Abstract
Lymphoepithelioma-like carcinoma, named after nasopharyngeal lymphoepithelioma and rarely seen at genitourinary malignancy, accounts for 1%–2% along the upper and lower urinary tract. For its rarity, no published guideline can be adhered to. Roles of surgery and chemotherapy are solid, and rich Programmed Death-Ligand 1 characteristics may furthermore light on the possible immunotherapy. This female case had it at both upper and lower urinary tract simultaneously. No involved regional lymph nodes and no distant metastasis were investigated. No adjuvant chemotherapy was given after robotics-assisted left nephroureterectomy and bladder cuff excision with partial cystectomy, and no recurrence was observed.
Keywords: Lymphoepithelioma-like carcinoma, Urinary tract tumor, Programmed death-ligand 1, Combined positive score
Introduction
Lymphoepithelioma, a malignant undifferentiated tumor commonly seen at nasopharynx,1 histologically is made up of twine of malignant epithelial parts and myeloid cells or lymphoid cells, such as histiocytes, eosinophils or neutrophils.2 Cancer histology of urothelial origin nearly makes up of 90% in majority along the upper urinary tract, also the lower urinary tract. Lymphoepithelioma is most seen at upper respiratory tract, said to be linked with Epstein-Barr virus (EBV) or Human Papillomavirus (HPV) infection, and carcinoma histology featuring alike but appearing at the sites other than nasopharynx will be termed as lymphoepithelioma-like carcinoma (LELC). The first published literature can be traced back to 1991 by Zukerberg et al. and, for its rare occurrence at urinary tract, for example only nearly 1%1,3 in bladder, no guidelines can be applied to treatment. Modern immunohistochemical studies provide us a more sophisticated looks into the component, and, from clinical to laboratory evidence, guide the surgeons to cope this rare type of disease.
Case presentation
A woman, aged 75 and with end-stage renal disease under hemodialysis, visited us first time for left flank pain and been examined of left hydronephrosis, accompanied with urinary tract infection. After ultrasound and lab assessment, computed tomography (CT) was done, and then tumors at bladder, left ureteral orifice, and left renal calyx were found, causing obstruction. Several enlarged lymph nodes were found around para-aortic site and gonadal vein. Subsequently, cystoscopy and left ureteroscopy biopsy were suggested. Prior to that, left percutaneous nephrostomy was indicated to control her infection and release the hydronephrosis and obstruction condition.
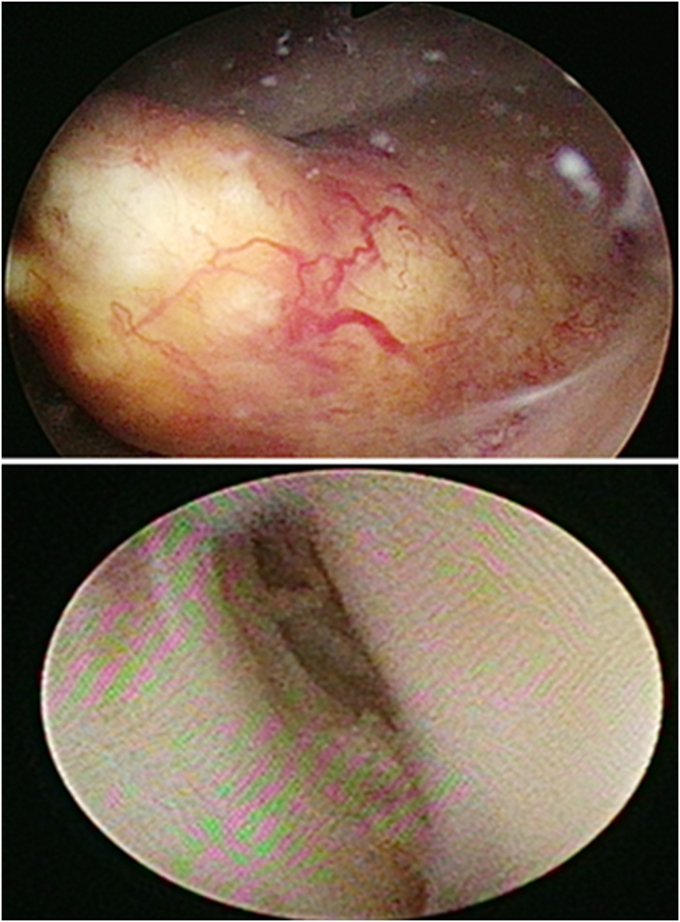
One week after, cystoscopy and left ureteroscopy biopsy were operated (Fig. 1) Intra-operatively, we observed total 4 visible tumors, one at urinary bladder, one at left lower third ureter, one at left upper third ureter, and the other at the left renal pelvic. All sessile in appearing ranged from 2 cm in width, minimally measured at left upper third ureter, to 10 cm in width, maximally measured at left renal pelvic. Pathology report 2 weeks after showed invasive LELC. Whole body bone scan and positron emission tomography were arranged and no signs of distant to organs and bone metastasis.
Fig. 1.
Tumors observed under cystoscopy (up) and ureteroscopy (down).
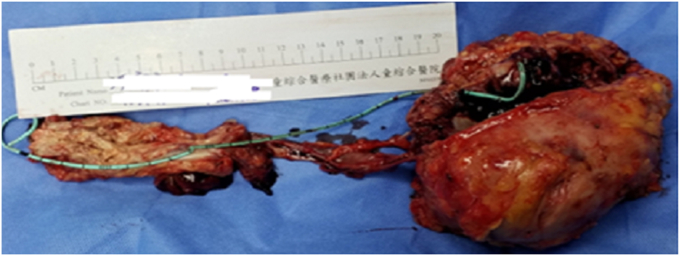
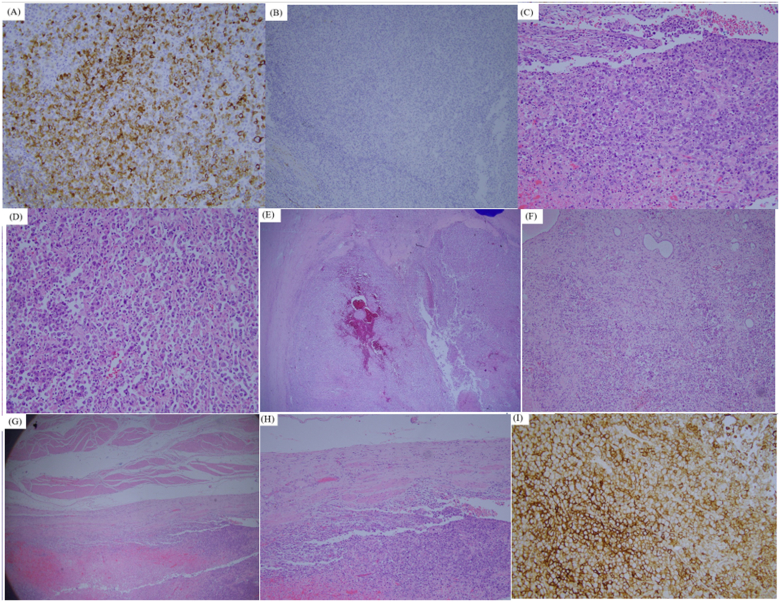
After discussion, we performed robotic-assisted left nephroureterectomy and bladder cuff excision with partial cystectomy. Total 4 tumors were seen in specimen (Fig. 2.), a 10.5 × 9.8 cm tumor at left renal pelvic, a 2.5 × 1.4 cm tumor at left upper third ureter, a 8.9 × 3.5 cm tumor at lower third ureter, and one 2.1 × 1.1 cm tumor at bladder. All of them were examined with pure cell type of invasive LELC, and all immunohistochemically express 100% Programmed Death-Ligand 1 (PD-L1) under Combined Positive Score (CPS) calculation, focally positive of CK and vimentin stain. Except for pT3 at left renal pelvic, the rest 3 all were pT2 (Fig. 3.).
Fig. 2.
Gross section of the surgical specimen.
Fig. 3.
(A) Positive CK stain, proved to be malignant. (B) Negative GATA-3 and CK7 stain. (C) Main bladder tumor (2.1 × 1.1 cm) under 40X magnitude (D) main bladder tumor under 400X magnitude (E) Malignancy at left renal pelvis, (10.5× 9.8 cm). Invasive lymphoepithelioma-like carcinoma, high grade, invasion to renal parenchyma layer (pT3) (F) 200X magnitude of tumor at left renal pelvis. (G) Left ureteral tumor is seen with muscle invasive type (2.5 × 1.4 cm). Invasive lymphoepithelioma-like carcinoma, high grade, invasion to muscle layer (pT2). (H) 40X of left invasive ureteral tumor. (I) High expression of 100% PD-L1 by CPS on main tumor at bladder.
No adjuvant chemotherapy was administered, and no recurrence was seen on the evidence from CT urography at the 3rd and 12th month, and urine cytology at the 6th and 12th month during follow-ups for one year after operation until now. We would stand by the plan of CT urography annually, and urine cytology every 6 months for the next 3 years. Plus, after referring her to our otorhinolaryngologist, investigation and examination both showed no nasopharynx tumor and neither the related familial history.
Discussion
LELC can be categorized into 3 types, mixed with other cancer histology or LELC-predominant (>50% LELC component), or pure (100% LELC component) of LELC. In terms of clinical outcome comparison, survival analysis, done by Antonio et al., in 2017,3 showed no differences with the conventional urothelial carcinoma (UC) at both upper and lower urinary tracts under designed treatment modality, and pathological T stage is the only meaningful parameter to prognosis. At the meantime, while the terminology is coined coherent to lymphoepithelioma under the basis of gross histology at nasopharynx, LELC expresses totally different characteristics under FISH analysis against it though.2 Immunohistochemical analysis by Sean Willliamson et al.2 expressed that more chromosomal abnormalities resembling to high grade UC and frequent p53 positivity but, in the other hand, hybridization with neither HPV nor EBV under immunostaining assessment are examined. In this way, most modern opinions deem LELC as a high grade invasive UC.1
Pathological specimen is composed undifferentiated epithelial cells trapped within lymphoid cells. For it features lymphoid cells or other inflammatory-related cells histologically, the assumption has been made that chemotherapy may be effective.1 Sean Willliamson et al.2 reported 28 cases and described that when Trans-Urethral Resection of Bladder tumor (TURBt) is added with intravesical chemotherapy, it will decrease the likelihood of recurrence. Until now, current published papers investigated the effect of chemotherapy the most often under the regimen combination from UC and produced profound outcome.4
Operations remain as the major curative method, except for conditions like bilateral urinary tract involvement, solitary or solitary functional kidney, chronic kidney disease, hereditary disease, or other conditions hampering surgeon from performing the surgery under the clinical evaluation. In the other hand, Andy Yang et al.4 in 2017 reviewed 140 cases of bladder LELC from published studies, concluding multi-modality treatment, the most frequent with radical cystectomy followed by chemotherapy, will lead to more disease-free survival and less mortality rates than TURBt alone.
Coalesce of the contemporary evidence, we could roughly picture that adhering to the treatment principles of urinary tract UC would yield better outcome for the LELC patients. Pure type LELC, at current studies on this topic in majority, is concluded to be with better outcome in terms of recurrence, mortality, disease-free survival and overall survival compared to mixed LELC.1 Ujjawal Manocha et al.5 researched through 16 LELC cases of bladder for immunohistochemical analysis, with 12 pure and 4 predominant, and concluded that 93% of them expressed positive PD-L1. Likelihood, there are studies telling high expression of PD-L1 in lung LELC under immunohistochemical studies, thus usage of immunotherapies like nivolumab is assumed,3 but still lack of the related information in urinary tract LELC.
Conclusions
It is reasonable to treat LELC like the ways we do in urinary tract UC. Chances still exist in distant metastasis especially those with muscle invasive type. Follow-up strategies are also required to be tailored. Immunotherapy should theoretically be feasible, but still need more evidence to concrete it.
Financial conflict of interest
None.
Declarations of competing interest
None.
Contributor Information
Che-Hsueh Yang, Email: b101098093@tmu.edu.tw.
Yi-Sheng Lin, Email: tung12197@gmail.com.
Wei-Chun Weng, Email: wcweng27@gmail.com.
Yen-Chuan Ou, Email: ycou228@gmail.com.
Chao-Yu Hsu, Email: sheujowyu@gmail.com.
Min-Che Tung, Email: tungminche@gmail.com.
References
- 1.Lopez-Beltrán A., Luque R.J., Vicioso L. Lymphoepithelioma-like carcinoma of the urinary bladder: a clinicopathologic study of 13 cases. Virchows Arch. 2001 Jun;438(6):552–557. doi: 10.1007/s004280000378. [DOI] [PubMed] [Google Scholar]
- 2.Williamson S.R., Zhang S., Lopez-Beltran A. Lymphoepithelioma-like carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, and molecular features. Am J Surg Pathol. 2011 Apr;35(4):474–483. doi: 10.1097/PAS.0b013e31820f709e. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Beltran A., Paner G., Blanca A. Lymphoepithelioma-like carcinoma of the upper urinary tract. Virchows Arch. 2017 Jun;470(6):703–709. doi: 10.1007/s00428-017-2117-z. [DOI] [PubMed] [Google Scholar]
- 4.Yang A.W., Pooli A., Lele S.M. Lymphoepithelioma-like, a variant of urothelial carcinoma of the urinary bladder: a case report and systematic review for optimal treatment modality for disease-free survival. BMC Urol. 2017 Apr 27;17(1):34. doi: 10.1186/s12894-017-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manocha U., Kardos J., Selitsky S. RNA expression profiling of lymphoepithelioma-like carcinoma of the bladder reveals a basal-like molecular subtype. Am J Pathol. 2020 Jan;190(1):134–144. doi: 10.1016/j.ajpath.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]