Abstract
During the last decade, the ‘precautionary principle’ health has gained importance. It is an approach to manage uncertain risks and to prevent any damage to the environment or human. A key element is to take action, even if some cause and effect relationships are not fully established scientifically. Although there are also critics of this principle, it is meanwhile, also increasingly implemented in medicine. An important subject is medicinal products of human or animal origin. Manifold official precaution‐guided regulations have been stated to improve their safety, particularly to avoid any infection by viruses and pathogens causing transmissible spongiform encephalopathies. In addition to numerous regulations and decisions, it is generally recommended to substitute animal and human‐derived products with adequate alternatives wherever possible. This is a great challenge for research and drug development. One option is recombinant proteins, which however, are not generally free of any risk of contamination. Therefore, the best strategy might be the development of synthetic, specifically acting drugs.
The most widely used medicinal product of animal origin at present is heparin. Although there has been no indication of any viral contamination, many other reasons suggest its substitution by alternative antithrombotics. These actually promoted the research on new anticoagulants. With the approval of fondaparinux, the first synthetic, selective factor Xa, a first alternative to the porcine‐derived heparin has become available. In addition, other synthetic antithrombotics are currently in clinical development. In principle, it is thus possible that the prophylaxis and therapy of thromboembolic diseases will become completely independent of animal‐derived drugs, which would be in line with the precautionary principle.
Keywords: Animal‐derived medicinal products, fondaparinux, heparin, precautionary principle, viral contamination, drug development
Introduction
Increasing scientific knowledge has led to far reaching advantages for the humankind like fertilization in farming, power generation by nuclear energy, or the application of drugs to aid patients’ recovery. But these benefits occasionally implicate damages and harms such as adverse effects of drugs, so that careful evaluation of the benefit–risk ratio is required. Sometimes, however, the potential risks are difficult to estimate and thus difficult to manage. At the worst, this can result in wrong decisions and actions. A historical case is the ‘swine flu affair’ in 1976 [1]. For fear of a swine flu epidemic in the USA, an expensive national immunization was rashly mounted. As a result of severe adverse events, the campaign had to be prematurely suspended, and the tragedy was that there wasn't any sign of an epidemic. A reverse example is the contamination of blood products with HIV in the early 1980s [1]. The risk of AIDS for patients treated with blood products was underestimated or deliberately downplayed, resp.. As a result of carelessness, thousands of HIV infections unnecessarily occurred. These two cases illustrate that neither to go all the way nor to do nothing at all is wise.
Today, the application of the so‐called ‘precautionary principle’ as a new type of action and responsibility is proposed to be suitable to manage such uncertain risks.
In the following, the development of the precautionary principle, its definition and the ongoing discussion on the subject will be shortly described. Examples will demonstrate that it is implemented not only in environmental and public health policy, but also in medicine. It will be focused on the area of medicinal products, where precautionary measures have gained importance regarding the risk of contamination of human and animal‐derived products with pathogens. Finally, anticoagulant drugs, i.e. the animal‐derived heparin and the new synthetic anticoagulants, are discussed under the aspect of the precautionary principle.
The ‘precautionary principle’
Development and definition of the ‘precautionary principle’
The key element of the precautionary principle is a ‘matter of activity in the face of uncertainty’, which means that immediate action must be taken to avoid serious consequences without waiting for scientifically established proof of danger [2]. This approach is characterized by slogans like ‘better safe than sorry’, ‘erring on the side of safety’, ‘goal‐oriented’, or ‘benefit of the doubt’. It has meanwhile gained international acceptance as a guiding principle for environmental and health decision making.
The precautionary principle has its seeds in the environmental sector (Table 1). In the early 1970s, the German ‘Vorsorgeprinzip’, or foresight principle, emerged and developed into a principle of German environmental law. It has since flourished in international policy statements and agreements – initially recognized in the World Charter for Nature, which was adopted by the UN General Assembly in 1982; and subsequently adopted in the First International Conference on Protection of the North Sea in 1984. At the Second International Conference on Protection of the North Sea in 1987, the European so‐called ‘generation target’, i.e. the elimination by year 2020 of discharging hazardous chemicals into the North Sea, was defined. The corresponding ministerial declaration embraced the ideology of the precautionary principle [3].
Table 1.
Chronicle of the development of the precautionary principle
| 1984 | First International Conference on Protection of the North Sea |
| 1992 | Rio Declaration on Environment and Development’ |
| 1992 | EU Treaty of Maastricht |
| 1998 | Recommendation of the extension to human health |
| 23–25 January, 1998 | Wingspread Conference, Racine, WI |
| 2 February, 2000 | EC Communication on the Precautionary Principle |
| 22–25 September, 2001 | International Summit on Science and the Precautionary Principle, Lowell, MA |
Following this conference, it was integrated into numerous international conventions and agreements such as the Rio Declaration on Environment and Development in 1992 [4]: ‘In order to protect the environment, the precautionary approach shall be widely applied by states according to their capability. Where there are threats of serious or irreversible damage, lack of full scientific certainty shall not be used as a reason for postponing cost‐effective measures to prevent environmental degradation.’ In the same year, it was also officially adopted by the EU Treaty in Maastricht and thereby became one of the ‘four pillars’ of EU environmental policy, which was reiterated and strengthened politically by the Amsterdam Treaty in 1998 [5]. In the late 1990s, legalists began to recommend that the precautionary approach should be extended to human health [6].
The actual coming out party of the precautionary principle in the United States was at the Wingspread Conference in 1998 [7]. Among the manifold, sometimes different definitions, the ‘Wingspread Statement on the Precautionary Principle’ has become its most frequently cited formulation (Table 2) [8]: ‘Where an activity raises threats of harm to the environment or human health, precautionary measures should be taken, even if some cause and effect relationships are not fully established scientifically’. It thus explicitly singles out the dose–response function. Further, it shifts the burden of proof from the public onto proponents of potentially harmful activities, also known as the ‘polluter pays’ principle. The third important characteristic of a precautionary approach is transparency and increased public participation in decision making, so that considerations of public health and environment should have priority over economic gain. Finally, the fourth central component is to explore a wide range of alternatives to possible harmful actions.
Table 2.
The essential points of the Wingspread statement of the Precautionary Principle (available at: http://www.gdrc.org/u-gov/precaution-3.html)
| ‘This statement was drafted and finalized at a conference at the Wingspread Conference Center, Racine, Wisconsin, which took place on 23–25 January 1998. |
| … Therefore it is necessary to implement the Precautionary Principle: |
| • Where an activity raises threats of harm to the environment or human health, precautionary measures should be taken even if some cause and effect relationships are not fully established scientifically. |
| • In this context, the proponent of an activity, rather than the public bears the burden of proof. |
| • The process of applying the precautionary principle must be open, informed and democratic, and must include potentially affected parties. |
| • It must also involve an examination of the full range of alternatives, including no action.…’ |
But also, this definition is not easy to understand, differently interpreted and provokes major criticism. In response to these challenges, on 2 February, 2000, the European Commission (EC) issued a communication on the precautionary principle to clarify it and to provide guidelines for its application [9]. According to this, experimentation should not proceed, if there is reasonable ground for concern (i.e. no evidence) that something might be harmful. The EC resolved that precautionary restrictions under its auspices would be ‘proportional to the chosen level of protection’, ‘nondiscriminatory in their application’, and ‘consistent with other similar measures’. The EC also avowed that persons responsible for making decisions on behalf of it would weigh ‘potential benefits and costs’.
To build better understanding of the important role of science in implementing the precautionary principle, an elaborate ‘International Summit on Science and the Precautionary Principle’ was held in Lowell, MA in September 2001 [10, 11]. Colley (2003) [12] summarized and explained six steps (1–6) and four criteria (A‐D) characterizing the application of the precautionary principle: (1) Identifying the possible threat; (2) listing the known and unknowns; (3) reframing the situation in ‘big picture’ terms; (4) vigorously assessing alternatives; (5) choosing a course of action; and (6) bearing the burden of demonstrating the risks going forward by the owner of the problem and the proposed solution. Further, honest, ethical and open practicing the precautionary principle includes (A) ‘transparency’ of information; (B) inclusion of stakeholders; (C) willingness to act in the face of uncertainty; and (D) accountability.
Opponents of the precautionary principle
Although precautionary approaches are going to play an increasing role in political decisions, especially in Europe, and partly proved wise and successful, the principle gives rise to major criticism. In the opinion of the sceptics it poses a serious threat to sound science, global commerce, consumer choice and technological progress [13, 14, 15, 16, 17].
The precautionary principle is regarded as a political idea characterized by a spectre of fear, emotions and ignorance, cloaked in the garb of pseudo science. The critics contend that the activists ignore the complexities of real world life and defy more than 300 years of scientific reasoning. Every choice is laden with risk, but instead of objectively establishing the risk–benefit relation and managing real risks, the precautionary principle leads to arbitrary decisions based on potential risks.
By focusing on ‘what we don't know’ and the conclusion ‘so let's err on the side of caution’, it is not possible to gain knowledge. Thus, the precautionary principle discourages progress (e.g. the development of life‐saving drugs) and is humanity's most powerful self‐imposed constraint. Sometimes, this strategy causes even more problems than it solves. As an example, the European Union's long‐running moratorium on genetically manipulated (GM) foods is mentioned, which has discouraged small farmers in Africa and Asia from using efficient GM agriculture, which would not only prevent starving, but also could be the basis of a flourishing export trade.
Another point of criticism concerns the demand ‘public participation in decision making’, which is considered not helpful for progress, as the opinion of the public is rather based on emotion than on knowledge. In this connection, the sceptics reproach the activists for manipulating and terrifying the public over hypothetical or minimal risks and thus exploiting their emotions.
It seems, however, that the debate between the proponents and opponents takes place rather on a theoretical level of two extreme ideologies being far away from the reality. Both parties are partly right and the best might be to steer a middle course.
Implementation of the precautionary principle in environmental and public health policy
Examples for the implementation of the precautionary principle in the environmental policy are: (1) water‐, air‐, and land pollution by discharging hazardous chemicals; (2) deletion of biodiversity; (3) fisheries degradation; (4) gene‐spliced crop plants, e.g. herbicide‐resistant ones, (5) climate change; (6) ozone hole, (7) chlorofluorocarbons; (8) ‘Y2K bug’; and (9) the new millennium preparation of computers. Further, it is applied to numerous public health concerns [6, 13–17] like: (1) potentially toxic chemicals, e.g. chlorinated water, asbestos, phthalate‐based plasticizers; (2) potentially carcinogenic compounds; (3) persistent chemicals in food chains, e.g. manmade pesticides; (4) antibiotics in animal food; (5) endocrine disrupting compounds, e.g. bovine growth hormone; (6) gene‐spliced food; (7) electric and magnetic field; (8) bioterrorism; and (9) severe acquired respiratory syndrome (SARS). An important issue is, for example, potentially carcinogenic compounds, which are the matter of the US National Toxicology Program (NTP). The NTP, a cooperative effort of three federal agencies, coordinates toxicological research and testing programs within the Department of Health and Human Services (DHHS), and through its annual Report on Carcinogens (RoC), it identifies and characterizes cancer hazards as the first step in quantitative risk assessment [18].
In contrast to the American governmental organizations, the European ones have widely implemented the precautionary principle in the area of food safety. For example, they erected strict rules regarding testing and commercialization of gene‐spliced food and prohibited the administration of bovine growth hormones to dairy cows [19], whereas the US and Canada still allow the use of the latter [2]. These opposed decisions reflect the continuous debate on endocrine‐disrupting chemicals in science and society, which is resulting from lack of knowledge and resulting to considerable uncertainty. For the same reason, potentially harmful effects by electric and magnetic fields such as mobile phones and power lines are intensely and controversially discussed [20].
Late‐breaking examples for precaution‐guided actions are measures like the present stockpiling of smallpox vaccine to obviate bioterrorism and all the efforts in the fight against the ‘severe acute respiratory syndrome’ (SARS) [21]. SARS may one day be an example of parties working successfully together for precautionary success [12].
Implementation of the precautionary principle in medicine
The precautionary principle plays not only a role in the prevention of damage to human health, but also has an impact on medical practice and medicinal products and devices.
Trilucent™ breast implants
A good example for a purely precautionary measure is Trilucent™ breast implants, which are no longer available following their preliminary worldwide withdrawal by the manufacturer in 1999 [22, 23]. Trilucent™ breast implants consist of a silicone elastomer shell with a lipid filler based on soybean oil and were for several years used as alternatives to silicone gel and saline filled breast implants. As a result of concerns of the Medical Device Agency (MDA) to long‐term safety data, the company voluntarily withdrew the product from the market in March 1999. The withdrawal was a precautionary measure until further information could be gathered about the biological safety and clinical experience with these implants. In May 2000, preliminary data of further analytical studies became available. The toxic degradation products found were considered as indication of the potentially hazardous nature of the filler and led to the MDA's Hazard Notice [HN2000(05)] with the recommendation to remove the Trilucent™ breast implants. The company initiated a reimbursement program for the implant replacement, further a clinical sample study (results expected in 2004) and is planning a large epidemiological study over the period of 9 years. So far, the continued research on the safety revealed no indication of toxic effects in implanted women. In this case, the official application of the precautionary principle can be regarded adequate, because it did not damage and even promoted research.
Porcine xenotransplants and the precautionary principle
In contrast to this, the opinions about xenotransplantation and the precautionary principle are divergent. For the activists, xenotransplantation represents an unacceptable risk, whereas the opponents fear that a life‐saving method is prevented by ill‐founded concerns.
Originally, xenotransplantation, i.e. the transplantation of animal, especially porcine cellular, tissue and organ grafts to humans, has been deemed a promising option to overcome the permanent shortage of donor organs. However, there are presently three important reasons being opposed to its introduction in practice: the immunological rejections against porcine xenotransplants; the long‐term physiological compatibility and thus functionality of xenotransplants in the human organism; and of late, the potential risk of damage by transmission of infectious agents present in pigs [24, 25]. Solving the well‐known problems of the rejection and of the compatibility is essential for the feasibility of xenotransplantation. But stepwise progresses such the production of new transgenic pigs [26] raise hope that this might be possible in the long run. In contrast to this, the question on the microbiological safety has been characterized by complete uncertainty of the risk.
At present, about 25 different viruses are identified such as swine hepatitis E virus, hendravirus, porcine circoviruses type I and II, two newly identified herpesviruses, porcine cytomegalovirus and in particular, pig endogenous retroviruses (PERVs) [27]. Whereas most of the microorganisms may be eliminated by specific pathogen‐free (SPF) animal husbandry and breed selection [25], PERVs represent a special problem.
PERVs are integrated in the porcine genome and are thus transmitted to the recipient. They are related to retrovirus known to induce leukaemia and immune deficiency syndromes. Consequently, the question arises whether PERVs might also become pathogenic in humans. Recent in vitro and animal studies suggest spreading of PERVs to tissues outside the transplant and replication [24, 28, 29, 30, 31]. Therefore, these agents could potentially reproduce in a transplant recipient, become pathogenic for him and infectious to contacts with that patient providing a source for a potential epidemic [25]. But to date, there are no clinical data indicating whether or how such agents would affect a patient.
Similarly, not much or nothing, resp., is known about the threat by other possibly latent or still unknown porcine viruses. Thus, porcine organs may be life saving for an individual, but implicate the virtual, unquantifiable risk to jeopardize the health of a wider population.
To minimize the risk, a framework of standards and strategies has to be developed. These include guidelines of good practice at each step of the xenotransplantation process such as the creation of a colony of SPF animals for xenotransplantation [32] and the establishment of an enhanced surveillance system [33]. But at present, most important is further intensified research to estimate the risk. For instance the Paul‐Ehrlich‐Institut (PEI), the German Federal Agency for Sera and Vaccines, has recently cloned the genome and functionally characterized infectious PERV and developed corresponding diagnostic tests [34].
Implementation of the precautionary principle in the area of medicinal products
A major field of concern of precautionary measures are medicinal products and devices of human or animal origin because of their potential risks by contamination with pathogens. Regarding the current strategies of health policy, it can be guessed that this is also going to influence future drug development. In the following discussions, a few significant examples and corresponding preventive measures are presented.
Viral contamination of blood and blood products
In the early 1980s, thousands of patients receiving blood transfusions or blood products like factor VIII and factor IX were infected with HIV, before the causal agent was identified. This AIDS pandemic actually happened, as missing knowledge and uncertainty provoked ignorance and no action. Taught by this experience, governmental agencies in the US and in Europe, in cooperation with the industry, made a considerable effort to develop new regulations for the manufacturing and control of blood derivatives [35, 36]. These, for instance, include careful selection and examination of blood donors, performance of HIV 1 and 2, hepatitis B and C virus tests and accurate documentation.
Nevertheless, iatrogenic transmission still occurs betimes as obvious, e.g. from the German ‘Stufenplanverfahren’ (i.e. officially regulated risk management procedure according to the German drug law) on the virus safety of erythrocyte concentrates in 1997 [37]. Moreover, potential risks may also arise from recently discovered viruses like the SARS causing coronavirus or still unknown viruses. Consequently, ongoing increased vigilance for the viral safety is justified, particularly because in the foreseeable future, it is not possible to replace blood and cellular blood products.
Spread of transmissible spongiform encephalopathies (TSE)
One of the most important topics is precautionary measures to prevent transmission of transmissible spongiform encephalopathies (TSE). They concern three types of TSE, which successively occurred: the human sporadic Creutzfeldt‐Jakob disease (CJD), the bovine spongiform encephalopathy (BSE) and as the latest the human variant CJD.
Creutzfeldt‐Jakob disease
Creutzfeldt‐Jakob disease (CJD) is a rare yet rapidly fatal neurological disorder with a prevalence of about one case per million people per year [38]. Besides the sporadic (85%) and familial forms, iatrogenic cases by transmission are known for a long time [39]. In the 1980s, the growing use of therapeutic products derived from human tissues was associated with a rising incidence of iatrogenic CJD [40]. Cases of CJD through human growth hormone, dura mater grafts, corneal transplants, stereotactic equipment and also from exposure in the laboratory have been documented.
After the accumulation of CJD cases following application of hormone concentrate isolated from hypophysis of human cadavers, its distribution was immediately discontinued as a precautionary measure [41], although the cause–effect relationship was unknown yet [42]. Because growth hormone from other mammalians cannot be used to treat idiopathic infantilism, an urgent need for an alternative arose. This problem was resolved by the instant approval of recombinant human growth hormone [43].
To increase the safety of human grafts, criteria for proper processing including, e.g. the use of inactivation methods, were defined and cataloguing by tissue banks was introduced [40]. Despite the small number of about 10 known surgical procedures related with accidental inoculation of patients with prions, a number of guidelines on safe working and prevention of infection in hospital were issued supplemented by training of the medical personnel in the practice of universal precautions [44].
Bovine spongiform encephalopathy and consequences for medicinal products
Since the appearance of BSE in the late 1980s in the United Kingdom and especially with its expansion to other European countries, and recently also to USA and Canada, transmissible spongiform encephalopathies (TSE) have captured increased attention. The initial burst of BSE in the United Kingdom has actually been man‐made [45]: in 1973, the manufacturing procedure of meat and bone meal (MBM) was changed for economic reasons. This was, however, at the expense of safety as recognized in 1988, when MBM was identified as the cause of BSE. Despite a first official feed ban of MBM in 1988, the disease could not be stopped.
Since the risk of transmission was unknown, it has been considered important to eliminate any contaminated material from the food chain by measures like holocaust of infected cattle, MBM feed ban or certification systems for beef. The current rules for the prevention, control and eradication of certain TSE are defined in the Regulation (EC) no. 999/2001 [46], which however, do not apply to medicinal products, medicinal devices and cosmetics.
Concerning animal‐derived medicinal products, the Committee for Proprietary Medicinal Products (CPMP) (from June 2004 replaced by the Committee for Medicinal Products for Human Use (CHMP)) and the Committee for Veterinary Medicinal Products (CVMP) adopted a ‘Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products’, whose second revision is applicable from 1 July, 2004 [47]. This guideline is concerned with materials derived from ‘TSE‐relevant animal species’ (but not from humans) used for the production of medicinal products and sets out the scientific principles for risk minimizing. Particular attention has been placed on the sourcing of animals used for the manufacture of medicinal products and the categorization of tissues. In addition, regulations on the design and control of the manufacturing process are included. The combination of all these measures gives assurance on product safety. Some measures are clearly stated as precautionary as a result of missing knowledge and corresponding benefit/risk assessments are demanded regarding the use of materials derived from ‘TSE‐relevant animals’. But research on TSE is promoted and the guidelines are permanently supplemented and revised to take into account advances in scientific knowledge as, e.g. obvious from the present elaboration of the third revision.
A general requirement is to replace materials from ‘TSE‐relevant animal species’ in the manufacture of any biological or pharmaceutical product by those obtained from other starting materials, wherever possible [47, 48, 49]. As a consequence, for example, heparin used in Europe and in North America is no longer isolated from bovine lung, but only from porcine intestinal mucosa. Similarly, pancreatic enzymes are presently exclusively obtained from swine organs, and in Germany, the distribution of bovine insulin has been discontinued in 2002.
Variant CJD and consequences for medicinal products
In 1996, the first vCJD case in connection with consumption of contaminated food was reported. Meanwhile, the number of vCJD cases has increased to about 140. The variant form of CJD has convincingly been shown to be caused by the BSE pathogen. This evidence influenced the permanent work on the above mentioned ‘Note for guidance’ as well as intensified research on TSE. However, there is still a high degree of uncertainty, e.g. concerning the infectivity of various tissues, the required dose, the incubation time, the individual liability. Accordingly, estimates of the expected incidence range between dozens up to several thousands. Up to now, there is neither a suitable test nor a therapeutic measure or a vaccine.
As obvious from vCJD, the transmission of the pathogen from ruminants to humans is possible. This led to the precaution‐based question as to whether the vCJD‐pathogen may also be transmitted by other human tissues, especially blood or blood products [50]. Although there is hitherto no indication of a direct transmission between humans by blood or blood products, the time to gain enough experience is too short to draw a conclusion.
A further reason for precaution is that the tissue distribution of the assumed pathogenic prion protein (PrPSC) causing vCJD differs from that associated with classical CJD: it does not only occur in the central nervous system, but also in lymphatic tissues such as lymph nodes, tonsils and appendix [39]. As recently published by Zanusso et al. [51], PrPSC was also detected in the cytoplasm and dendrites of olfactory receptor neurons in postmortem specimens of olfactory mucosa from all of the nine patients with sporadic CJD. Moreover, according to a study in mice, infectivity showed to be associated with the ‘buffy coat’ and was also found in the plasma [52].
Until infectivity of blood and blood products is disproved, the European Agency for the Evaluation of Medicinal Products (EMEA) recommends corresponding precautionary measures [53, 54]: Because testing each individual blood collection is a successful basic method to rule out the transmission of pathogens by blood [55], the development of detection techniques of the vCJD pathogen should be intensified. As long as a suitable screening test is not available, a first important preventive strategy is the exclusion of blood from donors who were exposed to a possible risk of infection. Thus, blood plasma from the UK must not be used in the manufacture of blood derivatives any longer. Furthermore, persons are excluded as potential blood donors who have spent a total of more than one year in the UK or Northern Ireland between 1980 and 1996. To expand the exclusion to individuals who have themselves received blood transfusion before is opined to be not acceptable in view of the prevailing blood shortage [56]. As a second measure, blood and cellular blood components have to be depleted from the ‘buffy coat’ (leucoreduction), to remove potentially present vCJD pathogens. Although the real effect of leucoreduction on TSE infectivity still has to be proven, the removal of leukocytes is anyway an advantage because of the adverse effects caused by leucocytes. Concerning plasma‐derived medicinal products, the manufacturing process should be optimized to the effect that the infectivity of plasma is further reduced as it is already indicated by available data. It is further suggested to recall batches of plasma‐derived medicinal products where a donor to a plasma pool subsequently develops vCJD. But regarding these measures, it has to be considered that the thinkable risk of a vCJD transmission is not exchanged for the real risk caused by a shortage of life‐saving blood and blood products. To guarantee the long‐term stability of blood supply, suitable promotion and motivation campaigns for recruiting blood donors should be carried out. In addition, Germany started an initiative for ‘optimum use’ of blood products in 1999 [54], which is now generally recommended. This means to avoid the use of blood and blood products wherever possible and represents the best way to reduce a potential risk on transmission. Finally, the substitution of plasma‐derived medicinal products with alternative products such as recombinant proteins as well as the development of substitutes for albumin is encouraged.
The case Metrodin HP
The far‐reaching impact of these precautionary measures on practice becomes obvious from the withdrawal of Metrodin HP (High purity) in UK in February 2003 [57]. Metrodin HP is a follicle‐stimulating hormone and is predominantly used for strong stimulation of the ovary in women undergoing in vitro fertilisation (IVF). It is prepared from urine collected in Italy, where one case of vCJD has been recently confirmed. Although up to now there has been no indication of transmission of vCJD via urine or products derived from urine [47], the Committee on Safety of Medicines (CSM) has advised that Metrodin HP should no longer be used as a precaution against the remote possibility it might be infected with the vCJD pathogen. This recommendation is substituted by the availability of adequate alternative products such as recombinant proteins [58].
It has to be waited whether this is possibly an original precedence for withdrawals of other medicinal products of human or animal origin, where alternatives are available. Besides other gonadotrophins, such candidates are for instance insulin, blood coagulation factors FVIII and FIX, or urokinase. But discontinuation of these products would not be a drastic change for medical practice, because the use of the recombinant analogues is anyway expanding.
Recombinant proteins
Recombinant proteins as alternatives to mammalian proteins
The trend to substitute animal and human‐derived proteins used in medicine with recombinant alternatives is not only the result of a reduced contamination risk, but they have some other pivotal advantages. Their production is independent of any limited disposability of the natural raw material. Some of them have even become only available by recombinant technologies (Table 4). They have a higher degree of purity, which reduces the risk of adverse drug reactions (ADRs) caused by concomitant proteins and other substances, and are better standardized than natural concentrates (e.g. gonadotrophins). As known from insulin, human‐identical proteins are better tolerated and induce less immunological reactions than those that are animal‐derived. In addition, recombinant technology enables specific modifications resulting in improved pharmacological profiles (Table 3).
Table 3.
Recombinant therapeutic enzymes, hormones and cytokines approved in the European Union with the date of first approval
| Recombinant proteins as substitutes for natural proteins | Drugs only available as recombinant proteins | Specifically modified recombinant proteins | |||
|---|---|---|---|---|---|
| Human insulin | 12/1987 | IL‐2 (Adesleukin) | 12/1989 | ||
| Somatotropin | 02/1991 | ||||
| Glucagon | 03/1992 | Epoetin beta | 05/1992 | ||
| Interferon gamma‐1b | 1992 | ||||
| Factor VIII | 07/1993 | Interferon alfa‐2b | 03/1993 | ||
| Epoetin alpha | 04/1993 | ||||
| GM‐CSF (Molgramostim) | 04/1993 | ||||
| Interferon alpha‐2a | 04/1993 | ||||
| glyc. G‐CSF (Lenograstim) | 10/1993 | ||||
| Dornase alfa † | 09/1994 | t‐PA (Alteplase) | 04/1994 | Imiglucerase * | 06/1994 |
| G‐CSF (Filgastrim) | 08/1994 | ||||
| Follitropin alpha | 10/1995 | Interferon bata‐1b | 11/1995 | ||
| Follitropin beta05/1996 | 10/1996 | Factor VII | 02/1996 | Insulin lispror‐PA (Reteplase) | 05/1996 11/1996 |
| Factor IX | 08/1997 | Interferon bata‐1a | 03/1997 | ||
| Lepirudin | 03/1997 | ||||
| Desirudin | 03/1997 | ||||
| Calcitonin | 01/1999 | r‐hPDGF (Becalpermin) | 03/1999 | Interferon alfacon‐1 | 02/1999 |
| TNF alfa‐1a | 04/1999 | Moroctocog alpha ‡ | 04/1999 | ||
| Thyreotropin alfa (TSH) | 07/1999 | Insulin aspart | 09/1999 | ||
| Peginterferon alfa‐2b | 05/2000 | ||||
| Insulin glargin | 06/2000 | ||||
| Choriogonadotropin alpha | 02/2001 | Rasburicase | 02/2001 | Etanercept § | 02/2001 |
| Lutropin alpha | 02/2001 | Tenecteplase | 02/2001 | ||
| Algalsidase alfa ¶ | 08/2001 | Darbepoetin alfa | 06/2001 | ||
| Algalsidase beta ¶ | 08/2001 | ||||
| Anakinra ** | 03/2002 | Pegfilgastrim †† | 08/2002 | ||
| Drotrecogin alfa ‡‡ | 08/2002 | ||||
| Dibotermin alfa §§ | 09/2002 | ||||
| Laronidase ¶¶ | 06/2003 | ||||
Glucocerebrosidase.
human DNAse.
Factor VIII without B domain.
Recombinant fusion protein of human TNF receptor and p75cl.
Alpha‐galactosidase.
Interleukin‐1 receptor antagonist.
Covalent conjugate of recombinant methionyl human G‐CSF (Filgastrim) and monomethoxypolyethylene glycol.
Activated protein C.
Bone morphogenetic protein.
Glycosaminoglycan alpha‐L‐iduronohydrolase.
Recombinant proteins as a subject of the precautionary principle
Nevertheless, recombinant proteins are also a matter of precautionary approach. Accordingly, for the approval of a recombinant protein, special requirements on quality and safety have to be met, which are both time‐consuming and extremely costly and consequently may even be constraining the medicinal progress. For example, although there are more efficient expression systems than those mostly used, such as the Baculovirus expression system or Sf 9 insect cells, they could not prevail yet because of the required expensive safety studies [59].
Further, even recombinant proteins are not generally free of any risk of viral contamination. There are manifold potential sources of contamination ranging from viruses endogenously present in the production cell line (e.g. Parvovirus or Hantavirus in CHO cells) or viruses used for inducing gene expression over reagents and additives applied in production and purification such as culture media, trypsin, growth factors or monoclonal antibodies up to drug formulation excipients like serum and albumin [60]. For instance, the initial optimism to escape the risk of virus (HBV, HCV and HIV) infection by plasma‐derived factor VIII with the recombinant analogue was dampened with the observation of seroconversion for parvovirus B19 after application of recombinant factor VIII, which was resulting from parvovirus B19‐contaminated albumin used as excipient [61, 62].
The case ‘epoetin alfa’
Albumin used as an excipient is also considered as a potential risk factor of vCJD transmission [38]. As a preventive measure, the development of substitutes for plasma‐derived albumin is recommended. According to this, the stabilizer human serum albumin (HAS) contained in the epoetin alfa (Eprex®/Erypo®) formulation was replaced by polysorbate 80 and glycine in 1998. From that time, an increasing incidence of pure red cell aplasia (PRCA) in patients with chronic renal failure after subcutaneous administration of epoetin alfa was observed [63]. This severe adverse event was caused by neutralizing antierythropoietin antibodies [64]. It is suggested that as a result of the changed formulation the protein is no more adequately stabilized resulting in the formation of immunogenic aggregates. Consequently, the application of a precautionary measure to this recombinant protein had no benefit, but has lead to a real threat to patients.
Regarding this and also other cases like the recently reported anaphylactic reactions caused by the thrombin inhibitor, lepirudin, a recombinant hirudin [65], the question arises whether the recent euphoria about recombinant proteins as the drugs of the future is really justified. In fact, pharmaceutical research conforms to the principle ‘small is beautiful’ and is focusing on the identification of targets and the rational design of specifically acting drugs like the small synthetic thrombin inhibitor melagatran.
Viral contamination of animal derived medicinal products
Pigs and birds, which are animal species of particular interest for the production of medicinal products, are not naturally susceptible to infection via the oral route. Therefore they are not ‘TSE‐relevant animal species’ according to the ‘Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents’[47]. Recently, however, porcine viruses, especially as contaminants of xenotransplants, attracted attention.
Porcine parvoviruses and consequences for medicinal products
In 2002, the safety of another porcine medicinal product was under consideration, namely pancreatic enzyme preparations, which are indicated in pancreatic enzyme insufficiency such as cystic fibrosis [66]. The reason was a potential contamination with the porcine parvovirus (PPP).
Parvoviruses are very small envelopeless DNA‐viruses, which can lead either to a specific disease or to complex syndromes in a susceptible host. Despite their high species‐specificity under normal conditions, all parvoviruses are considered to be able to cross species barriers [67]. Because of their outstanding physicochemical resistance, they are widespread contaminants in biological and pharmaceutical products, and it is difficult to remove or to inactivate them. The practical relevance becomes obvious from the above mentioned Parvovirus B19‐contaminated human serum albumin in a factor VIII preparation and the corresponding seroconversion in some patients [61, 62].
However, neither the probability of a transmission of PPP to humans nor its pathogenicity is known. Although porcine pancreatic enzymes are used for many years, up to now no clinical case of PPP infection has been known. Therefore, as a result of missing alternatives to pancreatic enzymes, their distribution was finally allowed to be continued. Thus, this case exemplarily demonstrates that consideration of the precautionary principle also implicates an accurate benefit/risk analysis.
Heparin and synthetic alternatives under the aspect of the precautionary principle
Heparin, the most widely used swine‐derived medicinal product
At present, the most widely used medicinal product isolated from porcine material is heparin (here referring to both unfractionated heparin (UFH) and the various low molecular weight heparins (LMWH)), which is still the most applied drug for prophylaxis and therapy of thromboembolic diseases [68]. Up to now, there is no clinical case of any viral contamination, which would make to question the viral safety of porcine heparin. But regardless of the recent precaution‐guided official decisions concerning animal‐derived drugs, there are some other reasons making an alternative to heparins desirable.
After the discontinuation of bovine heparin as a result of the BSE problem, all the heparins used in Europe and North America are isolated from porcine intestinal mucosa. But there is a sizeable shortage of raw material [69]. Presently, the mucosa of more than 200 million pigs is needed yearly for the production of heparin, to treat more than 20 million people worldwide. In US, Germany and France, 312 million heparin doses were applied in 2003, whereby the percentage of LMWH amounted to 40%, 79% and 86%, respectively (data from IMS/MIDAS). The need is still increasing, as heparin is used in more and more countries, in a growing number of indications and increasingly for longer periods. For example, the sales volume of heparins in Germany increased from 45 million € in 1986 to 150 million € in 2002 (corresponding to 87 million standard doses), and the sales of heparin doses in the US increased from 129 million in 2002 to 139 million doses in 2003 (data from IMS/MIDAS). Furthermore, for the production of the prevailing LMWH even more raw heparin is required. According to business reports of the pharmaceutical industry of the last few years, the sales volume of LMWH increases by 10–20% per year.
Another point is that the natural product heparin is a polydisperse mixture of molecules showing wide variations in its composition, and its preparations generally contain some dermatan and heparin sulfate to a varying extent [70]. The respective composition of a heparin preparation is dependent on the individual manufacturing processes. Manifold parameters ranging from the used pig subspecies and the conditions of the animal husbandry over the processing of the mucosa and the extraction of raw heparin up to the final purification influence the final heparin constitution. As a result, there are large differences between various heparin preparations as well as considerable batch‐to‐batch variations, which ultimately become manifested in differences in their biological activities [71, 72].
The biological activities comprise not only the acceleration of the antithrombin‐mediated inhibition of thrombin and factor Xa, which is thought to be mainly responsible for its antithrombotic activity, but heparin exhibits a wide range of biological effects [73]. Thus, it is not a specifically acting drug, but rather a multivalent biomodulator.
With the development of the LMWH, some of the drawbacks of UFH, like its unfavourable pharmacokinetic profile could be improved. However, regarding efficacy and safety, none of the heparins can be considered as optimal [74].
New antithrombotics as potential alternatives to heparin
Despite all the limitations of heparin, it had to be used because of a missing suitable alternative antithrombotic drug. With the approval of fondaparinux (Fig. 1), this situation is now changed. Fondaparinux is the first one of a new class of antithrombotic agents, i.e. the selective inhibitors of factor Xa. It is a fully synthetically produced, chemically defined pentasaccharide, which specifically binds to antithrombin with high affinity and in this way selectively inhibits FXa [75].
Figure 1.
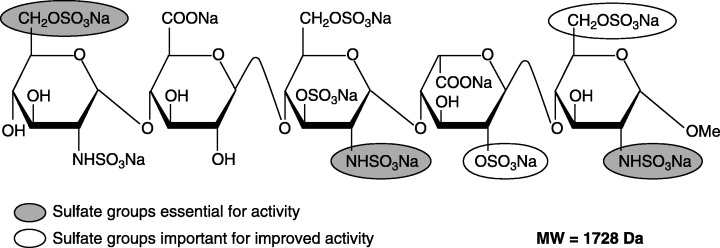
Chemical structure of the selective factor Xa‐inhibitor fondaparinux, a pentasaccharide binding specifically with high affinity to antithrombin.
The efficacy and safety of fondaparinux have been examined in several phase II and III clinical trials. Four phase III studies on short‐term prophylaxis in surgery involved 7344 patients undergoing major orthopaedic surgery of the lower limbs. Fondaparinux reduced the risk of venous thromboembolism (VTE) and of proximal deep vein thrombosis by more than 50% compared with the most common LMWH, enoxaparin, based on phlebographically detected thrombosis as the internationally accepted the efficacy endpoint [76, 77]. The benefit of extended thromboprophylaxis with fondaparinux in hip fracture surgery patients was also demonstrated by the 89% reduction of symtpomatic VTE from 2·7% to 0·3%[77]. Fondaparinux also showed benefit in the prevention of vVTE in other surgical and medical settings and in the treatment of patients with VTE. According to the clinical studies, fondaparinux therapy was as well‐tolerated as currently available treatments [75]. After the treatment of 300 000 patients with fondaparinux, no case of heparin‐induced thrombocytopenia (HIT) type II has so far been reported and no cross‐reactivity with plasma of HIT‐patients containing HIT‐associated antibodies was found [78]. Consequently, fondaparinux that is used according to its approved regimen provides an effective and safe postoperative regimen for all orthopaedic surgery patients, which is additionally simple and easy to use [68].
Independent of its clinical profile, the characteristics of fondaparinux concerning its pharmaceutical quality are advantageous compared to heparin. In addition, fondaparinux is in line with the current efforts of health policy to implement the precautionary principle, as because of its generation by total chemical synthesis, both potential risk of contamination and batch‐to‐batch variability are excluded.
Furthermore, there are some other synthetic antithrombotics in development. Under them, the synthetic thrombin inhibitor, melagatran and its orally available prodrug, ximelagatran have completed the mutual recognition procedure in Europe in May 2004, and has been introduced in Germany in June of the same year. Although the intensive research on new antithrombotic drugs during the last decades was certainly driven mainly by the clinical need of more effective and safer drugs, the current trends in drug development also consider the precaution‐guided suggestion by the health authorities to replace animal‐derived products. If the health care systems are willing to pay the costs combined with such innovative therapies, we could become independent of the use of animal products for antithrombotic therapy.
Conclusion
During the last decade, the precautionary principle has gained in importance. It is an approach to manage uncertain risks and to prevent any damage to the environment or human health. A key element is to take action, even if some cause and effect relationships are not fully established scientifically. In the meantime, it is increasingly implemented in medicine. An important subject is potential threats by medicinal products of human or animal origin. Manifold official precaution‐guided regulations have been stated to improve their safety and particularly to avoid any infection by viruses and TSE pathogens by animal and human‐derived medicinal products. The best preventive measure is to substitute them with alternative products whenever possible. In contrast to the opinion of sceptics, this promotes research and science. The new antithrombotic drug, fondaparinux, exemplary demonstrates that it is principally possible to become independent on animal‐derived products in the future. But it has to be considered that ultimately the official precaution‐oriented requirements can only be met, if the healthcare systems and the public are willing to pay the costs for corresponding innovative medicinal products.
Pharmaceutical Institute, Christian‐Albrechts‐University of Kiel, Germany (S. Alban).
References
- 1. Stoto MA. The precautionary principle and emerging biological risks: Lessons from swine flu and HIV in blood products. Public Health Rep 2002;117: 456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeFur PL, Kaszuba M. Implementing the precautionary principle. Sci Total Environ 2002;288: 155–65. [DOI] [PubMed] [Google Scholar]
- 3. Ministerial declaration calling for the reduction of pollution. Second International Conference on the Protection of North Sea. November 25 1987: 27 ILM 835.
- 4. Beliczy B. Science, ethics, responsibility. the principle of precaution In: Cetto AM, editor. World On Science: Science For The Twenty‐First Century – A New Commitment. London, UK: Banson; 2000,pp. 212–4. [Google Scholar]
- 5. Bro‐Rasmussen F. Risk, uncertainties and precautions in chemical legislation International Summit on Science and the Precautionary Principle. Lowell, MA; September 2022, 2001.. Available at: http://sustainableproduction.org/precaution/ (accessed: 30 June, 2004). [Google Scholar]
- 6. David G. The principle of precaution: its impact in medicine. Ann Otolaryngol Chir Cervicofac 2001;118: 208–14. [PubMed] [Google Scholar]
- 7. Pollan M. The precautionary principle. Altern Ther Health Med 2002;8: 38. [PubMed] [Google Scholar]
- 8. Raffensperger C, Tickner JA, Jackson W. Protecting Public Health and the Environment: Implementing the Precautionary Principle. Washington, DC: Island Press; 1999. [Google Scholar]
- 9. Commission of the European Communities. Communication from the Commission of the Precautionary Principle. Brussels: Commission of the European Communities; 2000:. Report number COM (2000), 1. [Google Scholar]
- 10. Tickner JA, editor. Precaution, Environmental Science, and Preventive Public Policy. Washington DC: Island Press; 2002. [DOI] [PubMed] [Google Scholar]
- 11. International Summit on Science and the Precautionary Principle. Lowell, MA; September 2022, 2001. Available at: http://sustainableproduction.org/precaution/ (accessed: 30 June, 2004). [DOI] [PubMed] [Google Scholar]
- 12. Cooley PE. The precautionary principle: Practical applications for real organizations, 2003. Available at: http://www.asq-eed.org/conferences/papers/CooleyPaper.doc (accessed: 30 June, 2004).
- 13. Lupien JR. The precautionary principle and other non‐tariff barriers to free and fair international food trade. Crit Rev Food Sci Nutr 2002;42: 403–15. [DOI] [PubMed] [Google Scholar]
- 14. Guldberg H. Challenging the precautionary principle. Spiked, July 1, 2003.. Available at: http://www.spiked-online.com/Articles/00000006DE2F.htm (accessed: 30 June, 2004).
- 15. Miller HI, Conko G. Precaution without principle. Nat Biotechnol 2001;19: 302–3. [DOI] [PubMed] [Google Scholar]
- 16. LaFee S. A matter of principle: Should threat of harm prompt action even if risk is uncertain? The SanDiego‐Union Tribune, April 16th, 2003.. Available at: http://www.environmentalhealth.org/News_Precautionary%20Principle.htm. (accessed: 30 June, 2004).
- 17. Collection of assays of prominent contrahentes of the precautionary principle, 2003. Available at: http://www.techcentralstation.com/risk.html. (accessed: 30 June, 2004).
- 18. Moure‐Eraso R. Primary prevention and precaution in hazard identification in the NIEHS/NTP: body in the morgue approach. Public Health Rep 2002;117: 564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Council of EU Commission. Council Decision of 17 December 1999 concerning the placing on the market and administration of bovine somatotrophin (BST) and repealing. Decision 90/218/EEC Off J Eur Commun 1999;L331: 71–2. [Google Scholar]
- 20. Kheifets LI. The Precautionary Principle and EMF. WHO, Meeting on EMF Biological Effects + Standards Harmonization in Asia & Oceania. Seoul, South Korea; October 22–24, 2001. Available at: http://www.who.International/peh-emf/meetings/southkorea/Leeka_Kheifets_principle_pdf (accessed: 30 June, 2004).
- 21. United Nations Agencies Joint Med Service Conference on Severe Acute Respiratory Syndrome (SARS). April 9th, 2003. Available at: http://www.who.international/csr/sars/press2003-04-09/en/ (accessed: 30 June, 2004).
- 22. MDA. Breast implants (last modified 27/06/02). Available at: http://www.medical-devices.gov.uk/mda/mdawebsitev2.nsf/webvwKeyTopics?OpenView&count%9999 (accessed: 30 June, 2004).
- 23. BfArM. Risikobewertung des BfArM von Silikongel‐gefüllten Brustimplantaten (Februar 2004). Available at: http://www.bfarm.de/de/Medizinprodukte/mp_akt/index.php (accessed: 30 June, 2004).
- 24. Denner J. Stellungnahme der GfV in Bezug auf Chancen und Risiken der Xenotransplantation 2002; Available at: http://www.g-f-v.org/inhalt_de.php?lmnop=1&modul=NEWS&action=DETAILS&id=13 (accessed: 30 June, 2004).
- 25. Michie C. Xenotransplantation, endogenous pig retroviruses and the precautionary principle. Trends Mol Med 2001;7: 62–3. [DOI] [PubMed] [Google Scholar]
- 26. Kolber‐Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML et al. Production of alpha‐1,3‐galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA 2004;101: 7335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoo D, Giulivi A. Xenotransplantation and the potential risk of xenogeneic transmission of porcine viruses. Can J Vet Res 2000;64: 193–203. [PMC free article] [PubMed] [Google Scholar]
- 28. Van Der Laan LJ, Lockey C, Griffeth BC, Frasier FS, Wilson CA, Onions DE et al. Infection by porcine endogenous retrovirus after islet xenotransplantation in SCID mice. Nature 2000;407: 90–4. [DOI] [PubMed] [Google Scholar]
- 29. Deng YM, Tuch BE, Rawlinson WD. Transmission of porcine endogenous retroviruses in severe combined immunodeficient mice xenotransplanted with fetal porcine pancreatic cells. Transplantation 2000;70: 1010–6. [DOI] [PubMed] [Google Scholar]
- 30. Wilson CA, Wong S, VanBrocklin M, Federspiel MJ. Extended analysis of the in vitro tropism of porcine endogenous retrovirus. J Virol 2000;74: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tacke SJ, Kurth R, Denner J. Porcine endogenous retroviruses inhibit human immune cell function: risk for xenotransplantation? Virology 2000;268: 87–93. [DOI] [PubMed] [Google Scholar]
- 32. Julvez J, Vannier P, Wadham L. Microbiological hazards of xenotransplantation. 2. Facing Risk Pathol Biol (Paris) 2000;48: 436–9. [PubMed] [Google Scholar]
- 33. Laderoute MP. Xenotransplantation surveillance in Canada. Can Communicable Dis Report 2001;2753: 34–6. [Google Scholar]
- 34. Niebert M, Tönjes RR. Molecular cloning and functional characterization of infectious PERV and development of diagnostic tests In: Salomon DR, Wilson C, editors. Current Topics in Microbiology and Immunology. Volume Xenotransplantation No. 278., Berlin, Heidelberg, New York: Springer; 2003,pp. 217–37. [DOI] [PubMed] [Google Scholar]
- 35. Heiden M, Seitz R. Zulassung von Blutprodukten zur Transfusion. Bundesgesundheitsbl – Gesundheitsforsch – Gesundheitsschutz 1999;42: 150–5. [Google Scholar]
- 36. SCMPMD. Opinion on quality and safety of blood. February 16, 2000.. Available at: http://europa.eu.int/comm/food/fs/sc/scmp/out29_en.pdf (accessed: 30 June, 2204).
- 37. PEI. Bekanntmachung über die Ergebnisse des Stufenplanverfahrens zur Verminderung des Risikos von Hepatitis B‐, Hepatitis C und HIV‐Infektionen bei Empfängern von Erythrozytenkonzentraten February 25, 1998.. BAnz 1998;53: 3935–6. [Google Scholar]
- 38. Tyler KL. Creutzfeldt‐Jakob Disease. N Engl J Med 2003;348: 681–2. [DOI] [PubMed] [Google Scholar]
- 39. Rappaport EB. Iatrogenic Creutzfeldt‐Jakob disease. Neurology 1987;37: 1520–2. [DOI] [PubMed] [Google Scholar]
- 40. Mocsny N. Precautions prevent spread of Creutzfeldt‐Jakob disease. J Neurosci Nurs 1991;23: 116–9. [DOI] [PubMed] [Google Scholar]
- 41. FDA. Human growth hormone distribution discontinued. FDA Drug Bull 1985;15: 17–8. [PubMed] [Google Scholar]
- 42. Owen R. The human growth hormone Creutzfeld Jakob disease litigation. Med Leg J 1997;65: 46–64. [DOI] [PubMed] [Google Scholar]
- 43. Glasbrenner K. Technology spurt resolves growth hormone problem, ends shortage. JAMA 1986;255 (581–4):587. [DOI] [PubMed] [Google Scholar]
- 44. Dealler S (consultant editor). Hospital and laboratory safety concerning TSEs Last updated January, 2004. Available at: http://www.priondata.org/data/A_control.html (accessed: 30 June, 2004).
- 45. N.N. Meat and bone meal. Available at: http://www.priondata.org/data/A_mbm.html (accessed: 24 March, 2003).
- 46. European Parliament and European Council. Rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies (Regulation (EC), 999/2001, 22 May 2001). Off J Eur Commun 2001;C24: 6–19. [Google Scholar]
- 47. CPMP, CVMP. Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products. (EMEA/410/01 Rev 2 October 2003). Off J Eur Union 2004;L147: 1–40. [Google Scholar]
- 48. WHO. Spongiform encephalopathies: New recommendations on medical products. Press Release WHO/27, March 27, 1997. Available at: http://www.who.International/archives/inf-pr-1997/en/pr97-27.html (accessed: 30 June, 2004).
- 49. WHO. Guidelines on transmissible spongiform encephalopathies in relation to biological and pharmaceutical products. (February 2003). Available at: http://www.who.international/biologicals/Guidelines/General.htm (accessed: 30 June, 2004).
- 50. PEI. Overview article of BSE‐safety from blood and other tissues: Transmission of vCJD from blood and other tissues November 2000.. Available at. http://www.pei.de/english/bse/vcjd_blood_review.pdf (accessed: 30 June, 2004).
- 51. Zanusso G, Ferrari S, Cardone F, Zampieri P, Gelati M, Fiorini M et al. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt‐Jakob disease. N Engl J Med 2003;348: 711–9. [DOI] [PubMed] [Google Scholar]
- 52. Brown P, Cervenakova L, McShane LM, Barber P, Rubenstein R, Drohan WN. Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit Creutzfeldt‐Jakob disease in humans. Transfusion 1999;39: 1169–78. [DOI] [PubMed] [Google Scholar]
- 53. EMEA. Report of EMEA expert workshop on human TSEs and plasma‐derived medicinal products. (EMEA/CPMP/BWP/1244/00; July 26, 2000). Available at: http://www.emea.eu.international/pdfs/human/regaffair/124400en.pdf (accessed: 30 June, 2004).
- 54. EMEA. CPMP position statement on Creutzfeldt‐jakob disease and plasma‐derived and urine‐derived medicinal products. (EMEA/CPMB/BWP/2879/02; February 20, 2003). Available at: http://www.emea.eu.international/pdfs/human/press/pos/287902en.pdf (accessed: 30 June, 2004).
- 55. PEI. Report of the working group: Overall blood supply strategy with regard to vCJD August 2001. Available at: http://www.pei.de/english/bse_blood_engl.pdf (accessed: 30 June, 2004).
- 56. RKI, PEI. Overall strategy blood supplies in view of the BSE crisis. Joint Press Release; October 15, 2001.. Available at: http://www.pei.de (accessed: 30 June, 2004).
- 57. Department of Health. Withdrawal of metrodin high purity (HP). February 10, 2003.. Available at: http://medicines.mhra.gov.uk/aboutagency/regframework/csm/csmhome.htm (accessed: 30 June, 2004).
- 58. CSM. The withdrawal of metrodin high purity: Questions and answers. Available at: http://medicines.mhra.gov.uk/aboutagency/regframework/csm/csmhome.htm (accessed: 30 June, 2004).
- 59. Dingermann T, Hänsel R, Zündorf I, editors. Pharmazeutische Biologie: Molekulare Grundlagen und Klinische Anwendung. Berlin, Heidelberg, New York: Springer; 2002,p.189. [Google Scholar]
- 60. Aranha H. Viral clearance strategies for biopharmaceutical safety. Part 1: General considerations. BioPharm 2001;. January: 28–35. Available at: http://www.biopharm-mag.com/biopharm/issue/issueList.jsp?id=48andhttp://www.pall.com/pdf/biopharm_pdf_bp5560.pdf. (accessed: 30 June, 2004).
- 61. Aygoren‐Pursun E, Scharrer I. A multicenter pharmacosurveillance study for the evaluation of the efficacy and safety of recombinant factor VIII in the treatment of patients with hemophilia A. German Kogenate Study Group. Thromb Haemost 1997;78: 1352–6. [PubMed] [Google Scholar]
- 62. Eis‐Hubinger AM, Sasowski U, Brackmann HH. Parvovirus B19 DNA contamination in coagulation factor VIII products. Thromb Haemost 1999;81: 476–7. [PubMed] [Google Scholar]
- 63. Breckenridge A. Eprex® (epoetin alfa) and pure red cell aplasia– contraindication of subcutaneous administration to patients with chronic renal disease. CEM/CMO/2002/17, December 12, 2002. Available at: http://199.228.212.132/doh/embroadcast.nsf/vwDiscussionAll/35595645F6304BFA80256C8D00456DCD (accessed: June 2004).
- 64. Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian JJ, Martin‐Dupont P, et al. Pure red‐cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. N Engl J Med 2002;346: 469–75. [DOI] [PubMed] [Google Scholar]
- 65. EMEA. Public statement on Refludan (INN: lepirudin) – fatal anaphylactic reactions. EMEA/27717/02; October 29, 2002.. Available at: http://www.emea.eu.international/pdfs/human/press/pus/2771702en.pdf (accessed: 30 June, 2004).
- 66. AFSSAPS. Sécurité virale des médicaments contenant des extraits de poudres de pancréas d’origine animale July 11, 2002.. Available at: http://afssaps.sante.fr/htm/10/pancreas/staquest.htm. (accessed: 30 June, 2004).
- 67. Siegl G. Significance of parvoviruses as contaminants in products of mammalian origin. Euroconference: Pharmaceuticals and viral safety: A global view. March 2–3, 2000. Available at: http://www.pasteur.fr/applications/euroconf/safety/safety-Abstract.html (accessed: 30 June, 2004).
- 68. Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R et al. Heparin and low‐molecular‐weight heparin: Mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001;119 (1 Suppl.):64S–94S. [DOI] [PubMed] [Google Scholar]
- 69. Demir M, Iqbal O, Dietrich CP, Hoppensteadt DA, Ahmad S, Daud AN et al. Anticoagulant and antiprotease effects of a novel heparinlike compound from shrimp (Penaeus brasiliensis) and its neutralization by heparinase I. Clin Appl Thromb Hemost 2000;7: 44–52. [DOI] [PubMed] [Google Scholar]
- 70. McGinnis DM, Outschoorn AS. Problems of component activities of heparin. Pharmacopeial Forum 1991;5: 2438–41. [Google Scholar]
- 71. Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost 1999;25 (Suppl. 3):5–16. [PubMed] [Google Scholar]
- 72. Alban S, Gastpar R. Plasma levels of total and free tissue factor pathway inhibitor (TFPI) as individual pharmacological parameters of various heparins. Thromb Haemost 200;85: 824–9. [PubMed] [Google Scholar]
- 73. Linhardt RJ, Toida T. Heparin oligosaccharides: New analogues development and applications In: Witczak ZJ, Nieforth KA, editors. Carbohydrates in Drug Design. New York: Marcel Dekker; 1997,pp. 277–341. [Google Scholar]
- 74. Geerts WH, Heit JA, Clagett GP, Pineo GF, Colwell CW, Anderson FA Jr et al. Prevention of venous thromboembolism. Chest 2001;119 (1 Suppl.):132S–75S. [DOI] [PubMed] [Google Scholar]
- 75. Turpie AG. Fondaparinux: a Factor Xa inhibitor for antithrombotic therapy. Expert Opin Pharmacother 2004;5: 1373–84. [DOI] [PubMed] [Google Scholar]
- 76. Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: A meta‐analysis of four randomized double‐blind studies. Arch Intern Med 2002;162: 1833–40. [DOI] [PubMed] [Google Scholar]
- 77. Turpie AG. The design of venous thromboembolism prophylaxis trials: fondaparinux is definitely more effective than enoxaparin in orthopaedic surgery. Int J Clin Pract 2004;58: 483–93. [DOI] [PubMed] [Google Scholar]
- 78. Summary of Product Characteristics. Fondaparinux Sodium, Arixtra. EMEA. January 2004.


