Abstract
Recent events have led to a revision in ED equipment, preparedness and training for disasters. However, clinicians must still decide when, and what level of personal protection is required when a toxic threat exists. If possible, clear, simple and achievable protocols are required in such situations. Following an off‐site Australasian chemical biological or radiological incident, current evidence indicates that the initial receiving ED staff will be adequately protected from all known chemical biological and radiological inhalational threats by wearing a properly fitted P2 (N95) mask, or its equivalent. Protection from serious contact injury is offered by wearing double gloves, disposable fluid‐repellent coveralls or gown, eye protection, surgical mask, and ideally, a cap and shoe covers; in conjunction with universal precautions and procedures.
Keywords: chemical biological and radiological, disaster planning, disasters, emergencies, personal protective equipment, protective clothing
Introduction
Chemical biological and radiological (CBR) events are not new. The developing world experienced several massive natural and man‐made CBR events through the 1980s, which included the Iraqi use of mustard gas and tabun against the Iranian military and Kurdish civilians, the 1984 methyl isocyanate release at Bhopal in India that leads to at least 15 000 deaths and the 1986 limnic eruption of carbon dioxide from Lake Nyos in Cameroon killing 1800 people.
More recently, the developed world has had a series of smaller‐scale CBR events including the 1995 Japanese Aum Shinrikyo sect releases of sarin killing 12 commuters, the 2001 USA domestic anthrax strain releases by a person/s unknown killing five politicians and media workers and the 2002 Russian special forces release of KOLOKOL‐1 (an opiate derivative) into the Moscow theatre accidentally killing 120 hostages.
Much has been published recently regarding the use of personal protective equipment (PPE) in an ED, following a CBR event. This includes which staff might participate, 1 protocols for contaminated ED patients, 2 the hospital response 3 and its incorporation into the wider health system disaster plan. 4 The use of PPE with air‐purifying respirators for hospital decontamination is controversial, 5 , 6 and forms part of what is termed ‘Level C’ PPE in some countries. The US Occupational Safety and Health Administration and Environmental Protection Agency definition of Level C PPE includes a full‐face air‐purifying respirator, inner and outer chemical‐resistant gloves and disposable chemical‐resistant outer boots, and a hard hat if appropriate. 7
Despite this, questions regarding what level of PPE is required for ED staff, and under what circumstances it should be worn, have sometimes remained unanswered, or left up to clinicians at the time, when rapid clear thinking about potentially unknown or unquantified threats will be difficult.
The objective of the present paper is to review the current evidence for the level of PPE required to safely protect ED staff following off‐site CBR incidents in Australasia. The issue of any potential physician role and PPE requirements at a CBR release site will not form part of this review.
Methods
An extensive literature search was undertaken. Advanced Medline searches for the years 1966 to present were performed using the terms ‘protective clothing’ and ‘emergency’ with ‘chemical’‘biological’‘radiological’‘cbr’ and, a title search of the term ‘ppe’. All potentially relevant subheadings were exploded, and each term itself was included in a keyword search. Results were limited to English language and human studies. These terms were also searched at the Cochrane Library. Following analysis of the returns, a follow‐up Medline search was conducted in an attempt to locate return deficiencies identified from the first search. These terms were ‘sarin’ and ‘emergency’, ‘Chernobyl’ and ‘radiation’, ‘nosocomial’ and ‘hydrocarbon’; similar limits were placed on these returns.
Returns were included if of relevance to the use of PPE in an ED following CBR incidents, and classified into chemical, biological, radiological, general CBR issues, or general PPE issues. Returns were excluded if referring to PPE use in prehospital CBR incidents. A manual search of the reference lists of these papers was then performed to access the original papers upon which recommendations have been based. Unpublished details from the original papers were also obtained following personal communication with original authors via email. Government and non‐government policy and guideline documents were accessed using the Google search engine.
Results
Following abstract screening, reference list searching and the exclusion of articles that dealt with only prehospital care or issues, 72 articles were selected for inclusion in the review. This final set of papers comprised 22 retrospective case series, 17 policies or guidelines, 12 reviews, 8 prospective observational studies, 5 institutional reports, 3 questionnaires, 2 case reports and 2 commentaries.
Discussion
Following the (non‐CBR) attacks in the USA on 11 September 2001 there has been a quantum leap in media, political, public and academic interest in disaster preparedness, including for CBR incidents. Locally the Australian Federal Government has committed millions of dollars to the further development of disaster preparedness, mitigation and response, including to its CBR capability. 8 , 9 Many Australasian ED have upgraded their CBR preparedness. Similar deficiencies have been identified in both the USA and UK. 10 , 11
Chemical
An ED usually receives chemically contaminated patients as the result of overdose or industrial accident. The risk to ED staff in chemical incidents is primarily from either inhalation, or direct contact with the chemical. The clinical reality is that victims who have survived to arrival at a hospital are unlikely to be sufficiently contaminated to pose a threat of serious injury to hospital workers.
An analysis was performed of 2562 victims contaminated in non‐petroleum hazardous material (HAZMAT) events following transportation to the ED in the USA from 1995 to 2001. It found a total of 15 staff had been injured by secondary contamination, mainly with respiratory and eye irritation. None of the staff had been wearing any form of protection at the time of the injury, despite this; none of them required admission to hospital. 12
Chemical warfare agents (CWA) still exist despite their being prohibited in the 1925 Geneva Protocol, and the 1993 Chemical Weapons Convention, which as of February 2006, only eight world states had either not acceded or not signed. 13 , 14 Both accidental and deliberate release of these CWA has occurred in the past. 15 They are commonly classified into four chemical groups: asphyxiants, respiratory irritants, vesicants and acetylcholinesterase (AChE) inhibitors (Table 1). The initial management of all these agents will in part be determined by their volatility.
Table 1.
Evaporation time constants for chemical warfare agents and selected toxic industrial chemicals 17
| Agent symbol | Agent | Action | MW (g/mol) | τ† (min) Evaporation time constant | |
|---|---|---|---|---|---|
| Higher volatility | Cl | Chlorine | Respiratory irritant | 71 | 0.04 |
| CG | Phosgene | Respiratory irritant | 99 | 0.1 | |
| AC | Hydrogen cyanide | Asphyxiant | 27 | 0.9 | |
| High volatility | MIC | Methyl isocyanate | Respiratory irritant | 57 | 1 |
| H2O | Water | – | 18 | 44 | |
| GB | Sarin | AChE inhibitor | 140 | 45 | |
| Low volatility | GD | Soman | AChE inhibitor | 182 | 256 |
| HD | Sulphur mustard | Vesicant | 159 | 1 087 | |
| GA | Tabun | AChE inhibitor | 162 | 1 639 | |
| GF | GF | AChE inhibitor | 180 | 1 721 | |
| Lower volatility | VX | VX | AChE inhibitor | 267 | 100 000 |
τ, evaporation time constant (the time for approximately 63% of the current amount of material to leave the surface). MW, molecular weight.
High‐volatility agents
This includes the respiratory irritants (e.g. chlorine, phosgene, methyl isocyanate), the asphyxiants (e.g. hydrogen cyanide) and the high‐volatility group of AChE inhibitors (most ‘G‐series’ nerve agents, e.g. soman and tabun). High‐volatility agents pose an inhalational risk for anyone at the chemical release site, or in the path of the chemical plume prior to its dispersal. This is due to their rapid evaporation if still in the liquid form. Patients arriving from a distant site do not pose any extra health risk to staff.
Low‐volatility agents
This includes the vesicants (e.g. sulphur mustard), and most AChE inhibitors (e.g. organophosphate insecticides and the V‐series of nerve agents). Low‐volatility agents are oily liquids, designed to stay on weeds, vegetation, or the skin and clothing of enemy military personnel, for hours or days. They evaporate slowly.
They do not pose a significant inhalational threat; although the highly volatile hydrocarbon solvents they might be dissolved in (e.g. toluene, xylene) can cause non‐specific symptoms via inhalation. Current evidence suggests that the Australasian College for Emergency Medicine‐recommended precautions when managing organophosphate poisonings 16 are appropriate for any low‐volatility CWA. These recommendations include good ventilation and regular rotation of staff, universal precautions (gloves, gowns and eye protection), early external decontamination (preferably self‐decontamination) and the immediate and thorough washing of affected areas if staff contact contaminated material.
Sarin the ‘medium’ volatility agent
The theoretical exception to the above principles will be an agent such as sarin. Sarin has an evaporation rate similar to water. It has been modelled as the potential worst‐case scenario agent for hospital staff not at the scene of a CBR release. 17 Other high‐volatility nerve agents will have dispersed prior to arrival at a hospital, whereas with lower‐volatility agents, the risk can be controlled through barrier methods, as long as these agents are not aerosolized during patient care.
In the 1995 Tokyo sarin subway attacks, initial reports from the metropolitan fire agency were of ‘a gas explosion in the Tokyo subway’. 18 ED staff prepared to receive patients with ‘burns, inhalation injuries, and carbon monoxide poisoning’. 19 The incident was then initially managed with no decontamination, and no special PPE at both the hospitals that recorded their experiences.
St Luke’s International Hospital received 641 victims (five of them in arrest). One hundred of 472 hospital staff developed symptoms. 20 One staff member was admitted for observation. 3 No staff received serious injuries, and none required antidote therapy. Staff symptoms were managed ‘by improving ventilation and by rotation of affected staff to other locations within the hospital’. 19
Meanwhile, the Keio University Hospital received 113 victims from the sarin attack (one in arrest). Thirteen of 15 emergency doctors developed symptoms including: dim vision, miosis, rhinorrhoea, shortness of breath or chest tightness, and cough. Although five of these affected doctors were given intramuscular atropine (and one given intravenous pralidoxime), none of them appeared to be suffering from severe respiratory embarrassment, or symptomatic bradycardia. These symptomatic staff were not rotated or removed from the affected area. Six of the eight most affected doctors were involved in either 40 min of CPR with the second patient to arrive, or patient clothing removal. Initial care was performed with staff wearing their usual work attire; this consisted of gloves, an apron and a surgical mask (Nozaki H, pers. comm.). In fact, all doctors continued to work, and of two doctors tested for serum cholinesterase, both returned normal levels. 18 No hospital staff were seriously injured.
Although miosis is a definitive cholinergic sign of AChE inhibitor contact, the signs of significant contact that will require ventilatory support are cyanosis, fasciculations, altered conscious state or coma. A low plasma cholinesterase level is also significant. 21
The sarin attack in Tokyo was the worst known example of ED staff dealing with an unsuspected mass chemical event. Staff were affected as the result of no patient decontamination, a lack of full basic level protection, and – at one centre – a failure to rotate or stand down staff once they became symptomatic. Despite this, no staff member became seriously ill.
Other chemicals
Some chemicals have the potential to permeate thin latex and polyvinyl chloride gloves within seconds with potentially fatal results. 22 Although the risks are dramatically reduced once distant from a chemical spill site, awareness should be maintained for chemicals that have the potential to permeate the PPE being worn. No one style of glove provides absolute protection from all chemicals. 23 When the properties of a chemical are unknown, staff should minimize direct contact, contact duration, and use double gloving. The patient should remove any soiled clothing immediately, and wash the affected area thoroughly.
Differential diagnosis of mass casualty or staff poisonings
On occasion ED have been closed because of an unpleasant smell or non‐specific symptoms developing in staff. 24 However, if there is a lack of evidence for any significant intoxication in staff following a CBR event, two other syndromes should also be considered in the differential: solvent fume exposure and epidemic hysteria.
Solvent fume exposure. 17, 25 Some chemicals, including AChE inhibitors might be dissolved in highly volatile organic hydrocarbon solvents. Symptoms of inhalation might include disorientation, headache, giddiness, dizziness, euphoria, eye irritation, nausea, lacrimation and cough. If deliberately abused by sniffing or ingestion, this might progress from confusion to unconsciousness, paralysis, convulsions and death from respiratory or cardiovascular arrest from arrhythmias. Treating staff might experience a fruity or unpleasant odour, or mild self‐limiting symptoms when managing affected patients. This should be managed by explanation, proper ventilation and rotation of affected staff.
Epidemic hysteria. 26, 27 The other major differential to consider is a hysterical reaction. Common triggers include unusual odours or an unknown chemical. The diagnosis should also be considered in mass outbreaks of non‐specific symptoms when all tests are unable to find an aetiological agent and no other cause has been found. 28 It is usually a diagnosis of exclusion. Patients usually present with headache, dizziness, weakness, stomach upset or nausea.
Risk factors for the diagnosis of epidemic hysteria include a preponderance of women or children, transmission of illness by sight and sound, rapid onset and offset with relapses, lack of clinical or laboratory pathology, a triggering stressful event or environment, and the presence and actions of emergency responders or media whose actions might add to the confusion and excitement associated with the event.
ED should not be closed on the basis of a strange odour alone. Senior ED staff should take steps to identify, then remove or reduce the source of the emissions, minimize contact, increase ventilation, rotate staff and continue to work. Senior staff can be a very steadying influence on junior staff in this situation if no real threat has been identified.
Biological
Decontamination of victims is not usually required following biological incidents, unless there is evidence of gross visible contact exposure to contaminated substances. 29 However, biological PPE is required to maintain respiratory and contact isolation, and meet infection control requirements. 30
The mortality rate from potentially life‐threatening infections such as severe adult respiratory syndrome (SARS) and avian influenza can be as high as 15–50%. 31 , 32 The major risk to health personnel in these cases is from unprotected contact with infected patients. 33
The level of PPE required varies if close contact (less than 1 m) is being made, or if an aerosol generating procedure is being preformed. Risk increases with the use of nebulizers, 34 bag‐mask ventilation, 35 suctioning, intubation and manipulation of the oxygen mask. 36 There is dispute about whether non‐invasive positive pressure ventilation (NIPPV) might increase the risk to staff. 37 , 38 , 39 , 40 This might relate to whether a patient’s expired air was filtered or isolated during NIPPV. Simple hand washing after attending each patient has again been identified as significantly reducing risk. 41
P2 masks (Fig. 1) are the currently recommended Australasian standard for biological inhalational hazard protection; 42 they have a filter efficiency of at least 94% when tested with sodium chloride aerosol (AS/NZS1716). They are equivalent to the USA occupational health‐approved N95 mask. (The ‘N’ means ‘Not resistant to oil’. The ‘95’ refers to a 95% filter efficiency for 0.3 µm particles.) These masks should be inspected and tested for fit prior to use by trained staff. If these masks are unavailable, an in vivo study using multiple surgical masks showed they do not confer the same level of protection as an N95 mask, but they are better than no mask at all. 43
Figure 1.
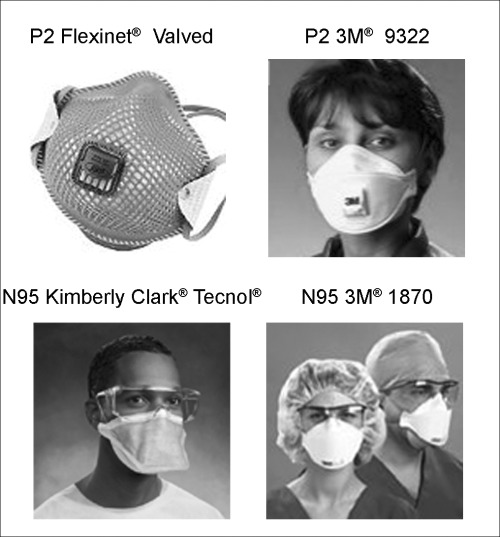
Examples of P2 and N95 masks.
There is other evidence for reducing the risk to carers during the management of patients with suspected or proven SARS or avian flu. 30 , 44 , 45 , 46 It primarily involves measures to reduce the aerosolization of infectious secretions. The patient should also wear a P2 (N95) mask and ideally be managed in an isolation room with negative pressure. Nebulizers should not be used, instead using inhalers or spacers if appropriate. When possible, respiratory isolation should be maintained by inserting respiratory filters to oxygen masks with exhalation ports, and NIPPV outlets (if deployed). If intubation is required, rapid sequence induction should be used to prevent coughing, and a closed suction system following intubation used for airway toilet. Staff rotations through the area should be minimized in these circumstances, and as always, other standard precautions for infection control should be maintained.
Table 2 shows the current Pandemic Influenza PPE Guidelines for Australia, as of June 2006. 42 All staff coming into contact with suspected or probable cases of SARS are adequately protected by use of a correctly fitted 47 appropriate disposable mask/respirator; 48 specifically, a P2 mask or equivalent (N95). The use of gloves is essential, but still does not replace the need for hand hygiene after attending a patient. The gown should ideally cover clothes, be disposable, long‐sleeved, cuffed and fluid‐repellent. Goggles or a face shield are not routine unless there is anticipated contact with infectious spray, splatter, or aerosol generating procedures. If aerosol generating procedures are to be performed, staff should also wear a disposable theatre‐type cap. If splashing of infected materials is anticipated, a fluid‐repellent gown should be worn or a plastic apron can be used as an alternative.
Table 2.
Summary of personal protective equipment for influenza pandemic patients in health‐care settings 42
| Entering patient room but no close patient contact | Close patient contact (<1 m) | Aerosol generating procedure being performed | |
|---|---|---|---|
| P2 mask | No | Yes | Yes, or PAPR |
| Surgical mask | Yes | Only if P2 unavailable | NA |
| Gown | No† | Yes | Yes |
| Gloves | No† | Yes | Yes |
| Eyewear | No | Yes, if body fluid exposure anticipated | Yes |
| Cap | No | No | Yes |
| Apron | No | Yes, if splashing possible and impermeable gown not available | Yes, if impermeable gown not available |
Note: Any cleaners who have to enter the room of an infectious patient should wear a gown and gloves, in addition to a surgical mask. This is because cleaning activities are likely to bring their hands and clothes into contact with potentially contaminated surfaces. They should also be advised to maintain a distance of at least 1 m from the patient if possible. NA, not applicable; PAPR, powered air purifying respirator.
The correct sequence for removing gloves, hair and shoe covers, eye protection, gown and mask is disputed. At least six national and international guidelines on this topic have been published for staff caring for SARS patients. 49 There is no available evidence to favour one sequence of PPE removal over another. The interim Australian guidelines for the removal of PPE for SARS are listed in Table 3. 50
Table 3.
Interim Australian infection control guidelines for SARS: removal of personal protective equipment (PPE) 50
| The steps in PPE removal are: |
| 1. Remove gloves by rolling back from the wrist, do not touch skin. |
| 2. Remove goggles/visor/shield and wipe with an alcohol wipe. |
| 3. Remove gown and fold carefully with contaminated side in and place in covered linen bin. |
| 4. Remove P2 (N95) mask/respirator by touching the tapes only, not the front of the mask, discard in bin. |
| Immediately decontaminate hands VERY WELL using soap and water or an alcohol rub. |
Personal protection equipment must be removed in a way that does not allow transmission of SARS coronavirus to the wearer. Gloves are likely to be heavily contaminated and should be removed first.
During the 2001 release of anthrax spores into the US community, no person‐to‐person transmission was observed. 51 In Australia, there was a wave of media interest, hundreds of false alarms, several hoaxes, and community disquiet that proved to be the greatest drain on resources. 52
We should also distinguish between biological warfare and biological terrorism issues. The response appropriate for each will be different. In regard to bioterrorism, ‘as noted repeatedly by the World Health Organisation, elaborate measures to defend populations against specific agents are likely to be wasteful, and may distract from the continuing importance of prevention’. 53 Long held standards of universal precautions, together with at least a P2 (N95) level mask, and the minimization and isolation from aerosolized secretions, provide adequate protection for hospital staff from biological threats.
Radiological
The average natural background radiation is 2 mSv per annum in Australia. The Australian National Occupational Health and Safety Commission’s standard for workers is a maximum effective dose of 50 mSv in any year (or 20 mSv/year averaged over 5 years). 54
In 1999, at the Japanese Tokai‐mura nuclear reactor criticality accident, three patients were massively irradiated (two died) with doses up to 20 Gy (∼Sv). However, of the 260 emergency personnel involved in the response, the maximum dose received by personnel was 9 mSv. 55 Irradiated patients pose no additional health risks to staff unless they are also significantly contaminated.
Following the nuclear accident at Chernobyl the maximal whole body dose received by hospital workers has been reported as between 10 and 50 mSv. 56 , 57 A subsequent study of the general population in the area found that self‐reported health problems correlated more with one’s risk perception and sense of control. 58
Australian standards on the control of radioactive contamination of a hospital recommend that for trained staff ‘In general, the normal clothing used in theatres should suffice – surgical gowns, masks, caps, gloves and overshoes’. Double gloving should be used. The clothing should ideally be waterproof including the overshoes. Alternatively, plastic aprons might be worn, and plastic bags taped over shoes. 59
The risk of trained hospital staff located near high‐grade nuclear material being subjected to significant amounts of ionizing radiation by contaminated patients remains small. This risk is further reduced if the hospital is not located near a nuclear reactor, nuclear powered transport, or a nuclear weapons stockpile. In Australasia there is no evidence of hospital staff being at risk from radiological injury provided they follow standard procedures. Their exposure should be monitored with radiation dosimeters, with staff rotation if required.
PPE – confounding factors
Higher levels of PPE are provided by use of a correctly fitted full‐face air‐purifying respirator. They provide an increased level of inhalational protection against nearly all toxins, most gases, but not low‐oxygen environments. However, use of this level of equipment also presents its own risks. Studies on the military and medical use of PPE have raised concerns regarding its potential for causing increases in user temperature, fatigue, unsteadiness; 60 and visual field restrictions with poor speech intelligibility even at minimal distances. 61 It might be more difficult to perform skilled procedures in experienced hands, 62 although a more recent Australian study has found otherwise. 63
Other studies point out the potential for harm to both patient and staff, through such risks as needle‐stick injury. 64 Also, butyl and nitrile gloves have theoretical laboratory‐based protection levels quoted by manufacturers that are not achievable in real‐world settings. 65 These problems can persist when using the equipment regularly.
Problems with the hospital‐based care of ‘victims’ during mass casualty CBR exercises using PPE with respirators have been documented in the UK. 66 In Australia, even without the use of this level of PPE, significant problems with crowd control and decontamination have again been documented recently. Some regions have chosen not to adopt the introduction ‘Level C’ PPE with full‐face air‐purifying respirators into their ED. 67
CBR – confounding factors
Together with clinical requirements, a number of other stakeholders have helped determine the allocation of resources for disasters. This has included occupational health and safety, environmental protection and hospital accreditation concerns, together with the partial adoption of external military and HAZMAT models directly to the ED setting.
The 2005 US Occupational Safety and Health best practice document for hospital‐based first receivers following HAZMAT incidents carries a non‐obligatory recommendation that when a substance is unknown, workers in the hospital decontamination zone (including, but not limited to: decontamination team members, clinicians, set‐up crew, clean‐up crew, security staff and patient tracking clerks) should wear a minimum of an air‐purifying respirator, chemical‐resistant suit, double‐layer gloves, tape seals. It also states that if the ED were to become secondarily contaminated, all staff in the ED should then wear this level of protection. 68 It is difficult to imagine any ED being able to competently deliver this level of protection, and a need for this level of protection has not been established from any known incident in the literature.
To minimize risk in the USA, ED are also expected to comply with the Environmental Protection Agency and the Joint Commission on Accreditation of Healthcare Organizations (hospital standards) requirements during a disaster. As a result, Level C PPE has been recommended for staff in the ED response to out‐of‐hospital events following a 10 min lag time. The adequacy of lower levels of PPE protection was not studied. 16 This is in part because some protocols have turned to pre‐existing military and HAZMAT models for their management, many of the assumptions made in non‐hospital settings will not be applicable to a hospital with civilian casualties. 6 , 69 An untrained, unprepared, uninformed and frightened civilian population in a real life setting will not respond in a similar way to injured professional armed services personnel when greeted by ED staff wearing higher levels of PPE.
Although the risk of a major industrial accident persists, there are no known Australasian communities living near a chemical, biological or nuclear weapons storage facility, as is the case in other countries. 70 The direct transferring to Australasia of overseas standards, and resource allocation requirements, might not be appropriate.
Specific recommendations
The following recommendations are based on what the evidence suggests will adequately protect our ED staff from any serious injury if any of these worst‐case out‐of‐hospital CBR incidents were to occur in our community.
Following an off‐site Australasian CBR incident, current evidence indicates that the initial receiving ED staff should, whenever possible, remain uphill and upwind or in a well ventilated area, with no direct patient contact, minimal close contact, and use universal precautions or infection control procedures as appropriate to the type of agent involved. They will be adequately protected from all documented inhalational CBR threats by wearing a P2 (N95) mask or its equivalent. Protection from serious contact injury is offered by wearing double gloves, low‐level fluid‐repellent or waterproof disposable coveralls or gown, eye protection, surgical mask, and ideally hair and shoe covers.
If external decontamination is required, it is primarily achieved by clothing removal, and aided by subsequent washing or showering. Able patients should decontaminate themselves. This should ideally occur prior to entry into the ED especially when multiple patients are involved. If a patient is unable to decontaminate, ED staff should assist using this same equipment with splash protection when undressing and washing the patient. Decontamination is usually not required for biological incidents, or for irradiated patients who have not been contaminated.
Trained staff should be rotated regularly if a non‐biological threat persists, and any staff member who develops symptoms should be removed from the area. Staff making direct contact with contaminants should wash the affected area immediately.
This simplification and uniformity of what is required for any type of CBR incident could deliver many benefits: simplified training, regional uniformity, cost savings and the ability to respond more rapidly and more efficiently to unexpected events. Other potential benefits would include easier communication and crowd control in mass events, and a reduction in the level of anxiety in both our patients and staff by wearing more conventional occupational clothing. The risks to patients and staff will be reduced.
Conclusions
Our ability to respond to any CBR event, including natural pandemics, and industrial accidents, has been improved in recent years following terrorist acts, world events, political priorities and the higher profile of risk management issues. This has been good for our ability to respond to any type of CBR event. However, there is no evidence to support the need for higher‐level PPE in Australasian ED, and much to indicate that its introduction would be problematic at best. Our community should also continue to assist the prevention of the humanitarian and health‐related risk factors for terrorism.
‘The use of these weapons threatens our society with not only widespread death and disease, but also with fear, panic, and societal disruption, the prime motivators of terrorists.’ 69 Preventive measures should also be directed at helping address some of the root causes of terrorism, which include enduring failed states, war, poverty, inequality, human rights abuses, dispossession and environmental degradation. 71 , 72 Prevention is better, cheaper, less stressful and more rewarding, than fighting this social disease.
Competing interests
None declared.
Guy W Sansom, MB BS, FACEM, AdvDipMM, Area Medical Coordinator.
References
- 1. Australasian College for Emergency Medicine. Policy document: emergency department hazardous material response plan: staff participation. Emerg. Med. Australas. 2004; 16: 177. [DOI] [PubMed] [Google Scholar]
- 2. Burgess JL, Kirk M, Borron SW, Cisek K. Emergency department hazardous materials protocol for contaminated patients. Ann. Emerg. Med. 1999; 34: 205–12. [DOI] [PubMed] [Google Scholar]
- 3. Okumura T, Suzuki K, Fukuda A et al. The Tokyo subway sarin attack: disaster management, Part 2: hospital response. Acad. Emerg. Med. 1998; 5: 618–24. [DOI] [PubMed] [Google Scholar]
- 4. Council on Scientific Affairs. Medical Preparedness for Terrorism and Other Disasters. Chicago: American Medical Association, June 2000. Available from URL: http://www.ama-assn.org/ama/pub/category/14313.html[Accessed 12 August 2006]. [Google Scholar]
- 5. Hick JL. Protective equipment for health care facility decontamination personnel: regulations, risks, and recommendations. Ann. Emerg. Med. 2003; 42: 370–80. [DOI] [PubMed] [Google Scholar]
- 6. Macintyre AG, Christopher GW, Eitzen EJ et al. Weapons of mass destruction events with contaminated casualties. JAMA 2000; 283: 242–9. [DOI] [PubMed] [Google Scholar]
- 7. US Environmental Protection Agency. Emergency Response Program: Personal Protective Equipment. Last updated on Thursday, 2 March 2006. Available from URL: http://www.epa.gov/superfund/programs/er/hazsubs/equip.htm[Accessed 10 August 2006].
- 8. Joint Standing Committee on Foreign Affairs, Defence and Trade. Watching Brief on the War on Terrorism. (Roundtable.) Hansard. Monday 9 December, 2002. Available from URL: http://www.aph.gov.au/house/committee/jfadt/Terrorism/media.htm[Accessed 28 July 2006].
- 9. Joint Standing Committee on Foreign Affairs, Defence and Trade. Watching Brief on the War on Terrorism. (Roundtable.) Hansard. Wednesday 19 November, 2003. Available from URL: http://www.aph.gov.au/house/committee/jfadt/Terrorism/media.htm[Accessed 28 July 2006].
- 10. Wetter DC, Daniell WE, Treser CD. Hospital preparedness for victims of chemical or biological terrorism. Am. J. Public Health 2001; 91: 710–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horby P, Murray V, Cummins A, Mackway‐Jones K, Euripidou R. The capability of accident and emergency departments to safely decontaminate victims of chemical incidents. J. Accid. Emerg. Med. 2000; 17: 344–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horton DK, Berkowitz Z, Kaye WE. Secondary contamination of ED personnel from hazardous materials events, 1995–2001. Am. J. Emerg. Med. 2003; 21: 199–204. [DOI] [PubMed] [Google Scholar]
- 13. Technical Secretariat of the Organisation for the Prohibition of Chemical Weapons. Convention on the prohibition of the development, production stockpiling and use of chemical weapons and use of chemical weapons and on their destruction. Organisation for the prohibition of chemical weapons. 29 April 1997. Depositary: UN Secretary‐General, 2005. Available from URL: http://nti.org/e_research/official_docs/inventory/pdfs/cwc.pdf#search?%22%22Convention%20on%20the%20prohibition%20of%20the%20development%2C%20production%22%20chemical%22[Accessed 12 August 2006].
- 14. Organisation for the Prohibition of Chemical Weapons. States that Have neither Signed nor Acceded to the Chemical Weapons Convention. 23 February 2006. Available from URL: http://www.opcw.org/html/db/members_nonsig.html[Accessed 12 August 2006].
- 15. Kales SN, Christiani DC. Acute chemical emergencies. N. Engl. J. Med. 2004; 350: 800–8. [DOI] [PubMed] [Google Scholar]
- 16. Little M, Murray L. Consensus statement: risk of nosocomial organophosphate poisoning in emergency departments. Emerg. Med. Australas. 2004; 16: 456–8. [DOI] [PubMed] [Google Scholar]
- 17. Georgopoulos PG, Fedele P, Shade P et al. Hospital response to chemical terrorism: personal protective equipment, training, and operations planning. Am. J. Ind. Med. 2004; 46: 432–45. [DOI] [PubMed] [Google Scholar]
- 18. Nozaki H, Hori S, Shinozawa Y et al. Secondary exposure of medical staff to sarin vapour in the emergency room. Intensive Care Med. 1995; 21: 1032–5. [DOI] [PubMed] [Google Scholar]
- 19. Okumura T, Takasu N, Ishimatsu S et al. Report on 640 victims of the Tokyo subway sarin attack. Ann. Emerg. Med. 1996; 28: 129–35. [DOI] [PubMed] [Google Scholar]
- 20. Ohbu S, Yamashina A, Takasu N et al. Sarin poisoning on Tokyo subway. South. Med. J. 1997; 90: 587–93. [DOI] [PubMed] [Google Scholar]
- 21. Goswamy R, Chauduri A, Mahashur AA. Study of respiratory failure in organophosphate and carbamate poisoning. Heart Lung 1994; 23: 466–72. [PubMed] [Google Scholar]
- 22. Blayney MB. The need for empirically derived permeation data for personal protective equipment: the death of Dr Karen E. Wetterhahn. Appl. Occup. Envir. Hyg. 2001; 61: 233–6. [DOI] [PubMed] [Google Scholar]
- 23. UCI Physical Sciences Chemical Hygiene Plan, Appendix G, Chemical Protection Glove Selection. University of California, Irvine Environmental Health and Safety, 4600 Bison Avenue, Irvine, CA, USA. ©2003–2005. Available from URL: http://www.ehs.uci.edu/programs/pschp/APPG.PDF#search?%22%22UCI%20Physical%20Sciences%20Chemical%20Hygiene%20Plan%22%20appendix%22[Accessed 12 August 2006].
- 24. Stacey R, Morfey D, Payne S. Secondary contamination in organophosphate poisoning: analysis of an incident. QJM 2004; 97: 75–80. [DOI] [PubMed] [Google Scholar]
- 25. Victoria MS, Nangia BS. Hydrocarbon poisoning: a review. Ped. Emerg. Care 1987; 3: 184–6. [DOI] [PubMed] [Google Scholar]
- 26. Baker P, Selvey D. Malathion‐induced epidemic hysteria in an elementary school. Vet. Hum. Toxicol. 1992; 34: 156–60. [PubMed] [Google Scholar]
- 27. Selden BS. Adolescent epidemic hysteria presenting as a mass casualty, toxic exposure incident. Ann. Emerg. Med. 1989; 18: 892–5. [DOI] [PubMed] [Google Scholar]
- 28. Emergency Services Commissioner. A Report of the Response to an Emergency at Melbourne Airport on 21 February 2005. Department of Premier and Cabinet, Victoria, Australia. Available from URL: http://www.dpc.vic.gov.au/CA256D800027B102/Lookup/Melbourne_Airport_Emergency_Review/$file/Melbourne%20Airport%20Review.pdf#search?%22A%20Report%20of%20the%20Response%20to%20an%20Emergency%20at%20%22Melbourne%20Airport%22%22[Accessed 12 August 2006].
- 29. English JF, Cundiff MY, Malone JD et al. Bioterrorism Readiness Plan: A Template for Healthcare Facilities. Atlanta: Centers for Disease Control and Prevention, 1999. [Google Scholar]
- 30. Keim M, Kaufmann AF. Principles for emergency response to bioterrorism. Ann. Emerg. Med. 1999; 34: 177–82. [DOI] [PubMed] [Google Scholar]
- 31. Chan‐Yeung M, Ooi GC, Hui DS et al. Severe acute respiratory syndrome. Int. J. Tuberc. Lung Dis. 2003; 7: 1117–30. [PubMed] [Google Scholar]
- 32. Hung S‐W, Lin I‐Y, Wang T‐L. Emerging infectious disease (1): avian influenza. Ann. Disaster Med. 2005; 3 (Suppl. 2): S40–6. [Google Scholar]
- 33. Verbeek PR, McClelland IW, Silverman AC, Burgess RJ. Loss of paramedic availability in an urban emergency medical services system during a severe acute respiratory syndrome outbreak. Acad. Emerg. Med. 2004; 11: 973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization. Avian Influenza, including Influenza A (H5N1), in Humans: WHO Interim Infection Control Guideline for Health Care Facilities.[Most recent amendment 24 April 2006]. Available from URL: http://www.wpro.who.int/NR/rdonlyres/EA6D9DF3-688D-4316-91DF-5553E7B1DBCD/0/InfectionControlAIinhumansWHOInterimGuidelinesfor2b_0628.pdf[Accessed 10 August 2006].
- 35. Christian MD, Loutfy M, McDonald C et al. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg. Infect. Dis. 2004; 10: 287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong TKS, Chung JWY, Li Y et al. Effective PPC for health care workers attending patients with severe acute respiratory syndrome (SARS). Am. J. Infect. Control. 2004; 32: 90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dwosh HA, Hong HHL, Austgarden D, Herman S, Schabas R. Identification and containment of an outbreak of SARS in a community hospital. CMAJ 2003; 168: 1415–20. [PMC free article] [PubMed] [Google Scholar]
- 38. Li H, Nie L, Wang G et al. [Clinical observation of non‐invasive positive pressure ventilation (NIPPV) in the treatment of severe acute respiratory syndrome (SARS).] Beijing Da Xue Xue Bao 2003; 35 (Suppl.): 41–3. [Article in Chinese, only English abstract viewed]. [PubMed] [Google Scholar]
- 39. Cheung TMT, Yam LYC, So LKY et al. Effectiveness of non‐invasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest 2004; 126: 845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fowler RA, Guest CB, Lapinsky SE et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am. J. Respir. Crit. Care Med. 2002; 169: 1198–202. [DOI] [PubMed] [Google Scholar]
- 41. Teleman MD, Boudville IC, Heng BH, Heng BH, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among health‐care workers in Singapore. Epidemiol. Infect. 2004; 132: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Australian Government, Department of Health and Aging. Interim Infection Control Guidelines for Pandemic Influenza in Healthcare and Community Settings (June 2006). Annex to Australian Health Management Plan for Pandemic Influenza. Important Information for All Australians. Available from URL: http://www.health.gov.au/internet/wcms/publishing.nsf/content/d164719adcc496f7ca25717d00080b37/$file/pandemicinfec-gl.pdf[Accessed 18 August 2006].
- 43. Derrick JL, Gomersall CD. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J. Hosp. Infect. 2005; 59: 365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Somogyi R, Vesely AE, Azami T et al. Dispersal of respiratory droplets with open vs closed oxygen delivery masks – implications for the transmission of severe acute respiratory syndrome. Chest 2004; 125: 1155–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lapinsky SE, Hawryluck L. ICU management of severe acute respiratory syndrome. Intensive Care Med. 2003; 29: 870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chan YM, Yu WC. Outbreak of severe acute respiratory syndrome in Hong Kong Special Administrative Region: case report. BMJ 2003; 326: 850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ofner M, SARS Investigative Team, CDC. Cluster of severe acute respiratory syndrome cases among protected health care workers – Toronto, Canada, April 2003. MMWR Morb. Mortal. Wkly Rep. 2003; 52: 433–6. [PubMed] [Google Scholar]
- 48. Loeb M, McGeer A, Henry B et al. SARS among critical care nurses, Toronto. Emerg. Infect. Dis. 2004; 10: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Puro V, Nicastri E. SARS and the removal of personal protective equipment. CMAJ 2004; 170: 930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Australian Government, Department of Health and Aging. Interim – Australian Infection Control Guidelines for Severe Acute Respiratory Syndrome (SARS): Section 5 Appendices. Last updated on 25 April 2004. Available from URL: http://www.hrdgp.org.au/gp_files/SARS%20IC%20Section%205%20Appendix%2016%20May.pdf[Accessed 18 August 2006].
- 51. Inglesby TV, O’Toole T, Henderson DA et al. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 2002; 287: 2236–52. [DOI] [PubMed] [Google Scholar]
- 52. Smallwood RA, Merianos A, Mathews JD. Editorial. Bioterrorism in Australia: how real is the threat, and how prepared are we? Med. J. Aust. 2002; 176: 251–2. [DOI] [PubMed] [Google Scholar]
- 53. Cherry CL, Kainer MA, Ruff TA. Biological weapons preparedness: the role of physicians. Intern. Med. J. 2003; 33: 242–53. [DOI] [PubMed] [Google Scholar]
- 54. Recommendations for Limiting Exposure to Ionizing Radiation and National Standard for Limiting Occupational Exposure to Ionizing Radiation. Radiation Protection Series No. 1. Australian Radiation Protection and Nuclear Safety Agency, March 2002.
- 55. Tanaka S‐I. Summary of JCO criticality accident in Tokai‐mura and a dose assessment. J. Radiat. Res. 2001; 42 (Suppl.): S1–9. [DOI] [PubMed] [Google Scholar]
- 56. Department of Homeland Security Working Group on Radiological Dispersal Device (RDD) Preparedness. Medical Preparedness and Response Sub‐Group. Page 7, 1 May 2003. Available from URL: http://www1.va.gov/emshg/docs/Radiologic_Medical_Countermeasures_051403.pdf#search?%22%22Department%20of%20Homeland%20Security%20Working%20Group%20on%20Radiological%20Dispersal%20Device%20(RDD)%20Preparedness%22%22[Accessed 8 August 2006].
- 57. Linnemann RE. Soviet medical response to the Chernobyl nuclear accident. JAMA 1987; 258: 637–43. [PubMed] [Google Scholar]
- 58. Havenaar JM. Perception of risk and subjective health among victims of the Chernobyl disaster. Soc. Sci. Med. 2003; 56: 569–72. [DOI] [PubMed] [Google Scholar]
- 59. Swindon TN, Soloman S, McKean N, Drummond R, Tai KH. Manual on the Medical Management of Individuals Involved in Radiation Accidents. Australian Radiation Protection and Nuclear Safety Agency, August 2000. Available from URL: http://www.arpansa.gov.au/pubs/tr/tr131a.pdf#search=%22%22Manual%20on%20the%20Medical%20Management%20of%20Individuals%20Involved%20in%20Radiation%20Accidents%22%22[Accessed 2 August 2006].
- 60. Kincl LD, Bhattacharya A, Succop PA, Clark CS. Postural sway measurements: a potential safety monitoring technique for workers wearing personal protective equipment. Appl. Occup. Environ. Hyg. 2002; 4: 256–66. [DOI] [PubMed] [Google Scholar]
- 61. Bishop J, Bahr RH, Gelfer MP. Near‐field speech intelligibility in chemical‐biological warfare masks. Mil. Med. 1999; 164: 543–50. [PubMed] [Google Scholar]
- 62. Goldik Z, Bornstein J, Eden A, Abraham RB. Airway management by physicians wearing anti‐chemical warfare gear: comparison between laryngeal mask airway and endotracheal intubation. Eur. J. Anaesthesiol. 2002; 19: 166–9. [DOI] [PubMed] [Google Scholar]
- 63. Knott J, Udayasiri R, Papson J, Leow F, Hassan F, Taylor D. Emergency department staff can effectively resuscitate in level C personal protective equipment. Emerg. Med. Australas. 2006; 18 (Suppl. 1): A1–17. [DOI] [PubMed] [Google Scholar]
- 64. West K. A common sense review of personal protective equipment. Safety smarts. J. Emerg. Med. Serv. 1998; 23: 54–61. [PubMed] [Google Scholar]
- 65. Evans PG, McAlinden JJ, Griffin P. Personal protective equipment and dermal exposure. Appl. Occup. Environ. Hyg. 2001; 16: 334–7. [DOI] [PubMed] [Google Scholar]
- 66. Al‐Damouk M, Bleetman A. Impact of the Department of Health initiative to equip and train acute trusts to manage chemically contaminated casualties. Emerg. Med. J. 2005; 22: 347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Edwards NA, Caldicott DG, Eliseo T, Pearce A. Truth hurts – hard lessons from Australia’s largest mass casualty exercise with contaminated patients. Emerg. Med. Australas. 2006; 18: 185–95. [DOI] [PubMed] [Google Scholar]
- 68. OSHA Best Practices for Hospital‐Based First Receivers of Victims from Mass Casualty Incidents Involving the Release of Hazardous Substances. Occupational Safety and Health Administration, USA, 2005.
- 69. Richards CF, Burstein JL, Waeckerle JF, Hutson HR. Emergency physicians and biological terrorism. Ann. Emerg. Med. 1999; 34: 183–90. [DOI] [PubMed] [Google Scholar]
- 70. Centres for Disease Control and Prevention. CDC recommendations for civilian communities near chemical weapons depots. Fed. Regist. 1995; 60: 33308–12. [PubMed] [Google Scholar]
- 71. Wilson N, Lush D. Bioterrorism in the Northern Hemisphere and potential impact on New Zealand. N. Z. Med. J. 2002; 115: 247–51. [PubMed] [Google Scholar]
- 72. Horton R. Public health: a neglected counterterrorist measure [Commentary]. Lancet 2001; 358: 1112–13. [DOI] [PubMed] [Google Scholar]


