Abstract
Background
Ovarian cancer (OC) is one of the leading causes of cancer-related mortality worldwide. The clinical outcome of EOC remains unsatisfactory with current therapeutic approaches such as surgery and platinum/taxane-based chemotherapy. Therefore, novel prognostic markers and personalized therapies targeting specific molecules are urgently needed. Here, we explored whether RNF126, an E3 ubiquitin ligase, is a potential biomarker for epithelial ovarian cancer (EOC).
Material/Methods
This was a retrospective cohort study of 122 EOC patients. The chi-square test was used to assess correlations between RNF126 level and clinical characteristics of enrolled patients. Univariate and multivariate analyses were performed to monitor the prognosis of enrolled patients. In addition, proliferation and invasion assays were conducted to assess the cellular effects of RNF126 on SKOV3 cell progression.
Results
Immunohistochemistry analysis (IHC) revealed that RNF126 was upregulated in EOC tissues compared to adjacent ovarian tissues. In addition, RNF126 expression was remarkably associated with LN metastasis, pathological differentiation, and FIGO stage. RNF126 protein level was found to be an independent biomarker for predication of prognosis in ovarian cancer patients. Cellular results showed that RNF126 enhanced the proliferation and invasion abilities of SKOV3 cells.
Conclusions
Upregulated protein level of RNF126 in EOC tissues is a biomarker predicting poor outcomes of EOC patients.
MeSH Keywords: Biological Markers, Cell Proliferation, Ovarian Neoplasms
Background
Ovarian cancer (OC) is one of the leading causes of cancer-related mortality worldwide [1,2], and the incidence rate has been rising in recent decades [3]. As the second most lethal gynecological cancer in women [3], 90 out of 100 tumors of the ovary are epithelial ovarian cancer (EOC) [4,5]. The histologic subtypes of EOC consist of clear cell, endometrioid, mucinous adenocarcinomas, and serous carcinoma [6–8], which present different characteristics and require different therapeutic treatments [9,10]. However, the same therapeutic approaches have been recommended for different histologic subtypes of EOC in clinical settings, including surgery and platinum/taxane-based chemotherapy [11]. Although these approaches have improved EOC patient quality of life, the clinical prognosis in these patients is still poor [11]. It was reported that survival rates of EOC patients varied widely depending on the stage at diagnosis [12]. EOC patients diagnosed at early stages show remarkably better overall survival than those who were diagnosed at advanced stages (28.8%) [3]. Due to the lack of early clinical symptoms and early diagnostic biomarkers for EOC, about 70% of EOC patients are diagnosed at late stages [12], which results in poor prognosis. Therefore, finding effective biomarkers would improve early detection and clinical prognosis of EOC.
RING finger protein 126 (RNF126) belongs to the E3 ligase family, which modulates cellular processes by regulating protein ubiquitination [13–15]. RNF126 targets p21, which is essential for proper cell-cycle progression and ubiquitin-mediated degradation and is an important protein for cell growth [13]. It was also reported to be the ligase that specifically mediates frataxin ubiquitination and enhances its degradation, thereby making it a potential treatment target for Friedreich ataxia (FRDA) [16]. RNF126 regulates the ubiquitin-dependent retrograde sorting of the cation-independent mannose 6-phosphate receptor (CI-MPR) and the intracellular trafficking of EGFR [17,18]. Furthermore, RNF126 promotes the completion of nonhomologous end joining-mediated DNA repair by ubiquitinating Ku80 [19] and plays a role in homologous recombination (HR)-mediated DNA double-strand break repair by upregulating E2F1 [15]. Information on the biological functions of RNF126 is rapidly growing.
Of note, recent studies revealed that RNF126 plays a role in oncogenesis [15,20–22]. In oral cancer, RNF126 promotes tumor progression by activating the AKT signaling pathway, and RNF126 can be regulated by ERK and subsequently modulates anoikis resistance in tumor cells. Higher levels of RNF126 also predict unfavorable outcomes of breast cancer. However, the expression and function of RNF126 in ovarian cancer are unreported. In the present study is the first to assess RNF126 expression in 122 glioma patients by immunohistochemistry (IHC). The clinical value of RNF126 was assessed by analyzing the association between RNF126 and other clinicopathologic factors. In addition, the prognostic significance of RNF126 was assessed by univariate analysis (log-rank test) and multivariate analysis (Cox regression model). Finally, we assessed the cellular role of RNF126 in ovarian cancer cells.
Material and Methods
Patients and samples
This retrospective study enrolled 122 patients diagnosed with EOC at the Affiliated Hospital of North Sichuan Medical College (Nanchong, China). Clinical resected tissues were collected from the Department of Pathology. Written informed consent was obtained from each patient. This study was approved by the Affiliated Hospital of North Sichuan Medical College Research Ethics Committee.
The mean age of the 122 enrolled patients was 59±10 years and the median age was 57 years old. They were all histologically graded and staged according to the International Federation of Gynecology and Obstetrics (FIGO) criteria [23]. There were 39 well-differentiated (G1) patients, 43 moderately-differentiated (G2) patients, and 40 poorly-differentiated (G3) patients. For FIGO stage, 59 (48.4%) and 63 (51.6%) patients were in FIGO stage I/II and stage III/IV, respectively (Table 1). The follow-up time for patients was 8–114 months, with a median follow-up of 55 months.
Table 1.
Expression of RNF126 in EOC tissues.
| Variables | Cases | RNF126 expression | P value | |
|---|---|---|---|---|
| (n=122) | Low (n=63) | High (n=59) | ||
| Age (years) | 122 | 59±25 | 60±23 | 0.284 |
| LN metastasis | 0.032* | |||
| Negative | 72 | 43 | 29 | |
| Positive | 50 | 20 | 30 | |
| Pathological differentiation | 0.161* | |||
| Well | 39 | 25 | 14 | |
| Moderate | 43 | 19 | 24 | |
| Poor | 40 | 19 | 21 | |
| FIGO stage | <0.001* | |||
| I–II | 59 | 43 | 16 | |
| III–IV | 63 | 20 | 43 | |
EOC – epithelial ovarian cancer; LN – lymph node metastasis; FIGO – International Federation of Gynecology and Obstetrics; RNF126 – RING finger protein 126.
Immunohistochemistry (IHC) and IHC evaluation
Expressions of RNF126 in clinical resected tissues were detected with IHC staining as described before [24,25]. In brief, tissue specimens were embedded in paraffin after being fixed with 10% neutral buffered formalin. Then, they were cut into 4-μm sections and placed on silane-coated slides. Before antigen retrieval, the slides were deparaffinized and slide sections were then treated with 3% H2O2 followed by blocking with BSA. Next, sections were treated with RNF126 primary antibody at 4°C overnight. The following day, slides were incubated with horseradish peroxide-conjugated secondary antibody for 30 min at 37°C, followed by staining with DAB solution. Hematoxylin was used for counterstaining.
The level of RNF126 was assessed by staining intensity and percentage of positively stained cells. Examination and scoring were independently performed by 2 pathologists. The percentage of positively stained cells was scored as: 1 for less than 10%, 2 for 10–40%, 3 for 40–70%, and 4 for more than 70%. The final score was calculated by multiplying the 2 scores above, with a range of 1–12. Tissues with final score no more than 6 were regarded as low-expression cases and the others were regarded as RNF126 high-expression cases.
Quantitative real-time PCR
RNA was isolated from different FIGO-staged EOC tissues and adjacent ovarian tissues with TRIzol reagent following the manufacturer’s instructions [26]. The cDNA template for quantitative real-time PCR was created by reverse-transcription PCR. GAPDH was selected as the reference gene. The comparative Ct method (ΔCt) was utilized to calculate the relative mRNA levels. We used the following primers:
RNF126 forward, 5′-TATCGAGGAGCTTCCGGAAGAGA-3′,
RNF126 reverse, 5′-AAAGCAAACTGTCCGTAGCCCT-3′;
GAPDH forward, 5′-AGGGCTGCTTTTAACTCTGGT-3′;
GAPDH reverse, 5′-CCCCACTTGATTTTGGAGGGA-3′.
Cell culture
The SKOV3 human ovarian carcinoma cell line was purchased from the Shanghai Branch of the Chinese Academy of Science. SKOV3 were cultured with Dulbecco’s modified Eagle’s medium (DMEM) with 10% Hyclone FBS, 100IU/mL penicillin, and 100 μg/mL streptomycin. Cells were cultured in a humidified incubator with 5% CO2 at 37°C.
Virus infection of SKOV3 cells
Recombinant adenovirus expressing human RNF126 (Ad-RNF126) and short hairpin RNA (shRNA) targeting RNF126 gene (target sequence: TGCATGGTTTGTGGCGGAAGA) were all generated by Shanghai GenePharma Co. SKOV3 cells at about 80% confluence were infected with Ad-RNF126 or shRNA-RNF126 at 100 MOI (multiplicity of infection) for at least 8 h before further experiments.
Western blot
Cells were lysed in RIPA buffer containing protease inhibitors and phosphatase inhibitors, and proteins were resolved by 10% SDS-PAGE. PVDF membranes were used for immunoblotting. Before incubating with anti-GAPDH and anti-RNF126 primary antibodies overnight at 4°C, PVDF was blocked using 5% BSA at room temperature for at least 1 h. PVDF was then incubated with the secondary antibody on the following day. Electrogenerated chemiluminescence (ECL) imaging was finally performed [27]. Each experiment was repeated independently 3 times.
Cell proliferation, migration, and invasion assays
Cell proliferation ability was assessed by MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium), as described by others [28]. Transwell assays with or without Matrigel (BD Biosciences, San Jose, CA, USA) were performed to assess cell migration and invasion abilities as described previously [29,30]. Each experiment was repeated independently 3 times.
Statistics
SPSS software (version 17; SPSS, Inc., Chicago, IL, USA) was utilized for statistical analysis. Chi-square tests were performed to test correlations between RNF126 level and clinicopathological characteristics of EOC patients. Kaplan-Meier method was used to generate survival curves, which were further subjected to log-rank test, including factors such as age, LN metastasis, pathological differentiation, FIGO stage, and RNF126 expression [31]. The significant factors revealed by univariate analyses were further subjected to multivariate analysis with the Cox regression model for identification of independent prognostic factors. A P value less than 0.05 was set as statistically significant.
Results
Expression of RNF126 is upregulated in EOC tissues
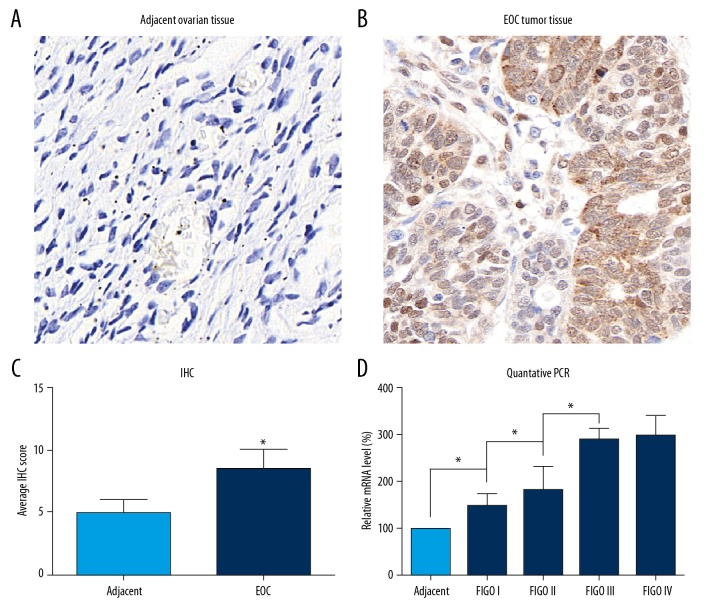
To determine the expression levels of RNF126 in EOC tissues, we collected the adjacent ovarian tissues and the EOC tissues from 122 EOC patients. The clinicopathologic parameters of EOC patients are presented in Table 1. Protein levels of RNF126 were determined by immunostaining (Figure 1A, 1B). The RNF126 level was obviously upregulated in EOC tissues compared to that in adjacent ovarian tissues. To analyze this relationship, we conducted the IHC evaluation by scoring the staining as described above in the Methods section. The average IHC score for EOC tissues was remarkably higher than the average IHC score for adjacent ovarian tissue (Figure 1C). Thus, we confirmed that RNF126 was highly expressed in EOC tissues but not in the adjacent ovarian tissues. Further, we examined the RNF126 levels in different FIGO-staged EOC tissues, setting the adjacent ovarian tissue as the control. The expression of RNF126 increased as the FIGO stage advanced (Figure 1D), indicating that RNF126 plays critical roles in the progression of EOC.
Figure 1.
Expression of RNF126 in adjacent ovarian tissues and EOC tissues. (A) Representative immunohistochemical expression of RNF126 in adjacent ovarian tissues, 100× magnification. (B) Representative immunohistochemical expression of RNF126 in EOC tissues, 100× magnification. (C) IHC result of RNF126 in adjacent ovarian tissues and EOC tissues were evaluated. (D) The mRNA level of RNF126 in adjacent non-tumorous ovarian tissues and different FIGO-staged tissues were assessed by Q-PCR. Data are expressed as mean±SD from 3 independent experiments (* p<0.05).
RNF126 protein level is related to the clinicopathological characteristics of EOC patients
Since RNF126 is higher in EOC, we next assessed the correlations between RNF126 expression and clinicopathological features of EOC patients. By performing IHC evaluation, EOC patients were divided into an RNF126 high-expression group with IHC scores over 6 and an RNF126 low-expression group with IHC scores equal to or less than 6. The analysis revealed that high RNF126 protein level was significantly correlated with LN metastasis, pathological differentiation, and FIGO stage. No obvious relationship between RNF126 expression and patient age was found.
RNF126 expression is associated with worse survival of EOC patients
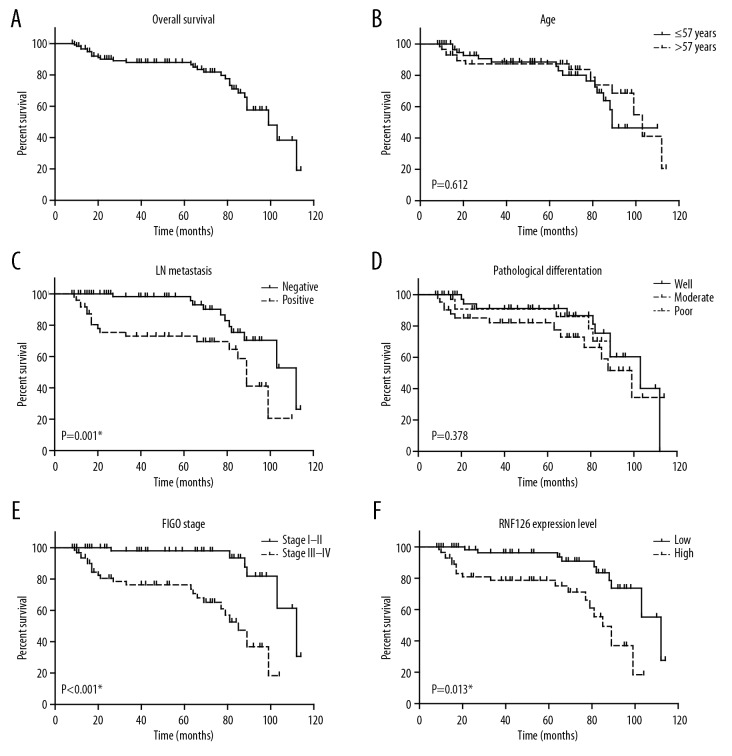
To assess whether RNF126 expression is associated with overall survival of EOC patients, we assessed overall survival times of enrolled EOC patients by utilizing Kaplan-Meier survival analyses (Figure 2A–2E), finding that FIGO stage, LN metastasis, and RNF126 level were significantly correlated with clinical outcomes of EOC patients (Table 2, Figure 2), but patient age and pathological differentiation were not significantly correlated with survival time. Of note, EOC patients with high RNF126 expression had shorter survival times than those with lower RNF126 expression (Figure 2F), indicating that high RNF126 expression was associated to worse prognosis of EOC.
Figure 2.
Kaplan-Meier analysis of overall survival. Kaplan-Meier results exhibited the relationships of: (A) overall survival of EOC patients with (B) age, (C) LN metastasis, (D) pathological differentiation, (E) FIGO stage, (F) RNF126 expression level; * p<0.05.
Table 2.
Kaplan-Meier survival analyses of EOC patients.
| Variables | Cases (n=122) | 5-year OS (%) | OS time (months) | P value |
|---|---|---|---|---|
| Age (years) | 0.612 | |||
| ≤57 | 64 | 90.5% | 89.1±4.3 | |
| >57 | 58 | 87.3% | 91.1±5.0 | |
| LN metastasis | 0.001* | |||
| Negative | 72 | 97.4% | 101.1±3.3 | |
| Positive | 50 | 73.0% | 74.4±5.9 | |
| Pathological differentiation | 0.378 | |||
| Well | 39 | 94.1% | 95.6±5.0 | |
| Moderate | 43 | 82.1% | 82.8±6.5 | |
| Poor | 40 | 90.8% | 83.9±4.3 | |
| FIGO stage | <0.001* | |||
| I–II | 59 | 98.0% | 103.8±3.3 | |
| III–IV | 63 | 78.0% | 73.7±4.7 | |
| RNF126 expression | 0.013* | |||
| Low | 63 | 98.1% | 102.0±3.4 | |
| High | 59 | 76.9% | 73.6±4.9 |
EOC – epithelial ovarian cancer; LN – lymph node metastasis; FIGO – International Federation of Gynecology and Obstetrics; RNF126 – RING finger protein 126.
RNF126 protein level is a novel independent prognostic biomarker for EOC patients
To determine whether RNF126 could be a novel independent prognostic biomarker for EOC patients, we used the multivariate Cox model for further analysis. The statistically significant risk factors as revealed by univariate analyses, including LN metastasis, FIGO stage, and RNF126 expression, were entered into the Cox regression model (Table 3). As expected, RNF126 was an independent risk factor (HR=1.713, 95% CI 1.024–4.514, P=0.044). Other independent risk factors included LN metastasis and FIGO stage (Table 3). In addition to LN metastasis and FIGO stage, which are widely-accepted independent risk factors for prognosis of EOC patients, RNF126 level now could serve as a predictor of EOC outcomes.
Table 3.
Cox regression analysis of EOC patients.
| Variables | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| LN metastasis | 2.338 | 1.050~5.206 | 0.038* |
| FIGO stage | 3.344 | 1.242~9.007 | 0.017* |
| RNF126 expression | 1.713 | 1.024~4.514 | 0.044* |
EOC – epithelial ovarian cancer; LN – lymph node metastasis; FIGO – International Federation of Gynecology and Obstetrics; RNF126 – RING finger protein 126.
RNF126 regulated the progression of ovarian carcinoma cells
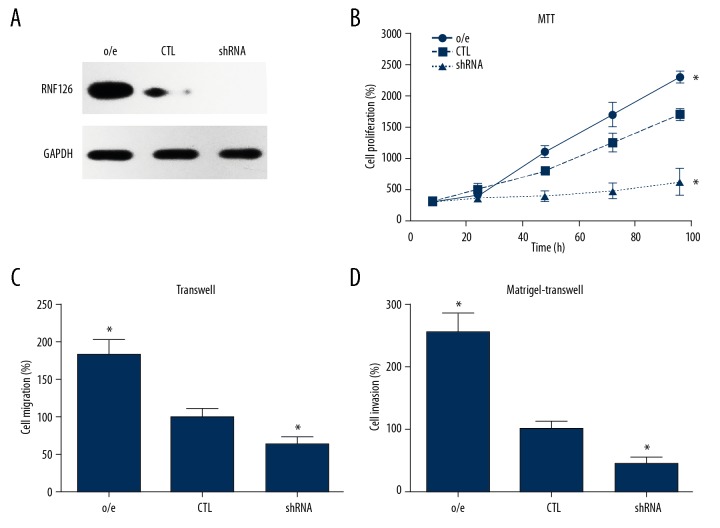
To define the molecular basis of RNF126 in predicting the unfavorable prognosis of EOC patients, we further explored the roles of RNF126 in the various cellular processes in the EOC cell line. We utilized the human ovarian carcinoma cell line SKOV3 and generated recombinant adenovirus expressing human RNF126 and lentivirus-mediated short hairpin RNA targeting the RNF126 gene. After infection of SKOV3 cells with Ad-RNF126 and shRNA-RNF126 for 24 h, Western blot analysis was carried out to examine the efficiency of the infections, showing that RNF126 was successfully overexpressed by Ad-RNF126 and was knocked down by shRNA-RNF126 (Figure 3A). We then conducted cellular experiments to explore the role of RNF126 in the proliferation and invasion processes of SKOV3 cells. MTT assay confirmed that overexpressing RNF126 induced the proliferation of SKOV3 cells, while silencing RNF126 inhibited SKOV3 proliferation (Figure 3B), and Transwell assays revealed a similar tendency. Overexpression of RNF126 significantly increased the migration and invasion of SKOV3 cells, while RNF126 knockdown dramatically decreased the SKOV3 migration and invasion capacities (Figure 3C, 3D), all indicating the important roles of RNF126 in EOC progression.
Figure 3.
RNF126 regulated the proliferation and invasion of SKOV3 cells. (A) Infection efficiencies of Ad-RNF126 and shRNA-RNF126 were tested by measuring protein levels of RNF126 with Western blot analysis. (B) MTT data showed that overexpressing (o/e) RNF126 enhanced proliferation of SKOV3 cells but silencing RNF126 inhibited the proliferation of SKOV3 cells. (C) Transwell cell migration assay and (D) Transwell Matrigel assay demonstrated that overexpressing RNF126 enhanced tumor migration and invasion. Data are expressed as mean±SD from 3 independent experiments (* p<0.05).
Discussion
Due to lack of early-stage biomarkers, most OC patients are diagnosed at late stages, which results in poor prognosis [12]. Therefore, we explored the potential biomarkers for EOC in a retrospective analysis of 122 patients. We focused on RNF126, an E3 ubiquitin ligase that was previously shown to play roles in tongue cancer [20]. By performing a series of analyses, we found that: 1) RNF126 is upregulated in EOC tissues, 2) higher RNF126 expression is an independent biomarker of unfavorable prognosis in EOC patients and a possible prognostic marker of EOC, and 3) RNF126 promotes EOC progression by increasing proliferation and migration abilities of tumor cells.
We found that RNF126 was upregulated in the cancerous tissues compared with the adjacent non-tumorous tissues, indicating a possible function of RNF126 in cancer development, which agrees with the findings of a previous study in breast cancer [22]. We also analyzed the potential relationship between RNF126 and the clinicopathological features. As reported by others [32,33], we found that LN metastasis and FIGO stage were both significantly associated with EOC prognosis. We also found that RNF126 expression can help predict survival of EOC patients, and high RNF126 expression in EOC patients was associated with shorter survival time than in patients with low RNF126 expression. Furthermore, multivariate analysis indicated that RNF126 protein level was an independent prognostic biomarker, as were LN metastasis and FIGO stages. Recent evidence also shows that RNF126 has an oncogenic role in breast cancer. Whether RNF126 could serve as a common biomarker for various cancers, like LN metastasis and FIGO stages, requires more clinical studies.
As an E3 ubiquitin ligase, RNF126 participates in many cellular processes that are involved in oncogenesis, including, but not limited to, cell cycle and DNA repair [13,15]. On the one hand, RNF126 shares a similar protein structure with BCA2 E3 ligases, which was shown to be associated with outcomes of breast cancer [34,35], and it was later discovered to be a pro-proliferation protein that promotes p21 degradation [13]. On the other hand, RNF126 was reported to promote HR-mediated DNA double-strand break repair, which is critical in tumor suppression and drug resistance. It can also promote E2F1-dependent BRCA1 expression, which is an critical tumor suppressor in several tumor types [15]. The anti-tumor role of RNF126 is in sharp contrast to previous reports highlighting the clinical relevance of RNF126 in tumorigenesis. Thus, to gain a better understanding of the roles of RNF126 in cancer, more studies are urgently needed.
Conclusions
We found that RNF126 promotes tumorigenesis in human ovarian carcinoma cells, which is consistent with other cancer studies [20,22]. RNF126 increased the proliferation and migration abilities of ovarian cancer cells. Further studies of the mechanisms involved will be invaluable for drug discovery.
Footnotes
Data availability
Data are available upon request.
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou S, Du P, Wang P, et al. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin Transl Oncol. 2017;19(9):1107–16. doi: 10.1007/s12094-017-1646-x. [DOI] [PubMed] [Google Scholar]
- 6.Kobel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: Implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):x111–17. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 8.Ma M, Yu N. Ubiquitin-specific protease 7 expression is a prognostic factor in epithelial ovarian cancer and correlates with lymph node metastasis. Onco Targets Ther. 2016;9:1559–69. doi: 10.2147/OTT.S100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tothill RW, Tinker AV, George J, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 10.Tan W, Pan M, Liu H, et al. ergosterol peroxide inhibits ovarian cancer cell growth through multiple pathways. Onco Targets Ther. 2017;10:3467. doi: 10.2147/OTT.S139009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y, Yu Y, Chen X, et al. Low expression of protocadherin-8 promotes the progression of ovarian cancer. Int J Gynecol Cancer. 2018;28(2):346–54. doi: 10.1097/IGC.0000000000001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren F, Shen J, Shi H, et al. Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta. 2016;1866(2):266–75. doi: 10.1016/j.bbcan.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhi X, Zhao D, Wang Z, et al. E3 ubiquitin ligase RNF126 promotes cancer cell proliferation by targeting the tumor suppressor p21 for ubiquitin-mediated degradation. Cancer Res. 2013;73(1):385–94. doi: 10.1158/0008-5472.CAN-12-0562. [DOI] [PubMed] [Google Scholar]
- 14.Delker RK, Zhou Y, Strikoudis A, et al. Solubility-based genetic screen identifies RING finger protein 126 as an E3 ligase for activation-induced cytidine deaminase. Proc Natl Acad Sci USA. 2013;110(3):1029–34. doi: 10.1073/pnas.1214538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Deng O, Feng Z, et al. RNF126 promotes homologous recombination via regulation of E2F1-mediated BRCA1 expression. Oncogene. 2016;35(11):1363–72. doi: 10.1038/onc.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benini M, Fortuni S, Condo I, et al. E3 Ligase RNF126 directly ubiquitinates frataxin, promoting its degradation: identification of a potential therapeutic target for Friedreich ataxia. Cell Rep. 2017;18(8):2007–17. doi: 10.1016/j.celrep.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith CJ, Berry DM, McGlade CJ. The E3 ubiquitin ligases RNF126 and Rabring7 regulate endosomal sorting of the epidermal growth factor receptor. J Cell Sci. 2013;126(Pt 6):1366–80. doi: 10.1242/jcs.116129. [DOI] [PubMed] [Google Scholar]
- 18.Smith CJ, McGlade CJ. The ubiquitin ligase RNF126 regulates the retrograde sorting of the cation-independent mannose 6-phosphate receptor. Exp Cell Res. 2014;320(2):219–32. doi: 10.1016/j.yexcr.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Ishida N, Nakagawa T, Iemura SI, et al. Ubiquitylation of Ku80 by RNF126 promotes completion of nonhomologous end joining-mediated DNA repair. Mol Cell Biol. 2017;37(4) doi: 10.1128/MCB.00347-16. pii: e00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Wang X, Zhao Y, et al. E3 Ubiquitin ligase RNF126 regulates the progression of tongue cancer. Cancer Med. 2016;5(8):2043–47. doi: 10.1002/cam4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshino S, Hara T, Nakaoka HJ, et al. The ERK signaling target RNF126 regulates anoikis resistance in cancer cells by changing the mitochondrial metabolic flux. Cell Discov. 2016;2:16019. doi: 10.1038/celldisc.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Pan Y, Qiu Z, et al. RNF126 as a biomarker of a poor prognosis in invasive breast cancer and CHEK1 inhibitor efficacy in breast cancer cells. Clin Cancer Res. 2018;24(7):1629–43. doi: 10.1158/1078-0432.CCR-17-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho KR, Shih Ie M. Ovarian cancer. Ann Rev Pathol. 2009;4:287–313. doi: 10.1146/annurev.pathol.4.110807.092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Melo Rego MJ, Cordeiro MF, de Cavalcanti CL, et al. Immunohistochemiluminescence detection: A quantitative tool in breast cancer HER-2 status evaluation. Dis Markers. 2013;34(5):373–77. doi: 10.3233/DMA-130981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Liu Z, Li K, et al. TBL1XR1 predicts isolated tumor cells and micrometastasis in patients with TNM stage I/II colorectal cancer. J Gastroenterol Hepatol. 2017;32(9):1570–80. doi: 10.1111/jgh.13749. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Yang X, Li Z, et al. Sprouty2 correlates with favorable prognosis of gastric adenocarcinoma via suppressing FGFR2-induced ERK phosphorylation and cancer progression. Oncotarget. 2017;8(3):4888–900. doi: 10.18632/oncotarget.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Xu Y, Zhang Q, et al. Correlations between TBL1XR1 and recurrence of colorectal cancer. Sci Rep. 2017;7:44275. doi: 10.1038/srep44275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delrue I, Pan Q, Baczmanska AK, et al. Determination of the selection capacity of antibiotics for gene selection. Biotechnol J. 2018;13(8) doi: 10.1002/biot.201700747. e1700747. [DOI] [PubMed] [Google Scholar]
- 29.Jiang K, Chen H, Tang K, et al. Puerarin inhibits bladder cancer cell proliferation through the mTOR/p70S6K signaling pathway. Oncol Lett. 2018;15(1):167–74. doi: 10.3892/ol.2017.7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song Y, Li X, Wang D, et al. Transcription factor Kruppel-like factor 2 plays a vital role in endothelial colony forming cells differentiation. Cardiovasc Res. 2013;99(3):514–24. doi: 10.1093/cvr/cvt113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Liu H, Xu Y, Zhang Q, et al. Prognostic significance of TBL1XR1 in predicting liver metastasis for early stage colorectal cancer. Surg Oncol. 2017;26(1):13–20. doi: 10.1016/j.suronc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Xiao N, Li Z, Wang Q. Expression of inorganic pyrophosphatase (PPA1) correlates with poor prognosis of epithelial ovarian cancer. Tohoku J Exp Med. 2017;241(2):165–73. doi: 10.1620/tjem.241.165. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Wei H, Zhang S. Dickkopf-4 is frequently overexpressed in epithelial ovarian carcinoma and promotes tumor invasion. BMC Cancer. 2017;17(1):455. doi: 10.1186/s12885-017-3407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burger AM, Gao Y, Amemiya Y, et al. A novel RING-type ubiquitin ligase breast cancer-associated gene 2 correlates with outcome in invasive breast cancer. Cancer Res. 2005;65(22):10401–12. doi: 10.1158/0008-5472.CAN-05-2103. [DOI] [PubMed] [Google Scholar]
- 35.Burger A, Amemiya Y, Kitching R, Seth AK. Novel RING E3 ubiquitin ligases in breast cancer. Neoplasia. 2006;8(8):689–95. doi: 10.1593/neo.06469. [DOI] [PMC free article] [PubMed] [Google Scholar]