Abstract
Aims
The main mechanism behind caffeine's ergogenicity lies in its tendency to bind to adenosine receptors, although other mechanisms might be involved. The aim of this investigation was to analyse the effects of caffeine on muscle oxygen saturation during exercise of increasing intensity.
Methods
Thirteen healthy and active individuals volunteered to participate in a randomized, double blind, placebo‐controlled crossover trial. During 2 different trials, participants either ingested a placebo (cellulose) or 3 mg/kg of caffeine. After waiting for 60 min to absorb the substances, participants underwent a maximal ramp cycle ergometer test (25 W/min). Near infrared spectrometers were positioned on each leg's vastus lateralis to monitor tissue O2 saturation. Blood lactate concentration was measured 1 min after the end of the exercise test.
Results
In comparison to the placebo, the ingestion of caffeine improved the maximal wattage (258 ± 50 vs 271 ± 54 W, respectively, P < .001, effect size [ES] = 0.27; 95% confidence interval [CI] 0.14–0.35) and blood lactate concentration (11.9 ± 3.8 vs 13.7 ± 3.5 mmol/L, P = .029, ES = 0.38; 95% CI 0.14–0.75) at the end of the test. Caffeine increased muscle oxygen saturation at several exercise workloads with a main effect found in respect to the placebo (F = 6.28, P = .029; ES = 0.30 to 0.54; 95% CI 0.01–0.78). Peak pulmonary ventilation (124 ± 29 vs 129 ± 23 L/min, P = 0.035, ES = 0.25; 95% CI 0.07–0.40) and peak oxygen uptake (3.18 ± 0.70 vs 3.33 ± 0.88 L/min, P = 0.032, ES = 0.26; 95% CI 0.08–0.51) were also increased with caffeine.
Conclusion
Acute ingestion of 3 mg/kg of caffeine improved peak aerobic performance and increased peak pulmonary ventilation. In addition, caffeine induced changes in muscle oxygen saturation during submaximal workloads, suggesting that this mechanism might also contribute to caffeine's ergogenic effect.
Keywords: cycling, high intensity exercise, muscle oxygenation, near infrared spectroscopy, VO2max
What is already known about this subject
The main mechanism behind caffeine's ergogenicity lies in its tendency to bind to adenosine A1 and A2A receptors
However, caffeine is a xanthine that acts on a wide range of molecular targets. Therefore, other mechanisms might contribute to caffeine's ergogenicity.
Caffeine augments endothelium‐dependent vasodilation due to increased nitric oxide production. Then, caffeine might lead to increased tissue blood flow and oxygen supply to the exercising muscle during exercise.
What this study adds
The acute ingestion of 3 mg of caffeine per kg of body mass was effective in increasing the maximal wattage obtained in a graded cycling test.
This ergogenic effect was accompanied by increased peak oxygen uptake, blood lactate concentration, and peak pulmonary ventilation.
Furthermore, a higher caffeine‐induced muscle oxygen saturation was found in low‐to‐moderate workloads, which allowed the obtaining of the end point for muscle oxygen saturation associated to fatigue at higher exercise intensity. This outcome indicates caffeine's ability to enhance oxygen availability in the exercising muscle.
1. INTRODUCTION
Caffeine (1,3,7‐trimethylxanthine) is a substance naturally found in coffee, tea and cocoa. However, its potent ability to enhance physical performance and wakefulness has favoured the inclusion of this stimulant in several over‐the‐counter medications and dietary supplements.1 Caffeine has the capacity to improve performance in a wide‐variety of exercise activities when ingested at low‐to‐moderate doses (3–9 mg/kg body mass2, 3). Perhaps, this is the reason why caffeine is ingested by ~80% of competitive athletes.4 While the ergogenic effect of caffeine to enhance sports performance is well‐recognized,5 the physiological origin of caffeine's ergogenicity is poorly understood. The hydrophobic nature of caffeine results in a post‐absorption distribution of the substance to all tissues of the body, making it difficult to accurately quantify its key mechanism of action during exercise.6
There is a consensus about caffeine antagonism of the adenosine receptors7 as the main mechanism behind the performance‐enhancing effect of this substance.8 Briefly, evidence in animal9 and human models10 supports the ability of caffeine to act as an adenosine A1 and A2A receptor antagonist, reducing the adenosine‐induced effect on neurotransmission and creating a greater dopaminergic drive.8 However, the influence of caffeine on exercise performance cannot be only explained by its effects on the brain, as several other central and peripheral mechanisms can aid in producing a more potent ergogenic effect. Other mechanisms, such as reduced muscle pain and perceived exertion,11 central stimulation of the respiratory medullary complex,12 fatty acid mobilization and oxidation,13 and local changes within the exercising muscle such as potassium ion attenuation in the interstitium and calcium iron release from the sarcoplasmic reticulum,6, 14 have also been proposed to explain caffeine effects on physical performance.
Caffeine also produces an indirect increase in serum adenosine concentration by competitively blocking adenosine receptors.15 The increased availability of adenosine causes a generalized stimulation of chemoreceptors distributed throughout circulation and creates an increase in the sympathetic tone and the upsurge of circulating catecholamines.16 Although the direct effects of adenosine on the different vascular systems depend on the type of receptor that is stimulated,17 the main vascular effect of adenosine is vasodilation of the different blood beds via A2A stimulation. In addition, acute administration of caffeine augments endothelium‐dependent vasodilation by way of increased nitric oxide production.18 Thus, caffeine might directly and indirectly produce vasodilation in the endothelium and in the vascular smooth muscle cells, which leads to increased tissue blood flow and oxygen supply to the exercising muscle during exercise. To the best of our knowledge, there are no investigations that have measured the effect of caffeine on tissue oxygen saturation during exercise. Thus, the aim of the current investigation was to analyse the effects of caffeine on oxygen saturation of the vastus lateralis during cycling of increasing intensity.
2. MATERIALS AND METHODS
2.1. Participants
Thirteen healthy and active (>4 days of training per week; >45 min per day) individuals volunteered to participate in this investigation. They had a mean ± standard deviation age of 32.5 ± 6.5 years, height of 171 ± 8 cm, weight of 65.2 ± 11.4 kg and peak oxygen uptake (VO2peak) of 49.7 ± 8.5 mL/kg/min. There were 7 women in the sample who participated in the entire experiment in their luteal phase. All the participants were light caffeine consumers (<50 mg of caffeine per day), nonsmokers, and did not report any previous history of cardiopulmonary diseases nor musculoskeletal injuries in the previous 3 months. One week prior to the study, the participants were fully informed of the experimental procedures and gave their informed written consent to participate in the investigation. The study was approved by the Camilo José Cela University Research Ethics Committee.
2.2. Experimental design
A randomized, double blind, placebo‐controlled and crossover experimental design was used in this study. Each participant took part in 2 identical trials that were conducted 48 h apart to allow time for recovery and substance elimination. The participants were randomly assigned to ingest an unidentifiable capsule either filled with 3 mg of caffeine per kg of body mass (Bulk Powders, UK) or with the same amount of cellulose as a placebo (Guinama, Spain). The assigned capsule for each trial was administered with 150 mL of tap water 60 min before the onset of the experimental trials. Each trial consisted of a graded maximal exercise test on a cycle ergometer (SNT Medical, Cardgirus, Spain) until volitional fatigue. Ventilatory variables, heart rate, and muscle oxygen saturation were continuously measured during exercise to assess the effect of caffeine on these variables. An alphanumeric code was assigned to each trial by an individual who was not involved in the study. Investigators and participants were not aware of the assignment of the trials or of the substances under investigation. All trials were performed in a laboratory with constant ambient conditions (21.5 ± 0.3°C and 45 ± 2% relative humidity). The drug/molecular target nomenclature used in this investigation conforms to the Guide to Pharmacology Nomenclature Classification of the International Union of Basic and Clinical Pharmacology (IUPHAR) and the British Pharmacological Society (BPS).7, 19
2.3. Experimental protocol
A week prior to the onset of the experiments, participants were familiarized with all the research protocols twice and their body mass was measured (±50 g, Radwag, Poland) to calculate proper caffeine dosage. During the familiarization protocols, skinfold thickness (Holtain Ltd, Bryberian, Crymmych, Pembrokeshire, UK) was measured in the biceps, triceps, subscapular and supra‐iliac areas to calculate body fat,20 and on the vastus lateralis of both legs (right limb = 5.7 ± 2.5 mm, left limb = 5.6 ± 2.0 mm). The day before each experimental trial, participants refrained from all sources of dietary caffeine, from strenuous exercise and alcohol, and adopted a standardized diet and fluid intake. All these standardizations were recorded in a diary during the first trial and later replicated in the second trial.
On the day of the trials, participants arrived at the laboratory at 09.00 in a fed state (at least 3 h have passed after their last meal) and the assigned experimental capsule was provided in an unidentifiable bag. They immediately ingested the capsule with water. Then, they changed into a T‐shirt, shorts and cleated shoes, and had a heart rate belt (Wearlink, Polar, Finland) attached to their chest. At this time, a near infrared spectrometer (Moxy, Fortiori Design LLC, Minnesota, USA) was positioned longitudinally on the musculus vastus lateralis of each lower limb, halfway between the greater trochanter and lateral epicondyle of the femur, to monitor tissue O2 saturation. This device has been shown to be reliable in measuring local oxygen saturation during exercise (intraclass correlation coefficient of 0.77–0.9921). The position of each spectrometer was marked with an indelible marking pen to assure inter‐day positioning. In addition, the spectrometers were firmly attached to the skin with an elastic tubular net bandage positioned around the thigh (Vendafix, Favesam, Spain). The lack of spectrometer movement was tested during the warm‐up. The vastus lateralis was chosen as the location for the spectrometers because it is a part of the knee extensor group, which is the primary contributor to force production during the down stroke of the pedal22 and it is a typical location used to assess muscle oxygenation during incremental cycling exercise.23 After this step, the participants rested on a stretcher in a supine position for 60 min to allow for the experimental substance to be absorbed.
After the resting period, participants performed a 10‐min standardized warm‐up on the cycle ergometer at 50 W and then exercise intensity was progressively increased by 25 W/min (ramp test) until volitional fatigue. The pedalling frequency was individually chosen (between 75 and 90 rpm) but maintained during the whole graded exercise test and replicated in both experimental trials. The seat and handlebar positions on the cycle ergometer were chosen in the familiarization trials and replicated for each individual in both experimental trials. Standardized encouragement and feedback were given to the participants in all trials by the same researcher who was blinded to the treatments.
During the exercise test, pulmonary ventilation, end‐tidal oxygen partial pressure and oxygen uptake (VO2), and heart rate were continuously measured and recorded by means of a breath‐by‐breath analyser (Metalyzer 3B, Cortex, Germany). Certified calibration gases (16.0% O2; 5.0% CO2, Cortex, Germany) and a 3‐L syringe were used to calibrate the gas analyser and the flow meter before each trial. In the graded exercise test, maximal wattage (Wmax) was recorded as the exercise load on the cycle ergometer at the moment that participants abruptly stopped pedalling or when an individual's pedalling frequency was lower than 50 rpm. VO2peak was defined as the highest VO2 value obtained during the test. The absolute value of VO2peak in the placebo trial was used to normalize the exercise intensity that represented each workload. For this normalization, the VO2 of each workload was divided by the individual VO2peak in the placebo trial, and the relative load (i.e. % of placebo VO2peak) was then allocated to the nearest load by using 5% intervals. At each workload, all variables were averaged every 15 s and the last 15 s of each stage were used as a representative value of the workload. The exercise test was considered maximal and valid when the following end criteria were reached at the end of the test: VO2 stabilized despite increases in ergometric power, the respiratory exchange ratio was >1.10, participant's rating of perceived exertion (6‐to‐20 point Borg scale) was >19 points and peak heart rate was >80% of the age‐adjusted estimate of maximal heart rate.24 One minute after the end of the graded test, a blood sample was obtained from a participants' fingertip to analyse blood lactate concentration (Lactate Pro 2, Arkay, Japan).
2.4. Statistical analysis
The results of each trial were blindly introduced into the statistical package SPSS v 20.0 for later analysis. Differences between the caffeine vs placebo protocols were determined by a 2‐way analysis of variance (substance × workload) with repeated measures. After a significant F test (Geisser–Greenhouse correction for the assumption of sphericity), differences between the means were identified using Tukey's HSD post hoc. The difference in peak values of caffeine vs placebo for all variables was identified with the Student t‐test for paired samples. The significance level was set at P < .05 and all data were presented as means ± standard deviation. The effect size (ES) and 95% confidence intervals (CI) was also calculated in all pairwise comparisons in which a statistical difference was found, following the formula proposed by Cohen.25
3. RESULTS
In comparison to the placebo, the ingestion of caffeine improved Wmax at the end of the ramp test by mean ± standard deviation 5.2 ± 3.8% (258 ± 50 vs 271 ± 54 W, respectively, P < .001, ES = 0.27; 95% CI 0.14–0.35). In addition, 1 min after the end of the ramp test, blood lactate concentration was increased by 14.3 ± 3.6% with the ingestion of caffeine (11.9 ± 3.8 vs 13.7 ± 3.5 mmol/L, P = .029, ES = 0.38; 95% CI 0.14–0.75). However, the rating of perceived exertion at the end of exercise was very similar, regardless of whether a placebo or caffeine was ingested (19.3 ± 0.9 vs 19.2 ± 1.0, P = .800).
During exercise, there was a main effect of caffeine on muscle oxygen saturation (F = 6.28, P = .029). The pairwise comparison detected differences between caffeine and placebo at 29 ± 3 (P = .049, ES = 0.45; 95% CI 0.01–0.78), 39 ± 3 (P = .046, ES = 0.40; 95% CI 0.02–0.70), 51 ± 2 (P = .018, ES = 0.43; 95% CI 0.11–0.69) and 61 ± 3% (P = .035, ES = 0.30; 95% CI 0.10–0.46) of placebo VO2peak (Figure 1). Nevertheless, the lowest value of muscle oxygen saturation, obtained at the end of exercise, was not different between treatments (26.8 ± 14.5 vs 26.9 ± 14.5%, P = .295). In pulmonary ventilation, a main effect of caffeine was not detected (F = 0.60, P = .460) but peak pulmonary ventilation was higher with caffeine by 6.1 ± 8.5% (124 ± 29 vs 129 ± 23 L/min, P = .035, ES = 0.25; 95% CI 0.07–0.40; Figure 2). In end‐tidal O2 partial pressure, there was no main effect of caffeine found (F = 0.10, P = .759) and peak O2 partial pressure remained unchanged with caffeine (115 ± 5 vs 115 ± 4 mmHg, P = .278). In VO2, there was no detected main effect of caffeine (F = 0.31, P = .589) but VO2peak was increased by 4.5 ± 10.6% with caffeine (3.18 ± 0.70 vs 3.33 ± 0.88 L/min, P = .032; ES = 0.26; 95% CI 0.08–0.51; Figure 2). In regards to heart rate, there was no main effect of caffeine (F = 3.77, P = .110) and peak heart rate remained unchanged with caffeine (173 ± 11 vs 173 ± 11 beats/min, P = .403).
Figure 1.
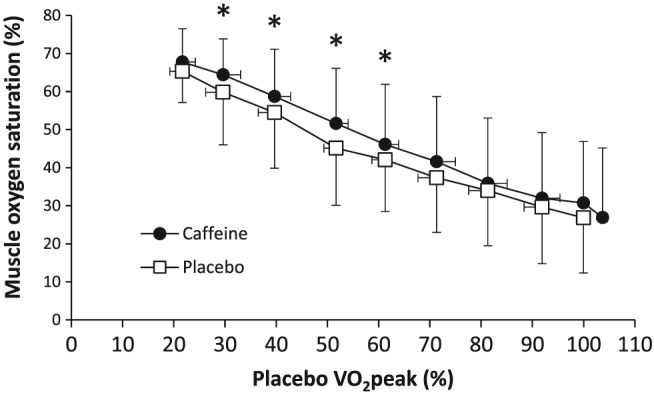
Muscle oxygen saturation during a maximal graded cycling test after the ingestion of 3 mg/kg of caffeine or a placebo. Data are mean ± standard deviation for 13 healthy and active individuals (*) Caffeine different from placebo at P < .05
Figure 2.
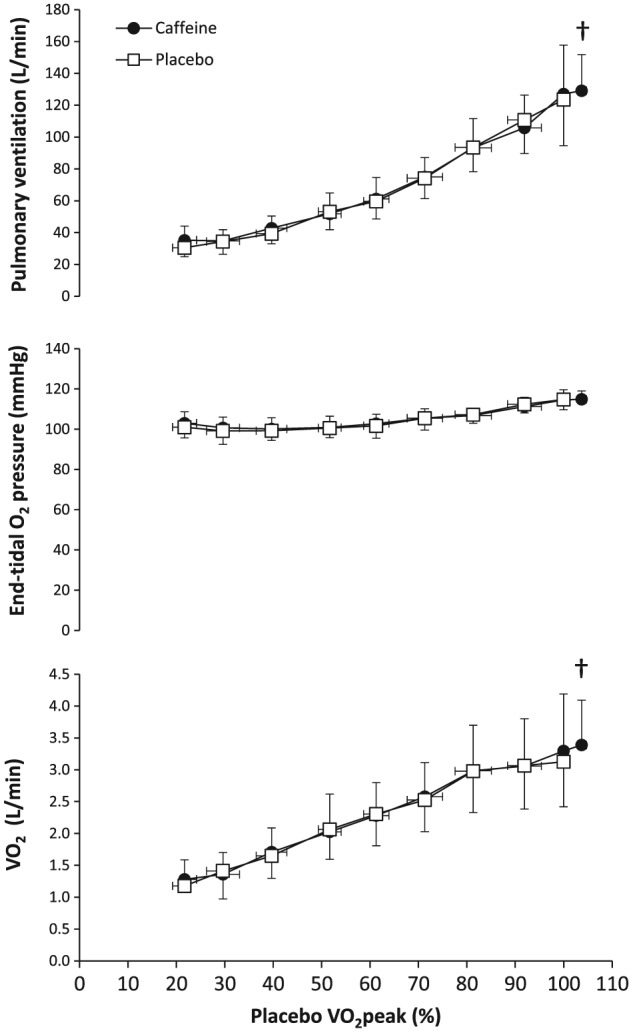
Pulmonary ventilation, end‐tidal O2 partial pressure, and O2 uptake during a maximal graded cycling test after the ingestion of 3 mg/kg of caffeine or a placebo. Data are mean ± standard deviation for 13 healthy and active individuals. (†) Peak value with caffeine different from peak value with placebo at P < .05
4. DISCUSSION
The aim of the investigation was to analyse the effects of caffeine on muscle oxygen saturation during a graded maximal cycling test in healthy individuals. This aim was designed to ascertain whether caffeine's ergogenicity during endurance exercise is produced, at least in part, via increased oxygen supply to the exercising muscle, in addition to the well‐contrasted mechanism via blockade of adenosine receptors in the brain.8 The main outcomes of this investigation indicate that caffeine increased Wmax while also enhancing muscle oxygen saturation at 30–60% of VO2peak. Although the caffeine–placebo comparison did not show an effect on muscle oxygen saturation at the highest workloads, the end‐point value for muscle oxygen saturation, which characterizes muscle fatigue during cycling,23 was later obtained and at a higher exercise intensity with caffeine (i.e. 104.5% of placebo VO2peak, Figure 1). The acute ingestion of caffeine also increased VO2peak, peak pulmonary ventilation and postexercise blood lactate concentration, suggesting that the ergogenic effect of caffeine was also accompanied by respiratory and metabolic changes induced by the increased wattage. These results suggest that caffeine's ergogenicity during an incremental cycling exercise relies, at least in part, on an enhanced oxygen availability in the exercising muscle during submaximal workloads drive by this substance. The effect of caffeine to peak aerobic performance and to modulate physiological responses at submaximal exercise intensity probably explains why caffeine has the capacity to increase performance in such a wide range of endurance exercise activities.1, 26
The benefits of caffeine ingestion on high‐intensity endurance cycling tests have been reported in the literature through original investigations27, 28, 29 and meta‐analysis.1, 26, 30 The magnitude of caffeine's ergogenicity is typically higher in investigations that used time‐to‐exhaustion endurance protocols than in maximal graded or time trials.1, 30 Furthermore, it seems that the effect of caffeine on endurance performance is of similar magnitude in men and women27 and may last for up to 15 days when the substance is ingested daily.29 Despite the consistency in the investigations that have reported an ergogenic effect of acute caffeine intake on endurance activities, there is a disparity of findings regarding the mechanism(s) behind the effects of caffeine. Shen et al., 1 through a meta‐analysis of 40 articles, have reported that caffeine's ergogenicity increases along with exercise duration. This finding is consistent with that of Silveira et al., 28 who indicated that caffeine effects on endurance performance might be linked to an enhanced maintenance of maximal metabolic oxidative pathways. However, other investigations have found caffeine‐induced effects on several variables associated with anaerobic energy systems31, 32, 33 and a direct effect of caffeine on ventilation.12 In the majority of these investigations, caffeine‐induced changes have been related to an effect on the central nervous system via the direct competitive blockade of the adenosine receptors in the brain that inhibits the deleterious effects of adenosine and permits more external work.9 Alternatively, caffeine has also been related to a direct effect on increasing muscle force production by way of calcium release from the sarcoplasmic reticulum during muscle contractions and delayed potassium accumulation.6
The current manuscript presents an additional mechanism of action that might help to understand the ergogenic effect of caffeine on endurance exercise. In the caffeine trial, muscle oxygen saturation was enhanced with caffeine at 30–60% of VO2peak. Although the statistical significance of this effect disappeared at higher workloads, there was a main effect of caffeine on muscle oxygen saturation in the caffeine trial that indicated higher oxygen availability in the exercising muscle. While endurance training habitually yields enhanced oxygen utilisation within the muscle, which is translated into lower muscle oxygen saturation,34 the ingestion of caffeine produced higher muscle oxygen saturation, which reflected enhanced blood oxygen supply to the exercising muscle. Interestingly, the end‐point of muscle oxygen saturation, obtained in the moment of volitional fatigue, was similar in caffeine and placebo trials despite the workload was significantly higher with caffeine, suggesting that the margin of improved tissue oxygenation due to caffeine allowed participants to cycle longer and at a higher exercise intensity in the caffeine trial. Although the causes for the higher muscle oxygen saturation with caffeine are not evident from our data, the unchanged values of pulmonary ventilation, end‐tidal oxygen partial pressure, and VO2 at submaximal workloads suggest that the load of oxygen at the alveolar level and the oxidative capacity of the exercising muscles were not modified with this stimulant. If these 2 factors were likely to be unchanged with caffeine ingestion, the alternative hypothesis for the physiological process that induced higher muscle oxygen saturation might be related to an improved blood flow to the muscle. In fact, this theory has scientific support due to the potential vasodilation effects of caffeine at the endothelial level18 and on smooth muscle cells,17 or indirectly through the increased concentration of adenosine once caffeine blocks its receptors in the brain.35 This is the first investigation that shows an effect of caffeine on muscle oxygenation during exercise and requires further investigation.
The current investigation presents some limitations, which should be discussed in order to understand the practical application of the results. First, we used a ramp exercise test to determine the effect of caffeine on muscle oxygen saturation during endurance exercise. However, this protocol of increasing exercise intensity is not representative of any endurance competition. Thus, the efficacy of caffeine in increasing tissue oxygen saturation should be confirmed by using exercise routines more applicable to sports before this mechanism is used to explain the ergogenic effect of caffeine in endurance sports. Second, we placed the near‐infrared spectrometers on the vastus lateralis, which only represents a small portion of the muscles involved in pedalling. To assure the effect of caffeine on tissue oxygenation during cycling, the measurement of muscle oxygen content should also be made in other leg muscles. Although the spectrometer used in this investigation is a valid and reliable tool for assessing local oxygen saturation, it has been found that its reliability is reduced along with exercise intensity.21 This lower reliability at higher exercise intensities might explain the lack of effect of caffeine on this variable at exercise intensities >60% VO2peak. Last, we used only 1 dose of caffeine (i.e. 3 mg/kg) and thus, we are unable to determine whether there is a dose–response effect of caffeine on muscle oxygen saturation. Despite these limitations, this investigation is innovative and can be used to further the understanding caffeine's ergogenic effect on endurance exercise performance.
In summary, the results of this investigation indicate that the acute ingestion of 3 mg of caffeine per kg of body mass was effective in increasing the maximal wattage obtained in a graded cycling test by 5.2 ± 3.8%. This ergogenic effect was accompanied by increased VO2peak, blood lactate concentration, and peak pulmonary ventilation, which represent effects found in previous investigations.29, 36 Furthermore, a higher caffeine‐induced muscle oxygen saturation was found in low‐to‐moderate workloads, which allowed the obtaining of the end point for muscle oxygen saturation associated to fatigue at higher exercise intensity. The results of this investigation suggest that higher VO2peak, blood lactate concentration, and peak pulmonary ventilation values with caffeine were the result of the higher wattage obtained in this trial rather than a direct effect of caffeine on these variables. However, caffeine might have the ability to enhance oxygen availability in the exercising muscle during submaximal workloads, which serves as a potential explanation for the well‐evidenced ergogenic effect of caffeine on endurance performance. Further investigation is necessary to determine whether this effect of caffeine is present during endurance exercise sports or in high‐intensity intermittent disciplines.
CONTRIBUTORS
Carlos Ruiz‐Moreno (1,2,3,4,5), Beatriz Lara (1,2,3,6), Diego Brito de Souza (4,6), Angel Cuéllar‐Rayo (4,6), Jorge Gutiérrez‐Hellín (3,6) and Juan Del Coso (1,2,3,4,5).
(1) Formulated the research question; (2) designed the study; (3) carried it out; (4) analysed the data; (5) wrote the article; (6) revised the article.
COMPETING INTERESTS
The authors of this study have not received any support from any organizations for the submitted work. They do not have any financial relationships with any organizations that might have had an interest in the submitted work in the last 3 years. Lastly, the authors have not been involved in any relationships or activities that could seem to have influenced the submitted work.
ACKNOWLEDGEMENTS
The authors wish to thank the subjects for their invaluable contribution to the study.
Ruíz‐Moreno C, Lara B, Brito de Souza D, et al. Acute caffeine intake increases muscle oxygen saturation during a maximal incremental exercise test. Br J Clin Pharmacol. 2020;86:861–867. 10.1111/bcp.14189
The authors confirm that the Principal Investigator for this paper is Juan del Coso and he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Shen JG, Brooks MB, Cincotta J, Manjourides JD. Establishing a relationship between the effect of caffeine and duration of endurance athletic time trial events: a systematic review and meta‐analysis. J Sci Med Sport. 2019;22(2):232‐238. 10.1016/j.jsams.2018.07.022 [DOI] [PubMed] [Google Scholar]
- 2. Salinero JJ, Lara B, Del Coso J. Effects of acute ingestion of caffeine on team sports performance: a systematic review and meta‐analysis. Res Sports Med. 2019;27(2):238‐256. 10.1080/15438627.2018.1552146 [DOI] [PubMed] [Google Scholar]
- 3. Grgic J, Mikulic P, Schoenfeld BJ, Bishop DJ, Pedisic Z. The influence of caffeine supplementation on resistance exercise: a review. Sports Med. 2019;49(1):17‐30. 10.1007/s40279-018-0997-y [DOI] [PubMed] [Google Scholar]
- 4. Aguilar‐Navarro M, Muñoz G, Salinero JJ, et al. Urine caffeine concentration in doping control samples from 2004 to 2015. Nutrients. 2019;11(2):286 https://www.ncbi.nlm.nih.gov/pubmed/?term=Urine+Caffeine+Concentration+in+Doping+Control+Samples+from+2004+to+2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maughan RJ, Burke LM, Dvorak J, et al. IOC consensus statement: dietary supplements and the high‐performance athlete. Br J Sports Med. 2018;52(7):439‐455. 10.1136/bjsports-2018-099027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tallis J, Duncan MJ, James RS. What can isolated skeletal muscle experiments tell us about the effects of caffeine on exercise performance? Br J Pharmacol. 2015;172(15):3703‐3713. 10.1111/bph.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Union of Basic and Clinical Pharmacology (IUPHAR) and the British Pharmacological Society (BPS). caffeine|Ligand page|IUPHAR/BPS Guide to PHARMACOLOGY. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=407. Accessed October 3, 2019.
- 8. Meeusen R, Roelands B, Spriet LL. Caffeine, Exercise and the Brain. Nestle Nutr Inst Workshop Ser. 2013;76:1‐12. 10.1159/000350223 [DOI] [PubMed] [Google Scholar]
- 9. Davis JM, Zhao Z, Stock HS, Mehl KA, Buggy J, Hand GA. Central nervous system effects of caffeine and adenosine on fatigue. Am J Physiol Integr Comp Physiol. 2003;284(2):R399‐R404. 10.1152/ajpregu.00386.2002 [DOI] [PubMed] [Google Scholar]
- 10. Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A. Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo quantification with 18F‐CPFPX and PET. J Nucl Med. 2012;53(11):1723‐1729. 10.2967/jnumed.112.105114 [DOI] [PubMed] [Google Scholar]
- 11. Glaister M, Gissane C. Caffeine and physiological responses to submaximal exercise: a meta‐analysis. Int J Sports Physiol Perform. 2018;13(4):402‐411. 10.1123/ijspp.2017-0312 [DOI] [PubMed] [Google Scholar]
- 12. Chapman RF, Stager JM. Caffeine stimulates ventilation in athletes with exercise‐induced hypoxemia. Med Sci Sports Exerc. 2008;40(6):1080‐1086. 10.1249/MSS.0b013e3181667421 [DOI] [PubMed] [Google Scholar]
- 13. Gutiérrez‐Hellín J, Del Coso J. Effects of p‐Synephrine and caffeine ingestion on substrate oxidation during exercise. Med Sci Sports Exerc. 2018;50(9):1899‐1906. 10.1249/MSS.0000000000001653 [DOI] [PubMed] [Google Scholar]
- 14. Davis JK, Green JM. Caffeine and anaerobic performance: ergogenic value and mechanisms of action. Sports Med. 2009;39(10):813‐832. 10.2165/11317770-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 15. Conlay LA, Conant JA, deBros F, Wurtman R. Caffeine alters plasma adenosine levels. Nature. 1997;389(6647):136‐136. 10.1038/38160 [DOI] [PubMed] [Google Scholar]
- 16. Biaggioni I, Olafsson B, Robertson RM, Hollister AS, Robertson D. Cardiovascular and respiratory effects of adenosine in conscious man. Evidence for chemoreceptor activation. Circ Res. 1987;61(6):779‐786. http://www.ncbi.nlm.nih.gov/pubmed/3677336 [DOI] [PubMed] [Google Scholar]
- 17. Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A. Caffeine's vascular mechanisms of action. Int J Vasc Med. 2010;2010:1‐10. 10.1155/2010/834060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Umemura T, Ueda K, Nishioka K, et al. Effects of acute Administration of Caffeine on vascular function. Am J Cardiol. 2006;98(11):1538‐1541. 10.1016/j.amjcard.2006.06.058 [DOI] [PubMed] [Google Scholar]
- 19. Alexander SP, Kelly E, Marrion NV, et al. The concise guide to pharmacology 2017/18: overview. Br J Pharmacol. 2017;174:S1‐S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stewart A, Marfell‐Jones M, International Society for Advancement of Kinanthropometry. International Standards for Anthropometric Assessment. Rev. 2006. Potchefstroom South Africa: International Society for the Advancement of Kinanthropometry; 2011. https://www.worldcat.org/title/international-standards-for-anthropometric-assessment/oclc/869687146. . [Google Scholar]
- 21. Crum EM, O'Connor WJ, Van Loo L, Valckx M, Stannard SR. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur J Sport Sci. 2017;17(8):1037‐1043. 10.1080/17461391.2017.1330899 [DOI] [PubMed] [Google Scholar]
- 22. Raasch CC, Zajac FE, Ma B, Levine WS. Muscle coordination of maximum‐speed pedaling. J Biomech. 1997;30(6):595‐602. [DOI] [PubMed] [Google Scholar]
- 23. Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Front Physiol. 2014;5:142 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3990045/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edvardsen E, Hem E, Anderssen SA. End criteria for reaching maximal oxygen uptake must be strict and adjusted to sex and age: a cross‐sectional study. PLoS ONE. 2014;9(1):e85276 10.1371/journal.pone.0085276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second; LAWRENCE ERLBAUM ASSOCIATES; 1988. https://content.taylorfrancis.com/books/download?dac=C2010-0-30830-5&isbn=9781134742707&format=googlePreviewPdf. . [Google Scholar]
- 26. Southward K, Rutherfurd‐Markwick KJ, Ali A. The effect of acute caffeine ingestion on endurance performance: a systematic review and meta–analysis. Sports Med. 2018;48(8):1913‐1928. 10.1007/s40279-018-0939-8 [DOI] [PubMed] [Google Scholar]
- 27. Skinner TL, Desbrow B, Arapova J, et al. Women experience the same ergogenic response to caffeine as men. Med Sci Sports Exerc January 2019:1 10.1249/MSS.0000000000001885, 6, 1195, 1202 [DOI] [PubMed] [Google Scholar]
- 28. Silveira R, Andrade‐Souza VA, Arcoverde L, et al. Caffeine increases work done above critical power, but not anaerobic work. Med Sci Sports Exerc. 2018;50(1):131‐140. 10.1249/MSS.0000000000001408 [DOI] [PubMed] [Google Scholar]
- 29.Lara B, Ruiz‐Moreno C, Salinero JJ, Del Coso J. Time course of tolerance to the performance benefits of caffeine. Sandbakk Ø, ed. PLoS One. 2019;14(1):e0210275. 10.1371/journal.pone.0210275 [DOI] [PMC free article] [PubMed]
- 30. Doherty M, Smith PM. Effects of caffeine ingestion on exercise testing: a meta‐analysis. Int J Sport Nutr Exerc Metab. 2004;14(6):626‐646. http://www.ncbi.nlm.nih.gov/pubmed/15657469 [DOI] [PubMed] [Google Scholar]
- 31. Doherty M. The effects of caffeine on the maximal accumulated oxygen deficit and short‐term running performance. Int J Sport Nutr. 1998;8(2):95‐104. http://www.ncbi.nlm.nih.gov/pubmed/9637189 [DOI] [PubMed] [Google Scholar]
- 32. Simmonds MJ, Minahan CL, Sabapathy S. Caffeine improves supramaximal cycling but not the rate of anaerobic energy release. Eur J Appl Physiol. 2010;109(2):287‐295. 10.1007/s00421-009-1351-8 [DOI] [PubMed] [Google Scholar]
- 33. Lara B, Ruiz‐Vicente D, Areces F, et al. Acute consumption of a caffeinated energy drink enhances aspects of performance in sprint swimmers. Br J Nutr. 2015;114(6):908‐914. 10.1017/S0007114515002573 [DOI] [PubMed] [Google Scholar]
- 34.Kime R, Niwayama M, Kaneko Y, et al. Muscle Deoxygenation and Its Heterogeneity Changes After Endurance Training. In: Advances in Experimental Medicine and Biology. Vol 923. ; 2016:275–281. 10.1007/978-3-319-38810-6_37 [DOI] [PubMed]
- 35. Ballard HJ. ATP and adenosine in the regulation of skeletal muscle blood flow during exercise. Acta Physiol Sin. 2014;66(1):67‐78. [PubMed] [Google Scholar]
- 36. Chapman RF, Mickleborough TD. The effects of caffeine on ventilation and pulmonary function during exercise: an often‐overlooked response. Physician Sport Med. 2009;37(4):97‐103. 10.3810/psm.2009.12.1747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


