Abstract
Viral lower respiratory tract infections (VLRTI) remain one of the most common causes of morbidity and mortality worldwide. For many years, the diagnosis of VLRTI was based on laboratory techniques such as viral isolation in cell culture, antigen detection by direct fluorescent antibody staining, and rapid enzyme immunoassay. Radiological imaging and morphology also play an important role in diagnosing these infections. Exfoliative cytology provides a simple, rapid, inexpensive, and valuable means to diagnose and manage VLRTI. Here we review viral‐associated cytomorphological changes seen in exfoliated cells of the lower respiratory tract. Diagn. Cytopathol. 2017;45:614–620. © 2017 Wiley Periodicals, Inc.
Keywords: viruses, lower respiratory tract infections, diagnosis, exfoliative cytology
Viral lower respiratory tract infections (VLRTI) remain a common cause of morbidity and mortality worldwide.1 Although in the majority of people these infections are largely self‐limited, in certain groups such as children, the elderly, those with chronic respiratory diseases, on mechanical ventilation in intensive care and immunocompromised patients (e.g., organ transplant recipients, those with AIDS or hematological malignancies), these infections can be fatal if undetected and not promptly treated.2 For many years, the diagnosis of VLRTI was based on techniques such as viral isolation in cell culture, antigen detection by direct fluorescent antibody staining, and rapid enzyme immunoassay. Currently, nucleic acid based amplification techniques constitute the gold standard for viral detection due to advantages such as high sensitivity and specificity, rapidity, viral quantitation, rapid TAT, and increased cost‐effectiveness compared to single PCR.3
Radiological imaging and morphology also play an important role in diagnosing VLRTI. Exfoliative cytology provides a simple, rapid, inexpensive, and valuable means to diagnose and manage VLRTI.4 Ancillary studies such as special histochemical and fluorescent stains, immunocytochemistry, in situ hybridization, and molecular studies can also be applied to cytology samples, thereby increasing their diagnostic specificity and sensitivity.5 Sputum collection (spontaneous or induced), bronchial brushings, tracheal, or bronchial aspirations, bronchial washings, and bronchoalveolar lavages (BAL) can provide respiratory tract samples for diagnosis. For lesions that are centrally located and involve major airways, sputum samples and bronchial brushings/washings may yield diagnostic material, whereas a BAL may be more useful to assess infectious processes in lung periphery. Depending on the age and condition of the patient (e.g., ICU setting), each of these methods has its indications and associated limitations, advantages, and diagnostic accuracy.6
Here we review the viral‐associated cytomorphological changes seen in exfoliated cells of the lower respiratory tract.
Viral Cytopathic Effect
Viruses are too small to be observed with light microscopy. Instead, it is possible to observe virus‐infected cells by means of cellular alterations referred to as viral‐induced “cytopathic effect” (CPE).7 Cells often respond in different ways to viral infection. Several viral‐induced cytomorphological changes are listed in Table 1. It is important to mention that not all respiratory viruses induce morphological changes in cells of the lower respiratory tract. Characteristic CPE is often used to indicate the presence of inoculated viruses in tissue culture cells.
Table 1.
Viral‐Induced Cytopathologic Effect
| ‐ Cytomegaly |
| ‐ Inclusion bodies |
| ‐ Multinucleation |
| ‐ Alteration in chromatin pattern |
| ‐ Syncytial formation |
| ‐ Cytoplasmic vacuolation |
| ‐ Ciliocytophthoria |
Apart from cell death, a common morphological change induced by viral infection is cellular enlargement (cytomegaly) and/or fusion of neighboring cells to form multinucleated syncytia (or polykaryocytes). Syncytium formation is induced by surface expression of viral fusion proteins. Inclusion bodies are aggregated virions and/or viral products that may be observed in the cytoplasm or nucleus of infected cells (Fig. 1A). The cellular location, appearance, and staining pattern of these intracellular inclusions are helpful for cytopathologists to diagnose specific respiratory viral infections (Table 2).8 Such inclusions may sometimes mimic normal structures, or degenerative changes (so‐called “retroplasia”) in cells.9 For example, it is important not to mistake intranuclear inclusions with macronucleoli of malignant cells (Fig. 1B). A helpful clue is the existence of a clear halo surrounding viral inclusion bodies.
Figure 1.
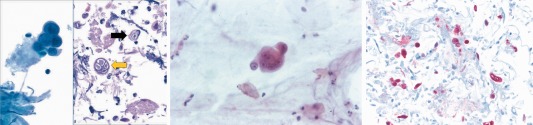
(A) Herpes simplex virus (HSV) inclusions in a bronchial washing cytology specimen. The left image shows infected bronchial epithelial cells with nuclei that have a glassy appearance due to viral cytopathic effect (Papanicolaou stain, 400×). The right image shows a cell block preparation of this sample demonstrating both Cowdry type A (black arrow) and Cowdry type B (yellow arrow) intranuclear inclusions (H&E stain, 400×). (B) Lung adenocarcinoma in a sputum specimen. The cluster of carcinoma cells shown has prominent nucleoli that may mimic viral infection (Papanicolaou stain, 400×). (C) Immunohistochemistry for HSV shows several scattered infected cells (positive red stain) in this lung sample from a lymphoma patient (immunohistochemical stain, 200×). [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Viral Inclusion Bodies
| Intranuclear | Intracytoplasmatic |
|---|---|
| Herpes Simplex Virus (e) | Parainfluenza (e) |
| Cytomegalovirus (a) | Measles (e) |
| Adenovirus (b) | Respiratory Syncytial Virus (b) |
a = amphophilic; b = basophilic; e = eosinophilic.
Most changes related to viral CPE can be readily identified with routine stains Papanicolaou (Pap) and/or hematoxylin & eosin (H&E) stains. In selected cases, special stains may be helpful. For example, cytomegalovirus (CMV) inclusions are magenta when stained with a Feulgen stain and measles can be more easily diagnosed using Lendrum's phloxine‐tartazine stain. More recently, immunocytochemsitry using monoclonal antibodies (Fig. 1C) and in‐situ hybridization using designed probes have become commercially available to detect viruses (e.g., adenovirus, CMV, herpes simplex virus 1 and 2, and respiratory syncytial virus). Certain monoclonal antibodies (e.g., pan‐adenovirus marker) may be reactive to all serotypes of a virus, whereas with others, viral subtypes may not be detected by specific antibody clones.
Ciliocytophthoria (CCT) or “detached ciliary tufts” is a term coined by Dr Papanicolaou in 1956 to describe a degenerative change observed in ciliated bronchial cells in association with viral infection and bronchial carcinoma.10, 11, 12 In sputum and other respiratory samples, CCT is recognized by the presence of cellular fragments without nuclei, presence of eosinophilic granules in the cytoplasm, and cilia attached to a terminal bar (Fig. 2A). The presence of CCT in sputum smears may be mistaken for multiflagellated protozoa or ciliated parasites.13, 14
Figure 2.
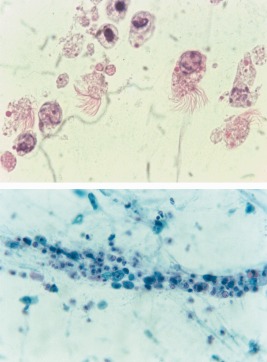
(A) Ciliocytophthoria due to viral infection in a respiratory sample showing several detached ciliary tufts attached to terminal bars, along with a few associated degenerated cells (Papanicolaou stain, 1000×). (B) Cellular necrosis due to viral infection (Papanicolaou stain, 200×). [Color figure can be viewed at http://wileyonlinelibrary.com]
In cytology samples from patients with a VLRTI, in addition to CPE, nonspecific findings such as intense cellular desquamation, cellular degenerative changes (e.g., nuclear pyknosis and karyorrhexis), an inflammatory infiltrate, and a granular or necrotic background (Fig. 2B) may be observed. It is important to be aware that the malignant tumors, such as bronchial squamous cell carcinoma and small cell carcinoma, can exhibit similar findings. As these viral infections are frequently found in immunosuppressed individuals, viral CPE may sometimes be seen together with other opportunistic pathogens such as Pneumocystis jirovecii.
Herpes Simplex Virus (HSV)
HSV is a large, enveloped DNA virus belonging to the Herpesviridae family. There are two subtypes of HSV, called HSV‐1 and HSV‐2. Although both types can affect the respiratory tract, HSV‐1 is the most frequently associated with these infections. VLRTI is caused through close contact with an infected individual. Focal HSV pneumonia likely results from contiguous spread of herpesvirus to lung parenchyma, whereas diffuse interstitial pneumonia may be a manifestation of hematogenous dissemination of virus. While primary infection is often accompanied by a self‐limited period of clinical illness, long‐term latency is symptom‐free. One feature of HSV infection is reactivation from the sensory nervous system of latently infected humans, although all the triggers for this are not well defined.15 Colonization of the lower respiratory tract by HSV may occur by means of aspiration from a reactivation of infection of the upper respiratory tract. HSV can infect the lower respiratory tract in both immunologically normal patients and the immunocompromised host.16
VLRTI is mainly associated with necrotizing tracheobronchitis and bronchopneumonia. On CT scan, infection manifests with areas of diffuse or multifocal ground‐glass attenuation and/or predominant areas of multifocal peribronchial consolidation. Predisposing factors for HSV tracheobronchial infection and pneumonia include immunosuppression, debilitation, severe burns, underlying malignancy, advanced age, and/or prolonged mechanical intubation. The latter has special relevance in patients admitted to the intensive care unit (ICU), usually reflecting viral reactivation during mechanical ventilation without lung parenchymal involvement.17 ICU and hospital length of stay can be significantly longer in such HSV‐positive patients. Disseminated HSV infection can also cause VLRTI in neonates.
Cytology samples may be more efficacious than tissue biopsy in establishing the diagnosis of HSV infection. Cytological features of HSV infection seen in respiratory samples include enlarged and multinucleated cells, with nuclear molding (conformity of adjacent nuclei to one another) and a chromatin characterized by an opaque or ground‐glass smudged appearance (so‐called Cowdry type B inclusions) (Fig. 3A) that is clumped at the nuclear borders (i.e., peripheral condensation of chromatin, also referred to as nuclear margination). The single or multiple nuclei often occupy the entire cell. Eosinophilic intranuclear inclusions (so‐called Cowdry type A inclusions) are centrally placed and surrounded by a clear halo (Fig. 3B). These inclusions are named after the cytologist Edmund Vincent Cowdry (1888–1975). The background may show associated acute inflammatory cells and necrosis as well as the presence of atypical keratinized and hyperchromatic squamous cells, atypical repair, and a necroinflammatory background18, 19 are pitfalls that can mimick malignancy in HSV‐affected samples. Also, in response to reactive changes, normal bronchial cells may become multinucleated and could hence mimic an HSV.
Figure 3.
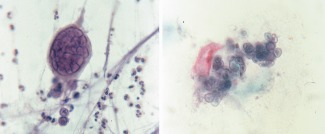
(A) Cowdry type B inclusion of Herpes simplex virus (HSV). There is multinucleation in this infected cell, molding of these nuclei, and chromatin margination beneath the nuclear membrane (Papanicolaou stain, 400×). (B) Cowdry type A inclusion of HSV. Note the characteristic eosinophilic intranuclear inclusion surrounded by a clear zone in these infected cells (Papanicolaou stain, 400×). [Color figure can be viewed at http://wileyonlinelibrary.com]
In questionable cases, immunocytochemistry and/or quantitative detection of HSV DNA can be diagnostic.20 In general, testing for viruses on BAL fluid should be restricted to immunocompromised patients with acute respiratory diseases and/or those with unexplained ground‐glass attenuations on CT scan.
Cytomegalovirus (CMV)
CMV, another DNA enveloped virus of the Herpesviridae family, commonly infects most people at some point. Primary infection is usually inapparent. As with other herpes viruses, CMV remains latent within its host, reactivating and shedding when the host's immune system becomes compromised. CMV is spread by means of blood transfusion, organ transplant, respiratory droplets, saliva, sexual contact, and urine. Newborns, especially those with congenital infection,21 and immunosuppressed patients such as those with AIDS and transplant recipients,22, 23 are the most susceptible population. CT findings of CMV pneumonia are nonspecific. However, CMV pneumonia may rarely mimic malignancy, especially in patients who present with a cavitary lung mass.
Infected lungs show interstitial pneumonitis with sparse mononuclear inflammation and characteristic nuclear inclusions. Cytological features associated with CMV include marked enlargement of infected cells with a large, homogeneous, round intra‐nuclear inclusion (Fig. 4A) surrounded by a clear halo (creating an “owl's eye” appearance). There is also margination of chromatin toward the nucleus periphery. These inclusions may be observed in airway epithelium, alveolar pneumocytes, macrophages, endothelium, and interstitial cells. Small cytoplasmic granular, basophilic inclusions may also be observed aggregating near the cell membrane. Multinucleation may occur, but is a rare finding. With severe infection neutrophil infiltrates, necrosis, hemorrhage, hyaline membranes, and diffuse alveolar damage (DAD) may also be observed.
Figure 4.
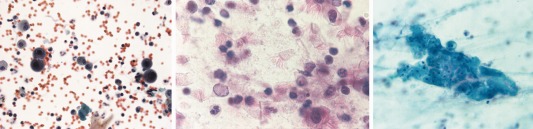
(A) Bronchoalveolar lavage specimen showing four cells on either side of the image with cytomegalovirus (CMV) intranuclear inclusions. Note the large central inclusions surrounded by a clear halo within the nuclei of infected cells (Papanicolaou stain, high magnification). (B) Cytological features of respiratory adenovirus infection are shown including dark stained “smudgy” nuclei and ciliocytophthoria (Papanicolaou stain, 1000×). (C) Enlarged syncytial cell with intracytoplasmic inclusions due to parainfluenza virus infection (Papanicolaou stain, 400×). [Color figure can be viewed at http://wileyonlinelibrary.com]
Although CMV DNA probes have been shown to be more sensitive than conventional cytological procedures, most cases with characteristic CMV‐induced cellular changes do not require ancillary studies such as immunocytochemistry or polymerase chain reaction (PCR) to confirm the diagnosis. In some cases, immunohistochemistry utilizing monoclonal antibodies to immediate‐early and early CMV nuclear antigens may indicate the development of CMV pneumonitis before cytopathic changes are evident. Moreover, while CMV may be frequently recovered from BAL specimens, this result does not necessarily indicate acute CMV pulmonary disease. In certain settings, BAL CMV cultures can also have high negative and low positive predictive value for CMV pneumonia.
Adenoviruses
Adenoviruses are DNA nonenveloped virus members of the Adenoviridae family. There are currently over 60 human adenoviruses types in seven species named human adenovirus A–G.24 Respiratory infections occur following contact with infectious material (e.g., respiratory secretions) from another individual or a fomite. Epidemic respiratory disease may be concentrated in populations of military recruits during winter months. Adenovirus infection typically causes respiratory illnesses such as a common cold, croup, bronchitis, or pneumonia.
Infection causes necrotizing inflammation affecting the bronchial and bronchiolar mucosa. In tissue sections, distinct intranuclear basophilic inclusions, surrounded by a small halo or often filling the entire nucleus and obscuring the nuclear membrane (referred to as “smudge” cells) may be seen.25 Electron microscopy reveals that these inclusion bodies consist of arrays of particles of adenovirus. CCT is a common, but nonspecific, feature of adenovirus infection (Fig. 4B), often found in sputum smears.26 The direct association of CCT with adenovirus infection was established when studying infected patients from whom sputum as well as serum specimens were obtained. Significant increases in adenovirus antibody occurred in 48 (80%) of 60 individuals whose sputum specimens were positive for CCT.27
Available ancillary studies include immunocytochemistry for adenovirus, monoclonal antibody‐based enzyme immunoassay on fresh specimens, immunofluorescence assay, tissue culture, and PCR.
Parainfluenza Virus
Human parainfluenza is an enveloped RNA virus in the Paramyxoviridae family. Genetically and antigenically, they are divided into four types (1 to 4). They are ubiquitous and infect most people during childhood (especially type 3 virus). The principal clinical syndromes are acute laryngotracheobronchitis, bronchiolitis, and pneumonia.28
The classic pathologic finding in severe parainfluenza infection is alveolar epithelial damage, focal or diffuse, with hyaline membranes. The healing phase is characterized by an interstitial lymphocytic infiltrate and bronchial squamous metaplasia. Multinucleation, enlarged syncytial cells with irregular intracytoplasmic basophilic inclusions, and vacuolated cytoplasm are the major cytological features (Fig. 4C).
Available ancillary studies include immunocytochemistry, PCR, multiplex nucleic acid sequence‐based amplification (NASBA) assay, and tissue culture.
Measles
Measles is caused by a single‐stranded, enveloped RNA virus with 1 serotype. It is classified as a member of the genus Morbillivirus in the Paramyxoviridae family. Humans are the only natural hosts of the measles virus.29 Infection is characterized by a prodrome of fever and malaise, cough, coryza, conjunctivitis, and a pathognomonic enanthem (Koplik spots) followed by a maculopapular rash that spreads over the entire body. Measles virus is highly contagious and spreads through the air with coughing and sneezing. Measles frequency has decreased dramatically as the introduction of a vaccine in the 1960s.
Important respiratory complications of measles infection are tracheitis in breastfeeding babies and giant cell (Hecht's) pneumonia in adults, especially those who are immunocompromised. Measles pneumonia is characterized by an obliteration of airspaces with a mixed inflammatory infiltrate (especially CD8+ T‐cells), proliferation of alveolar epithelial cells, and the formation of multinucleated epithelial cells. Some cases of bronchiolitis obliterans organizing pneumonia related to measles virus have also been reported.30 Cytology specimens show large multinucleated giant cells with cytoplasmic and intranuclear inclusions resembling those of HSV (Fig. 5A). The intranuclear inclusions have a glassy, eosinophilic appearance. Cytoplasmic inclusions are best demonstrated with the Lendrum phloxine tartrazine stain, which appear red on a yellow background. The differential diagnosis includes other viral infections characterized by multinucleated giant cells, benign noninfectious entities associated with giant cells such as hard‐metal pneumoconiosis, and circulating megakaryocytes. Ancillary studies include PCR and viral culture.
Figure 5.
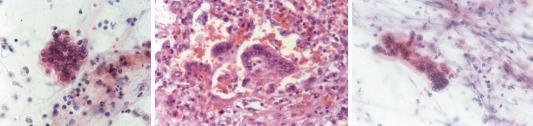
(A) Enlarged multinucleated cell indicative of measles pneumonia (Papanicolaou stain, 400×). (B) Lung parenchyma with respiratory syncytial virus (RSV) infection characterized by several syncytial multinucleated cells (H&E stain, 400×). (C) RSV‐induced multinucleated giant cell (Papanicolaou stain, 400×). [Color figure can be viewed at http://wileyonlinelibrary.com]
Respiratory Syncytial Virus (RSV)
RSV is an enveloped RNA virus in the Paramyxoviridae family and Pneumovirus genus. Although RSV causes acute respiratory tract illness in people of all ages, it is the most common cause of lower respiratory infection in children younger than 1 year.31 While healthy people typically experience mild, cold‐like symptoms if infected and recover in a week or two. RSV can be serious, particularly in infants and the elderly.32 High‐risk groups such as premature infants, immunocompromised children, or those with chronic pulmonary disease or congenital cardiac disease are more likely to have a severe disease process, including bronchiolitis and/or pneumonia. RSV infection is considered to be a major public health problem and economic burden in most countries.33 Epidemiologic, experimental, and clinical links between RSV infection and asthma have been reported.34, 35
Extensive neutrophil accumulation in the lungs and occlusion of small airways by DNA‐rich mucus plugs are characteristic of severe infection, contributing to airway obstruction.36 In autopsy series, the distribution of virus‐infected cells ranged from clusters of cells in large cartilaginous airways to more circumferential infection of epithelium in the smallest airways. This suggests a viral tropism for cells of the distal airway, where there are more homogeneous cell populations, thereby allowing the virus to efficiently spread from cell to cell with significant syncytium formation (Fig. 5B) in bronchiolar epithelium.37 The presence of large syncytial cell aggregates with indistinct eosinophilic cytoplasmic inclusions is typical of RSV infection in cytology samples (Fig. 5C). It has also been suggested that that a high rate of “Creola bodies” in sputum (Fig. 6A) may be present in infants with RSV bronchiolitis, which are typically associated with disease progression to recurrent wheezing and asthma.38 A Creola body is a rounded cluster of reactive ciliated bronchial epithelial cells. Other viruses that cause wheezing in children are rhinovirus, human metapneumovirus, and influenza viruses.39
Figure 6.
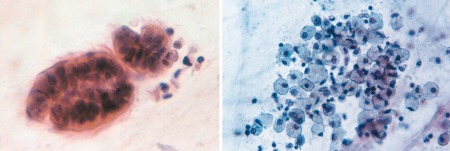
(A) Creola body (Papanicolaou stain, 400×). (B) Multiple foamy macrophages are shown associated with SARS (Papanicolaou stain, high magnification). [Color figure can be viewed at http://wileyonlinelibrary.com]
The cytomorphologic differential diagnosis of RSV infection includes other viral infections such as human metapneumonia and measles, and reactive multinucleated bronchial cells with clusters of bronchial hyperplasia. Additional studies include the BinaxNOW RSV card test, an in vitro immunochromatographic assay used to detect viral antigen in nasal wash and nasopharyngeal swab specimens from symptomatic patients, as well as PCR, shell vial culture, and tissue culture.
SARS Coronavirus
Severe acute respiratory syndrome coronavirus (SARS‐CoV) emerged several years ago as an important cause of SARS in humans. The predominant pathological features of SARS‐CoV infection include diffuse alveolar damage (DAD), atypical pneumonia with dry cough, persistent fever, progressive dyspnea, and sometimes abrupt deterioration of lung function.40
In postmortem studies, viral particles have been detected in pneumocytes, suggesting that they are likely the primary target of infection.41 Additional morphological alterations include the presence of multinucleated pneumocytes and fibrogranulation tissue proliferation in small airways and air spaces. In sputum smears submitted for cytology examination, loose aggregates of macrophages were commonly seen with morphological changes such as cytoplasmic foaminess and distinct vacuoles (Fig. 6B), multinucleation, and ground glass appearance of the nucleus.42
Conclusion
There is a need to establish a specific diagnosis of LRTI to allow accurate targeted therapy. For this purpose, the collection of lung samples is important to establish a diagnosis of VLRTI, especially in very ill patients where rapid detection is important and one cannot wait for culture results. Invasive procedures such as open lung biopsy may not always be necessary, as many viral infections can be diagnosed with exfoliative cytology. While viral CPE can be pathognomic, morphological overlap among these infections does exist (e.g., giant cell formation and multinucleation). When examining lung cytology samples, it is important to be cognizant of potential pitfalls including mimics, especially cancer, as well as the presence of concomitant infections in immunosuppressed persons. Finally, as the field evolves, it is imperative for researchers to report findings of potential CPE with emerging VLRTI such as Avian Influenza A and Middle East respiratory Syndrome Coronavirus (MERS‐CoV).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1. Singanayagam A, Joshi PV, Mallia P, Johnston SL. Viruses exacerbating chronic pulmonary disease: the role of immune modulation. BMC Med 2012;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavia AT. What is the role of respiratory viruses in community‐acquired pneumonia? What is the best therapy for influenza and other viral causes of community‐acquired pneumonia? Infect Dis Clin North Am 2013;27:157–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prachayangprecha S, Schapendonk CM, Koopmans MP, et al. Exploring the potential of next‐generation sequencing in detection of respiratory viruses. J Clin Microbiol 2014;52:3722–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erozan YS, Ramzy I. Respiratory infections In: Rosenthal Dorothy L., editor. Pulmonary cytopathology. New York: Springer; 2014. p 5383. [Google Scholar]
- 5. Khalbuss WE, Laucirica R, Pantanowitz L. Pulmonary infections In: Rosenthal Dorothy L., editor. Cytopathology of infectious diseases. New York: Springer; 2011. p 121159. [Google Scholar]
- 6. Suen KC, Abdul‐Karim FW, Kaminsky DB, et al. Guidelines of the Papanicolaou Society of Cytopathology for the examination of cytologic specimensobtained from the respiratory tract. Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1999;21:61–69. [DOI] [PubMed] [Google Scholar]
- 7. Wagner RR. Cytopathic effects of viruses: A general survey. In: Fraenkel‐Conrat Heinz. and Wagner Robert R., editors. New York: Springer Science; 1984. p 150. [Google Scholar]
- 8. Koprowska I. Intranuclear inclusion bodies in smears of respiratory secretions. Acta Cytol 1961;5:219–228. [PubMed] [Google Scholar]
- 9. Ariyasu S, Hirokawa M, Kanahara T. Retroplasia of bronchial columnar cells mimicking intranuclear viral inclusions. Acta Cytol 2000;44:100–101. [DOI] [PubMed] [Google Scholar]
- 10. Papanicolaou GN. Degenerative changes in ciliated cells exfoliating from the bronchial epithelium as a cytologic criterion in the diagnosis of diseases of the lung. NY State J Med 1956;56:2647–2650. [PubMed] [Google Scholar]
- 11. Pierce CH, Hirsch JG. Ciliocytophthoria: relationship to viral respiratory infections of humans. Proc Soc Exp Biol Med 1958;98:489–492. [DOI] [PubMed] [Google Scholar]
- 12. Kim CJ, Ko I, Bukantz SC. Ciliocytophthoria (CCP) in asthmatic children, with references to viral respiratory infection and exacerbation of asthma. Preliminary report. J Allergy 1964;35:159–168. [DOI] [PubMed] [Google Scholar]
- 13. Hadziyannis E, Yen‐Lieberman B, Hall G, Procop GW. Ciliocytophthoria in clinical virology. Arch Pathol Lab Med 2000;124:1220–1223. [DOI] [PubMed] [Google Scholar]
- 14. Martínez‐Girón R, Doganci L, Ribas A. From the 19th century to the 21st, an old dilemma: ciliocytophthoria, multiflagellated protozoa, or both? Diagn Cytopathol 2008;36:609–611. [DOI] [PubMed] [Google Scholar]
- 15. Traylen CM, Patel HR, Fondaw W, et al. Virus reactivation: a panoramic view in human infections. Future Virol 2011;6:451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schuller D, Spessert C, Fraser VJ, Goodenberger DM. Herpes simplex virus from respiratory tract secretions: epidemiology, clinical characteristics, and outcome in immunocompromised and nonimmunocompromised hosts. Am J Med 1993;94:29–33. [DOI] [PubMed] [Google Scholar]
- 17. Bruynseels P, Jorens PG, Demey HE, et al. Hepes simplex virus in the respiratory tract of critical care patients: a prospective study. Lancet 2003;362:1536–1541. [DOI] [PubMed] [Google Scholar]
- 18. Lapkus O, Elsheikh TM, Ujevich BA, Liu YL, Silverman JF. Pitfalls in the diagnosis of herpes simplex infection in respiratory cytology. Acta Cytol 2006;50:617–620. [DOI] [PubMed] [Google Scholar]
- 19. Martínez‐Girón R. Degenerate herpes cells in bronchial secretions mimicking malignancy. Diagn Cytopathol 2012;40:1035–1036. [DOI] [PubMed] [Google Scholar]
- 20. Gooskens J, Templeton KE, Claas EC, van Bussel MJ, Smit VT, Kroes AC. Quantitative detection of herpes simplex virus DNA in the lower respiratory tract. J Med Virol 2007;79:597–604. [DOI] [PubMed] [Google Scholar]
- 21. Weisblum Y, Panet A, Haimov‐Kochman R, Wolf DG. Models of vertical cytomegalovirus (CMV) transmission and pathogenesis. Semin Immunopathol 2014;36:615–625. [DOI] [PubMed] [Google Scholar]
- 22. Drew WL. Cytomegalovirus disease in the highly active antiretroviral therapy era. Curr Infect Dis Rep 2003;5:257–265. [DOI] [PubMed] [Google Scholar]
- 23. Coussement J, Steensels D, Nollevaux MC, et al. When polymerase chain reaction does not help: cytomegalovirus pneumonitis associated with very low or undetectable viral load in both blood and bronchoalveolar lavage samples after lung transplantation. Transpl Infect Dis 2016;18:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Robinson CM, Singh G, Lee JY, et al. Molecular evolution of human adenoviruses. Sci Rep 2013;3:1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaki Sr, Paddock CD. Viral infections of the lung In: Goldblum John, editor. Pulmonary pathology. Philadelphia: Churchill Livingstone Elsevier; 2008. p 245288. [Google Scholar]
- 26. French CA. Respiratory tract and mediastinum In: Cibas Edmund S. and Ducatman Barbara S., editors. Cytology. Diagnostic principles and clinical correlates. Philadelphia: Elsevier Sounders; 2014. p 59104. [Google Scholar]
- 27. Pierce CH, Knox AW. Ciliocytophthoria in sputum from patients with adenovirus infections. Proc Soc Exp Biol Med 1960;104:492–495. [DOI] [PubMed] [Google Scholar]
- 28. Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev 2003;16:242–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moss WJ, Strebel P. Biological feasibility of measles eradication. J Infect Dis 2011;204 (Suppl 1):S47–S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casas Maldonado F, Gallardo Medina M, Franco Campos MA, Conde Valero A, Pérez Chica G, Cruz Molina JM. Bronchiolitis obliterans with organized pneumonia associated with measles virus. [Article in Spanish]. Arch Bronconeumol 1997;33:541–544. [PubMed] [Google Scholar]
- 31. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. N Engl J Med 2005;352:1749–1759. [DOI] [PubMed] [Google Scholar]
- 33. Bohmwald K, Espinoza JA, Rey‐Jurado E, et al. Human respiratory syncytial virus: Infection and pathology. Semin Respir Crit Care Med 2016;37:522–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohapatra SS, Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev 2008;21:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh AM, Moore PE, Gern JE, Lemanske RF, Jr , Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene‐virus interactions in asthma causation. Am J Respir Crit Care Med 2007;175:108–119. [DOI] [PubMed] [Google Scholar]
- 36. Cortjens B, de Boer OJ, de Jong R, et al. Neutrophil extracelular traps cause airway obstruction during respiratory syncytial virus disease. J Pathol 2016;238:401–411. [DOI] [PubMed] [Google Scholar]
- 37. Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007;20:108–119. [DOI] [PubMed] [Google Scholar]
- 38. Yamada Y, Yoshihara S. Creola bodies in infancy with respiratory syncytial virus bronchiolitis predict the development of asthma. Allergol Int 2010; 59:375–380. [DOI] [PubMed] [Google Scholar]
- 39. Inoue Y, Shimojo N. Epidemiology of virus‐induced wheezing/asthma in children. Front Microbiol 2013;4:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lau SK, Chan JF. Coronaviruses: emerging and re‐emerging pathogens in humans and animals. Virol J 2015;12:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tse GM, To KF, Chan PK, et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J Clin Pathol 2004;57:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tse GM, Hui PK, Ma TK, et al. Sputum cytology of patients with severe acute respiratory syndrome (SARS). J Clin Pathol 2004;57:256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]


