Abstract
Aims
Two phase 1, open‐label studies were conducted to investigate the effect of renal impairment (RI) and organic anion transporter (OAT) inhibition on pharmacokinetics (PK) and safety of relebactam (REL) plus imipenem/cilastatin (IMI).
Methods
Study PN005 evaluated the PK of REL (125 mg) plus IMI (250 mg) in participants with RI vs healthy controls. Study PN019 evaluated the PK of REL (250 mg) and imipenem (500 mg; dosed as IMI) with/without probenecid (1 g; OAT inhibitor) in healthy adults.
Results
Geometric mean ratios (RI/healthy matched controls) of area under the concentration–time curve from time 0 to infinity (AUC0‐∞; 90% confidence interval) for REL, imipenem and cilastatin increased as RI increased from mild (1.6 [1.1, 2.4], 1.4 [1.1, 1.8] and 1.6 [1.0, 2.5], respectively) to severe (4.9 [3.4, 7.0], 2.5 [1.9, 3.3] and 5.6 [3.6, 8.6], respectively). For all 3 analytes, plasma and renal clearance decreased and corresponding plasma apparent terminal half‐life increased with increasing RI. Geometric mean ratios ([probenecid+IMI/REL]/[IMI/REL]) of plasma exposure for REL and imipenem were 1.24 (1.19, 1.28) and 1.16 (1.13, 1.20), respectively. The dose fraction excreted (fe) in the urine decreased progressively from mild to severe RI. Probenecid reduced renal clearance of REL and imipenem by 25 and 31%, respectively. Compared with IMI/REL, coadministration of IMI/REL with probenecid yielded lower fe for REL and imipenem. In both studies, treatment was well tolerated; there were no serious adverse events or discontinuations due to adverse events.
Conclusion
RI increased plasma exposure and similarly decreased clearance of REL, imipenem and cilastatin; IMI/REL dose adjustment (fixed‐ratio) will be required for patients with RI. Probenecid had no clinically meaningful impact on the PK of REL or imipenem.
Keywords: antibioticsdrug transporterspharmacokineticsphase 1
What is already known about this subject
Urinary excretion is the major route of elimination for relebactam (REL), imipenem and cilastatin.
Active secretion contributes to ~30% of total REL clearance; coadministration with probenecid increased imipenem exposure by 15%.
REL, imipenem and cilastatin have comparable apparent terminal half‐lives and no drug–drug interactions among individual components, supporting coadministration.
What this study adds
REL, imipenem and cilastatin exposure exhibited similar pharmacokinetics alterations with renal impairment and haemodialysis.
Probenecid had no clinically meaningful impact on the pharmacokinetics of REL or imipenem.
These data support an imipenem/REL fixed‐dose combination for individuals with renal impairment and demonstrate a lack of clinically relevant interactions with organic anion transporter inhibitors.
1. INTRODUCTION
Gram‐negative pathogens are a common cause of intra‐abdominal infections, urinary tract infections, pneumonia and bacteraemia.1, 2 Resistance to antibacterial agents is considered a global health threat by the World Health Organization and is associated with increased mortality, hospital length of stay, hospital readmission and cost.3, 4, 5, 6, 7 New β‐lactam/β‐lactamase inhibitor combinations are being developed in an effort to outpace increasing antibacterial resistance.
Carbapenems are effective against many Gram‐negative pathogens, including several extended‐spectrum β‐lactamase producers.8 Imipenem is a broad‐spectrum carbapenem antibiotic that is coformulated with cilastatin, a dehydropeptidase inhibitor used to prevent degradation of imipenem.9, 10 Resistance to imipenem/cilastatin (IMI) and rates of infection with carbapenem‐resistant bacteria are increasing, highlighting the need for novel agents.8, 11
Relebactam (REL) is a novel class A and C β‐lactamase inhibitor that is in development in combination with IMI for treatment of Gram‐negative bacterial infections. The IMI/REL combination has been evaluated in 2 completed phase 2 clinical trials (NCT01505634 and NCT01506271), a completed phase 3 clinical trial (NCT02452047) and 1 ongoing phase 3 trial (NCT02493764).12, 13, 14, 15 The addition of REL has been shown to restore the in vitro activity of imipenem against the majority of carbapenem‐nonsusceptible isolates of Pseudomonas aeruginosa and Enterobacteriaceae (new taxonomy: Enterobacterales).16, 17 In phase 2 clinical studies, REL coadministered with IMI (IMI/REL) for the treatment of participants with complicated urinary tract infection and intra‐abdominal infection was efficacious and well tolerated and had a safety profile similar to that of IMI alone.12, 13
Several studies have contributed to what is known of the pharmacokinetic (PK) profile of the IMI/REL combination. A PK, safety and tolerability study of IMI/REL reported no drug–drug interactions among the 3 individual components and that the apparent terminal half‐lives (t1/2) of REL, imipenem and cilastatin were comparable, providing evidence that coadministration is appropriate.18 It was also demonstrated that urinary excretion was the major route of elimination for REL, similar to what was observed for imipenem and cilastatin,19, 20 providing rationale for characterizing the impact of renal impairment (RI) on the PK of REL and the IMI/REL combination. In addition, both REL and imipenem undergo active renal secretion and REL was shown to be a substrate of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1027)21; therefore, it is important to determine if potential drug–drug interactions with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4357, a prototypic OAT inhibitor, have an impact on exposure.22, 23, 24 In a previous PK study, coadministration of imipenem with probenecid resulted in a mild increase (15%) in drug exposure.25 The impact of probenecid on REL and imipenem exposure when administered as IMI/REL was therefore evaluated.
We present the results of 2 phase 1 studies that investigated the PK of a single intravenous (IV) dose of IMI/REL in participants with impaired renal function (Study PN005) and the impact of probenecid on the PK of REL and imipenem when coadministered with IMI/REL in healthy adults (Study PN019).
2. METHODS
2.1. Study designs
Study PN005 was an open‐label, single‐dose, 2‐period study that compared the PK of a 125‐mg dose of REL in combination with a 250‐mg IV dose of IMI in participants with impaired renal function with that of healthy controls (matched for age, sex and body mass index [BMI]). Participants were grouped according to degree of RI as follows: mild RI (estimated glomerular filtration rate [eGFR] >50 to <80 mL/min/1.73m2), moderate RI (eGFR 30 to 50 mL/min/1.73m2), severe RI (eGFR <30 mL/min/1.73m2), end‐stage renal disease (ESRD) requiring haemodialysis (HD) and healthy controls (eGFR ≥80 mL/min/1.73m2). Confirmatory analyses were conducted with participants grouped according to the National Kidney Foundation Dialysis Outcome Initiative (K/DOQI) renal function classification with additional confirmatory analyses conducted with participants stratified by absolute GFR (not normalized to body surface area), according to European Medicines Agency (EMA) Guidance (Supplemental Table 1).26
The primary objective of Study PN005 was to investigate the effect of RI on plasma PK of REL and the extent to which REL was removed from plasma by HD, following IMI/REL administration. Secondary objectives were to evaluate the safety and tolerability of a single dose of IMI/REL in participants with RI; obtain plasma PK data for IMI following IMI/REL administration in participants with RI compared with healthy adults; evaluate urinary excretion of REL, imipenem and cilastatin in participants with RI compared with healthy adults; and evaluate the relationship between eGFR and PK parameters.
Study PN005 consisted of 8 panels of 6 participants: Panel A, mild RI; Panel B, healthy controls; Panel C, moderate RI; Panel D, healthy controls; Panel E, severe RI; Panel F, healthy controls; Panel G, ESRD‐HD; Panel H, healthy controls. Participants in Panels A to F and H were treated with a single dose of IMI/REL followed by 14 h of plasma sampling for PK analysis (Figure 1). Participants in Panel G participated in 2 dosing periods. In Period 1, participants with ESRD‐HD received a single dose of IMI/REL immediately following their normally scheduled HD with up to 14 h of plasma collection for PK measurements. In Period 2, the same 6 participants with ESRD‐HD received a single dose of IMI/REL. Study drug infusion started 30 min prior to their normally scheduled HD. Plasma samples and dialysate were collected predialysis, postdialysis and every 30 min during HD.
Figure 1.
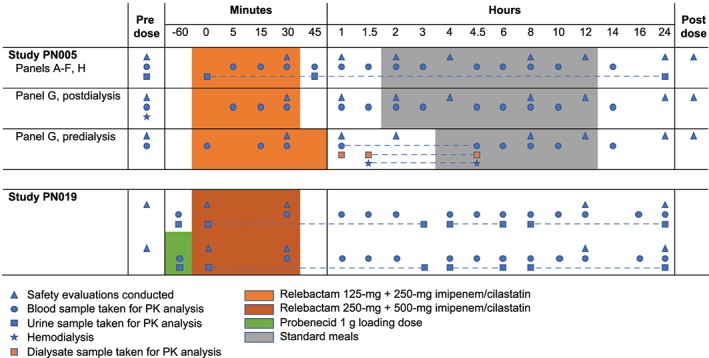
Study designs. Healthy control: estimated glomerular filtration rate (eGFR) ≥80 (panels B, D, F, H); mild renal insufficiency: 50 < eGFR < 80 mL/min/1.73m2 (panel A); moderate renal insufficiency: 30 ≤ eGFR ≤ 50 mL/min/1.73m2 (panel C); severe renal insufficiency: eGFR < 30 mL/min/1.73m2 (panel E); end‐stage renal disease requiring haemodialysis: eGFR < 15 mL/min/1.73m2 (panel G)
Study PN019 was an open‐label, randomized, 2‐period, crossover study consisting of 14 participants that compared the PK of a single IMI/REL (500 mg/250 mg) dose with or without a prior 1‐g dose of probenecid (Figure 1). On Day 1 of each period, participants were randomized to receive a single IV dose of IMI/REL or a single oral dose of probenecid 1 h prior to a single IV dose of IMI/REL. Each IV infusion was administered over 30 min, following an overnight fast of ≥8 h. Washout was 7 days between each period.
The primary objective of Study PN019 was to determine the potential impact of OAT3 inhibition on the renal secretion of REL, an OAT3 substrate, by evaluating the effect of probenecid (an OAT1/3 inhibitor) on the plasma PK of REL (administered as IMI/REL), as measured by area under the concentration–time curve from time 0 to infinity (AUC0‐∞) in healthy adults.21 Probenecid is a commonly used probe for determining the PK effect of OAT1/OAT3 inhibition on investigational compounds and was selected based on US Food and Drug Administration guidance.27 Secondary endpoints included the effect of probenecid coadministered with IMI/REL on plasma PK of imipenem, renal clearance of REL and imipenem and safety.
These studies were conducted using standards established by the International Conference on Harmonisation E6 Good Clinical Practice Guidelines, and in compliance with all local and/or national regulations and directives. Study PN005 was given initial institutional review board approval of the protocol and informed consent form on 21 December 2010, by Independent Investigational Review Board (IIRB Inc., Plantation, FL, USA). Study PN019 was granted approval of the protocol and informed consent form on 7 September 2016, by Chesapeake institutional review board (Columbia, MD, USA). IUPHAR/BPS nomenclature classification is used in this publication.28
2.2. Participants
In Study PN005, eligible participants included adults (aged 18–75 years) who weighed ≥60 kg with a BMI ≤40 kg/m2, were in stable health (good health for healthy controls) and had no clinically significant abnormality on electrocardiogram (ECG). For Panels A–D, participants could be smokers, but smoking was limited to 10 cigarettes/d. For Panels E–H, participants had to be nonsmokers. Participants in Panels A, C, E and G had to have a clinical diagnosis of renal insufficiency and have mild RI (Panel A), moderate RI (Panel C), severe RI (Panel E), or ESRD‐HD (Panel G). Healthy controls had to have BMI within 5 kg/m2 and age within 10 years of the matched renal participant. Women were allowed to participate if they were postmenopausal or agreed to use birth control. Participants were excluded if they had: history of stroke, chronic seizures or major neurological disorders; clinically significant endocrine, gastrointestinal, cardiovascular, haematological, hepatic, immunological, renal (healthy controls only), respiratory or genitourinary abnormalities or disease; history of malignant neoplastic disease; history of multiple and/or severe allergies or had an anaphylactic reaction or intolerance to prescription or nonprescription drugs or food; personal history of hypersensitivity to IMI or other β‐lactam antibiotics; or without adequate venous access were excluded. Nursing mothers, participants who had a kidney removed or had a renal transplant, participants who consume excessive amounts of alcohol (>3 glasses/d) or caffeine (>6 servings/d), and participants who regularly use illicit drugs were also excluded.
In Study PN019, eligible participants included healthy adults (aged 18–55 years) with a BMI between 18.5 and 32.0 kg/m2, haemoglobin ≥12 g/dL for men and ≥11 g/dL for women and were nonsmokers. Women of childbearing potential and nonvasectomized men could enrol if they followed permitted birth control methods.
2.3. PK samples
Whole blood samples were collected at the protocol‐specified time points into K2EDTA collection tubes, inverted 6 times and processed for the analysis of REL, imipenem and cilastatin plasma concentrations.18 Immediately after collection, the blood‐containing tubes were placed on ice and the samples were centrifuged within 45 min of collection between 1000–1300 g at 4°C for 10 min. Immediately after separation of the whole blood, the plasma (1.0 mL) was transferred into an ice‐cold 3.6‐mL internally threaded Nunc cryotube containing an equal amount of plasma stabilizer solution (2‐[N‐morpholino]ethanesulfonic acid). The cryotube was capped and inverted at least 6 times to mix the plasma with the stabilizer. The tube was then stored at –70°C until shipment. Freezing of the plasma sample occurred within 60 min of the blood draw.18 In Study PN005, in all panels except Panel G (ESRD‐HD), urine samples were collected at selected timed intervals over 24 h in each treatment period. The entire void was collected and weighed, and the weight was recorded. A 1.0‐mL aliquot of the weighed urine specimen was transferred to a 3.6‐mL Nunc cryotube containing 1.0 mL of urine stabilizer solution and mixed. For Panel G Period 2, dialysate samples were obtained predialysis, postdialysis and every 30 min during HD. A 4‐mL sample of dialysate was promptly separated and placed in round‐bottom polypropylene tubes with screw caps. A 1‐mL aliquot of dialysate was transferred to a 3.6‐mL Nunc cryotube containing 1.0 mL of urine stabilizer solution. Both urine and dialysate samples were stored frozen at –70°C until shipment to the research laboratories of Merck & Co., Inc. (West Point, PA, USA) for determination of REL, imipenem and/or cilastatin.
In Study PN019, whole blood samples were collected at predose and at selected timed intervals over 24 h following study drug administration. Urine samples were collected prior to the start of infusion and 0–3, 3–6 and 6–24 h following the start of infusion. Blood samples for creatinine clearance (CrCl) were taken at midpoint of each 24‐h urine collection (i.e. approximately 12 h following the start of infusion for each period). Blood and urine samples were processed in the same manner as for Study PN005.
A validated high‐performance liquid chromatographic tandem mass spectrometric method was used for determination of plasma, urine and dialysate concentrations of REL, imipenem and cilastatin. For plasma and urine, the analytical range was 0.25–100 μg/mL and the lower limit of quantification (LLOQ) for all analytes was 0.25 μg/mL. For dialysate, the analytical range was 10.0–2500 ng/mL and the LLOQ for all analytes was 10.0 ng/mL. Details of the accuracy and precision of the assays for each of the analytes are reported in Supplemental Table 2.
2.4. Safety
In both studies, all safety and tolerability parameters were monitored through the duration of dosing and continued through approximately 14 days after the last dose. In Study PN005, safety and tolerability of REL was monitored by clinical assessment of adverse events (AEs) and by repeated measurements of vital signs, physical examinations, 12‐lead ECG, assessment of local IV tolerability and standard laboratory safety tests (haematology, chemistry and urinalysis). Laboratory parameters that indicate renal function (serum/urine creatinine, serum urea, urine protein and urine albumin) and hepatic function (serum bilirubin, alanine transaminase, aspartate transaminase) were carefully monitored during the study. In Study PN019, safety assessments included all types of AEs and were determined by repeated measurements of vital signs, physical examinations, 12‐lead ECG and standard laboratory safety tests (haematology, chemistry and urinalysis) obtained at prespecified time points.
2.5. PK parameter analyses
Plasma and urine PK parameters of REL, imipenem and cilastatin were calculated as previously described.18 Briefly, a noncompartmental approach using Phoenix WinNonlin version 6.3 (Certara LP, Princeton, NJ, USA) was used to assess concentration–time data. The linear‐up/log‐down trapezoidal method was used to determine AUC. Plasma concentration–time data were summarized using the concentration at the end of infusion (CEOI); AUC0‐∞ (AUC0‐∞ was calculated as the sum of AUC0‐last and estimated last measurable concentration [Cest,last]/λz, where AUC0‐last is the AUC from time 0 to the time of the last measurable concentration, Cest,last is the estimated last measurable concentration and λz is the apparent first‐order terminal elimination rate constant calculated from the slope of the linear regression of the terminal log‐linear portion of the plasma concentration–time profile); t1/2 (calculated as quotient of the natural log of 2 and the apparent terminal rate constant [λz]); clearance (calculated as dose/AUC0‐∞); and volume of distribution (calculated as AUC0‐∞ × λz). Urine PK data were summarized using the amount of unchanged drug excreted in urine during a collection interval (Ae), fraction of dose excreted in urine (fe; calculated as Ae/dose) and renal clearance (calculated as Ae/AUCτ, where AUCτ is the AUC from 0 to t h [0–24 h for single dose]). Drug elimination through dialysis was summarized using dialysis clearance and the extraction coefficient for REL, imipenem and cilastatin.
In Study PN019, plasma REL and imipenem concentrations were summarized using the following PK parameters: AUC0‐∞, AUC0‐last, CEOI, clearance, time to last quantifiable concentration, time to maximum concentration, t1/2, apparent volume of distribution estimated at steady state and volume of distribution. Urine concentrations of REL and imipenem were summarized using the following PK parameters: Ae from 0–3 h, from 3–6 h, from 6–24 h, from 0–6 h, from 0–24 h, renal clearance and fe. Meaningful changes in cilastatin PK are generally expected to result in alterations in the renal clearance of imipenem, as cilastatin inhibits the metabolism of imipenem in the kidney to maintain effective imipenem concentrations in urine. As alterations of cilastatin PK with the administration of probenecid would thus be reflected in imipenem PK, cilastatin PK was not analysed in PN019. For the purposes of these 2 phase 1 studies, meaningful changes in PK were considered exposures that would be expected to have a discernible pharmacodynamic impact from a clinical perspective.
2.6. Statistical analysis
In Study PN005, a linear fixed‐effects model was used for analysis of REL, imipenem and cilastatin natural log transformed values of AUC0‐∞ after a single dose of IMI/REL. The model contained factors for type (mild RI, moderate RI, severe RI, ESRD dosed prior to HD, ESRD dosed after HD and healthy matched controls), categorical covariates for sex, and continuous covariates for age and BMI. Point estimates and 90% confidence intervals (CIs) for the AUC0‐∞ ratio of the geometric least‐squares means (GMR; RI/healthy controls) was computed from the above model. Geometric mean (GM) and 95% CIs were provided for each type. Other PK parameters (CEOI, clearance, volume of distribution) were evaluated similarly to AUC0‐∞. Summary statistics, including GM and % coefficient of variation (CV), were provided for t1/2.
In Study PN019, a linear mixed‐effects model with fixed‐effects terms for treatment and period was used for REL and imipenem natural log transformed values for AUC0‐∞ after a single dose of IMI/REL with or without probenecid. A 90% CI was constructed for the difference in treatment least‐squares means ([IMI/REL + probenecid] – IMI/REL alone) on the log scale for AUC0‐∞. Exponentiation of the log‐scale 90% CI provided a 90% CI for the AUC0‐∞ GMR (IMI/REL + probenecid/IMI/REL alone).
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
3.1. Participants
Participants (n = 49) enrolled in Study PN005 had a median age of 64 years, 71% were white and 57% were men (Table 1). Patient characteristics were similar across panels, with the exception of Panels G and H, which were comprised of younger patients of black race. The healthy adults (n = 14) enrolled in Study PN019 had a median age of 46 years, 93% were white and 50% were men (Table 1).
Table 1.
Study PN005 and Study PN019 demographic and baseline characteristics
| Age, y | Sex, n (%) | Ethnicity, n (%) | Race, n (%) | eGFR, mL/min/1.73m2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Panel | n | Mean (SD) | Median (range) | Male | Female | Not Hispanic or Latino | Hispanic or Latino | White | Black | Asian | Mean (range) |
| PN005 | Aa | 7f | 71.1 (3.4) | 72.0 (66–75) | 4 (57.1) | 3 (42.9) | 3 (42.9) | 4 (57.1) | 7 (100) | 0 | 0 | 63.4 (54.0–71.8) |
| Bb | 6 | 64.5 (3.9) | 64.5 (58–70) | 4 (66.7) | 2 (33.3) | – | – | 6 (100) | 0 | 0 | 94.6 (75.2–111.5) | |
| Cc | 6 | 68.3 (5.0) | 68.5 (60–75) | 3 (50.0) | 3 (50.0) | 5 (83.3) | 1 (16.7) | 5 (83.3) | 1 (16.7) | 0 | 38.0 (30.1–49.0) | |
| Db | 6 | 64.2 (5.7) | 63.5 (57–74) | 3 (50.0) | 3 (50.0) | – | – | 5 (83.3) | 1 (16.7) | 0 | 91.3 (65.7–123.4) | |
| Ed | 6 | 62.8 (7.5) | 63.5 (52–74) | 3 (50.0) | 3 (50.0) | 2 (33.3) | 4 (66.7) | 6 (100) | 0 | 0 | 20.5 (10.9–28.6) | |
| Fb | 6 | 60.5 (6.4) | 58 (54–69) | 3 (50.0) | 3 (50.0) | – | – | 6 (100) | 0 | 0 | 92.6 (83.7–103.4) | |
| Ge | 6 | 44.5 (7.9) | 46.0 (34–54) | 4 (66.7) | 2 (33.3) | 6 (100) | 0 | 0 | 6 (100) | 0 | 8.0 (5.8–11.0) | |
| Hb | 6 | 41.5 (11.5) | 45 (26–52) | 4 (66.7) | 2 (33.3) | – | – | 0 | 6 (100) | 0 | 98.1 (81.0–119.9) | |
| PN019 | 14 | 43.8 (8.8) | 45.5 (23–55) | 7 (50.0) | 7 (50.0) | 5 (35.7) | 9 (64.3) | 13 (92.9) | 0 | 1 (7.1) | N/A | |
Mild renal insufficiency: 50 < eGFR < 80 mL/min/1.73m2.
Healthy control: eGFR ≥ 80 mL/min/1.73m2.
Moderate renal insufficiency: 30 ≤ eGFR ≤ 50 mL/min/1.73m2.
Severe renal insufficiency: eGFR < 30 mL/min/1.73m2.
End‐stage renal disease requiring haemodialysis: eGFR <15 mL/min/1.73m2.
One additional participant was enrolled but was discontinued without having received a dose.
eGFR, estimated glomerular filtration rate; N/A, not available; SD, standard deviation.
3.2. Plasma PK
3.2.1. Effect of renal impairment
In Study PN005, PK parameters were assessed following a 30‐min infusion of a single 125‐mg dose of REL coadministered with a 250‐mg IV dose of IMI in participants with impaired renal function and healthy matched controls (Table 2 , Figure 2). For REL, exposure (AUC0‐∞) increased significantly and progressively as degree of RI increased from mild RI to ESRD‐HD, but was relatively constant across groups of healthy matched controls. The AUC0‐∞ GMR (90% CI) for RI/healthy matched controls was 1.6 (1.1, 2.4) for mild RI, 2.2 (1.5, 3.2) for moderate RI and 4.9 (3.4, 7.0) for severe RI. For ESRD‐HD patients, the AUC0‐∞ GMR (90% CI) was 9.3 (6.5, 13.5) postdialysis and 1.8 (1.2, 2.6) predialysis.
Table 2.
Study PN005: plasma pharmacokinetic parameters following a single intravenous dose of 125 mg relebactam with 250 mg imipenem/cilastatin in patients with impaired renal function and matched healthy controls
| Panel | RI status | n | CEOI, μMa | AUC0‐∞, μM ha | t1/2, hb | CL, mL/mina | Vz, La |
|---|---|---|---|---|---|---|---|
| REL | |||||||
| A | Mildc | 6 | 22.4 (13.0, 38.7) | 73.5 (50.0, 108) | 2.63 (26.4) | 81.3 (55.3, 119) | 21.4 (17.6, 26.1) |
| B | Normald | 6 | 20.4 (12.8, 32.7) | 45.0 (32.4, 62.6) | 1.75 (16.6) | 133 (95.5, 185) | 21.6 (18.2, 25.5) |
| C | Moderatee | 6 | 23.5 (14.0, 39.4) | 115 (79.8, 165) | 4.51 (25.7) | 52.1 (36.2, 74.9) | 22.2 (18.4, 26.7) |
| D | Normald | 6 | 22.5 (14.1, 35.9) | 52.3 (37.7, 72.7) | 2.10 (31.0) | 114 (82.3, 159) | 21.9 (18.5, 25.9) |
| E | Severef | 6 | 23.6 (15.0, 37.2) | 236 (171, 325) | 8.65 (31.0) | 25.3 (18.4, 34.9) | 20.1 (17.1, 23.7) |
| F | Normald | 6 | 18.1 (11.6, 28.4) | 48.5 (35.3, 66.4) | 2.00 (10.4) | 123 (90.0, 169) | 22.4 (19.0, 26.3) |
| G, postdialysish | ESRD‐HDg | 6 | 53.1 (30.7, 91.9) | 414 (280, 612) | 15.6 (103.1) | 14.4 (9.77, 21.3) | 16.2 (13.3, 19.8) |
| G, predialysisi | ESRD‐HDg | 6 | 19.3 (11.2, 33.4) | 78.0 (50.3, 121) | 10.5 (100.6) | 76.6 (49.4, 119) | 55.7 (44.5, 69.7) |
| H | Normald | 6 | 22.7 (12.5, 41.0) | 44.5 (29.1, 67.9) | 1.79 (13.9) | 135 (88.1, 206) | 17.0 (13.6, 21.1) |
| Imipenem | |||||||
| A | Mildc | 6 | 40.7 (22.7, 73.2) | 77.3 (58.9, 101) | 1.54 (15.2) | 180 (137, 236) | 21.1 (15.8, 28.2) |
| B | Normald | 6 | 35.3 (21.3, 58.5) | 55.0 (43.5, 69.5) | 1.24 (10.8) | 253 (200,320) | 26.1 (20.4, 33.5) |
| C | Moderatee | 6 | 45.6 (26.2, 79.5) | 101 (77.9, 130) | 2.18 (12.8) | 138 (107, 179) | 22.3 (17.0, 29.3) |
| D | Normald | 6 | 42.6 (25.8, 70.4) | 66.0 (52.2, 83.3) | 1.40 (21.1) | 211 (167, 266) | 23.4 (18.3, 30.0) |
| E | Severef | 6 | 46.9 (28.7, 76.5) | 160 (127, 201) | 2.78 (11.9) | 87.0 (69.4, 109) | 20.0 (15.7, 25.4) |
| F | Normald | 6 | 35.5 (21.9, 57.6) | 63.8 (51.0, 79.9) | 1.32 (5.8) | 218 (174, 273) | 24.8 (19.6, 31.5) |
| G, postdialysish | ESRD‐HDg | 6 | 103 (57.1, 186) | 223 (169, 293) | 3.24 (18.7) | 62.5 (47.5, 82.1) | 20.5 (15.4, 27.4) |
| G, predialysisi | ESRD‐HDg | 6 | 35.9 (19.9, 64.7) | 71.2 (54.2, 93.6) | 3.20 (47.8) | 195 (149, 257) | 63.3 (47.3, 84.6) |
| H | Normald | 6 | 41.8 (22.1, 79.1) | 71.8 (53.4, 96.5) | 1.21 (13.7) | 194 (144, 261) | 24.9 (18.2, 34.1) |
| Cilastatin | |||||||
| A | Mildc | 6 | 43.4 (25.9, 72.7) | 71.7 (45.8, 112) | 1.43 (31.9) | 162 (104, 254) | 19.2 (14.8, 24.9) |
| B | Normald | 6 | 34.8 (22.3, 54.4) | 44.8 (30.5, 65.9) | 1.08 (27.8) | 259 (176, 381) | 23.9 (19.1, 30.0) |
| C | Moderatee | 6 | 48.7 (29.8, 79.4) | 100 (65.4, 153) | 2.11 (20.5) | 116 (76.1, 178) | 19.4 (15.2, 24.9) |
| D | Normald | 6 | 42.9 (27.5, 66.8) | 53.6 (36.6, 78.6) | 1.19 (28.5) | 217 (148, 318) | 21.4 (17.1, 26.7) |
| E | Severef | 6 | 53.3 (34.6, 82.0) | 300 (207, 436) | 5.08 (59.2) | 38.7 (26.7, 56.2) | 16.9 (13.6, 21.0) |
| F | Normald | 6 | 35.8 (23.4, 54.8) | 53.7 (37.2, 77.6) | 1.09 (10.7) | 217 (150, 313) | 21.2 (17.1, 26.2) |
| G, postdialysish | ESRD‐HDg | 6 | 111 (65.9, 186) | 777 (493, 1220) | 12.2 (118.5) | 15.0 (9.50, 23.6) | 15.9 (12.2, 20.7) |
| G, predialysisi | ESRD‐HDg | 6 | 41.7 (24.8, 70.0) | 205 (123, 343) | 12.2 (131.5) | 56.6 (33.9, 94.4) | 59.1 (43.9, 79.6) |
| H | Normald | 6 | 44.5 (25.4, 78.0) | 56.5 (34.5, 92.6) | 1.14 (26.1) | 206 (126, 337) | 21.0 (15.8, 28.0) |
Back‐transformed least squares mean and 95% confidence interval from linear fixed‐effects model performed on natural log‐transformed values.
Geometric mean and percent geometric coefficient of variation (CV) reported for apparent t1/2.
50 < eGFR < 80 mL/min/1.73m2.
eGFR ≥ 80 mL/min/1.73m2.
30 ≤ eGFR ≤ 50 mL/min/1.73m2.
eGFR < 30 mL/min/1.73m2.
eGFR <15 mL mL/min/1.73m2, requiring haemodialysis.
For the postdialysis value, participants with ESRD‐HD received a single dose of imipenem/cilastatin/REL immediately following their normally scheduled HD and plasma collection for PK measurements occurred up to 14 h postdialysis.
For the predialysis value, the same 6 participants with ESRD‐HD received a single dose of imipenem/cilastatin/REL and plasma collection for PK measurements occurred at 4 time points prior to scheduled HD.
AUC, area under the concentration–time curve; CEOI, concentration at end of infusion; CL, clearance; eGFR, estimated glomerular filtration rate; ESRD‐HD, end‐stage renal disease requiring haemodialysis; GM, geometric mean; GMR, geometric mean ratio; REL, relebactam; RI, renal impairment; SD, standard deviation; t1/2, apparent terminal half‐life; Vz, volume of distribution.
Figure 2.
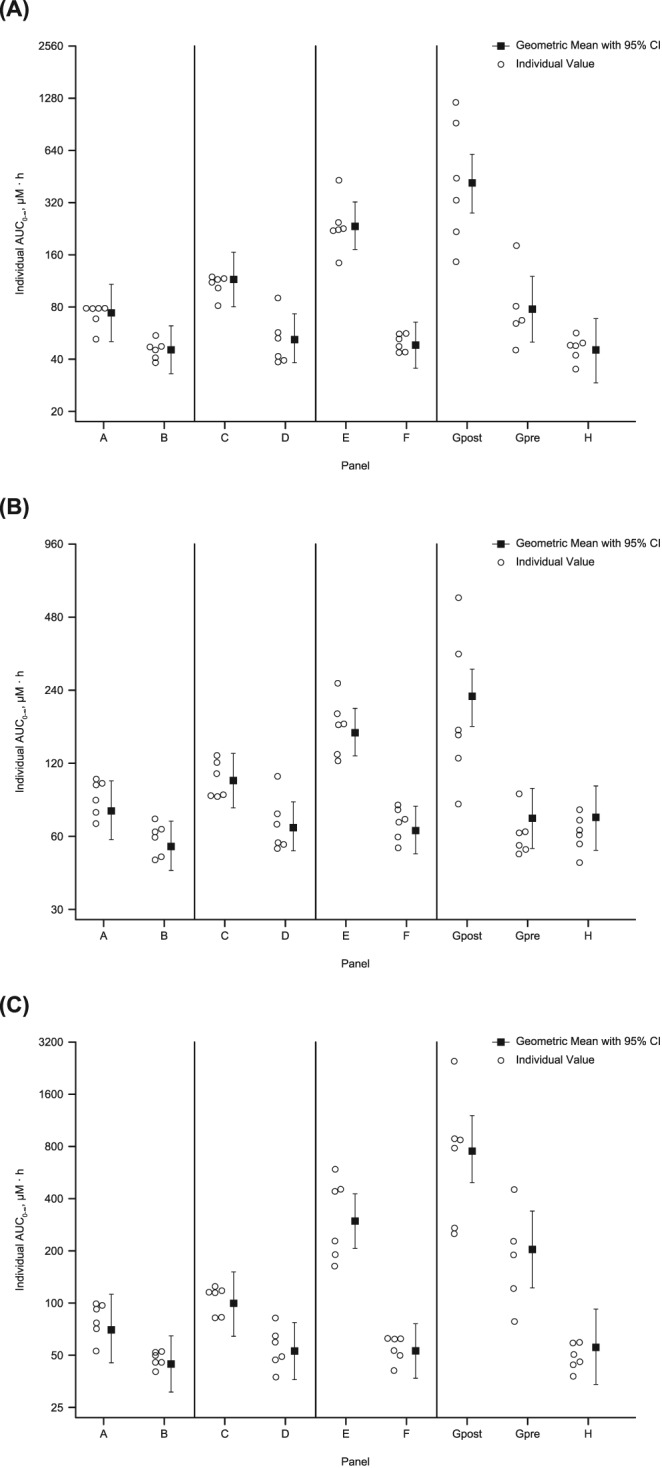
Study PN005. Plasma exposures vs time profiles following administration of a single 125‐mg dose of relebactam (REL) with 250‐mg imipenem/cilastatin. Plasma exposures of (A) REL, (B) imipenem and (C) cilastatin. A, mild renal impairment (RI): 50 < estimated glomerular filtration rate (eGFR) < 80 mL/min/1.73m2; B, healthy participants matched to mild RI: eGFR ≥80 mL/min/1.73m2; C, moderate RI: 30 ≤ eGFR ≤ 50 mL/min/1.73m2; D, healthy participants matched to moderate RI: eGFR ≥ 80 mL/min/1.73m2; E, severe RI: eGFR < 30; F, healthy participants matched to severe RI: eGFR ≥ 80 mL/min/1.73m2; Gpost, end‐stage renal disease requiring haemodialysis (post‐dialysis): eGFR < 15 mL/min/1.73m2; Gpre, end‐stage renal disease requiring haemodialysis (pre‐dialysis): eGFR < 15 mL/min/1.73m2; H, healthy participants matched to end‐stage renal disease: eGFR ≥80 mL/min/1.73m2; RI, renal impairment; CI, confidence interval
Imipenem and cilastatin exposure also significantly and progressively increased with increasing degree of RI, but was relatively constant across groups of healthy matched controls (Table 2 , Figure 2). For imipenem, the AUC0‐∞ GMR (90% CI) for RI/healthy matched controls was 1.4 (1.1, 1.8) for mild RI, 1.5 (1.2, 2.0) for moderate RI and 2.5 (1.9, 3.3) for severe RI. For ESRD‐HD patients, the AUC0‐∞ GMR (90% CI) was 3.1 (2.4, 4.0) postdialysis and 1.0 (0.8, 1.3) predialysis. For cilastatin, the AUC0‐∞ GMR (90% CI) for RI/healthy matched controls was 1.6 (1.0, 2.5) for mild RI, 1.9 (1.2, 2.9) for moderate RI and 5.6 (3.6, 8.6) for severe RI. For ESRD‐HD patients, the AUC0‐∞ GMR (90% CI) was 13.8 (9.0, 21.1) postdialysis and 3.6 (2.3, 5.7) predialysis.
For all 3 analytes, plasma clearance decreased and corresponding t1/2 increased with increasing degree of RI (Table 2). RI had no clinically relevant effect on CEOI or volume of distribution for any of the analytes. Similar results were obtained when PK parameters were assessed using the K/DOQI renal function classification (Supplemental Table 3) and EMA guidance (Supplemental Table 4).
3.2.2. Effect of probenecid coadministration
In Study PN019, the PK of REL and imipenem were not meaningfully changed when dosed as IMI/REL alone or in combination with a 1‐g dose of probenecid (Table 3 , Figure 3). The AUC0‐∞ GMR (90% CI) for IMI/REL + probenecid/IMI/REL comparison was 1.24 (1.19, 1.28) for REL and 1.16 (1.13, 1.20) for imipenem, indicating probenecid coadministration did not meaningfully alter REL or imipenem PK.
Table 3.
Study PN019 plasma pharmacokinetic parameters following administration of a single dose of 250 mg relebactam and 500 mg imipenem/cilastatin with or without probenecid
| Parameter | |||||||
|---|---|---|---|---|---|---|---|
| n | CEOI, μMa , b | AUC0‐∞, μM·hb | t1/2, hc | CL, L/hb | Vz, Lc | ||
| REL | |||||||
| GM (95% CI) | IMI/REL | 13 | 47.7 (43.6, 52.2) | 78.9 (71.9, 86.5) | 1.72 (17.0) | 9.10 (8.29, 9.99) | 22.8 (20.4) |
| IMI/REL + P | 14 | 50.6 (45.2, 56.5) | 97.5 (87.2, 109) | 2.04 (13.0) | 7.36 (6.59, 8.23) | 21.7 (21.2) | |
| GMR (90% CI) | IMI/REL + P/IMI/REL | 1.06 (1.00, 1.12) | 1.24 (1.19, 1.28) | – | 0.81 (0.78, 0.84) | – | |
| Imipenem | |||||||
| GM (95% CI) | IMI/REL | 13 | 104 (94.5, 115) | 131 (118, 145) | 1.14 (8.9) | 12.8 (11.5, 14.2) | 21.3 (17.8) |
| IMI/REL + P | 14 | 112 (99.0, 127) | 152 (135, 172) | 1.28 (12.2) | 11.0 (9.74, 12.4) | 20.3 (21.1) | |
| GMR (90% CI) | IMI/REL + P/IMI/REL | 1.07 (1.01, 1.13) | 1.16 (1.13, 1.20) | – | 0.86 (0.83, 0.89) | – | |
Concentrations for this study are expressed in μM; to convert to mg h/L, multiply by 0.34848 for REL and 0.29937 for imipenem.
Back‐transformed least squares mean and confidence interval from linear mixed‐effect model performed on natural‐log transformed values.
GM (percent coefficient of variation) reported for t1/2 and Vz.
AUC, area under the concentration–time curve; CEOI, concentration at end of infusion; CI, confidence interval; CL, clearance; GM, geometric least‐squares mean; GMR, geometric least‐squares mean ratio; IMI/REL, REL coadministered with imipenem/cilastatin; IMI/REL + P, IMI/REL coadministered with probenecid; REL, relebactam; Vz, volume of distribution.
Figure 3.
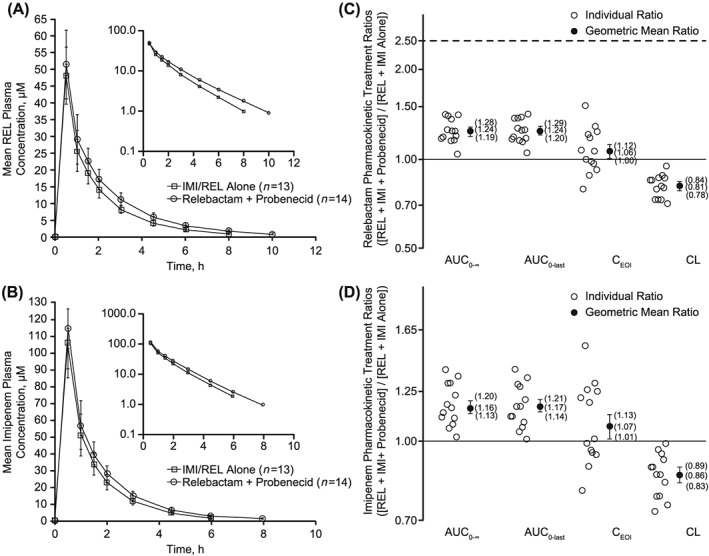
Study PN019. Arithmetic mean plasma concentration of (A) relebactam (REL) or (B) imipenem over time following administration of a single 250‐mg intravenous (IV) dose of REL coadministered with 500 mg IMI with or without 1 g probenecid in healthy adults, and plasma pharmacokinetic treatment ratios (REL + imipenem/cilastatin + probenecid/REL + imipenem/cilastatin alone) for (C) REL and (D) imipenem. AUC, area under the concentration–time curve; CEOI, concentration at end of infusion; CL, clearance; IMI, imipenem/cilastatin; REL, relebactam
The GMRs (90% CIs) for CEOI and plasma clearance were 1.06 (1.00, 1.12) and 0.81 (0.78, 0.84), respectively, for REL and 1.07 (1.01, 1.13) and 0.86 (0.83, 0.89), respectively, for imipenem. The observed GM apparent t1/2 of REL was slightly prolonged following probenecid coadministration (2.04 h) compared with IMI/REL alone (1.72 h), whereas the t1/2 of imipenem was comparable between both treatments (~1 h). The GM volume of distribution was similar between both treatments REL (~22 L) and imipenem (~20 L).
3.3. Urinary PK
3.3.1. Effect of renal impairment
In Study PN005, the urine PK results for REL, imipenem and cilastatin confirmed the effect of RI on plasma PK, with renal clearance decreasing progressively from mild to severe RI (Table 4). As expected, the renal clearance for REL, imipenem and cilastatin in RI was consistently lower than in healthy matched controls. The fe in the urine also decreased progressively from mild to severe RI.
Table 4.
Summary of urine pharmacokinetic parameters following administration of a single dose of 125 mg relebactam with 250 mg imipenem/cilastatin (Study PN005), or a single dose of 250 mg REL with 500 mg imipenem/cilastatin with or without coadministration with probenecid (Study PN019)
| Parameter, GM (%CV) | ||||||
|---|---|---|---|---|---|---|
| Panela | RI status | n | CLR, mL/min | Fe, % | Ae, mg | |
| REL | ||||||
| Study PN005 | A | Mildb | 6 | 69.8 (16.5) | 79.4 (7.4) | 99.2 (7.4) |
| B | Normalc | 6 | 118 (19.8) | 83.3 (17.6) | 104 (17.6) | |
| C | Moderated | 6 | 38.4 (28.8) | 60.3 (28.9) | 75.4 (28.9) | |
| D | Normalc | 6 | 110 (36) | 86.2 (15.3) | 108 (15.3) | |
| E | Severee | 6 | 22.3 (47.2) | 59.1 (20.7) | 73.9 (20.7) | |
| F | Normalc | 6 | 107 (20.9) | 81.8 (10.1) | 102 (10.1) | |
| H | Normalc | 6 | 110 (20) | 78.0 (3.9) | 97.5 (3.9) | |
| Study PN019 | IMI/REL | – | 12 | 7.82 (7.09, 8.63) | – | – |
| IMI/REL + P | – | 14 | 5.87 (4.89, 7.05) | – | – | |
| IMI/REL + P/IMI/REL | – | 0.75 (0.68, 0.83) | – | – | ||
| Imipenem | ||||||
| Study PN005 | A | Mildb | 6 | 75.0 (14.6) | 45.0 (12) | 112 (12) |
| B | Normalc | 6 | 115 (18.4) | 46.5 (25.3) | 116 (25.3) | |
| C | Moderated | 6 | 41.1 (23.8) | 28.9 (38.4) | 72.3 (38.4) | |
| D | Normalc | 6 | 109 (29.2) | 51.0 (14.6) | 128 (14.6) | |
| E | Severee | 6 | 17.4 (44.7) | 20.2 (17.1) | 50.6 (17.1) | |
| F | Normalc | 6 | 104 (10.5) | 48.9 (11.6) | 122 (11.6) | |
| H | Normalc | 6 | 99.1 (22.3) | 42.2 (11.4) | 105 (11.4) | |
| Study PN019 | IMI/REL | – | 12 | 6.81 (6.06, 7.64) | – | – |
| IMI/REL + P | – | 14 | 4.72 (3.72, 5.99) | – | – | |
| IMI/REL + P/IMI/REL | – | 0.69 (0.60, 0.80) | – | – | ||
| Cilastatin | ||||||
| Study PN005 | A | Mildb | 6 | 99.4 (29.1) | 67.1 (9.5) | 168 (9.5) |
| B | Normalc | 6 | 144 (21.9) | 56.7 (26.4) | 142 (26.4) | |
| C | Moderated | 6 | 59.6 (29.4) | 52.9 (25.8) | 132 (25.8) | |
| D | Normalc | 6 | 136 (23.9) | 63.1 (21.2) | 158 (21.2) | |
| E | Severee | 6 | 24.5 (50.5) | 54.3 (12.2) | 136 (12.2) | |
| F | Normalc | 6 | 140 (24.4) | 64.9 (16.8) | 162 (16.8) | |
| H | Normalc | 6 | 146 (18.6) | 60.7 (2.4) | 152 (2.4) | |
Panel G consisted of participants with ESRD‐HD (eGFR <15 mL/min/1.73m2) who did not have a urine collection, given the limitations in producing urine.
50 < eGFR < 80 mL/min/1.73m2.
eGFR ≥80 mL/min/1.73m2.
30 ≤ eGFR ≤ 50 mL/min/1.73m2.
eGFR < 30 mL/min/1.73m2.
Ae, amount excreted; CLR, renal clearance; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; ESRD‐HD, end stage renal disease requiring haemodialysis; fe, fraction excreted; GM, geometric mean; IMI, imipenem/cilastatin; IMI/REL, REL coadministered with imipenem/cilastatin; IMI/REL + P, IMI/REL coadministered with probenecid; REL, relebactam; RI, renal impairment.
For participants with ESRD‐HD, the extraction coefficient of REL, imipenem and cilastatin was measured at various timepoints during the HD session, and calculated based on predialysis and postdialysis plasma samples. Overall, the extraction coefficient was consistent over time and indicated that all 3 analytes were efficiently removed through HD (Table 5).
Table 5.
Study PN005 summary of dialysis clearance and extraction coefficient for relebactam, imipenem and cilastatin following administration of a single dose of 125 mg relebactam with 250 mg imipenem/cilastatin
| Parameter, GM (%CV) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time, h | n | REL CLd, mL/mina | Imipenem CLd, mL/mina | Cilastatin CLd, mL/mina | REL extraction Coefficientb | Imipenem extraction Coefficientb | Cilastatin extraction Coefficientb | |
| Study PN005 | 1 | 3c | 172 (3.5) | 174 (5.0) | 132 (17.2) | 73 (1.8) | 73 (3.8) | 56 (15.6) |
| 1.5 | 3c | 158 (9.4) | 157 (9.9) | 108 (24.1) | 67 (9.5) | 66 (10.1) | 46 (22.6) | |
| 2 | 4d | 170 (5.9) | 169 (7.2) | 115 (17.8) | 73 (4.5) | 72 (5.8) | 49 (15.6) | |
| 2.5 | 4d | 166 (7.0) | 163 (7.2) | 109 (8.6) | 71 (5.6) | 69 (6.3) | 47 (6.3) | |
| 3 | 6 | 171 (5.4) | 167 (7.8) | 118 (12.5) | 73 (4.1) | 71 (7.0) | 50 (11.1) | |
| 3.5 | 6 | 177 (15.7) | 186 (19.1) | 118 (9.8) | 76 (15.7) | 79 (19.0) | 50 (8.2) | |
| 4 | 6 | 204 (20.2) | 204 (20.3) | 114 (11.5) | 87 (19.6) | 87 (19.7) | 48 (9.6) | |
| 4.5 | 6e | 198 (23.0) | 194 (25.5) | 111 (11.0) | 84 (22.2) | 83 (24.7) | 47 (9.0) | |
CLd = (1 – Hct) × QB × [(predialysis concentration – postdialysis concentration) / (predialysis concentration)], where QB = 350 mL/min and Hct = haematocrit.
Extraction Coefficient = ABS[100 × (postdialysis concentration – predialysis concentration) /predialysis concentration].
Three patients did not have samples available for assay due to mislabelling.
Two patients were excluded due to mislabelling of samples at 2 h and 2 patients were excluded at 2.5 h because the CLd, result was negative (due to a higher concentration in postdialysis compared to predialysis sample).
One patient for REL and imipenem CLd had values below the limit of quantitation and was excluded from the analysis.
CLd, dialysate clearance; CV, coefficient of variation; GM, geometric mean; REL, relebactam.
3.3.2. Effect of probenecid coadministration
In Study PN019, renal clearance of REL and imipenem was reduced by approximately 25 and 31%, respectively, when IMI/REL was coadministered with probenecid compared with when IMI/REL was administered alone (Table 4). The GM of the fe over the 24‐h collection interval was slightly lower for REL and lower for imipenem in healthy adult participants who received IMI/REL administered with a single oral dose of 1 g probenecid compared with participants who received IMI/REL alone (data not shown).
3.4. Safety
3.4.1. Adverse events
In Study PN005, 6 AEs were reported by 6 participants (12.2%), including 3 AEs that were considered drug‐related (Table 6). Drug‐related AEs were infusion site swelling, headache and musculoskeletal discomfort. All AEs were mild in intensity, with the exception of 1 case of severe abdominal pain reported by a patient in the ESRD‐HD group, which was deemed not related to the study drug. No serious AEs were reported and no participants discontinued due to an AE.
Table 6.
Adverse events (AEs) following administration of a single dose of 125 mg relebactam with 250 mg imipenem/cilastatin (Study PN005), or a single dose of 250 mg REL with 500 mg imipenem/cilastatin with or without coadministration with probenecid (Study PN019)
| Study PN005 | Study PN019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| REL + Imipenem/Cilastatin | REL + Imipenem/Cilastatin ± probenecid | |||||||||
| Mild RIa | Moderate RIb | Severe RIc | ESRD‐HDd | Healthy controlse | Total N | IMI/REL | Probenecid | REL/IMI + probenecid | Total N | |
| n f | 7 | 6 | 6 | 6 | 24 | 49 | 13 | 14 | 14 | 14 |
| AE, g n (%) | ||||||||||
| Gastrointestinal disorders | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (2.0) | 1 (7.7) | 0 | 1 (7.1) | 2 (14.3) |
| Abdominal pain | 0 | 0 | 0 | 1 (16.7) | 0 | 1 (2.0) | 0 | 0 | 0 | 0 |
| Dyspepsia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (7.1) |
| General disorders and administration site conditions | 1 (14.3) | 0 | 0 | 0 | 0 | 1 (2.0) | 1 (7.7) | 0 | 1 (7.1) | 2 (14.3) |
| Infusion site swelling | 1 (14.3) | 0 | 0 | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 0 |
| Malaise | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Vessel puncture site paraesthesia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (7.1) |
| Infections and infestations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Tooth infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Musculoskeletal and connective tissue disorders | 0 | 1 (16.7) | 1 (16.7) | 0 | 0 | 2 (4.1) | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Back pain | 0 | 0 | 1 (16.7) | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 0 |
| Musculoskeletal discomfort | 0 | 1 (16.7) | 0 | 0 | 0 | 1 (2.0) | 0 | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Nervous system disorders | 1 (14.3) | 0 | 0 | 1 (16.7) | 0 | 2 (4.1) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Headache | 1 (14.3) | 0 | 0 | 1 (16.7) | 0 | 2 (4.1) | ‐‐ | ‐‐ | ‐‐ | ‐‐ |
| Respiratory, thoracic and mediastinal disorders | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Oropharyngeal pain | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
| Skin and subcutaneous tissue disorders | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 1 (7.1) | 2 (14.3) |
| Alopecia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (7.1) |
| Erythema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) |
50 < eGFR < 80 mL/min/1.73m2.
30 ≤ eGFR ≤ 50 mL/min/1.73m2.
eGFR < 30 mL/min/1.73m2.
eGFR < 15 mL/min/1.73m2, requiring haemodialysis.
eGFR ≥ 80 mL/min/1.73m2.
n values reflect total number of patients administered a particular dose; N reflects the total number of patients in the particular cohort.
Every participant was counted a single time for each AE category, so total values in the total AE categories may be less than the sum of the individual AEs.
AE, adverse event; eGFR, estimated glomerular filtration rate; IMI, imipenem; IMI/REL, REL coadministered with imipenem/cilastatin; REL, relebactam; RI, renal impairment.
In Study PN019, 9 AEs were reported by 7 participants, including 2 AEs that were considered drug related (Table 6). No serious AEs were reported and no participants discontinued due to AEs. One patient who experienced a drug‐related AE of erythema in Period 1 was discontinued by the investigator prior to dosing in Period 2.
3.4.2. Abnormal laboratory values
For both studies, there were no clinically important variations in laboratory values as assessed by the investigator. In Study PN005, no clinically meaningful changes were observed. Participants with RI had some abnormalities in laboratory values at baseline and during the study that were consistent with their disease (e.g. blood urea nitrogen, creatinine, phosphorus, glucose, alkaline phosphatase, haemoglobin and haematocrit). A single dose of IMI/REL did not appear to have any clinically relevant impact on these values.
4. DISCUSSION
The 2 phase 1 PK studies discussed here are important for understanding the clinical pharmacology profile of IMI/REL in RI subpopulations and to understand the potential of OAT‐mediated drug interactions.
Study PN005 was designed and conducted early in phase 1 to evaluate and understand the effect of RI on the plasma PK of IMI/REL in order to guide dosing recommendations for patients with RI, including those requiring HD in subsequent phase 2 and 3 trials. In this analysis, clinically relevant increases in exposures (AUC0‐∞) of REL, imipenem and cilastatin were observed as renal function decreased, which was expected as all 3 drugs are primarily renally cleared.18 The magnitude of REL plasma clearance and fe decrease (and corresponding t1/2 and AUC0‐∞ increase) observed was consistent with the expected proportional change in magnitude of eGFR in patients with RI (mild, moderate, severe and ESRD‐HD). Similar proportional changes were observed for imipenem and cilastatin. In patients with ESRD‐HD, all 3 compounds were efficiently removed by HD. These data support a dose adjustment in patients with RI to maintain systemic exposures similar to patients with normal renal function; patients with ESRD‐HD should receive IMI/REL after haemodialysis sessions. The results of this study also support the use of the IMI/REL fixed‐dose combination (FDC), as all 3 compounds exhibited comparable PK alterations in patients with mild, moderate and severe renal disease, and were similarly removed by HD. Actual dose adjustments have been developed by population PK analysis for imipenem and REL using integrated phase 1, phase 2 and phase 3 PK data; the details of this renal PK study support that the ratio of the individual components in the combination can be maintained, as RI affected imipenem and REL to a similar extent.
While observed exposures with REL are somewhat higher than those with imipenem in study participants with severe RI and ESRD, these results still support the development of an FDC of REL with imipenem/cilastatin. All 3 compounds have adequate safety margins to allow for differential changes in the PK of each in the setting of renal insufficiency. IMI has been authorized for decades at doses of up to 1000 mg every 6 h and has a well‐established safety profile in patients with renal disease, while REL was generally well tolerated at single doses of 1150 mg and multiple doses of 625 mg every 6 h for 7 days.18 An FDC containing 500 mg imipenem, 500 mg cilastatin and 250 mg REL is currently in phase 3 clinical development; based on the results of PN005, RI is unlikely to result in clinically significant changes in exposures that would preclude the development of an FDC formulation.
These results are consistent with the previously reported PK data for both imipenem and cilastatin in patients with RI, which showed that the t1/2 of imipenem increased from 1.02 h in healthy controls to 3.96 h in HD patients, and the t1/2 of cilastatin increased from 0.86 h in healthy controls to 17.08 h in HD patients.29 In addition, there was no change in the volume of distribution for either drug, and both imipenem and cilastatin were removed by HD.
In Study PN019, probenecid, an OAT inhibitor, did not cause clinically meaningful changes in plasma exposure or renal clearance of REL or imipenem. The REL result is consistent with active renal secretion contributing to only ~30% of the total clearance of REL in humans.16 Thus, if all the active renal secretion were to be inhibited, the increase in REL systemic AUC exposure would be <1.5‐fold and thus would have minimal clinical significance. In the study, probenecid decreased the renal clearance of REL by 25%. The effect of probenecid coadministration on imipenem exposure is consistent with historical data from coadministration of IMI with probenecid, which demonstrated only a slight increase (~15%) in the plasma exposure of imipenem.25
For both studies PN005 and PN019, IMI/REL administered alone or in combination with probenecid was generally well tolerated in healthy adults as well as participants with RI. Most AEs were mild and transient. In participants with RI, changes in laboratory values were consistent with the underlying disease.
5. CONCLUSION
In conclusion, RI was associated with reduced plasma and renal clearance and increased plasma exposure and t1/2 for REL, imipenem and cilastatin, indicating that dose adjustment is required for patients with RI. Overall, IMI/REL was well tolerated in patients with RI. Probenecid had no clinically meaningful impact on the PK of REL or imipenem. Taken together, these data support maintaining an FDC of IMI with REL in individuals with RI and demonstrate a lack of clinically relevant interactions with OAT inhibitors.
COMPETING INTERESTS
K.L.'s institution (Clinical Pharmacology of Miami) and T.M.'s institution (Orlando Clinical Research Center) received research support from MSD. F.C.G., Y.L., J.W., S.S.X., G.G., P.J., M.L.R., M.L., E.G.R., J.R.B. and K.B. are employees of MSD and may own stock and/or hold stock options in the company. P.B. is a former employee of MSD and may own stock and/or hold stock options in the company.
CONTRIBUTORS
All authors are responsible for the work described in this paper. K.L. and T.M. served as the Principal Investigators for these studies and acquired the data presented herein. P.B., F.C.G., Y.L., J.W., S.S.X., G.G., P.J., M.L.R., M.L., E.G.R., J.R.B. and K.B. designed the study protocols and analysed the data. All authors contributed to the drafting of the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
TABLE S1 Renal function groups used for confirmatory analyses
TABLE S2 Precision and accuracy of relebactam, cilastatin and imipenem in quality control samples in stabilized human plasma for PN005 and PN019
TABLE S3 Plasma pharmacokinetic parameters following a single intravenous dose of 125 mg relebactam with 250 mg imipenem/cilastatin in patients with impaired renal function (using K/DOQI classification) and matched healthy controls
TABLE S4 Plasma pharmacokinetic parameters following a single intravenous dose of 125 mg relebactam with 250 mg imipenem/cilastatin in patients with impaired renal function (using EMA Guidance classification) and matched healthy controls
ACKNOWLEDGEMENTS
The authors would like to thank all investigators who contributed to the study. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD). Medical writing and/or editorial assistance was provided by Amy Agbonbhase, PhD and Alanna Kennedy, PhD, CMPP, of The Lockwood Group, Stamford, CT, USA. This assistance was funded by MSD.
Bhagunde P, Colon‐Gonzalez F, Liu Y, et al. Impact of renal impairment and human organic anion transporter inhibition on pharmacokinetics, safety and tolerability of relebactam combined with imipenem and cilastatin. Br J Clin Pharmacol. 2020;86:944–957. 10.1111/bcp.14204
The authors confirm that the Principal Investigators for this paper are Kenneth Lasseter and Thomas Marbury (Study PN005) and Jeffrey Zacher (Study PN019) and that they had direct clinical responsibility for patients. Dr Zacher contributed to the collection of data but not to the writing of the manuscript.
DATA AVAILABILITY STATEMENT
MSD is committed to providing qualified scientific researchers access to anonymized patient‐level data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. The company is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The process includes submission of data requests to the MSD data sharing website (available at http://engagezone.merck.com/ds_documentation.php). Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing the requested data.
REFERENCES
- 1. Morrissey I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A review of ten years of the study for monitoring antimicrobial resistance trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013;6(11):1335‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thaden JT, Pogue JM, Kaye KS. Role of newer and re‐emerging older agents in the treatment of infections caused by carbapenem‐resistant Enterobacteriaceae. Virulence. 2017;8(4):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiner LM, Fridkin SK, Aponte‐Torres Z, et al. Vital signs: preventing antibiotic‐resistant infections in hospitals ‐ United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(9):235‐241. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Worldwide country situation analysis: response to antimicrobial resistance. 2015. http://www.who.int/drugresistance/documents/situationanalysis/en/. Accessed October 9, 2017.
- 5. Messina JA, Cober E, Richter SS, et al. Hospital readmissions in patients with carbapenem‐resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2016;37(3):281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans HL, Lefrak SN, Lyman J, et al. Cost of gram‐negative resistance. Crit Care Med. 2007;35(1):89‐95. [DOI] [PubMed] [Google Scholar]
- 7. Lautenbach E, Synnestvedt M, Weiner MG, et al. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect Control Hosp Epidemiol. 2010;31(1):47‐53. [DOI] [PubMed] [Google Scholar]
- 8. Nicolau DP. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother. 2008;9(1):23‐37. [DOI] [PubMed] [Google Scholar]
- 9. Chiodini PL, Geddes AM, Smith EG, Conlon CP, Farrell ID. Imipenem/cilastatin in the treatment of serious bacterial infections. Rev Infect Dis. 1985;7(Suppl 3):S490‐S495. [DOI] [PubMed] [Google Scholar]
- 10. Kahan FM, Kropp H, Sundelof JG, Birnbaum J. Thienamycin: development of imipenen‐cilastatin. J Antimicrob Chemother. 1983;12(Suppl D):1‐35. [DOI] [PubMed] [Google Scholar]
- 11. Thaden JT, Fowler VG, Sexton DJ, Anderson DJ. Increasing incidence of extended‐spectrum β‐lactamase‐producing Escherichia coli in community hospitals throughout the southeastern United States. Infect Control Hosp Epidemiol. 2016;37(1):49‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose‐ranging study of relebactam with imipenem‐cilastatin in subjects with complicated intra‐abdominal infection. Antimicrob Agents Chemother. 2016;60(10):6234‐6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double‐blind, phase 2 dose‐ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72:2616‐2626. [DOI] [PubMed] [Google Scholar]
- 14. Motsch J, de Oliveira C, Stus V, et al. RESTORE‐IMI 1: a multicenter, randomized, double‐blind trial comparing efficacy and safety of imipenem/relebactam versus colistin plus imipenem in patients with imipenem‐nonsusceptible bacterial infections. Clin Infect Dis. 2019. Aug 10. 10.1093/cid/ciz530. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ClinicalTrials.gov . Imipenem/relebactam/cilastatin versus piperacillin/tazobactam for treatment of participants with bacterial pneumonia (MK‐7655A‐014) (RESTORE‐IMI 2). Accessed February 5, 2019.
- 16. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of Imipenem with relebactam against gram‐negative pathogens from New York City. Antimicrob Agents Chemother. 2015;59(8):5029‐5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blizzard TA, Chen H, Kim S, et al. Discovery of MK‐7655, a β‐lactamase inhibitor for combination with Primaxin®. Bioorg Med Chem Lett. 2014;24:780‐785. [DOI] [PubMed] [Google Scholar]
- 18. Rhee EG, Rizk ML, Calder N, et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of relebactam, a β‐lactamase inhibitor, in combination with imipenem and cilastatin in healthy participants. Antimicrob Agents Chemother. 2018;62:e00280‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toon S, Hopkins KJ, Garstang FM, Aarons L, Rowland M. Pharmacokinetics of imipenem and cilastatin after their simultaneous administration to the elderly. Br J Clin Pharmacol. 1987;23(2):143‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. PRIMAXIN® (imipenem and cilastatin) prescribing information. Whitehouse Station, NJ: Merck & Co., Inc.; 2019. [Google Scholar]
- 21. Chan G, Houle R, Lin M, et al. Role of transporters in the disposition of a novel β‐lactamase inhibitor: relebactam (MK‐7655). J Antimicrob Chemother. 2019;74(7):1894‐1903. [DOI] [PubMed] [Google Scholar]
- 22. Lim SC, Im YB, Bae CS, Han SI, Kim SE, Han HK. Protective effect of morin on the imipenem‐induced nephrotoxicity in rabbits. Arch Pharm Res. 2008;31:1060‐1065. [DOI] [PubMed] [Google Scholar]
- 23. Mor AL, Kaminski TW, Karbowska M, Pawlak D. New insight into organic anion transporters from the perspective of potentially important interactions and drugs toxicity. J Physiol Pharmacol. 2018;69(3):307‐324. [DOI] [PubMed] [Google Scholar]
- 24. Wolman AT, Gionfriddo MR, Heindel GA, et al. Organic anion transporter 3 interacts selectively with lipophilic β‐lactam antibiotics. Drug Metab Dispos. 2013;41(4):791‐800. [DOI] [PubMed] [Google Scholar]
- 25. Norrby SR, Bjornegard B, Ferber F, Jones KH. Pharmacokinetics of imipenem in healthy volunteers. J Antimicrob Chemother. 1983;12(Suppl D):109‐124. [DOI] [PubMed] [Google Scholar]
- 26. European Medicines Agency . Guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function. London, UK: Committee for Medicinal Products for Human Use (CHMP); 2015. [Google Scholar]
- 27. U.S. Department of Health and Human Services Food and Drug Administration . Guidance for industry: drug interaction studies — study design, data analysis, implications for dosing, and labeling recommendations. Silver Spring, MD: Center for Drug Evaluation and Research (CDER); 2012. [Google Scholar]
- 28. Alexander SP, Kelly E, Marrion NV, et al. The concise guide to pharmacology 2017/18. Br J Pharmacol. 2017;174(Suppl 1):S1‐S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson TP, Demetriades JL, Bland JA. Imipenem/cilastatin: pharmacokinetic profile in renal insufficiency. Am J Med. 1985;78(6A):54‐61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Renal function groups used for confirmatory analyses
TABLE S2 Precision and accuracy of relebactam, cilastatin and imipenem in quality control samples in stabilized human plasma for PN005 and PN019
TABLE S3 Plasma pharmacokinetic parameters following a single intravenous dose of 125 mg relebactam with 250 mg imipenem/cilastatin in patients with impaired renal function (using K/DOQI classification) and matched healthy controls
TABLE S4 Plasma pharmacokinetic parameters following a single intravenous dose of 125 mg relebactam with 250 mg imipenem/cilastatin in patients with impaired renal function (using EMA Guidance classification) and matched healthy controls
Data Availability Statement
MSD is committed to providing qualified scientific researchers access to anonymized patient‐level data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. The company is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The process includes submission of data requests to the MSD data sharing website (available at http://engagezone.merck.com/ds_documentation.php). Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing the requested data.


