Summary
The AKP (Anna Karenina principle), which refers to observations inspired by the opening line of Leo Tolstoy's Anna Karenina, “all happy families are all alike; each unhappy family is unhappy in its own way,” predicts that all “healthy” microbiomes are alike and each disease-associated microbiome is “sick” in its own way in human microbiome-associated diseases (MADs). The AKP hypothesis predicts the rise of heterogeneity/stochasticity in human microbiomes associated with dysbiosis due to MADs. We used the beta-diversity in Hill numbers and stochasticity analysis to detect AKP and anti-AKP effects. We tested the AKP with 27 human MAD studies and discovered that the AKP, anti-AKP, and non-AKP effects were exhibited in approximately 50%, 25%, and 25% of the MAD cases, respectively. Mechanistically, AKP effects are primarily influenced by highly dominant microbial species and less influenced by rare species. In contrast, all species appear to play equal roles in influencing anti-AKP effects.
Subject Areas: Microbiology, Microbiome, In Silico Biology
Graphical Abstract

Highlights
-
•
About a half of microbiome-associated diseases follow AKP (Anna Karenina principle)
-
•
AKP effects are primarily influenced by highly dominant microbial species
-
•
About one-fourth of microbiome-associated diseases follow the anti-AKP
-
•
All species appear to play equal roles in influencing the anti-AKP effects
Microbiology; Microbiome; In Silico Biology
Introduction
The mostly peaceful coexistence of human microbiomes with hosts and the tolerance by our immune system is still poorly understood in human biology and modern biomedicine. In fact, the interaction between the immune system and microbiome is bidirectional. On the one hand, the immune system certainly plays a critical role in shaping and maintaining the human microbiome; on the other hand, gut microbiome of infants may help to train the full development of their immune systems (Rooks and Garrett, 2016, Levy et al., 2017, Thaiss et al., 2016). There is a hypothesis that animal regulation of immunity evolved to not only defend against pathogens but also carefully regulate symbiotic microbes (Giongo et al., 2010, Zaneveld et al., 2017, Rizzetto et al., 2018, Lotter and Altfeld, 2019). In consideration of the significance of immune system, Zaneveld et al. (2017) argued that the so-termed AKP (Anna Karenina principle) effects in animal microbiomes are ubiquitous and significant and frequently linked to deteriorating host health. The AKP refers to observations derived from the opening line of Leo Tolstoy's Anna Karenina: “all happy families are all alike; each unhappy family is unhappy in its own way.” In terms of the microbiome-associated diseases (MADs), it may be translated into a hypothesis: all “healthy” microbiomes (of healthy individuals) are alike; each “diseased” microbiome (of a patient with MAD) is “sick” in its own way.
Studies on animal and human microbiomes have suggested that microbiome stability is a hallmark of healthy host physiology, which is consistent with the evolution of animals (humans) in a sea of microbes (Zaneveld et al., 2017, Ma and Ellison, 2019). For example, it has been found that compromised host immunity can induce AKP effects (e.g., reviews by Williams et al., 2016, Zaneveld et al., 2017) and gut microbiome has been found deeply involved in allergenic and autoimmune disorders (Giongo et al., 2010, Halfvarson et al., 2017). AKP effects imply that microbiome instability (dysbiosis) can only be observed in comparison with normal variation (heterogeneity) (Brüssow, 2016, Zaneveld et al., 2017). Furthermore, a hallmark of AKP effects is the rising heterogeneity and/or stochasticity in community composition and assembly, which can be measured with beta-diversity (Zaneveld et al., 2017). We perform a meta-analysis with a big dataset of 27 MAD case studies that cover all five major microbiome habitats (airway, oral, gut, skin, and vaginal) and include most high-profile MADs such as obesity, inflammatory bowel disease (IBD), diabetes, and neurodegenerative diseases. Methodologically, we take advantages of the Hill numbers, which have been recognized as the most appropriate alpha-diversity metrics, and their multiplicative partition of beta-diversity is found to be superior to other existing beta-diversity measures (Chao et al., 2014, Chao et al., 2019, Ma, 2017, Ma and Li, 2018). In addition, we use a very recent framework for assessing and interpreting ecological stochasticity (Ning et al., 2019) to cross-verify the findings from the beta-diversity measures, given that rising stochasticity is considered as another hallmark of the AKP (Zaneveld et al. 2017). Both the approaches essentially measure similarity/dissimilarity among microbiome samples, each with unique advantages. The Hill numbers present the so-termed diversity profile, which offer a series of diversity measures corresponding to different diversity order (q = 0, 1, 2, …), weighted differently by species abundances. Therefore, the diversity profile in Hill numbers provides comprehensive diversity metrics, on the whole spectrum of commonness versus rarity in terms of species abundance distribution (SAD) (level). Since the SAD is well known to be a highly skewed long-tail distribution, the Hill numbers hence can comprehensively capture the characteristics of community diversity (similarity) at different sections of SAD and produce not only a more comprehensive but also an accurate assessment than traditional diversity measures such as species richness and Shannon entropy.
Ning et al. (2019) recently developed a new null-model based framework for assessing and interpreting ecological stochasticity. With sophisticated computational procedures and algorithms, the framework was actually presented as a simple index—normalized stochasticity ratio (NSR), which makes its application simple but provides a powerful tool for disentangling the relative importance of stochastic and deterministic forces in shaping community diversity. An underlying principle in devising the NSR framework Ning et al. (2019) adopted is that deterministic forces drive the community more similar to dissimilar than null expectation. In the present study, we take advantage of its similarity metrics to detect the AKP effects since the similarity metrics can directly address our problem—determining whether or not the divergence (difference) between the similarity of intra-healthy individuals and the similarity of intra-diseased individuals in their microbiome compositions is statistically significant. If the similarity of “unhappy families” is significantly lower than that of “happy families,” we may declare the presence of an AKP effect. In the Ning et al. (2019) framework, they recommended using the Ružička similarity metrics, which is a true distance function based on species abundance (Ružička, 1958), and we use this similarity metric to cross-verify the AKP test results from the beta-diversity approach in the Hill numbers.
Results
Detecting the AKP Effects with Beta-Diversity in Hill Numbers
Table S1 summarized brief descriptions on the 27 MAD (microbiome-associated disease) studies used in this study. We first computed the pairwise beta-diversity for all samples in the healthy and diseased treatments, respectively, for each study, based on Equations (1–4) (Table S2) and then computed the average beta-diversity in the Hill numbers for each treatment of the 27 MAD cases, as well as the p values from Wilcoxon tests for the differences in the beta-diversity between the healthy (H) and diseased (D) treatments for each case study (Table S3).
Specifically, Table S2 listed the pairwise beta-diversity for each treatment (either H or D treatment), i.e., computing a beta-diversity value for each pair of samples within each treatment. The pairwise beta-diversity values of samples within each H treatment represent the heterogeneity within the H treatment (intra-H treatment). The pairwise beta-diversity values of samples within each D treatment represent the heterogeneity within the D treatment (intra-D treatment). By conducting Wilcoxon test with the intra-H treatment beta-diversity and intra-H treatment beta-diversity, we can detect the existence of AKP effects. Table S3 exhibited the summary statistics (mean, standard error) of beta-diversity and p values from the Wilcoxon test. The threshold of p value = 0.05 is used to determine whether a particular MAD case satisfies the AKP (D > H), anti-AKP (D < H), or non-AKP (D ≈ H). Table 1 excerpted portion of the results from Table S3, and Table 2 (Figure 1) summarized the test results from Table S3. From Table 2 and Table S3, we summarize the following three findings:
-
(1)
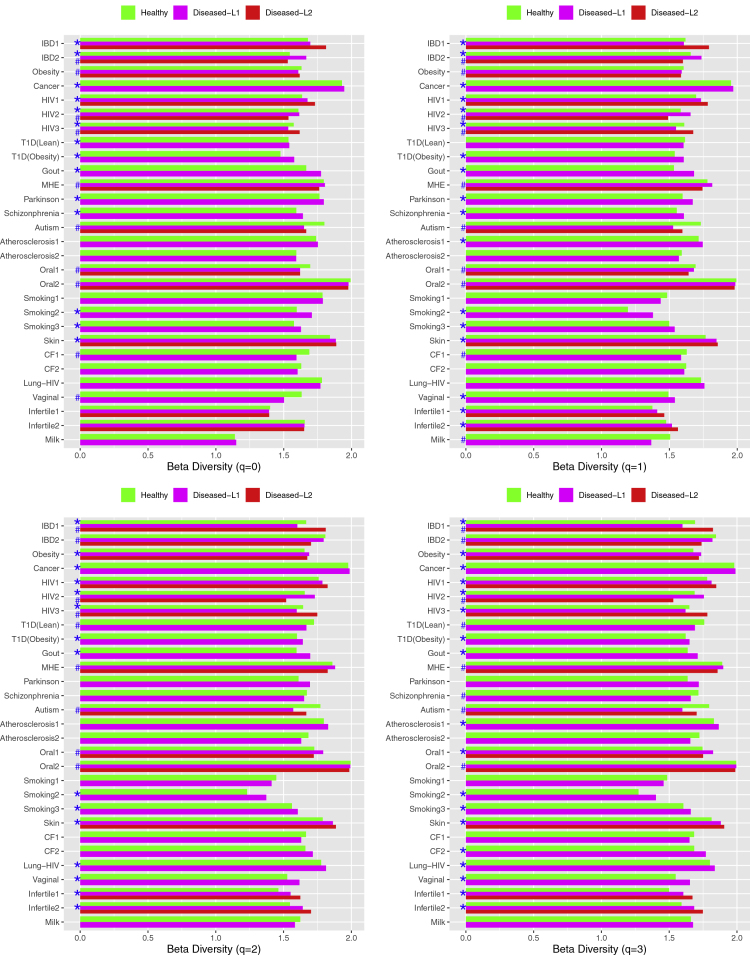
The proportions (percentages) of the 27 MAD studies exhibiting AKP effects varied from 38.1% to 57.1% depending on the diversity order (q), with q = 0 (species richness) having the lowest proportion and q = 3 having the highest proportion. This suggests that the AKP effects are more sensitive to highly dominant species (q = 3) than to common species (q = 1) or total species numbers (q = 0). In other words, what matter more are the highly dominant species, rather than rare species, in terms of displaying the AKP effects.
-
(2)
The proportions of the 27 MAD studies exhibiting anti-AKP are relatively stable across diversity orders (q = 0–3), ranged from 26.2% to 33.3%, with q = 3 (very dominant species) exhibiting the lowest proportion and q = 1 (common species) and q = 0 (total species or species richness) exhibiting the highest proportion. As expected, the pattern of anti-AKP is opposite to that of the AKP. That is, what matter less are the highly dominant species, rather than rare species, in terms of displaying the anti-AKP effects.
-
(3)
The proportions of the 27 MAD studies exhibiting non-AKP are relatively stable across diversity orders (q = 0–3), ranged from 23.8% to 28.6%, with q = 2–3 (dominant species) exhibiting the equal proportion (23.8%, also the lowest) and q = 0 (species richness) exhibiting the highest proportion. Hence, it appears that the continuum (spectrum) of commonness versus rarity in species abundances does not influence the proportion of non-AKP pattern, given that the continuum determines the weights (of species abundance distribution) used for computing the diversity (Hill numbers) at different diversity orders.
Table 1.
Wilcoxon Tests for the AKP Effects in the 27 MAD (Microbiome-Associated Diseases) Case Studies Based on the Beta-Diversity in Hill Numbers, Portion of Results Excerpted from Table S3
| Microbiome | Treatments (Healthy vs. Diseased) | Diversity Order | Average of Healthy (H) Treatments | Average of Diseased (D) Treatments | p Value (H≠D) | p value (H < D) AKP |
p value (H > D) Anti-AKP |
|---|---|---|---|---|---|---|---|
| IBD1 | Healthy vs. CD | q=0 | 1.678 | 1.696 | 0.036 | 0.018 | 0.982 |
| q=1 | 1.619 | 1.607 | 0.850 | 0.575 | 0.425 | ||
| q=2 | 1.667 | 1.602 | 0.033 | 0.983 | 0.017 | ||
| q=3 | 1.691 | 1.598 | 0.012 | 0.994 | 0.006 | ||
| Healthy vs. UC | q=0 | 1.678 | 1.812 | 0.000 | 0.000 | 1.000 | |
| q=1 | 1.619 | 1.792 | 0.000 | 0.000 | 1.000 | ||
| q=2 | 1.667 | 1.811 | 0.000 | 0.000 | 1.000 | ||
| q=3 | 1.691 | 1.823 | 0.000 | 0.000 | 1.000 | ||
| Obesity | Lean vs. Overweight | q=0 | 1.631 | 1.608 | 0.000 | 1.000 | 0.000 |
| q=1 | 1.605 | 1.591 | 0.091 | 0.955 | 0.045 | ||
| q=2 | 1.655 | 1.689 | 0.037 | 0.019 | 0.981 | ||
| q=3 | 1.678 | 1.736 | 0.008 | 0.004 | 0.996 | ||
| Lean vs. Obese | q=0 | 1.631 | 1.618 | 0.000 | 1.000 | 0.000 | |
| q=1 | 1.605 | 1.585 | 0.000 | 1.000 | 0.000 | ||
| q=2 | 1.655 | 1.675 | 0.001 | 0.000 | 1.000 | ||
| q=3 | 1.678 | 1.719 | 0.000 | 0.000 | 1.000 | ||
| CRC | Healthy vs. CRC | q=0 | 1.929 | 1.946 | 0.000 | 0.000 | 1.000 |
| q=1 | 1.954 | 1.971 | 0.000 | 0.000 | 1.000 | ||
| q=2 | 1.977 | 1.987 | 0.000 | 0.000 | 1.000 | ||
| q=3 | 1.980 | 1.989 | 0.000 | 0.000 | 1.000 | ||
| HIV1 | Negative vs. ART | q=0 | 1.635 | 1.675 | 0.000 | 0.000 | 1.000 |
| q=1 | 1.697 | 1.733 | 0.000 | 0.000 | 1.000 | ||
| q=2 | 1.759 | 1.787 | 0.005 | 0.002 | 0.998 | ||
| q=3 | 1.780 | 1.813 | 0.002 | 0.001 | 0.999 | ||
| Negative vs. Non-ART | q=0 | 1.635 | 1.730 | 0.000 | 0.000 | 1.000 | |
| q=1 | 1.697 | 1.783 | 0.000 | 0.000 | 1.000 | ||
| q=2 | 1.759 | 1.825 | 0.000 | 0.000 | 1.000 | ||
| q=3 | 1.780 | 1.847 | 0.000 | 0.000 | 1.000 | ||
| Type 1 Diabetes (T1D) and Obesity | Normal Healthy vs. Normal T1D |
q=0 | 1.535 | 1.541 | 0.007 | 0.003 | 0.997 |
| q=1 | 1.615 | 1.605 | 0.546 | 0.727 | 0.273 | ||
| q=2 | 1.724 | 1.669 | 0.014 | 0.993 | 0.007 | ||
| q=3 | 1.759 | 1.690 | 0.006 | 0.997 | 0.003 | ||
| Obesity Healthy vs. Obesity T1D | q=0 | 1.476 | 1.577 | 0.000 | 0.000 | 1.000 | |
| q=1 | 1.539 | 1.606 | 0.027 | 0.014 | 0.987 | ||
| q=2 | 1.599 | 1.642 | 0.010 | 0.005 | 0.995 | ||
| q=3 | 1.622 | 1.650 | 0.019 | 0.010 | 0.990 | ||
| Gout | Healthy vs. Gout | q=0 | 1.666 | 1.775 | 0.000 | 0.000 | 1.000 |
| q=1 | 1.533 | 1.682 | 0.000 | 0.000 | 1.000 | ||
| q=2 | 1.596 | 1.696 | 0.000 | 0.000 | 1.000 | ||
| q=3 | 1.636 | 1.710 | 0.000 | 0.000 | 1.000 | ||
| ▪▪▪ | ▪▪▪ | ▪▪▪ | ▪▪▪ | ▪▪▪ | ▪▪▪ | ▪▪▪ | ▪▪▪ |
CD, Crohn's Disease; UC, Ulcerative Colitis; IBD, Inflammatory Bowel Disease; CRC, Colorectal Cancer; ART, Antiretroviral Therapy.
Table 2.
The Mean and Standard Error of the Beta-Diversity (at Different Diversity Order q = 0–3) for the Healthy and Diseased Treatments of the Three Categories (AKP, Anti-AKP, Non-AKP), Respectively, Summarized from Table S3; See the Bottom Section for the Percentages of AKP, anti-AKP, and non-AKP
| Category, Treatment & Statistics | q = 0 | q = 1 | q = 2 | q = 3 | ||
|---|---|---|---|---|---|---|
| AKP | Healthy | Mean | 1.646 | 1.584 | 1.635 | 1.674 |
| Std. Err. | 0.029 | 0.037 | 0.035 | 0.030 | ||
| Diseased | Mean | 1.707 | 1.656 | 1.714 | 1.753 | |
| Std. Err. | 0.028 | 0.033 | 0.029 | 0.025 | ||
| Anti-AKP | Healthy | Mean | 1.707 | 1.688 | 1.776 | 1.811 |
| Std. Err. | 0.032 | 0.032 | 0.030 | 0.029 | ||
| Diseased | Mean | 1.641 | 1.620 | 1.707 | 1.731 | |
| Std. Err. | 0.033 | 0.038 | 0.038 | 0.041 | ||
| Non-AKPa | Healthy | Mean | 1.637 | 1.664 | 1.699 | 1.720 |
| Std. Err. | 0.067 | 0.044 | 0.043 | 0.042 | ||
| Diseased | Mean | 1.634 | 1.642 | 1.695 | 1.726 | |
| Std. Err. | 0.067 | 0.053 | 0.049 | 0.047 | ||
| Total | Healthy | Mean | 1.663 | 1.632 | 1.689 | 1.715 |
| Std. Err. | 0.024 | 0.024 | 0.023 | 0.022 | ||
| Diseased | Mean | 1.664 | 1.644 | 1.707 | 1.736 | |
| Std. Err. | 0.024 | 0.023 | 0.021 | 0.020 | ||
| The percentage for each of the three categories (AKP, Anti-AKP, and Non-AKP), summarized fromTable S3and computed based on Wilcoxon tests of the differences between the intra-H and intra-D treatments in their beta-diversity with Hill numbers | ||||||
| Category | q = 0 | q = 1 | q = 2 | q = 3 | ||
| Percentage of AKP | 38.1 | 50.0 | 50.0 | 57.1 | ||
| Percentage of Anti-AKP | 33.3 | 33.3 | 28.6 | 26.2 | ||
| Percentage of Non-AKP | 28.6 | 26.2 | 23.8 | 23.8 | ||
Figure 1.
Beta Diversity
The mean beta-diversity (at each diversity order q = 0–3) for each of the 27 MAD (microbiome-associated disease) case studies used for detecting the AKP (Anna Karenina principle): the cases detected with AKP effects are marked with ∗; the cases detected with Anti-AKP effects are marked with #.
Detecting the AKP Effects with the Ning et al. Framework for Quantifying Ecological Similarity and Stochasticity
Table S4 listed the results of similarity (C) and p value from Wilcoxon tests for the differences in the similarity (C) between the H and D treatments of 27 MAD case studies. As introduced previously, if the similarity of H treatment is lower (higher) than that of D treatment, then the test indicates the existence of AKP (anti-AKP) effects; otherwise no AKP effects exist. Table 3 is excerpted and summarized from Table S4. It is shown that the percentages of MAD studies with AKP effects and anti-AKP effects were 50% and 30%, respectively. There were approximately 27% of MAD cases without displaying AKP effects. These numbers are rather close to the previous AKP test results from the beta-diversity approach. Both approaches cross-verified each other's findings, but the beta-diversity approach offered more comprehensive results obviously. Hence, in the remainder of this article, we focus on the findings from beta-diversity approach.
Table 3.
The Means of the Similarity (C) for the Intra-healthy Treatment, Intra-diseased Treatment, as well as Wilcoxon Tests for Detecting the AKP Effects: C(H)>C(D) Indicating AKP Effects, C(H)<C(D) Indicating Anti-AKP Effects, C(H) = C(D) Indicating Non-AKP Effects
| Site | Disease Case Study | Index | Treatments |
Similarity (C) |
p Value of Wilcoxon Test |
||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy (H) | Diseased (D) | Healthy (H) | Diseased (D) | ≠ | > | < | |||
| Gut | IBD (Inflammatory Bowel Disease) | 1 | Healthy | CD | 0.124 | 0.128 | 0.776 | 0.612 | 0.388 |
| Healthy | UC | 0.124 | 0.060 | 0.000 | 0.000 | 1.000 | |||
| 2 | Healthy | CD | 0.108 | 0.065 | 0.000 | 0.000 | 1.000 | ||
| Healthy | UC | 0.108 | 0.110 | 0.001 | 1.000 | 0.000 | |||
| Obesity | 3 | Lean | Overweight | 0.121 | 0.125 | 0.273 | 0.864 | 0.136 | |
| Lean | Obesity | 0.121 | 0.132 | 0.000 | 1.000 | 0.000 | |||
| Cancer | 4 | Healthy | Cancer | 0.011 | 0.007 | 0.000 | 0.000 | 1.000 | |
| HIV | 5 | Negative | Treatment | 0.091 | 0.082 | 0.004 | 0.002 | 0.998 | |
| Negative | Non-treat | 0.091 | 0.064 | 0.000 | 0.000 | 1.000 | |||
| 6 | Negative | Treatment | 0.135 | 0.107 | 0.000 | 0.000 | 1.000 | ||
| Negative | Non-treat | 0.135 | 0.173 | 0.001 | 0.999 | 0.001 | |||
| 7 | Negative | Treatment | 0.136 | 0.158 | 0.012 | 0.994 | 0.006 | ||
| Negative | Non-treat | 0.136 | 0.095 | 0.002 | 0.001 | 0.999 | |||
| T1D (Lean) | 8 | H | T1D | 0.119 | 0.131 | 0.274 | 0.863 | 0.137 | |
| HO | T1DO | 0.156 | 0.143 | 0.881 | 0.560 | 0.440 | |||
| Gout | 9 | Healthy | Gout | 0.164 | 0.102 | 0.000 | 0.000 | 1.000 | |
| MHE | 10 | Healthy | MHE | 0.062 | 0.072 | 0.043 | 0.979 | 0.021 | |
| Control | MHE | 0.058 | 0.072 | 0.000 | 1.000 | 0.000 | |||
| Parkinson's Disease | 11 | Healthy | PD | 0.131 | 0.104 | 0.000 | 0.000 | 1.000 | |
| Schizophrenia | 12 | Healthy | Diseased | 0.141 | 0.117 | 0.000 | 0.000 | 1.000 | |
| Autism | 13 | Healthy | Autism | 0.092 | 0.160 | 0.000 | 1.000 | 0.000 | |
| Healthy | Neurotypical | 0.092 | 0.132 | 0.000 | 1.000 | 0.000 | |||
| Atherosclerosis | 14 | Healthy | Diseased | 0.080 | 0.071 | 0.062 | 0.031 | 0.969 | |
| Intra-H (healthy) treatments | Mean | 0.138 | NA | ||||||
| Std. Err. | 0.015 | ||||||||
| Intra-D (diseased) treatments | Mean | 0.118 | |||||||
| Std. Err. | 0.010 | ||||||||
| % With significant differences between intra-H and intra-D treatments | NA | 72.5 (29/40) | 50.0 (20/40) | 30.0 (12/40) | |||||
| % Without significant differences between intra-H and intra-D treatments | 27.5 (11/40) | 50.0 (20/40) | 70.0 (28/40) | ||||||
| AKP (%) | 50% (20/40) | ||||||||
| Anti-AKP (%) | 30% (12/40) | ||||||||
| Non-AKP (%) | 27.5% (11/40) | ||||||||
CD, Crohn's Disease; UC, Ulcerative Colitis; T1D, Type-1 Diabetes; T1DO, Type-1 Diabetes)(Obese); MHE, Minimal Hepatic Encephalopathy; PD, Parkinson’s Disease.
Summarized from Table S4.
Conclusions and Discussion
In summary, our tests based on the beta-diversity profiles (Hill numbers) and a big dataset of 27 MAD studies demonstrate that the AKP effects exist in 50% or more of the MAD cases, except for the species richness (q = 0). Furthermore, the effects seem more significant (sensitive) for highly dominant species (OTUs) (57%) and less significant (sensitive) at species richness level (38%). In other words, the far-reaching changes occurred with the very abundant (dominant) species in the microbiome associated with diseases, but the total species number (species richness) is less sensitive to MAD. Therefore, we postulate that the AKP effects can be primarily attributed to highly abundant species and less to rare species. In contrast with the AKP effects, the anti-AKP effects, i.e., lower beta-diversity associated with MAD, are demonstrated in approximately one-fourth of the studied cases. The remaining one-fourth of the MAD cases show no changes in the beta-diversity, namely, the non-AKP cases. Interestingly, the patterns of anti-AKP and non-AKP appear relatively stable across diversity orders. In other words, all species appear to play equal roles in the anti-AKP and non-AKP effects, unlike in the AKP effects, in which highly dominant species can play a more important role.
The approximately 50% or more of positive cases of AKP effects echo the finding in a previous shared-species analysis (SSA) by Ma et al. (2019), which used the same dataset as this study. In the SSA, it was found that, in approximately 50% of the studied cases, shared species between the healthy and diseased microbiome samples were lower than that expected by chance. That is, MADs are associated with increased heterogeneity in species compositions, which is manifested by the reduced number of shared species between the healthy and diseased treatments. In the same study, it was found that, in only approximately one-third of the studied cases, there were significant diversity-disease relationships (DDRs), i.e., significant differences between the healthy and diseased treatments in term of the alpha-diversity. In contrast with the 50% of AKP effects, the DDR (measured with alpha-diversity) seems less sensitive. This also indicates that AKP effects, measured by beta-diversity, are more sensitive to MADs than the DDR measured with alpha-diversity. Hence, we argue that the AKP effects should be more promising for developing personified diagnosis indicators for diseases, as suggested by Zaneveld et al. (2017), than the routinely computed alpha-diversity indexes in the DDR analysis (Ma et al., 2019).
A key element of the AKP is the bidirectional interactions between the immune system and human microbiome. The notion that stability is associated with the healthy microbiome or host physiology mirrors the notion that dysbiosis (or rising heterogeneity) is often associated with the diseased microbiome or abnormal host physiology. The disruption or breakdown modes of the bidirectional interactions between the immune system and symbiotic microbiotas can vary from case to case (of diseases), and therefore, AKP effects are unlikely to emerge in all MAD cases. In our opinion, a demonstration of 50% or more positive AKP effects should be a strong piece of evidence supporting the AKP hypothesis in the human MADs.
The AKP hypothesis predicts the rise of stochasticity in animal/human microbiomes due to stress, diseases, and immune system dysfunctions. However, it does not negate the importance of deterministic changes in community composition (Zaneveld et al., 2017). In the human microbiome research, the effects of deterministic forces have been well recognized. One such example is the community state types (CSTs) of human vaginal microbiomes (Ravel et al., 2011, Gajer et al., 2012, Doyle et al., 2018). According to the four processes (mechanisms) synthesis of community dynamics (Vellend, 2016, Hanson et al., 2012), which states that it is the four processes (i.e., selection, drift, dispersal, and speciation) that drive the community dynamics, CST should primarily be shaped by deterministic selection forces (such as host genome). Nevertheless, one of the CSTs, the CST-IV that is almost exclusively associated with BV (bacterial vaginosis) disease, showed significant intra-type heterogeneity (variability). For example, the initial classification of the CST-IV (Ravel et al., 2011) was further classified into two sub-types to accommodate more diverse communities associated with BV (Gajer et al., 2012); yet, the classification system could not fully cover more diverse communities (Doyle et al., 2018, Li and Ma, 2019). This example of human vaginal microbiome and associated BV suggests that deterministic selection forces and stochastic disturbances such as BV are often interwoven and it is their joint forces that drive the dynamics of human vaginal microbiome, possibly leading to the rising heterogeneity of community compositions and even displaying AKP effects.
It should be noted that, to increase the robustness of the statistical analyses performed for detecting the AKP, anti-AKP effects, we conducted two additional statistical analyses. One was to apply the FDR (false discovery rate) control to the test results (p values) of the AKP/anti-AKP effects with non-parametric Wilcoxon test exhibited in Table S3, and the FDR-adjusted results were exhibited in Table S5. The FDR control was applied to control the expected proportions of “discoveries” (rejected null hypotheses) that are false (i.e., wrong rejections) during multiple tests. In other words, FDR control was designed to increase the test power to detect true positives, while still controlling the proportion of type I errors (i.e., false positives) at a specified level (Korthauer et al., 2019). Another alternative test we adopted was to use the “Effect Size test” based on Cohen (1988) d-statistic in place of Wilcoxon test, and the results from the alternative test were displayed in Table S6. The advantage of using the effect size test can be to alleviate or even eliminate the potential influence of different sample sizes on the test results. We observed that the differences these two additional tests made were less than 5% on average and did not cause any change of the conclusions we inferred in previous sections.
Limitations of the Study
It is suggested that future tests of AKP theory should be expanded in two frontiers: one is to use temporal data and another is to simultaneously assess and interpret the balance and relative importance of deterministic versus stochastic forces. The former is necessary for investigating the diversity-stability relationship (DSR) (the other side of disease-associated dysbiosis) (Ma and Ellison, 2019), which can be simultaneously utilized to assess the temporal heterogeneity associated with AKP effects. The latter can be much more challenging and requires both methodological innovations and more sophisticated datasets. Obviously, the recent development of the normalized stochasticity ratio framework by Ning et al. (2019) presented a promising methodological advance, and yet, sufficient datasets from longitudinal studies are still rare. Still, as suggested by one anonymous expert reviewer of this article, a third frontier for investigating the AKP theory can be to perform “cross-diseases” tests by asking the question: “do microbiomes associated with different diseases demonstrate greater heterogeneity than control healthy microbiomes across the same studies?” For example, Duvallet et al. (2017) demonstrated that there are both universal and disease-specific signatures in the gut microbiome. With more extensive datasets (e.g., sufficiently large numbers of disease cases for all microbiome types such as gut and skins), the proposed approach used in this study should also be useful for performing cross-diseases AKP tests.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
I appreciate two anonymous expert reviewers and Dr. Stefano Tonzani, the iScience Lead Editor, for their insightful comments and suggestions, which helped to improve my manuscript significantly. I am also indebted to Mr. L.W. Li and Miss Wendy Li of the Chinese Academy of Sciences for their computational support to this study. This study received funding from the following sources: A National Natural Science Foundation of China (NSFC) Grant (No. 31970116) on Medical Ecology of Human Microbiome; The Cloud-Ridge Industry Technology Leader Award; An International Cooperation Grant (YNST) on Genomics & Metagenomics Big Data. The funders played no roles in interpreting the results.
Author Contributions
Z.S.M. designed and performed the study and wrote the manuscript.
Declaration of Interests
The author declares no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101007.
Data and Code Availability
All datasets analyzed in this study are available in public domain and see Table S1 for the access information.
Supplemental Information
References
- Brüssow H. How stable is the human gut microbiota? And why this question matters. Environ. Microbiol. 2016;18:2779–2783. doi: 10.1111/1462-2920.13473. [DOI] [PubMed] [Google Scholar]
- Chao A., Chiu C.H., Jost L. Unifying species diversity, phylogenetic diversity, functional diversity and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014;45:297–324. [Google Scholar]
- Chao A., Colwell R.K., Gotelli N.J., Thorn S. Proportional mixture of two rarefaction/extrapolation curves to forecast biodiversity changes under landscape transformation. Ecol. Lett. 2019;22:1913–1922. doi: 10.1111/ele.13322. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. of the American Statistical Association; 1988. The Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- Doyle R., Gondwe A., Fan Y., Maleta K., Ashorn P., Klein N., Harris K. A lactobacillus-deficient vaginal microbiota dominates postpartum women in rural Malawi. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02150-17. e02150–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvallet C., Gibbons S.M., Gurry T., Irizarry R.A., Alm E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017;8:1784. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P., Brotman R.M., Bai G., Sakamoto J., Schütte U.M., Zhong X., Koenig S.S., Fu L., Ma Z.S., Zhou X. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012;132:132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giongo A., Gano K.A., Crabb D.B., Mukherjee N., Novelo L.L., Casella G., Drew J.C., Ilonen J., Knip M., Hyöty H. Toward defining the autoimmune microbiome for type-1diabetes. ISME J. 2010;5:82–91. doi: 10.1038/ismej.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C.A., Fuhrman J.A., Horner-Devine M.C., Martiny J.B.H. Beyond biogeographic patterns: processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012;10:497–506. doi: 10.1038/nrmicro2795. [DOI] [PubMed] [Google Scholar]
- Halfvarson J., Brislawn C.J., Lamendella R., Vázquez-Baeza Y., Walters W.A., Bramer L.M., D'Amato M., Bonfiglio F., McDonald D., Gonzalez A. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korthauer K., Kimes P.K., Duvallet C., Reyes A., Subramanian A., Teng M., Shukla C., Alm E.J., Hicks S.C. A practical guide to methods controlling false discoveries in computational biology. Genome Biol. 2019;20:118. doi: 10.1186/s13059-019-1716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M., Kolodziejczyk A.A., Thaiss C.A., Elinav E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017;17:219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- Li W.D., Ma Z.S. Diversity scaling of human vaginal microbial communities. Zoolog. Res. 2019;40:587–594. doi: 10.24272/j.issn.2095-8137.2019.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotter H., Altfeld M. Sex differences in immunity. Semin. Immunopathol. 2019;41:133–135. doi: 10.1007/s00281-018-00728-x. [DOI] [PubMed] [Google Scholar]
- Ma Z.S. Measuring microbiome diversity and similarity with Hill Numbers. Chapter 8. In: Muniyandi Nagarajan., editor. Metagenomics. Elsevier; 2017. [DOI] [Google Scholar]
- Ma Z.S., Li L.W. Measuring metagenome diversity and similarity with Hill numbers. Mol. Ecol. Resour. 2018;18:1339–1355. doi: 10.1111/1755-0998.12923. [DOI] [PubMed] [Google Scholar]
- Ma Z.S., Li L.W., Gotelli N.J. Diversity-disease relationships and shared species analyses for human microbiome-associated diseases. ISME J. 2019;13:1911–1919. doi: 10.1038/s41396-019-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.S., Ellison A.M. Dominance network analysis provides a new framework for studying the diversity-stability relationship. Ecol. Monogr. 2019;89:e01358. [Google Scholar]
- Ning D., Deng Y., Tiedje J.M., Zhou J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. U S A. 2019;116:16892–16898. doi: 10.1073/pnas.1904623116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S.K., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U S A. 2011;108(Suppl. 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto L., Fava F., Tuohy K.M., Selmi C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J. Autoimmun. 2018;92:12–34. doi: 10.1016/j.jaut.2018.05.008. [DOI] [PubMed] [Google Scholar]
- Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ružička M. Anwendung mathematisch-statistischer methoden in der Geobotanik (Synthetische Bearbeitung von Aufnahmen) Biológia, Bratislava. 1958;13:647–661. [Google Scholar]
- Thaiss C.A., Zmora N., Levy M., Elinav E. The microbiome and innate immunity. Nature. 2016;535:65–74. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Vellend M. Princeton University Press; 2016. The Theory of Ecological Communities. [Google Scholar]
- Williams B., Landay A., Presti R.M. Microbiome alterations in HIV infection a review. Cell. Micobiol. 2016;18:645–651. doi: 10.1111/cmi.12588. [DOI] [PubMed] [Google Scholar]
- Zaneveld J.R., Mcminds R., Vega Thurber R. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat. Microbiol. 2017;2:17121. doi: 10.1038/nmicrobiol.2017.121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets analyzed in this study are available in public domain and see Table S1 for the access information.