Abstract
In this study, β-cyclodextrin was covalently bonded on carbon fibers and its influence through host-guest complex formation was analyzed. Since cyclodextrins act as host molecules for aromatic compounds, complex formations can be performed in carbon fiber reinforced epoxy resins, between the carbon fiber surface and the aromatic compounds of the surrounding plastic. This interface design leads to improved adhesion between fiber and plastic. An increase of the interfacial shear strength from 12 MPa to 38 MPa was detected. It was possible to increase the interfacial shear strength even further, to 41 MPa, through a prior complex formation with one of the plastics components. In addition to the micromechanical analysis, energy-dispersive x-ray spectroscopy and contact angle measurements were performed to confirm the covalent coating of cyclodextrin on the carbon fiber surface.
Keywords: Materials science, Materials chemistry, Carbon fiber, Cyclodextrin, Pull-out tests, Improved interfacial shear strength, Carbon fiber reinforced plastics, Interface design
Materials science, Materials chemistry, Carbon fiber, Cyclodextrin, Pull-out tests, Improved interfacial shear strength, Carbon fiber reinforced plastics, Interface design.
1. Introduction
Today's technological designs combine innovation with sustainability, and focus in the overall transportation industry not just on alternative fuel sources but also on lightweight construction. The idea of weight reduction of structural components describes lower fuel consumption and therefore, lower CO2 emissions. One of the most promising materials for lightweight designs are carbon fiber reinforced plastics. Carbon fibers show impressive properties in terms of low density and high strength [1, 2, 3, 4] and are therefore, a popular choice for lightweight construction in industries such as aerospace [5, 6], automotive [7, 8, 9], construction [10, 11, 12] and sports [13, 14, 15].
The task distribution within carbon fiber reinforced plastics (CFRP) is defined clearly: the carbon fibers absorb mechanical loads and the plastic forms the composite's shape and secures the fibers' position within the hybrid structure. CFRP composites combine two rigid and brittle materials to a hybrid structure, which is seemingly less rigid and brittle than its single structures. This reinforcement effect is caused by the structure of the composite and its influence on crack behavior and load distribution [1]. A crack can grow unhindered in a pure material, but in a fiber reinforced material, the crack propagation stops at every single fiber and the mechanical load is then being redistributed to several neighboring fibers. More specifically, a crack does not break a fiber immediately, but has a notch effect on it. Depending on the severity of the induced stress on the fiber, the crack propagation stops or the fiber breaks and the crack continues growing until it reaches the next fiber. This failure behavior has two advantages: first, even if a few fibers break, it does not mean, that the entire composite fails; and second, the crack propagation is slowed down, so that even brittle matrices do not fail abruptly [16].
The visible component separation between CFRP laminates is called delamination. The detachment of fibers and matrix can be caused by brittleness and rigidity of the single structures, low adhesive forces between fiber and matrix, external high-impact loads and high internal stress in the composite. The fiber-matrix interface has direct influence on the energy transfer between both components: the stronger the adhesion between them, the more energy is necessary for their separation. This means, strong adhesive forces between fiber and matrix lower the speed of crack propagation and lower the possibility of fiber-matrix separation and delamination.
The improvement of interfacial fiber-matrix adhesion is promised by sizing of carbon fibers. This process describes a coating of the carbon fiber with a polymer. The sizing protects the carbon fibers from environmental influences and stress due to transportation and processing. However, the use of polymers as sizing agents grants the incorporation of the carbon fiber surface into the polymeric network of the final CFRP composite. Meaning that, the new created interphase can be tailored precisely to the adhesive requirements of fiber and plastic. Typical sizing agents are epoxy resins [17, 18, 19], polyurethane [19, 20, 21], vinyl ester resins [22, 23], polyamide [19, 21], polyimide [21], acrylic acid [24, 25], polymethylmethacrylate [26] and polystyrene [27]. Next to polymer coatings, particle sizing is possible and introduces materials like nickel particles [28], carbon nanotubes [29, 30, 31, 32, 33, 34] and graphene oxide [35, 36]. Interesting approaches combine different sizings, e.g. carbon nanotubes with silane coating and zinc oxide nanorods [37], incorporate sensors and damage detection or self-healing mechanisms [38, 39, 40].
In this study, β-cyclodextrin was covalently bonded on carbon fibers and its influence through host-guest complex formation was analyzed. Cyclodextrins are cyclic oligosaccharides and can form complexes with aromatic compounds (Figure 1). They have a hydrophilic exterior and a hydrophobic interior, which makes them soluble in water and able to incorporate hydrophobic compounds into their cavity [41, 42]. Since they can act as host molecules for aromatic compounds like the monomers of epoxy resins, polymerizations can be performed with location specifity. The complex formation was performed between the cyclodextrin, which was bonded covalently on the carbon fibers, and the epoxy resin system.
Figure 1.
(a) Molecular structure of β-cyclodextrin. (b) Schematic illustration of cyclodextrin conus and (c) inclusion complex of aromatic compound.
2. Material and methods
2.1. Materials
β-Cyclodextrin (Sigma Aldrich, 98 %) was grafted onto PAN-based, high strength, unsized carbon fibers (Sigrafil C30 T050 Uns SGL Carbon) and its reactivity was improved by p-toluenesulfonyl chloride (ABCR, 98 %). The carbon fiber surface was functionalized beforehand by t etraethylenepentamine (Merck, ≥ 95 %).
For the preparation of the pull-out tests, a solvent-free, liquid epoxy resin BECKOPOX EP140 and EH637 by Allnex was used. The samples were cured for two weeks at room temperature.
2.2. Oxidation and coating procedure
Coating of carbon fibers with cyclodextrin requires a four-step synthesis. First, the carbon fiber surface had to be activated through oxidation with nitric acid, following a treatment with tetraethylenepentamine for the introduction of amine groups. Since β-cyclodextrin has a low reactivity, it was tosylated with p-toluenesulfonyl chloride. This way, a good leaving group is available instead of the less reactive hydroxyl group. The coating process describes the reaction between aminated carbon fibers and tosylated β-cyclodextrin.
The oxidation of unsized carbon fibers was performed in concentrated nitric acid at 120 °C for 3 h (based on Pittman's instructions [43, 44]). Deactivation of nitrous gases was performed with a gas washing bottle filled with a saturated solution of iron sulfate. After the reaction was completed, the acid solution was poured onto ice and the oxidized fibers were washed with distilled water until a neutral pH was reached. For the amination, the oxidized carbon fibers were mixed into tetraethylenepentamine and heated to 200 °C for 4 h (based on Pittman's instructions [43, 44]). After the mixture cooled down, the fibers were washed with distilled water. The resulting fibers contain reactive amine groups on the fiber surface and can be used directly for the coating with tosylated β-cyclodextrin.
For every 0.1 g aminated carbon fibers, 1.14 g (1 mmol) β-cyclodextrin were tosylated (based on Fujita's instructions [45]). β-cyclodextrin was dissolved in 50 ml water and 0.2 g (1 mmol) p-toluenesulfonyl chloride was added. The mixture was stirred at room temperature for 2 h and the resulting gas of hydrochloric acid had to be removed constantly. After the tosylation was completed, the mixture was poured into dichloromethane. The excess solvent was removed from the precipitated solid and the product was dissolved in dimethylformamide.
The coating process was performed in DMF at temperatures between 80 and 120 °C for 4 h. The coated fibers were then washed three times with 50 ml DMF and dried at 80 °C. Two additional samples were then prepared for the beforehand complex formation between the coated β-cyclodextrin and the aromatic parts of the resin system. One of the samples describes a beforehand complex formation between the coated β-cyclodextrin and the epoxy resin binder and for the other sample, the amine adduct was used for the complex formation. The beforehand complex formation was performed in water, where β-cyclodextrin coated carbon fibers and either the epoxy binder or the amine curing agent were added. The mixture was stirred at room temperature for 24 h and later on dried at 80 °C.
2.3. Characterization: scanning electron microscopy
The topography and elemental composition of the carbon fiber surface were analyzed with scanning electron microscopy (SEM) with a Zeiss Neon 40 including energy-dispersive X-ray spectroscopy (EDX). The carbon fibers were glued onto aluminium stubs with an electrically conductive adhesive. To obtain electric conductivity, the coated fibers were sputtered with a film thickness of 3 nm. SEM was performed at a working distances of 7 mm and an accelerating voltage of 2 kV. The EDX measurements were performed at an acceleration voltage of 5 kV and a working distance of 5 mm.
2.4. Contact angle analysis
A Krüss Tensiometer K100SF was used to perform contact angle measurements of single carbon fibers. Using an adapted Wilhelmy method, the measurements provide information about surface energy, polarity and wettability.
2.5. Characterization: pull-out tests
A test machine MTS Criterion C45.105 with a HBM load cell S2M (10 N) was used to perform the micromechanical analysis. Single carbon fibers were embedded in epoxy resin droplets and pulled out after curing of the resin. The measurements were performed at room temperature, with speeds of 0.05 mm/min and free fiber lengths of 10 mm. The detailed sample preparation and test procedure were published previously [46]. Pull-out tests are used to determine the bond strength between fibers and resin and deliver quantitative results concerning chemical and physical interactions in the fiber-resin interface. The IFSS is defined as force per interface area (Eq. (1)) and describes the adhesion between carbon fiber and matrix.
| (1) |
-
•
IFSS interfacial shear strength
-
•
Fmax recorded maximal force
-
•
l embedded length of the fiber
-
•
d diameter of the fiber
3. Results and discussion
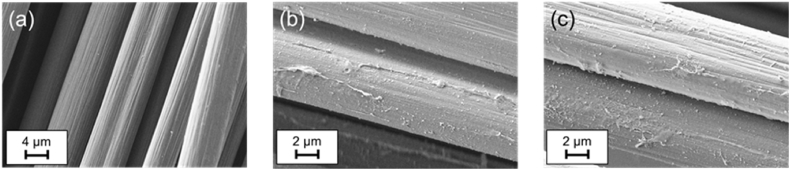
The β-cyclodextrin coating on carbon fibers was visually analyzed via scanning electron microscopy, including energy-dispersive x-ray spectroscopy. The coating can be observed via SEM, as seen in Figure 2. Even though the coating is not homogenous, the covered area is high.
Figure 2.
Exemplary SEM images of (a) unsized carbon fibers, and (b) & (c) covalently bonded β-cyclodextrin on carbon fibers.
The chemical composition was analyzed with energy-dispersive x-ray spectroscopy and is listed in Table 1. The Kα lines for carbon is at 0.277 keV, for nitrogen at 0.392 keV, for oxygen at 0.525 keV and for sulfur at 2.307 keV. The reactivity of the carbon fiber surface was improved by a treatment with tetraethylenepentamine; therefore, an increase in nitrogen content was detected. The following coating with β-cyclodextrin increased the oxygen content. P-toluenesulfonyl chloride was used as a coupling agent, since it describes a good leaving group and the primary hydroxyl groups of β-cyclodextrin are low in reactivity. Sulfur was detected through EDX measurements and leads to the conclusion, that even though the reaction was set up stoichiometric, some β-cyclodextrin molecules got tosylated multiple times. The maximum amount of tosylations of β-cyclodextrin is seven times.
Table 1.
Element composition determined by EDX measurements. [∗ - calculated without hydrogen and chloride content].
| sample | C [at%] | N [at%] | O [at%] | S [at%] |
|---|---|---|---|---|
| carbon fiber | 85.0 | 6.9 | 8.1 | |
| cyclodextrin coated carbon fiber | 68.0 | 11.3 | 18.0 | 2.7 |
| cyclodextrin ∗ | 54.5 | 45.5 | ||
| tetraethylenepentamine ∗ | 61.5 | 38.5 | ||
| p-toluenesulfonyl chloride ∗ | 70.0 | 20.0 | 10.0 |
Furthermore, contact angle measurement of single β-cyclodextrin coated fibers were performed. Coating the fiber surface changes the chemical composition of the surface and leads to changes in polarity. Table 2 summarizes the advancing contact angles. Unsized carbon fibers contain mainly carbon, but also have a few oxygen and nitrogen containing functions on the surface. The expected wettability [47, 48, 49] matches the measured contact angle of 89°. The coating process of the carbon fiber surface with β-cyclodextrin increases the hydrophilicity and therefore, decreases the contact angle, due to the hydrophilic exterior of β-cyclodextrin. The complex formation with both, epoxy binder and amine curing agent, was detected with contact angle measurements, since just the aromatic parts are part of the inclusion complex and the remaining parts of the molecules stick out and increase the hydrophobicity.
Table 2.
Comparison of contact angles of carbon fibers and β-cyclodextrin coated carbon fibers.
| sample | θ [°] |
|---|---|
| carbon fiber | 88.8 ± 7.3 |
| β-cyclodextrin coated carbon fiber | 54.5 ± 3.1 |
| β-cyclodextrin complex with epoxy resin coated carbon fiber | 74.2 ± 4.8 |
| β-cyclodextrin complex with amine curing agent coated carbon fiber | 71.6 ± 16.8 |
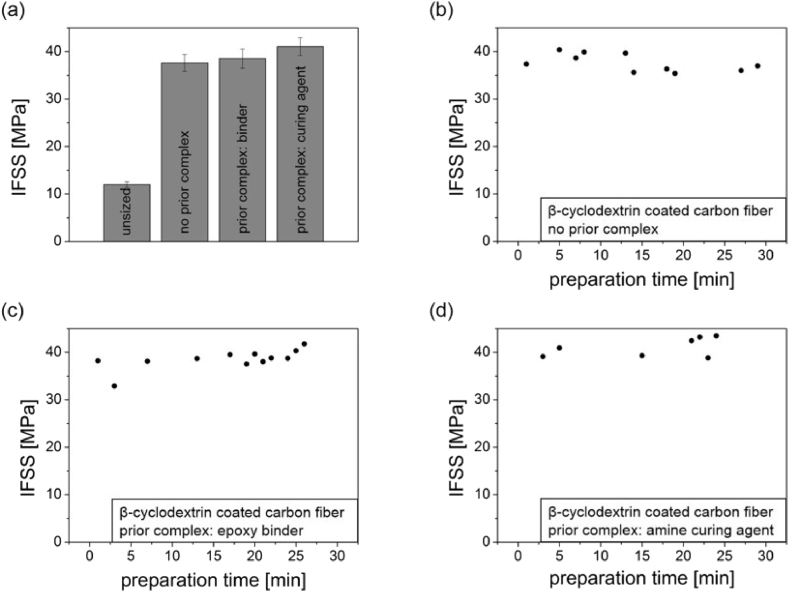
The coating with β-cyclodextrin increases the interfacial shear strength between carbon fibers and epoxy resin drastically (Figure 3). Unsized carbon fibers have interfacial shear strengths of about 12 ± 0.6 MPa, while β-cyclodextrin coated carbon fibers reach IFSS values of 37.6 ± 1.8 MPa. Using prior complex formation with the epoxy binder or the amine coupling agent, increases the IFSS even further, to 41.0 ± 1.9 MPa. Meaning, the beforehand complex formation has a positive influence on the increase of the interfacial shear strength. These high values in adhesion cannot be explained by just functional groups, since oxidized carbon fibers (compared to the epoxy binder complex) and aminated carbon fibers (compared to the amine coupling agent complex) show IFSS values of 16.2 ± 1.1 MPa and 21.3 ± 2.8 MPa. Meaning, the bonding mechanism and complex formation have a significant impact on the adhesion between fiber and resin.
Figure 3.
Pull-out tests of β-cyclodextrin coated carbon fibers. (a) Summary of the interfacial shear strength of fibers without and with a beforehand complex formation. (b) Summary of the measurement series of β-cyclodextrin coated carbon fibers without beforehand complex formation. (c) Summary of the measurement series of β-cyclodextrin coated carbon fibers with beforehand complex formation with epoxy resin and (d) summary of the measurement series of β-cyclodextrin coated carbon fibers with beforehand complex formation with amine coupling agent.
4. Summary
The interface of CFRPs was designed with host-guest complex formation by the successful use of β-cyclodextrin. The complex formation was done with the inclusion of either the epoxy binder or the amine curing agent, since both components are partly aromatic.
The ability of β-cyclodextrin to include aromatic guest molecules in its cavity has a significant impact on the interfacial shear strength of carbon fiber reinforced plastics. The complex formation itself lead to an increase of the fiber-resin adhesion from IFSS values of 12 MPa–38 MPa. It was possible to increase the interfacial shear strength even further, to 41 MPa, through a prior complex formation with one of the plastics components.
It can be stated, that the complex formation of β-cyclodextrin and the plastic components in the interface of CFRPs has a huge impact on the improvement of the interfacial shear strength. At this, an emergence can be observed, since the increase in adhesion cannot be explained by the covalent use of the single components.
Declarations
Author contribution statement
Anna Becker-Staines: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Wolfgang Bremser, Thomas Tröster: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Schürmann H. Konstruieren mit Faser-Kunststoff-Verbunden. second edition. Springer-Verlag; Berlin Heidelberg: 2007. [Google Scholar]
- 2.Minus M.L., Kumar S. The processing, properties, and structure of carbon fibers. J. Miner. Met. Mater. Soc. 2005;57:52–58. [Google Scholar]
- 3.Mohanty A.K., Misra M., Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: an overview. Macromol. Mater. Eng. 2000;276–277:1–24. [Google Scholar]
- 4.Heim M., Keerl D., Scheibel T. Spider silk: from soluble protein to extraordinary fiber. Angew. Chem. Int. Ed. 2009;48:3584–3596. doi: 10.1002/anie.200803341. [DOI] [PubMed] [Google Scholar]
- 5.Soutis C. Carbon fiber reinforced plastics in aircraft construction. Mater Sci Eng A. 2005;412:171–176. [Google Scholar]
- 6.Botelho E.C., Silva R.A., Pardini L.C., Rezende M.C. A review on the development and properties of continuous fiber/epoxy/aluminum hybrid composites for aircraft structures. Mater. Res. 2006;9:247–256. [Google Scholar]
- 7.Stewart R. Rebounding automotive industry welcome news for FRP. Reinforc. Plast. 2011;55:38–44. [Google Scholar]
- 8.Wilson A. Vehicle weight is the key driver for automotive composites. Reinforc. Plast. 2017;61:100–102. [Google Scholar]
- 9.Meng F., McKechnie J., Pickering S.J. An assessment of financial viability of recycled carbon fibre in automotive applications. Compos Part A Appl Sci Manuf. 2018;109:207–220. [Google Scholar]
- 10.Meiarashi S., Asce M., Nishizaki I., Kishima T. Life-cycle cost of all-composite suspension bridge. J. Compos. Construct. 2002;6:206–215. [Google Scholar]
- 11.Shehata L.A.E.M., Carneiro L.A.V., Shehata L.C.D. Strength of short concrete columns confined with CFRP sheets. Mater. Struct. 2002;35:50–58. [Google Scholar]
- 12.Orton S.L. The University of Texas at Austin (U.S); 2007. Development of a CFRP System to Provide Continuity in Existing Reinforced Concrete Buildings Vulnerable to Progressive Collapse; p. 3277579. [Google Scholar]
- 13.Zhang Q. The “black revolution” of sports equipment: application of carbon fiber reinforced plastics (CFRP) Appl. Mech. Mater. 2013;440:69–73. [Google Scholar]
- 14.Sun G.H., Wang J.J. The applied research of fiber reinforced composites materials in sports equipments. Adv. Mater. Res. 2012;485:506–509. [Google Scholar]
- 15.Zhang J. Study on carbon fiber composite materials in sports equipment. Appl. Mech. Mater. 2013;329:105–108. [Google Scholar]
- 16.Maimí P., Camanho P.P., Mayugo J.A., Turon A. Matrix cracking and delamination in laminated composites. Part II: evolution of crack density and delamination. Mech. Mater. 2011;43:194–211. [Google Scholar]
- 17.Fernández B., Arbelaiz A., Valea A., Mujika F., Mondragon I. A comparative study on the influence of epoxy sizings on the mechanical performance of woven carbon fiber-epoxy composites. Polym. Compos. 2004;25:319–330. [Google Scholar]
- 18.Ren P., Liang G., Zhang Z. Influence of epoxy sizing of carbon-fiber on the properties of carbon fiber/cyanate ester composites. Polym. Compos. 2006;27:591–598. [Google Scholar]
- 19.Gnädinger F., Middendorf P., Fox B. Interfacial shear strength studies of experimental carbon fibres, novel thermosetting polyurethane and epoxy matrices and bespoke sizing agents. Compos. Sci. Technol. 2016;133:104–110. [Google Scholar]
- 20.Dilsiz N., Wightman J.P. Effect of acid-base properties of unsized and sized carbon fibers on fiber/epoxy matrix adhesion. Colloids Surfaces A Physicochem Eng Asp. 2000;164:325–336. [Google Scholar]
- 21.Karsli N.G., Ozkan C., Aytac A., Deniz V. Effects of sizing materials on the properties of carbon fiber-reinforced polyamide 6,6 composites. Polym. Compos. 2013;34:1583–1590. [Google Scholar]
- 22.Wonderly C., Grenestedt J., Fernlund G., Cěpus E. Comparison of mechanical properties of glass fiber/vinyl ester and carbon fiber/vinyl ester composites. Compos. B Eng. 2005;36:417–426. [Google Scholar]
- 23.Tekalur S.A., Shivakumar K., Shukla A. Mechanical behavior and damage evolution in E-glass vinyl ester and carbon composites subjected to static and blast loads. Compos. B Eng. 2008;39:57–65. [Google Scholar]
- 24.Li J., Fan Q., Chen Z., Huang K., Cheng Y. Effect of electropolymer sizing of carbon fiber on mechanical properties of phenolic resin composites. Trans Nonferrous Met Soc China. 2006;16:457–461. [Google Scholar]
- 25.Kettle A.P., Beck A.J., O’Toole L., Jones F.R., Short R.D. Plasma polymerisation for molecular engineering of carbon-fibre surfaces for optimised composites. Compos. Sci. Technol. 1997;57:1023–1032. [Google Scholar]
- 26.Drzal L.T., Raghavendran V.K. Adhesion of thermoplastic matrices to carbon fibers: effect of polymer molecular weight and fiber surface chemistry. J. Thermoplast. Compos. Mater. 2003;16:21–30. [Google Scholar]
- 27.Bismarck A., Pfaffernoschke M., Springer J., Schulz E. Polystyrene-grafted carbon fibers: surface properties and adhesion to polystyrene. J. Thermoplast. Compos. Mater. 2005;18:307–331. [Google Scholar]
- 28.Yadav A.K., Banerjee S., Kumar R., Kar K.K., Ramkumar J., Dasgupta K. Mechanical analysis of nickel particle-coated carbon fiber-reinforced epoxy composites for advanced structural applications. ACS Appl Nano Mater. 2018;1:4332–4339. [Google Scholar]
- 29.Zhang F.H., Wang R.G., He X.D., Wang C., Ren L.N. Interfacial shearing strength and reinforcing mechanisms of an epoxy composite reinforced using a carbon nanotube/carbon fiber hybrid. J. Mater. Sci. 2009;44:3574–3577. [Google Scholar]
- 30.Mei L., Li Y., Wang R., Wang C., Peng Q., He X. Multiscale carbon nanotube-carbon fiber reinforcement for advanced epoxy composites with high interfacial strength. Polym. Polym. Compos. 2011;19:107–112. [Google Scholar]
- 31.Lv P., Feng Y.Y., Zhang P., Chen H.M., Zhao N., Feng W. Increasing the interfacial strength in carbon fiber/epoxy composites by controlling the orientation and length of carbon nanotubes grown on the fibers. Carbon N Y. 2011;49:4665–4673. [Google Scholar]
- 32.Qian H., Bismarck A., Greenhalgh E.S., Shaffer M.S.P. Carbon nanotube grafted carbon fibres: a study of wetting and fibre fragmentation. Compos Part A Appl Sci Manuf. 2010;41:1107–1114. [Google Scholar]
- 33.Lee S.B., Choi O., Lee W., Yi J.W., Kim B.S., Byun J.H. Processing and characterization of multi-scale hybrid composites reinforced with nanoscale carbon reinforcements and carbon fibers. Compos Part A Appl Sci Manuf. 2011;42:337–344. [Google Scholar]
- 34.Guo J., Lu C., An F., He S. Preparation and characterization of carbon nanotubes/carbon fiber hybrid material by ultrasonically assisted electrophoretic deposition. Mater. Lett. 2012;66:382–384. [Google Scholar]
- 35.Zhang X., Fan X., Yan C., Li H., Zhu Y., Li X. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide. ACS Appl. Mater. Interfaces. 2012;4:1543–1552. doi: 10.1021/am201757v. [DOI] [PubMed] [Google Scholar]
- 36.Ma Y., Yan C., Xu H., Liu D., Shi P., Zhu Y. Enhanced interfacial properties of carbon fiber reinforced polyamide 6 composites by grafting graphene oxide onto fiber surface. Appl. Surf. Sci. 2018;452:286–298. [Google Scholar]
- 37.Kim B.J., Cha S.H., Kong K., Ji W., Park H.W., Park Y Bin. Synergistic interfacial reinforcement of carbon fiber/polyamide 6 composites using carbon-nanotube-modified silane coating on ZnO-nanorod-grown carbon fiber. Compos. Sci. Technol. 2018;165:362–372. [Google Scholar]
- 38.Jones A.R., Cintora A., White S.R., Sottos N.R. Autonomic healing of carbon fiber/epoxy interfaces. ACS Appl. Mater. Interfaces. 2014;6:6033–6039. doi: 10.1021/am500536t. [DOI] [PubMed] [Google Scholar]
- 39.Thostenson E.T., Chou T.W. Carbon nanotube networks: sensing of distributed strain and damage for life prediction and self healing. Adv. Mater. 2006;18:2837–2841. [Google Scholar]
- 40.Thostenson E.T., Chou T.W. Real-time in situ sensing of damage evolution in advanced fiber composites using carbon nanotube networks. Nanotechnology. 2008;19 doi: 10.1088/0957-4484/19/21/215713. [DOI] [PubMed] [Google Scholar]
- 41.Szejtli J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998;98:1743–1754. doi: 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- 42.Del Valle E.M.M. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–1046. [Google Scholar]
- 43.Pittman C.U., He G.R., Wu B., Gardner S.D. Chemical modification of carbon nanofiber surfaces by nitric acid oxidation followed by reaction with tetraethylenepentamine. Carbon N Y. 1997;35:317–331. [Google Scholar]
- 44.Pittman C.U., Wu Z., Jiang W., He G.R., Wu B., Li W. Reactivities of amine functions grafted to carbon fiber surfaces by tetraethylenepentamine. Designing interfacial bonding. Carbon N Y. 1997;35:929–943. [Google Scholar]
- 45.Kahee F., Nagamura S., Imoto T. Convenient preparation and effective separation of the C-2 and C-3 tosylates of α-cyclodextrin. Tetrahedron Lett. 1984;25:5673–5676. [Google Scholar]
- 46.Becker-Staines A., Bremser W., Tröster T. Poly(dimethylsiloxane) as interphase in carbon fiber-reinforced epoxy resin: topographical analysis and single-fiber pull-out tests. Ind. Eng. Chem. Res. 2019;58:23143–23153. [Google Scholar]
- 47.Application Report AR271e . 2013. Wettability of Carbon Fibers Using Single-Fiber Contact Angle Measurements – a Feasibility Study. Hamburg. [Google Scholar]
- 48.Wang J., Qiu S., Fuentes C.A., Zhang D., Wang X., Vuure AW Van. 2017. Application Report AR284: Determining the Wettability of Carbon Fiber Tows from Single Fiber Contact Angle Data. Hamburg. [Google Scholar]
- 49.Wang J., Fuentes C.A., Zhang D., Wang X., Van Vuure A.W., Seveno D. Wettability of carbon fibres at micro- and mesoscales. Carbon N Y. 2017;120:438–446. [Google Scholar]