Abstract
Prostate cancer is the most common malignancy and the second leading cause of cancer-related death in men. Radiotherapy is a curative option that is administered via external beam radiation, brachytherapy, or in combination. Erectile, ejaculatory and orgasm dysfunction(s) is/are known potential and common toxicities associated with prostate radiotherapy. Our multidisciplinary team of physicians and/or scientists have written a three (3) part comprehensive review of the pathogenesis and management radiation-induced sexual dysfunction. Part I reviews pertinent anatomy associated with normal sexual function and then considers the pathogenesis of prostate radiation-induced sexual toxicities. Next, our team considers the associated radiobiological (including the effects of time, dose and fractionation) and physical (treatment planning and defining a novel Organ at Risk (OAR)) components that should be minded in the context of safe radiation treatment planning. The authors identify an OAR (i.e., the prostatic plexus) and provide suggestions on how to minimize injury to said OAR during the radiation treatment planning process.
Keywords: Prostate cancer, Erectile dysfunction, Ejaculatory dysfunction, Orgasmic dysfunction, Radiation therapy, Organ at risk
1. Background
Prostate cancer (PCa) is the number one cancer diagnosed in men with 183,529 new cases diagnosed in 2015 alone; it is the second leading cause of cancer-related deaths in men with 28,848 men dying as a result of prostate cancer in 2015. Prostate cancer trailed only lung and bronchial cancer in this regard.1
Sexual function is divided into three phases: desire (i.e., central activation/response), arousal (i.e., peripheral physiological response to various internal/external cues), and orgasm (i.e., a central and peripheral physiological climax resulting from sexual stimulation). Sexual dysfunction occurs when any of these phases are impeded secondary to physical and/or psychological causes [ASRM DEF - 50]. Erectile dysfunction (ED) is the inability to achieve or maintain a penile erection suitable for satisfactory sexual relations [ISSM DEF - 51]. After prostate radiotherapy, patients often report impaired sexual function; including ED, increased time until orgasm, decreased intensity of orgasm and/or dry ejaculation. Tactile sensation of the penis, scrotum, perineum, and anal regions generally remains intact.2
ED becomes more prevalent with increasing age as a result of vascular comorbidities (e.g. diabetes, obesity, and hypertension). These conditions impair endothelial function and may be accelerated by radiotherapy.3,4 Androgen deprivation therapy (ADT), commonly used to treat PCa in conjunction with radiotherapy, has well-known effects on desire, creating a situation of iatrogenic deficiency of testosterone. In long-term ADT exposure, structural damage occurs to the cavernoal smooth muscle with collagenization resulting in irreversible ED.
The psychological impact of a cancer diagnosis may also lead to decreased sexual desire,5 as anxiety and depressive symptoms (e.g., anhedonia, adynamia, asthenia, hypersomnia/insomnia) can lower a man’s sexual desire. A partner’s reaction to the cancer diagnosis or cancer treatment can have a major impact on either alleviating and/or aggravating a man’s sexual dysfunction. Additionally, many commonly prescribed antidepressants, particularly the tricyclic antidepressants, are known to impede sexual function as one of their adverse effects.6
Innovations in screening and treatment (whether radiation or surgical therapy) of PCa are translating into a steadily increasing population of survivors. However, this survivor population must contend with the long-term side effects of their treatment (e.g., urinary and/or sexual problems), necessitating development of methods to mitigate these toxicities and maintain their quality of life (QOL).7, 8, 9 Patients with PCa report significantly decreased QOL owing to decreased sexual function and/or sexual toxicities related to definitive treatment.10,11 The PCa patient’s experience can extend to impairing the QOL of the partner as well,12 ultimately leading to relationship difficulties and increased regret after PCa treatment. To this end, Singer et al., reported that men are willing to accept a 10% decrease in overall survival if the inferior treatment regimen would optimize preservation of their erectile function.13
Thus, the patients’ multidisciplinary team must be cognizant of these biopsychosocial issues in order to initiate preventive measures for, and the lead management of, sexual dysfunction. These measures can be instituted as early as the initial consultation and the informed consent process for radiotherapy. As the frontline physicians for PCa patients, radiation oncologists and urologists, should consider referring patients to appropriate mental health specialists (e.g., psychologists, psychiatrists, therapists) to improve disease perception, acceptance, treatment experiences and outcomes in order to improve QOL in PCa survivors.
2. Functional anatomy & physiology
2.1. Penile anatomy
The penis is composed of three cylinders; two corpora cavernosa forming the dorsal aspect of the phallus while the ventral aspect is comprised of the corpora spongiosum that contains the urethra. The corpora cavernosa contain a network of sinusoids, lined by endothelium organized within smooth muscle trabeculae that are supported by the tunica albuginea.14 The tunica albuginea is a meshwork of elastin and collagen fibers composed of 2 distinct layers. The inner layer is arranged circumferentially along the entire length of the corpora and acts by restricting girth. The outer layer of longitudinal fibers provides additional support which restricts length.15
2.2. Penile vascular supply
The penis receives its blood supply via the internal pudendal arteries that give rise to the common penile artery, which subsequently divides into the bulbourethral and common penile arteries, the latter dividing itself into the cavernous and dorsal arteries of the penis.16 Smooth muscle within the corpora regulates blood flow into the sinusoids. Increased blood flow into the corpora causes an expansion of the tunica albuginea which is ultimately responsible for the increased intracavernosal pressure required for erectile rigidity. Sinusoidal blood travels through venules to a subtunical venous plexus that exits the corpora cavernosa via emissary veins penetrating the tunica albuginea. Elevated intracorporal pressure compresses the subtunical and emissary veins, reducing venous outflow, and enabling maintenance of an erection. Diminished blood flow or inability to restrict blood outflow will impair erectile rigidity.
2.3. Penile autonomic nervous system
The penis receives both sympathetic and parasympathetic innervation (Fig. 1). Parasympathetic pathways are primarily responsible for initiating erections and regulating blood flow into the corporal bodies, while stimulation of the sympathetic pathways results in detumescence.
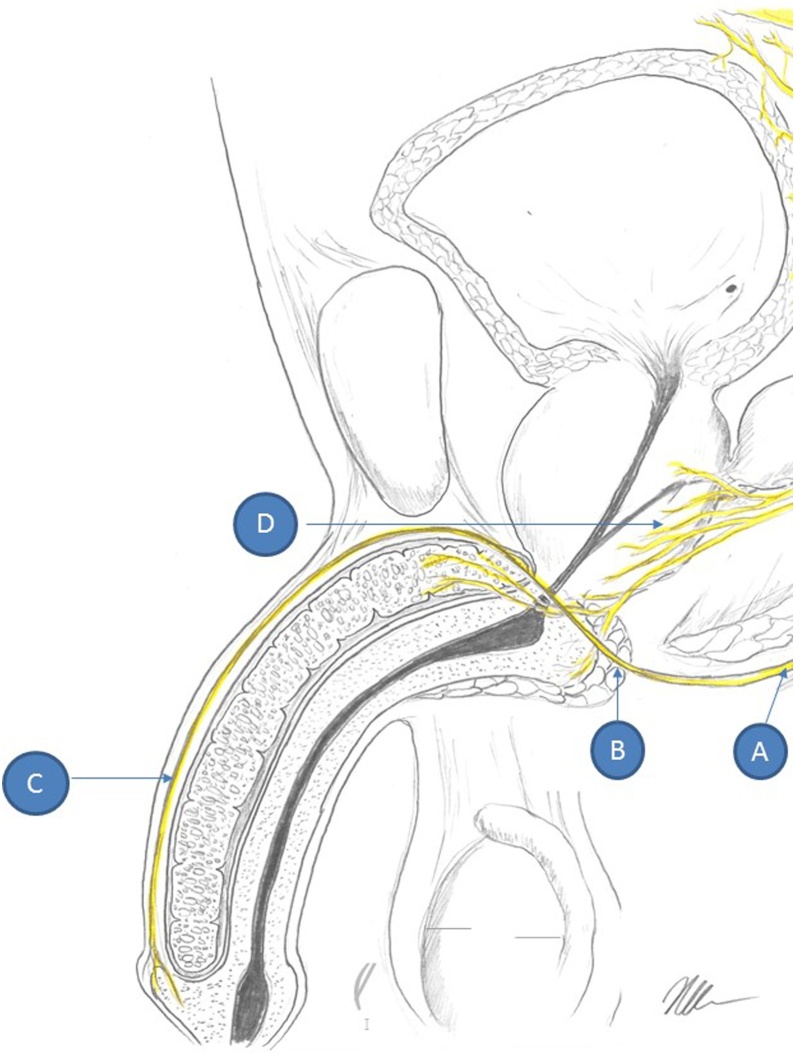
Fig. 1.
Schematic drawing of male genitourinary system and the nerves that are in or in proximity of radiation treatment fields for prostate cancer. A) is the sacral nerve root that gives rise to the (B) pudendal nerve and eventually (C) the dorsal penile nerve that is important for sensation during intercourse. (D) is the prostatic plexus that lies within the prostatic fascia and is most susceptible to radiation damage. The prostatic plexus gives rise to the cavernosal nerves that help control the neural input for erections.
Parasympathetic nerves originate from the intermediolateral cell columns of the spinal cord at S2-S4 levels, travel through the pelvic splanchnic nerves, enter into the inferior hypogastric plexus and then into the prostatic plexus, eventually giving rise to the cavernous nerves of the penis. These structures run along the lateral aspect of the prostate, forming the neurovascular bundles (at the 5 and 7 o’clock positions) and entering into the corpora cavernosa.
Sympathetic fibers arise from the intermediolateral cell column at T10-L2 levels of the spinal cord, synapse on sympathetic chain ganglia, join parasympathetic fibers in the hypogastric plexus, and traverse the prostatic plexus posteriorly to innervate smooth muscle cells of the penis.
Different pathways for erectile function have been described: reflexogenic erections, psychogenic erections, and nocturnal erections. Reflexogenic erections are triggered by direct sensory stimulation of the genitalia. The sensation travels through afferent fibers to the spinal cord, where part of the signal travels up to the brain and the sensation is perceived, while the rest of the signal stimulates the S1-S4 parasympathetic center. The parasympathetic innervation to the penis promotes cavernous nerve nitric oxide (NO) release, activating nitrergic neurons on smooth muscle that increase cyclic GMP (cGMP) levels, ultimately resulting in smooth muscle relaxation and increased blood flow into the sinusoidal tissue.17 Cyclic GMP is inactivated to 5′GMP via phosphodiesterase type 5 (PDE-5), the target of PDE-5 inhibitors (PDE5i), allowing increased blood flow to the sinusoids.
Psychogenic erections are triggered by sexual thoughts, fantasies, or erotic visual or auditory stimuli. Brain activation, especially in the medial preoptic area and in the paraventricular nucleus of the thalamus, in turn, modulates the activity at the T11-L2 and S1-S4 centers.18 The resulting erection is mediated by both a decrease in tonic sympathetic signal from T11-L2 and an increase in parasympathetic activity from S1-S4. When patients have complete spinal cord lesions above the T10 level, the connection from the brain to the T11-L2 erection center is severed and psychogenic erections are lost. Sensory information from the genitalia cannot travel up to the brain, so the perception of sensation is also lost. However, since sensory afferent fibers stimulate the S1-S4 centers directly, reflexogenic erections are maintained. Conversely, patients with spinal cord damage at the sacral levels still have preservation of the psychogenic erection center at T11-L2 and, thus, are still able to achieve erections, but these will not be as robust due to the loss of the parasympathetic stimulation from the S1-S4 reflexogenic centers.19 Nocturnal erections arise from rapid eye movement (REM) sleep. The neural mechanisms for these are not completely understood yet.
Detumescence is regulated by the alpha-adrenergic nerve fibers of the sympathetic nervous system via norepinephrine release.20 Venous intracavernosal blood pressure during the flaccid state is approximately 35 mm Hg.21 Endothelial cells also release endothelin-1, prostaglandin FA2, and angiotensin II, which act in concert to promote vasoconstriction and expulsion of blood from the sinusoids during detumescence.
2.4. Penile somatosensory innervation
Penile sensation is innervated by the pudendal nerve that originates from S2-S4 and runs beneath the levator ani muscle in the pudendal canal and its course lies outside the primary radiation fields for PCa (Fig. 1). The dorsal nerve of the penis is the most distal portion of the pudendal nerve and after traveling through the pudendal canal it courses under the inferior pubic ramus and onto the dorsum of the penis. The dorsal nerve of the penis provides sensation to the penile shaft and glans during sexual activity. Afferent terminations are mostly free nerve endings, Pacinian corpuscles, and Ruffini’s corpuscles are located in the stratum granulosum; there are also mechanoreceptors located in the muscle spindles/Golgi tendons of ischiocavernosus and bulbospongiosus muscles, tunica albuginea, and corpora.
The pudendal nerve branches into the deep and superficial perineal nerves, as well as the posterior scrotal nerves. The anterior scrotum is innervated by a branch of the ilioinguinal nerve that courses through the inguinal canals.22 The spinothalamic and spinoreticular pathways ultimately relay sensory information from the spinal cord to the thalamus, hypothalamus, sensory cerebral cortex and other cerebral parts.
2.5. Ejaculatory system
The ejaculatory ducts connect the inferior portion of the seminal vesicle and the ampullae of the vasa to the urethra. The ducts pass through the prostate gland, posterior to the median lobe. The ductal wall is a single layer of easily compressible endothelial cells with an approximately 1 mm diameter and 2 cm in length. These ducts terminate lateral to the prostate utricle on the verumontanum of the urethral ridge within the prostatic central zone.
Ejaculation consists of two distinct phases: 1) emission, the release of semen into the posterior urethra from the ejaculatory ducts, and 2) expulsion, the forceful discharge of semen from the penis. These complex processes are mediated and orchestrated by the sympathetic and somatic nervous systems and are out of the scope of this review.23,24 Emission relies on sympathetic nerves from the superior and inferior hypogastric plexuses that course through the prostatic plexus (within the primary radiation fields for PCa). Expulsion relies on a spinal reflex arc, Onuf’s nucleus, and the perineal branch of the pudendal nerve (mostly outside the primary radiation fields for PCa).25 A patent urethra is necessary for the antegrade movement of seminal fluid.
2.6. Orgasm
The psychoneuroendocrine processes involved in orgasm have been researched in various contexts of sexual dysfunction. There is no standard definition of orgasm. Orgasm and ejaculation, while often associated, are two distinct physiological processes. Typically, orgasm involves a constellation of responses including increased heart rate, breathing, and blood pressure elevation along with penile/pelvic floor contractions.18 The psychogenic aspects of sexual arousal and eventual orgasm are complex and modulated by the relationship status, sexual orientation, self-confidence, masculine self-esteem, libido, genital morphology, and other factors.
Testosterone levels are associated with sexual drive, but the frequency of sexual activity is moderated by the partner relationship and the man’s psychiatric characteristics. Plasma testosterone levels increase and temporal lobe regions associated with sexual drive are activated, both result as a response to sexual stimuli. Increased testosterone levels are associated with sexual partner gratification and frequency of intercourse or with frequency of masturbation in men without partners.26
Prolactin concentrations can significantly increase for over one hour after orgasm, but are notably unchanged following sexual arousal without orgasm.27 It is suggested that quantitative measures of serum prolactin after orgasm serve as a neuroendocrine index of sexual satiety. Intercourse-induced orgasms produce 400% more prolactin compared to masturbation-induced orgasms.28 The importance of these findings is still debated in the literature; however, it is believed that the elevated prolactin plays a role in the male refractory period after orgasm.
Ultimately, the exact underlying mechanisms causing orgasm are incompletely understood. Studies of the brain using positron emission tomography have found increased blood flow to the mesodiencephalic transition zones and decreased flow in the entorhinal cortex and the amygdala. These alterations are still being studied but are likely important to the overall sensation of an orgasm.18
3. Pathophysiology of radiation-induced sexual dysfunction
Radiation-induced sexual dysfunction results from a concert of neurological, vascular and endocrine perturbations.29 The majority of the work composing our current understanding of this topic was performed in the 3D conformal radiation therapy (3D-CRT) era when it was reasonable to assume that specific toxicities were directly caused by localized doses to the affected anatomy (e.g., ED resulting from a threshold corporal cavernosal dose; dose to the penile bulb is measured as a surrogate for the corporal cavernosal dose). However, appreciable rates of sexual toxicities are still reported in the intensity modulated radiation therapy (IMRT) era, as well as among those who undergo brachytherapy. The predominant structures at risk leading to sexual dysfunction are the ejaculatory ducts within the prostate and the prostatic neurovascular plexus (Fig. 2). The prostatic plexus is adherent to the posterior prostate, making it unavoidable during radiotherapy planning and treatment by any current radiotherapeutic modality or fractionation scheme. The prostatic plexus is the source of the cavernous nerves that are important for increased blood flow for erections and sympathetic nerves that regulate expulsion of semen into the posterior urethra from the ejaculatory ducts.
Fig. 2.
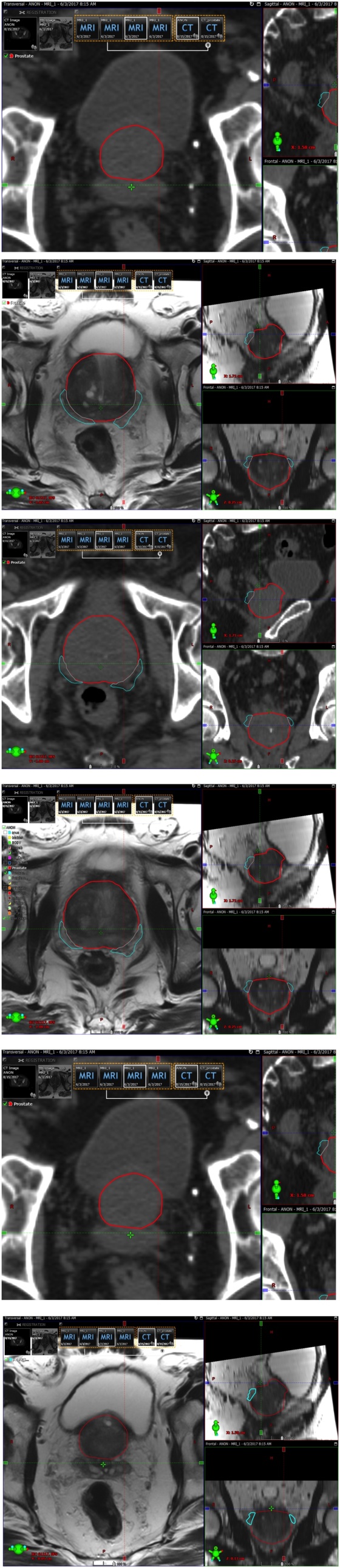
Contour of the prostatic plexus as an Organ at Risk during the radiation treatment planning.
According to the NCCN guidelines, a cumulative dose of 75.6–79.2 Gy in conventional fractions of external beam radiation treatment (EBRT) for patients that receive definitive, or ablative radiation, is appropriate for a low risk patient group. For patients with intermediate or high-risk disease, doses up to 81 Gy will provide improved disease control. A median brachial plexus dose of >69 Gy, a maximum dose of >75 Gy to 2 cm, and the presence of plexopathy before irradiation are each independent predictors of brachial plexopathy.30 Drawing an analogy of brachial plexopathy to prostatic plexopathy, it would be helpful to contour the prostatic plexus as an organ at risk (OAR) and try to avoid hot-spot(s) inside the prostatic plexus during the treatment planning process (Fig. 2).
When a patient undergoes EBRT to the prostate, a series of pro-inflammatory cytokines (e.g., TGF-β, TNF-α) are released that result in a circumferential cascade of inflammation beyond the prescription isodose lines of prostatic dose deposition. The extent of inflammation is directly proportional to the amount of prostatic tissue that is irradiated. While the benefit of covering regional lymph nodes outweighs the risks in patients with advanced disease, this will extend the field of injury to the sacral nerve roots and pelvic floor. Salvage radiation post-prostatectomy further extends the treatment field and subsequent inflammation to the pubic rami. Interstitial brachytherapy results in a more circumscribed region of peri-prostatic inflammation; however, the significantly increased biological effective dose affecting the prostatic plexus can result in a more severe acute neurovascular toxicity and late prostatic plexopathy. Inflammatory spread is also influenced by fraction delivery time, patient set-up errors, and use of rectal sparing protocols (e.g., rectal balloon, enemas, Space OAR).31, 32, 33 Longer fraction delivery time allows for more prostatic movement which can now be accommodated with MRI-guided or surface tracking treatment platforms, such as the ViewRay and VisionRT.34,35
The internal pudendal artery, and anastomoses between the deep dorsal, main cavernous and bulbar veins and the prostatic venous plexus receive a significant portion of the full radiation prescription dose.34 Accelerated atherosclerosis is a known phenomenon related to increased inflammation in patients that have received radiotherapy and has been primarily described in head and neck cancer patients.36 Similar toxicities have been reported with prostatic irradiation, and atherosclerosis of the small cavernosal vessels correlate with ED.37 Goldstein et al. reported that 15 of 23 men who underwent EBRT experienced ED after treatment. Arteriography on 2 of these men demonstrated arterial occlusive disease in the pudendal artery immediately adjacent to the irradiated field.29 Other vascular studies on men who had undergone radiotherapy reported that between 40–85% had abnormal blood flow.38,39
Preclinical models have shown that prostatic irradiation results in decreased conduction times of the pudendal40 and cavernosal nerves.41 The damage to the prostatic plexus is clear, given that it resides within the irradiated field. While the pudendal nerve lies beneath the levator ani, it comes close to the field as it enters onto the dorsum of the penis and is a likely area of some damage. Therefore, the entire penile nervous system will be receiving damage with any form of prostatic irradiation and can induce downstream small-nerve neuropathies causing the commonly encountered late sexual toxicities (e.g., erectile dysfunction, orgasmic dysfunction, ejaculatory dysfunction). However, our current understanding is limited to anecdotal evidence and extrapolations from radiation-induced neuropathies in other parts of the body; there is controversy amongst clinicians as to whether or not small-nerve neuropathies affect ejaculation and orgasm. More specific clinical studies are needed to fully elucidate the extent of involvement of small-nerve neuropathies in the development of sexual dysfunction.
The single-cell layer comprising the ejaculatory ducts is subjected to doses up to 150–200% of the prescription dose; these ducts are particularly susceptible to collapse and fibrotic fixation.42 Rates of antegrade ejaculatory dysfunction after radiotherapy vary widely, ranging from 11%–91%.43, 44, 45, 46 Anejaculation or “dry ejaculation” rates of 16%, 69%, and 89% at 1, 3, and 5 years, respectively, are indicative of a late reaction of atrophy, fibrosis, and scarring of the ejaculatory ducts within the prostate. Prior ADT is an independent predictor of ejaculatory dysfunction.44 Urethral stricture disease resulting from RT may predispose patients to develop obstruction, retrograde ejaculation or decreased force of ejaculation; however, obstructive urinary symptoms would typically be present as well.
Orgasm is a complex topic with a dearth of studies delineating the impact of radiotherapy.18 Another study of 52 men completing International Index of Erectile Function (IIEF) orgasm domain questionnaires reported a progressive decline from 7.4–5.4 to 3.2 to 2.8 at baseline and at 1, 2, and 3 years from treatment, respectively. There was a trend that patients who had undergone brachytherapy had slightly worse scores.44 While these findings correlate with the decreased conduction times associated with prostatic plexopathy, the extent to which small-nerve neuropathy is involved in the pathogenesis of orgasm dysfunction is not known.
3.1. Androgen deprivation therapy
While patients undergoing radical prostatectomy generally forego ADT, it is part of the standard of care in conjunction with radiotherapy for intermediate and high-risk prostate cancer.47 The reported ED rates seen with combination therapy (radiation and ADT) are likely synergistic, involving both peripheral and central components.
The majority of data evaluating ADT monotherapy on sexual function is in the metastatic setting. ADT directly lowers testosterone, inducing an iatrogenic testosterone deficiency that manifests as decreased sexual desire, decreased erectile function, anejaculation, and delayed orgasm.48,49 Decreased sexual activity is measurable within six months, and the loss of spontaneous erections occurs progressively, approximately 6–12 months after starting ADT.50, 51, 52, 53 ED occurs in 80–100% of men undergoing ADT alone.54 A prospective study of 44 men with adequate baseline sexual function reported that 70% experienced decreased sexual desire, 60% were unable to develop an erection with erotic imagery, and 19% of those still able to maintain erections during sexual activity had decreased tumescence.54
Erectile dysfunction rates are increased with concurrent ADT and radiotherapy.55 Several studies show men undergoing IMRT had significantly higher rates of ED if they also received ADT, compared to patients who did not.56,57 A prospective trial of 152 men undergoing radiotherapy with or without ADT (average duration 3.8 ± 1.8 months) reported an average IIEF score of 19 ± 7 for the RT alone group, compared to 14 ± 4 in the combination group. The response to sildenafil was also significantly better in the RT alone group (61% vs. 47%).58
The Radiation Therapy Oncology Group (RTOG) protocol 0215 was a randomized controlled cross-over study of 115 men who completed radiotherapy with ADT for at least 6 months. The men were randomly assigned to 12 weeks of sildenafil or placebo with 1-week washout followed by 12 weeks of another intervention. Approximately 36% responded to sildenafil but not to placebo, with men under-going ADT for less than 120 days demonstrating better response rates.59 Thus, the duration of ADT may potentiate the adverse effects of radiotherapy on sexual function. With regard to orgasms, a retrospective review of 109 patients undergoing radiotherapy and ADT demonstrated that 24% experienced anorgasmia, 44% reported decreased intensity of orgasm, and 40% noted an increased latency period before orgasm, indicating that testosterone levels directly effect the quality of orgasms.45
Importantly, studies have shown that the scatter dose to the testicles from EBRT alone (i.e., without neoadjuvant, concurrent or adjuvant ADT) is sufficient to significantly decrease serum testosterone levels to approximately 10% and 80% below baseline at 3 and 6 months, respectively.60,61 Therefore, even patients that forgo ADT with EBRT are at risk of developing testosterone decrease related sexual dysfunction.
3.2. 3D conformal radiotherapy
3D conformal radiotherapy (3DCRT) was utilized more in the earlier days of prostatic irradiation. As previously described in this review, it was initially thought that the development of sexual toxicities was correlated to the radiation doses absorbed by the penile bulb and proximal corporal bodies (versus the prostatic plexus). For that reason, a significant amount of conflicting literature was published on these dose-volume effects.
For example,62 RTOG 9406 reported that a mean dose greater than 52.2 Gy to the penile bulb was significantly associated with an increased risk of developing ED two years after radiotherapy (hazard ratio = 5.0).63 Another study demonstrated a permanent loss of erections in 30–40% of men receiving 3D conformal radiation.64
In these days of 3DCRT, the conceptualization of “sexual dysfunction” from the radiation oncological perspective seemed to be mostly limited to the degree of ED developed by a patient in follow-up visits. In an effort to consolidate the published data on dose-volume effect, the published literature on the penile bulb dose as it correlated with development ED was conducted; a reliability score was established.65 This group found that their analysis, based on a reliability score supported the Quantitative Analysis of Normal Tissue Effects in Clinic recommendations to maintain the mean dose to 95% of the penile bulb, less than 50 Gy (i.e., that 95% of the penile bulb should receive no more than 50 Gy) in order to decrease risk of ED.65 This group also recognized that it was unlikely for the penile bulb to be the OAR involved in the pathophysiology of ED.65
Literature from this period that was written with urological insight, incorporated the dose-volume effects of radiation more comprehensively on symptoms of ED, orgasmic dysfunction and pain.66 This group analyzed 328 sexually active men with treated PCa that either had or had not received pelvic-irradiation. These investigators measured the absorbed radiation doses as the Mean Dose (Dmean) and the Maximum Dose (Dmax) received by the corpora cavernosa and the penile bulb; they found that the strongest predictors for development of: 1) ED was the additive Dmax to the corpora cavernosa and the penile bulb (P = 0.001–0.03); 2) Orgasmic dysfunction was the Dmean to the corpora cavernosa and/or the penile bulb (i.e., absorbed dose to each OAR equally contributed to developing orgasmic dysfunction) (P = 0.03 and 0.02–0.05, respectively), and 3) Pain was additive Dmean to the corpora cavernosa and the penile bulb (P = 0.02–0.03).66
3.3. Intensity modulated radiotherapy — conventional fractionation
Dose-escalated IMRT with image-guidance is now the standard of care for low risk, intermediate risk and high risk PCa. 67 The gross tumor volume (GTV) is defined as the region containing a known disease (i.e., entire prostate) as seen in the planning CT (i.e., CT simulation). Clinical target volume (CTV) is the GTV plus an extension of the prostate contour to include regions that are likely involved with microscopic disease. In treatment planning, a third extension is made to the CTV to create the planning target volume (PTV) margins. PTV expansion margins are based on set-up uncertainty and internal organ motion so as to ensure that the CTV receives the full prescription dose during each fraction. Motion estimates are derived from Calypso data with and without use of a rectal balloon. The resultant margin is designed to contain the CTV > 95% of the time during a fraction-time of 6 min.34 For patients with nodal involvement, the CTV4500 (by convention annotated in cGy (i.e., 0.01 Gy)) includes the prostate with a 3 mm expansion (excluding the rectum) and the proximal 2.5 cm of seminal vesicles; the seminal vesicles are generally contoured in a bowtie-shaped volume that accounts for seminal vesicle movement and includes the first echelon peri-prostatic and obturator lymphatic drainage. The CTV7920 includes the prostate only. The CTV to PTV expansions vary according to the individual practice guidelines of different treatment centers. With conventional fractionation, the prescription dose is delivered in 1.8–2 Gy fractions.
The ProtecT trial was a multi-institutional randomized trial comparing active surveillance, radical prostatectomy and dose-escalated, conventionally fractionated, IMRT in localized PCa. Men received 3–6 months of neoadjuvant ADT in the IMRT arm. 67% of men reported erections firm enough for intercourse at baseline. At 6 months, 22%, 12% and 52% of patients reported having erections firm enough for intercourse in the radiotherapy, prostatectomy and active surveillance groups, respectively. In the radiotherapy group, the number of men with erections firm enough for intercourse rose between 6–12 months, and then declined again to 27% at 6 years. In the immediate post-intervention setting, IMRT demonstrates superior erectile function preservation over prostatectomy; perhaps because the injuries associated with radiation therapy are irritative/inflammatory versus the direct (and possible irreversible) trauma that may occur during a prostatectomy. The ProtecT trial demonstrated a 40% decline in erectile function with dose-escalated radiation therapy.68
3.4. Hypofractionation
Hypofractionation has proven to be non-inferior to conventional fractionation for low risk and intermediate risk disease in multiple phase 3 trials.69 Hypofractionation involves the delivery of fewer fractions with larger doses per fraction. In RTOG 0938, arm 1 delivered 36.25 Gy in 5 fractions of 7.25 Gy while arm 2 delivered 51.6 Gy in 12 daily fractions of 4.3 Gy. Given the greater dose per fraction, tighter prostatic margins are used to limit dose deposition and subsequent toxicities in regional normal tissues (e.g., corpora cavernosa, penile bulb). For a 60 Gy in 20-fraction regimen, CTV4800 included the prostate and proximal 1 cm of seminal vesicles with 3 mm expansion (excluding the rectum). The CTV6000 includes the prostate only. PTV expansions to account for set-up error and prostatic motion are similar to those used with conventional fractionation. Besides linear accelerators, the Cyberknife system [Accuray Incorporated; Munich, Germany] has been utilized for the advantage of a sharper dose fall off in the periphery of the target volume; for the sake of brevity, the utility of Cyberknife will not be discussed further within this manuscript.
Several trials have investigated the safety and efficacy of hypofractionated radiotherapy on intermediate and high-risk PCa patients. The CHHiP trial is a randomized three-arm, non-inferiority, phase 3 study comparing 74 Gy in 37 fractions, 60 Gy in 20 fractions, and 57 Gy in 19 fractions with a primary endpoint of clinical or biochemical failure. Most patients also received 3–6 months of neoadjuvant and concurrent ADT. The authors reported similar long-term sexual side-effects in the hypofractionated groups compared with the conventional group with no significant differences in either the proportion or cumulative incidence of side-effects 5 years after treatment using clinician and patient-reported outcome measures. There was no significant difference in late grade 2 or worse toxicity at 2 years in the 74 Gy group compared to the 60 Gy (46% vs. 49%, p = 0.34) and 57 Gy (46% vs. 46%, p = 0.90). The 5 year incidence of RTOG grade 2 or worse bowel and bladder adverse events was 9.1% in the 74 Gy group, 11.7% in the 60 Gy group, and 6.6% in the 57 Gy group, respectively.69 The hypofractionated versus conventionally fractionated radiotherapy for patients with localized prostate cancer (HYPRO) trial was a randomized superiority study comparing 64.6 Gy in 19 fractions to 78 Gy in 39 fractions with relapse-free survival as a primary endpoint. 66% of participants also received ADT. At 5 years, genitourinary toxicity was not non-inferior.70 The PROFIT trial was a randomized non-inferiority study comparing 78 Gy in 39 fractions to 60 Gy in 20 fractions to the prostate and seminal vesicles without ADT. Late grade 2 toxicities were 19% for 78 Gy compared to 20% for the 60 Gy arm; notably, late toxicities occurred within the first 48 months of treatment.71
Unfortunately, the aforementioned hypofractionation trials do not specifically report on the sexual function-specific toxicities such as ED. The gentitourinary toxicities reported are used as surrogates for sexual toxicities for the purpose of comparing the anticipated effects of fractionation. Gentitourinary toxicities were previously found to be predictive of erectile dysfunction HR = 1.64 in a retrospective analysis of 1002 men that underwent dose-escalated, standardly fractionated irradiation to 86.4 Gy.64
Sexual dysfunction is considered to be a late toxicity of radiation treatment. When discussing radiation toxicities from a radiobiological standpoint, α/β ratios (pertaining to the linear and quadratic parameters of the cell survival curve for a given cell type) are assigned to tissues to indicate whether the effects of radiation are generally acute (occurring within days or weeks of irradiation) or late (occurring after ∼ 6 months latency) for that specific tissue. Tissues with high α/β ratios are early responding (e.g., skin, gastrointestinal mucosa, most non-prostate solid tumors). Tissues with low α/β ratios are late responding tissues. To quantify toxicity expectations and to compare different fractionation schemes (e.g., more fractions of lower dose per fraction compared to fewer fractions with larger dose per fraction) a Biologically Effective Dose (BED) is calculated.72
Given low α/β ratios of the late responding tissues (i.e., α/β = 3) involved, such as the neurovascular prostatic plexus residing on the posterior prostate, the probability of developing late sexual dysfunction should theoretically be increased with hypofractionation (i.e., larger dose per fraction). However, the calculated BED for late toxicities is lower with hypofractionation schemes used (e.g., for 60 Gy in 20 fractions, BED is 120 GyBED(α/β=3)), compared to conventional fractionation (e.g., 79.2 Gy in 44 fractions, BED is 126.72 GyBED(α/β=3)). The lower BED of the hypofractionation regimen in PCa may explain why increased rates of genitourinary toxicity were not seen in the ‘CHHiP’, HYPRO, or PROFIT trials as compared to conventional fractionation. Therefore, it is reasonable to speculate that the rate of sexual toxicities will not vary drastically between hypofractionation and standard fractionation, as the data on sexual toxicities from hypofractionation matures.30 of late responding normal tissue toxicities for development of sexual toxicities given that the structure at risk (i.e., prostatic plexus) receives the full prescription dose in both fractionation regimens.
3.5. Proton therapy
Proton irradiation has been utilized in a variety of different solid tumors (particularly in the pediatric population) for the advantage of a sharper dose fall off in the periphery of the target treatment volume. Toxicity data on proton therapy in PCa is relatively limited compared to published EBRT data. However, preliminary effects of proton therapy on erectile function and serum testosterone have been published. The long-term outcomes of 254 men under 60 years that had received proton therapy alone (at 76−82 Gy at 2 Gy/fraction or 70–72.5 Gy at 2.5 Gy/fraction showed that erectile potency (i.e., erections firm enough for penetration) decreased from 90% at baseline to 72% and 67% at 1 and 5-year follow-up, respectively.73
Unlike photon radiation, passive-scatter proton therapy does not appear to significantly decrease serum testosterone levels (presumably due to less out-of-field scatter radiation).74 These findings suggest that the decrease in serum testosterone seen with photon therapy is not negligible, but also insufficient to result in one parameter of sexual dysfunction (i.e., ED). A comparative study between men that undergo traditional photon therapy or proton therapy that accounts for serum testosterone as a variable could help elucidate more accurate dose-volume effects related to the prostatic plexus as the proposed OAR for the development of sexual dysfunction.
3.6. Brachytherapy
Very low dose rate (VLDR) irradiation, such as is delivered with 103Pd and 125I (short range emitters), allows for the delivery of more ablative doses with only a modest increase in normal tissue toxicity. Presumably this clinical reality is explained by VLDR increased therapeutic ratio and allowance for split dose recovery. The Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT) trial was a study of PCa patients treated with neoadjuvant and concurrent ADT for 8 months and pelvic EBRT to 46 Gy in 23 fractions randomized to a conformal EBRT boost to the prostate of 36 Gy in 16 fractions or 125I low-dose-rate (LDR) brachytherapy boost prescribed to a minimum peripheral dose of 115 Gy. At 9 years, relapse free survival was significantly higher in the brachytherapy boost arm compared to the EBRT arm (83% versus 62%; p < 0.001). At 5 years the cumulative incidence of grade 3 genitourinary events was 18.4% for LDR brachytherapy cohort, versus 5.2% for EBRT cohort (p < 0.001). Among men reporting adequate baseline erections, 45% of LDR brachytherapy patients reported similar erectile function at 5 years, versus 37% after EBRT (p = 0.30). Thus, a LDR brachytherapy boost significantly improved biochemical control compared to an EBRT boost, but with higher genitourinary toxicity and no difference in erectile function.75,76 Late genitourinary toxicities generally manifest as urethral strictures which are associated with ejaculatory dysfunction; however, ejaculatory function is not generally measured in a clinical trial setting.
4. Conclusions
Prostate cancer is the second leading cause of cancer related deaths among men in the United States despite it being by far the most commonly diagnosed cancer. In the current era of radical radiotherapy, there is growing population of PCa survivors. Sexual toxicities induced by radiotherapy is a relatively fundamental and often neglected problem for patients with expected long-term survival.
To best help these patients, and their partners, a multi-specialty approach is the most effective. Our review demonstrates that similar to surgery, radiation treatment of PCa can have significant negative impacts on multiple aspects of a man’s sexual function. Our review attempts to consolidate available data on radiation-induced sexual toxicities in order to better understand the pathogenesis of a wide range of dysfunctions that patients commonly develop within the realm of sexual toxicities. To this end, we have identified the prostatic plexus as a new “organ at risk” that should be evaluated during the radiation treatment planning process. The prostatic plexus cannot be avoided in the radiation treatment of prostate cancer, but efforts can be made to eliminate radiation hot spots within the plexus.
To better understand late sexual toxicities associated with radiation therapy, measurements of and/or questionnaires to assess ejaculatory and orgasm toxicities in addition to erectile dysfunction should be incorporated into the clinical trial design. Sexual toxicity information from large, prospective, randomized, clinical trials could improve our understanding of vascular, neuronal, and ductal damage, which in turn could help focus and improve efforts to prevent and treat radiation-induced sexual toxicities.
The management of radiation-induced sexual toxicities are reviewed in Part(s) II and III of this series. Part II provides a focus on patient assessment through physical examination and validated questionnaires, followed by a comprehensive review of surgical and pharmaceutical or medical device-based therapeutic options for the management of sexual dysfunction. Part III reviews the importance and role of psychosexual therapy prior to, during and after PCa irradiation. This latter review includes innovative strategies to help increase a long-term PCa survivor’s masculine self-esteem
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.https://gis.cdc.gov/Cancer/USCS/DataViz.html obtained 9/29/2018.
- 2.Teloken C. Management of erectile dysfunction secondary to treatment for localized prostate cancer. Cancer Control. 2001;8(6):540–545. doi: 10.1177/107327480100800609. [DOI] [PubMed] [Google Scholar]
- 3.Feldman H.A., Goldstein I., Hatzichristou D.G., Krane R.J., McKinlay J.B. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151(1):54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 4.Shrader-Bogen C.L., Kjellberg J.L., McPherson C.P., Murray C.L. Quality of life and treatment outcomes: prostate carcinoma patients’ perspectives after prostatectomy or radiation therapy. Cancer. 1997;79(10):1977–1986. doi: 10.1002/(sici)1097-0142(19970515)79:10<1977::aid-cncr20>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Potosky A.L., Reeve B.B., Clegg L.X. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002;94(6):430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 6.Braun I.M., Rao S.R., Meyer F.L., Fedele G. Patterns of psychiatric medication use among nationally representative long-term cancer survivors and controls. Cancer. 2015;121(1):132–138. doi: 10.1002/cncr.29014. [DOI] [PubMed] [Google Scholar]
- 7.Achille M.A., Rosberger Z., Robitaille R. Facilitators and obstacles to sperm banking in young men receiving gonadotoxic chemotherapy for cancer: the perspective of survivors and health care professionals. Hum Reprod. 2006;21(12):3206–3216. doi: 10.1093/humrep/del307. [DOI] [PubMed] [Google Scholar]
- 8.Fugl-Meyer A.R., Lodnert G., Branholm I.B., Fugl-Meyer K.S. On life satisfaction in male erectile dysfunction. Int J Impot Res. 1997;9(3):141–148. doi: 10.1038/sj.ijir.3900269. [DOI] [PubMed] [Google Scholar]
- 9.Olweny C.L., Juttner C.A., Rofe P. Long-term effects of cancer treatment and consequences of cure: cancer survivors enjoy quality of life similar to their neighbours. Eur J Cancer. 1993;29A(6):826–830. doi: 10.1016/s0959-8049(05)80418-6. [DOI] [PubMed] [Google Scholar]
- 10.Forbat L., White I., Marshall-Lucette S., Kelly D. Discussing the sexual consequences of treatment in radiotherapy and urology consultations with couples affected by prostate cancer. BJU Int. 2012;109(1):98–103. doi: 10.1111/j.1464-410X.2011.10257.x. [DOI] [PubMed] [Google Scholar]
- 11.Korfage I.J., Hak T., de Koning H.J., Essink-Bot M.L. Patients’ perceptions of the side-effects of prostate cancer treatment—a qualitative interview study. Soc Sci Med. 2006;63(4):911–919. doi: 10.1016/j.socscimed.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien R., Rose P., Campbell C. "I wish I'd told them": a qualitative study examining the unmet psychosexual needs of prostate cancer patients during follow-up after treatment. Patient Educ Couns. 2011;84(2):200–207. doi: 10.1016/j.pec.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Singer P.A., Tasch E.S., Stocking C., Rubin S., Siegler M. Sex or survival: trade-offs between quality and quantity of life. J Clin Oncol. 1991;9(2):328–334. doi: 10.1200/JCO.1991.9.2.328. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein A.M., Padma-Nathan H. The microarchitecture of the intracavernosal smooth muscle and the cavernosal fibrous skeleton. J Urol. 1990;144(5):1144–1146. doi: 10.1016/s0022-5347(17)39677-5. [DOI] [PubMed] [Google Scholar]
- 15.Hsu G.L., Brock G., Martinez-Pineiro L., von Heyden B., Lue T.F., Tanagho E.A. Anatomy and strength of the tunica albuginea: its relevance to penile prosthesis extrusion. J Urol. 1994;151(5):1205–1208. doi: 10.1016/s0022-5347(17)35214-x. [DOI] [PubMed] [Google Scholar]
- 16.Droupy S., Benoit G., Giuliano F., Jardin F. Penile arteries in humans. Origin--distribution--variations. Surg Radiol Anat. 1997;19(3):161–167. doi: 10.1007/BF01627967. [DOI] [PubMed] [Google Scholar]
- 17.Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20(1):17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- 18.Alwaal A., Breyer B.N., Lue T.F. Normal male sexual function: emphasis on orgasm and ejaculation. Fertil Steril. 2015;104(5):1051–1060. doi: 10.1016/j.fertnstert.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean R.C., Lue T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379–395. doi: 10.1016/j.ucl.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson K.E. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol Rev. 2011;63(4):811–859. doi: 10.1124/pr.111.004515. [DOI] [PubMed] [Google Scholar]
- 21.Sattar A.A. Cavernous oxygen tension and smooth muscle fibers: relation and function. J Urol. 1995;154(5):1736–1739. [PubMed] [Google Scholar]
- 22.Colombel M., Droupy S., Paradis V., Lassau J.P., Benoit G. Caverno-pudendal nervous communicating branches in the penile hilum. Surg Radiol Anat. 1999;21(4):273–276. doi: 10.1007/BF01631399. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano F. Neurophysiology of erection and ejaculation. J Sex Med. 2011;8(Suppl 4):310–315. doi: 10.1111/j.1743-6109.2011.02450.x. [DOI] [PubMed] [Google Scholar]
- 24.Tajkarimi K., Burnett A.L. The role of genital nerve afferents in the physiology of the sexual response and pelvic floor function. J Sex Med. 2011;8(5):1299–1312. doi: 10.1111/j.1743-6109.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 25.Clement P., Giuliano F. Physiology and pharmacology of ejaculation. Basic Clin Pharmacol Toxicol. 2016;119(Suppl 3):18–25. doi: 10.1111/bcpt.12546. [DOI] [PubMed] [Google Scholar]
- 26.Zitzmann M., Nieschlag E. Testosterone levels in healthy men and the relation to behavioural and physical characteristics: facts and constructs. Eur J Endocrinol. 2001;144(3):183–197. doi: 10.1530/eje.0.1440183. [DOI] [PubMed] [Google Scholar]
- 27.Kruger T.H., Haake P., Hartmann U., Schedlowski M., Exton M.S. Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci Biobehav Rev. 2002;26(1):31–44. doi: 10.1016/s0149-7634(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 28.Brody S., Kruger T.H. The post-orgasmic prolactin increase following intercourse is greater than following masturbation and suggests greater satiety. Biol Psychol. 2006;71(3):312–315. doi: 10.1016/j.biopsycho.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein I., Feldman M.I., Deckers P.J., Babayan R.K., Krane R.J. Radiation-associated impotence. A clinical study of its mechanism. JAMA. 1984;251(7):903–910. doi: 10.1001/jama.251.7.903. [DOI] [PubMed] [Google Scholar]
- 30.Amini A., Yang J., Williamson R. Dose constraints to prevent radiation-induced brachial plexopathy in patients treated for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82(3):e391–8. doi: 10.1016/j.ijrobp.2011.06.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamstra D.A., Mariados N., Sylvester J. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 32.Krol R., McColl G.M., Hopman W.P.M., Smeenk R.J. Anal and rectal function after intensity-modulated prostate radiotherapy with endorectal balloon. Radiother Oncol. 2018;128(2):364–368. doi: 10.1016/j.radonc.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 33.South C.P., Khoo V.S., Naismith O., Norman A., Dearnaley D.P. A comparison of treatment planning techniques used in two randomised UK external beam radiotherapy trials for localised prostate cancer. Clin Oncol (R Coll Radiol) 2008;20(1):15–21. doi: 10.1016/j.clon.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Both S., Wang K.K., Plastaras J.P. Real-time study of prostate intrafraction motion during external beam radiotherapy with daily endorectal balloon. Int J Radiat Oncol Biol Phys. 2011;81(5):1302–1309. doi: 10.1016/j.ijrobp.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 35.Mariados N., Sylvester J., Shah D. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Abayomi O.K. Neck irradiation, carotid injury and its consequences. Oral Oncol. 2004;40(9):872–878. doi: 10.1016/j.oraloncology.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Levine F.J., Greenfield A.J., Goldstein I. Arteriographically determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol. 1990;144(5):1147–1153. doi: 10.1016/s0022-5347(17)39678-7. [DOI] [PubMed] [Google Scholar]
- 38.Mulhall J., Ahmed A., Parker M., Mohideen N. The hemodynamics of erectile dysfunction following external beam radiation for prostate cancer. J Sex Med. 2005;2(3):432–437. doi: 10.1111/j.1743-6109.2005.20362.x. [DOI] [PubMed] [Google Scholar]
- 39.Zelefsky M.J., Eid J.F. Elucidating the etiology of erectile dysfunction after definitive therapy for prostatic cancer. Int J Radiat Oncol Biol Phys. 1998;40(1):129–133. doi: 10.1016/s0360-3016(97)00554-3. [DOI] [PubMed] [Google Scholar]
- 40.Nolan M.W., Marolf A.J., Ehrhart E.J. Pudendal nerve and internal pudendal artery damage may contribute to radiation-induced erectile dysfunction. Int J Radiat Oncol Biol Phys. 2015;91(4):796–806. doi: 10.1016/j.ijrobp.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood J., Connors C.Q., Alexander A.A. Cavernous nerve injury by radiation therapy may potentiate erectile dysfunction in rats. Int J Radiat Oncol Biol Phys. 2017;99(3):680–688. doi: 10.1016/j.ijrobp.2017.06.2449. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez-Fort M.K., Meier B., Feily A., Cooper S.L., Lange C.S. Adjuvant irradiation to prevent keloidal fibroproliferative growth should be standard of care. Br J Dermatol. 2017;177(6):e327–e328. doi: 10.1111/bjd.15667. [DOI] [PubMed] [Google Scholar]
- 43.Helgason A.R., Fredrikson M., Adolfsson J., Steineck G. Decreased sexual capacity after external radiation therapy for prostate cancer impairs quality of life. Int J Radiat Oncol Biol Phys. 1995;32(1):33–39. doi: 10.1016/0360-3016(95)00542-7. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan J.F., Stember D.S., Deveci S., Akin-Olugbade Y., Mulhall J.P. Ejaculation profiles of men following radiation therapy for prostate cancer. J Sex Med. 2013;10(5):1410–1416. doi: 10.1111/jsm.12101. [DOI] [PubMed] [Google Scholar]
- 45.Frey A., Pedersen C., Lindberg H., Bisbjerg R., Sonksen J., Fode F. Prevalence and predicting factors for commonly neglected sexual side effects to external-beam radiation therapy for prostate cancer. J Sex Med. 2017;14(4):558–565. doi: 10.1016/j.jsxm.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Choo R., Long J., Gray R., Morton G., Gardner S., Danjoux C. Prospective survey of sexual function among patients with clinically localized prostate cancer referred for definitive radiotherapy and the impact of radiotherapy on sexual function. Support Care Cancer. 2010;18(6):715–722. doi: 10.1007/s00520-009-0675-6. [DOI] [PubMed] [Google Scholar]
- 47.D’Amico A.V., Manola J., Loffredo M., Renshaw A.A., DellaCroce A., Kantoff P.W. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 48.Itoh Y., Ando M., Ohmura M., Kondo A., Miyake K., Saito M. Effects of luteinizing hormone-releasing hormone (LH-RH) analogue on the function of the isolated rabbit corpus cavernosum. Hinyokika Kiyo. 1996;42(3):215–220. [PubMed] [Google Scholar]
- 49.Smith D.B., Babaian R.J. The effects of treatment for cancer on male fertility and sexuality. Cancer Nurs. 1992;15(4):271–275. [PubMed] [Google Scholar]
- 50.Schroder F.H., Collette L., de Reijke T.M. Prostate cancer treated by anti-androgens: is sexual function preserved? EORTC Genitourinary Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 2000;82(2):283–290. doi: 10.1054/bjoc.1999.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker L.M., Santos-Iglesias P., Robinson J. Mood, sexuality, and relational intimacy after starting androgen deprivation therapy: Implications for couples. Support Care Cancer. 2018;26(11):3835–3842. doi: 10.1007/s00520-018-4251-9. [DOI] [PubMed] [Google Scholar]
- 52.Donovan K.A., Gonzalez B.D., Nelson A.M. Effect of androgen deprivation therapy on sexual function and bother in men with prostate cancer: a controlled comparison. Psychooncology. 2018;27(1):316–324. doi: 10.1002/pon.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benedict C., Traeger L., Dahn J.R. Sexual bother in men with advanced prostate cancer undergoing androgen deprivation therapy. J Sex Med. 2014;11(10):2571–2580. doi: 10.1111/jsm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rousseau L., Dupont A., Labrie F., Couture M. Sexuality changes in prostate cancer patients receiving antihormonal therapy combining the antiandrogen flutamide with medical (LHRH agonist) or surgical castration. Arch Sex Behav. 1988;17(1):87–98. doi: 10.1007/BF01542054. [DOI] [PubMed] [Google Scholar]
- 55.Hollenbeck B.K., Wei J.T., Sanda M.G., Dunn R.L., Sandler H.M. Neoadjuvant hormonal therapy impairs sexual outcome among younger men who undergo external beam radiotherapy for localized prostate cancer. Urology. 2004;63(5):946–950. doi: 10.1016/j.urology.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 56.Zelefsky M.J., Chan H., Hunt M., Yamada Y., Shippy A.M., Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176(4 Pt 1):1415–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Bruner D.W. Quality of life and cost-effectiveness outcomes of androgen deprivation therapy for prostate cancer. Sonoma, CA: report, First Sonoma conference on Prostate Cancer. ONC News International. 1997;6(supplement 3)(11) [Google Scholar]
- 58.Teloken P.E., Ohebshalom M., Mohideen N., Mulhall J.P. Analysis of the impact of androgen deprivation therapy on sildenafil citrate response following radiation therapy for prostate cancer. J Urol. 2007;178(6):2521–2525. doi: 10.1016/j.juro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Watkins Bruner D., James J.L., Bryan C.J. Randomized, double-blinded, placebo-controlled crossover trial of treating erectile dysfunction with sildenafil after radiotherapy and short-term androgen deprivation therapy: results of RTOG 0215. J Sex Med. 2011;8(4):1228–1238. doi: 10.1111/j.1743-6109.2010.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pickles T., Graham P., I. Members of the British Columbia Cancer Agency Prostate Cohort Outcomes What happens to testosterone after prostate radiation monotherapy and does it matter? J Urol. 2002;167(6):2448–2452. [PubMed] [Google Scholar]
- 61.Zagars G.K., Pollack A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 1997;39(1):85–89. doi: 10.1016/s0360-3016(97)00311-8. [DOI] [PubMed] [Google Scholar]
- 62.Mulhall J.P., Yonover P., Sethi A., Yasuda G., Mohideen N. Radiation exposure to the corporeal bodies during 3-dimensional conformal radiation therapy for prostate cancer. J Urol. 2002;167(2 Pt 1):539–542. doi: 10.1016/S0022-5347(01)69081-5. [DOI] [PubMed] [Google Scholar]
- 63.Roach M., Winter K., Michalski J.M. Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. 2004;60(5):1351–1356. doi: 10.1016/j.ijrobp.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 64.Spratt D.E., Pei X., Yamada J., Kollmeier M.A., Cox B., Zelefsky M.J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85(3):686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rivin del Campo E., Thomas K., Weinberg V., Roach M. Erectile dysfunction after radiotherapy for prostate cancer: a model assessing the conflicting literature on dose-volume effects. Int J Impot Res. 2013;25(5):161–165. doi: 10.1038/ijir.2013.28. [DOI] [PubMed] [Google Scholar]
- 66.Thor M., Olsson C.E., Oh J.H. Radiation dose to the penile structures and patient-reported sexual dysfunction in long-term prostate cancer survivors. J Sex Med. 2015;12(12):2388–2397. doi: 10.1111/jsm.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zietman A.L., DeSilvio M.L., Slater J.D. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 68.Donovan J.L., Hamdy F.C., Lane J.A. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375(15):1425–1437. doi: 10.1056/NEJMoa1606221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dearnaley D., Syndikus I., Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Incrocci L., Wortel R.C., Alemayehu W.G. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061–1069. doi: 10.1016/S1470-2045(16)30070-5. [DOI] [PubMed] [Google Scholar]
- 71.Catton C.N., Lukka H., Gu C.S. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884–1890. doi: 10.1200/JCO.2016.71.7397. [DOI] [PubMed] [Google Scholar]
- 72.Jones B., Dale R.G., Deehan C., Hopkins K.I., Morgan D.A. The role of biologically effective dose (BED) in clinical oncology. Clin Oncol (R Coll Radiol) 2001;13(2):71–81. doi: 10.1053/clon.2001.9221. [DOI] [PubMed] [Google Scholar]
- 73.Ho C.K., Bryant C.M., Mendenhall N.P. Long-term outcomes following proton therapy for prostate cancer in young men with a focus on sexual health. Acta Oncol. 2018;57(5):582–588. doi: 10.1080/0284186X.2018.1427886. [DOI] [PubMed] [Google Scholar]
- 74.Nichols R.C., Morris C.G., Bryant C. Serum testosterone 60 months after passive-scatter proton therapy for localized prostate cancer. Cancer Invest. 2019;37(2):85–89. doi: 10.1080/07357907.2019.1565766. [DOI] [PubMed] [Google Scholar]
- 75.Morris W.J., Tyldesley S., Rodda S. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-Rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. doi: 10.1016/j.ijrobp.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 76.Rodda S., Tyldesley S., Morris W.J. ASCENDE-RT: an analysis of treatment-related morbidity for a randomized trial comparing a low-dose-rate brachytherapy boost with a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]