Abstract
Aims: Inflammatory cytokines, particularly tumour necrosis factor‐α (TNFα), are thought to promote arterial disease through a variety of mechanisms leading to arteriosclerosis and atherosclerosis. We reviewed the existing evidence of the effect of anti‐TNFα treatment on arteriosclerosis and atherosclerosis in chronic inflammatory disease.
Methods: We performed a systematic review of studies examining effects of monoclonal antibodies against TNFα on subclinical measures of arteriosclerosis (arterial pulse wave velocity) and atherosclerosis (endothelial function measured by flow‐mediated dilation or forearm blood flow responses to endothelium‐dependent agonists, and common carotid intima‐media thickness).
Results: We identified 60 studies (of 854 potential studies identified using a systematic search) in which effects of anti‐TNFα biologics on these measures were assessed in patients receiving anti‐TNFα therapy for a clinical indication (usually an inflammatory disease such as an inflammatory arthritis, psoriasis or inflammatory bowel disease). Of these, only 6 were randomised clinical controlled trials. Whilst many observational studies and noncontrolled studies reported positive findings, positive finding were reported in only 1 of 6 randomised clinical controlled trials.
Conclusions: There is no strong evidence for an effect of anti‐TNFα biologics on the subclinical measures of arteriosclerosis or atherosclerosis examined in this review. This does not exclude a positive effect of TNFα biologics on clinical outcomes through alternate pathways including those induced by remission of the primary inflammatory disease.
Keywords: inflammation, atherosclerosiscardiovascular, systematic review
What is already known about this subject
Proinflammatory cytokines such as tumour necrosis factor‐α (TNFα) are associated with arterial disease, particularly in the context of chronic inflammatory diseases.
What this study adds
This paper reviews the existing evidence of the effect of anti‐TNFα monoclonal antibodies on subclinical measures of arterial disease.
There is no strong evidence from randomised trials that anti‐TNFα treatment causes durable improvement in such measures.
TNFα antagonism could still have positive effects on clinical outcomes in this population through other mechanisms.
1. INTRODUCTION
Morbidity and mortality from circulatory disease is thought to arise from both atherosclerosis and arteriosclerosis. Atherosclerosis is characterized by development of lipid‐laden, fibro‐muscular plaques within the intima that give rise to acute clinical events through rupture and subsequent thrombosis occluding the arterial lumen—atherothrombosis. 1 Arteriosclerosis is the stiffening of the media of the arterial wall as a result of degeneration of connective tissue, particularly elastin.2, 3 Clinical events in arteriosclerosis are thought to be driven by the systolic hypertension that results from aortic stiffening as well as other adverse haemodynamic effects. Whilst both atherosclerosis and arteriosclerosis commonly occur together, they are thought to have differing aetiology and to be influenced to different degrees by classical risk factors.4 However, inflammatory cytokines, particularly tumour necrosis factor‐α (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074) are thought to play a role in both conditions5, 6 and could represent a novel therapeutic target for preventing or treating arterial disease. Key evidence cited for this in humans derives from studies on patients with various inflammatory diseases (who are at increased risk of arterial disease and often have subclinical arterial disease) treated with anti‐TNFα monoclonal antibodies. However, most studies that have examined specific aspects of arterial disease (rather than focusing on clinical events that could result from many aspects of arterial disease and/or thrombosis), have been small scale and uncontrolled. We therefore performed a systematic review of studies examining effects of anti‐TNFα treatments on subclinical measures of arterial disease. These included endothelial function measured by flow‐mediated dilation (FMD) and as a forearm blood flow (FBF) response to an endothelium‐dependent vasodilator and common carotid intima‐media thickening (CIMT), measures of subclinical atherosclerosis7, 8 and pulse wave velocity (PWV), a measure of arterial stiffening and arteriosclerosis.9 We also included results of studies examining augmentation index (AIx), which has been widely used as a measure of arterial stiffening, but which is probably influenced by other haemodynamic factors.10
2. METHODS
A systematic search (see appendix) was performed using MEDLINE and EMBASE databases from inception to October 2018. Filters included articles in the English language only. Once relevant publications were identified, a manual search of citation lists was performed.
2.1. Selection criteria
Articles were initially screened and rejected if the reviewer could determine from the title that the article was not relevant to the search question. Publications were rejected if they: (i) did not use an anti‐TNFα treatment; (ii) did not measure at least 1 of the following; PWV, AIx, FMD, FBF, cIMT; (iii) were review articles; or (iv) were conference abstracts.
2.2. Data extraction
Variables extracted from each study included: (i) the chronic inflammatory disease investigated and the number of patients and/or controls; (ii) the study design, including type of anti‐TNFα administered, duration of study and time points of the measurements; (iii) type of measurement used to determine arterial function/structure and endothelial function; and (iv) outcome variables following anti‐TNFα treatment: changes reported in PWV, CIMT, FMD and endothelium‐dependent FBF.
2.3. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY.
3. RESULTS
The initial search identified 854 potentially relevant articles. Of these, 220 were duplicates, non‐English language or conference proceedings leaving 634 articles which were screened based on the title and abstract; 549 were identified as not relevant and the remaining 85 publications were retrieved in full for detailed review. After review, a further 25 publications were identified as not fulfilling the inclusion criteria, leaving 60 papers included in the final analysis (Figure 1).
Figure 1.
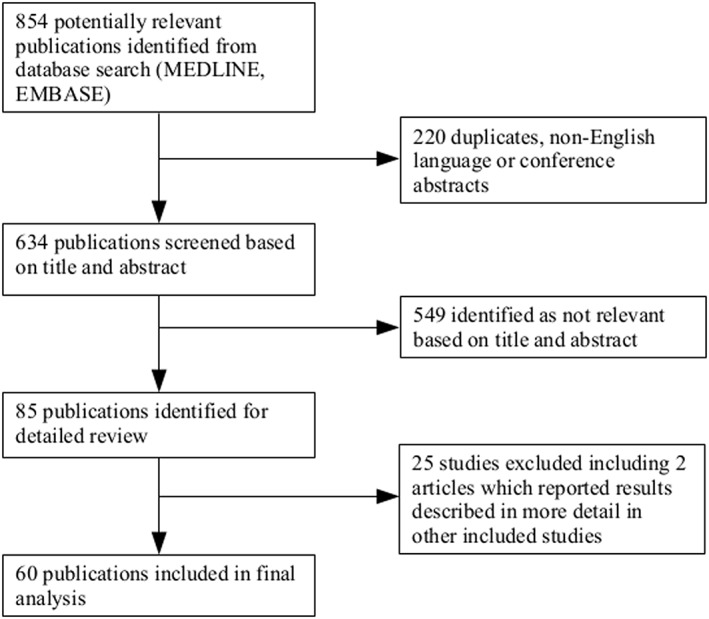
Summary of search results and review process
3.1. Data analysis
The identified studies were heterogenous in size and methodology, with many small scale and uncontrolled studies. In total, 1626 patients were treated with anti‐TNFα across the 60 studies included. Seven studies were cross‐sectional, of which 4 included a control group. Thirty studies were uncontrolled longitudinal studies with a mean sample size of 19 (median 14) and mean follow up of 33 weeks (median 12). Twenty‐four were longitudinal controlled studies; of these only 6 were randomised. The controlled studies were on average larger, with a mean sample size of 47 (median 29), and of longer duration, with a mean follow up of 41 weeks (median 28). Mann–Whitney U tests showed that this difference was statistically significant for both duration of follow up (W = 521.5, P = .0221) and sample size (W = 567.5, P = .00243). The majority of studies were in patients with rheumatoid arthritis (33), ankylosing spondylitis (8), psoriasis (6) and psoriatic arthritis (2). Seven studies included multiple different types of inflammatory arthritides. Two were in systemic vasculitis and 2 in IBD. In terms of anti‐TNFα therapy, 30 studies included patients taking multiple different TNFα antagonists. Fifteen assessed the effect of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5004 alone, 7 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6789, 7 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4860 and 2 studies assessed the effect of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6776. Many studies did not break down the results according to the individual TNFα antagonist used.
3.2. Arterial stiffness
In total, 27 studies investigated some measure of arterial stiffness (Table 1). Twenty‐two measured PWV, of which 15 used carotid‐femoral PWV, 5 used some other technique (e.g. a carotid‐radial PWV) and 2 measured both. Nineteen studies measured AIx. Of the 22 studies measuring PWV, 2 were cross‐sectional, 10 were uncontrolled longitudinal studies, 6 were controlled and 4 were randomised controlled trials. Of these studies, 9 suggested a beneficial effect of anti‐TNFα (i.e. a reduction in PWV in treated patients), with 13 showing no effect or suggesting a negative effect. Of the studies finding a statistically significant effect of anti‐TNFα treatment on PWV, the magnitude (decrease in PWV) ranged from 0.46 to 2.6 m s−1 (median 0.73 m s−1). All 4 of the randomised controlled trials measuring PWV showed no benefit of anti‐TNFα agents. Of the 19 studies measuring AIx, 1 was cross sectional, 8 were uncontrolled longitudinal studies, 5 were controlled and 5 were randomised controlled trials. Only 3 studies showed an improvement in AIx associated with anti‐TNFα treatment; 1 of these a randomised trial,24 which measured only AIx and not PWV. The other 4 randomised controlled trials showed no beneficial effect of anti‐TNFα on AIx.
Table 1.
Studies evaluating the effects of anti‐TNFα treatment on arterial stiffness in patients with CID
|
Reference |
CID | Study design | Outcome measures and results |
|---|---|---|---|
| 11 | 35 RA, PsA or AS | 35 patients given either etanercept, adalimumab or infliximab. 25 patients delayed anti‐TNFα treatment (controls). Measurements at baseline and 3 months. |
1. cfPWV decreased significantly in anti‐TNF group but remained unchanged in control group after 3 months (P = .002). 2. AIX remained unchanged in both groups. |
| 12 | 17 RA, PsA or AS | Patients treated with infliximab for 12 months. Measurements taken before every infliximab infusion and thereafter every 10th day until their next infusion. No controls. |
1. cfPWV did not change after infliximab treatment. 2. AIX did not change after infliximab treatment. |
| 13 | 41 RA, PsA or AS | Either etanercept, adalimumab or infliximab administered for 1 year to 41 patients compared to a nontreatment group (19 patients). |
1. cfPWV improved in treatment group vs. controls (P = .004). 2. No difference in AIX or AIX@75. |
| 14 | 14 psoriasis | Patients received adalimumab for 12 weeks. No controls. | 1. Adalimumab therapy had no effect on cfPWV. |
| 15 | 34 AS | Cross‐sectional study comparing 34 AS patients on anti‐TNFα, 33 treated with NSAIDs and 34 healthy controls. |
1. cfPWV significantly lower in healthy controls compared to AS patients (P = .004). 2. No significant difference in cfPWV between AS treatment groups. |
| 16 | 15 RA | Cross‐sectional study comparing 15 patients on infliximab with 73 RA patients on other therapy and 87 healthy controls. |
1. bPWV reduced in infliximab group (P = .004) vs. controls. 2. AIX remained unchanged post‐infliximab therapy vs. controls. |
| 17 | 26 RA | 21 treated with MTX, 26 treated with etanercept. Follow‐up at 2 and 4 months. | 1. AIX@75 significantly improved in etanercept group at 2 and 4 months (P = .025). No change in MTX group. |
| 18 | 21 psoriasis | 21 psoriasis patients randomised to receive either etanercept 20 mg SC twice weekly or placebo for 12 weeks (n not specified). cfPWV and AIX measured at baseline, 12 and 24 weeks. | 1. No significant change in cfPWV or AIX at 12 or 24 weeks. |
| 19 | 50 psoriasis | 150 patients randomised to either ustekinumab (n = 50), cyclosporine (n = 50) or etanercept (n = 50). cfPWV and AIX measured at baseline and 16 weeks. | 1. No significant change in cfPWV or AIX at 16 weeks. |
| 20 | 28 RA | Patients treated with etanercept for 6 mo and compared with 20 RA controls on standard DMARD therapy. Measurements at 3 and 6 months. | 1. No significant change in cfPWV at 6 months. |
| 21 | 28 AS | Treated with anti‐TNFα as per ASAS guidelines. cfPWV and AIX measured at baseline, 24 weeks and 2 years. No controls. | 1. No significant change in cfPWV or AIX at 24 weeks or 2 years. |
| 22 | 8 RA | RA patients received 40 mg subcutaneous adalimumab every 2 weeks. Measurements taken up to week 24. | 1. No significant change in cfPWV at week 24. |
| 23 | 15 RA | RA subjects received inflixmab infusions at 0, 2 and 6 weeks at 3 mg kg–1 with measurements taken at each infusion. No controls. | 1. cfPWV remained unchanged. |
| 24 | 42 RA | Patients were randomly assigned to receive either tocilizumab (n = 22), etanercept (n = 21) or adalimumab (n = 21) for 24 weeks.. | 1. AIX@75 significantly improved after anti‐TNFα therapy at wk 24 vs. baseline (P < .05). |
| 25 | 9 RA | Patients received etanercept and were assessed at weeks 0, 4 and 12 after therapy onset. No controls. |
1. AIX % did not change 2. bPWV did not change 3. cfPWV significantly reduced at weeks 4 and 12 (P = .0003). |
| 26 | 17 RA | Patients assessed before and after 8 weeks of etanercept or adalimumab treatment. No controls. |
1. cfPWV significantly reduced post‐treatment (P = .04). 2. bPWV showed a trend to improve post‐treatment (P = .06). |
| 27 | 18 AS | Treatment with either infliximab, etanercept or adalimumab. Measurements taken at baseline and week 14. No controls. | 1. AIX remained unchanged post–anti‐TNFα treatment. |
| 28 | 49 AS | Received either etanercept, infliximab or adalimumab. Measurements taken at 0, 24 and 52 weeks. No controls. |
1. AIX did not improve post‐treatment. 2. cfPWV did not improve post‐treatment. |
| 29 | 20 RA, 7 AS, 5 PsA | 32 CID patients treated with anti‐TNFα for 6 months and compared with 8 RA controls. Measurements taken at baseline and 3 months. | 1. Significant decrease in aPWV (as measured by CMR) at 3 months in treatment group (P < .001). |
| 30 | 17 RA and 13 AS | Patients received infliximab infusions at weeks 0, 2 and 6 at 3 mg kg–1 in RA and 5 mg kg–1 in AS. No controls. | 1. AIX significantly increased collectively (P = .03) and in RA group (P = .01). |
| 31 | 29 psoriasis | Patients treated with 40 mg SC adalimumab 2 weekly for 6 months. No controls. | 1. Significant improvement in cfPWV at 6 months (P = .003). |
| 32 | 20 RA | Patients randomly assigned to receive either MTX alone (n = 20) or MTX and infliximab (n = 20). All patients followed up at 6 months and some up to 12 months. |
1. cfPWV improved in MTX group vs. infliximab and MTX (P = .044) after 6 months, but did not remain significant at 12 months. 2. No change in AIX@75 at 6 or 12 months. |
| 33 | 20 AS | Randomized double blinded RCT comparing golimumab at 50 mg mo–1 (n = 20) vs. placebo (n = 21) for 12 months in AS patients. Most placebo patients switched to golimumab at 6 months (n = 17). |
1. No significant difference in bPWV or AIX between placebo and treatment groups at 6 or 12 months. 2. Greater progression in bPWV in placebo group at 6 months (P = .028). |
| 34 | 14 RA | Either etanercept, adalimumab or infliximab for 6 weeks. No controls. | 1. No change in AIX |
| 35 | 18 RA | Patients treated with adalimumab 40 mg SC 2 weekly for 12 weeks, compared to controls receiving methotrexate. Measurements at baseline and 12 weeks. | 1. Significant decrease in cfPWV at 12 weeks (P = .0006) in treatment group only. No change in AIX. |
| 36 | 26 RA | Administration of infliximab for 56 weeks. No controls. |
1. cfPWV reduced significantly over 56 weeks (P = .004). 2. AIX did not change post‐therapy. |
| 37 | 11 IBD | 11 IBD patients treated with anti‐TNFα, compared to 14 matched controls treated with salicylates, 11 with DMARDS and 30 healthy matched controls. Measurements taken at baseline and follow up, mean duration 3.4 years. |
1. No change in cfPWV in anti‐TNFα group at follow up, compared to significant increase in salicylate treated group and healthy controls. 2. No change in AIX. |
CID: chronic inflammatory disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AS: ankylosing spondilitis; IBD: inflammatory bowel disease; anti‐TNFα: anti‐tumour necrosis factor α; PWV: pulse wave velocity; aPWV: aortic PWV; bPWV: brachial PWV; cfPWV: carotid–femoral PWV; AIX: augmentation index; AIX@75: augmentation index adjusted for 75 beats min–1; ASAS: Association of SpondyloArthritis international Society.
3.3. Carotid intima‐media thickness
Twenty‐three studies reported the effect of anti‐TNFα therapy on CIMT (Table 2). Four were cross‐sectional studies, 7 uncontrolled longitudinal studies, 8 controlled studies and 4 randomised controlled trials. Overall, 7 studies showed a positive effect of anti‐TNFα therapy on CIMT (i.e. a reduction in CIMT), whilst 16 showed either no effect or a worsening of CIMT. Observed decreases in cIMT in response to anti‐TNFα treatment were generally small, ranging from 0.06 to 0.16 mm.
Table 2.
Studies evaluating the effects of anti‐TNFα treatment on carotid atherosclerosis in patients with CID
| Reference | CID | Study design | Outcome measures and results |
|---|---|---|---|
| 13 | 41 RA, PsA or AS | Either etanercept, adalimumab or infliximab administered for 1 year to 41 patients compared to a nontreatment group (19 patients). | 1. CIMT progression reduced in treatment group vs. controls (P = .01). |
| 38 | 14 RA | Adalimumab 40 mg 2 weekly for 3 months with CIMT measured at baseline and conclusion. No controls. | 1. No significant change in CIMT. |
| 15 | 34 AS | Cross‐sectional study comparing 34 AS patients on anti‐TNFα, 33 treated with NSAIDs and 34 healthy controls. | 1. No significant difference in CIMT between groups. |
| 39 | 30 RA | RA patients received either infliximab or etanercept for 1 year. 10 RA patients remained on standard treatment. Carotid ultrasound performed at 0 and 12 months. | 1. Significant CIMT reduction on right and left sides (P < .0001 for both sides) post‐infliximab/etanercept therapy. CIMT was also reduced in treatment group compared to controls (P = 0.002). |
| 40 | 7 RA | 7 RA patients treated with infliximab and standard therapy for 1 year compared to 7 RA controls on standard therapy only. IMT measured at 0 and 12 months. | 1. IMT worsened post‐infliximab treatment (P = .026) compared to baseline. No difference in IMT in control group. |
| 41 | 120 PsA | Cross‐sectional study comparing 120 PsA patients on anti‐TNFα and 104 on DMARDs. | 1. CIMT was higher in those on DMARDs compared to those on anti‐TNFα (P < .001). |
| 42 | 40 RA | 119 RA patients recruited and commenced on MTX. After 3 months 40 non‐responders to MTX therapy received either infliximab/etanercept or adalimumab. The rest remained on MTX. | 1. CIMT decreased significantly in anti‐TNFα treatment group after 2‐year follow‐up (p < 0.001) compared to no change in CIMT in MTX treated group. |
| 43 | 34 RA | Patients received 40 mg adalimumab every other week from day 0 to month 12 . Measurements taken at day 0, day 14 and month 12. No controls. | 1. No significant change in CIMT at 12 months. |
| 44 | 16 psoriasis | Etanercept/infliximab/adalimumab for 6 months with measurements at baseline and 6 months. No controls. | 1. Reduction in cIMT in 13 patients without plaque disease (P = .0002); no change in 3 patients with established atherosclerosis. |
| 22 | 8 RA | RA patients received 40 mg subcutaneous adalimumab every 2 weeks. Measurements up to week 24. No controls. | 1. CIMT decreased post‐treatment at week 24 vs. baseline (P = .002). |
| 24 | 42 RA | Patients were randomly assigned to receive either tocilizumab (n = 22), etanercept (n = 21) or adalimumab (n = 21) for 24 weeks. | 1. No change in CIMT post–anti‐TNFα therapy. |
| 45 | 13 psoriasis | 53 psoriasis patients recruited, of whom 13 were treated with anti‐TNFα agents. Compared with 30 DMARD patients and 10 on other biological drugs. Assessment at baseline and 8 months. | 1. No significant change in cIMT at 8 months. |
| 46 | 23 RA and PsA | 3 groups, treated with either infliximab (n = 13) etanercept (n = 10) or DMARDs (n = 13). | 1. No significant difference in CIMT between treatment groups. |
| 47 | 32 PsA | Etanercept/infliximab/adalimumab for 2 years. No controls. | 1. Progression of cIMT at 2 years (P < .0005). |
| 48 | 12 RA | Patients taking infliximab or adalimumab. Measurements performed at baseline and every 3 months up to 18 months. No controls. | 1. No change in cIMT at 18 months. |
| 32 | 20 RA | Patients randomly assigned to receive either MTX alone (n = 20) or MTX and infliximab (n = 20). All patients followed up at 6 months and some up to 12 months. | 1. No change in IMT at 6 or 12 months. |
| 33 | 20 AS | Randomized double blinded RCT comparing golimumab at 50 mg month–1 (n = 20) vs. placebo (n = 21) for 12 months in AS patients. Most placebo patients switched to golimumab at 6 months (n = 17). |
1. No significant difference in IMT between groups at 6 or 12 months. 2. Greater IMT progression in placebo group at 6 months. |
| 49 | 10 RA | RA patients assigned to receive MTX (n = 10) or adalimumab 40 mg every other wk subcutaneously (n = 10). Measurements at baseline and 18 months. | 1. No change in CIMT in either treatment group post‐therapy. |
| 50 | 81 AS | 70 treated with etanercept, 11 with adalimumab. Measurements taken at baseline and 5 years. No controls. | 1. No significant change in cIMT at 5 years, but a trend towards increase in patients who discontinued anti‐TNFα. |
| 51 | 290 RA | Placebo + MTX (n = 102), golimumab + placebo (n = 98), golimumab 50 mg + MTX (n=91) or golimumab 100 mg + MTX (n = 101) with assessments at baseline, 24 and 52 weeks. |
1. No effect of golimumab compared to placebo. 2. Increase in cIMT compared to placebo at 24 weeks in the MTX + golimumab 100 mg group (P = .005). 3. Increase in cIMT compared to placebo at 52 weeks in the MTX + golimumab 50 mg group (P = .029). |
| 52 | 13 RA | Cross sectional study comparing 13 RA patients on anti‐TNFα therapy (adalimumab/infliximab/etanercept) and 12 on DMARDs. | 1. No significant difference in cIMT between groups. |
| 36 | 26 RA | Infliximab for 56 weeks. No controls. | 1. No significant change in cIMT. |
| 53 | 14 AS | 14 AS patients treated with anti‐TNFα for at least 2 years compared with 14 healthy controls. | 1. Significantly reduced cIMT in AS patients compared to healthy controls. |
CID: chronic inflammatory disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AS: ankylosing pondylitis; anti‐TNFα: anti‐tumour necrosis factor α; cIMT: carotid intima media thickening; NSAIDs: nonsteroidal anti‐inflammatory drugs; DMARDs: disease modifying antirheumatic drugs; MTX: methotrexate.
3.4. Endothelial dysfunction
The majority of studies identified in this review measured endothelial function either by FMD or FBF response in the brachial artery. Twenty two studies measured FMD (Table 3) and, of these, 19 reported an improvement in FMD after anti‐TNFα treatment. However, these were largely short‐term studies, with a mean duration of follow up of 24 weeks and a median of 12 weeks. Only 9 studies followed up patients for more than 12 weeks Of these longer duration studies only 4 showed sustained improvement with 2 showing transient effects and 3 showing no effect, including the only randomised trial measuring FMD.18 In 3 studies demonstrating an initial improvement in FMD, this was not sustained at later measurements 46, 55, 58 and, in 1 study, sustained improvement was seen only with adalimumab and no other anti‐TNFα treatments.57 Statistically significant changes in FMD were more variable than PWV or cIMT with absolute increases in FMD in response to anti‐TNFα treatment ranging from 0.9 to 16.23% (median 3.61%).
Table 3.
Studies evaluating the effects of anti‐TNFα treatment on endothelial function measured by FMD in patients with CID
| Reference | CID | Study design | Outcome measures and results |
|---|---|---|---|
| 14 | 14 psoriasis | Patients received adalimumab for 12 weeks. No controls. | 1. FMD improved significantly after 12 weeks (P = .01). |
| 54 | 9 RA | 9 RA patients treated with etanercept/infliximab for 36 weeks, compared to 5 RA patients on conventional treatment. | 1. FMD significantly improved in anti‐TNFα treatment group vs. controls (P = .02) after 36 weeks. |
| 55 | 10 RA | Infliximab administered at wks 0, 2, 6, 14 at 3 mg kg–1. No controls. | 1. FMD improved significantly in RA after each infliximab infusion (P < .05), but no sustained change seen. |
| 56 | 10 RA | 5 treated with infliximab, 5 with etanercept and assessed at 1 day, 1, 2 and 6 weeks. | 1. FMD improved from baseline up to wk 6 (P < .05). |
| 57 | 24 RA | Patients treated with either infliximab, etanercept or adalimumab. Endothelial function measured at day 0, week 1, 2 and 6, and every 6 months. No controls. | 1. FMD improved significantly at 6 weeks (P < .05) post–anti‐TNFα treatment. After 2 years only patients on adalimumab maintained FMD improvement. |
| 58 | 7 RA | 7 RA patients with prior infliximab treatment for at least 1 year had FMD measured weekly for 3 weeks post‐infusion. No controls. | 1. FMD improved significantly up to day 7 post‐infliximab infusion (P = .02), but by day 28 values returned to baseline. |
| 59 | 8 RA | Patients taking infliximab for at least 1 year were switched to adalimumab due to loss of efficacy. Patients assessed prior to (day 0) and day 2, week 2 and 12 after onset of adalimumab therapy. No controls. | 1. Rapid increase in FMD up to wk 12 compared to baseline (P = .012). |
| 43 | 34 RA | Patients received 40 mg adalimumab every other week from day 0 (onset of study) to month 12 (end of the study). Measurements taken at day 0, day 14 and month 12. No controls. | 1. FMD significantly improved at day 14 (P = .03) and month 12 vs. baseline (P < .001). |
| 18 | 21 psoriasis | 21 psoriasis patients randomised to receive either etanercept 20 mg SC twice weekly or placebo for 12 weeks (n not specified). Measurements at baseline, 12 and 24 weeks. | 1. No significant change in FMD at 12 or 24 weeks. |
| 60 | 11 RA | Treatment with infliximab at week 0, 2 and 6 at 3 mg kg–1 measurements taken at baseline and 12 weeks. No controls. | 1. FMD improved significantly 12 weeks post‐treatment (P = .018). |
| 61 | 10 RA | Treatment with infliximab at week 0, 2 and 6 at 3 mg kg–1. No controls. | 1. FMD increased post‐treatment at each week (P < .05). |
| 22 | 8 RA | RA patients received 40 mg subcutaneous adalimumab every 2 weeks. Measurements taken up to week 24. No controls. | 1. FMD improved post‐treatment up to week12 (P < .05). |
| 25 | 9 RA | Patients received etanercept and were assessed at week 0, 4 and 12 after therapy onset. No controls. | 1. FMD significantly increased at weeks 0, 4 and 12 (P = .003). |
| 26 | 17 RA | Patients assessed before and after 8 weeks of etanercept or adalimumab treatment. No controls. | 1. FMD improved after 8 weeks of anti‐TNFα therapy (P = .003). |
| 46 | 23 RA and PsA | 3 groups, treated with either infliximab (n = 13) etanercept (n = 10) or DMARDs (n = 13). Measurements for FMD taken at 1 h and 8–12 wk post‐treatment. | 1. FMD improved significantly at 1 hour post–anti‐TNFα treatment but no significant change at 12 weeks. |
| 31 | 29 psoriasis | Psoriasis patients received 40 mg subcutaneous adalimumab every 2 weeks. Measurements taken at baseline and 6 months. No controls. | 1. FMD improved significantly after 26 weeks of treatment (P = .008). |
| 47 | 32 PsA | Etanercept/infliximab/adalimumab for 2 years. No controls. | 1. No change in FMD at 2 years. |
| 62 | 23 RA | Treated with either infliximab, etanercept or adalimumab. Assesments at baseline, 2 weeks and 3 months. No controls. | 1. No change in FMD post‐treatment. |
| 48 | 12 RA | Patients taking infliximab or adalimumab. Measurements performed at baseline and every 3 months up to 18 months. No controls. | 1. FMD improved significantly at 18 months vs. baseline (P = .026). |
| 63 | 12 AS | Measurements taken at baseline and at 12 weeks, after administration with a single infusion of infliximab at 5 mg kg–1 | 1. FMD improved significantly after 12 weeks (p < 0.001). |
| 64 | 11 RA | Etanercept injected subcutaneously at 2 × 25 mg wk–1 for 12 weeks. No controls. | 1. FMD improved significantly after 12 weeks of anti‐TNF (P = .04). |
| 52 | 13 RA | Cross sectional study comparing 13 RA patients on anti‐TNFα therapy (adalimumab/infliximab/etanercept) and 12 on DMARDs. | 1. Significantly higher FMD in anti‐TNFα group than in DMARDs (p < 0.001). |
CID: chronic inflammatory disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AS: ankylosing spondilitis; AASV: antibody associated systemic vasculitis; CD: Crohn's disease; UC: ulcerative colitis; anti‐TNFα: anti‐tumour necrosis factor α; FMD: flow‐mediated‐dilatation; MTX: methotrexate.
Some studies investigated FBF responses to acetylcholine (endothelium dependent) and glyceryl‐trinitrate (as an endothelium‐independent control) via plethysmography as measures of endothelial function (Table 4). No study showed any difference in endothelium‐independent FBF response (results not shown). Regarding endothelium‐dependent FBF there are overall 7 studies in Table 4 reporting on FBF responses to anti‐TNFα therapy. Five of these reported an improvement after anti‐TNFα therapy, which was only transient in 1 study; none of these studies were randomised controlled trials.
Table 4.
Studies evaluating the effects of anti‐TNFα treatment on endothelial function measured by FBF in patients with CID
| Reference | CID | Study design | Outcome measures and results |
|---|---|---|---|
| 65 | 10 antibody associated systemic vasculitis | Intravenous infusion of infliximab alone or as additional therapy in AASV patients. Measurements taken at baseline and 12 weeks and compared to 21 healthy controls. | 1. No change in FBF compared to control group. |
| 66 | 10 RA | Intravascular administration of infliximab at 200 μg min–1 for 60 min. FBF response measured every 30 min and compared to response to saline infusion. | 1. Improved FBF response to ACh (P = .004) but not to SNP. |
| 67 | 30 RA | 30 RA patients treated with either etanercept/adalimumab and 21 with MTX. Follow‐up at 2 and 4 months. | 1. No improvement in FBF response. |
| 68 | 8 RA | Etanercept for 2 weeks at 2 × 25 mg doses. No controls. | 1. No change in FBF response. |
| 23 | 15 RA | RA subjects received infliximab infusions at week 0, 2 and 6 at 3 mg kg–1. No controls. | 1. Significant improvement in FBF response post‐infliximab (P < .05). |
| 69 | 3 primary systemic vasculitis | 10 infusions of infliximab at 5 mg kg–1. Measurements taken at day 0,1,2,5 and 14. No controls. | 1. FBF to ACh improved significantly within 24 hours of inflimab infusion, but was not maintained at day 14. No change in SNP dilation. |
| 62 | 23 RA | Treated with either infliximab, etanercept or adalimumab. Assessments at baseline, 2 weeks and 3 months. No controls. | 1. ACh mediated dilation improved at week 2 vs. baseline only (P = .001) but no change in SNP dilation. |
| 70 | 12 CD and 12 UC | Cross‐sectional study comparing the response to infliximab infusion for 60 min. IBD groups compared with each other and healthy controls. |
1. Infliximab improved ACh vasodilation in CD vs. controls (P = .005), but not in UC. 2. No change in SNP vasodilation in either CD or UC. |
CID: chronic inflammatory disease; RA: rheumatoid arthritis; PsA: psoriatic arthritis; AS: ankylosing spondilitis; IBD: inflammatory bowel disease; CD: Crohn's disease; UC: ulcerative colitis; anti‐TNF: anti‐tumour necrosis factor; FBF: forearm blood flow; ACh: acetylcholine (endothelium dependant dilation); SNP: sodium nitroprusside (independent dilation); GTN: glyceryl trinitrate; MTX: methotrexate.
4. DISCUSSION
This systematic review identified 60 studies that investigated the effects of treatment with TNFα antagonists on subclinical measures of arteriosclerosis and atherosclerosis in a range of chronic inflammatory diseases. In general, these studies reported a short‐term improvement in FMD in response to anti‐TNFα, with a less pronounced effect on FBF. The results were mixed with regards to PWV and largely negative with regards to AIx and CIMT. These differences may reflect various experimental factors such as the importance of technique and inter‐observer variability in FMD measurement.71 It may also reflect the underlying quality of the studies, which were mostly small scale, with relatively small sample sizes and short follow up.
Importantly, there is a clear dissociation between the results of uncontrolled and controlled studies; the latter were both on average larger and had a longer duration, and were more likely to be negative. The 5 randomised controlled trials in this review were all negative apart from 1,24 which showed a positive effect on AIx. Unlike other measures examined in this review, it is uncertain whether AIx is a measure of arteriosclerosis or atherosclerosis.10 Differences between controlled and uncontrolled studies could represent differences in statistical power, but given that our results have highlighted multiple small studies with positive results and larger negative controlled studies, such differences are more likely to be due to publication bias. It is notable that, with regards to studies measuring FMD, of 22 total studies, the 17 uncontrolled studies were mostly positive whereas all 5 of the controlled studies were negative. Given the heterogenous quality and the negative controlled studies, we find no convincing evidence for an effect of anti‐TNFα treatment on subclinical measures of atherosclerosis and arteriosclerosis.
A previous meta‐analysis by Vlachopoulos et al. looking at effects of anti‐TNFα treatment on measures of arterial stiffness in rheumatoid arthritis6 concluded that there was a positive effect of such treatment on both PWV and AIx. However, they did not distinguish between controlled and uncontrolled studies. Of the 14 studies included, 3 were uncontrolled and 11 were controlled. It is notable that of the only 2 randomised controlled trials the first24 only measured augmentation index and had no placebo control group, whilst the second32 showed a statistically significant improvement in carotid–femoral PWV at 6 months, but not at 12 months; it is thus included as a negative study in our review. The broader scope of our review and distinction between controlled and uncontrolled trials thus probably explains the difference in results.
The above meta‐analysis6 also suggested a greater efficacy of adalimumab and etanercept compared to infliximab. With regards to individual agents, there were no clear differences observable in our review; the majority of the studies included multiple different agents and results were rarely reported individually. However, among single agent studies, some patterns may be of interest. Two studies showed a positive effect of infliximab on arterial stiffness with 4 showing no effect or a negative effect. This compares to 2 positive studies for adalimumab and etanercept, with 2 and 3 negative studies for these 2 agents, respectively. For cIMT, all studies of infliximab were negative, whilst for adalimumab there was 1 positive and 3 negative studies. There were no individual studies of the effects of etanercept on cIMT. For studies on endothelial function, all 5 studies of adalimumab were positive; the 5 studies on infliximab included 3 positive and 2 negative findings. Results for etanercept were positive in 2 studies and negative in 1 study. The 3 studies of golimumab were all negative. The results of multiple agent studies were roughly evenly split between negative and positive. It could thus be argued that our results support Vlachopoulos et al.'s finding of better efficacy of adalimumab and etanercept compared to infliximab, but small sample sizes and variable trial design are a significant confounder and limit the conclusions that can be drawn from these observations.
It is important to distinguish between the effect of anti‐TNFα treatment on subclinical arterial disease and the effect on cardiovascular outcomes. PWV is a predictor of all‐cause mortality in hypertensive patients,9 and is a predictor of future cardiovascular events independent of traditional cardiovascular risk factors.72, 73, 74 There is good evidence that increases in CIMT are associated with an increased risk of future cardiovascular events,75, 76, 77, 78 but there have been conflicting results as to whether CIMT improves performance of traditional risk factor based models,79, 80, 81 with several studies finding little to no improvement. Some authors explain this based on differences in CIMT measurement technique, and suggest that when carotid plaque is measured, CIMT measurement can improve risk prediction 8 Furthermore, there is evidence that CIMT scores normalised for age, sex and ethnicity do provide additional predictive power compared to a traditional model.82 Measures of endothelial function such as FMD are less reproducible, and there is less evidence linking them to clinical end points. There have been 2 positive meta‐analyses of FMD as a predictor of cardiovascular events,7, 83 but both noted that the underlying quality of studies was poor and publication bias likely contributed to the results. FMD does not yet appear to improve prediction compared to traditional vascular risk factors.84
This review did not focus on clinical endpoints, but instead subclinical measures, which can provide mechanistic insight. Thus, our results do not mean that anti‐TNFα treatment could not result in an improvement in outcomes due to other mechanisms; for example, a reduction in prothrombotic tendency 85, 86 or through improvement in quality of life facilitating lifestyle change. Furthermore, this study only addresses anti‐TNFα biologics. The CANTOS trial showed that targeting https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974 can reduce the incidence of cardiovascular endpoints 87 but, as discussed above, this could be due to a variety of mechanisms.
5. CONCLUSION
These results highlight a dissociation in findings between controlled and uncontrolled studies of the effect of anti‐TNFα treatment on subclinical measures of arteriosclerosis and atherosclerosis. Overall, there is no convincing evidence for an effect of anti‐TNFα treatment on these measures.
ACKNOWLEDGEMENTS
This research was supported by the National Institute for Health Research (NIHR) Clinical Research Facility at Guy's & St Thomas' NHS Foundation Trust and NIHR Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London.
COMPETING INTEREST
The authors have no conflicts of interest to declare.
APPENDIX A.
The keywords, operators and terms used systematic search were as follows:
Medline:
| 1 | (Tumo?r necrosis factor alpha adj2 antagonist).Mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 2 | (Tumo?r necrosis factor alpha adj2 antibody).Mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 3 | (Tumo?r necrosis factor alpha adj2 inhibitor).Mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | Anti tumo?r necrosis factor.Mp. |
| 5 | Anti tumo?r necrosis factor alpha.Mp. |
| 6 | (anti TNF or anti‐TNF alpha).Mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | Adalimumab.Mp. |
| 8 | Certolizumab pegol.Mp. |
| 9 | Etanercept.Mp. |
| 10 | Golimumab.Mp. |
| 11 | Infliximab.Mp. |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
| 13 | Exp vascular stiffness/ |
| 14 | Arterial stiffness.Mp. |
| 15 | Arterial function.Mp. |
| 16 | Arterial compliance.Mp. |
| 17 | Vascular function.Mp. |
| 18 | Vascular compliance.Mp. |
| 19 | Exp pulse wave analysis/ |
| 20 | Pulse wave velocity.Mp. |
| 21 | Augmentation index.Mp. |
| 22 | Endothelial dysfunction.Mp. |
| 23 | Endothelial function.Mp. |
| 24 | Flow mediated dilation.Mp. |
| 25 | Flow mediated dilatation.Mp. |
| 26 | Exp carotid intima‐media thickness/ |
| 27 | 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 |
| 28 | (chronic disease adj2 inflammat*).Mp. [mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 29 | Chronic inflammatory disease*.Mp. |
| 30 | Exp arthritis, rheumatoid/ |
| 31 | Exp arthritis, psoriatic/ |
| 32 | Exp spondylitis, ankylosing/ |
| 33 | Exp inflammatory bowel diseases/or exp colitis, ulcerative/or exp crohn disease/ |
| 34 | Exp enteritis/ |
| 35 | Exp dermatitis/ |
| 36 | Exp psoriasis/ |
| 37 | Chronic inflammatory skin disease*.Mp. |
| 38 | Exp skin diseases, vesiculobullous/or exp pemphigoid, bullous/ |
| 39 | Exp Vasculitis/ |
| 40 | Exp lupus erythematosus, systemic/ |
| 41 | Exp pulmonary disease, chronic obstructive/ |
| 42 | Exp bronchitis, chronic/ |
| 43 | 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 |
| 44 | 12 and 27 and 43 |
Embase:
| 1 | Exp tumor necrosis factor alpha antibody/ |
| 2 | Exp tumor necrosis factor antibody/ |
| 3 | Tumo?r necrosis factor antagonist.Mp. |
| 4 | Tumo?r necrosis factor alpha antagonist.Mp. |
| 5 | Exp tumor necrosis factor alpha inhibitor/ |
| 6 | Anti tumo?r necrosis factor.Mp. |
| 7 | Anti tumo?r necrosis factor alpha.Mp. |
| 8 | (anti‐TNF or anti‐TNF alpha).m_titl. |
| 9 | Adalimumab/ |
| 10 | Certolizumab pegol/ |
| 11 | Etanercept/ |
| 12 | Infliximab/ |
| 13 | Golimumab/ |
| 14 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 |
| 15 | Arterial stiffness/ |
| 16 | Exp artery compliance/ |
| 17 | Arter* function.Mp. |
| 18 | Exp pulse wave/ |
| 19 | Pulse wave velocity.Mp. |
| 20 | Pulse wave analysis.Mp. |
| 21 | Exp augmentation index/ |
| 22 | Endothelial dysfunction/ |
| 23 | Endotheli* function*.Mp. |
| 24 | Exp vasodilatation/ |
| 25 | Flow mediated dilat*.Mp. |
| 26 | Exp blood vessel compliance/ |
| 27 | Vascular compliance.Mp. |
| 28 | Exp carotid intima media thickness/ |
| 29 | Carotid intima media thick*.Mp. |
| 30 | Intima media thick*.Mp. |
| 31 | 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 |
| 32 | (chronic disease* adj2 inflammat*).Mp. [mp = title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word, candidate term word] |
| 33 | Chronic inflammatory disease*.Mp. |
| 34 | Exp rheumatoid arthritis/ |
| 35 | Exp psoriatic arthritis/ |
| 36 | Exp ankylosing spondylitis/ |
| 37 | Inflammatory bowel disease*.Mp. |
| 38 | Exp Crohn disease/ |
| 39 | Colitis/or exp ulcerative colitis/ |
| 40 | Exp enteritis/ |
| 41 | Chronic inflammatory skin disease*.Mp. |
| 42 | Exp dermatitis/ |
| 43 | Psoriasis vulgaris/or psoriasis/ |
| 44 | Bullous skin disease/or bullous pemphigoid/ |
| 45 | Exp vasculitis/ |
| 46 | Exp systemic lupus erythematosus/ |
| 47 | Exp chronic obstructive lung disease/ |
| 48 | Chronic obstructive pulmonary disease.Mp. |
| 49 | Exp chronic bronchitis/ |
| 50 | 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 |
| 51 | 14 and 31 and 50 |
Knowles L, Nadeem N, Chowienczyk PJ. Do anti‐tumour necrosis factor‐α biologics affect subclinical measures of atherosclerosis and arteriosclerosis? A systematic review. Br J Clin Pharmacol. 2020;86:837–851. 10.1111/bcp.14215
Laurence Knowles and Nida Nadeem, Equal contributions
REFERENCES
- 1. Ross R. Atherosclerosis‐‐an inflammatory disease. N Engl J Med. 1999;340(2):115‐126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- 2. Lee H‐Y, Oh B‐H. Aging and arterial stiffness. Circ J. 2010;74(11):2257‐2262. [DOI] [PubMed] [Google Scholar]
- 3. Tsamis A, Krawiec JT, Vorp DA. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10(83). 10.1098/rsif.2012.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54(6):1328‐1336. 10.1161/HYPERTENSIONAHA.109.137653 [DOI] [PubMed] [Google Scholar]
- 5. Szekanecz Z, Kerekes G, Soltész P. Vascular effects of biologic agents in RA and spondyloarthropathies. Nat Rev Rheumatol. 2009;5(12):677‐684. 10.1038/nrrheum.2009.219 [DOI] [PubMed] [Google Scholar]
- 6. Vlachopoulos C, Gravos A, Georgiopoulos G, et al. The effect of TNF‐a antagonists on aortic stiffness and wave reflections: a meta‐analysis. Clin Rheumatol. 2018;37(2):515‐526. 10.1007/s10067-017-3657-y [DOI] [PubMed] [Google Scholar]
- 7. Charakida M, Masi S, Loukogeorgakis SP, Deanfield JE. The role of flow‐mediated dilatation in the evaluation and development of antiatherosclerotic drugs. Curr Opin Lipidol. 2009;20(6):460‐466. 10.1097/MOL.0b013e3283330518 [DOI] [PubMed] [Google Scholar]
- 8. Naqvi TZ, Lee M‐S. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025‐1038. 10.1016/j.jcmg.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236‐1241. [DOI] [PubMed] [Google Scholar]
- 10. Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse‐wave velocity in healthy men. Hypertension. 2001;37(6):1429‐1433. 10.1161/01.HYP.37.6.1429 [DOI] [PubMed] [Google Scholar]
- 11. Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor‐alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. 2010;55(2):333‐338. 10.1161/HYPERTENSIONAHA.109.143982 [DOI] [PubMed] [Google Scholar]
- 12. Angel K, Provan SA, Hammer HB, Mowinckel P, Kvien TK, Atar D. Changes in arterial stiffness during continued infliximab treatment in patients with inflammatory arthropathies. Fundam Clin Pharmacol. 2011;25(4):511‐517. 10.1111/j.1472-8206.2010.00872.x [DOI] [PubMed] [Google Scholar]
- 13. Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1‐year anti‐TNF‐α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25(6):644‐650. 10.1038/ajh.2012.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avgerinou G, Tousoulis D, Siasos G, et al. Anti‐tumor necrosis factor α treatment with adalimumab improves significantly endothelial function and decreases inflammatory process in patients with chronic psoriasis. Int J Cardiol. 2011;151(3):382‐383. 10.1016/j.ijcard.2011.06.112 [DOI] [PubMed] [Google Scholar]
- 15. Capkin E, Kiris A, Karkucak M, et al. Investigation of effects of different treatment modalities on structural and functional vessel wall properties in patients with ankylosing spondylitis. Joint Bone Spine. 2011;78(4):378‐382. 10.1016/j.jbspin.2010.09.023 [DOI] [PubMed] [Google Scholar]
- 16. Cypiene A, Laucevicius A, Venalis A, et al. Non‐invasive assessment of arterial stiffness indices by applanation tonometry and pulse wave analysis in patients with rheumatoid arthritis treated with TNF‐alpha blocker remicade (infliximab). Proc West Pharmacol Soc. 2007;50:119‐122. [PubMed] [Google Scholar]
- 17. Galarraga B, Khan F, Kumar P, Pullar T, Belch JJF. Etanercept improves inflammation‐associated arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford). 2009;48(11):1418‐1423. 10.1093/rheumatology/kep251 [DOI] [PubMed] [Google Scholar]
- 18. Hayek SS, Neuman R, Kavtaradze N, et al. Tumor necrosis factor‐alpha antagonism with etanercept improves endothelial progenitor cell counts in patients with psoriasis: etanercept, vascular function and endothelial progenitor cells in psoriasis. Int J Cardiol. 2015;182:387‐389. 10.1016/j.ijcard.2014.12.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ikonomidis I, Papadavid E, Makavos G, et al. Lowering Interleukin‐12 activity improves myocardial and vascular function compared with tumor necrosis factor‐a antagonism or cyclosporine in psoriasis. Circ Cardiovasc Imaging. 2017;10(9). 10.1161/CIRCIMAGING.117.006283 [DOI] [PubMed] [Google Scholar]
- 20. Daïen CI, Fesler P, du Cailar G, et al. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):881‐887. 10.1136/annrheumdis-2012-201489 [DOI] [PubMed] [Google Scholar]
- 21. Karkucak M, Çapkin E. Ki˙ri˙ş a, et al. arterial stiffness and anti‐tumor necrosis factor‐alpha therapy in ankylosing spondylitis: results with long‐term two year‐follow‐up. Arch Rheumatol. 2014;29(4):250‐256. 10.5606/ArchRheumatol.2014.4261 [DOI] [Google Scholar]
- 22. Kerekes G, Soltész P, Szucs G, et al. Effects of adalimumab treatment on vascular disease associated with early rheumatoid arthritis. Isr Med Assoc J. 2011;13(3):147‐152. [PubMed] [Google Scholar]
- 23. Komai N, Morita Y, Sakuta T, Kuwabara A, Kashihara N. Anti‐tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod Rheumatol. 2007;17(5):385‐390. 10.1007/s10165-007-0605-8 [DOI] [PubMed] [Google Scholar]
- 24. Kume K, Amano K, Yamada S, Hatta K, Ohta H, Kuwaba N. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open‐label randomized controlled trial. J Rheumatol. 2011;38(10):2169‐2171. 10.3899/jrheum.110340 [DOI] [PubMed] [Google Scholar]
- 25. Mäki‐Petäjä KM, Hall FC, Booth AD, et al. Rheumatoid arthritis is associated with increased aortic pulse‐wave velocity, which is reduced by anti‐tumor necrosis factor‐alpha therapy. Circulation. 2006;114(11):1185‐1192. 10.1161/CIRCULATIONAHA.105.601641 [DOI] [PubMed] [Google Scholar]
- 26. Mäki‐Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti‐tumor necrosis factor‐α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473‐2480. 10.1161/CIRCULATIONAHA.112.120410 [DOI] [PubMed] [Google Scholar]
- 27. Mathieu S, Joly H, Baron G, et al. Trend towards increased arterial stiffness or intima‐media thickness in ankylosing spondylitis patients without clinically evident cardiovascular disease. Rheumatology (Oxford). 2008;47(8):1203‐1207. 10.1093/rheumatology/ken198 [DOI] [PubMed] [Google Scholar]
- 28. Mathieu S, Pereira B, Couderc M, Rabois E, Dubost J‐J, Soubrier M. No significant changes in arterial stiffness in patients with ankylosing spondylitis after tumour necrosis factor alpha blockade treatment for 6 and 12 months. Rheumatology (Oxford). 2013;52(1):204‐209. 10.1093/rheumatology/kes272 [DOI] [PubMed] [Google Scholar]
- 29. Ntusi NAB, Francis JM, Sever E, et al. Anti‐TNF modulation reduces myocardial inflammation and improves cardiovascular function in systemic rheumatic diseases. Int J Cardiol. 2018;270:253‐259. 10.1016/j.ijcard.2018.06.099 [DOI] [PubMed] [Google Scholar]
- 30. Pieringer H, Stuby U, Pohanka E, Biesenbach G. Augmentation index in patients with rheumatoid arthritis and ankylosing spondylitis treated with infliximab. Clin Rheumatol. 2010;29(7):723‐727. 10.1007/s10067-010-1388-4 [DOI] [PubMed] [Google Scholar]
- 31. Pina T, Corrales A, Lopez‐Mejias R, et al. Anti‐tumor necrosis factor‐alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6‐month prospective study. J Dermatol. 2016;43(11):1267‐1272. 10.1111/1346-8138.13398 [DOI] [PubMed] [Google Scholar]
- 32. Tam L‐S, Shang Q, Li EK, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis ‐‐ a randomized trial. J Rheumatol. 2012;39(12):2267‐2275. 10.3899/jrheum.120541 [DOI] [PubMed] [Google Scholar]
- 33. Tam L‐S, Shang Q, Kun EW, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis—a randomized, placebo‐controlled pilot trial. Rheumatology (Oxford). 2014;53(6):1065‐1074. 10.1093/rheumatology/ket469 [DOI] [PubMed] [Google Scholar]
- 34. Van Doornum S, McColl G, Wicks IP. Tumour necrosis factor antagonists improve disease activity but not arterial stiffness in rheumatoid arthritis. Rheumatology (Oxford). 2005;44(11):1428‐1432. 10.1093/rheumatology/kei033 [DOI] [PubMed] [Google Scholar]
- 35. Vassilopoulos D, Gravos A, Vlachopoulos C, et al. Adalimumab decreases aortic stiffness independently of its effect in disease activity in patients with rheumatoid arthritis. Clin Rheumatol. 2015;34(2):359‐364. 10.1007/s10067-014-2718-8 [DOI] [PubMed] [Google Scholar]
- 36. Wong M, Oakley SP, Young L, et al. Infliximab improves vascular stiffness in patients with rheumatoid arthritis. Ann Rheum Dis. 2009;68(8):1277‐1284. 10.1136/ard.2007.086157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zanoli L, Rastelli S, Inserra G, et al. Increased arterial stiffness in inflammatory bowel diseases is dependent upon inflammation and reduced by immunomodulatory drugs. Atherosclerosis. 2014;234(2):346‐351. 10.1016/j.atherosclerosis.2014.03.023 [DOI] [PubMed] [Google Scholar]
- 38. Bergström U, Jovinge S, Persson J, Jacobsson LTH, Turesson C. Effects of treatment with Adalimumab on blood lipid levels and atherosclerosis in patients with rheumatoid arthritis. Curr Ther Res Clin Exp. 2018;89:1‐6. 10.1016/j.curtheres.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Del Porto F, Laganà B, Lai S, et al. Response to anti‐tumour necrosis factor alpha blockade is associated with reduction of carotid intima‐media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford). 2007;46(7):1111‐1115. 10.1093/rheumatology/kem089 [DOI] [PubMed] [Google Scholar]
- 40. Di Micco P, Ferrazzi P, Librè L, et al. Intima‐media thickness evolution after treatment with infliximab in patients with rheumatoid arthritis. Int J Gen Med. 2009;2:141‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Minno MND, Iervolino S, Peluso R, Scarpa R, Di Minno G. CaRRDs study group. Carotid intima‐media thickness in psoriatic arthritis: differences between tumor necrosis factor‐α blockers and traditional disease‐modifying antirheumatic drugs. Arterioscler Thromb Vasc Biol. 2011;31(3):705‐712. 10.1161/ATVBAHA.110.214585 [DOI] [PubMed] [Google Scholar]
- 42. Ferrante A, Giardina AR, Ciccia F, et al. Long‐term anti‐tumour necrosis factor therapy reverses the progression of carotid intima‐media thickness in female patients with active rheumatoid arthritis. Rheumatol Int. 2009;30(2):193‐198. 10.1007/s00296-009-0935-2 [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez‐Juanatey C, Vazquez‐Rodriguez TR, Miranda‐Filloy JA, et al. Anti‐TNF‐alpha‐adalimumab therapy is associated with persistent improvement of endothelial function without progression of carotid intima‐media wall thickness in patients with rheumatoid arthritis refractory to conventional therapy. Mediators Inflamm. 2012;2012:674265 10.1155/2012/674265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jókai H, Szakonyi J, Kontár O, et al. Impact of effective tumor necrosis factor‐alfa inhibitor treatment on arterial intima‐media thickness in psoriasis: results of a pilot study. J am Acad Dermatol. 2013;69(4):523‐529. 10.1016/j.jaad.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 45. Martinez‐Lopez A, Blasco‐Morente G, Perez‐Lopez I, Tercedor‐Sanchez J, Arias‐Santiago S. Studying the effect of systemic and biological drugs on intima‐media thickness in patients suffering from moderate and severe psoriasis. J Eur Acad Dermatol Venereol. 2018;32(9):1492‐1498. 10.1111/jdv.14841 [DOI] [PubMed] [Google Scholar]
- 46. Mazzoccoli G, Notarsanto I, de Pinto GD, et al. Anti‐tumor necrosis factor‐α therapy and changes of flow‐mediated vasodilatation in psoriatic and rheumatoid arthritis patients. Intern Emerg Med. 2010;5(6):495‐500. 10.1007/s11739-010-0458-6 [DOI] [PubMed] [Google Scholar]
- 47. Ramonda R, Puato M, Punzi L, et al. Atherosclerosis progression in psoriatic arthritis patients despite the treatment with tumor necrosis factor‐alpha blockers: a two‐year prospective observational study. Joint Bone Spine. 2014;81(5):421‐425. 10.1016/j.jbspin.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 48. Sidiropoulos PI, Siakka P, Pagonidis K, et al. Sustained improvement of vascular endothelial function during anti‐TNFalpha treatment in rheumatoid arthritis patients. Scand J Rheumatol. 2009;38(1):6‐10. 10.1080/03009740802363768 [DOI] [PubMed] [Google Scholar]
- 49. Turiel M, Tomasoni L, Sitia S, et al. Effects of long‐term disease‐modifying antirheumatic drugs on endothelial function in patients with early rheumatoid arthritis. Cardiovasc Ther. 2010;28(5):e53‐e64. 10.1111/j.1755-5922.2009.00119.x [DOI] [PubMed] [Google Scholar]
- 50. van Sijl AM, van Eijk IC, Peters MJL, et al. Tumour necrosis factor blocking agents and progression of subclinical atherosclerosis in patients with ankylosing spondylitis. Ann Rheum Dis. 2015;74(1):119‐123. 10.1136/annrheumdis-2013-203934 [DOI] [PubMed] [Google Scholar]
- 51. Wasko MC, Hsia EC, Kirkham B, et al. Effect of golimumab on carotid atherosclerotic disease measures and cardiovascular events in inflammatory arthritides. J Clin Rheumatol. 2014;20(1):1‐10. 10.1097/RHU.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 52. Watanabe T, Takemura M, Sato M, et al. Clinical significance of brachial flow‐mediated dilation in patients with rheumatoid arthritis. Int J Rheum Dis. 2014;17(1):26‐33. 10.1111/1756-185x.12021 [DOI] [PubMed] [Google Scholar]
- 53. Zardi EM, Pipita ME, Giorgi C, Lichinchi D, Zardi DM, Afeltra A. Differences in carotid atherosclerosis between patients with ankylosing spondylitis treated with tumor necrosis factor‐α antagonists and healthy matched controls. Medicine (Baltimore). 2018;97(27):e11250 10.1097/MD.0000000000011250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bilsborough W, Keen H, Taylor A, O'Driscoll GJ, Arnolda L, Green DJ. Anti‐tumour necrosis factor‐alpha therapy over conventional therapy improves endothelial function in adults with rheumatoid arthritis. Rheumatol Int. 2006;26(12):1125‐1131. 10.1007/s00296-006-0147-y [DOI] [PubMed] [Google Scholar]
- 55. Bosello S, Santoliquido A, Zoli A, et al. TNF‐alpha blockade induces a reversible but transient effect on endothelial dysfunction in patients with long‐standing severe rheumatoid arthritis. Clin Rheumatol. 2008;27(7):833‐839. 10.1007/s10067-007-0803-y [DOI] [PubMed] [Google Scholar]
- 56. Capria A, De Nardo D, De Sanctis G, et al. Endothelial dysfunction in rheumatoid arthritis is improved by anti‐tumor necrosis Factorα treatment. Eur J Inflamm. 2004;2(3):113‐118. 10.1177/1721727X0400200303 [DOI] [Google Scholar]
- 57. Capria A, De Nardo D, Baffetti FR, et al. Long‐term anti‐TNF‐alpha treatments reverse the endothelial dysfunction in rheumatoid arthritis: the biological coherence between synovial and endothelial inflammation. Int J Immunopathol Pharmacol. 2010;23(1):255‐262. 10.1177/039463201002300123 [DOI] [PubMed] [Google Scholar]
- 58. Gonzalez‐Juanatey C, Testa A, Garcia‐Castelo A, Garcia‐Porrua C, Llorca J, Gonzalez‐Gay MA. Active but transient improvement of endothelial function in rheumatoid arthritis patients undergoing long‐term treatment with anti‐tumor necrosis factor alpha antibody. Arthritis Rheum. 2004;51(3):447‐450. 10.1002/art.20407 [DOI] [PubMed] [Google Scholar]
- 59. Gonzalez‐Juanatey C, Llorca J, Sanchez‐Andrade A, Garcia‐Porrua C, Martin J, Gonzalez‐Gay MA. Short‐term adalimumab therapy improves endo‐thelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol. 2006;24(3):309‐312. [PubMed] [Google Scholar]
- 60. Hürlimann D, Forster A, Noll G, et al. Anti‐tumor necrosis factor‐alpha treatment improves endothelial function in patients with rheumatoid arthritis. Circulation. 2002;106(17):2184‐2187. [DOI] [PubMed] [Google Scholar]
- 61. Irace C, Mancuso G, Fiaschi E, Madia A, Sesti G, Gnasso A. Effect of anti TNFalpha therapy on arterial diameter and wall shear stress and HDL cholesterol. Atherosclerosis. 2004;177(1):113‐118. 10.1016/j.atherosclerosis.2004.04.031 [DOI] [PubMed] [Google Scholar]
- 62. Sandoo A, van Zanten JJCSV, Toms TE, Carroll D, Kitas GD. Anti‐TNFα therapy transiently improves high density lipoprotein cholesterol levels and microvascular endothelial function in patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord. 2012;13(127). 10.1186/1471-2474-13-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Syngle A, Vohra K, Sharma A, Kaur L. Endothelial dysfunction in ankylosing spondylitis improves after tumor necrosis factor‐alpha blockade. Clin Rheumatol. 2010;29(7):763‐770. 10.1007/s10067-010-1402-x [DOI] [PubMed] [Google Scholar]
- 64. Tikiz H, Arslan O, Pirildar T, Tikiz C, Bayindir P. The effect of anti‐tumor necrosis factor (TNF)‐alpha therapy with etanercept on endothelial functions in patients with rheumatoid arthritis. Anadolu Kardiyol Derg. 2010;10(2):98‐103. 10.5152/akd.2010.031 [DOI] [PubMed] [Google Scholar]
- 65. Booth AD, Jayne DRW, Kharbanda RK, et al. Infliximab improves endothelial dysfunction in systemic vasculitis: a model of vascular inflammation. Circulation. 2004;109(14):1718‐1723. 10.1161/01.CIR.0000124720.18538.DD [DOI] [PubMed] [Google Scholar]
- 66. Cardillo C, Schinzari F, Mores N, et al. Intravascular tumor necrosis factor alpha blockade reverses endothelial dysfunction in rheumatoid arthritis. Clin Pharmacol Ther. 2006;80(3):275‐281. 10.1016/j.clpt.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 67. Galarraga B, Belch JJF, Pullar T, Ogston S, Khan F. Clinical improvement in rheumatoid arthritis is associated with healthier microvascular function in patients who respond to antirheumatic therapy. J Rheumatol. 2010;37(3):521‐528. 10.3899/jrheum.090417 [DOI] [PubMed] [Google Scholar]
- 68. Hänsel S, Lässig G, Pistrosch F, Passauer J. Endothelial dysfunction in young patients with long‐term rheumatoid arthritis and low disease activity. Atherosclerosis. 2003;170(1):177‐180. [DOI] [PubMed] [Google Scholar]
- 69. Raza K, Carruthers DM, Stevens R, Filer AD, Townend JN, Bacon PA. Infliximab leads to a rapid but transient improvement in endothelial function in patients with primary systemic vasculitis. Ann Rheum Dis. 2006;65(7):946‐948. 10.1136/ard.2005.043638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schinzari F, Armuzzi A, De Pascalis B, et al. Tumor necrosis factor‐alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83(1):70‐76. 10.1038/sj.clpt.6100229 [DOI] [PubMed] [Google Scholar]
- 71. Bruno RM, Bianchini E, Faita F, Taddei S, Ghiadoni L. Intima media thickness, pulse wave velocity, and flow mediated dilation. Cardiovasc Ultrasound. 2014;12:34 10.1186/1476-7120-12-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J am Coll Cardiol. 2014;63(7):636‐646. 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69(6):1045‐1052. 10.1161/HYPERTENSIONAHA.117.09097 [DOI] [PubMed] [Google Scholar]
- 74. Zhong Q, Hu M‐J, Cui Y‐J, et al. Carotid‐femoral pulse wave velocity in the prediction of cardiovascular events and mortality: an updated systematic review and meta‐analysis. Angiology. 2018;69(7):617‐629. 10.1177/0003319717742544 [DOI] [PubMed] [Google Scholar]
- 75. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987‐1993. Am J Epidemiol. 1997;146(6):483‐494. 10.1093/oxfordjournals.aje.a009302 [DOI] [PubMed] [Google Scholar]
- 76. Lorenz MW, Schaefer C, Steinmetz H, Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten‐year results from the carotid atherosclerosis progression study (CAPS). Eur Heart J. 2010;31(16):2041‐2048. 10.1093/eurheartj/ehq189 [DOI] [PubMed] [Google Scholar]
- 77. O'Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the cardiovascular health study. The CHS collaborative research group. Stroke. 1992;23(12):1752‐1760. [DOI] [PubMed] [Google Scholar]
- 78. Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb. 1991;11(5):1245‐1249. [DOI] [PubMed] [Google Scholar]
- 79. Den Ruijter HM, Peters SAE, Anderson TJ, et al. Common carotid intima‐media thickness measurements in cardiovascular risk prediction: a meta‐analysis. JAMA. 2012;308(8):796‐803. 10.1001/jama.2012.9630 [DOI] [PubMed] [Google Scholar]
- 80. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115(4):459‐467. 10.1161/CIRCULATIONAHA.106.628875 [DOI] [PubMed] [Google Scholar]
- 81. van den Oord SCH, Sijbrands EJG, ten Kate GL, et al. Carotid intima‐media thickness for cardiovascular risk assessment: systematic review and meta‐analysis. Atherosclerosis. 2013;228(1):1‐11. 10.1016/j.atherosclerosis.2013.01.025 [DOI] [PubMed] [Google Scholar]
- 82. Polak JF, Szklo M, O'Leary DH. Carotid intima‐media thickness score, positive coronary artery calcium score, and incident coronary heart disease: the multi‐ethnic study of atherosclerosis. J am Heart Assoc. 2017;6(1). 10.1161/JAHA.116.004612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26(6):631‐640. 10.1007/s10554-010-9616-1 [DOI] [PubMed] [Google Scholar]
- 84. Yeboah J, McClelland RL, Polonsky TS, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate‐risk individuals. JAMA. 2012;308(8):788‐795. 10.1001/jama.2012.9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beinsberger J, Heemskerk JWM, Cosemans JMEM. Chronic arthritis and cardiovascular disease: altered blood parameters give rise to a prothrombotic propensity. Semin Arthritis Rheum. 2014;44(3):345‐352. 10.1016/j.semarthrit.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 86. Ferraccioli G, Gremese E. Thrombogenicity of TNF alpha in rheumatoid arthritis defined through biological probes: TNF alpha blockers. Autoimmun Rev. 2004;3(4):261‐266. 10.1016/j.autrev.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 87. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. New England Journal of Medicine. 2017;377(12):1119‐1131. 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]


