This nonrandomized controlled trial evaluates the feasibility and tolerability of local injections of autologous adipose tissue–derived stromal vascular fraction in patients with scarred vocal folds.
Key Points
Question
Is autologous adipose tissue–derived stromal vascular fraction feasible as a new treatment for scarred vocal folds?
Findings
In this nonrandomized controlled trial of 8 patients (7 women and 1 man) with severe dysphonia due to vocal fold scarring, only anticipated adverse events associated with liposuction and adipose tissue–derived stromal vascular fraction injection occurred but resolved spontaneously.
Meaning
The findings suggest that injection of autologous adipose tissue–derived stromal vascular fraction in scarred vocal folds is feasible and deserves further evaluation in a randomized clinical trial.
Abstract
Importance
Patients with scarred vocal folds, whether congenitally or after phonosurgery, often exhibit dysphonia that negatively affects daily life and is difficult to treat. The autologous adipose tissue–derived stromal vascular fraction (ADSVF) is a readily accessible source of cells with angiogenic, anti-inflammatory, immunomodulatory, and regenerative properties.
Objective
To evaluate the feasibility and tolerability of local injections of autologous ADSVF in patients with scarred vocal folds.
Design, Setting, and Participants
CELLCORDES (Innovative Treatment for Scarred Vocal Cords by Local Injection of Autologous Stromal Vascular Fraction) is a prospective, open-label, single-arm, single-center, nonrandomized controlled trial with a 12-month follow-up and patient enrollment from April 1, 2016, to June 30, 2017. Eight patients with severe dysphonia attributable to vocal fold scarring associated with a congenital malformation or resulting from microsurgical sequelae (voice handicap index score >60 of 120) completed the study. Data analysis was performed from September 1, 2018, to January 1, 2019.
Interventions
Injection of ADSVF into 1 or 2 vocal folds.
Main Outcomes and Measures
The primary outcomes were feasibility and the number and severity of adverse events associated with ADSVF-based therapy. The secondary outcomes were changes in vocal assessment, videolaryngostroboscopy, self-evaluation of dysphonia, and quality of life at 1, 6, and 12 months after cell therapy.
Results
Seven women and 1 man (mean [SD] age, 44.6 [10.4] years) were enrolled in this study. Adverse events associated with liposuction and ADSVF injection occurred; most of them resolved spontaneously. One patient received minor treatment to drain local bruising, and another experienced a minor contour defect at the liposuction site. At 12 months, the voice handicap index score was improved in all patients, with a mean (SD) improvement from baseline of 40.1 (21.5) points. Seven patients (88%) were considered to be responders, defined as improvement by 18 points or more in the voice handicap index score (the minimum clinically important difference).
Conclusions and Relevance
The findings suggest that autologous ADSVF injection in scarred vocal folds is feasible and tolerable. The findings require confirmation in a randomized clinical trial with a larger population.
Trial Registration
ClinicalTrials.gov Identifier: NCT02622464
Introduction
The microstructure of the vocal folds is complex1,2 in particular because of their foliated organization. The proportions and organization of the extracellular matrix components in each layer determine the mechanical properties of the vocal folds. Vocal fold scarring is sometimes observed after laryngeal microsurgery because of the partial disappearance of the lamina propria, with the superficial and/or intermediate layer replaced by fibrous tissue, thereby inducing vibration disorder. This scarring is often accompanied by loss of volume and a glottal defect. Noniatrogenic scar tissue owing to congenital issues or acquired as a result of trauma or chronic inflammatory phenomena is also found.
Such scarring can be disabling, especially for communication professionals. Patients usually present with long-lasting dysphonia, loss of vocal control, vocal fatigue, and a breathy voice that is frequently too high-pitched and has difficulty sustaining tone.
Current therapeutic options are limited in part because of the high complexity of the microstructure of the vocal folds.1 In cases in which an insufficient glottic closure is the predominant issue, medialization procedures have proved to be effective.1,3 When rigidity is the major feature, other techniques have been described (eg, scar resection, microflap,4 and hyaluronic acid5,6) but are often disappointing, especially in the long term,7,8,9 and may worsen the situation by leading to additional scarring. Thus, development of innovative strategies to improve the vibrational mechanical properties of the vocal folds is an important clinical challenge.1,3
The autologous adipose tissue–derived stromal vascular fraction (ADSVF) is a heterogeneous population of cells that comprise mesenchymal stem cells (MSCs) or adipose-derived stem cells (ADSCs), fibroblasts, leukocytes, endothelial cells, progenitors, and pericytes.10 The ADSCs can be used alone as a homogeneous cell product, but their culture requires several weeks. However, ADSVF is a heterogeneous cell product; its components exert a synergistic effect, and it does not require culture. Furthermore, its preparation based on enzymatic digestion of adipose tissue takes merely hours, facilitating its autologous use.
The ADSVF is a readily accessible source of cells with angiogenic, anti-inflammatory, immunomodulatory, and regenerative properties.11 Recent experimental and clinical reports also supported the antifibrotic potential of ADSVF, which was mainly attributed to the ADSC subset.
A preclinical study12 found that cultured ADSCs are associated with the processes underlying vocal fold scarring. Coculture of ADSCs with scar tissue fibroblasts was associated with decreased proliferation and expression of α–smooth muscle actin (a marker of myofibroblast differentiation) and with production of an extracellular matrix with less collagen and more hyaluronic acid.13,14 Some in vivo animal studies14 reported significant improvements in vocal fold scarring after injection of ADSCs immediately after injury or after a longer interval, as evidenced by the macroscopic and histologic structures of the vocal folds15,16,17,18,19,20,21,22,23,24 along with their viscoelastic properties.16,18,22,25 We hypothesized that use of whole ADSVF has advantages over ADSCs alone. The complementary properties of its various components could optimize the healing process.
The safety and efficacy of ADSVF cells have been examined in various conditions, including those with vascular or immune components, such as burn wound healing,26 lower-limb ischemia,27 myocardial infarction,28 graft-vs-host disease,29 and systemic sclerosis.30 However, extrapolation of the benefits of ADSVF to the vocal folds requires caution because the actors in the scarring process, including fibroblasts, extracellular matrix components, and growth factors, differ depending on the tissue.31 Nevertheless, the outcomes of the second patient treated (who was included in this study) encouraged us to evaluate ADSVF in a larger number of patients.32 We evaluated the feasibility and tolerability and performed a preliminary assessment of the potential efficacy of local injections of autologous ADSVF as an innovative therapeutic strategy for patients with scarred vocal folds.
Methods
Study Design and Eligibility Criteria
CELLCORDES (Innovative Treatment for Scarred Vocal Cords by Local Injection of Autologous Stromal Vascular Fraction) is a prospective, open-label, single-arm, single-center, nonrandomized controlled trial designed to evaluate the feasibility and tolerability of local injection of autologous ADSVF into scarred vocal folds (trial protocol in Supplement 1). Eight patients with scarred vocal folds were enrolled in the study from April 1, 2016, to June 30, 2017 (Figure 1). Data analysis was performed from September 1, 2018, to January 1, 2019. The study was approved by the French National Agency for Medicines and Health Products Safety and the French ethics committee (Comite de Protection des Personnes Sud Mediterranee V) and was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent. A data and safety monitoring board reviewed and evaluated the accumulated study data in terms of participants’ safety, study conduct, and progress. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Figure 1. CONSORT Flow Diagram for the Single-Arm, Open-Label, Phase 1 Clinical Trial.
In addition to a thorough medical history, videolaryngostroboscopy was used for diagnosis based on the following major clinical features1: a spindle-shaped glottis during phonation (insufficient glottic closure and air leakage) and impaired vocal fold vibration (reduced amplitude and mucosal wave). In 4 patients, prior interventions had failed (scar resection [n = 4] and corticosteroid injection [n = 1]). All participants had received extensive voice therapy without improvement before enrollment. All the patients fulfilled the inclusion criteria, and none met the exclusion criteria (Box).
Box. Inclusion and Exclusion Criteria for the CELLCORDES Clinical Trial.
Inclusion Criteria
Subscription to the French Social Security System
Signed informed consent
Voice Handicap Index score >60 of 120
Scarred vocal folds, congenital (sulcus) or after phonosurgery
Scarred middle third in stroboscopy
At least 1 year of dysphonia after initial surgery if appropriate
Aged between 18 and 65 years
Good general condition
Negative pregnancy test result and contraception for women of childbearing age
Exclusion Criteria
Specific
Refusal of speech therapy
History of malignant lesion or severe dysplasia of the scarred vocal fold
Contraindication to anesthesia
Anticoagulant treatment
Coagulation disorders (prothrombin time <65%, kaolin-activated partial thromboplastin time >1.2 seconds)
Active infectious diseases
Positive serologic test results for HIV, hepatitis B virus, hepatitis C virus, human T-lymphotropic virus, or syphilis
Necessity of intraoperative prophylactic antibiotics
Nonspecific
Persons having taken an investigational medicinal product in the last 3 months
Refusal or inability to comply with study procedures
Pregnant and lactating women
Patients under curatorship or guardianship
Persons living in public health facilities or residential care
Minors
Persons without French Social Security System coverage
Persons deprived of liberty or detainees
Persons in high-risk and emergency situations
Collection of Adipose Tissue Samples and Preparation of ADSVF
Adipose tissue samples were collected with the patient under conscious sedation with local anesthesia. The fat harvesting site was determined during the first consultation with the plastic surgeon based on the patient’s adipose tissue distribution (in order of preference: subumbilical region and flank-peritrochanteric area). The adipose tissue sample was harvested using a 10-mL syringe in a closed circuit with a 3-mm Khouri cannula and a 500-mL collection bag. At least 100 mL of lipoaspirate was collected, immediately transported to the authorized cell therapy unit, and transferred into the Celution 800/CRS system (Cytori Therapeutics Inc). Collected lipoaspirate was washed and enzymatically digested to obtain ADSVF in accordance with the European regulations and Good Manufacturing Practice for advanced therapy medicinal products. Cells were concentrated, washed, aseptically recovered, and resuspended in 5 mL of lactated Ringer solution using Good Manufacturing Practice–grade reagents. At least 1 mL was obtained for injection, and the remaining cells were used for biological characterization32,33 (eMethods 1 in Supplement 2). During the preparation of ADSVF (4 hours), patients were transported to the recovery room.
Treatment Procedure
The second surgical step consisted of reinjection of ADSVF while the patient was under general anesthesia (suspension direct laryngoscopy). The ADSVF suspension was injected using a 14-gauge needle into the anterior and/or middle third of the scarred vocal fold(s). Fibrous tissue in the area was not resected to avoid increasing the atrophy of the vocal fold. All ADSVF injections were performed by 1 laryngologist (A.G.).
Patients were allowed to leave the hospital in the evening (outpatient surgery) or on the following day with a prescription for 1 g of acetaminophen to be taken 4 times a day for 7 days in case of pain and 1 g of amoxicillin and 125 mg of clavulanic acid to be taken 3 times a day for 2 days. The standard postoperative speech therapy was prescribed after surgery.
Clinical Assessment
The primary end points were feasibility of subepithelial injection without epithelial rupture (sufficient product fluid) during surgery and adverse events and the status of the injection site and airway using videolaryngoscopy after injection. The assessments were performed at day 7 (±4 days) and months 1 (±7 days), 6 (±30 days), and 12 (±30 days). The secondary end point was potential efficacy at months 1, 6, and 12 (except for the Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36], which was evaluated at 6 and 12 months).
The vocal assessment included the following: (1) self-evaluation of dysphonia using the Voice Handicap Index (VHI); (2) self-evaluation of quality of life using the SF-36; (3) videolaryngostroboscopy assessment of glottal closure, regularity, and vibration (mucosal wave)34; (4) perceptual analysis using the Hirano simplified GRB scale35 (G, global; R, roughness; and B, breathiness); (5) acoustic analysis, including the signal to noise ratio and vocal range; and (6) aerodynamic analysis, including maximum phonation time, oral airflow, and estimated subglottic pressure at the phonatory threshold (eMethods 2 in Supplement 2).34
The VHI is composed of 3 subscales (emotional, functional, and physical), with lower scores indicating better voice perception. Patients were considered to be responders if they achieved the minimal clinically important difference, defined by Jacobson et al36 as a shift in the total VHI score of 18 points or more.
The SF36 is a generic quality-of-life scale that consists of 36 items that describe 8 dimensions37 and 2 summary measures (ie, the physical and mental component summary scores).38 The score for each dimension ranges from 0 to 100, with higher scores indicating better perceived state of health.
Videolaryngoscopic records at baseline and month 12 were analyzed by the same experienced laryngologist (A.G.) in a randomized and blinded manner.34 For the perception analysis, all vocal records were rated at baseline, month 6, and month 12 by a jury that comprised 3 experienced speech therapists (including J.R. and C.G.) assigned in a randomized and blinded manner.
Statistical Analysis
No formal sample size calculation was performed. The sample size was fixed at 8 patients based on empirical considerations, as in similar early-phase studies39: this was a phase 1 study without a formal hypothesis, with tolerability as the primary end point. In addition, the clinical situation is relatively rare, and studies on innovative therapies typically have small populations. Efficacy end points were deemed to be exploratory. The tolerability analysis was descriptive and based on the reported treatment-emergent adverse events (AEs). The AEs were coded according to an established and validated adverse reaction dictionary.40 Continuous data are shown as mean (SD) or as median (range); 95% CIs were calculated when differences were clinically relevant. Statistical analysis was performed using SPSS software, version 20.0 (IBM Corp).
Results
Characteristics of the Patients and the ADSVF
Seven women and 1 man (mean [SD] age, 44.6 [10.4] years) were enrolled in this study. The demographic characteristics of the 8 patients and the ADSVF are given in Table 1. The median body mass index (calculated as weight in kilograms divided by height in meters squared) was 25.6 (range, 20.4-28.1). Adipose tissue samples were harvested from abdominal fat in 6 patients and from crural fat in 2 patients. All enrolled patients underwent surgery; there were no dropouts or patients lost to follow-up. All subepithelial injections were completed without epithelial rupture, demonstrating the feasibility of the approach. One patient did not undergo postoperative speech therapy. The patients received a median of 7.5 × 106 (range, 2.2-13.6 × 106) viable nucleated cells. The characteristics of the cell subsets in ADSVF as determined by flow cytometry are given in eTable 1 in Supplement 2. There was no association between potential efficacy and the number or type of injected ADSVF cells.
Table 1. Patient Demographics and Treatment Doses.
| Patient No. | Cause | Disease Duration, ya | Volume Injected, mL | VNCs Injected, Cells ×106 | ||
|---|---|---|---|---|---|---|
| Left Vocal Fold | Right Vocal Fold | Left Vocal Fold | Right Vocal Fold | |||
| 1 | Phonosurgery | 8 | 0.30 | 0.70 | 4.1 | 9.5 |
| 2 | Phonosurgery | 4 | 0.45 | 0.45 | 6.1 | 6.1 |
| 3 | Sulcus | >20 | 0.50 | 0.75 | 1.1 | 1.6 |
| 4 | Phonosurgery | 13 | 0.50 | 0.50 | 3.6 | 3.6 |
| 5 | Phonosurgery | 6 | 0.00 | 1.00 | 0.0 | 10.3 |
| 6 | Sulcus | >20 | 0.50 | 0.50 | 3.2 | 3.2 |
| 7 | Phonosurgery | 3 | 0.40 | 0.60 | 0.9 | 1.3 |
| 8 | Phonosurgery | 1 | 0.30 | 0.70 | 2.3 | 5.5 |
| Mean (SD) | NA | 5.8 (4.3) | 0.37 (0.17) | 0.65 (0.18) | 2.7 (2.0) | 5.1 (3.4) |
| Median (range) | NA | 5 (1-13) | 0.42 (0.00-0.45) | 0.65 (0.50-1.00) | 2.8 (0.0-6.1) | 4.5 (1.3-10.3) |
Abbreviation: VNCs, viable nucleated cells.
Statistical analysis performed only for noncongenital origins (n = 6).
Adverse Event Profile of Autologous ADSVF Injection
All patients were discharged from the hospital within 24 hours of surgery. No serious AEs linked to ADSVF injection occurred during follow-up, and videolaryngoscopy showed only edema 1 week after injection. Two severe AEs were declared to pharmacovigilance: acute pancreatitis and mammary prosthesis rupture; neither was deemed to be associated with the study procedures or ADSVF. Minor AEs deemed to be potentially associated with the procedure (doubtful or possible) are given in Table 2. Most of these AEs resolved spontaneously. One patient received minor treatment for persistent crural pain. One patient experienced a cosmetic contour defect associated with liposuction (fat defect with subcutaneous depression). This contour defect was corrected without sequelae by crural lipofilling 6 months after the initial surgery. Abdominal lipoaspiration sample points healed in less than 7 days after surgery. Self-evaluated dysphonia did not worsen for any patient.
Table 2. Adverse Events Potentially Associated With the Procedure for Each Patient and Adverse Event Duration.
| Adverse Eventa | Adverse Event Duration | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
| Odynophagia | 3 d | NA | NA | NA | 4 d | NA | NA | NA |
| Mouth ulceration | NA | NA | NA | 9 d | NA | NA | NA | NA |
| Asthenia | NA | NA | NA | 8 d | NA | NA | NA | NA |
| Postprocedural hematoma or ecchymosis | 1 mo | 7 d | 1 mo | 19 d | 22 d | 5 d | 7 d | 15 d |
| Procedural pain | NA | 1 mo | NA | NA | NA | NA | 1 mo | 12 mo |
| Pain in jaw | NA | 5 d | 8 mob | NA | NA | NA | NA | NA |
| Dysgeusia | NA | NA | 1 mo | NA | NA | NA | NA | NA |
| Headache | NA | NA | 1 mob | NA | NA | NA | NA | NA |
| Tremor | NA | NA | 2 mob | NA | NA | NA | NA | NA |
| Hemoptysisc | NA | NA | 7 d | NA | NA | NA | 3 d | NA |
| Productive cough | NA | NA | 1 mo | NA | NA | NA | NA | NA |
| Rhinorrhea | NA | NA | NA | NA | NA | NA | NA | NA |
| Allergic dermatitisd | NA | NA | NA | 16 d | NA | NA | NA | NA |
| Lipodystrophye | NA | NA | NA | 6 mo | NA | NA | NA | NA |
Abbreviation: NA, not applicable.
Preferred term according to MedDRA MSSO.40
Patient 3 reported symptoms associated with the recovery of a high-intensity voice: a feeling of resonance in the skull, cheeks, and even lower limbs.
Hemoptysis consisted of rusty sputum.
Dermatitis allergic to plaster.
Patient 4 had a cosmetic contour defect.
Preliminary Efficacy Profile of Autologous ADSVF Injection
Self-evaluated Dysphonia
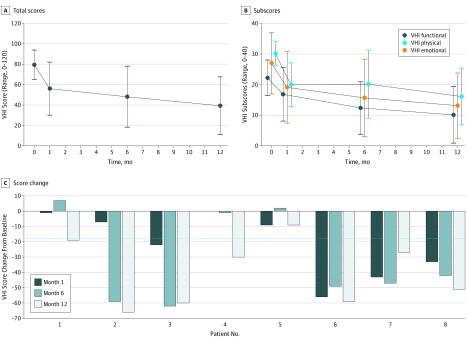
The mean improvement in dysphonia at 12 months was a decrease of 40.1 points (95% CI, −58.1 to −22.2 points) in the VHI score compared with baseline (Figure 2). In addition, each of the emotional, functional, and physical subscales was improved (Figure 2). Seven of the 8 treated patients (88%) were considered to be responders (improvement ≥18 points) (eTable 2 and eTable 3 in Supplement 2).
Figure 2. Voice Handicap Index (VHI) Scores During 12-Month Follow-up After Adipose Tissue–Derived Stromal Vascular Fraction (ADSVF) Injection.
A, Total mean (SD) VHI score during the 12-month follow-up after ADSVF injection. B, Mean (SD) physical, functional, and emotional VHI subscores. C, Changes in VHI scores from baseline during 12-month follow-up after ADSVF injection for each patient. The dotted blue line represents the minimal shift in the total VHI score required to be clinically significant (18 points).36 Sample sizes were 8 patients at baseline and months 6 and 12 and 7 patients at month 1 (data missing for patient 4).
Quality of Life
The physical and mental component summary SF-36 scores did not improve.
Videolaryngostroboscopy
Clinical improvement, as determined in videolaryngostroboscopy, in glottal closure was noted in 5 patients (62%) and in vocal fold vibration in 4 patients (50%). Video 1 shows the videolaryngostroboscopy results for patient 6 before and 12 months after injection. The data indicate an improvement in glottal closure and vibration of the middle third of the 2 vocal folds.
Video 1. Vocal Cord Scarring—Structural Response to Autologous Adipose Tissue–Derived Stromal Vascular Fraction (ADSVF) Injection.
Videolaryngostroboscopy in patient 6, whose vocal fold scarring was congenital (sulcus), documents vocal fold scarring with an absence of vibration in the middle third and insufficient glottic closure. A year after ADSVF injection, the vibration of the middle third of vocal folds was improved (mucosal wave was clearly visible) with near-complete glottic closure.
Perceptive, Acoustic, and Aerodynamic Assessments
A potentially clinically meaningful difference in roughness of voice was detected in the perceptual analysis at month 12 (mean difference, –0.5; 95% CI, −1.1 to 0.1). The voices of the responder patients were less hoarse, more stable, and without breathiness (Video 2). This improvement was also noticed during singing in patient 8. Another potentially clinically meaningful difference was observed in the signal to noise ratio at month 12 (mean difference, 2.6; 95% CI, −1.3 to 6.6). There was no improvement in the aerodynamic outcomes.
Video 2. Vocal Cord Scarring—Functional Response to Autologous Adipose Tissue–Derived Stromal Vascular Fraction (ADSVF) Injection.
Videolaryngostroboscopy in patient 6, whose vocal fold scarring was congenital (sulcus), documents vocal fold scarring with an absence of vibration in the middle third and insufficient glottic closure. A year after ADSVF injection, the vibration of the middle third of vocal folds was improved (mucosal wave was clearly visible) with near-complete glottic closure.
Discussion
To our knowledge, this nonrandomized controlled trial is the first evaluation of autologous ADSVF to treat vocal fold scarring in humans. To date, preclinical research and animal models have suggested the potential of stem cell injection into the vocal folds.12 This new cell-based therapy is particularly attractive because current therapeutic options are limited and do not fully resolve voice handicap.
The feasibility and tolerability of injection of autologous ADSVF into scarred vocal folds were demonstrated by the lack of treatment-related serious AEs. Treatment-related AEs were limited to those normally associated with lipoaspiration and direct laryngoscopy. This finding is consistent with the good tolerance profile of local injection of ADSVF in other clinical contexts, including those characterized by fibrosis, such as hand disability in patients with systemic sclerosis.30,41,42
The results also support the potential efficacy of ADSVF injection because VHI scores were reduced, including after 1 year. The VHI is the most widely used tool for self-assessment of dysphonia. The response rate of ADSVF treatment was 88% at 1 year, which is particularly encouraging given the long disease duration of the patients (5.8 [4.3] years for noncongenital origins and >20-year disease histories in 2 patients with congenital lesions). Improvement in the VHI score was associated with a potentially clinically meaningful improvement of vocal roughness. In some cases, typical rigid vocal folds recovered an almost normal amplitude and vibration regularity, suggesting that ADSVF injection is not limited to a simple volumizing outcome but may also be associated with regeneration of the lamina propria by replacing fibrotic tissue. The SF-36 scores were not improved, but 3 patients described major life changes after voice recovery (2 returned to work, and 1 returned to activity as a professional singer).
A variety of treatment options for vocal-fold scarring have been developed. Despite all efforts, complete restoration of altered vocal fold structure and vibration has not been reported. Surgical techniques, including manipulation of the lamina propria, have a risk of an unfavorable outcome with potential for worsening the situation by additional scarring. Thus, conservative therapies are considered to be first-line treatment modalities. Injection of hyaluronic acid, acellular matrix, calcium hydroxyapatite, or corticosteroids are candidates,5,6,43 but none has been adopted in daily practice. Welham et al5 reported no improvement in vocal function indices, including the VHI, and the case series described by Young et al,43 in which corticosteroids were used, revealed a mean improvement of 15 points in the VHI score with a follow-up of only 3 months after injection. In addition, the only cell-based approach reported to date is use of expanded autologous fibroblasts from the oral mucosa in 5 patients; 4 achieved an 18-point improvement in the VHI score at 1 year. However, this improvement was achieved after 3 injections in each vocal fold at 2-week intervals.39
Although the mechanisms of the association of ADSVF with scarred vocal folds are unknown, the MSCs are highly represented within ADSVF and may be important contributors. Although early research attributed the outcomes of MSC therapies primarily to their capacity for local engraftment and differentiation into multiple tissues, more recent studies16,44,45 have revealed that implanted cells do not survive for long and that the benefits are more likely attributable to the bioactive factors secreted by MSCs.46 These paracrine functions of MSCs play a key role in the regulation of immunosuppressive and inflammatory responses.47,48,49 The MSC secretome also includes an array of antiapoptotic and growth factors that promote regeneration of damaged tissue. Of importance, MSCs also exert an antifibrotic outcome that could be beneficial to reduce scar formation. The mechanisms of the antifibrotic outcomes of MSCs are unclear, but candidates include inhibition of the transforming growth factor β1 pathway, stimulation of hepatocyte growth factor expression, reduction of oxidative stress, and restoration of external matrix degradation.50 In preclinical animal models of scarred vocal folds, MSCs enhanced healing and reduced granuloma, inflammation, and fibrosis.12 The vocal folds became less atrophic, and their viscoelasticity and vibration amplitude recovered. Regarding the extracellular matrix, the authors reported reduced levels of collagen and fibronectin and increased levels of hyaluronic acid and elastin. Transforming growth factor β1 expression was reduced, as was inflammatory cell infiltration.51
However, ADSVF and ADSCs are markedly different products: ADSVF is an uncultured and minimally processed product that contains a heterogenous population of cells, whereas ADSCs are a purified and ex vivo expanded multipotent MSC population. Hirose et al52 recently found that ADSVF secretes higher levels of cytokines and soluble proteins compared with ADSCs, indicating that it is a source of more multifunctional cells. The hematopoietic cells in ADSVF, including macrophages of the anti-inflammatory M2 phenotype and regulatory T cells that express high levels of immunosuppressive cytokines, are emerging as important contributors to the immunomodulatory findings of ADSVF. Many elements of the innate and adaptive immune response participate in the differentiation and activation of fibroblasts, and acute inflammatory reactions play an important part in triggering fibrosis in a variety of organ systems.53 To our knowledge, there are no available data regarding the use of ADSVF in preclinical models of scarred vocal folds. However, ADSVF significantly reduced the clinical and histologic findings of hypertrophic scars in the epidermis and dermis compared with the control 8 weeks after injection in a humanized mouse model,54 suggesting an antifibrotic outcome. Therefore, the regenerative and antifibrotic properties of ADSVF are likely associated with the favorable outcomes. In this trial, the possibility that the association was independent of the cells and mediated only on the volumizing finding of the injection procedure is unlikely because, in such a scenario, the improvement would have rapidly decreased over time and mucosal vibration would not have been affected.
Limitations
The main limitations of this study are the small number of patients, imbalanced male-to-female ratio, and the different causes of vocal fold scarring. However, iatrogenic and noniatrogenic causes are based on the same anatomical description and are included in 1 classification.55 The absence of a control arm and the open-label protocol prevented determination of whether the positive changes in subjective factors were attributable to the placebo effect. In addition, speech therapy after surgery was not standardized and could have contributed to voice improvement. The patients received a wide range of viable nucleated cells, and although the number of cells injected was not associated with potential efficacy, it should be constant in a randomized clinical trial.
Conclusions
This single-center, nonrandomized controlled trial found that ADSVF treatment was feasible and well tolerated in patients with scarred vocal folds. Additional safety studies of ADSVF in scarred vocal folds after cordectomy for laryngeal neoplasia are warranted. Although we could not reach a conclusion regarding the efficacy of ADSVF injection, the observed data were encouraging. Longer-term, larger, multicenter, randomized, double-blind studies with a more homogeneous population are needed to evaluate the effect of ADSVF therapy on the long-term prognosis. In addition, future studies should delineate the contribution of each cell subpopulation in ADSVF and identify the mechanisms underlying its association with scarred vocal folds.
eMethods 1. Biological Characterization of the Adipose-Derived Stromal Vascular Fraction
eMethods 2. Definition of Vocal Assessment’s Parameters
eTable 1. Characteristics of Adipose Tissue Harvest and Adipose-Derived Stromal Vascular Fraction
eTable 2. Changes in the Self-evaluation, Videolaryngostroboscopy, Perceptive, Acoustic, and Aerodynamic Analyses After Injection of Autologous Adipose-Derived Stromal Vascular Fraction Injection
eTable 3. Individual Patient Data of Vocal Assessments and Quality of Life
References
- 1.Friedrich G, Dikkers FG, Arens C, et al. ; European Laryngological Society. Phonosurgery Committee . Vocal fold scars: current concepts and future directions: consensus report of the Phonosurgery Committee of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2013;270(9):2491-2507. doi: 10.1007/s00405-013-2498-9 [DOI] [PubMed] [Google Scholar]
- 2.Hirano M. Phonosurgery: basic and clinical investigation. Otologia (Fukuoka). 1975;21:239-440. [Google Scholar]
- 3.Graupp M, Bachna-Rotter S, Gerstenberger C, et al. The unsolved chapter of vocal fold scars and how tissue engineering could help us solve the problem. Eur Arch Otorhinolaryngol. 2016;273(9):2279-2284. doi: 10.1007/s00405-015-3668-8 [DOI] [PubMed] [Google Scholar]
- 4.Sataloff RT, Spiegel JR, Heuer RJ, et al. Laryngeal mini-microflap: a new technique and reassessment of the microflap saga. J Voice. 1995;9(2):198-204. doi: 10.1016/S0892-1997(05)80253-X [DOI] [PubMed] [Google Scholar]
- 5.Welham NV, Choi SH, Dailey SH, Ford CN, Jiang JJ, Bless DM. Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis. Laryngoscope. 2011;121(6):1252-1260. doi: 10.1002/lary.21780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molteni G, Bergamini G, Ricci-Maccarini A, et al. Auto-crosslinked hyaluronan gel injections in phonosurgery. Otolaryngol Head Neck Surg. 2010;142(4):547-553. doi: 10.1016/j.otohns.2009.12.035 [DOI] [PubMed] [Google Scholar]
- 7.Hertegård S, Hallén L, Laurent C, et al. Cross-linked hyaluronan versus collagen for injection treatment of glottal insufficiency: 2-year follow-up. Acta Otolaryngol. 2004;124(10):1208-1214. doi: 10.1080/00016480410017701 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Zheng Shu X, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26(23):4737-4746. doi: 10.1016/j.biomaterials.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Rossi F, Papa S, et al. Non-invasive in vitro and in vivo monitoring of degradation of fluorescently labeled hyaluronan hydrogels for tissue engineering applications. Acta Biomater. 2016;30:188-198. doi: 10.1016/j.actbio.2015.11.053 [DOI] [PubMed] [Google Scholar]
- 10.Magalon J, Velier M, Simoncini S, et al. Molecular profile and proangiogenic activity of the adipose-derived stromal vascular fraction used as an autologous innovative medicinal product in patients with systemic sclerosis. Ann Rheum Dis. 2019;78(3):391-398. doi: 10.1136/annrheumdis-2018-214218 [DOI] [PubMed] [Google Scholar]
- 11.Dykstra JA, Facile T, Patrick RJ, et al. Concise review: fat and furious: harnessing the full potential of adipose-derived stromal vascular fraction. Stem Cells Transl Med. 2017;6(4):1096-1108. doi: 10.1002/sctm.16-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattei A, Magalon J, Bertrand B, Philandrianos C, Veran J, Giovanni A. Cell therapy and scarred vocal folds. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;296(17):30103-30105. [DOI] [PubMed] [Google Scholar]
- 13.Kumai Y, Kobler JB, Park H, et al. Crosstalk between adipose-derived stem/stromal cells and vocal fold fibroblasts in vitro. Laryngoscope. 2009;119(4):799-805. doi: 10.1002/lary.20149 [DOI] [PubMed] [Google Scholar]
- 14.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VL, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120(2):330-337. [DOI] [PubMed] [Google Scholar]
- 15.Hiwatashi N, Hirano S, Mizuta M, et al. Adipose-derived stem cells versus bone marrow-derived stem cells for vocal fold regeneration. Laryngoscope. 2014;124(12):E461-E469. doi: 10.1002/lary.24816 [DOI] [PubMed] [Google Scholar]
- 16.Hiwatashi N, Hirano S, Suzuki R, et al. Comparison of ASCs and BMSCs combined with atelocollagen for vocal fold scar regeneration. Laryngoscope. 2016;126(5):1143-1150. doi: 10.1002/lary.25667 [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Hu R, Fan E, Han D. Adipose-derived mesenchymal stem cells in collagen-hyaluronic acid gel composite scaffolds for vocal fold regeneration. Ann Otol Rhinol Laryngol. 2011;120(2):123-130. doi: 10.1177/000348941112000209 [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Oh SH, Choi JS, et al. Adipose-derived stem cell-containing hyaluronic acid/alginate hydrogel improves vocal fold wound healing. Laryngoscope. 2014;124(3):E64-E72. doi: 10.1002/lary.24405 [DOI] [PubMed] [Google Scholar]
- 19.Kwon SK, Lee BJ. The combined effect of autologous mesenchymal stem cells and hepatocyte growth factor on vocal fold regeneration and fibrosis in vocal fold wound. J Tissue Eng Regen Med. 2008;5(4–6):735-742. [Google Scholar]
- 20.Shiba TL, Hardy J, Luegmair G, Zhang Z, Long JL. Tissue-engineered vocal fold mucosa implantation in rabbits. Otolaryngol Head Neck Surg. 2016;154(4):679-688. doi: 10.1177/0194599816628501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valerie A, Vassiliki K, Irini M, Nikolaos P, Karampela E, Apostolos P. Adipose-derived mesenchymal stem cells in the regeneration of vocal folds: a study on a chronic vocal fold scar. Stem Cells Int. 2016;2016:9010279. doi: 10.1155/2016/9010279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bonnecaze G, Chaput B, Woisard V, et al. Adipose stromal cells improve healing of vocal fold scar: morphological and functional evidences. Laryngoscope. 2016;126(8):E278-E285. doi: 10.1002/lary.25867 [DOI] [PubMed] [Google Scholar]
- 23.Hong SJ, Lee SH, Jin SM, et al. Vocal fold wound healing after injection of human adipose-derived stem cells in a rabbit model. Acta Otolaryngol. 2011;131(11):1198-1204. doi: 10.3109/00016489.2011.599816 [DOI] [PubMed] [Google Scholar]
- 24.Hu R, Ling W, Xu W, Han D. Fibroblast-like cells differentiated from adipose-derived mesenchymal stem cells for vocal fold wound healing. PLoS One. 2014;9(3):e92676. doi: 10.1371/journal.pone.0092676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BJ, Wang SG, Lee JC, et al. The prevention of vocal fold scarring using autologous adipose tissue-derived stromal cells. Cells Tissues Organs. 2006;184(3-4):198-204. doi: 10.1159/000099627 [DOI] [PubMed] [Google Scholar]
- 26.Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns. 2014;40(7):1375-1383. doi: 10.1016/j.burns.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 27.Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16(2):245-257. doi: 10.1016/j.jcyt.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 28.Leblanc AJ, Touroo JS, Hoying JB, Williams SK. Adipose stromal vascular fraction cell construct sustains coronary microvascular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2012;302(4):H973-H982. doi: 10.1152/ajpheart.00735.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashmi S, Ahmed M, Murad MH, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016;3(1):e45-e52. doi: 10.1016/S2352-3026(15)00224-0 [DOI] [PubMed] [Google Scholar]
- 30.Granel B, Daumas A, Jouve E, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 2015;74(12):2175-2182. doi: 10.1136/annrheumdis-2014-205681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karbiener M, Darnhofer B, Frisch MT, Rinner B, Birner-Gruenberger R, Gugatschka M. Comparative proteomics of paired vocal fold and oral mucosa fibroblasts. J Proteomics. 2017;155:11-21. doi: 10.1016/j.jprot.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattei A, Magalon J, Bertrand B, et al. Autologous adipose-derived stromal vascular fraction and scarred vocal folds: first clinical case report. Stem Cell Res Ther. 2018;9(1):202. doi: 10.1186/s13287-018-0842-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641-648. doi: 10.1016/j.jcyt.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dejonckere PH, Bradley P, Clemente P, et al. ; Committee on Phoniatrics of the European Laryngological Society (ELS) . A basic protocol for functional assessment of voice pathology, especially for investigating the efficacy of (phonosurgical) treatments and evaluating new assessment techniques: guideline elaborated by the Committee on Phoniatrics of the European Laryngological Society (ELS). Eur Arch Otorhinolaryngol. 2001;258(2):77-82. doi: 10.1007/s004050000299 [DOI] [PubMed] [Google Scholar]
- 35.Hirano M. Psycho-acoustic evaluation of voice: GRBAS Scale for evaluating the hoarse voice In: Hirano M, ed. Clinical Examination of Voice. Wien, Germany: Springer Verlag; 1981:81-84. [Google Scholar]
- 36.Jacobson B, Johnson A, Grywalski C, et al. The Voice Handicap Index (VHI): development and validation. Am J Speech Lang Pathol. 1997;6:66-70. doi: 10.1044/1058-0360.0603.66 [DOI] [Google Scholar]
- 37.Ware JE, Kosinski M, Gandek B. SF-36® Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Inc; 1993. [Google Scholar]
- 38.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 39.Chhetri DK, Berke GS. Injection of cultured autologous fibroblasts for human vocal fold scars. Laryngoscope. 2011;121(4):785-792. doi: 10.1002/lary.21417 [DOI] [PubMed] [Google Scholar]
- 40.MedDRA MSSO Medical Dictionary for Regulatory Activities Version 21.0. https://www.meddra.org/. Accessed November 1, 2018.
- 41.Daumas A, Magalon J, Jouve E, et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Curr Res Transl Med. 2017;65(1):40-43. doi: 10.1016/j.retram.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 42.Guillaume-Jugnot P, Daumas A, Magalon J, et al. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology (Oxford). 2016;55(2):301-306. doi: 10.1093/rheumatology/kev323 [DOI] [PubMed] [Google Scholar]
- 43.Young WG, Hoffman MR, Koszewski IJ, Whited CW, Ruel BN, Dailey SH. Voice outcomes following a single office-based steroid injection for vocal fold scar. Otolaryngol Head Neck Surg. 2016;155(5):820-828. doi: 10.1177/0194599816654899 [DOI] [PubMed] [Google Scholar]
- 44.Svensson B, Nagubothu SR, Nord C, et al. Stem cell therapy in injured vocal folds: a three-month xenograft analysis of human embryonic stem cells. Biomed Res Int. 2015;2015:754876. doi: 10.1155/2015/754876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hertegård S, Cedervall J, Svensson B, et al. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. Laryngoscope. 2006;116(7):1248-1254. doi: 10.1097/01.mlg.0000224548.68499.35 [DOI] [PubMed] [Google Scholar]
- 46.Rohban R, Pieber TR. Mesenchymal stem and progenitor cells in regeneration: tissue specificity and regenerative potential. Stem Cells Int. 2017;2017:5173732. doi: 10.1155/2017/5173732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):E1852. doi: 10.3390/ijms18091852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8(9):268-278. doi: 10.4252/wjsc.v8.i9.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017;74(13):2345-2360. doi: 10.1007/s00018-017-2473-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Y, Peng C, Lv S, et al. Adipose-derived stem cells ameliorate renal interstitial fibrosis through inhibition of EMT and inflammatory response via TGF-β1 signaling pathway. Int Immunopharmacol. 2017;44:115-122. doi: 10.1016/j.intimp.2017.01.008 [DOI] [PubMed] [Google Scholar]
- 51.Hiwatashi N, Bing R, Kraja I, Branski RC. Mesenchymal stem cells have antifibrotic effects on transforming growth factor-β1-stimulated vocal fold fibroblasts. Laryngoscope. 2017;127(1):E35-E41. doi: 10.1002/lary.26121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirose Y, Funahashi Y, Matsukawa Y, et al. Comparison of trophic factors secreted from human adipose-derived stromal vascular fraction with those from adipose-derived stromal/stem cells in the same individuals. Cytotherapy. 2018;20(4):589-591. doi: 10.1016/j.jcyt.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 53.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028-1040. doi: 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Domergue S, Bony C, Maumus M, et al. Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars. PLoS One. 2016;11(5):e0156161. doi: 10.1371/journal.pone.0156161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hantzakos A, Dikkers FG, Giovanni A, et al. Vocal fold scars: a common classification proposal by the American Laryngological Association and European Laryngological Society. Eur Arch Otorhinolaryngol. 2019;276(8):2289-2292. doi: 10.1007/s00405-019-05489-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Biological Characterization of the Adipose-Derived Stromal Vascular Fraction
eMethods 2. Definition of Vocal Assessment’s Parameters
eTable 1. Characteristics of Adipose Tissue Harvest and Adipose-Derived Stromal Vascular Fraction
eTable 2. Changes in the Self-evaluation, Videolaryngostroboscopy, Perceptive, Acoustic, and Aerodynamic Analyses After Injection of Autologous Adipose-Derived Stromal Vascular Fraction Injection
eTable 3. Individual Patient Data of Vocal Assessments and Quality of Life