studies identified. Review search dates updated, minor copy edits made to text. Conclusions not changed. |
| 17 January 2013 | New search has been performed | Searches re‐run but no new studies identified. Review search dates updated. |
| 24 September 2008 | New search has been performed | Searches re‐run and no new studies found. One additional report (Klomp 2006) added to ESES study. Correction made to the analysis results reported in the last paragraph of "Effects of Interventions", Limb salvage. Plain language summary added. |
| 8 July 2008 | Amended | Converted to new review format. |
| 24 June 2005 | New citation required but conclusions have not changed | No new trials found at last search. Review updated with minor formatting changes and correction to the numbers of amputations in the ESES trial, in accordance with the Comment submitted by Klomp and Steyerberg. |
| 16 November 2004 | Amended | Synopsis added. |
| 4 November 2004 | Feedback has been incorporated | Feedback and response to feedback added. |
Acknowledgements
We thank Mrs Heather Maxwell for her helpful and prompt assistance in the preparation of the protocol and review, Mrs Astrid Goossens (AG) for her assistance in the quality assessment and the Bakken Research Center Maastricht for supplying the data from the European SCS‐EPOS trial.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Arteriosclerosis] this term only | 890 |
| #2 | MeSH descriptor: [Arteriolosclerosis] this term only | 0 |
| #3 | MeSH descriptor: [Arteriosclerosis Obliterans] this term only | 71 |
| #4 | MeSH descriptor: [Atherosclerosis] this term only | 382 |
| #5 | MeSH descriptor: [Arterial Occlusive Diseases] this term only | 753 |
| #6 | MeSH descriptor: [Intermittent Claudication] this term only | 710 |
| #7 | MeSH descriptor: [Ischemia] this term only | 751 |
| #8 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees | 2146 |
| #9 | MeSH descriptor: [Vascular Diseases] this term only | 379 |
| #10 | MeSH descriptor: [Leg] explode all trees and with qualifiers: [Blood supply ‐ BS] | 1069 |
| #11 | MeSH descriptor: [Femoral Artery] explode all trees | 719 |
| #12 | MeSH descriptor: [Popliteal Artery] explode all trees | 250 |
| #13 | MeSH descriptor: [Iliac Artery] explode all trees | 151 |
| #14 | MeSH descriptor: [Tibial Arteries] explode all trees | 29 |
| #15 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 16775 |
| #16 | (arter*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 4842 |
| #17 | (vascular) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1368 |
| #18 | (vein*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 706 |
| #19 | (veno*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 974 |
| #20 | (peripher*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1352 |
| #21 | peripheral near/3 dis* | 3203 |
| #22 | arteriopathic | 9 |
| #23 | (claudic* or hinken*) | 1427 |
| #24 | (isch* or CLI) | 16663 |
| #25 | dysvascular* | 13 |
| #26 | leg near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 176 |
| #27 | limb near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 227 |
| #28 | (lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 136 |
| #29 | (aort* or iliac or femoral or popliteal or femoro* or fempop* or crural) near/3 (obstruct* or occlus*) | 324 |
| #30 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 | 39111 |
| #31 | MeSH descriptor: [Electric Stimulation Therapy] explode all trees | 4045 |
| #32 | spin* near stimulat* | 388 |
| #33 | column* near stimulat* | 11 |
| #34 | epidur* near stimulat* | 79 |
| #35 | MeSH descriptor: [Electric Stimulation] explode all trees | 1460 |
| #36 | #31 or #32 or #33 or #34 or #35 | 5657 |
| #37 | #30 and #36 in Trials | 427 |
Appendix 2. Keywords used in search strategy
Spinal cord stimulation Dorsal column stimulation Epidural spinal stimulation Epidural electrical stimulation
Critical limb isch(a)emia Lower limb isch(a)emia Leg isch(a)emia Peripheral vascular disease Peripheral arterial (occlusive) disease Atherosclerosis
Non‐reconstructable Intractable Inoperable
Limb salvage Amputation Pain relief Wound / ulcer healing Quality of life Complications Cost
Data and analyses
Comparison 1. Limb survival.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amputations (12 months) | 6 | 433 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.20, ‐0.02] |
| 2 Amputation rate in non‐hypertensives (18 months) | 1 | Risk Difference (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1 Limb survival, Outcome 1 Amputations (12 months).
1.2. Analysis.

Comparison 1 Limb survival, Outcome 2 Amputation rate in non‐hypertensives (18 months).
Comparison 2. Pain relief.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in VAS score (0‐100; where 100 is worst) after 12 months | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Change in Pain Rating Index (overall; where higher is worse) after 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in VAS score (0‐100; where 100 is worst) after 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.
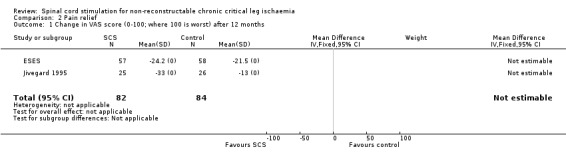
Comparison 2 Pain relief, Outcome 1 Change in VAS score (0‐100; where 100 is worst) after 12 months.
2.2. Analysis.

Comparison 2 Pain relief, Outcome 2 Change in Pain Rating Index (overall; where higher is worse) after 12 months.
2.3. Analysis.

Comparison 2 Pain relief, Outcome 3 Change in VAS score (0‐100; where 100 is worst) after 3 months.
Comparison 3. Clinical improvement.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reaching Fontaine stage II | 2 | 124 | Risk Difference (M‐H, Fixed, 95% CI) | 0.33 [0.19, 0.47] |
| 2 Reaching Fontaine stage III | 2 | 206 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.24, 0.38] |
| 3 Ulcer healing and hypertension | 2 | 72 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.54 [‐0.73, ‐0.35] |
| 3.1 Hypertensives | 1 | 44 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.57 [‐0.80, ‐0.33] |
| 3.2 Non‐hypertensives | 1 | 28 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.5 [‐0.82, ‐0.18] |
3.1. Analysis.

Comparison 3 Clinical improvement, Outcome 1 Reaching Fontaine stage II.
3.2. Analysis.

Comparison 3 Clinical improvement, Outcome 2 Reaching Fontaine stage III.
3.3. Analysis.

Comparison 3 Clinical improvement, Outcome 3 Ulcer healing and hypertension.
Comparison 4. Ankle/brachial index.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ankle/brachial pressure index increase after 12 months | 2 | 137 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
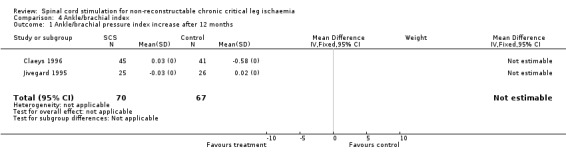
Comparison 4 Ankle/brachial index, Outcome 1 Ankle/brachial pressure index increase after 12 months.
Comparison 5. Quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nottingham Health Profile (0‐100; where 100 is worst) after 12 months | 1 | 189 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.20, 2.20] |
| 1.1 Mobility score amputees | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Overall | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.20, 2.20] |
| 1.3 Mobility score non‐amputees | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Euroqol (0‐100; where 100 is worst) after 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.1. Analysis.
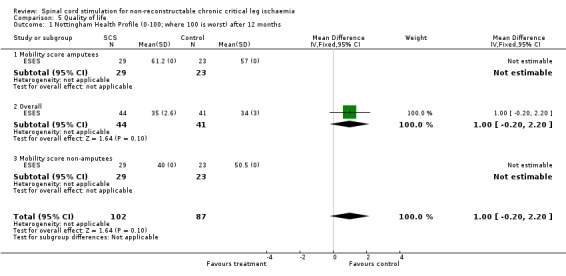
Comparison 5 Quality of life, Outcome 1 Nottingham Health Profile (0‐100; where 100 is worst) after 12 months.
5.2. Analysis.

Comparison 5 Quality of life, Outcome 2 Euroqol (0‐100; where 100 is worst) after 12 months.
Comparison 6. TcpO2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 TcpO2 after 12 months | 2 | 197 | Mean Difference (IV, Random, 95% CI) | 1.39 [‐15.66, 18.44] |
6.1. Analysis.

Comparison 6 TcpO2, Outcome 1 TcpO2 after 12 months.
Comparison 7. Complications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in stimulation requiring reintervention (within 12 months) | 6 | 412 | Risk Difference (M‐H, Random, 95% CI) | 0.12 [0.04, 0.20] |
| 2 Infection requiring removal | 6 | 412 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.00, 0.06] |
| 3 Battery: end of life | 6 | 412 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.07] |
| 4 Overall complications requiring re‐intervention | 5 | 375 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.03, 0.33] |
| 5 Initial implantation problems (not included in overall complications) | 2 | 232 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.06, 0.23] |
7.1. Analysis.

Comparison 7 Complications, Outcome 1 Change in stimulation requiring reintervention (within 12 months).
7.2. Analysis.

Comparison 7 Complications, Outcome 2 Infection requiring removal.
7.3. Analysis.

Comparison 7 Complications, Outcome 3 Battery: end of life.
7.4. Analysis.

Comparison 7 Complications, Outcome 4 Overall complications requiring re‐intervention.
7.5. Analysis.
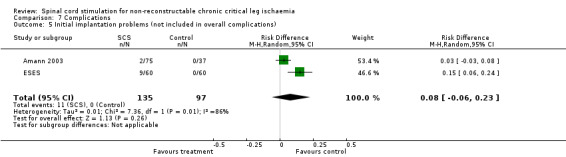
Comparison 7 Complications, Outcome 5 Initial implantation problems (not included in overall complications).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amann 2003.
| Methods | Controlled clinical trial. | |
| Participants | Country: 17 European centres. Number of participants: 112. Age: SCS group = 68 ± 13 SD; Control group = 67 ± 9 SD. Sex: M=75; F=37. Inclusion criteria: Non‐reconstructable CLI. Fontaine stages III; persistent pain at rest > 2 weeks or ischaemic skin lesions. Exclusion criteria: Extensive necrosis; infected gangrene; terminally ill. | |
| Interventions | Treatment group: SCS + best medical therapy (n=73). Control group: Best medical therapy (n=39). Duration: 18 months follow up. | |
| Outcomes | Limb survival, pain relief, QoL, Fontaine stage, complications. | |
| Notes | TcpO2 selection. Test stimulation. Raw data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | D ‐ Not used |
Claeys 1996.
| Methods | Randomised controlled trial. | |
| Participants | Country: Germany. Number of participants: 86. Age: SCS group = 67.7 ± 11.9 SD; Control group = 69.9 ± 10.2 SD. Sex: M=49; F=37. Inclusion criteria: Non‐reconstructable CLI; Fontaine stage IV; ulcers or gangrene present for minimum of 3 weeks; ankle pressure < 50mmHg. Exclusion criteria: Mixed type of ulceration; local infection; patients suitable for reconstructive procedures. | |
| Interventions | Treatment group: SCS + PGE1 (n=45). Control group: Conservative medical therapy ‐ PGE1 (n=41). Duration: 1 year follow up. | |
| Outcomes | Limb survival, pain relief, ABPI, Fontaine stage, ulcer healing, complications. | |
| Notes | TcpO2. Test stimulation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ESES.
| Methods | Randomised controlled trial. Randomisation by random‐numbers table, stratified by diabetes, ankle pressure, and hospital. Treatment allocation performed in an independent institute. | |
| Participants | Country: The Netherlands, 17 centres. Number of participants: 120. Age: SCS group = 73.1 ± 9.8 SD; Control group = 721.1 ± 10.6 SD. Sex: M=70; F=50. Inclusion criteria: Non‐reconstructable CLI, persistent pain at rest > 2 weeks or ischaemic skin lesions, ankle systolic pressure < 50mmHg. Exclusion criteria: Extensive necrosis, infected gangrene, terminally ill. | |
| Interventions | Treatment group: SCS + best medical therapy (n=60). Control group: Best medical therapy (n=60). Duration: 2 years. | |
| Outcomes | Limb survival, pain relief, Fontaine stage, QoL, ABPI complications. | |
| Notes | TcpO2 selection. Raw data available in Ubbink 1999b reference. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Jivegard 1995.
| Methods | Randomised controlled trial. Method of randomisation: unsure. | |
| Participants | Country: Sweden, 2 university hospitals. Number of participants: 51. Age: SCS group = 73 ± 12 SD; Control group = 73 ± 12 SD. Inclusion criteria: Severe chronic (> 2 weeks duration) lower limb ischaemia. non‐reconstructable CLI. Exclusion criteria: Rapidly progressing ischaemia, gangrene of more than one toe, extensive infection and/or non‐healing ischaemic ulcerations. | |
| Interventions | Treatment group: SCS + oral analgesics (n=25). Control group: Oral analgesic (n=26). Duration: 18 months follow up. | |
| Outcomes | Limb survival, pain relief, ABPI, toe pressure, complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Spincemaille 2000a.
| Methods | Randomised controlled trial (preliminary results). | |
| Participants | Country: The Netherlands. Number of participants: 37. Age: SCS group: 72; Control group: 70. Range: 56 to 87. Sex: M=28; F=9. Inclusion criteria: Non‐reconstructable CLI; persistent pain at rest, or ischaemic skin lesions. Exclusion criteria: infected gangrene, terminally ill, ulcer surface > 3 cm. | |
| Interventions | Treatment group: SCS+ best medical therapy (n=19). Control group: Best medical therapy: analgesic and vasoactive drugs (n=18). Duration: 2 years. | |
| Outcomes | Limb survival, pain relief. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Suy 1994.
| Methods | Randomised controlled trial. | |
| Participants | Country: Belgium, 3 university hospitals. Number of participants: 38. Age: SCS group 66 ± 11 SD; Control group 65 ± 9 SD. Inclusion criteria: chronic ischaemic pain related to PAOD; non‐reconstructable CLI (n=27) and Buerger (n=11 excluded). | |
| Interventions | Treatment group: SCS (n=20). Control group: Conservative therapy (n=18). Duration: follow up 20 ± 15 months. | |
| Outcomes | Limb survival, pain relief, Fontaine stage, complications. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ABPI: Ankle Brachial Pressure Index Hg: Mercury CLI: Critical limb ischaemia PGE1: Prostaglandin E1 QoL: Quality of life SCS: Spinal cord stimulation TcpO2: Transcutaneous oxygen pressure
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Claeys 1997a | Double publication. |
| Claeys 1997b | No controls. |
| Claeys 1998b | Double publication. |
| Claeys 1999 | Double publication. |
| Guarnera 1994 | Inclusion of reconstructable CLI patients. |
| Palombo 1995 | No controls. |
| Petrakis 2000 | No controls. |
| Tallis 1983 | No controls. |
Contributions of authors
DU identified potentially relevant papers, decided upon eligibility and quality of trials, extracted data, and wrote the review.
HV identified potentially relevant papers, discussed their eligibility with DU, decided upon the quality of trials, and extracted data.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
Bakken Research Centre sponsored the principal investigator (DU) as 'quality officer' for the duration of the Spinal Cord Stimulation European Peripheral Artery Disease Outcomes Study (SCS‐EPOS). The Research Centre belongs to the Medtronic Company, which is the worldwide manufacturer of the neurostimulators for SCS treatment. Medtronic did not, however, sponsor the preparation of this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Amann 2003 {unpublished data only}
- Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. Spinal cord stimulation in the treatment of non‐reconstructable stable critical leg ischaemia: results of the European peripheral vascular disease outcomes study (SCS‐EPOS). European Journal of Vascular and Endovascular Surgery 2003;26(3):280‐6. [DOI] [PubMed] [Google Scholar]
Claeys 1996 {published data only}
- Claeys LG, Horsch S. Transcutaneous oxygen pressure as predictive parameter for ulcer healing in endstage vascular patients treated with spinal cord stimulation. International Angiology 1996;15(4):344‐9. [PubMed] [Google Scholar]
ESES {published data only}
- Klomp HM, Spincemaille GH, Steyerberg EW, Berger MY, Habbema JD, Urk H. Design issues of a randomised controlled clinical trial on spinal cord stimulation in critical limb ischaemia. ESES Study Group. European Journal of Vascular and Endovascular Surgery 1995;10(4):478‐85. [DOI] [PubMed] [Google Scholar]
- Klomp HM, Spincemaille GH, Steyerberg EW, Habbema JD, Urk H. Spinal‐cord stimulation in critical limb ischaemia: a randomised trial. ESES Study Group. Lancet 1999;353(9158):1040‐4. [DOI] [PubMed] [Google Scholar]
- Klomp HM, Steyerberg EW, van UH, Habbema JD, ESES Study Group. Spinal cord stimulation is not cost‐effective for non‐surgical management of critical limb ischaemia. European Journal of Vascular and Endovascular Surgery 2006;31(5):500‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JD. Pain and quality of life in patients with critical limb ischaemia: results of a randomized controlled multicentre study on the effect of spinal cord stimulation. ESES study group. European Journal of Pain 2000;4(2):173‐84. [DOI] [PubMed] [Google Scholar]
- Spincemaille GH, Klomp HM, Steyerberg EW, Urk H, Habbema JD, ESES Study Group. Technical data and complications of spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotactic and Functional Neurosurgery 2000;74(2):63‐72. [DOI] [PubMed] [Google Scholar]
- Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. Journal of Vascular Surgery 1999;30(2):236‐44. [DOI] [PubMed] [Google Scholar]
Jivegard 1995 {published data only}
- Jivegard LE, Augustinsson LE, Holm J, Risberg B, Ortenwall P. Effects of spinal cord stimulation (SCS) in patients with inoperable severe lower limb ischaemia: a prospective randomised controlled study. European Journal of Vascular and Endovascular Surgery 1995;9(4):421‐5. [DOI] [PubMed] [Google Scholar]
Spincemaille 2000a {published data only}
- Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JD. Spinal cord stimulation in patients with critical limb ischemia: A preliminary evaluation of a multicentre trial. Acta Chirurgica Austriaca 2000;32:49‐51. [Google Scholar]
Suy 1994 {published data only}
- Suy R, Gybels J, Damme H, Martin D, Maele R, Delaporte C. Spinal cord stimulation for ischemic rest pain. The Belgian randomized study. In: Horsch S, Claeys L editor(s). Spinal Cord Stimulation: An innovative method in the treatment of PVD. Springer, 1994:197‐202. [Google Scholar]
References to studies excluded from this review
Claeys 1997a {published data only}
- Claeys LGY, Horsch S. Effects of spinal cord stimulation on ischemic inflammatory pain and wound healing in patients with peripheral arterial occlusive disease Fontaine Stage IV. Pain Digest 1997;7(4):200‐3. [Google Scholar]
Claeys 1997b {published data only}
- Claeys LG. Improvement of microcirculatory blood flow under epidural spinal cord stimulation in patients with nonreconstructible peripheral arterial occlusive disease. Artificial Organs 1997;21(3):201‐6. [DOI] [PubMed] [Google Scholar]
Claeys 1998b {published data only}
- Claeys LGY, Horsch S. Epidural spinal cord stimulation following intravenous prostaglandin E1 therapy in patients with ischaemic pain (peripheral vascular disease Fontaine stage IV). Preliminary results of a controlled randomized study. Pain Clinic 1998;10(3):165‐72. [Google Scholar]
Claeys 1999 {published data only}
- Claeys LGY, Horsch S. Spinal cord stimulation (SCS) following intravenous prostaglandin E1 (PGE1) therapy in non‐reconstructible peripheral vascular disease (PVD): Fontaine stage IV. Pain Clinic 1999;11(3):235‐43. [Google Scholar]
Guarnera 1994 {published data only}
- Guarnera G, Furgiuele S, Camilli S. Spinal cord electric stimulation vs. femoro‐distal bypass in critical ischemia of the legs. Preliminary results in a randomized prospective study [Elettrostimulazione midollare vs bypass femoro‐distale nell'ischemia critica degli arti inferiori]. Minerva Cardioangiologica 1994;42(5):223‐7. [PubMed] [Google Scholar]
Palombo 1995 {published data only}
- Palombo D, Porta C, Brustia P, Peinetti F, Udini M, Antico A, et al. Limb salvage in critical ischemia. Our experience [Salvataggio d'arto nell'ischemia critica. Nostra esperienza.]. Minerva Chirurgica 1995;50(3):263‐8. [PubMed] [Google Scholar]
Petrakis 2000 {published data only}
- Petrakis E, Sciacca V. Prospective study of transcutaneous oxygen tension (TcPO2) measurement in the testing period of spinal cord stimulation in diabetic patients with critical lower limb ischaemia. International Angiology 2000;19(1):18‐25. [PubMed] [Google Scholar]
Tallis 1983 {published data only}
- Tallis RC, Illis LS, Sedgwick EM. Spinal cord stimulation in peripheral vascular disease. Journal of Neurology, Neurosurgery, and Psychiatry 1983;46(6):478‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Augustinsson 1985
- Augustinsson LE, Carlsson CA, Holm J, Jivegard L. Epidural electrical stimulation in severe limb ischemia. Pain relief, increased blood flow, and a possible limb‐saving effect. Annals of Surgery 1985;202(1):104‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Broseta 1986
- Broseta J, Barbera J, Vera JA, Barcia‐Salorio JL, Garcia‐March G, Gonzalez‐Darder J, et al. Spinal cord stimulation in peripheral arterial disease. A cooperative study. Journal of Neurosurgery 1986;64(1):71‐80. [DOI] [PubMed] [Google Scholar]
Bunt 1991
- Bunt TJ, Holloway GA, Lawrence P, Cherney D, Malone J. Experience with epidural spinal stimulation in the treatment of end‐stage peripheral vascular disease. Seminars in Vascular Surgery 1991;4:215‐20. [Google Scholar]
Carlsson 1983
- Carlsson AM. Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain 1983;16(1):87‐101. [DOI] [PubMed] [Google Scholar]
Claeys 1998
- Claeys LGY, Ktenidis K, Horsch S. Effects of spinal cord stimulation on ischemic pain in patients with Buerger's disease. Pain Digest 1998;7(3):138‐41. [Google Scholar]
Cook 1976
- Cook AW, Oygar A, Baggenstos P, Pacheco S, Kleriga E. Vascular disease of extremities. Electrical stimulation of spinal cord and posterior roots. New York State Journal of Medicine 1976;76(3):366‐8. [PubMed] [Google Scholar]
Deeks 1998
- Deeks J. When can odds ratios mislead? Odds ratios should be used only in case control studies and logistic regression analyses. BMJ 1998;317(7166):1155‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutch CC
- Dutch Cochrane Center. Therapy checklist (extended version). http://www.amc.uva.nl/EN/OtherOrganisations/dcc/RCT.pdf.
Fiume 1989
- Fiume D, Palombi M, Sciassa V, Tamorri M. Spinal cord stimulation (SCS) in peripheral ischemic pain. Pacing and Clinical Electrophysiology 1989;12(4 Part 2):698‐704. [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstörungen]. Helvetica Chirurgica Acta 1954;5/6:491‐533. [PubMed] [Google Scholar]
Galley 1994
- Galley D, Pavy O, Elharrar C, et al. The use of TcpO2 in assessing patients with spinal cord stimulation for peripheral vascular disease of the lower limbs. In: Herreros J, Lazorthes Y, Boccalon H, et al. editor(s). Spinal cord stimulation for peripheral vascular diseases. Advances and controversies. Madrid, Spain: Editorial libro del ano, 1994:93‐8. [Google Scholar]
Gersbach 1997
- Gersbach P, Hasdemir MG, Stevens RD, Nachbur B, Mahler F. Discriminative microcirculatory screening of patients with refractory limb ischaemia for dorsal column stimulation. European Journal of Vascular and Endovascular Surgery 1997;13(5):464‐71. [DOI] [PubMed] [Google Scholar]
Horsch 1994
- Horsch S, Claeys L. Epidural spinal cord stimulation in the treatment of severe peripheral arterial occlusive disease. Annals of Vascular Surgery 1994;8(5):468‐74. [DOI] [PubMed] [Google Scholar]
Jacobs 1990
- Jacobs MJ, Jorning PJ, Beckers RC, Ubbink DT, Kleef M, Slaaf DW, et al. Foot salvage and improvement of microvascular blood flow as a result of epidural spinal cord electrical stimulation. Journal of Vascular Surgery 1990;12(3):354‐60. [PubMed] [Google Scholar]
Klomp 1999
- Klomp HM, Spincemaille GHJJ, Steyerberg EW, Habbema JD, Urk H. Spinal‐cord stimulation in critical limb ischaemia: a randomised trial. Lancet 1999;353(9158):1040‐4. [DOI] [PubMed] [Google Scholar]
Klomp 2006
- Klomp HM, Steyerberg EW, van UH, Habbema JD, ESES Study Group. Spinal cord stimulation is not cost‐effective for non‐surgical management of critical limb ischaemia. European Journal of Vascular and Endovascular Surgery 2006;31(5):500‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kumar 1997
- Kumar K, Toth C, Nath RK, Verma AK, Burgess JJ. Improvement of limb circulation in peripheral vascular disease using epidural spinal cord stimulation: a prospective study. Journal of Neurosurgery 1997;86(4):662‐9. [DOI] [PubMed] [Google Scholar]
Linderoth 1992
- Linderoth B. Dorsal column stimulation and pain: experimental studies on putative neurochemical and neurophysiological mechanisms. Thesis. Karolinska Institute, Stockholm 1992.
Masters‐Steed 1992
- Masters‐Steedman S, Middaugh SJ, Kee WG, Carson DS, Harden RN, Miller MC. Chronic‐pain medications: equivalent levels and methods of quantifying usage. Clinical Journal of Pain 1992;8(3):204‐14. [PubMed] [Google Scholar]
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1(3):277‐99. [DOI] [PubMed] [Google Scholar]
Mingoli 1993
- Mingoli A, Sciacca V, Tamorri M, Fiume D, Sapienza P. Clinical results of epidural spinal cord electrical stimulation in patients affected with limb‐threatening chronic arterial obstructive disease. Angiology 1993;44(1):21‐5. [DOI] [PubMed] [Google Scholar]
Rickman 1994
- Rickman S, Wuebbels BH, Holloway GA Jr. Spinal cord stimulation for relief of ischemic pain in end‐stage arterial occlusive disease. Journal of Vascular Nursing 1994;12(1):14‐20. [PubMed] [Google Scholar]
Spiegelmann 1991
- Spiegelmann R, Friedman WA. Spinal cord stimulation: a contemporary series. Neurosurgery 1991;28(1):65‐70; discussion 70‐1. [PubMed] [Google Scholar]
Spincemaille 2000b
- Spincemaille GH, Klomp HM, Steyerberg EW, Habbema JD. Pain and quality of life in patients with critical limb ischaemia: results of a randomized controlled multicentre study on the effect of spinal cord stimulation. ESES study group. European Journal of Pain 2000;4(2):173‐84. [DOI] [PubMed] [Google Scholar]
Spincemaille 2000c
- Spincemaille GH, Klomp HM, Steyerberg EW, Urk H, Habbema JD. ESES Study Group. Technical data and complications of spinal cord stimulation: data from a randomized trial on critical limb ischemia. Stereotactic and Functional Neurosurgery 2000;74(2):63‐72. [DOI] [PubMed] [Google Scholar]
Spincemaille 2001
- Spincemaille GH, Vet HC, Ubbink DT, Jacobs MJ. The results of spinal cord stimulation in critical limb ischaemia: a review. European Journal of Vascular and Endovascular Surgery 2001;21(2):99‐105. [DOI] [PubMed] [Google Scholar]
TASC 2000a
- TASC Working Group. Management of peripheral arterial disease. TransAtlantic inter‐Society Consensus. International Angiology 2000;19 Suppl 1:23‐6. [PubMed] [Google Scholar]
TASC 2000b
- TASC Working Group. Management of peripheral arterial disease. TransAtlantic inter‐Society Consensus. International Angiology 2000;19 Suppl 1:208‐20. [PubMed] [Google Scholar]
TASC 2000c
- TASC Working Group. Management of peripheral arterial disease. TransAtlantic inter‐Society Consensus. International Angiology 2000;19 Suppl 1:185. [PubMed] [Google Scholar]
Tesfaye 1996
- Tesfaye S, Watt J, Benbow SJ, Pang KA, Mile J, MacFarlane IA. Electrical spinal cord stimulation for painful diabetic peripheral neuropathy. Lancet 1996;348(9043):1698‐701. [DOI] [PubMed] [Google Scholar]
Ubbink 1999
- Ubbink DT, Spincemaille GH, Prins MH, Reneman RS, Jacobs MJ. Microcirculatory investigations to determine the effect of spinal cord stimulation for critical leg ischemia: the Dutch multicenter randomized controlled trial. Journal of Vascular Surgery 1999;30(2):236‐44. [DOI] [PubMed] [Google Scholar]
Ware 1998
- Ware JE Jr, Gandek B. Overview of the SF‐36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. Journal of Clinical Epidemiology 1998;51(11):903‐12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ubbink 2003
- Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical leg ischaemia. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004001] [DOI] [PubMed] [Google Scholar]
Ubbink 2005
- Ubbink DT, Vermeulen H. Spinal cord stimulation for non‐reconstructable chronic critical leg ischaemia. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004001.pub2] [DOI] [PubMed] [Google Scholar]


