Abstract
Background
Primary sclerosing cholangitis is a chronic cholestatic disease of intrahepatic and extrahepatic biliary ducts, characterised by chronic periductal inflammation and sclerosis of the ducts, which results in segmental stenoses of bile ducts, cholestasis, fibrosis, and ultimately, liver cirrhosis. Patients with primary sclerosing cholangitis are at higher risk of cholangiocarcinoma as well as of colonic neoplasia, since primary sclerosing cholangitis is associated with inflammatory bowel disease in more than 80% of the patients. Several therapeutic modalities have been proposed for primary sclerosing cholangitis, like ursodeoxycholic acid, glucocorticosteroids, and immunomodulatory agents, but none has been successful in reversing the process of the disease. To date, liver transplantation is the only definite therapeutic solution for patients with advanced primary sclerosing cholangitis with liver cirrhosis.
Objectives
To assess the beneficial and harmful effects of glucocorticosteroids for patients with primary sclerosing cholangitis.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials in The Cochrane Library, MEDLINE, EMBASE, and LILACS from their inception until September 2009, as well as reference lists.
Selection criteria
Randomised clinical trials comparing any dose or duration of glucocorticosteroids versus placebo, no intervention, or other immunosuppressive agents. We included trials irrespective of language, blinding, or publication status.
Data collection and analysis
Authors extracted data independently and assessed the methodological quality by the generation of the allocation sequence, allocation concealment, double blinding, follow‐up, incomplete outcome data reporting, selective reporting, baseline imbalance, and early stopping. The results of the meta‐analyses were presented as relative risks (RR) or mean difference (MD), both with 95% confidence intervals (CI). The primary outcome measures were mortality and liver‐related morbidity.
Main results
Two randomised clinical trials were eligible for inclusion. One trial compared biliary lavage with hydrocortisone versus saline in 17 patients. Hydrocortisone tended to increase adverse events (pancreatitis, cholangitis with septicaemia, paranoid ideas, fluid retention) (RR 3.43, 95% CI 0.51 to 22.9) and had no cholangiographic improvement, which led to termination of the trial. The other trial compared budesonide versus prednisone in 18 patients. Patients had statistically significant higher serum bilirubin concentration after treatment with prednisone compared with budesonide (MD 10.4 µmol/litre, 95% CI 1.16 to 19.64 µmol/litre). No other statistically significant effects on clinical or biochemical outcomes were reported on any of the evaluated interventions.
Authors' conclusions
There is no evidence to support or refute peroral glucocorticosteroids for patients with primary sclerosing cholangitis. The intrabiliary application of corticosteroids via nasobiliary tube seems to induce severe adverse effects.
Plain language summary
Insufficient evidence to support or refute glucocorticosteroids for primary sclerosing cholangitis
Primary sclerosing cholangitis is a chronic cholestatic disease of intrahepatic and extrahepatic biliary ducts, characterised by chronic periductal inflammation and sclerosis of the ducts, which results in segmental stenoses of bile ducts, cholestasis, fibrosis, and, ultimately, liver cirrhosis. Patients with primary sclerosing cholangitis are at higher risk of cholangiocarcinoma as well as of colonic neoplasia, since primary sclerosing cholangitis is associated with inflammatory bowel disease in more than 80% of patients. Several therapeutic modalities have been proposed for primary sclerosing cholangitis, like ursodeoxycholic acid, glucocorticosteroids and immunomodulatory agents, but none has been successful in reversing the process of the disease. To date, liver transplantation is the only definite therapeutic solution for patients with advanced primary sclerosing cholangitis with liver cirrhosis.
Two trials on glucocorticosteroids for primary sclerosing cholangitis were identified. One trial compared biliary lavage with hydrocortisone versus saline. This trial was stopped due to adverse events. The other trial compared oral administration of budesonide versus prednisone. No statistically significant effects were found on mortality, serum activity of alkaline phosphatases, serum bilirubin, and adverse events for any of the evaluated intervention regimens.
Background
Primary sclerosing cholangitis is a slowly progressive chronic cholestatic disease of unknown etiology characterised by progressive segmental obliterative fibrosis of the biliary tree (LaRusso 1984; Chapman 2003; Cullen 2005; Silveira 2008). The disease may lead to liver fibrosis, portal hypertension, and premature death from liver failure (Wee 1985a; Silveira 2008a). The risk of liver‐related mortality and morbidity depends on genetic or immune factors, portal bacteraemia (associated with inflammatory bowel disease), viral infections, toxins, and ischaemic injury (Quigley 1983; Wee 1985b; Donaldson 1991; Olerup 1995; LaRusso 1999; Poupon 2000).
Primary sclerosing cholangitis can be classified as a large‐duct or a small‐duct variant (Ludwig 1981). The large‐duct variant involves the extrahepatic bile ducts and parts of the intrahepatic ductal system, which can be visualised cholangiographically and sometimes through histological findings. The small‐duct variant involves the intrahepatic bile ducts and can only be visualised through histological findings (Ludwig 1986; Ludwig 1991; Charatcharoenwitthaya 2006; Gordon 2008). Primary sclerosing cholangitis is diagnosed using biochemical, radiological, and hepatic histological criteria. The diagnostic criteria include elevated alkaline phosphatases (Lindor 1986; Grijm 1986) and cholangiographic demonstration of diffusely distributed and irregular multifocal strictures of the bile ducts (Chen 1984). The severity of the histological findings (Wiesner 1985; Ludwig 1986) are categorised as stage 1 (cholangitis or portal hepatitis), stage 2 (periportal fibrosis and hepatitis), stage 3 (septal fibrosis or necrosis), or stage 4 (biliary cirrhosis).
Both surgical and medical interventions have been suggested for treatment of primary sclerosing cholangitis (Lindor 1987; Grijm 1986; Vacca 2007). The suggested surgical interventions include balloon dilatation of dominant strictures, biliary tract reconstructive procedures, liver transplantation, and proctocolectomy in patients with concomitant ulcerative colitis (Lindor 1986; Lindor 1987; Stiehl 1997; LaRusso 1999). Based on cohort studies, liver transplantation is regarded to prolong survival. None of the surgical interventions have been evaluated in randomised clinical trials (Ahrendt 1998). The suggested medical interventions include various immunosuppressive agents, colchicine, D‐ penicillamine, and ursodeoxycholic acid (LaRusso 1999). The immunosuppressive agents include glucocorticosteroids, cyclosporine, tacrolimus (FK 506), and methotrexate. Following evaluations in four small cohort studies (Burgert 1984; Lindor 1991; Wolfhagen 1994; Fracchia 2000) and one randomised clinical trial (van Hoogstraten 2000), glucocorticosteroids may be used both topically and systemically. The studies suggest that glucocorticosteroids may improve the clinical course. On the other hand, long‐term use of glucocorticosteroids is also associated with adverse events including osteoporosis (Long 1978). We have been unable to identify systematic reviews or meta‐analyses on glucocorticosteroids for patients with primary sclerosing cholangitis.
Objectives
To evaluate the beneficial and harmful effects of glucocorticosteroids for patients with primary sclerosing cholangitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (parallel group and cross‐over trials) were included irrespective of blinding, publication status, or language. Quasi‐randomised trials (eg, trials using date of birth or similar for allocating patients) and observational studies were excluded.
Types of participants
Patients with primary sclerosing cholangitis diagnosed by biochemical, radiological, and hepatic histological criteria.
The biochemical criteria include elevated alkaline phosphatases, gamma‐glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, and bilirubin.
The radiological criteria include cholangiographic demonstration of irregular, annular strictures with intervening segments of normal or ectatic ducts, short and band‐like strictures, and diverticulum‐like outpouchings.
The hepatic histological criteria include cholangitis, portal hepatitis, periportal fibrosis and hepatitis, septal fibrosis, septal necrosis, and cirrhosis.
Types of interventions
Randomised comparisons of any dose or duration of glucocorticosteroid administration versus placebo, no intervention, or other immunosuppressive agents such as cyclosporine, tacrolimus (FK 506), and methotrexate. We allowed co‐interventions if administered equally to the intervention arms.
Types of outcome measures
All outcomes were evaluated at the maximum follow‐up. The primary outcome measures were: 1. All cause mortality. 2. Liver‐related morbidity including liver transplantation, biopsy‐proven cirrhosis, adenocarcinoma of the bile duct, variceal bleeding, ascites, or other signs of liver failure.
The secondary outcome measures were: 3. Clinical symptoms including pruritus or fatigue. 4. Biochemical criteria (as defined above) evaluated by absolute values and number of patients with abnormal values. The upper limit of normal was set to 120 IU/litre for alkaline phosphatases and 17 µmol/litre for serum bilirubin. 5. Deterioration of radiological findings. 6. Deterioration of histological findings. 7. Adverse events defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment, but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997). Severe adverse events were defined as any event that would increase mortality; is life‐threatening; requires inpatient hospitalisation; results in a persistent or significant disability; or any important medical event, which may jeopardise the patient or require intervention to prevent it. 8. Quality of life.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2009), The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and LILACS. Search strategies with the time span of the searches are given in Appendix 1. We also identified trials through reference lists of the identified studies.
Data collection and analysis
We performed the review following the recommendations of The Cochrane Collaboration (Higgins 2008) and The Cochrane Hepato‐Biliary Group Module (Gluud 2009).
All authors independently identified trials and extracted data, evaluated whether trials fulfilled our inclusion criteria, and listed excluded trials with the reasons for exclusion. Disagreements were resolved by discussion. From each randomised clinical trial we extracted the following data: primary author, number of patients randomised, patient inclusion and exclusion criteria, methodological quality, sample‐size calculation, use of intention‐to‐treat analysis, intervention regimens, mean age, proportion of men, proportion of patients with cirrhosis, proportion of patients with large‐duct and small‐duct cholangitis, proportion of patients with inflammatory bowel disease, duration of follow‐up, whether cost‐effectiveness analyses were performed, and all outcome measures. We wrote to the authors of included trials for additional information when we were unable to extract data from the original reports.
Assessment of methodological quality Methodological quality of the randomised clinical trials was assessed using eight components (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008). Hence, the risk of bias was assessed using the following domains:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice was considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. Such quasi‐randomised studies were excluded.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or serially numbered, sealed, and opaque envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised. The latter studies were excluded.
Blinding
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo or active drugs.
Unclear, if the trial was described as double blind, but the method of blinding was not described.
Not performed, if the trial was not double blind.
Incomplete outcome data reporting
Adequate, the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, the number or reasons for dropouts and withdrawals were not described.
Selective outcome reporting
Adequate, pre‐defined, or clinically relevant and reasonably expected outcomes are reported on.
Unclear, not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not.
Inadequate, one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Baseline imbalance
Adequate, if there was no baseline imbalance in important characteristics.
Unclear, if the baseline characteristics were not reported.
Inadequate, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation.
Early stopping
Adequate, if sample size calculation was reported and the trial was not stopped, or the trial was stopped early by formal stopping rules at a point where the likelihood of observing an extreme intervention effect due to chance was low.
Unclear, if sample size calculation was not reported and it is not clear whether the trial was stopped early or not.
Inadequate, if the trial was stopped early due to informal stopping rules or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high.
Other biases
Adequate, the trial appears to be free of other components that could put it at risk of bias.
Unclear, the trial may or may not be free of other components that could put it at risk of bias.
Inadequate, there are other factors in the trial that could put it at risk of bias, eg, no sample size calculation made, inappropriate industry involvement (ie, data owned or influenced by industry), publication bias, academic bias, etc.
Statistical methods The analyses were performed in Review Manager 5. When possible, all patients were included in the analyses irrespective of compliance or follow‐up (intention‐to‐treat). For patients with missing data, we used 'carry forward' of the last observed response. Binary outcome measures were presented as relative risks (RR) and continuous outcome measures as mean differences (MD), both with 95% confidence intervals (CI). Since we have made comparisons where only one trial was included, we have checked the RevMan 5 results with the more correct analyses with Fisher's exact test, chi‐square test, and t‐test and have in all instances found identical or very similar results. We did not perform subgroup or sensitivity analyses because only two trials were included.
Results
Description of studies
Six‐hundred and thirty‐eight trials were identified through the initial searches. We excluded 614 duplicates or clearly irrelevant studies by reading abstracts. The remaining 24 references were 17 review articles, which were included in Additional references section, and five non randomised case series that were excluded (Excluded studies). Two trials met our inclusion criteria (Allison 1986; van Hoogstraten 2000), and those were the two trials included in the previous version of this review by Chen 2004. There were no new trials that met the inclusion criteria.
One trial (Allison 1986) compared biliary lavage with hydrocortisone plus saline versus saline. Biliary lavage was continued for two weeks via a nasobiliary tube. The dose of hydrocortisone was 100 mg per day in one litre or saline per day, and the dose of saline was one litre per day. The trial included 17 patients, but only 11 were randomised. Four patients were excluded because the nasobiliary tubes could not be inserted in the biliary tree. Another two patients were excluded because the nasobiliary tubes fell out of the biliary tree. Ten of the patients had strictures in the extrahepatic biliary tree. Eight of the patients had ulcerative colitis. Eleven patients were followed for ten weeks after the end of treatment. The evaluated outcomes were mortality, serum activity of alkaline phosphatases, serum bilirubin, and adverse events.
The other trial (van Hoogstraten 2000) compared oral administration of budesonide versus prednisone for eight weeks. Included patients were randomised to budesonide 3 mg/day, budesonide 9 mg/day, or prednisone 10 mg/day. Patients in the two budesonide groups were combined in all analyses. Patients in the budesonide group received prednisone placebo tablets and patients in the prednisone group received budesonide placebo tablets. The trial included 18 patients with characteristic radiographic and histological findings in combination with elevated serum alkaline phosphatases. All patients had inflammatory bowel disease and received ursodeoxycholic acid for at least five months before entering the trial. All patients were followed until the end of treatment. The evaluated outcomes were pruritus, fatigue, serum bilirubin, serum alkaline phosphatases, and adverse events at the end of treatment.
Risk of bias in included studies
Both trials were considered to have low risk of bias. The Allison 1986 trial had adequate generation of the allocation sequence, allocation concealment, and incomplete data addressed. The trial was unblinded. Sample‐size calculations and intention‐to‐treat analysis were not performed. These caveats may be considered as risk factors for bias (systematic error overestimating beneficial effects and underestimating harmful effects). However, when we look at the results of the trial, this bias seems remote. The van Hoogstraten 2000 trial had adequate generation of the allocation sequence, allocation concealment, blinding, follow‐up, incomplete data addressed, it was free of selective reporting, and reported intention‐to‐treat analyses (Figure 1; Figure 2). Sample‐size calculations were not performed.
1.
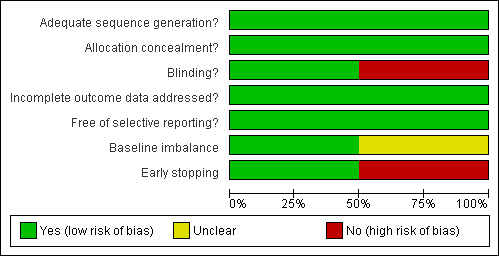
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
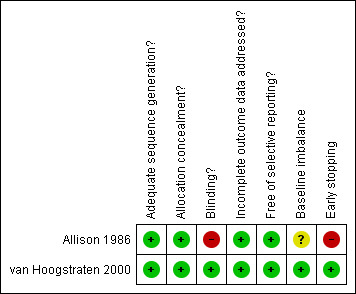
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
Biliary lavage with hydrocortisone plus saline versus saline (Analyses 1.1 to 1.4) One of the seven patients randomised to hydrocortisone died due to variceal haemorrhage. None of the six patients in the control group died. Hydrocortisone had no significant effect on mortality (RR 2.63, 95% CI 0.13 to 54.6) (Analysis 1.1). We found no significant effects of hydrocortisone on serum activity of alkaline phosphatases (MD ‐35 IU/litre, 95% CO ‐213 to 143) or serum bilirubin (MD 17.4 IU/litre, 95% CI ‐13.0 to 47.8) (Analysis 1.2; Analysis 1.3). All patients (n = 11) in both intervention groups developed bacterial colonisation of the biliary tree. In two patients in the hydrocortisone group three episodes of cholangitis with septicaemia occurred. Another two hydrocortisone‐treated patients developed fluid retention or paranoid ideas, respectively. One patient in the saline control group developed pancreatitis. Accordingly, hydrocortisone tended to increase the occurrence of adverse events, but not significantly (RR 3.43, 95% CI 0.51 to 22.9) (Analysis 1.4). In addition to the adverse events described above, two of the patients in whom insertion of nasobiliary tube failed developed pancreatitis. This make the proportion of patients with adverse events raise to 7/17 (41%).
1.1. Analysis.
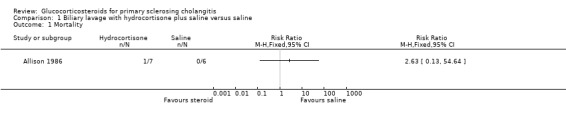
Comparison 1 Biliary lavage with hydrocortisone plus saline versus saline, Outcome 1 Mortality.
1.2. Analysis.
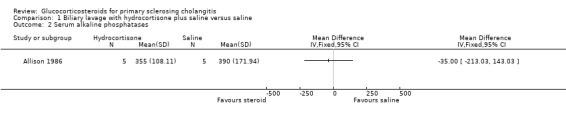
Comparison 1 Biliary lavage with hydrocortisone plus saline versus saline, Outcome 2 Serum alkaline phosphatases.
1.3. Analysis.
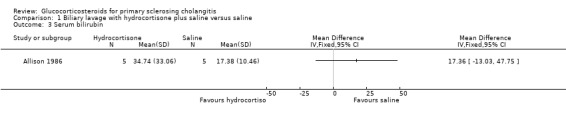
Comparison 1 Biliary lavage with hydrocortisone plus saline versus saline, Outcome 3 Serum bilirubin.
1.4. Analysis.
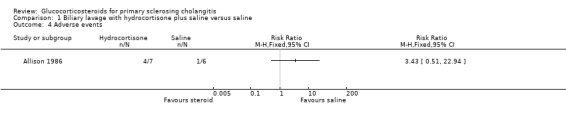
Comparison 1 Biliary lavage with hydrocortisone plus saline versus saline, Outcome 4 Adverse events.
Budesonide versus prednisone (Analyses 2.1 to 2.5) Two of the 12 patients in the budesonide group and 3 out of the 6 patients in the prednisone group had pruritus. Six of 12 patients in the budesonide group and 2/6 patients in the prednisone group suffered from fatigue. The evaluated treatments had no significantly different effects on pruritus (RR 0.33, 95% CI 0.07 to 1.49) or fatigue (RR 1.50, 95% CI 0.42 to 5.32) (Analysis 2.1; Analysis 2.2). Serum bilirubin concentrations were significantly lower in the budesonide compared with the prednisone group (MD ‐10.4 µmol/litre, 95% CI ‐19.6 to ‐1.2 µmol/litre) (Analysis 2.3). The serum alkaline phosphatase activities were not significantly different in the two groups (MD 17 IU/litre, 95% CI ‐112 to 146) (Analysis 2.4). In the budesonide group, two patients developed autoimmune hepatitis, acne, and nausea. In the prednisone group, one patient developed impaired vision and itching eyes. The proportions of adverse events in two groups were not significantly different (RR 1.50, 95% CI 0.20 to 11.54) (Analysis 2.5).
2.1. Analysis.
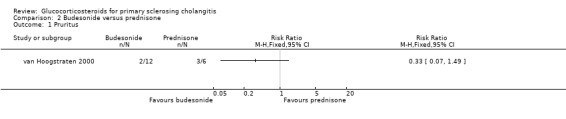
Comparison 2 Budesonide versus prednisone, Outcome 1 Pruritus.
2.2. Analysis.
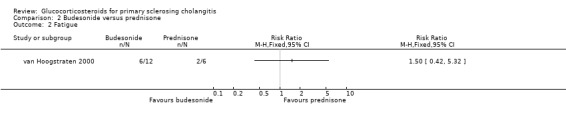
Comparison 2 Budesonide versus prednisone, Outcome 2 Fatigue.
2.3. Analysis.
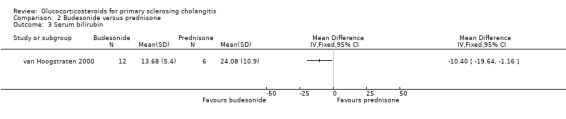
Comparison 2 Budesonide versus prednisone, Outcome 3 Serum bilirubin.
2.4. Analysis.
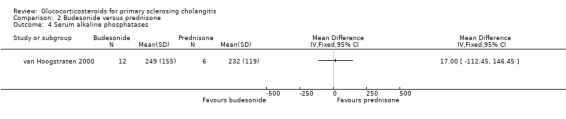
Comparison 2 Budesonide versus prednisone, Outcome 4 Serum alkaline phosphatases.
2.5. Analysis.
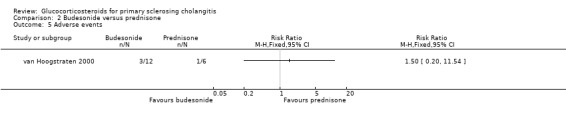
Comparison 2 Budesonide versus prednisone, Outcome 5 Adverse events.
Discussion
We found no evidence to support or refute glucocorticosteroids for patients with primary sclerosing cholangitis. Only two randomised clinical trials with small sample sizes and short treatment durations were available. The wide confidence intervals warrant caution when interpreting the results. Also, the administration route of corticosteroids in the two trials differed; therefore, this fact should be taken into account. Considering the nature of the disease treatment regimens of considerably longer duration seem warranted. Randomised clinical trials on glucocorticosteroids versus placebo are needed before glucocorticosteroids can be recommended for primary sclerosing cholangitis.
One of the identified trials (Allison 1986) evaluated the effect of biliary lavage with hydrocortisone. This treatment was associated with several severe adverse events including pancreatitis, bacterial cholangitis, and fluid retention. The other trial (van Hoogstraten 2000) compared different types and doses of glucocorticosteroids. We found no significant differences between the evaluated interventions, but the primary analyses in the published trial report suggest that prednisone 10 mg/day may be associated with reduction in pruritus. A similar effect of glucocorticosteroids on pruritus has been found for primary biliary cirrhosis (Mitchison 1989; van Hoogstraten 2000). The effect may be related to induction of cytochrome P450‐3A activity (Pichard 1992) as it has been found for the antipruritic drug rifampicin. When comparing the serum bilirubin levels before and after treatment, serum bilirubin did not change significantly in the budesonide group, but was significantly increased in the prednisone group. Considering the small sample size of the trial, additional information is needed to estimate whether this difference is a chance finding. Ursodeoxycholic acid may improve biochemical variables in some patients with primary sclerosing cholangitis (Chen 2003). The lack of effects of glucocorticosteroids on biochemical variables may theoretically be related to the inclusion criteria because all patients had no response to previous ursodeoxycholic acid. On the other hand, the unresponsiveness of patients to ursodeoxycholic acid may have been an advantage for glucocorticosteroids to exhibit their effect, but that effect did not take place. Theoretically, following this assumption, the evidence for no significant effect of glucocorticosteroids in primary sclerosing cholangitis may stand even firmer ground.
Long‐term treatment with glucocorticosteroids may aggravate bone calcium loss. Previous studies suggest that the bone density in patients who receive prednisone is lower than patients who are not treated (Lindor 1991; Charatcharoenwitthaya 2006 ). Calcium loss seems to occur in patients with primary sclerosing cholangitis (Angulo 2000) as well as in primary biliary cirrhosis (Mitchison 1989). Long‐term treatment with glucocorticosteroids must be carefully monitored.
Authors' conclusions
Implications for practice.
There is insufficient evidence either to support or refute glucocorticosteroids for primary sclerosing cholangitis. Therefore, we cannot recommend the use of glucocorticosteroids outside randomised clinical trials. Biliary lavage in patients with primary sclerosing cholangitis seems to be associated with prevalent and serious adverse events. Adverse events in such studies must be very closely monitored.
Implications for research.
We need randomised clinical trials comparing oral administration of glucocorticosteroids versus placebo for patients with primary sclerosing cholangitis. Because the disease is rare, multicentre trials will be necessary. Future trials should have sufficient statistical power, low risk of bias, adequate follow‐up, intensive monitoring of adverse events, and should be reported according to the CONSORT guidelines (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 17 February 2010 | Amended | Peer reviewers' names added. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 29 September 2009 | New citation required but conclusions have not changed | No new trials were identified for this update. |
| 29 September 2009 | New search has been performed | This review is an update of the review prepared by Chen 2004. |
Acknowledgements
We thank the patients who participated in, and the researches who designed and conducted the included trials. We thank the previous first author of the review, Wendong Chen, for the firm base for us to update this review on. Also, we extend our gratitude to Dimitrinka Nikolova and Sarah Louise Klingenberg from The Cochrane Hepato‐Biliary Group, for assistance during the process of preparation of this review.
Peer Reviewers: Peter Madsen, Denmark; Paul Paul Schlichting, Denmark, Lise Lotte Gluud, Denmark. Contact Editor: Jian Ping Liu, China.
Appendices
Appendix 1. Search Strategies
| Database | Date of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | September 2009. | ('primary sclerosing cholangit*' OR PSC) AND (corticosteroid* OR glucocortico* OR predniso* OR budenosid* OR betamethason* OR methylpredniso* OR hydrocortison* OR dexamethason*) |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 3, 2009. | #1 LIVER‐CHOLANGITIS‐BILIARY*:ME #2 (PRIMARY and (SCLEROSING and CHOLANGITIS)) or PSC) #3 ((((((((GLUCOCORTICO* or CORTICO*) or PREDNISO*) or METHYLPREDNISO*) or BUDESONIDE) or BECLMETASON) or BETAMETASON) or HYDROCORTISON) or IMMUNOSUPPRES*) #4 ADRENAL‐CORTEX‐HORMONES*:ME #5 (#3 or #4) #6 (#1 and #5) #7 (#2 and #5) #8 (#6 or #7) #9 ((RANDOM* or PLACEBO*) OR (DOUBLE and BLIND*)) #10 (#8 and #9) |
| MEDLINE (Ovid SP) | 1950 to September 2009. | 1. Cholangitis/ or exp Cholangitis, Sclerosing/ or primary‐sclerosing‐cholangitis.mp. 2. ("primary sclerosing cholangitis" or PSC).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. "sclerosing cholangitis".mp. [mp=title, original title, abstract, name of substance word, subject heading word] 4. psc.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 5. cholangitis.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. 1 or 2 or 3 or 4 or 5 7. Anti‐Inflammatory Agents/ or exp Glucocorticoids/ or exp Dexamethasone/ or glucocorticosteroids.mp. or exp Hydrocortisone/ 8. glucocortico*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. cortico*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 10. steroid*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 11. predniso*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 12. methylpredniso*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 13. budesonid*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 14. dexamethason*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 15. hydrocortison*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 16. immunosuppress*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 17. adrenal cortex hormones.mp. or exp Adrenal Cortex Hormones/ 18. 11 or 7 or 9 or 17 or 12 or 15 or 14 or 8 or 16 or 10 or 13 19. 6 and 18 20. randomised controlled trial.mp. or exp Randomized Controlled Trial/ 21. random*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 22. placebo*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 23. control*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 24. blind*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 25. ("randomised controlled trial" or rct).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 26. rct.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 27. 25 or 22 or 21 or 24 or 26 or 23 or 20 28. 27 and 19 |
| EMBASE (Ovid SP) | 1980 to September 2009. | 1. primary sclerosing cholangitis.mp. or exp primary sclerosing cholangitis/ 2. glucocorticosteroids.mp. or exp glucocorticoid/ 3. exp fludrocortisone/ or exp hydrocortisone/ or exp budesonide plus formoterol/ or exp glucocorticoid/ or exp steroid/ or exp dexamethasone/ or glucocortico*.mp. or exp methylprednisolone/ or exp corticosteroid/ 4. budesonid*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 5. predniso*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 6. methylpredniso*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. hydrocortison*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 8. dexamethason*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. immunosuppress*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 10. 8 or 6 or 4 or 3 or 7 or 9 or 2 or 5 11. 1 and 10 12. randomised controlled trial/ 13. random*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 14. placebo*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 15. blind*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 16. double blind.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 17. rct.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 18. randomised controlled trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 19. randomised controlled trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 20. 17 or 12 or 15 or 14 or 18 or 13 or 16 or 19 21. 11 and 20 |
| LILACS | September 2009. | (RCT or "randomised controlled trial") AND ("primary sclerosing cholangitis" or psc) AND (glucocorticosteroids or glucortico* or corticosteroid* or predniso* or budesonid* or betamethason* or methylpredniso* or predniso*) |
Data and analyses
Comparison 1. Biliary lavage with hydrocortisone plus saline versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Serum alkaline phosphatases | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Serum bilirubin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Budesonide versus prednisone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Serum bilirubin | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Serum alkaline phosphatases | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allison 1986.
| Methods | Randomised parallel group trial. | |
| Participants | Country: United Kingdom.
Publication language: English. Inclusion criteria: patients with primary sclerosing cholangitis of the intrahepatic ducts. Exclusion criteria: patients who had a biliary bypass procedure or ascending cholangitis in the previous month. Participants: 1. Hydrocortisone plus saline group (n = 7) Mean age (years +/‐ SD): 46.3 +/‐ 15.5. Male/female: 6/1. Site of disease: Intrahepatic bile duct 5. Intrahepatic bile duct and main hepatic duct 2. Mean bilirubin level at start of lavage (µmol/l +/‐ SD): 72.57 +/‐ 98.04. 2. Saline group (n = 6) Mean age (years +/‐ SD): 58.0 +/‐ 9.6. Male/female: 4/2. Site of disease: Intrahepatic bile duct 3. Generalised 2. Intrahepatic bile duct and common bile duct 1. Mean bilirubin level at start of lavage (µmol/l +/‐ SD): 30.00 +/‐ 37.64. |
|
| Interventions | Hydrocortisone plus saline group:
biliary lavage with one litre per day normal saline plus 100 mg per day hydrocortisone for two weeks. Saline group: biliary lavage with one litre per day normal saline for two weeks. |
|
| Outcomes | Mortality at the end of follow‐up. Serum alkaline phosphatases activity at the end of follow‐up. Serum bilirubin level at the end of follow‐up. Adverse events. | |
| Notes | Follow‐up time: 12 weeks. We obtained additional information from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated list. |
| Allocation concealment? | Low risk | Sealed envelopes. |
| Blinding? All outcomes | High risk | Not performed. |
| Incomplete outcome data addressed? All outcomes | Low risk | One patient in each group dropped out due to technical failures. |
| Free of selective reporting? | Low risk | All outcomes reported. |
| Baseline imbalance | Unclear risk | Not reported. |
| Early stopping | High risk | The trial was stopped early because of no clear benefit. |
van Hoogstraten 2000.
| Methods | Randomised parallel group trial. | |
| Participants | Country: Netherlands.
Publication language: English. Inclusion criteria: 1. Patients with primary sclerosing cholangitis based on the findings on endoscopic retrograde cholangiography and typical histological lesions (pericholangiolar onion‐skin fibrosis) in combination with both a serum alkaline phosphatase level elevated to more than twice the upper limit of normal and the presence of inflammatory bowel disease. 2. All patients had been treated with ursodeoxycholic acid (mean dose, 12 mg/kg body weight/day) for at least five months without achieving biochemical remission. Excluded criteria: Age over 65 years old, use of immunosuppressive drugs such as corticosteroids, azathioprine, cyclosporin, or methotrexate, pregnancy, evidence of primary sclerosing cholangitis associated autoimmune hepatitis, previous cholecystectomy, presence of a biliary stent, and cirrhosis with a Child‐Pugh score over six. Participants: 1. Budesonide (9 mg/day) group (n = 6) Mean age (years +/‐ SD): 46.4 +/‐ 9.7. Male/female: 4/2. Inflammatory bowel disease: 4. 2. Budesonide (3 mg/day) group (n = 6) Mean age (years +/‐ SD): 38.9 +/‐ 12.3. Male/female: 4/2. Inflammatory bowel disease: 4. 3. Prednisone (10 mg/day) group (n = 6) Mean age (years +/‐ SD): 44.5 +/‐ 10.9. Male/female: 6/0. Inflammatory bowel disease: 4. |
|
| Interventions | Budesonide (9 mg/day) group:
9 mg/day budesonide (three 3‐mg budesonide capsules and two placebo tablets of 5 mg prednisone) for eight weeks. Budesonide (3 mg/day) group: 3 mg/day budesonide (one 3‐mg budesonide capsule, two placebo capsules of 3‐mg budesonide, and two placebo tablets of 5 mg prednisone) for eight weeks. Prednisone (10 mg/day) group: 10 mg/day prednisone (two 5‐mg tablets of prednisone and three 3‐mg placebo capsules budesonide) for eight weeks. |
|
| Outcomes | Serum alkaline phosphatases activity at the end of treatment. Serum bilirubin level at the end of treatment. Adverse events. | |
| Notes | Follow‐up time: eight weeks. We obtained additional information from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random table. |
| Allocation concealment? | Low risk | Coded medication. |
| Blinding? All outcomes | Low risk | Identical placebos. |
| Incomplete outcome data addressed? All outcomes | Low risk | No attrition. |
| Free of selective reporting? | Low risk | No selective reporting. |
| Baseline imbalance | Low risk | No imbalance. |
| Early stopping | Low risk | The trial was carried out as planned. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angulo 2000 | This is a case series. Twenty‐one primary sclerosing cholangitis patients received 9 mg budesonide orally each day for one year. Liver biochemistry was marginally better, but at a cost of marked bone loss. |
| Boberg 2003 | A retrospective study of primary sclerosing cholangitis that were treated with corticosteroids. The authors compared responders with non‐responders to corticosteroids. They concluded that certain subgroups of patients that respond to corticosteroids may have improved long‐term survival. |
| Burgert 1984 | This is a case series. Ten primary sclerosing cholangitis patients received at least six months prednisone therapy. The mean initial prednisone dosage was 39 mg/day, which was tapered to 23 mg/day by six months. Seven of ten patients showed improvement in liver biochemistry variables and liver histology. Three patients with pruritus had relief within one month. |
| Grijm 1986 | A case series of 21 patients with primary sclerosing cholangitis that were treated with biliary lavage with prednisolone. |
| Lindor 1991 | This is a case series. Twelve primary sclerosing cholangitis patients received a combination of low‐dose prednisone (10 mg/day) and colchicine (0.6 mg bid) for two years. Their course of disease was compared with that of a group of historical controls. At six and 12 months, there was significantly more improvement in liver test results over baseline values in the patients receiving prednisone and colchicine than in the untreated controls. At 24 months, however, no significant differences in biochemical tests were observed between the treated and untreated patients. |
| Schramm 1999 | A case series of 15 patients treated with combination of azathioprine, prednisolone and ursodeoxycholic acid. Follow‐up from 3 to 81 months. A benefit was shown in histopathology and biochemistry. |
bid = twice daily
Differences between protocol and review
None.
Contributions of authors
Vanja Giljaca revisited the protocol for this review, drafted the review, developed the search strategy and performed searches, extracted data and wrote the update of the review. Goran Poropat independently developed the search strategy, performed searches, and validated the data. Davor Stimac and Christian Gluud formulated the idea of the review, validated the data and revised the review.
Sources of support
Internal sources
Copenhagen Trial Unit, Centre for Clinical Intervention Research, H:S Rigshospitalet, Denmark.
External sources
Danish Medical Research Council Grant on Getting Research into Practice (GRIP), Denmark.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Allison 1986 {published data only}
- Allison MC, Burroughs AK, Noone P, Summerfield JA. Biliary lavage with corticosteroids in primary sclerosing cholangitis. A clinical, cholangiographic and bacteriological study. Journal of Hepatology 1986;3(1):118‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Hoogstraten 2000 {published data only}
- Hoogstraten HJ, Vleggaar FP, Boland GJ, Steenbergen W, Griffioen P, Hop WCJ, et al. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomised double‐blind pilot study. Belgian‐Dutch PSC Study Group. The American Journal of Gastroenterology 2000;95(8):2015‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Angulo 2000 {published data only}
- Angulo P, Batts KP, Jorgensen RA, LaRusso NA, Lindor KD. Oral budesonide in the treatment of primary sclerosing cholangitis. American Journal of Gastroenterology 2000;95(9):2333‐7. [DOI] [PubMed] [Google Scholar]
Boberg 2003 {published data only}
- Boberg KM, Egeland T, Schrumpf E. Long‐term effect of corticosteroid treatment in primary sclerosing cholangitis patients. Scandinavian Journal of Gastroenterology 2003;38(9):991‐5. [DOI] [PubMed] [Google Scholar]
Burgert 1984 {published data only}
- Burgert SL, Brown BP, Kirkpatrick RB, LaBrecque DR. Positive corticosteroid response in early sclerosing cholangitis. Gastroenterology 1984;86(5):1037. [Google Scholar]
Grijm 1986 {published data only (unpublished sought but not used)}
- Grijm R, Huibregtse K, Bartelsman J, Mathus‐Vliegen EMH, Dekker W, Tytgat GN. Therapeutic investigations in primary sclerosing cholangitis. Digestive Diseases and Sciences 1986;31(8):793‐8. [DOI] [PubMed] [Google Scholar]
Lindor 1991 {published data only}
- Lindor KD, Wiesner RH, Colwell LJ, Steiner B, Beaver S, LaRusso NF. The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. The American Journal of Gastroenterology 1991;86(1):57‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Schramm 1999 {published data only}
- Schramm C, Schirmacher P, Helmreich‐Becker I, Gerken G, Meyer zum Büschenfelde KH, Lohse AW. Combined therapy with azathioprine, prednisolone and ursodiol in patients with primary sclerosing cholangitis. Annals of Internal Medicine 1999;131:943‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Ahrendt 1998
- Ahrendt SA, Pitt HA, Kalloo AN, Venbrux AC, Klein AS, Herlong HF, et al. Primary sclerosing cholangitis: resect, dilate, or transplant?. Annals of Surgery 1998;227(3):412‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgert 1984
- Burgert SL, Brown BP, Kirkpatrick RB, LaBrecque DR. Positive corticosteroid response in early sclerosing cholangitis. Gastroenterology 1984;86(5):1037. [Google Scholar]
Chapman 2003
- Chapman RW. The management of primary sclerosing cholangitis. Current Gastroenterology Reports 2003;5:9‐17. [DOI] [PubMed] [Google Scholar]
Charatcharoenwitthaya 2006
- Charatcharoenwitthaya P, Lindor KD. Pirmary sclerosing cholangitis: diagnosis and management. Current Gastroenterology Reports 2006;8:75‐82. [DOI] [PubMed] [Google Scholar]
Chen 1984
- Chen LY, Goldberg HI. Sclerosing cholangitis: broad spectrum of radiographic features. Gastrointestinal Radiology 1984;9(1):39‐47. [DOI] [PubMed] [Google Scholar]
Chen 2003
- Chen W, Gluud C. Bile acids for primary sclerosing cholangitis (Cochrane review). Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003626] [DOI] [PubMed] [Google Scholar]
Cullen 2005
- Cullen SN, Chapman RW. Review article: current management of primary sclerosing cholangitis. Alimentary Pharmacology and Therapeutics 2005;21:933‐48. [DOI: 10.1111/j.1365-2036.2005.02407.x] [DOI] [PubMed] [Google Scholar]
Donaldson 1991
- Donaldson PT, Farrant JM, Wilkinson ML, Hayllar K, Portmann BC, Williams R. Dual association of HLA DR2 and DR3 with primary sclerosing cholangitis. Hepatology 1991;13(1):129‐33. [MEDLINE: ] [PubMed] [Google Scholar]
Fracchia 2000
- Fracchia M, Secreto P, Tabone M, Zaffino C, Pera A, Galatola G. Serum interferon gamma in primary biliary cirrhosis: effect of ursodeoxycholic acid and prednisone therapy alone and in combination. European Journal of Gastroenterology & Hepatology 2000;12(4):463‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gluud 2009
- Gluud C, Nikolova D, Klingenberg SL, Whitfield K, Alexakis N, Als‐Nielsen B, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2009, Issue 4. Art. No.: LIVER.
Gordon 2008
- Gordon FD. Primary sclerosing cholangitis. Surgical Clinics of America 2008;88:1385‐407. [DOI: 10.1016/j.suc.2008.07.010] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Colloboration, 2008. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. Code of Federal Regulations & International Conference on Harmonization Guidelines. Media: Parexel Barnett, 1997. [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LaRusso 1984
- LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL. Current concepts. Primary sclerosing cholangitis. The New England Journal of Medicine 1984;310(14):899‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LaRusso 1999
- LaRusso NF, Wiesner RH, Ludwig J. Sclerosing cholangitis. In: Bircher J, Benhamou JP, McIntyre N, Rizzetto M, Rodes J editor(s). Oxford Textbook of Clinical Hepatology. 2. Vol. II, Oxford: Oxford University Press, 1999:1121‐1130. [Google Scholar]
Lindor 1986
- Lindor KD, Wiesner RH, LaRusso NF, Dickson ER. Chronic active hepatitis: overlap with primary biliary cirrhosis and primary sclerosing cholangitis. In: Czaja AJ, Dickson ER editor(s). Chronic active hepatitis: the Mayo Clinic experience. New York: Marcel Dekker, 1986:171‐85. [Google Scholar]
Lindor 1987
- Lindor KD, Wiesner RH, LaRusso NF. Recent advances in the management of primary sclerosing cholangitis. Seminars in Liver Disease 1987;7(4):322‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lindor 1991
- Lindor KD, Wiesner RH, Colwell LJ, Steiner B, Beaver S, LaRusso NF. The combination of prednisone and colchicine in patients with primary sclerosing cholangitis. The American Journal of Gastroenterology 1991;86(1):57‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Long 1978
- Long RG, Meinhard E, Skinner RK, Varghese Z, Wills MR, Sherlock S. Clinical, biochemical, and histological studies of osteomalacia, osteoporosis, and parathyroid function in chronic liver disease. Gut 1978;19(2):85‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ludwig 1981
- Ludwig J, Barham SS, LaRusso NF, Elveback LR, Wiesner RH, McCall JT. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology 1981;1(6):632‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ludwig 1986
- Ludwig J, LaRusso NF, Wiesner RH. Primary sclerosing cholangitis. In: Peters RL, Craig JR editor(s). Liver pathology‐contemporary issues in surgical pathology. New York: Churchill Livingstone, 1986:193‐213. [Google Scholar]
Ludwig 1991
- Ludwig J. Small‐duct primary sclerosing cholangitis. Seminars in Liver Disease 1991;11(1):11‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitchison 1989
- Mitchison HC, Bassendine MF, Malcolm AJ, Watson AJ, Record CO, James OF. A pilot, double‐blind, controlled 1‐year trial of prednisolone treatment in primary biliary cirrhosis: hepatic improvement but greater bone loss. Hepatology 1989;10(4):420‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olerup 1995
- Olerup O, Olsson R, Hultcrantz R, Broome U. HLA‐DR and HLA‐DQ are not markers for rapid disease progression in primary sclerosing cholangitis. Gastroenterology 1995;108(3):937‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pichard 1992
- Pichard L, Fabre I, Daujat M, Domergue J, Joyeux H, Maurel P. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Molecular Pharmacology 1992;41(6):1047‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Poupon 2000
- Poupon R, Chazouilleres O, Poupon RE. Chronic cholestatic diseases. Journal of Hepatology 2000;32(Suppl 1):129‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quigley 1983
- Quigley EM, LaRusso NF, Ludwig J, MacSween RN, Birnie GG, Watkinson G. Familial occurrence of primary sclerosing cholangitis and ulcerative colitis. Gastroenterology 1983;85(5):1160‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0.. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schulz 1995
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silveira 2008
- Silveira MG, Lindor KD. Clinical features and management of primary sclerosing cholangitis. World Journal of Gastroenterology 2008;14(21):3338‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silveira 2008a
- Silveira MG, Lindor KD. Primary sclerosing cholangitis. Canadian Journal of Gastroenterology 2008;22(8):689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiehl 1997
- Stiehl A, Rudolph G, Sauer P, Benz C, Stremmel W, Walker S, et al. Efficacy of ursodeoxycholic acid treatment and endoscopic dilation of major duct stenoses in primary sclerosing cholangitis. An 8‐year prospective study. Journal of Hepatology 1997;26(3):560‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vacca 2007
- Vacca M, Krawczyk M, Petruzzelli M, Sasso RC, Erpecum KJ, Palasciano G, et al. Current treatments of primary sclerosing cholangitis. Current Medical Chemistry 2007;14:2081‐94. [DOI] [PubMed] [Google Scholar]
van Hoogstraten 2000
- Hoogstraten HJ, Vleggaar FP, Boland GJ, Steenbergen W, Griffioen P, Hop WC, et al. Budesonide or prednisone in combination with ursodeoxycholic acid in primary sclerosing cholangitis: a randomised double‐blind pilot study. American Journal of Gastroenterology 2000;95(8):2015‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wee 1985a
- Wee A, Ludwig J, Coffey RJ Jr, LaRusso NF, Wiesner RH. Hepatobiliary carcinoma associated with primary sclerosing cholangitis and chronic ulcerative colitis. Human Pathology 1985;16(7):719‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wee 1985b
- Wee A, Ludwig J. Pericholangitis in chronic ulcerative colitis: primary sclerosing cholangitis of the small bile ducts?. Annals of Internal Medicine 1985;102(5):581‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wiesner 1985
- Wiesner RH, LaRusso NF, Ludwig J, Dickson ER. Comparison of the clinicopathologic features of primary sclerosing cholangitis and primary biliary cirrhosis. Gastroenterology 1985;88(1 Pt 1):108‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wolfhagen 1994
- Wolfhagen FH, Buuren HR, Schalm SW. Combined treatment with ursodeoxycholic acid and prednisone in primary biliary cirrhosis. The Netherlands Journal of Medicine 1994;44(3):84‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schuly KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chen 2004
- Chen W, Gluud C. Clucocorticosteroids for primary sclerosing cholangitis. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004036.pub2.] [DOI] [PubMed] [Google Scholar]


