Abstract
Aims
To promote effective methods to improve overutilization patterns of acid‐suppressive therapy in hospitalized patients and to evaluate the impact of multidisciplinary team efforts to reduce inappropriate use of stress ulcer prophylaxis in low‐risk patients.
Methods
A multidisciplinary quality improvement initiative incorporating education, medication use reviews and reconciliation, and pharmaceutical intervention was implemented in June 2018 for surgical patients hospitalized via emergency department. For the pre‐post analysis and time series analysis, patients admitted during April and May were classified into the pre‐intervention cohort and those admitted during July and August into the post‐intervention cohort.
Results
Three hundred and seventeen patients were included in this study (153 and 164 in the pre‐ and post‐intervention cohorts, respectively). The multidisciplinary program was effective in reducing overuse of stress ulcer prophylaxis and healthcare expenses associated with it. Biweekly education on risk factors warranting stress ulcer prophylaxis was provided for clinicians, and acid‐suppressive therapy was removed from a preset list of admission orders. The incidence of inappropriate prophylaxis use declined substantially following intervention in overall patients (OR = 0.51, P = 0.01) and a significant decrease was primarily observed among non‐ICU patients (OR = 0.50, P = 0.01). Interrupted time series analysis confirmed the significant decline in inappropriate use post intervention (coefficient = −0.63, P < 0.001). The total healthcare expenses associated with such overuse decreased by 58.5% from US$ 19.39 to US$ 8.04 per 100 patient‐days.
Conclusions
Our multidisciplinary team efforts were associated with improvement in stress ulcer prophylaxis overuse patterns, resulting in a substantial decrease in the incidence of inappropriate use, especially in general wards, and associated healthcare costs.
Keywords: acid suppression, multidisciplinary, quality improvement, stress ulcer prophylaxis
What is already known about this subject
Acid suppressant‐based stress ulcer prophylaxis has been over‐utilized in low‐risk patients worldwide.
Latest clinical practice guidelines provided specific indications warranting stress ulcer prophylaxis, which have largely been underutilized in real‐world practice.
Medically important, albeit still controversial, adverse drug events and drug interactions have been linked to long‐term use of acid suppressive therapy.
What this study adds
Our multidisciplinary quality improvement initiative incorporating biweekly in‐service education, medication use review on daily rounds, pharmaceutical interventions and medication reconciliation at care transition was effective in reducing inappropriate use of stress ulcer prophylaxis in hospitalized patients.
Multidisciplinary collaboration not only provided cost‐saving opportunities for both hospitals and patients by decreasing unnecessary use of stress ulcer prophylaxis in low‐risk patients but also lowered the risk of unwarranted prolonged acid suppression, which may lead to potential adverse events and drug interactions in affected patients.
1. INTRODUCTION
Stress ulceration or stress‐related mucosal disease (SRMD) is characterized as an acute erosive mucosal damage in the upper gastrointestinal (GI) tract in critically ill patients, commonly detected endoscopically within 24 hours of intensive care unit (ICU) admission in 75% of patients.1, 2, 3 The spectrum of clinical presentations ranges from asymptomatic superficial lesions to clinically significant or overt bleedings secondary to mucosal damage.2 The prevalence of stress ulceration is variable among studies depending on the definition, the severity of illness, and the outcome parameters used in different clinical settings. However, there is no doubt that clinically significant bleeding induced by stress ulceration, although the incidence is rare, remarkably increases morbidity, mortality and length of stay in ICU patients.2, 4, 5
The incidence of stress ulcer has decreased notably in the past two to three decades with advanced therapeutic monitoring and management of critically ill patients.2, 6 Nevertheless, stress ulcer prophylaxis (SUP) with acid‐suppressive therapy (AST), either with proton pump inhibitors (PPIs) or histamine‐2 receptor antagonists (H2RAs), is inevitable in patients at high risk of bleeding.7, 8 An updated guideline published in 2006 identified strong independent risk factors for clinically significant bleeding which require SUP: coagulopathy, respiratory failure requiring mechanical ventilation for longer than 48 hours and history of GI bleeding or ulceration within the past year.9 Furthermore, SUP is recommended in patients with multiple risk factors, such as major trauma, severe brain injury, spinal cord injury, multiple organ failure, major burns associated with more than 25–30% of total body surface area, major surgical procedures, etc.7, 8, 10 However, overutilization of SUP is frequently noticed in actual clinical practices, as indicated by a high percentage of patients receiving AST without SUP indications or continuing SUP after transition of care from the ICU to a general ward.11, 12, 13, 14, 15 Moreover, 18–34% of discharged patients continued to be on SUP despite no medical indications.1
Although H2RAs or PPIs are considered safe and tolerable, overutilization of SUP has become a prominent issue in healthcare because inappropriate AST use not only increases medical costs but also puts patients at risk of developing drug‐induced complications, such as infections or malabsorption of nutrients.16, 17 SUP overutilization is also a critical issue in Korea, where more than 50% of non‐ICU patients were prescribed with AST for SUP and continued the treatment after discharge.1 Thus, the aim of this study is to assess inappropriate SUP use and evaluate the effectiveness of a multidisciplinary quality improvement initiative in preventing SUP overutilization in a single centre in Korea.
2. METHODS
2.1. Study design and cohorts
A multidisciplinary quality improvement initiative to promote appropriateness of SUP use for inpatients was first implemented in June 2018 by the Acute Care Surgery (ACS) team of Ajou University Medical Center. The team was composed of intensivists, acute care surgeons and physician assistants, along with an academic pharmacist and faculty member of the study institution‐affiliated college of pharmacy. The initiative adopted a four‐pronged strategy: biweekly in‐service education for clinicians on SUP indications per the latest guidelines, prescription reviews and discussion on daily rounds, interventions when appropriate, and medication reconciliations at care transition by the multidisciplinary team members. To evaluate the improvement in SUP use patterns before and after the implementation of this multidisciplinary project as well as to investigate its clinical and economic impact on medical costs and patient outcomes, a retrospective cohort study was designed. Eligible patients were categorized into two cohorts: surgical patients admitted via emergency department in April and May 2018 were designated as the pre‐intervention cohort and those admitted in July and August 2018 were designated as the post‐intervention cohort. The predetermined exclusion criteria were as follows: (a) pre‐existing conditions as summarized in Table S1 that require AST and (b) H2RAs or PPIs listed as home medication prior to hospital admission. The protocol of this study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB‐MED‐MDB‐18‐308). Informed consent from study participants was waived as this project was initiated in the form of quality improvement efforts within the ACS team and relevant patient data were collected retrospectively and de‐identified prior to data analyses. No further ethics approval was necessary because the investigators were authorized by the study institution to assess patient data for research purposes.
2.2. Study outcomes
In this study, the appropriate use of SUP was defined when a PPI or a H2RA was prescribed for those patients with at least one of the major risk factors (coagulopathy, mechanical ventilation ≥48 hours and GI bleeding or ulceration within the past year prior to the current hospital encounter) or with multiple minor risk factors (sepsis, multiple organ failure, hepatic failure, renal insufficiency, ICU stay ≥7 days, hypotension or shock, organ transplantation, multiple trauma, burns >25–30% of body surface area, major surgery, occult GI bleed ≥6 days and use of anticoagulants, corticosteroids, or nonsteroidal anti‐inflammatory drugs (NSAIDs)). The frequency of acid suppressant utilization was assessed in all patients, and the appropriateness of SUP was then evaluated in individual patients according to the latest guidelines recommendations. The incidence and risk of inappropriate SUP use were analysed in all patients during the pre‐ and post‐intervention periods, and the risk analysis was repeated after stratification into two groups by the initial severity of their conditions (non‐ICU and ICU‐admitted patients). Additionally, the incidence of inappropriate SUP use per 100 patient‐days was estimated and the percentage change was determined by comparing SUP use patterns before and after intervention. The total costs associated with inappropriate SUP use during the pre‐ and post‐intervention periods as well as the percentage difference between the two periods were also calculated. The estimated total expenditure associated with inadequate use of SUP was obtained based on the patient‐specific regimen as well as the individual price of the SUP agent prescribed. For example, the total costs linked to a certain regimen were calculated by multiplying the unit price of the agent by the number of doses per day and the number of prescribed days. A US$–South Korean Won exchange rate of 1161.1 was used to convert currencies. Additionally, interrupted time series analysis was carried out using daily data of inappropriate SUP prescription to account for intervention‐independent trends in the use of SUP. With respect to the secondary outcomes, not only incident GI bleeding cases but also pneumonia and Clostridium difficile‐associated diarrhoea (CDAD) cases, the two most controversial safety concerns potentially associated with prolonged AST, were identified. The incidence rates in each outcome were compared between the pre‐ and post‐intervention study cohorts. Data on length of ICU and hospital stays were also extracted and compared between pre‐ and post‐intervention periods as a part of the safety assessments.
2.3. Patient attributes and covariates
Patient demographic data plus prespecified covariates were age, sex, ICU or non‐ICU admission, and comorbidity status, along with concomitant use of pharmacologic therapies that may increase bleeding risk (anticoagulants, steroids and NSAIDs). Charlson Comorbidity Index (CCI) scores were calculated for individual patients at study entry and the following pretreatment comorbidities were identified per relevant International Classification of Disease, Tenth Revision (ICD‐10) codes: cardiovascular disease, diabetes, cerebral vascular accident, renal disease, liver disease and dyslipidemia.
2.4. Statistical analysis
Following the evaluation of appropriateness of SUP in individual patients, we calculated incidence rates and odds ratios (ORs) along with 95% confidence intervals (CIs) for inappropriate use of SUP by comparing the post‐intervention cohort against the pre‐intervention cohort (reference). We also performed multiple logistic regression analysis to evaluate the impact of our intervention, with adjustment for potential confounding variables at baseline. With regard to interrupted time series analysis, the augmented Dickey–Fuller test was used to test for stationarity of the time series data. To fit the daily time series into an autoregressive integrated moving average (ARIMA) model, Box–Jenkins methodology was applied. The autocorrelation function (ACF) and the partial autocorrelation function (PACF) along with the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) for goodness of fit were examined in order to determine the best fit model for characterizing inappropriate SUP utilization series. With the best fit model selected, interrupted time series analysis was performed to evaluate the impact of the implementation of our multidisciplinary quality improvement initiative (intervention) on the inappropriate utilization patterns of SUP in all patients and ICU versus non‐ICU patients. The statistical analyses involving categorical variables were carried out by using Chi‐square or Fisher's exact tests. P values were two‐tailed and considered statistically significant if less than 0.05. Statistical analyses were performed using SAS 9.4 Software (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Characteristics of study patients
There were 362 patients admitted to the ACS team during the study period. Of those, 45 patients were excluded due to either the pre‐existing conditions requiring AST or their home medication including AST, resulting in 317 patients eligible for study inclusion (153 in the pre‐intervention cohort and 164 in the post‐intervention cohort). Table 1 summarizes the baseline characteristics of study patients. The mean age of patients was 44.2 ± 24.8 vs 40.9 ± 24.0 years in the pre‐ and the post‐intervention cohorts, respectively. ICU admission rates were 11.1% and 9.1% during the pre‐ and post‐intervention periods, respectively. Approximately 90% of overall patients were admitted to general wards after a brief stay in the emergency department. The mean CCIs were 0.3 ± 0.8 and 0.3 ± 0.9 in the pre‐ and post‐intervention cohorts, respectively, suggesting mild comorbid status in overall patients as well as minimal difference in comorbidity between cohorts. The most common comorbidity was cardiovascular disease (4.6% vs 6.7%), followed by diabetes (2.0% vs 2.4%) in the pre‐ and post‐intervention cohorts, respectively. In terms of concomitant use of other medications with potential bleeding risks, NSAIDs were most commonly utilized (81.7% vs 79.9%), followed by anticoagulants (11.8% vs 10.4%) and steroids (2.0% vs 3.7%) in the pre‐ and post‐intervention cohorts, respectively.
Table 1.
Baseline characteristics of study patients
| Pre‐intervention n = 153 | Post‐intervention n = 164 | |
|---|---|---|
| Age/years, mean ± SD | 44.2 ± 24.8 | 40.9 ± 24.0 |
| <18, n (%) | 29 (19.0) | 40 (24.4) |
| 18–39, n (%) | 40 (26.1) | 43 (26.2) |
| 40–64, n (%) | 45 (29.4) | 50 (30.5) |
| 65–79, n (%) | 25 (16.3) | 20 (12.2) |
| ≥80, n (%) | 14 (9.2) | 11 (6.7) |
| Female, n (%) | 73 (47.7) | 68 (41.5) |
| ICU admission, n (%) | 17 (11.1) | 15 (9.1) |
| Charlson comorbidity index, mean ± SD | 0.3 ± 0.8 | 0.3 ± 0.9 |
| ≤1, n (%) | 134 (88.0) | 150 (91.5) |
| 2, n (%) | 17 (11.1) | 6 (3.7) |
| ≥3, n (%) | 2 (1.3) | 8 (4.9) |
| Comorbidity, n (%) | ||
| Cardiovascular disease | 7 (4.6) | 11 (6.7) |
| Diabetes | 3 (2.0) | 4 (2.4) |
| Cerebral vascular accident | 2 (1.3) | 5 (3.0) |
| Renal disease | 3 (2.0) | 2 (1.2) |
| Liver disease | 2 (1.3) | 3 (1.8) |
| Dyslipidemia | 0 (0) | 2 (1.2) |
| Co‐medication, n (%) | ||
| Anticoagulants | 18 (11.8) | 17 (10.4) |
| Steroids | 3 (2.0) | 6 (3.7) |
| NSAIDs | 125 (81.7) | 131 (79.9) |
Abbreviations: SD, standard deviation; ICU, intensive care unit; NSAIDs, nonsteroidal anti‐inflammatory drugs.
3.2. Patterns of AST use
The incidence and risk of AST use post the multidisciplinary team intervention were assessed in all patients, irrespective of severity of conditions, and then separately in non‐ICU vs ICU‐admitted patients. ORs and 95% CIs were then adjusted for age category, sex and co‐medication with anticoagulant, steroid and/or NSAID therapy. The results are presented as crude and adjusted ORs with 95% CIs in Table 2. Rates of AST use decreased substantially after the intervention, by 22.9% from 78.4% to 55.5% in overall patients, and by 25.5% from 77.2% to 51.7% in non‐ICU patients: the adjusted OR was 0.32 (95% CI 0.19–0.54, P < .001) and 0.29 (95% CI 0.17–0.50, P < .001), respectively. Rates of AST use among ICU patients remained high throughout the study period at 88.2% before intervention and at 93.3% after intervention. Due to the institutional SUP protocols, H2RAs were more frequently prescribed than PPIs as the first‐line AST at the study institution; PPIs were reserved for high‐risk patients and when acid suppression with H2RAs was deemed insufficient or failed to prevent GI ulceration or bleeding.
Table 2.
Acid suppressive therapy use
| Pre‐intervention n = 153 | Post‐intervention n = 164 | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | |
|---|---|---|---|---|---|---|
| Acid suppressant use in overall patients, n (%) | 120 (78.4) | 91 (55.5) | 0.34 (0.21–0.56) | <0.001 | 0.32 (0.19–0.54) | <0.001 |
| None | 33 (21.6) | 73 (44.5) | ||||
| PPI | 7 (4.6) | 5 (3.1) | ||||
| H2RA | 100 (65.4) | 75 (42.9) | ||||
| Switch | 13 (8.5) | 11 (6.7) | ||||
| Acid suppressant use in non‐ICU patients, n (%) | 105/136 (77.2) | 77/149 (51.7) | 0.32 (0.19–0.53) | <0.001 | 0.29 (0.17–0.50) | <0.001 |
| None | 31/136 (22.8) | 72/149 (48.3) | ||||
| PPI | 3/136 (2.2) | 2/149 (1.3) | ||||
| H2RA | 95/136 (69.9) | 70/149 (47.0) | ||||
| Switch | 7/136 (5.1) | 5/149 (3.4) | ||||
| Acid suppressant use in ICU patients, n (%) | 15/17 (88.2) | 14/15 (93.3) | 1.87 (0.15–22.94) | 0.62 | 3.33 (0.12–93.13) | 0.48 |
| None | 2/17 (11.8) | 1/15 (6.7) | ||||
| PPI | 4/17 (23.5) | 3/15 (20.0) | ||||
| H2RA | 5/17 (29.4) | 5/15 (33.3) | ||||
| Switch | 6/17 (35.3) | 6/15 (40.0) |
Abbreviations: OR, odds ratio; CI, confidence interval; PPI, proton pump inhibitor; H2RA, histamine‐2 receptor antagonist; ICU, intensive care unit.
P values were estimated by Chi‐squire or Fisher's exact tests.
Statistically significant P values are highlighted in bold.
ORs and 95% CIs were adjusted for age category, sex and concomitant use of anticoagulant, steroid and/or nonsteroidal anti‐inflammatory drug therapy.
3.3. Evaluation of the indication for SUP
The evaluation of the appropriateness of SUP in individual patients was conducted in accordance with the latest guidelines recommendations. The distribution and frequency of each of the aforementioned major and minor risk factors among study patients along with the number of patients with a clear indication warranting SUP were compared between the pre‐ and post‐intervention cohorts (Table 3). Of the three major risk factors for stress ulcer and stress ulcer‐induced GI bleeding, history of GI bleeding or ulceration within the past year was the most common risk factor identified in study patients: 5.2% vs 4.9% of patients in the pre‐ and post‐intervention cohorts, respectively. Of the minor risk factors, the most frequently observed risk factor was NSAID use (81.7% vs 80.0%), followed by major surgery (20.9% vs 23.2%), active GI bleeding (17.0% vs 9.1%) and anticoagulant use (11.8% vs 10.4%) before and after intervention, respectively. Overall, the numbers of patients with a proper indication for SUP were assessed to be 64 (41.8%) and 60 (36.6%) during the pre‐ and post‐intervention periods, respectively. Of those, 11 patients in each cohort required SUP as they had at least one major risk factor regardless of minor risk factors, whereas 53 (34.6%) and 49 (29.9%) patients in the pre‐ and post‐intervention cohorts, respectively, met the indication criteria by having multiple minor risk factors but no major risk factors. Notably, although AST use decreased substantially by 22.9% post intervention as shown in Table 2, the proportion of patients with an appropriate SUP indication remained similar between the two cohorts, irrespective of our intervention and the level of care (non‐ICU vs ICU). The provision of SUP in 2 (1.3%) and 11 (6.7%) patients in the pre‐ and post‐intervention cohorts, respectively, was decided to be made by clinicians following a risk–benefit analysis, in consideration of short inpatient stay and low risk of GI bleeding.
Table 3.
Appropriateness of SUP use
| Pre‐intervention n = 153 | Post‐intervention n = 164 | |
|---|---|---|
| Patients with risk factors for stress ulcer, n (%) | ||
| Coagulopathy | 1 (0.7) | 0 (0.0) |
| Mechanical ventilation ≥48 h | 2 (1.3) | 3 (1.8) |
| History of GI bleed or ulceration | 8 (5.2) | 8 (4.9) |
| Head trauma | 0 (0.0) | 2 (1.2) |
| Active GI bleeding | 26 (17.0) | 15 (9.1) |
| Sepsis | 3 (2.0) | 3 (1.8) |
| Renal insufficiency | 7 (4.6) | 7 (4.3) |
| ICU stay ≥7 d | 6 (3.9) | 6 (3.7) |
| Major surgery | 32 (20.9) | 38 (23.2) |
| Steroid use | 3 (2.0) | 6 (3.7) |
| NSAID use | 125 (81.7) | 131 (80.0) |
| Anticoagulant use | 18 (11.8) | 17 (10.4) |
| Patients with appropriate indication for SUP per guidelines, n (%) | 64 (41.8) | 60 (36.6) |
| Patients with major risk factor | 11 (7.2) | 11 (6.7) |
| Patients with multiple minor risk factors | 53 (34.6) | 49 (29.9) |
| Non‐ICU patients | 51/136 (37.5) | 48/149 (32.2) |
| ICU patients | 13/17 (76.5) | 12/15 (80.0) |
| SUP held by clinical decision, n (%) | 2 (1.3) | 11 (6.7) |
Abbreviations: SUP, stress ulcer prophylaxis; GI, gastrointestinal; ICU, intensive care unit; NSAID, nonsteroidal anti‐inflammatory drug.
3.4. Inappropriate use of SUP
The incidence of inappropriate use of SUP declined by 12.3% from 58 (37.9%) patients prior to intervention to 42 (25.6%) patients post intervention and the adjusted OR indicated a statistically significant risk reduction: 0.51 (95% CI 0.31–0.86, P = .01) (Table 4): ORs and 95% CIs were adjusted for age category, sex and co‐medication with anticoagulant, steroid and/or NSAID therapy. The overuse reduction was more pronounced in non‐ICUs where inappropriate use rates decreased by 14.4% from 56 (41.2%) prior to intervention to 40 (26.8%) post intervention (adjusted OR 0.50, 95% CI 0.30–0.85, P = .01), whereas the incidence of inappropriate use in the ICUs was 11.8% before intervention and 13.3% after intervention. In consideration of varied total patient‐days during each bimester, the incidence per 100 patient‐days was also assessed and decreased by 12.1 from 28.7 before intervention to 16.6 after intervention. The total expenses incurred by unwarranted SUP therapy amounted to US$199.93 during April and May vs US$79.01 during July and August in 2018, indicating a relative decrease of about 60.5% post intervention. The expenses associated with inappropriate utilization of SUP in ICU showed a greater relative decrease post intervention, almost 92.4%, from US$55.33 to US$4.23. All Korean citizens have universal health coverage via the National Health Insurance Program, which provides a co‐payment rate of 20% for general inpatient expenditure including pharmaceuticals.18 Patient co‐payments thus accounted for approximately 20% of the total expenses, which also decreased from US$39.99 to US$15.80 following the multidisciplinary team intervention. To adjust for varied patient‐days in each period, the total and patient expenses per 100 patient‐days were calculated and both showed a substantial relative decrease by 58.5% from US$19.39 to US$8.04 and from US$3.88 to US$1.61, respectively.
Table 4.
Inappropriate use of SUP
| Pre‐intervention n = 153 | Post‐intervention n = 164 | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value | |
|---|---|---|---|---|---|---|
| Incidence of inappropriate use, n (%) | 58 (37.9) | 42 (25.6) | 0.56 (0.35–0.91) | 0.02 | 0.51 (0.31–0.86) | 0.01 |
| Non‐ICU | 56/136 (41.2) | 40/149 (26.8) | 0.52 (0.32–0.86) | 0.01 | 0.50 (0.30–0.85) | 0.01 |
| ICU | 2/17 (11.8) | 2/15 (13.3) | 1.15 (0.14–9.38) | 0.89 | 0.50 (0.02–13.85) | 0.68 |
| Total patient‐days | 1031 | 983 | NA | NA | NA | NA |
| Non‐ICU | 644 | 666 | ||||
| ICU | 387 | 317 | ||||
| Inappropriate patient‐days | 296 | 163 | NA | NA | NA | NA |
| Non‐ICU | 265 | 154 | ||||
| ICU | 31 | 9 | ||||
| Incidence per 100 patient‐days | 28.7 | 16.6 | NA | NA | NA | NA |
| Non‐ICU | 41.1 | 23.1 | ||||
| ICU | 8.0 | 2.8 | ||||
| Total cost (US$) | $199.93 | $79.01 | NA | NA | NA | NA |
| Non‐ICU | $144.61 | $74.78 | ||||
| ICU | $55.33 | $4.23 | ||||
| Patient cost (US$) | $39.99 | $15.80 | NA | NA | NA | NA |
| Total cost per 100 patient‐days (US$) | $19.39 | $8.04 | NA | NA | NA | NA |
| Patient cost per 100 patient‐days (US$) | $3.88 | $1.61 | NA | NA | NA | NA |
Abbreviations: SUP, stress ulcer prophylaxis; OR, odds ratio; CI, confidence interval; ICU, intensive care unit; NA, not applicable.
P values were estimated by Chi‐squire or Fisher's exact tests.
Statistically significant P values are highlighted in bold.
ORs and 95% CIs were adjusted for age category, sex and co‐medication with anticoagulant, steroid and/or nonsteroidal anti‐inflammatory drug therapy.
3.5. Time series analysis on inappropriate SUP use
Figure 1 shows the impact of our intervention concerning appropriate SUP use on the reduction in unwarranted SUP prescription. Daily time series data regarding inappropriate SUP utilization were found to be stationary. By examining ACF and PACF plots plus AIC and BIC values, we assessed that the ARIMA (1, 0, 0) model best represents our data. The signs and magnitudes of the impact attributable to our intervention are expressed as a coefficient (slope, ß), which is summarized in Table 5. A significant reduction in the incidence of inappropriate SUP use was observed after our intervention with a coefficient of −0.63 in both overall and non‐ICU patients and −1.00 in ICU patients (P < .001 for all time‐series analyses).
Figure 1.
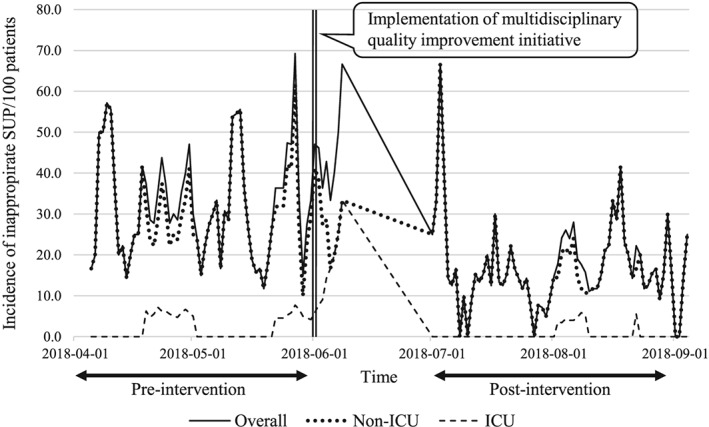
Daily incidence of inappropriate SUP use per 100 patients during pre‐ and post‐intervention periods. The vertical line denotes the implementation of the multidisciplinary quality improvement initiative concerning appropriate usage of SUP. Abbreviations: SUP, stress ulcer prophylaxis; ICU, intensive care unit
Table 5.
Autoregressive integrated moving average (ARIMA) model parameters for time series data on inappropriate SUP use
| Overall inappropriate SUP per 100 patients | Inappropriate SUP in non‐ICU per 100 patients | Inappropriate SUP in ICU per 100 patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope (ß) | SE | t value | P value | Slope (ß) | SE | t value | P value | Slope (ß) | SE | t value | P value | |
| Intervention | −0.63 | 0.07 | 8.98 | <0.001 | −0.63 | 0.07 | 8.93 | <0.001 | −1.00 | 0.01 | 114.35 | <0.001 |
Abbreviations: SUP, stress ulcer prophylaxis; ICU, intensive care unit; SE, standard error.
Slope (ß) is the estimate of direction and magnitude of the response to the intervention.
3.6. Safety outcomes: GI bleeding, pneumonia and length of stay
Although AST overuse and inappropriate SUP rates decreased substantially with the implementation of our multidisciplinary program, no increased risk of GI bleeding was observed during the post‐intervention bimester: GI bleeding occurred in 26 (17.0%) patients during April–May vs 15 (9.1%) patients during July–August in 2018 (Table 6). There was no CDAD cases encountered during the study period in both cohorts. The incidence of pneumonia was relatively low: 4 (2.6%) patients and 7 (4.3%) patients developed pneumonia during the pre‐ and post‐intervention periods, respectively. The mean length of ICU and hospital stay in days showed a tendency to decrease following our multidisciplinary team efforts, albeit statistically not significant, from 10.5 ± 16.0 to 10.3 ± 17.5 (P = .98) and 6.7 ± 9.1 to 6.0 ± 8.3 (P = .45) over the pre‐ vs post‐intervention periods, respectively. Caution is advised in interpreting these results because of the small sample size and the sparsity of outcome events during the study period. Further study is required to verify a potential association between AST overuse and adverse safety outcomes.
Table 6.
Incident GI bleeding and pneumonia
| Pre‐intervention n = 153 | Post‐intervention n = 164 | |
|---|---|---|
| GI bleeding, n (%) | 26 (17.0) | 15 (9.1) |
| Pneumonia, n (%) | 4 (2.6) | 7 (4.3) |
| Length of ICU stay (days), mean ± SD | 10.5 ± 16.0 | 10.3 ± 17.5 |
| Length of hospital stay (days), mean ± SD | 6.7 ± 9.1 | 6.0 ± 8.3 |
Abbreviations: GI, gastrointestinal; ICU, intensive care unit; SD, standard deviation.
No patients developed Clostridium difficile colitis during the study period.
4. DISCUSSION
In this study, we implemented the multidisciplinary quality improvement initiative to enhance appropriate utilization of SUP in patients hospitalized via emergency department. The initiative was composed of clinician education, medication use reviews and reconciliations, and pharmaceutical interventions when necessary. In April and May 2018, prior to the initiation of our team efforts, AST use rates were as high as 78.4% in study patients, which is partly because of the preset admission order lists established in the study institution. SUP was first removed from the admission order list by which medication sets were routinely prescribed for newly admitted patients. Our multidisciplinary team embarked on this quality improvement project in June 2018, and a 1‐month‐long transition period was allowed before designating those admitted to hospital in July and August as the post‐intervention cohort. Overall, not only the inappropriate use of SUP in patients, especially among non‐ICU patients, but also healthcare expenses associated with such overuse decreased substantially post intervention.
H2RAs and PPIs are typical AST agents for SUP. Intravenous cimetidine injection, however, is the only Food and Drug Administration (FDA) approved medication for prevention of GI bleeding, and other agents are prescribed as off‐label use according to guidelines.7, 8, 19 The first SUP guidelines, published by American Society of Health‐system Pharmacists (ASHP) in 1999, strongly recommend H2RAs as part of a prophylactic regimen in patients with high risk for stress ulcer‐induced bleeding.7, 20 Recently, the prescribing trends have shifted towards PPIs based on the study results demonstrating superior efficacy of PPIs to H2RAs in decreasing bleeding occasions as well as inhibiting acid secretions.6, 8, 20, 21 Nevertheless, H2RAs are more frequently used over PPIs for SUP in our institution due to institution‐specific clinical practices and insurance‐related issues. Currently, although various SUP guidelines are available, these do not provide clear standards regarding when to initiate, discontinue or withhold prophylactic AST, which may play as a contributing factor in rising incidences of SUP overutilization in clinical settings.7, 8, 10
The side effect profiles of both H2RAs and PPIs are generally considered safe and tolerable; however, inappropriate long‐term use of these agents definitely puts patients at risk of developing various medication‐related adverse events or complications.22, 23, 24 Although our study results demonstrated a low incidence of pneumonia before and after the intervention, patients receiving prolonged AST are prone to infections such as community‐ or hospital‐acquired pneumonia, or CDAD secondary to increased pH of the gastric tract.22, 23, 24, 25 Moreover, increased incidences of disease conditions resulted from malabsorption of essential nutrients, including vitamin B12, calcium, iron and magnesium, are commonly identified as drawbacks of long‐term AST.22, 26, 27 Additional concerns are raised with overutilization of PPIs because of possible incidences of osteoporosis, interstitial nephritis or drug interactions. PPIs inhibit CYP2C19, a key enzyme responsible for activating clopidogrel.22, 28 A meta‐analysis revealed a significantly higher rate of adverse cardiovascular events in patients concomitantly using PPIs and clopidogrel, and the US FDA issued a warning regarding concurrent administration of clopidogrel and omeprazole/esomeprazole in 2009.28, 29 As a result, individualized medication regimens along with appropriate monitoring based on risk–benefit approaches are recommended when PPIs are to be considered in clopidogrel users.
To date, the importance of appropriate SUP has been relatively underemphasized regardless of the profound risks of complications induced by long‐term AST use. The approved SUP indications involve critical conditions, such as the aforementioned major and minor risk factors.7, 8, 10 Theoretically, SUP should be initiated in ICU patients with approved indications and discontinued once risk factors for bleeding are resolved or patients are discharged from the ICU, based on the study results revealing no benefits of continuing SUP after ICU discharge.30 However, in the real world, the most common reason for inappropriate AST use was associated with SUP in low‐risk patients; continuation of SUP on hospital discharge was also prevalent.1, 11, 12, 13, 14, 15, 16 Insufficient education related to appropriate SUP use and limited time for healthcare providers to assess the risk factors for bleeding at transition of care may contribute as key factors to SUP overutilization. Therefore, this study was conducted to assess the impact of our multidisciplinary quality improvement initiative on resolution of the above issues.
The beneficial effects of a multidisciplinary team in managing inappropriate SUP have been demonstrated in previous studies conducted in different academic institutions.12, 31 The primary intervention was performed through adequate education of healthcare providers, which significantly decreased the number of inappropriate AST prescriptions. In addition, adopting a pharmacist‐involved collaborative approach in addition to medication use reviews on daily rounds and reconciliation services also improved SUP practices.12, 31, 32 As part of our multidisciplinary program, in‐service education on appropriate SUP use was offered biweekly for healthcare providers, which led to deletion of H2RAs and PPIs from the preset admission order list in the surgical ICUs and general wards. Moreover, the provision of SUP and its appropriateness in individual patients were assessed by clinicians on each transition of care, which provided opportunities for accomplishing medication reconciliation. This resource‐intensive task was only possible through effective leadership and systematic efforts within the team. Our multidisciplinary team efforts not only significantly reduced inappropriate use of SUP, especially in non‐ICU floors, but also showed cost‐saving effects. These results were consistent with the findings in previous studies where adequate education on appropriate SUP use led to a significant decrease of 10‐fold in most in healthcare costs associated with inappropriate use of SUP.13, 33
To the best of our knowledge, this is the first study to evaluate the beneficial impact of a multidisciplinary quality improvement initiative on reducing the inappropriate use of SUP in hospitalized patients in Korea. Our study results clearly demonstrate decreased inappropriate SUP use along with cost‐saving effects with the intervention. This study, however, did not have a chance to observe reduction in drug‐induced complications associated with inappropriate SUP use. Therefore, further studies assessing the impact of a multidisciplinary quality improvement initiative on reducing complications secondary to SUP overutilization are recommended to encourage multidisciplinary patient care practice as part of the institutional guidelines for appropriate SUP use and management.
5. LIMITATIONS
The study period consisted of two bimesters of pre‐intervention and post‐intervention, and this multidisciplinary initiative was implemented only in the patients admitted to the Department of Surgery via the emergency department. If we could have embarked on an institution‐wide program to cover the entire inpatients for a longer study period, the impact in terms of risk reduction of inappropriate SUP and the resultant cost savings would have been more substantial. As there is no specific ICD‐10 code assigned to stress ulcer or SRMD, an assumption was made that patients with no apparent pre‐existing disease state warranting H2RAs or PPIs were treated with the medications for prophylactic purposes. Because of the short duration of study, the low frequency of certain adverse events and the limited sample size, it was difficult to investigate a potential link between prolonged AST and adverse complications in exposed patients.
6. CONCLUSION
Our multidisciplinary team efforts incorporating education, medication use reviews and reconciliation, discussion on daily rounds, and prompt pharmaceutical interventions were associated with improvement in SUP prescription patterns, resulting in a substantial decrease in both inappropriate use of SUP in hospitalized patients, especially in non‐ICUs, and its associated healthcare costs.
CONTRIBUTORS
Y.J.C., J.S. and Y.T.J. contributed equally to this study.
COMPETING INTERESTS
There are no competing interests to declare.
Supporting information
Table S1. Pre‐existing conditions warranting acid‐suppressive therapy (exclusion criteria)
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (No. 2017R1C1B5015912) and by Ajou University research fund (S‐2019‐G0001‐00273).
Choi YJ, Sim J, Jung YT, Shin S. Impact of a multidisciplinary quality improvement initiative to reduce inappropriate usage of stress ulcer prophylaxis in hospitalized patients. Br J Clin Pharmacol. 2020;86:903–912. 10.1111/bcp.14197
Principal Investigator Statement: The authors confirm that the Principal Investigator for this paper is Sooyoung Shin.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available and shared due to privacy or ethical restrictions.
REFERENCES
- 1. Shin S. Evaluation of costs accrued through inadvertent continuation of hospital‐initiated proton pump inhibitor therapy for stress ulcer prophylaxis beyond hospital discharge: a retrospective chart review. Ther Clin Risk Manag. 2015;11:649‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Plummer M, Blaser A, Deane A. Stress ulceration: prevalence, pathology and association with adverse outcomes. Crit Care. 2014;18(2):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119(4):1222‐1241. [DOI] [PubMed] [Google Scholar]
- 4. Cook DJ, Fuller HD, Guyatt GH, et al. Lacroix, J, Griffith L, Willan a; Canadian critical care trials group. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian critical trials group. N Engl J Med. 1994;330(6):377‐381. [DOI] [PubMed] [Google Scholar]
- 5. Cook DJ, Griffith LE, Walter SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care. 2001;5(6):368‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alhazzani W, Alenezi F, Jaeschke RZ, Moayyedi P, Cook DJ. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta‐analysis. Crit Care Med. 2013;41(3):693‐705. [DOI] [PubMed] [Google Scholar]
- 7. ASHP Therapeutic Guidelines on Stress Ulcer Prophylaxis . ASHP Commissions on therapeutics and approved by the ASHP Board of Directions on November 14, 1998. Am J Health Syst Pharm. 1999;56(4):347‐379. [DOI] [PubMed] [Google Scholar]
- 8. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580‐637. [DOI] [PubMed] [Google Scholar]
- 9. Spirt M, Stanley S. Update on stress ulcer prophylaxis in critically ill patients. Crit Care Nurse. 2006;26:18‐20. 22‐8 [PubMed] [Google Scholar]
- 10. Guillamondegui OD, Gunter OL, Bonadies JA, Coates JE, Kurek SJ, De Moya MA, Sing RF, Sori AJ. Practice management guidelins for stress ulcer prophylaxis. https://www.east.org/education/practice-management-guidelines/stress-ulcer-prophylaxis Accessed on August 7, 2019.
- 11. Wohlt P, Hansen L, Fish J. Inappropriate continuation of stress ulcer prophylactic therapy after discharge. Ann Pharmacother. 2007;41(10):1611‐1616. [DOI] [PubMed] [Google Scholar]
- 12. Hatch J, Schulz L, Fish J. Stress ulcer prophylaxis: reducing non‐indicated prescribing after hospital discharge. Ann Pharmacother. 2010;44(10):1565‐1571. [DOI] [PubMed] [Google Scholar]
- 13. Masood U, Sharma A, Bhatti Z, et al. A successful pharmacist‐based quality initiative to reduce inappropriate stress ulcer prophylaxis use in an academic medical intensive care unit. Inquiry. 2018;55:46958018759116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy C, Stevens A, Ferrentino N, et al. Frequency of inappropriate continuation of acid suppressive therapy after discharge in patients who began therapy in the surgical intensive care unit. Pharmacotherapy. 2008;28(8):968‐976. [DOI] [PubMed] [Google Scholar]
- 15. Grube RR, May DB. Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64(13):1396‐1400. [DOI] [PubMed] [Google Scholar]
- 16. Durand C, Willett KC, Desilets AR. Proton pump inhibitor use in hospitalized patients: is overutilization becoming a problem? Clin Med Insights Gastroenterol. 2012;5:65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas L, Culley E, Gladowski P, Goff V, Fong J, Marche SM. Longitudinal analysis of the costs associated with inpatient initiation and subsequent outpatient continuation of proton pump inhibitor therapy for stress ulcer prophylaxis in a large managed care organization. J Manag Care Pharm. 2010;16(2):122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Health Insurance Service . National Health Insurance System of Korea. https://www.nhis.or.kr/static/html/wbd/g/a/wbdga0101.html. Accessed October 26, 2019.
- 19. Zeitoun A, Zeineddine M, Dimassi H. Stress ulcer prophylaxis guidelines: are they being implemented in Lebanese health care centers? World J Gastrointest Pharmacol Ther. 2011;2(4):27‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cook D, Guyatt G. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378(26):2506‐2516. [DOI] [PubMed] [Google Scholar]
- 21. Lin PC, Chang CH, Hsu PI, Tseng PL, Huang YB. The efficacy and safety of proton pump inhibitors vs histamine‐2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta‐analysis. Crit Care Med. 2010;38(4):1197‐1205. [DOI] [PubMed] [Google Scholar]
- 22. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton‐pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laheij R, Sturkenboom M, Hassing R, Dieleman J, Stricker BH, Jansen JB. Risk of community‐acquired pneumonia and use of gastric acid‐suppressive drugs. JAMA. 2004;292(16):1955‐1960. [DOI] [PubMed] [Google Scholar]
- 24. Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community‐acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population‐based case‐control study. Pharmacoepidemiol Drug Saf. 2009;18(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 25. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta‐analysis. Am J Gastroenterol. 2012;107(7):1011‐1019. [DOI] [PubMed] [Google Scholar]
- 26. Force R, Nahata M. Effect of histamine H2‐receptor antagonists on vitamin B12 absorption. Ann Pharmacother. 1992;26(10):1283‐1286. [DOI] [PubMed] [Google Scholar]
- 27. Vinnakota R, Brett A. Iron deficiency anemia associated with acid‐modifying medications: two cases and literature review. Am J Med Sci. 2019;357(2):160‐163. [DOI] [PubMed] [Google Scholar]
- 28. Bundhun PK, Teeluck AR, Bhurtu A, Huang WQ. Is the concomitant use of clopidogrel and proton pump inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty?: a systematic review and meta‐analysis of recently published studies (2012‐2016). BMC Cardiovasc Disord. 2017;17(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guerin A, Mody R, Carter V, et al. Changes in practice patterns of Clopidogrel in combination with proton pump inhibitors after an FDA safety communication. PLoS One. 2016;11(1):e0145504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam NP, Le PD, Crawford SY, Patel S. National survey of stress ulcer prophylaxis. Crit Care Med. 1999;27(1):98‐103. [DOI] [PubMed] [Google Scholar]
- 31. Tasaka CL, Burg C, VanOsdol SJ, et al. An interprofessional approach to reducing the overutilization of stress ulcer prophylaxis in adult medical and surgical intensive care units. Ann Pharmacother. 2014;48(4):462‐469. [DOI] [PubMed] [Google Scholar]
- 32. Buckley MS, Park AS, Anderson CS, et al. Impact of a clinical pharmacist stress ulcer prophylaxis management program on inappropriate use in hospitalized patients. Am J Med. 2015;128(8):905‐913. [DOI] [PubMed] [Google Scholar]
- 33. Erstad BL, Camamo JM, Miller MJ, Webber AM, Fortune J. Impacting cost and appropriateness of stress ulcer prophylaxis at a university medical center. Crit Care Med. 1997;25(10):1678‐1684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pre‐existing conditions warranting acid‐suppressive therapy (exclusion criteria)
Data Availability Statement
The data that support the findings of this study are not publicly available and shared due to privacy or ethical restrictions.


