Abstract
ABSTRACT: Although chloroquine, hydroxychloroquine and quinacrine were originally developed for the treatment of malaria, these medications have been used to treat skin disease for over 50 years. Recent clinical data have confirmed the usefulness of these medications for the treatment of lupus erythematosus. Current research has further enhanced our understanding of the pharmacologic mechanisms of action of these drugs involving inhibition of endosomal toll‐like receptor (TLR) signaling limiting B cell and dendritic cell activation. With this understanding, the use of these medications in dermatology is broadening. This article highlights the different antimalarials used within dermatology through their pharmacologic properties and mechanism of action, as well as indicating their clinical uses. In addition, contraindications, adverse effects, and possible drug interactions of antimalarials are reviewed.
Keywords: antimalarial drugs, dermatomyositis, lupus erythematosus, pharmacology, sarcoidosis
Introduction
Though primarily synthesized to treat malaria, antimalarials have now been extensively accepted and approved to treat cutaneous lupus. More interestingly, recent evidence has also shown these medications to lower lipid levels in systemic lupus erythematosus (LE) and to play a cardioprotective role in addition to lowering rates of thrombosis (1). Payne is credited with being the first in 1894 to prescribe an antimalarial, quinine, to treat patients with lupus (2). Quinine is a natural alkaloid isolated from the South American cinchona bark tree (3).
Subsequently synthetic antimalarials were produced. Quinacrine (atabrine, mepacrine, chinacrin), an acridine compound, was the first antimalarial to be synthesized (4). Quinacrine replaced quinine as an antimalarial compound in World War II when over 3 million American servicemen each took quinacrine for 4 years. As well, Page effectively treated 18 cutaneous lupus patients who were resistant to treatment with quinine (5). In the 1950s the 4‐aminoquinoline derivatives of quinine, such as chloroquine and hydroxychloroquine, became well‐accepted drugs for the management of skin lupus as they were found to be more effective with more tolerable side effects in lupus patients (6). Since then, antimalarials have been shown to be effective in treating a variety of skin conditions.
Pharmacology of the antimalarials used in dermatology
Chloroquine
Chloroquine is a 4‐amino quinoline (See FIG. 1). The isoquinoline nucleus has a double benzene‐like structure with a chloride atom at position 7 and an alkyl side chain at the 4‐amino site. It is a chiral molecule with D and L forms (7). The clinical relevance of the chiral isoforms remains a subject of debate (8). The drug is manufactured as a diphosphate (aralen phosphate, resochin) for oral administration or as a hydrochloride for intramuscular injection (in the therapy of malaria‐induced coma). Approximately 60% of the diphosphate represents the base. It is water soluble and readily absorbed from the gastrointestinal tract. The pharmacokinetics of the drug was reviewed by Krishna and White (9) and by Ducharme (10). After oral administration, peak plasma concentrations are reached within 8–12 hours. Approximately 60% of the drug in the plasma is bound to protein. Doses of 250 mg daily lead to stable plasma concentrations of 100–500 ng/mL (11). The drug is deposited in tissues with 200–20,000 times the plasma concentration found in the liver, spleen, kidney, and lung (12). Significant accumulation of the drug also occurs in leukocytes. Highest concentrations of the drug are found in melanin‐containing cells in the retina and skin. As a result of this widespread tissue deposition, there is a large volume or distribution (over 100 L/kg) (9). Chloroquine undergoes hepatic biotransformation into two active metabolites, desethylchloroquine and bisdesethylchloroquine via the cytochrome P450 enzymes (13, 14). Renal clearance accounts for half of the total systemic clearance and is increased by acidification of the urine. The pharmacokinetics is complex with plasma levels determined by the rate of distribution rather than by the rate of elimination. When the drug is discontinued after daily dosage for 2 weeks, plasma half‐life is initially 6–7 days with a gradual increase to 17 days after 4 weeks and a terminal half‐life of 30–60 days (7). Chloroquine remains in the skin for 6–7 months after cessation of therapy at a time when the drug is no longer detectable in the plasma (11).
Figure 1.
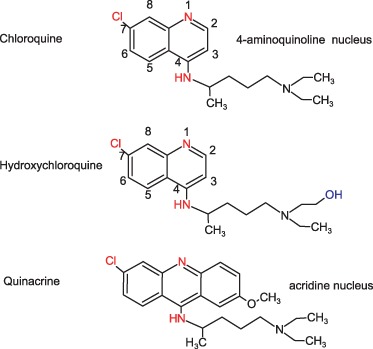
Structural formulas of the antimalarial drugs used in dermatology. Chloroquine and hydroxychloroquine are 4 amino‐quinolines. Quinacrine is an acridine dye.
Hydroxychloroquine
Hydroxychloroquine differs from chloroquine by the presence of a hydroxyl group at the end of the side chain: The N‐ethyl substituent is beta‐hydroxylated (FIG. 1). It is available for oral administration as hydroxychloroquine sulfate (plaquenil) of which 200 mg contains 155 mg base in chiral form. Hydroxychloroquine has similar pharmacokinetics to chloroquine with rapid gastrointestinal absorption, a large volume of distribution, and renal elimination. Hydroxychloroquine is N‐desethylated by cytochrome P450 enzymes CYP 2D6, 2C8, 3A4 and 3A5 into N‐desethylhydroxychloroquine (15). Whether genotype differences in these enzymes affect the efficacy or toxicity of hydroxychloroquine is still unknown. Maximal clinical efficacy may take up to 3–6 months to achieve. Dose loading, with administration of higher doses (up to 1200 mg/day) for the first 6 weeks, has been shown to accelerate clinical response in rheumatoid arthritis (16), albeit with an increase in incidence of gastrointestinal intolerance. Interestingly, the efficacy of this drug has been correlated with drug blood concentrations in rheumatoid arthritis (17) and in systemic LE. In a recent study, whole blood drug concentrations were measured in 143 unselected systemic lupus patients receiving 400 mg of hydroxychloroquine for at least 6 months (18). A large variability in whole blood drug concentrations was noted possibly due to poor compliance and as of yet unknown interindividual pharmacokinetic parameters. The mean whole blood concentrations in patients with inactive disease (1079 ng/mL) was significantly higher than in patients with active disease (694 ng/mL) and a low level of blood hydroxychloroquine was a predictor of exacerbation during follow‐up, leading the authors to propose individual dosing schedules to maintain whole drug concentrations above 1000 ng/mL. Similar studies are not available for chloroquine. It has been stated, based on therapeutic response in rheumatoid arthritis, that hydroxychloroquine in approximately two‐thirds as effective as chloroquine and half as toxic (19). Both chloroquine and hydroxychloroquine readily cross the placenta. Hydroxychloroquine blood levels in the fetus are similar to those in the mother and are excreted in small amounts in breast milk (20).
Quinacrine
Quinacrine has an extra benzine ring (and thus an acridine nucleus) when compared to the 4‐aminoquinoline chloroquine (FIG. 1). The alkyl side chain is identical. The pharmacokinetics of this drug is similar to the 4‐aminoquinolones (19). Skin deposition is readily visible as a yellow cast. Importantly, there is no cross‐reactivity between the 4‐aminoquinolines and quinacrine. Thus, an adverse reaction to one drug group does not preclude the use of the other (21).
Quinacrine was commonly used as an irritant for voluntary female nonsurgical sterilization in India due to its ability to produce tubal occlusion after intrauterine instillation (22). It has also been used as a vesicant for pleurodesis in the treatment of recurrent pneumothorax or malignant pleural effusion (23).
Mechanisms of action of antimalarials in skin disease
Potential mechanisms of action of interest to the dermatologist have been reviewed in depth within the past 10 years (24, 25). A summary and new concepts are presented here (FIG. 2). Antimalarials are lipophilic weak bases that pass easily through plasma membranes in the free base form to accumulate in acidic cytoplasmic vesicles such as lysosomes where they are trapped in a protonated, ionized state (26). Christian de Duve, the discoverer of lysosomes, termed drugs with such properties lysosomotropic agents (27). As such, partitioning results in concentrations of drug within the lysosome that are up to 1000 times greater than in the culture media. This results in a number of immunologic and nonimmunologic effects. The pH of the lysosome increases from 4 to 6 (28). The alteration in pH results in the inhibition of acidic proteases within the lysosomal compartment and generally diminishes proteolysis (29). This also results in the decreased intracellular processing, glycosylation, and secretion of proteins (30). Furthermore, the morphology of lysosomes is altered, reflecting altered maturation. In patients treated with chloroquine, an increased number of lamellar inclusions is noted within lymphocytes for example. This has been associated with decreased cell functioning such as chemotaxis, phagocytosis and superoxide production by neutrophils (31).
Figure 2.
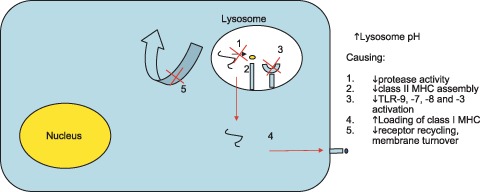
The major proposed mechanism of action of antimalarial drugs: Interference with lysosomal acidification. Consequences of this lysosomotropic effect within macrophages, dendritic cells and lymphocytes are felt to underlay the therapeutic antiinflammatory effect of these drugs.
Effect on antigen presentation
Antigen‐presenting cells such as monocytes, macrophages, and dendritic cells present exogenous antigens (that is, of extra‐cellular origin) to CD4 T cells on class II MHC molecules (32). The foreign antigens are digested within the lysosomal cellular compartment and loaded onto a peptide groove of the class II MHC molecule. Chloroquine interferes with the digestion of these antigens and the subsequent presentation of these antigens to T cells (33). Chloroquine also prevents the dissociation of the invariant chain from the class II MHC molecule. This dissociation must occur for peptide loading to proceed. Thus, chloroquine interferes with class II MHC antigenic peptide loading (34). As self‐peptides are thought to be of relatively low affinity to the class II MHC molecules (35), it has been proposed that this process might preferentially diminish responses to self (36). Whereas lysosomotropic agents such as chloroquine have been known to interfere with class II MHC‐restricted antigen presentation, they have recently been shown to increase the CD8 T‐cell responses against soluble antigens (37): A single 500‐mg dose of chloroquine, 1 day prior to hepatitis B vaccine booster dose immunization, increased the CD8 T‐cell response to the vaccine. It was shown that interference with soluble antigen degradation in the lysosomes increased export into the cytosol, where the soluble antigen could access the pathway leading to loading of class I MHC molecules for presentation to CD8 T cells. This dichotomous effect (inhibition of CD4 T‐cell stimulation and promotion of CD8 T‐cell stimulation), which still needs to be demonstrated with chronic antimalarial use, may explain in part the paucity of opportunistic infections during antimalarial therapy.
Cytokine effects
In vitro studies have shown that both chloroquine and hydroxychloroquine decrease the production of TNF‐α, IL‐6, and IFN‐γ by mitogen‐stimulated peripheral blood lymphocytes (38). A dose‐dependent inhibition of TNF‐α, IL‐1β, and IL‐6 by endotoxin‐stimulated whole blood was also noted (39). Although inhibition of T‐lymphocyte mediated cytokines such as IL‐2, IL‐4, and IFN‐γ is described (40), the major cellular target appears to be macrophages and monocytes whose IL‐1 and IL‐6 production is readily inhibited (41). Monotherapy of SLE patients with chloroquine results in a decrease in serum levels of IL‐6, IL‐18, and TNF‐α (42). It has been suggested that antimalarial‐induced inhibition of TNF‐α production, which mainly affects monocytes, may be independent of the lysosomotropic action of the drugs and related to nuclear effects (43). Chloroquine was shown to interfere with phosphorylation of extracellular signal‐regulated kinases (ERK)1/2 and the ERK‐activating kinases mitogen‐activating protein/ERK kinase (MEK)1/2 and thus inhibit TNF‐α production (44). However, many of these assays were conducted using concentrations at or above 10 µm, which are higher than attained in vivo and that may also result in mammalian cell growth inhibition.
Endosomal TLR inhibition
A more potent effect of the antimalarials has recently been noted on the inhibition of stimulation of the toll‐like receptor (TLR) 9 family receptors. TLRs are cellular receptors for microbial products that induce inflammatory responses through activation of the innate immune system (45). Nanomolar rather than micromolar concentrations of chloroquine were shown to potently prevent bacterial DNA‐induced IL‐6 production by human peripheral blood mononuclear cells (46), an effect now known to be the result of inhibition of TLR‐9 signaling. TLR‐9, ‐8, ‐7, and ‐3 are all found within the endosomal compartment of antigen‐presenting cells and are susceptible to inhibition by lysosomotropic agents. In a recent mouse model, rheumatoid factor production by B cells could be induced by chromatin‐containing immune complexes, and this effect required signaling through TLR‐9, which could be inhibited by chloroquine (47). In SLE, DNA and DNA‐associated auto‐antigens were shown to activate auto‐reactive B cells via sequential engagement of the B‐cell antigen receptor (BCR) and TLR‐9. Likewise, TLR‐7 signaling promotes the activation of autoreactive B cells by RNA and RNA‐associated autoantigens in SLE (48). Furthermore, necrotic synovial cells from RA patients induce pro‐inflammatory cytokine and chemokine production by synovial fibroblasts through a TLR‐3–mediated mechanism (49). DNA‐associated auto‐antigens also stimulate dendritic cells to produce inflammatory cytokines through TLR‐9–mediated signaling (50). All of these events are inhibited by low (and therapeutically relevant) concentrations of antimalarials. Taken together, these observations suggest that chloroquine and hydroxychloroquine have a potent effect on TLR signaling, which may explain their efficacy in the treatment of SLE, RA, and possibly other inflammatory diseases (51, 52). As this effect is primarily on antigen‐presenting cells which initiate immune reactions, this may account for their delayed onset of action in the clinic (in that previously activated T cells and perhaps B cells are less readily inhibited).
Inhibition of prostaglandin synthesis, lipid peroxidation, and other anti‐inflammatory effects
Antimalarials act as prostaglandin antagonists (53). This is secondary to inhibition of phospholipase A2 (54). Antimalarials affect membrane traffic and impair receptor recycling (55). This may alter the inflammatory cells’ responsiveness to mitogenic stimuli (56). Lysosomal effects may also underlie the observed decrease in cell mediated cytotoxicity and natural‐killer–cell mediated lysis noted in patients on antimalarials (57). Quinacrine, but not chloroquine has been shown to affect matrix metalloprotein production by leukocytes (58). Increased nitric oxide synthase activity is noted in endothelial cells following exposure to chloroquine in vitro (59). In whole animal models, this results in an increase in vasopressin release and in an increase in the glomerular filtration rate (60).
Antimalarials absorb UV light and can block cutaneous reactions induced by UV light when given topically or intradermally (61). Topical chloroquine can protect against UVB‐ and UVA‐induced erythema (62), but not against immediate pigment darkening, suggesting that UV absorption may not be the only, or even major, operant mechanism after topical application. Quinacrine was noted to increase the tolerance of lupus patients to light over 50 years ago (63). The increase in mean UVB erythema doses (MEDs) recently confirmed in lupus patients following 3 months of chloroquine therapy may be to the result of either the anti‐inflammatory or the photoprotective actions of the drug (42). Antimalarials have also been shown to enhance the protective early limb of the UV response through up‐regulation of c‐jun (64).
Ancillary effects
Inhibition of ERK activation by chloroquine has been shown to sensitize HeLa cells to Fas‐mediated apoptosis (44). Likewise, hydroxychloroquine potentiates Fas‐mediated apoptosis of synoviocytes (65). This may explain the increased sensitivity to apoptosis demonstrated by lymphocytes in vitro after exposure to hydroxychloroquine (66). These pro‐apoptototic qualities and a demonstrated blockade of ABC drug transporters (67) have made these drugs candidate antineoplastic agents (68). Antimicrobial effects upon HIV, SARS coronavirus, and influenza have been noted (69), related in part to interference with protein glycosylation. Antimalarials also have anticoagulant, lipid‐lowering, and hypoglycemic effects. Chloroquine inhibits platelet aggregation and adhesion in a clinically relevant manner (70). Hydroxychloroquine use has been associated with a 15–20% decrease in serum cholesterol, triglyceride, and LDL levels (71). Antimalarials also decrease insulin degradation and thus improve glucose tolerance (72).
Clinical use: indications
The clinical use of antimalarials have been reviewed in the literature (24, 73, 74, 75). An overview of these is provided with recent updates that have been reported regarding the use of antimalarials.
Lupus erythematosus
Lupus erythematosus‐specific skin conditions encompass acute cutaneous LE, subacute cutaneous LE, and chronic cutaneous LE (76). A double‐blind study of hydroxychloroquine for discoid LE established that it was more efficacious than placebo at 3 months and after 1 year of treatment (77). At crossover, hydroxychloroquine was more efficacious than placebo at 3 months. Similar results have been achieved in large, open clinical studies for patients with chronic cutaneous LE and subacute cutaneous LE (78, 79). Therapy also improves fatigue, arthralgia, myalgias, serositis, and mucous membrane ulceration SLE patients (80). Antimalarials have been shown to be effective in treating LE–non‐specific skin diseases such as oral mucosal ulcerations, calcinosis cutis, calcifying lupus panniculitis, and photosensitivity (81). In addition, the number of flare‐ups has been shown to be reduced in SLE patients taking hydroxychloroquine compared to placebo (82), and hydroxychloroquine has a protective effect on the survival of patients with SLE (83). Verrucous or hypertrophic plaques respond less effectively to antimalarial therapy.
Typically, patients with LE‐specific cutaneous manifestations are started on hydroxychloroquine at 400 mg/day for the average sized adult, and the maximum dosage given is 6.5 mg/kg lean bodyweight/day. Common formulas for the calculation of lean body weight include the method of James: lean body weight (men) = (1.10 × weight(kg)) – 128 (weight2/(100 × height(m))2); lean body weight (women) = (1.07 × weight(kg)) – 148 (weight2/(100 × height(m))2) (84). After a 2‐month trial of hydroxychloroquine if no improvements are seen then quinacrine is added at an initial dosage of 100 mg/day. Two studies indicate that such combination antimalarial use may be of benefit in skin disease (85, 86). Interestingly, the addition of quinacrine therapy to patients with stable systemic lupus on hydroxychloroquine and prednisone has been shown to decrease systemic activity scores, together with a lowering of serum levels of B lymphocyte activating factor (BLyS) and anticardiolipin antibody titers (87). With this antimalarial combination treatment, if clinical effect is achieved, then the hydroxychloroquine dosage may be lowered to 200 mg/day after 1 month, as well the quinacrine dosage may be lowered to reduce any side effects. Alternatively, chloroquine 250–500 mg daily (maximum dosage 4 mg/kg lean bodyweight/day) may be used in cases not responding to hydroxychloroquine and can also be combined with quinacrine. The combination of hydroxychloroquine and chloroquine are avoided due to the risk of retinal toxicity. Clinical improvements with cutaneous LE are not seen until several weeks later with the use of antimalarials; therefore, 2 months is considered the standard time frame before changing to another therapy (88). Intralesional and topical corticosteroids are more effective in providing a quick improvement.
Porphyria cutanea tarda
In patients with porphyria cutanea tarda (PCT) usual treatment involves phlebotomy. However, the use of antimalarial therapy may be indicated in patients that have contraindications or failures with receiving phlebotomy (89, 90, 91). Use of antimalarials in children may be preferred over using phlebotomy as the latter can be traumatizing to the patient (92, 93).
The discovery of the use of antimalarial therapy in PCT was made when patients were given hydroxychloroquine or chloroquine and resulted in acute exacerbation of hepatic disease (94, 95). However, recovery from the acute exacerbation resulted in long‐term clinical remission. Use of antimalarials may be initiated either in low dosage to prevent the acute exacerbation or given intentionally with a high dosage in hospitalized patients, as well antimalarial therapy can be combined with patients pretreated with one to four phlebotomies. Low‐dose therapy is started with either choroquine 125 mg twice weekly or hydroxychloroquine 100 mg two times weekly, which the dosage is increased depending on the clinical response (89). Patients are recommended by some authors to be given a test dose of 125–250 mg of chloroquine with examination of liver function tests initially (96). The use of high‐dose hydroxychloroquine consisting of 250 mg three times daily for 3 days has been reported to be used in 72 patients (97). An increase in serum transaminases was noted and patients receiving this regimen are advised to be hospitalized.
Polymorphous light eruption
Though not accepted as the first line of treatment in polymorphous light eruption (PMLE) antimalarial therapy has been shown to be effective in these patients (98). Two controlled efficacy trials that used hydroxychloroquine and chloroquine demonstrated increased sun tolerance, and a moderate clinical improvement was seen with a statistically significant skin rash reduction (90, 99). If first‐line treatment regimens are ineffective such as sun avoidance and failure or contraindications to receiving prophylactic UVB or PUVA phototherapy exist, then intermittent hydroxychloroquine 200–400 mg daily prior to increased sun exposure can be tried.
Dermatomyositis
Muscle involvement in dermatomyositis tends to responds well to use of systemic corticosteroids and other immunosuppressive agents. However, cutaneous lesions still persist (100). The authors of this article have found the use of hydroxychloroquine 200–400 mg/daily to improve cutaneous manifestations of the dermatomyositis.
In an open study containing seven patients with cutaneous lesions of dermatomyositis that failed to respond to other therapy, improvement was seen in all of these patients with use of hydroxychloroquine with three showing complete resolution (101). Another retrospective study looked at 12 patients with dermatomyositis having one lab criterion of muscle involvement but no muscle weakness (102). Five patients showed complete improvement and four showed partial improvement when receiving only hydroxychloroquine. In addition, when two patients were taken off of hydroxychloroquine, the cutaneous rash flared up and cleared after receiving treatment again. Hydroxychloroquine has also been shown to be effective in childhood dermatomyositis as shown in a study consisting of nine patients with incomplete response to corticosteroids or flaring up when corticosteroids were lowered (103). With hydroxychloroquine, these patients showed significant improvement in cutaneous symptoms at 3 and 6 months and also had a reduction of proximal and abdominal muscle weakness. Hydroxychloroquine is recommended in the treatment of dermatomyositis with patients having cutaneous lesions not responding to steroids and in patients with cutaneous involvement without any muscle involvement (termed amyopathic dermatomyositis). Recently, the use of combination antimalarial treatment (with this addition of quinacrine to the 4‐amino quinolone) has been shown to be of benefit in patients that do not respond to a single 4‐amino quinolone antimalarial alone (104).
Solar urticaria
The promotion of antimalarials in solar urticaria is based on only two anecdotal reports that demonstrated the effectiveness of therapy (24, 105, 106). Therefore, there have been no trials to compare the efficacy of antimalarials. Antimalarials therapy may be tried if antihistamines, phototherapy, photochemotherapy, and plasmapheresis are ineffective.
Sarcoidosis
The British Tuberculosis Association in 1967 evaluated the efficacy of chloroquine therapy given over 4 months in patients with sarcoidosis (107). The randomized double‐blind clinical trial with 57 patients showed significant improvements in patients after 4 and 6 months. After 12 months the two groups showed no difference, demonstrating that the therapy had a suppressive mechanism. In another study, 17 patients with cutaneous sarcoidosis were treated with hydroxychloroquine 200–400 mg daily (108). Four to 12 weeks of being on treatment resulted in improvement in skin lesions. In cutaneous sarcoidosis, antimalarials are thus an effective alternative to corticosteroid therapy.
Granuloma annulare
Granuloma annulare is known to be resistant to many forms of treatment. Several case reports have been published showing the effective treatment of granuloma annulare with use of antimalarials (109). An open trial was carried out in children with generalized granuloma annulare that resulted in complete clearance of lesions after 4–6 weeks of therapy (110, 111). Also, recently published are two children with generalized granuloma annulare clearing after receiving low‐dose antimalarials (111). Therefore, antimalarials may be considered an effective treatment choice if topical therapies fail in granuloma annulare.
Oral lichen planus
The clinical and histopathological resemblance of oral lesions of lichen planus to LE led to determine if antimalarial therapy is effective in oral lichen planus. Ten patients were enrolled in an open trial and received hydroxychloroquine 200–400 mg daily for 6 months (112). Excellent response was seen in nine patients, with effects being as early as 1 to 2 months. When therapy was discontinued in four patients, recurrence was seen after 3 months, whereas in the six patients with maintenance treatment no flare‐ups were observed. A 51‐year‐old woman with lichen planus of the lower lip was treated successfully with chloroquine (113). Although the first line of therapy of oral lichen planus is intralesional and topical corticosteroids, antimalarial therapy can be considered in resistant cases.
Panniculitis
In a study where 33 lupus panniculitis patients were treated with antimalarial therapy, 23 patients had clinical improvement within months, demonstrating the effectiveness of antimalarial therapy in these patients (114). As well several case reports have shown the complete resolution of lupus panniculitis lesions with antimalarial treatment (115, 116), although one case that was ANCA‐positive LE profundus showed no clearance of lesions with use of chloroquine, but improved with dapsone. Two cases of Weber‐Christian panniculitis have been shown to be treated successfully; one in a 62‐year‐old woman with oral chloroquine 250 mg daily (117), and the other was a 10‐year‐old boy being treated with hydroxychloroquine 4 mg/kg/day that allowed reduction in his systemic prednisone therapy (115). The use of hydroxychloroquine has been reported to be effective in chronic erythema nodosum (118). Recently, the use of antimalarials has been reported to treat lipoatrophic panniculitis (119). There is convincing evidence demonstrating antimalarials being effective in lupus panniculitis but in other forms of panniculitis further studies need to be performed to prove its effectiveness.
Miscellaneous
Chronic ulcerative stomatitis tends to be resistant to the use of local or systemic corticosteroids. However, antimalarials have shown to be effective and are considered as a first‐line agent in this rare condition (24). Antimalarials have shown to be effective in some cases of epidermolysis bullosa (120), atopic dermatitis (121), eosinophilic fasciitis (122), scleroderma (123), urticarial vasculitis (124) and reticular erythematous mucinosis (125).
Contraindications
An absolute contraindication for the use of antimalarials is hypersensitivity and prescribing to patients with a history of retinopathy is contraindicated. Caution should be used in patients with documented neuromuscular disorders such as myasthenia gravis, as well as in patients with psychotic disorders. Patients with glucose‐6‐phosphate deficiency should be carefully monitored although hemolysis does not occur at the recommended dosages of antimalarials for cutaneous diseases. As discussed in previous discussions, patients with porphyria cutanea tarda antimalarials are known to cause acute hepatitis. As both glucose‐6‐phosphate deficiency and porphyria cutanea tarda are rare, routine testing for these conditions are not recommended. Current evidence for use during pregnancy and lactation is discussed in succeeding discussions.
Pregnancy
Evidence of transplacental passage of hydroxychloroquine has been recently shown where levels of hydroxychloroquine exposure to the fetus are similar to maternal levels (20). However, a subsequent clinical study carried out by the same authors concluded hydroxychloroquine being overall beneficial in treatment of 123 pregnant lupus patients that were compared to controls (126). A randomized controlled trial with 20 pregnant lupus patients compared hydroxychloroquine to placebo treatment to assess effectiveness and safety (127). The hydroxychloroquine group consisted of eight patients with systemic lupus and two with discoid lupus, whereas the placebo group had nine and one patients with systemic lupus and discoid lupus, respectively. The hydroxychloroquine group had higher delivery age and apgar scores, and no congenital abnormalities, auditory defects or retinal toxicities were noted in the three years of follow up. No flare‐ups were seen when given hydroxychloroquine, in contrast to the three patients that flared in the placebo group. This study concluded that hydroxychloroquine can be started during pregnancy for treatment of lupus.
Another prospective study that evaluated lupus pregnancies in which patients received hydroxychloroquine from 1987 to 2002 observed similar results (128). When patients were examined that were receiving hydroxychloroquine and quinacrine during the first trimester of pregnancy, it was found that no congenital abnormalities with no eye or hearing defects were noted from the 14 deliveries examined (129). However, the authors of this study concluded that no long‐term effects of fetal exposure can be commented on. A British study retrospectively reviewed 36 patients receiving hydroxychloroquine (mean duration during pregnancy = 28.4 weeks) to 53 controls (130). The two different groups did not differ in obstetric outcome, and no congenital abnormalities were seen with hydroxychloroquine use, but the authors also commented that no difference was seen in disease activity as well between both groups.
If antimalarials are to be used during pregnancy, it is advised that hydroxychloroquine be utilized over other antimalarials for several reasons: (i) lower toxicity as it binds less avidly to tissues and unlike chloroquine is not associated with causing retinal toxicity in fetus (129), (ii) treatment of antiphospholipid antibody syndrome has been shown with hydroxychloroquine (131), (iii) hydroxychloroquine is found in decreased quantities in breast milk when compared to chloroquine (132). The evidence for usage of antimalarials during lactation is not well established, a recent follow‐up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation observed normal visual function and neurodevelopment outcome in early infancy (133).
Psoriasis
The mechanisms of action of antimalarials inducing psoriasis are currently believed to be twofold: (i) antimalarials block UV radiation (134); (ii) inhibition of transglutaminase with antimalarials comprises the epidermal barrier function (135). Speculatively, antimalarials may also indirectly enhance CD8 T‐cell activation through the increased shuttling of soluble antigens to class I MHC molecules (37).
Antimalarials have been used extensively in patients having psoriasis, as it effectively reduces inflamed joints in psoriatic arthritis. While being on this therapy it was noted that patients tended to flare up with their psoriasis; however, subsequent studies have shown the incidence of psoriasis while on antimalarials substantially varies (136). Forty‐eight military patients that received malaria prophylaxis weekly doses of 200 mg of chloroquine and 15 mg primaquine (137) were studied. Twenty of these patients had mild to moderate worsening of their psoriasis, and three patients became resistant to topical therapy. One study concluded that hydroxychloroquine was not associated with any psoriasis flare‐ups with patients being treated for psoriatic arthritis (138). However, pustular psoriasis is occasionally reported to occur in a similar setting (139).
One review has indicated that quinacrine is associated with higher frequencies of exfoliative erythroderma, and chloroquine had a higher chance of causing significant psoriasis flare‐ups (140). Hydroxychloroquine was shown to have lower rates of both of these reactions. Some authors claim that antimalarials do not induce psoriasis de novo but only triggers existing disease (136). A systematic review of these discrepant reports recently concluded that the evidence is insufficient to either refute or support a role of antimalarials in the exacerbation of psoriasis (141).
Adverse effects
Ocular
Antimalarials have been shown to cause adverse ocular effects that are reversible when the patient is taken off their antimalarial except for retinopathy. With chloroquine, patients occasionally experience blurry vision or diplopia when therapy is initiated; these symptoms usually resolve with time or the dosage can be decreased. This effect has not been observed with hydroxychloroquine (142).
Although corneal deposits are usually asymptomatic, patients can experience seeing halos around lights. The findings of corneal deposits under slit‐lamp examination are not a contraindication to continuing treatment (143). Depositions are related to dosage and usually appear after 1–1.5 months after therapy is initiated and are reversible with resolution occurring usually 1.5–2 months after therapy is discontinued. Easterbrook estimated that approximately 90% of patients with chloroquine 250 mg daily develop corneal deposits, in contrast with patients who received hydroxychloroquine 400 mg daily in whom corneal deposits were not observed (144, 145).
The most serious ocular toxicity with antimalarials is retinopathy and this is an absolute contradiction to continuing therapy. Retinopathy in its early stages is called premaculopathy, which is a reversible adverse effect characterized by changes in visual field or fundoscopic examination with no associated vision loss (146). With progression, a zone of depigmentation surrounds the pigmented part of the macula that is all encircled further with an area of pigment resulting in a “bull's eye” appearance.
In the 1960–70s retinal toxicity was seen in patients receiving high standard dosage antimalarial regimens, but more recent studies have shown that this toxicity can be limited with lower effective dosages and patient monitoring (147, 148). In a study with nine patients that received less than 6.5 mg/kg/day of hydroxychloroquine with a total cumulative dose of greater than 1000 g (1054–3923 g), no individuals acquired retinal toxicity (149). The second study being the largest retrospective study on hydroxychloroquine retinal toxicity contained 1207 patients being followed up for 2 years also observed no toxicity in the same dosage range (150). Mackenzie followed up over 900 patients with rheumatoid arthritis over 7 years to determine retinopathy associated with cumulative and daily dosage regimens (151). From these patients, those that received greater than 1 kg of chloroquine or hydroxychloroquine were further observed to find that the mean daily dosage were: (i) 3.65 mg/kg/day of chloroquine and 6.49 mg/kg/day of hydroxychloroquine in those that did not progress to retinopathy; (ii) 5.11 mg/kg/day of chloroquine and 7.77 mg/kg/day of hydroxychloroquine in those that developed retinopathy. Therefore, Mackenzie concluded in his study that recommended safe dosages for chloroquine is less than 4 mg/kg/day and for hydroxychloroquine is less than 6.5 mg/kg/day to prevent retinopathy. A review of the literature has shown that chloroquine 250 mg/day is more oculotoxic than hydroxychloroquine 400 mg/day, whereas quinacrine has a relative lack of retinal toxicity (152, 153). Only two questionable cases of retinotoxicity have been reported with quinacine use (154, 155), and thus most specialists do not recommend routine ophthalmologic follow‐up of patients on this drug.
The American Academy of Ophthalmology (AAO) screening guidelines recommend patients be placed on hydroxychloroquine ≤ 6.5 mg/kDa/day or chloroquine ≤ 3 mg/kg/day (156, 157, 158). Based on the guidelines, patients are divided into two categories: low‐risk patients who receive this lower dosage and have used the drug for ≤ 5 years, and higher‐risk patients who have received higher dosages or having other risk factors such as high body fat level, concomitant kidney or liver disease, concomitant retinal disease, or ≥ 60 years of age. Patients should have a baseline ophthalmologic examination within 1 year of starting these medications, which includes examination of the retina through a dilated pupil and testing of the central visual field sensitivity by either a self‐testing grid chart (Amsler grid) or an automated field tester (Humphrey 10‐2 testing). When patients are in the low‐risk category, no further testing is required for 5 years if the initial baseline examination is normal according to the AAO guidelines, whereas patients in the high‐risk category are examined annually. If any toxicity appears to develop then more elaborate tests are recommended such as multifocal electroretinography (159).
Neuromuscular
Antimalarials have been reported to stimulate the cerebral cortex in particular with quinacrine and to a lesser effect with chloroquine (75). The most frequent manifestation reported by patients include nightmares and headaches. Other side effects include irritability, nervousness, psychosis, seizures and hyperexcitability.
Musculoskeletal symptoms have been reported in the literature to occur with usage of antimalarials that resolved with cessation of therapy. Chloroquine can occasionally cause myalgia and fatigue with onset of treatment. As well, neuromyopathy has been reported in several patients while on chloroquine. In 156 SLE patients who were treated with hydroxychloroquine, two patients developed proximal myopathy with positive electromyography (160).
Hematologic
The use of antimalarials in patients with glucose‐6‐phosphate deficiency has been reported to cause hemolysis (161). Agranulocytosis and aplastic anemia have been uncommonly observed in patients that have received chloroquine or quinacrine (24, 88). Although rare, all the antimalarials have caused severe leukopenia in patients. The hematologic adverse effects caused by antimalarials are reversed with cessation of therapy. The risk of aplastic anemia has been reported in patients receiving quinacrine that require bone marrow transplantation, with the risk being estimated to be 1/50,000 (88). Patients are normally monitored with a complete blood count (CBC) at baseline, followed with a CBC monthly for 3 months and then every 4–6 months.
Cardiac
Cardiac side effects from prescribing antimalarials in treatment of cutaneous lesions are rare. However, a recent case was reported in a 59‐year‐old woman treated with antimalarials during 13 years for discoid LE who subsequently developed conduction disturbances and congestive heart failure (CHF) (162). This cardiotoxicity is more commonly seen in patients being treated for systemic LE. Usually, the antimalarial known to cause conduction disturbances and CHF is chloroquine and less frequently, hydroxychloroquine (163).
Gastrointestinal
The manifestation of gastrointestinal symptoms occurs in descending order with quinacrine (30%), chloroquine (20%), followed by hydroxychloroquine (10%) (75). Common symptoms include nausea, vomiting, and diarrhea. Some patients present with additional symptoms such as anorexia, heartburn, abdominal distension, and elevated transaminases. These manifestations are transient that resolve with time or with decreased dosage. Liver function tests are usually taken at baseline and then measured monthly for 3 months, followed by monitoring every 4–6 months.
Cutaneous
Blue‐gray to black pigmentation is observed in 10–30% of patients who receive long‐term antimalarial therapy (164, 165, 166). Darker pigmentation is associated with quinacrine compared to hydroxychloroquine or chloroquine. The pigmentation change is typically seen in the face, hard palate, forearms, and shins. Melanin granules and hemosiderin deposits are observed within the dermis with biopsy. In addition, the roots of the hair become bleached with successive streaking, and nailbeds may develop transverse bands (167, 168). These cutaneous manifestations resolve after several months of cessation of therapy.
Pruritus and other cutaneous eruptions occur in patients taking antimalarials. The cutaneous manifestations reported include morbilliform, exfoliative dermatitis, urticaria, eczematous, alopecia, photosensitivity, erythroderma, and erythema annulare centrifugum (75, 169). A recent report suggests that cutaneous adverse reactions to antimalarials may be more common in patients with dermatomyositis (where they were noted in roughly 30% of patients treated at one center) than in patients with lupus where the historical reaction rate is roughly 3–10% (170). Patients that took quinacrine during World War II developed lichenoid eruptions with rates of 1/2000 soldiers on 100 mg daily, and in 1/500 soldiers taking 200 mg daily of therapy (171). Three of these patients were observed to develop squamous cell carcinoma; however, two of these patients also had radiation therapy and the medication was continued for months to years despite the onset of the eruption. It has been proposed that such lichenoid tissue reactions may precede quinacrine bone marrow toxicity (171).
Drug interactions
Antimalarials exhibit several important drug interactions that are highlighted in this section. The combination of hydroxychloroquine treatment with chloroquine should not be given due to potential retinotoxicity (148). Levels of penicillamine or digoxin may be increased with hydroxychloroquine or chloroquine usage (21). Metoprolol levels have been shown to increase with usage of hydroxychloroquine (172). Chloroquine can reduce the bioavailability of ampicillin and increase cyclosporine levels (21). Kaolin and anti‐acids may decrease absorption of chloroquine and therefore the intake of these medications should not be within 4 hours of chloroquine therapy. Anti‐arrhythmic activity is possible with chloroquine as seen with amiodarone and chlorpromazine, and therefore caution should be used in prescribing these medications concurrently (21). Cimetidine tends to impair elimination of chloroquine. Simultaneous usage of chloroquine with hepatotoxic drugs increases the risk of hepatotoxicity and use with mefloquine with seizures (173, 174, 175). Antimalarials interfere with neuromuscular transmission and therefore should not be used in patients with disruption of neuromuscular transmission via predisposing conditions or secondary to medications. Smoking has been reported to inhibit the P450 enzyme system, decreasing the efficacy of antimalarial therapy (176). Furthermore, nicotine may inhibit the lysosomal uptake of antimalarials (177). A more recent study showed no difference in effectiveness in smokers; however, there were a lower number of patients in this study that could account for failing to detect a difference (178).
Conclusion
Antimalarials have been used by dermatologists for over 50 years, principally for the management of cutaneous LE. When compared with other immunomodulatory agents, antimalarials have a favorable safety profile. The major mode of action now appears to be inhibition of endosomal TLR signaling resulting in reduced B‐cell and dendritic‐cell activation. This mode of action has obvious relevance in these drugs’ efficacy in the treatment of LE. This mode of action may also explain the beneficial effects of these drugs in other T‐cell–mediated dermatoses such as lichen planus and granulomatous dermatoses such as sarcoidosis and granuloma annulare. Our new understanding of the toxicities and modes of action of these drugs may suggest new applications and modified treatment regimens.
References
- 1. Ruiz‐Irastorza G, Egurbide MV, Pijoan JI, et al Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006: 15: 577. [DOI] [PubMed] [Google Scholar]
- 2. Payne JF. A postgraduate lecture on lupus erythematosus. Clin J 1894: 4: 223–229. [Google Scholar]
- 3. Isaacson D, Elgart M, Turner ML. Anti‐malarials in dermatology. Int J Dermatol 1982: 21: 379–395. [DOI] [PubMed] [Google Scholar]
- 4. Hofheniz W, Merkli B. Quinine and quinine analogues In: Peters W, Richards WHG, eds. Antimalarial drugs II: current antimalarials and new drug development. New York: Springer‐Verlag, 1984: 61. [Google Scholar]
- 5. Page F. Treatment of lupus erythematosus with mepacrine. Lancet 1951: 2: 755–758. [DOI] [PubMed] [Google Scholar]
- 6. McChesney E, Fitch C. 4‐Aminoquinolines In: Peters W, Richards W, eds. Antimalarial drugs: II current antimalarials and new drug developments. Berlin: Springer‐Verlag, 1984: 3–60. [Google Scholar]
- 7. Rollo I. Drugs used in the chemotherapy of malaria In: Goodman A, Goodman L, Gilamn A, eds. The pharmacological basis of therapeutics, 6th ed. New York: MacMillan Publishing, 1980: 1038–1060. [Google Scholar]
- 8. Midha KK, McKay G, Rawson MJ, Hubbard JW. The impact of stereoisomerism in bioequivalence studies. J Pharm Sci 1998: 87: 797–802. [DOI] [PubMed] [Google Scholar]
- 9. Krishna S, White NJ. Pharmacokinetics of quinine, chloroquine and amodiaquine: clinical implications. Clin Pharmacokinet 1996: 30: 263–299. [DOI] [PubMed] [Google Scholar]
- 10. Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine: focus on recent advancements. Clin Pharmacokinet 1996: 31: 257–274. [DOI] [PubMed] [Google Scholar]
- 11. Sjolin‐Forsberg G, Berne B, Blixt C, Johansson M, Lindstrom B. Chloroquine phosphate: a long‐term follow‐up of drug concentrations in skin suction blister fluid and plasma. Acta Derm Venereol 1993: 73: 426–429. [DOI] [PubMed] [Google Scholar]
- 12. Koranda FC. Antimalarials. J Am Acad Dermatol 1981: 4: 650–655. [DOI] [PubMed] [Google Scholar]
- 13. Projean D, Baune B, Farinotti R, et al In vitro metabolism of chloroquine: identification of CYP2C8, CYP3A4, and CYP2D6 as the main isoforms catalyzing N‐desethylchloroquine formation. Drug Metab Dispos 2003: 31: 748–754. [DOI] [PubMed] [Google Scholar]
- 14. Kim KA, Park JY, Lee JS, Lim S. Cytochrome P450 2C8 and CYP3A4/5 are involved in chloroquine metabolism in human liver microsomes. Arch Pharm Res 2003: 26: 631–637. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka E, Taniguchi A, Urano W, Yamanaka H, Kamatani N. Pharmacogenetics of disease‐modifying anti‐rheumatic drugs. Best Pract Res Clin Rheumatol 2004: 18: 233–247. [DOI] [PubMed] [Google Scholar]
- 16. Furst DE, Lindsley H, Baethge B, et al Dose‐loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double‐blind six‐week trial with 18‐week extension. Arthritis Rheum 1999: 42: 357–365. [DOI] [PubMed] [Google Scholar]
- 17. Munster T, Gibbs JP, Shen D, et al Hydroxychloroquine concentration–response relationships in patients with rheumatoid arthritis. Arthritis Rheum 2002: 46: 1460–1469. [DOI] [PubMed] [Google Scholar]
- 18. Costedoat‐Chalumeau N, Amoura Z, Hulot JS, et al Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006: 54: 3284–3290. [DOI] [PubMed] [Google Scholar]
- 19. Wallace D. Antimalarial therapies In: Wallace D, Hahn B, eds. Dubois’ lupus erythematosus. Philadelphia: Lippincott Williams & Wilkins, 2007: 1152–1174. [Google Scholar]
- 20. Costedoat‐Chalumeau N, Amoura Z, Aymard G, et al Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum 2002: 46: 1123–1124. [DOI] [PubMed] [Google Scholar]
- 21. Griffin JP. Drug interactions with antimalarial agents. Adverse Drug React Toxicol Rev 1999: 18: 25–43. [PubMed] [Google Scholar]
- 22. Rynes RI. Antimalarial drugs in the treatment of rheumatological diseases. Br J Rheumatol 1997: 36: 799–805. [DOI] [PubMed] [Google Scholar]
- 23. Janzing HM, Derom A, Derom E, Eeckhout C, Derom F, Rosseel MT. Intrapleural quinacrine instillation for recurrent pneumothorax or persistent air leak. Ann Thorac Surg 1993: 55: 368–371. [DOI] [PubMed] [Google Scholar]
- 24. Wolf R, Wolf D, Ruocco V. Antimalarials: unapproved uses or indications. Clin Dermatol 2000: 18: 17–35. [DOI] [PubMed] [Google Scholar]
- 25. Wozniacka A, Carter A, McCauliffe DP. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus 2002: 11: 71–81. [DOI] [PubMed] [Google Scholar]
- 26. Kaufmann AM, Krise JP. Lysosomal sequestration of amine‐containing drugs: analysis and therapeutic implications. J Pharm Sci 2007: 96: 729–746. [DOI] [PubMed] [Google Scholar]
- 27. De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol 1974: 23: 2495–2531. [DOI] [PubMed] [Google Scholar]
- 28. Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 1978: 75: 3327–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohkuma S, Chudzik J, Poole B. The effects of basic substances and acidic ionophores on the digestion of exogenous and endogenous proteins in mouse peritoneal macrophages. J Cell Biol 1986: 102: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oda K, Koriyama Y, Yamada E, Ikehara Y. Effects of weakly basic amines on proteolytic processing and terminal glycosylation of secretory proteins in cultured rat hepatocytes. Biochem J 1986: 240: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hurst NP, French JK, Gorjatschko L, Betts WH. Chloroquine and hydroxychloroquine inhibit multiple sites in metabolic pathways leading to neutrophil superoxide release. J Rheumatol 1988: 15: 23–27. [PubMed] [Google Scholar]
- 32. Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol 1997: 15: 821–850. [DOI] [PubMed] [Google Scholar]
- 33. Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci USA 1982: 79: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nowell J, Quaranta V. Chloroquine affects biosynthesis of ia molecules by inhibiting dissociation of invariant (gamma) chains from alpha‐beta dimers in B cells. J Exp Med 1985: 162: 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gammon G, Sercarz EE, Benichou G. The dominant self and the cryptic self: shaping the autoreactive T‐cell repertoire. Immunol Today 1991: 12: 193–195. [DOI] [PubMed] [Google Scholar]
- 36. Fox R. Anti‐malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus 1996: 5 (Suppl. 1): S4–S10. [PubMed] [Google Scholar]
- 37. Accapezzato D, Visco V, Francavilla V, et al Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J Exp Med 2005: 202: 817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Den Borne BE, Dijkmans BA, De Rooij HH, Le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor‐alpha, interleukin 6, and interferon‐gamma production by peripheral blood mononuclear cells. J Rheumatol 1997: 24: 55–60. [PubMed] [Google Scholar]
- 39. Karres I, Kremer JP, Dietl I, Steckholzer U, Jochum M, Ertel W. Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Physiol 1998: 274: R1058–R1064. [DOI] [PubMed] [Google Scholar]
- 40. Landewe RB, Miltenburg AM, Breedveld FC, Daha MR, Dijkmans BA. Cyclosporine and chloroquine synergistically inhibit the interferon‐gamma production by CD4‐positive and CD8‐positive synovial T‐cell clones derived from a patient with rheumatoid arthritis. J Rheumatol 1992: 19: 1353–1357. [PubMed] [Google Scholar]
- 41. Sperber K, Quraishi H, Kalb TH, Panja AVS, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine. Inhibition of interleukin 1 alpha (IL‐1‐alpha) and IL‐6 in human monocytes and T cells. J Rheumatol 1993: 20: 803–808. [PubMed] [Google Scholar]
- 42. Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa‐Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006: 15: 268. [DOI] [PubMed] [Google Scholar]
- 43. Weber SM, Levitz SM. Chloroquine interferes with lipopolysaccharide‐induced TNF‐alpha gene expression by a nonlysosomotropic mechanism. J Immunol 2000: 165: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 44. Weber SM, Chen JM, Levitz SM. Inhibition of mitogen‐activated protein kinase signaling by chloroquine. J Immunol 2002: 168: 5303–5309. [DOI] [PubMed] [Google Scholar]
- 45. Takeda K, Kaisho T, Akira S. Toll‐like receptors. Annu Rev Immunol 2003: 21: 335–376. [DOI] [PubMed] [Google Scholar]
- 46. Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG‐oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol 1998: 160: 1122–1131. [PubMed] [Google Scholar]
- 47. Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak‐Rothstein A. Chromatin‐IgG complexes activate B cells by dual engagement of IgM and toll‐like receptors. Nature 2002: 416: 603–607. [DOI] [PubMed] [Google Scholar]
- 48. Lau CM, Broughton C, Tabor AS, et al RNA‐associated autoantigens activate B cells by combined B cell antigen receptor/toll‐like receptor 7 engagement. J Exp Med 2005: 202: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via toll‐like receptor 3. Arthritis Rheum 2005: 52: 2656–2665. [DOI] [PubMed] [Google Scholar]
- 50. Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody‐DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 2005: 115: 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kyburz D, Brentano F, Gay S. Mode of action of hydroxychloroquine in RA‐evidence of an inhibitory effect on toll‐like receptor signaling. Nat Clin Pract Rheumatol 2006: 2: 458–459. [DOI] [PubMed] [Google Scholar]
- 52. Lafyatis R, York M, Marshak‐Rothstein A. Antimalarial agents: closing the gate on toll‐like receptors? Arthritis Rheum 2006: 54: 3068–3070. [DOI] [PubMed] [Google Scholar]
- 53. Manku MS, Horrobin DF. Chloroquine, quinine, procaine, quinidine, tricyclic antidepressants, and methylxanthines as prostaglandin agonists and antagonists. Lancet 1976: 2: 1115–1117. [DOI] [PubMed] [Google Scholar]
- 54. Loffler BM, Bohn E, Hesse B, Kunze H. Effects of antimalarial drugs on phospholipase A and lysophospholipase activities in plasma membrane, mitochondrial, microsomal and cytosolic subcellular fractions of rat liver. Biochim Biophys Acta 1985: 835: 448–455. [DOI] [PubMed] [Google Scholar]
- 55. Gonzalez‐Noriega A, Grubb JHVT, Sly WS. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol 1980: 85: 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Labro MT, Babin‐Chevaye C. Effects of amodiaquine, chloroquine, and mefloquine on human polymorphonuclear neutrophil function in vitro. Antimicrob Agents Chemother 1988: 32: 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ausiello CM, Barbieri P, Spagnoli GC, Ciompi ML, Casciani CU. In vivo effects of chloroquine treatment on spontaneous and interferon‐induced natural killer activities in rheumatoid arthritis patients. Clin Exp Rheumatol 1986: 4: 255–259. [PubMed] [Google Scholar]
- 58. Stuhlmeier KM, Pollaschek C. Quinacrine but not chloroquine inhibits PMA induced up‐regulation of matrix metalloproteinases in leukocytes: quinacrine acts at the transcriptional level through a PLA2‐independent mechanism. J Rheumatol 2006: 33: 472–480. [PubMed] [Google Scholar]
- 59. Ghigo D, Aldieri E, Todde R, et al Chloroquine stimulates nitric oxide synthesis in murine, porcine, and human endothelial cells. J Clin Invest 1998: 102: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ahmed MH, Ashton N, Balment RJ. The effect of chloroquine on renal function and vasopressin secretion: a nitric oxide‐dependent effect. J Pharmacol Exp Ther 2003: 304: 156–161. [DOI] [PubMed] [Google Scholar]
- 61. Lester RS, Burnham TK, Fine G, Murray K. Immunologic concepts of light reactions in lupus erythematosus and polymorphous light eruptions. I the mechanism of action of hydroxychloroquine. Arch Dermatol 1967: 96: 1–10. [PubMed] [Google Scholar]
- 62. Sjolin‐Forsberg G, Lindstrom B, Berne B. Topical chloroquine applied before irradiation protects against ultraviolet B (UVB)‐ and UVA‐induced erythema but not against immediate pigment darkening. Photodermatol Photoimmunol Photomed 1992: 9: 220–224. [PubMed] [Google Scholar]
- 63. Bettley FR. Chilblain lupus erythematosus. Proc R Soc Med 1964: 57: 515–516. [PMC free article] [PubMed] [Google Scholar]
- 64. Nguyen TQ, Capra JD, Sontheimer RD. 4‐Aminoquinoline antimalarials enhance UV‐B induced c‐jun transcriptional activation. Lupus 1998: 7: 148–153. [DOI] [PubMed] [Google Scholar]
- 65. Kim WU, Yoo SA, Min SY, et al Hydroxychloroquine potentiates fas‐mediated apoptosis of rheumatoid synoviocytes. Clin Exp Immunol 2006: 144: 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meng XW, Feller JM, Ziegler JB, Pittman SM, Ireland CM. Induction of apoptosis in peripheral blood lymphocytes following treatment in vitro with hydroxychloroquine. Arthritis Rheum 1997: 40: 927–935. [DOI] [PubMed] [Google Scholar]
- 67. Vezmar M, Georges E. Reversal of MRP‐mediated doxorubicin resistance with quinoline‐based drugs. Biochem Pharmacol 2000: 59: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 68. Sotelo J, Briceno E, Lopez‐Gonzalez MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 2006: 144: 337–343. [DOI] [PubMed] [Google Scholar]
- 69. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis 2006: 6: 67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Rheum 1987: 30: 1435–1436. [DOI] [PubMed] [Google Scholar]
- 71. Wallace DJ, Metzger ALVS, Turnbull BA, Kern PA. Cholesterol‐lowering effect of hydroxychloroquine in patients with rheumatic disease. Reversal of deleterious effects of steroids on lipids. Am J Med 1990: 89: 322–326. [DOI] [PubMed] [Google Scholar]
- 72. Smith GD, Amos TA, Mahler R, Peters TJ. Effect of chloroquine on insulin and glucose homoeostasis in normal subjects and patients with non‐insulin‐dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987: 294: 465–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Callen JP, Camisa C. Antimalarial agents In: Wolverton SE, ed. Comprehensive dermatologic drug therapy, 2nd ed. Philadelphia, PA: Saunders Elsevier, 2007: 259–273. [Google Scholar]
- 74. Wozniacka A, McCauliffe DP. Optimal use of antimalarials in treating cutaneous lupus erythematosus. Am J Clin Dermatol 2005: 6: 1. [DOI] [PubMed] [Google Scholar]
- 75. Van Beek MJ, Piette WW. Antimalarials . Dermatol Clin 2001: 19: 147–160, ix. [DOI] [PubMed] [Google Scholar]
- 76. Gilliam JN, Sontheimer RD. Distinctive cutaneous subsets in the spectrum of lupus erythematosus. J Am Acad Dermatol 1981: 4: 471–475. [DOI] [PubMed] [Google Scholar]
- 77. Kraak JH, Van Ketel W, Prakken JR, Van Zwet W. The value of hydroxychloroquine (plaquenil) for the treatment of chronic discoid lupus erythematosus: a double blind trial. Dermatologica 1965: 130: 293–305. [DOI] [PubMed] [Google Scholar]
- 78. Callen JP. Chronic cutaneous lupus erythematosus. Clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol 1982: 118: 412–416. [DOI] [PubMed] [Google Scholar]
- 79. Furner BB. Treatment of subacute cutaneous lupus erythematosus. Int J Dermatol 1990: 29: 542–547. [DOI] [PubMed] [Google Scholar]
- 80. The Canadian Hydroxychloroquine Study Group . A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991: 324: 150–154. [DOI] [PubMed] [Google Scholar]
- 81. The Canadian Hydroxychloroquine Study Group . A long‐term study of hydroxychloroquine withdrawal on exacerbation in systemic lupus erythematosus. Lupus 1997: 7: 80–85. [DOI] [PubMed] [Google Scholar]
- 82. Fessler BJ, Alarcon GS, McGwin G Jr., et al Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005: 52: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 83. Alarcon GS, McGwin G Jr, Bertoli AM, et al Effect of hydroxychloroquine in the survival of patients with systemic lupus erythematosus: data from lumina, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007. [DOI] [PMC free article] [PubMed]
- 84. Hallynck TH, Soep HH, Thomis JA, Boelaert J, Daneels R, Dettli L. Should clearance be normalised to body surface or to lean body mass? Br J Clin Pharmacol 1981: 11: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Feldmann R, Salomon D, Saurat JH. The association of the two antimalarials chloroquine and quinacrine for treatment‐resistant chronic and subacute cutaneous lupus erythematosus. Dermatology. Basel: S. Karger; 1994: 189: 425. [DOI] [PubMed] [Google Scholar]
- 86. Lipsker D, Piette JC, Cacoub P, Godeau P, Frances C. Chloroquine‐quinacrine association in resistant cutaneous lupus. Dermatology 1995: 190: 257. [DOI] [PubMed] [Google Scholar]
- 87. Toubi E, Kessel A, Rosner I, Rozenbaum M, Paran D, Shoenfeld Y. The reduction of serum B‐lymphocyte activating factor levels following quinacrine add‐on therapy in systemic lupus erythematosus. Scand J Immunol 2006: 63: 299–303. [DOI] [PubMed] [Google Scholar]
- 88. Wallace DJ. Antimalarials – the “real” advance in lupus. Lupus 2001: 10: 385. [DOI] [PubMed] [Google Scholar]
- 89. Malkinson FD, Levitt L. Hydroxychloroquine treatment of porphyria cutanea tarda. Arch Dermatol 1980: 116: 1147–1150. [PubMed] [Google Scholar]
- 90. Murphy GM, Hawk JL, Magnus IA. Hydroxychloroquine in polymorphic light eruption: a controlled trial with drug and visual sensitivity monitoring. Br J Dermatol 1987: 116: 379–386. [DOI] [PubMed] [Google Scholar]
- 91. Ashton RE, Hawk JL, Magnus IA. Low‐dose oral chloroquine in the treatment of porphyria cutanea tarda. Br J Dermatol 1984: 111: 609–613. [DOI] [PubMed] [Google Scholar]
- 92. Bruce AJ, Ahmed I. Childhood‐onset porphyria cutanea tarda: successful therapy with low‐dose hydroxychloroquine (plaquenil). J Am Acad Dermatol 1998: 38: 810–814. [DOI] [PubMed] [Google Scholar]
- 93. Badcock NR, O’Reilly DA, Zoanetti GD, Robertson EF, Parker CJ. Childhood porphyrias: implications and treatments. Clin Chem 1993: 39: 1334–1340. [PubMed] [Google Scholar]
- 94. Baler R. Porphyria precipitated by hydroxychloroquine treatment of systemic lupus erythematosus. Cutis 1976: 17: 96–98. [PubMed] [Google Scholar]
- 95. Cripps D, Curtis A. Toxic effect of chloroquine on porphyria hepatica. Arch Dermatol 1962: 86: 575–581. [Google Scholar]
- 96. Valls V, Ena J, Enriquez‐De‐Salamanca R. Low‐dose oral chloroquine in patients with porphyria cutanea tarda and low‐moderate iron overload. J Dermatol Sci 1994: 7: 169–175. [DOI] [PubMed] [Google Scholar]
- 97. Petersen CS, Thomsen K. High‐dose hydroxychloroquine treatment of porphyria cutanea tarda. J Am Acad Dermatol 1992: 26: 614–619. [DOI] [PubMed] [Google Scholar]
- 98. Jansen CT. Oral carotenoid treatment in polymorphous light eruption. A cross‐over comparison with oxychloroquine and placebo. Photodermatol 1985: 2: 166–169. [PubMed] [Google Scholar]
- 99. Corbett MF, Hawk JL, Herxheimer A, Magnus IA. Controlled therapeutic trials in polymorphic light eruption. Br J Dermatol 1982: 107: 571–581. [DOI] [PubMed] [Google Scholar]
- 100. Quain RD, Werth VP. Management of cutaneous dermatomyositis: current therapeutic options. Am J Clin Dermatol 2006: 7: 341–351. [DOI] [PubMed] [Google Scholar]
- 101. Woo TY, Callen JP, Voorhees JJ, Bickers DR, Hanno R, Hawkins C. Cutaneous lesions of dermatomyositis are improved by hydroxychloroquine. J Am Acad Dermatol 1984: 10: 592–600. [DOI] [PubMed] [Google Scholar]
- 102. Cosnes A, Amaudric F, Gherardi R, et al Dermatomyositis without muscle weakness long‐term follow‐up of 12 patients without systemic corticosteroids. Arch Dermatol 1995: 131: 1381–1385. [See comment.] [DOI] [PubMed] [Google Scholar]
- 103. Olson NY, Lindsley CB. Adjunctive use of hydroxychloroquine in childhood dermatomyositis. J Rheumatol 1989: 16: 1545–1547. [PubMed] [Google Scholar]
- 104. Ang GC, Werth V. Combination antimalarials in the treatment of cutaneous dermatomyositis: a retrospective study. Arch Dermatol 2005: 141: 855–859. [DOI] [PubMed] [Google Scholar]
- 105. Epstein J, Vandenberg J, Wright W. Solar urticaria. Arch Dermatol 1963: 88: 135–141. [DOI] [PubMed] [Google Scholar]
- 106. Willis J, Epstein J. Solar‐ vs. heat‐induced urticaria. Arch Dermatol 1974: 110: 389–392. [PubMed] [Google Scholar]
- 107. British Tuberculous Assocation . Chloroquine in the treatment of sarcoidosis: a report of the research committee of the British Tuberculous Association. Tubercle 1967: 48: 257–272. [PubMed] [Google Scholar]
- 108. Jones E, Callen JP. Hydroxychloroquine is effective therapy for control of cutaneous sarcoidal granulomas. J Am Acad Dermatol 1990: 23: 487–489. [DOI] [PubMed] [Google Scholar]
- 109. Cannistraci C, Lesnoni La Parola I, Falchi M, Picardo M. Treatment of generalized granuloma annulare with hydroxychloroquine. Dermatology 2005: 211: 167–168. [DOI] [PubMed] [Google Scholar]
- 110. Simon M Jr, Von Den Driesch P. Antimalarials for control of disseminated granuloma annulare in children. J Am Acad Dermatol 1994: 31: 1064–1065. [DOI] [PubMed] [Google Scholar]
- 111. Masmoudi A, Abdelmaksoud W, Turki H, et al [Beneficial effects of antimalarials in the treatment of generalized granuloma annular in children] in French. Tunis Med 2006: 84: 125–127. [PubMed] [Google Scholar]
- 112. Eisen D. Hydroxychloroquine sulfate (plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol 1993: 28: 609–612. [DOI] [PubMed] [Google Scholar]
- 113. De Argila D, Gonzalo A, Pimentel J, Rovira I. Isolated lichen planus of the lip successfully treated with chloroquine phosphate. Dermatology 1997: 195: 284–285. [DOI] [PubMed] [Google Scholar]
- 114. Martens PB, Moder KG, Ahmed I. Lupus panniculitis: clinical perspectives from a case series. J Rheumatol 1999: 26: 68–72. [PubMed] [Google Scholar]
- 115. Chung HS, Hann SK. Lupus panniculitis treated by a combination therapy of hydroxychloroquine and quinacrine. J Dermatol 1997: 24: 569–572. [DOI] [PubMed] [Google Scholar]
- 116. Shelley WB. Chloroquine‐induced remission of nodular panniculitis present for 15 years. J Am Acad Dermatol 1981: 5: 168–170. [DOI] [PubMed] [Google Scholar]
- 117. Sorensen RU, Abramowsky C, Stern RC. Corticosteroid‐sparing effect of hydroxychloroquine in a patient with early‐onset Weber–Christian syndrome. J Am Acad Dermatol 1990: 23: 1172–1174. [DOI] [PubMed] [Google Scholar]
- 118. Alloway JA, Franks LK. Hydroxychloroquine in the treatment of chronic erythema nodosum. Br J Dermatol 1995: 132: 661–662. [DOI] [PubMed] [Google Scholar]
- 119. Manchanda Y, Sharma VK, Das S, Satish R. Lipoatrophic panniculitis treated with antimalarials. J Dermatol 2004: 31: 347–349. [DOI] [PubMed] [Google Scholar]
- 120. Baer TW. Epidermolysis bullosa hereditaria treated with antimalarials. Arch Dermatol 1961: 84: 503–504. [DOI] [PubMed] [Google Scholar]
- 121. Doring HF, Mullejans‐Kreppel U. Chloroquine – therapy of atopic dermatitis. Z Hautkr 1987: 62: 1205–1213. [PubMed] [Google Scholar]
- 122. Lakhanpal S, Ginsburg WW, Michet CJ, Doyle JA, Moore SB. Eosinophilic fasciitis: clinical spectrum and therapeutic response in 52 cases. Semin Arthritis Rheum 1988: 17: 221–231. [DOI] [PubMed] [Google Scholar]
- 123. Nagy E, Ladanyi E. Treatment of circumscribed scleroderma in childhood. Z Hautkr 1987: 62: 547–549. [PubMed] [Google Scholar]
- 124. Lopez LR, Davis KC, Kohler PF, Schocket AL. The hypocomplementemic urticarial‐vasculitis syndrome: therapeutic response to hydroxychloroquine. J Allergy Clin Immunol 1984: 73: 600–603. [DOI] [PubMed] [Google Scholar]
- 125. Cernea SS, Rivitti EA. Successful treatment of mucinosis with chloroquine. J Dermatol Treat 1990: 1: 163–165. [Google Scholar]
- 126. Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum 2003: 48: 3207–3211. [DOI] [PubMed] [Google Scholar]
- 127. Levy RA, Vilela VS, Cataldo MJ, et al Hydroxychloroquine (HCQ) in lupus pregnancy: double‐blind and placebo‐controlled study. Lupus 2001: 10: 401. [DOI] [PubMed] [Google Scholar]
- 128. Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006: 54: 3640–3647. [DOI] [PubMed] [Google Scholar]
- 129. Levy M, Buskila D, Gladman DD, Urowitz MB, Koren G. Pregnancy outcome following first trimester exposure to chloroquine. Am J Perin 1991: 8: 174–178. [DOI] [PubMed] [Google Scholar]
- 130. Khamashta MA, Buchanan NM, Hughes GR. The use of hydroxychloroquine in lupus pregnancy: the British experience. Lupus 1996: 5: S65–S66. [PubMed] [Google Scholar]
- 131. Petri M. Thrombosis and systemic lupus erythematosus: the Hopkins lupus cohort perspective. Scand J Rheumatol 1996: 25: 191–193. [DOI] [PubMed] [Google Scholar]
- 132. American Academy of Pediatrics Committee on Drugs . Transfer of drugs and other chemicals into human milk. Pediatrics 2001: 108: 776–789. [PubMed] [Google Scholar]
- 133. Motta M, Tincani A, Faden D, et al Follow‐up of infants exposed to hydroxychloroquine given to mothers during pregnancy and lactation. J Perinatol 2005: 25: 86–89. [DOI] [PubMed] [Google Scholar]
- 134. Werth VP, Dutz JP, Sontheimer RD. Pathogenetic mechanisms and treatment of cutaneous lupus erythematosus. Curr Opin Rheumatol 1997: 9: 400–409. [DOI] [PubMed] [Google Scholar]
- 135. Wolf R, Lo Schiavo A. Is transglutaminase the mediator between antimalarial drugs and psoriasis? Int J Dermatol 1997: 36: 10–13. [DOI] [PubMed] [Google Scholar]
- 136. Wolf R, Ruocco V. Triggered psoriasis. Adv Exp Med Biol 1999: 455: 221–225. [DOI] [PubMed] [Google Scholar]
- 137. Kuflik EG. Effect of antimalarial drugs on psoriasis. Cutis 1980: 26: 153–155. [PubMed] [Google Scholar]
- 138. Kammer GM, Soter NA, Gibson DJ, Schur PH. Psoriatic arthritis: a clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum 1979: 9: 75–97. [DOI] [PubMed] [Google Scholar]
- 130. Vine JE, Hymes SR, Warner NB, Cohen PR. Pustular psoriasis induced by hydroxychloroquine: a case report and review of the literature. J Dermatol 1996: 23: 357–361. [PubMed] [Google Scholar]
- 140. Slagel GA, James WD. Plaquenil‐induced erythroderma. J Am Acad Dermatol 1985: 12: 857–862. [DOI] [PubMed] [Google Scholar]
- 141. Herman SM, Shin MH, Holbrook A, Rosenthal D. The role of antimalarials in the exacerbation of psoriasis: a systematic review. Am J Clin Dermatol 2006: 7: 249. [DOI] [PubMed] [Google Scholar]
- 142. Easterbrook M. The ocular safety of hydroxychloroquine. Semin Arthritis Rheum 1993: 23: 62–67. [DOI] [PubMed] [Google Scholar]
- 143. Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol 1990: 25: 249–251. [PubMed] [Google Scholar]
- 144. Easterbrook M, Bernstein H. Ophthalmological monitoring of patients taking antimalarials: preferred practice patterns. J Rheumatol 1997: 24: 1390–1392. [PubMed] [Google Scholar]
- 145. Easterbrook M. An ophthalmological view on the efficacy and safety of chloroquine versus hydroxychloroquine. J Rheumatol 1999: 26: 1866–1868. [PubMed] [Google Scholar]
- 146. Maturi RK, Yu M, Weleber RG. Multifocal electroretinographic evaluation of long‐term hydroxychloroquine users. Arch Ophthalmol 2004: 122: 973–981. [DOI] [PubMed] [Google Scholar]
- 147. Mavrikakis I, Sfikakis PP, Mavrikakis E, et al The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology 2003: 110: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 148. Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol 2002: 133: 649–656. [DOI] [PubMed] [Google Scholar]
- 149. Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol 1987: 104: 139–144. [DOI] [PubMed] [Google Scholar]
- 150. Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1207 patients in a large multicenter outpatient practice. Arthritis Rheum 1997: 40: 1482–1486. [DOI] [PubMed] [Google Scholar]
- 151. Mackenzie AH. Antimalarial drugs for rheumatoid arthritis. Am J Med 1983: 75: 48–58. [DOI] [PubMed] [Google Scholar]
- 152. Houpt JB. A rheumatologist's verdict on the safety of chloroquine versus hydroxychloroquine liability in off‐label prescribing. J Rheumatol 1999: 26: 1864–1866. [PubMed] [Google Scholar]
- 153. Zuehlke RL, Lilis PJ, Tice A. Antimalarial therapy for lupus erythematosus: an apparent advantage of quinacrine. Int J Dermatol 1981: 20: 57–61. [DOI] [PubMed] [Google Scholar]
- 154. Browning DJ. Bull's‐eye maculopathy associated with quinacrine therapy for malaria. Am J Ophthalmol 2004: 137: 577–579. [DOI] [PubMed] [Google Scholar]
- 155. Carr RE, Henkind P, Rothfield N, Siegel IM. Ocular toxicity of antimalarial drugs long‐term follow‐up. Am J Ophthalmol 1968: 66: 738–744. [DOI] [PubMed] [Google Scholar]
- 156. Marmor MF. New American Academy of Ophthalmology recommendations on screening for hydroxychloroquine retinopathy. Arthritis Rheum 2003: 48: 1764. [DOI] [PubMed] [Google Scholar]
- 157. Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy. A report by the American Academy of Ophthalmology. Ophthalmology 2002: 109: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 158. Sfikakis PP, Mavrikakis M. Ophthalmologic monitoring for antimalarial toxicity. J Rheumatol 2004: 31: 1011–1012. [PubMed] [Google Scholar]
- 159. Lai TY, Chan WM, Li H, Lai RY, Lam DS. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol 2005: 140: 794–807. [DOI] [PubMed] [Google Scholar]
- 160. Wang C, Fortin PR, Li Y, Panaritis T, Gans M, Esdaile JM. Discontinuation of antimalarial drugs in systemic lupus erythematosus. J Rheumatol 1999: 26: 808–815. [PubMed] [Google Scholar]
- 161. Hochstein P. Glucose‐6‐phosphate dehydrogenase deficiency: mechanisms of drug‐induced hemolysis. Exp Eye Res 1971: 11: 389–395. [DOI] [PubMed] [Google Scholar]
- 162. Costedoat‐Chalumea N, Hulot N, Amoura J, et al Cardiomyopathy related to antimalarial therapy with illustrative case report. Cardiology 2007: 107: 73. [DOI] [PubMed] [Google Scholar]
- 163. Wozniacka A, Cygankiewicz I, Chudzik M, Sysa‐Jedrzejowska A, Wranicz JK. The cardiac safety of chloroquine phosphate treatment in patients with systemic lupus erythematosus. The influence on arrhythmia, heart rate variability and repolarization parameters. Lupus 2006: 15: 521–525. [DOI] [PubMed] [Google Scholar]
- 164. Conroy EA, Liranzo MO, McMahon J, Steck WD, Tuthill RJ. Quinidine‐induced pigmentation. Cutis 1996: 57: 425–427. [PubMed] [Google Scholar]
- 165. Dereure O. Drug‐induced skin pigmentation: epidemiology, diagnosis and treatment. Am J Clin Dermatol 2001: 2: 253–262. [DOI] [PubMed] [Google Scholar]
- 166. Kleinegger CL, Hammond HL, Finkelstein MW. Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000: 90: 189–194. [DOI] [PubMed] [Google Scholar]
- 167. Asch PH, Caussade P, Marquart‐Elbaz C, Boehm N, Grosshans E. Chloroquine‐induced achromotrichia: an ultrastructural study. Ann Dermatol Venereol 1997: 124: 552–556. [PubMed] [Google Scholar]
- 168. Daniel CR, 3rd Scher RK. Nail changes caused by systemic drugs or ingestants. Dermatol Clin 1985: 3: 491–500. [PubMed] [Google Scholar]
- 169. Khraishi MM, Singh G. The role of anti‐malarials in rheumatoid arthritis – the American experience. Lupus 1996: 5: S41–S44. [PubMed] [Google Scholar]
- 170. Pelle MT, Callen JP. Adverse cutaneous reactions to hydroxychloroquine are more common in patients with dermatomyositis than in patients with cutaneous lupus erythematosus. Arch Dermatol 2002: 138: 1231–1233. [DOI] [PubMed] [Google Scholar]
- 171. Bauer F. Quinacrine hydrochloride drug eruption (tropical lichenoid dermatitis): its early and late sequelae and its malignant potential. A review. J Am Acad Dermatol 1981: 4: 239–248. [DOI] [PubMed] [Google Scholar]
- 172. Somer M, Kallio J, Pesonen U, Pyykko K, Huupponen R, Scheinin M. Influence of hydroxychloroquine on the bioavailability of oral metoprolol. Br J Clin Pharmacol 2000: 49: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Wielgo‐Polanin R, Lagarce L, Gautron E, Diquet B, Laine‐Cessac P. Hepatotoxicity associated with the use of a fixed combination of chloroquine and proguanil. Int J Antimicrob Agents 2005: 26: 176–178. [DOI] [PubMed] [Google Scholar]
- 174. Haehner T, Refaie MO, Muller‐Enoch D. Drug‐drug interactions evaluated by a highly active reconstituted native human cytochrome P4503A4 and human NADPH‐cytochrome P450 reductase system. Arzneimittelforschung 2004: 54: 78–83. [DOI] [PubMed] [Google Scholar]
- 175. Ridtitid W, Wongnawa M, Mahatthanatrakul W, Raungsri N, Sunbhanich M. Ketoconazole increases plasma concentrations of antimalarial mefloquine in healthy human volunteers. J Clin Pharm Ther 2005: 30: 285–290. [DOI] [PubMed] [Google Scholar]
- 176. Rahman P, Gladman DD, Urowitz MB. Smoking interferes with efficacy of antimalarial therapy in cutaneous lupus. J Rheumatol 1998: 25: 1716–1719. [PubMed] [Google Scholar]
- 177. Polet H. The effects of lysosomotropic amines on protein degradation, migration of nonhistone proteins to the nucleus, and cathepsin D in lymphocytes. J Cell Physiol 1985: 122: 415–423. [DOI] [PubMed] [Google Scholar]
- 178. Lardet D, Martin S, Truchetet F, Cuny JF, Virion JM, Schmutz JL. Effect of smoking on the effectiveness of antimalarial drugs for cutaneous lesions of patients with lupus: assessment in a prospective study. Revue de Medecine Interne 2004: 25: 786–791. [DOI] [PubMed] [Google Scholar]


