Abstract
The iliofemoral ligament, which plays an important role in hip joint stability, is formed on the anterosuperior region of the hip joint capsule. Although the tendon and deep aponeurosis of the gluteus minimus and iliopsoas are partly connected to the same region of the capsule, the precise location of the connections between the joint capsule and the tendons and deep aponeuroses remains unclear. The locations of the tendinous and aponeurotic connections with the joint capsule may clarify whether the iliofemoral ligament can be regarded as the dynamic stabilizer. This study investigated the relationships between the anterosuperior region of the joint capsule and the tendon and deep aponeurosis of the gluteus minimus and iliopsoas. Fourteen hips from nine cadavers (five males; four females; mean age at death 76.7 years) were analyzed. Ten hips were macroscopically analyzed, and four were histologically analyzed. During macroscopic analysis, the joint capsule was detached from the acetabular margin and the femur, and its local thickness was measured using microcomputed tomography (micro‐CT). The gluteus minimus tendon was connected to the joint capsule, and the lateral end of this connection was adjoined with the tubercle of the femur at the superolateral end of the intertrochanteric line. The deep aponeurosis of the iliopsoas was also connected to the joint capsule, and the inferomedial end of its anterior border corresponded with the inferomedial end of the intertrochanteric line. In the micro‐CT analysis, capsular thickening was observed at the base of the connection to the gluteus minimus tendon and at the anterior border of the deep aponeurosis of the iliopsoas. A histological study showed that the gluteus minimus tendon and the deep aponeurosis of the iliopsoas were continuous with the hip joint capsule. Based on the morphology of the tendinous and aponeurotic connections, local capsular thickening and histological continuity, the transverse and descending parts of the iliofemoral ligament were the joint capsules, with fibers arranged according to the connection with the gluteus minimus tendon and the deep aponeurosis of the iliopsoas, respectively. Therefore, the so‐called iliofemoral ligament could be regarded as the dynamic stabilizer, with the ability to transmit the muscular power to the joint via the capsular complex. This anatomical knowledge provides a better understanding of the hip stabilization mechanism.
Keywords: gluteus minimus, hip joint capsule, iliofemoral ligament, iliopsoas
Based on the morphology of the tendinous and aponeurotic connections, local capsular thickening and histological continuity, the transverse and descending parts of the iliofemoral ligament comprised the hip joint capsule itself, with fibers arranged according to the connection with the gluteus minimus tendon and the deep aponeurosis of iliopsoas, respectively. Therefore, the so‐called iliofemoral ligament could be regarded as the dynamic stabilizer.

Introduction
The iliofemoral ligament plays a key role in hip joint stability. The origin of the iliofemoral ligament is the same as that of the capsular attachment inferior to the anterior inferior iliac spine, and that capsular attachment is highly adaptive to mechanical stress, on the basis of its osseous impression, attachment width and histological features (Tsutsumi et al. 2019b). Distally, the iliofemoral ligament comprises two parts: the transverse part, which extends to a tubercle of the femur at the superolateral end of the intertrochanteric line, and the descending part, which extends to the inferomedial end of the intertrochanteric line (Schäfer & Thane, 1894; Neumann, 2016).
According to previous reports, the gluteus minimus and the iliopsoas are located immediately superficial to the anterosuperior region of the joint capsule, and the tendon and deep aponeurosis are partly connected to the joint capsule (Ward et al. 2000; Walters et al. 2001; Tsutsumi et al. 2019b). However, the relationships between the iliofemoral ligament and the tendon and deep aponeuroses remain unclear because of a lack of knowledge of the precise location of the connections between the joint capsule and the tendons and deep aponeuroses. The precise location of the connection may clarify whether the iliofemoral ligament could be regarded as the dynamic stabilizer with the ability to transmit muscular power to the joint via the capsular complex.
In the present study, we investigated the morphological features of the connections between the anterosuperior region of the hip joint capsule and the tendon and deep aponeurosis of the gluteus minimus and iliopsoas, based on macroscopic findings, analysis of the local thickness, and histological features. We hypothesized that the tendon and deep aponeurosis of the gluteus minimus and iliopsoas were connected to the part of the joint capsule which was identical to the iliofemoral ligament.
Materials and methods
Cadaveric specimen preparations
Fifteen hips from nine Japanese cadavers (five males; four females; mean age at death 76.7 years) that were donated to our Department of Anatomy were used in this study. The study design was approved by the Ethics Committee at our institution.
All cadaver specimens were fixed in 8% formalin and preserved in 30% ethanol. During dissection of the hip joint capsule and the pericapsular muscles, the skin and subcutaneous tissues were removed. In one specimen, cancer tissue infiltrating the pericapsular muscles was identified macroscopically and excluded. Of the remaining 14 hips, 10 hips and four hips were randomly assigned to macroscopic analysis and histological analysis, respectively.
Macroscopic analysis: outer appearance of the hip joint capsule
During the macroscopic analysis of 10 hips, the pericapsular muscles were carefully reflected from both the anterior and posterior aspects to expose the outer surface of the joint capsule (Fig. 1A–C shows the anterior aspect; Fig. 1D–F shows the posterior aspect). First, the surface of the pericapsular muscles including the iliopsoas, gluteus minimus, rectus femoris, gemelli superior, gemelli inferior, obturator internus and obturator externus were exposed (Fig. 1A and 1). Second, these pericapsular muscles, except the rectus femoris, were detached from the pelvic bone and reflected distally, and the rectus femoris was reflected proximally (Fig. 1B and 1). During reflection of each muscle, the deep aponeuroses were also identified to check whether or not they connected to the joint capsule. Third, we removed the deep aponeurosis that did not connect to the outer surface of the joint capsule, and also removed the rectus femoris to expose the proximal attachment of the joint capsule (Fig. 1C and 1). Finally, the joint capsule was detached from the acetabular margin and removed from the pelvic bone. To expose and observe the outer appearance of the joint capsule, we checked whether the pericapsular muscles connected to the joint capsule or not and carefully removed them.
Figure 1.
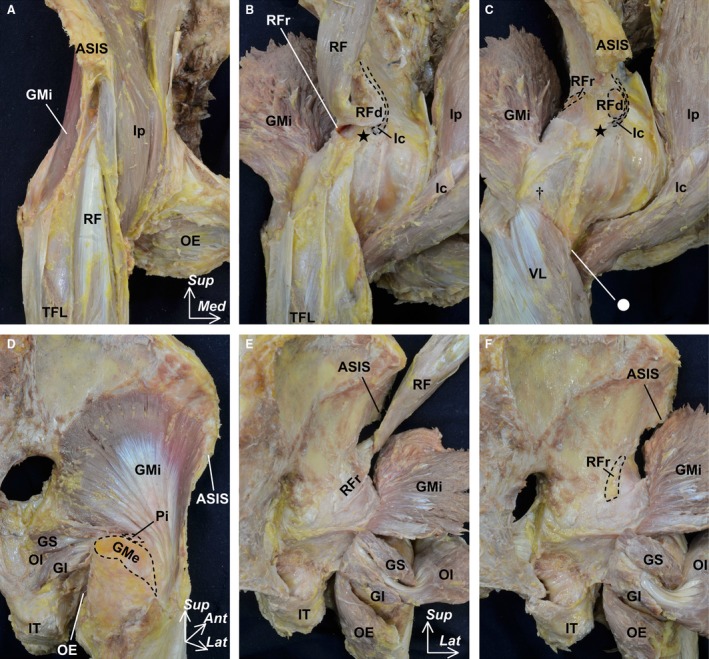
Macroscopic methods for exposing the outer surface of the hip joint capsule. The pericapsular muscles including the iliopsoas (Ip), gluteus minimus (GMi), rectus femoris (RF), gemelli superior (GS), gemelli inferior (GI), obturator internus (OI) and obturator externus (OE) were reflected from the anterior (A–C) and posterior aspects (D–F). After exposing the surface of the pericapsular muscles (A and D), these muscles were reflected to identify the deep aponeuroses (B and E). In addition, we removed the deep aponeurosis, which did not connect to the outer surface of the joint capsule and the rectus femoris (C and F). Dashed lines indicate the detachment region of the labeled muscle. Circle indicates the inferomedial end of the intertrochanteric line. Dagger indicates the superolateral end of the intertrochanteric line. Star indicates the inferior area of the anterior inferior iliac spine. Ant, anterior; ASIS, anterior superior iliac spine; GMe, gluteus medius; Ic, iliocapsularis; IT, ischial tuberosity; Lat, lateral; Med, medial; Pi, piriformis; RFd, direct head of the RF; RFr, reflected head of the RF; Sup, superior; TFL, tensor fasciae latae; VL, vastus lateralis.
Thickness distribution of the whole hip joint capsule on microcomputed tomography
After macroscopic analysis, the thickness distribution of the whole joint capsule was analyzed by using the previously verified method as follows (Tsutsumi et al. 2019a). The joint capsule, detached from the femur, was expanded flatly and examined using microcomputed tomography (micro‐CT; inspeXio smx‐100ct; SHIMADZU, Kyoto, Japan) with a 200‐μm resolution. The obtained images of the joint capsule were re‐sliced to be horizontal to the sheet of the joint capsule, and slice thickness was changed to 100 μm with use of OsiriX (Pixmeo, Benex, Switzerland). Re‐sliced image data were transferred to ImageJ software (version 1.52; National Institutes of Health, Bethesda, MD, USA) for visualization of the thickness distribution. First, binalization for all sequential frames was extracted from input micro‐CT images by specifying a CT intensity value. Second, an image stack was projected along the axis perpendicular to the image plane (the hip joint capsule thickness). The projection created a real image that was the sum of the binary slices in the stack. The joint capsule thickness was calculated according to slice unit length and number. On the basis of the calculated thickness, we compared the thicknesses of the two regions, the whole joint capsule and its anterosuperior region, which was defined by the bony attachment such as the inferior area of the anterior inferior iliac spine, superolateral and inferomedial end of the intertrochanteric line. Finally, a color look‐up table was created to demonstrate the specific joint capsule location.
Histological analysis of the cross‐section of the hip joint capsule
We performed histological examinations of the layer structure of the joint capsule in the four randomly selected hips. At first, the bony configuration of the pelvic bone and femur was examined using micro‐CT with 200‐μm resolution, and the three‐dimensional images were reconstructed using application software (VG Studio Max 2.0; Heidelberg, Germany). Then, we harvested en bloc specimens using a diamond saw in the following two small regions: the proximal cross‐section horizontal to the acetabular margin and the distal cross‐section horizontal to the intertrochanteric line of the femur. These regions were identified by three‐dimensional conformations of the micro‐CT images. After fixation, these en bloc specimens were dehydrated and embedded in paraffin solution. Subsequently, the blocks were serially sectioned (thickness 5 μm) and stained using Masson’s trichrome staining protocol.
Statistical analyses
Statistical tests were performed using JMP 14.0 (SAS Institute Inc., Cary, NC, USA). Statistical comparisons of the thicknesses of the hip joint capsules were performed using a paired t‐test, and the significance level was set at P < 0.001. We did not adjust the hip joint capsule thickness measurements for the size of the proximal femur or acetabulum. Data are provided as mean ± SD.
Results
Outer appearance of the hip joint capsule
On the anterior aspect of the joint capsule, the deep aponeurosis of the gluteus minimus, the iliopsoas, and the proximal aponeurosis of the rectus femoris were connected to the capsular surface (Fig. 2A). The gluteus minimus was adjoined to the joint capsule via its deep aponeurosis. To clarify the precise relationships between the gluteus minimus and the joint capsule, we removed the muscular portion of the gluteus minimus (Fig. 2B). The deep aponeurosis of the gluteus minimus merged into the gluteus minimus tendon, which inserted into the anterior facet of the greater trochanter. By detaching the insertion of the gluteus minimus tendon and reflecting it superiorly (Fig. 2C), we revealed that the joint capsule also merged into the gluteus minimus tendon and cut the base of this connection (Fig. 2D). The lateral end of this connection was adjoined with the tubercle of the femur at the superolateral end of the intertrochanteric line. In contrast, the iliopsoas could be completely detached from its deep aponeurosis and the lesser trochanter. The inferomedial end of the deep aponeurosis of the iliopsoas, at its anterior border, corresponded with the inferomedial end of the intertrochanteric line.
Figure 2.
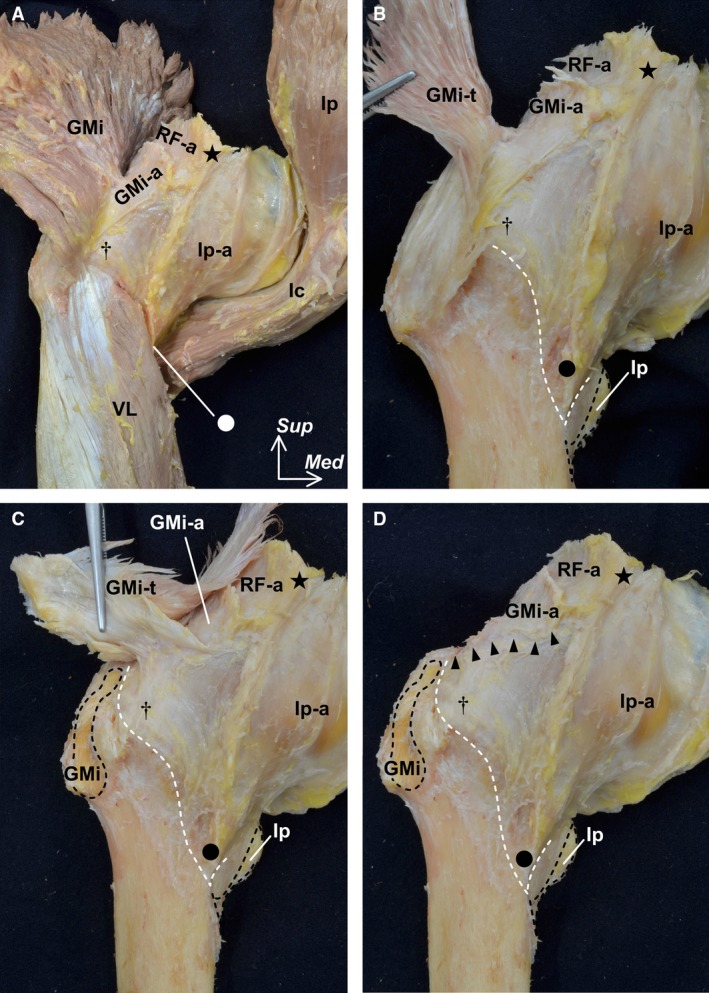
Outer appearance of the hip joint capsule (anterior aspect). The pelvic bone was removed. (A) The gluteus minimus (GMi) and iliopsoas (Ip) are reflected distally. The deep aponeuroses of the GMi (GMi‐a), Ip (Ip‐a), and the proximal aponeurosis of the rectus femoris (RF‐a) are connected to the joint capsule. (B) The muscular portion of the GMi was removed to reveal the tendinous portion (GMi‐t). The iliopsoas (Ip) was detached from its insertion (black dashed lines). The vastus lateralis (VL) was also removed to show the distal margin of the joint capsule on the intertrochanteric line (white dashed line). (C) After being detached from the insertion on the femur (black dashed line), the GMi‐t was reflected superiorly. (D) The connection between the GMi‐t and the joint capsule was cut. Arrow heads indicate the cut line. The lateral end of the connection was adjoined with the tubercle of the femur at the superolateral end of the intertrochanteric line (dagger). The inferomedial end of the anterior border of the Ip‐a corresponds to the inferomedial end of the intertrochanteric line (circle). Star indicates the inferior area of the anterior inferior iliac spine, Ic, iliocapsularis, Med, medial; Sup, superior
On the posterior aspect of the joint capsule, the deep aponeuroses of the gluteus minimus, the complex of the obturator internus and gemelli superior and inferior, and the obturator externus were also connected to its surface (Fig. 3A). However, the gemelli superior and inferior and obturator internus and externus could be completely detached from the trochanteric fossa (Fig. 3B).
Figure 3.
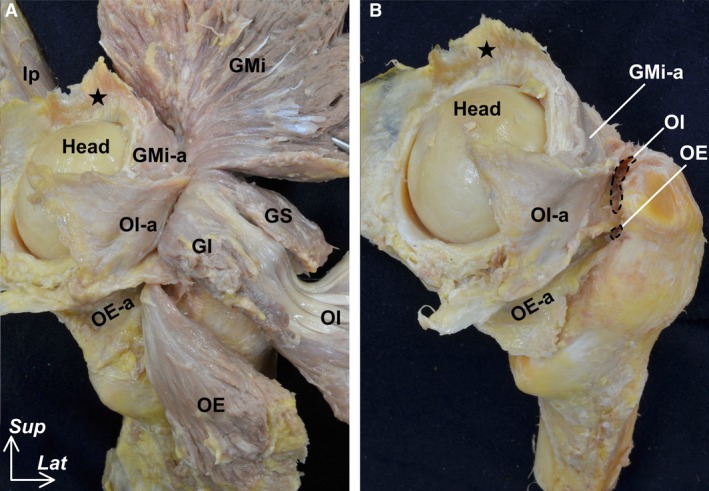
Outer appearance of the hip joint capsule (posterior aspect). Posterior aspect of Figure 2. (A) The gluteus minimus (GMi), gemelli superior (GS), gemelli inferior (GI), obturator internus (OI), and obturator externus (OE) are reflected distally. The deep aponeuroses of the GMi (GMi‐a), complex of the GS, GI and OI (OI‐a) and OE (OE‐a) were connected to the joint capsule. (B) Myotendinous unit of the GMi, OI and OE were removed to reveal their insertions (black dashed areas). Star indicates proximal detachment region corresponding to the inferior area of the anterior inferior iliac spine. Head, head of femur; Ip, iliopsoas; Lat, lateral; Sup, superior.
Appearance of the hip joint capsule and its thickness distribution
To observe the whole appearance of the joint capsule, we completely detached it from the femur (Fig. 4A). From the inner aspect of the joint capsule, its anterosuperior region, which was surrounded by the inferior area of the anterior inferior iliac spine and the intertrochanteric line (between its superolateral and inferomedial end), was identified (Fig. 4B). The zona orbicularis joined posteriorly to this anterosuperior region. From the outer aspect of the joint capsule, the borders among the deep aponeuroses connecting to the joint capsule were clearly identified (Fig. 4C). Based on the thickness distribution analysis using micro‐CT, these borders were identified as relatively thick regions (Fig. 4D). At the anterosuperior region of the joint capsule, two regions, the base of the connection to the gluteus minimus tendon and the anterior border of the deep aponeurosis of the iliopsoas, were relatively thick. The mean thickness of the anterosuperior region including these two thick regions was significantly greater than that of the whole joint capsule between the distal margin of the labrum and the proximal margin of the femoral attachment (P < 0.001; Table 1).
Figure 4.
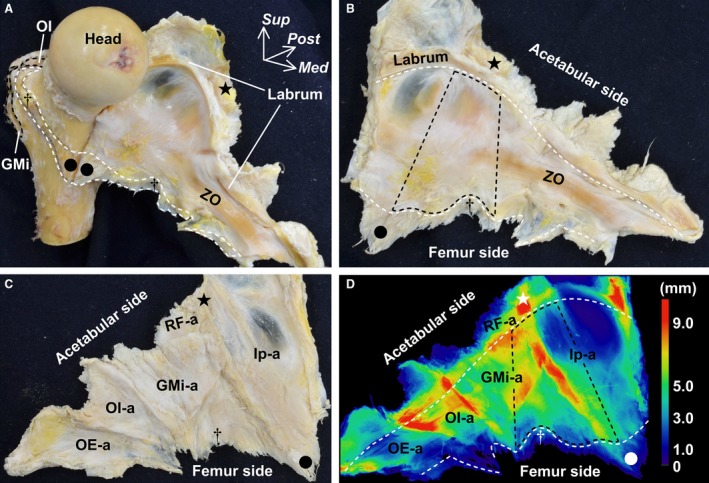
Appearance of the whole hip joint capsule and its thickness distribution. (A) Anteromedial aspect of the femur. The joint capsule is detached from the femur and reflected medially. White dashed line indicates the distal attachment of the joint capsule. Star indicates the inferior area of the anterior inferior iliac spine. Dagger indicates a tubercle of the femur at the superolateral end of the intertrochanteric line. Circle indicates the inferomedial end of the intertrochanteric line. (B) Inner aspect of the joint capsule. White dashed line on the acetabular side indicates the distal margin of the labrum. White dashed line on the femur side indicates the proximal margin of the capsular attachment. Black dashed area corresponds to the anterosuperior region of the joint capsule surrounded by the star, circle and dagger. (C) Outer aspect of the joint capsule. (D) Local thickness of the same joint capsule analyzed using microcomputed tomography and colored with ImageJ. Color bar represents the approximate thickness corresponding to the different colors. White and black dashed areas correspond to those of (B). GMi, gluteus minimus; GMi‐a, deep aponeurosis of the GMi; Head, head of femur; Ip, deep aponeurosis of the iliopsoas; Med, medial; OE‐a, deep aponeurosis of the obturator externus; OI, obturator internus; OI‐a, deep aponeurosis of the OI, gemelli superior and gemelli inferior; Post, posterior; RF‐a, deep aponeurosis of the rectus femoris; Sup, superior; ZO, zona orbicularis.
Table 1.
Measurements of the mean thickness of the hip joint capsule.
| Whole* | Anterosuperior region† | P | |
|---|---|---|---|
| Thickness, mm | 4.4 ± 0.4 | 5.9 ± 0.5 | < 0.001 |
Histological features of the cross‐section of the hip joint capsule
We performed a histological analysis of the cross‐section of the joint capsule at its proximal and distal portions, which were parallel to the acetabular margin and intertrochanteric line, respectively (Fig. 5A). At the proximal joint capsule, the deep aponeuroses of the rectus femoris and iliopsoas were continuous with the joint capsule (Fig. 5B), and these structures were composed of a dense connective tissue (Fig. 5C). Additionally, the iliocapsularis was surrounded by and connected to the deep aponeurosis of the iliopsoas. At the distal joint capsule, the deep aponeurosis of the iliopsoas was also continuous with the joint capsule (Fig. 5D) and composed of a dense connective tissue (Fig. 5E). The gluteus minimus tendon could not be separated from the joint capsule.
Figure 5.
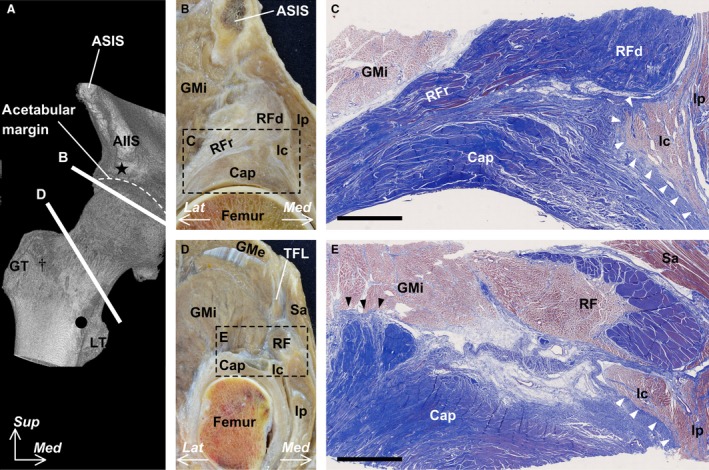
Histological analysis of the cross‐section at the proximal and distal portions of the hip joint capsule (Masson’s trichrome stain). (A) Microcomputed tomography image of the anterior aspect of the right hip. Star indicates the inferior area of the anterior inferior iliac spine (AIIS). Dagger indicates a tubercle of the femur at the superolateral end of the intertrochanteric line. Circle indicates the inferomedial end of the line. (B) Cross‐section along line B in (A). (C) Histological section of the boxed region in (B). White arrow heads indicate the connection between the muscular portion of the iliocapsularis (Ic) and the dense connective tissue of the joint capsule (Cap). (D) Cross‐section along line D in (A). (E) Histological section in the boxed region in (D). Black arrow heads indicate the connection between the muscular portion of the gluteus minimus (GMi) and the dense connective tissue of the joint capsule (Cap). Scale bar in (C) and (E) = 5 mm. ASIS, anterior superior iliac spine; GMe, gluteus medius; GT, greater trochanter; Ip, iliopsoas; Lat, lateral; LT, lesser trochanter; Med, medial; RF, rectus femoris; RFd, direct head of the RF; RFr, reflected head of the RF; Sa, sartorius; Sup, superior; TFL, tensor fasciae latae.
Discussion
The present study revealed that the gluteus minimus tendon was connected to the hip joint capsule, and that the lateral end of the base of this connection was adjoined with the tubercle of the femur at the superolateral end of the intertrochanteric line. Furthermore, the deep aponeurosis of the iliopsoas was also connected to the joint capsule, and the inferomedial end of its anterior border corresponded with the inferomedial end of the intertrochanteric line. Capsular thickening was observed at the base of the connection to the gluteus minimus tendon and at the anterior border of the deep aponeurosis of the iliopsoas. The mean thickness of the anterosuperior region of the joint capsule including these two regions was significantly greater than that of the whole joint capsule. A histological study showed that the gluteus minimus tendon and deep aponeurosis of the iliopsoas were continuous with the joint capsule.
Previous studies have focused on the relationship between the gluteus minimus and the joint capsule. According to Walters et al. (2001), the gluteus minimus tendon was attached to the joint capsule. Tsutsumi et al. (2019b) showed that the deep aponeurosis of the gluteus minimus was connected to the joint capsule. The present study revealed that the deep aponeurosis of the gluteus minimus was connected to the joint capsule and that this aponeurosis–capsule complex merged into the gluteus minimus tendon. Moreover, some anatomical textbooks have described the gluteus minimus tendon as being connected to the transverse part of the iliofemoral ligament (Henle, 1855; Fick, 1904). Our study showed that the lateral end of the base of the connection to the gluteus minimus tendon was adjoined with the tubercle at the superolateral end of the intertrochanteric line, namely, the insertion of the transverse part of the iliofemoral ligament (Schäfer & Thane, 1894; Neumann, 2016). Therefore, we inferred that the transverse part of the iliofemoral ligament was the joint capsule itself, with fibers that were arranged according to the connection with the gluteus minimus tendon.
The iliocapsularis was attached to the joint capsule as described by Ward et al. (2000). The present study showed that the deep aponeurosis of the iliopsoas was connected to the anterosuperior region of the joint capsule. Since the iliocapsularis occupied the deepest portion of the iliopsoas, we considered that the muscular portion of the iliocapsularis was attached to the joint capsule via the deep aponeurosis of the iliopsoas. Henle (1855) stated that a part of the iliacus, which is the same as the iliocapsularis, arose from the descending part of the iliofemoral ligament. The present study showed that the inferomedial end of the anterior border of its deep aponeurosis corresponded with the inferomedial end of the intertrochanteric line, namely, the insertion of the descending part of the iliofemoral ligament (Neumann, 2016). Based on the description by Henle (1855) and the present study, the descending part of the iliofemoral ligament was the joint capsule itself with fibers that were arranged according to the connection with the deep aponeurosis of the iliopsoas.
Few studies have measured the thickness of the hip joint capsule. Philippon et al. (2015) stated that the joint capsule was relatively thick at the region that corresponded to the location of the iliofemoral ligament. However, in their study, the thicknesses were measured at some points of the joint capsule, but not on the whole joint capsule. Using micro‐CT, we were able to show that, based on the thickness distribution of the whole joint capsule, the anterosuperior region, which was defined by the proximal and distal attachments of the iliofemoral ligament, was significantly thicker than the whole joint capsule. Furthermore, the anterosuperior region of the joint capsule was relatively thick at two regions, the base of the connection to the gluteus minimus tendon and the anterior border of the deep aponeurosis of the iliopsoas. Because the capsular ligament was defined as local thickening of the fibrous joint capsule (Adams, 2016), these two regions could be regarded as the capsular ligament owing to their thickness.
Although the histological features of the joint capsule have been investigated on many planes, including the axial, coronal sagittal and axial oblique planes (Wagner et al., 2012), the layer structures of the joint capsule at the proximal and distal cross‐sections horizontal to the acetabular margin and intertrochanteric line have never been shown in previous studies. Regarding the histological connection between the pericapsular muscles and the joint capsule, Walters et al. (2001) only showed the connection between the gluteus minimus tendon and the joint capsule. We showed the layer structures of the joint capsule, which cannot be separated from the gluteus minimus tendon and the deep aponeurosis of the iliopsoas. Moreover, the capsular complex including the connecting structures comprised dense connective tissue, identical to the histological features of the ligament (Fawcett & Bloom, 1986; Wigley, 2016).
The present study is clinically relevant because it provides useful information regarding hip joint stability. It is generally thought that the iliofemoral ligament contributes to hip joint stability as the main static stabilizer (Myers et al. 2011; Walters et al. 2014; van Arkel et al. 2015a,2015b). Tsutsumi et al. (2019b) showed that the origin of the iliofemoral ligament was identical to that of the capsular attachment inferior to the anterior inferior iliac spine. We showed that the transverse and descending parts of the iliofemoral ligament were the joint capsules, with fibers arranged according to the connection with the gluteus minimus tendon and the deep aponeurosis of iliopsoas, respectively. Therefore, the so‐called iliofemoral ligament could be regarded as the dynamic stabilizer, able to transmit muscular power to the joint via the capsular complex. In other words, the iliofemoral ligament might be able to maintain its tension via the contraction force of the gluteus minimus and iliopsoas to some extent, even in hip positions in which the ligament is often considered to be becoming loose.
This study had some limitations. First, it was a purely anatomical investigation and was limited to uninjured specimens, therefore, we cannot prove the mechanism of hip joint stability, and whether the iliofemoral ligament truly stabilizes the joint by transmitting muscle power to the joint was more an induction than a finding substantiated by the evidence of the study. Second, measurements were not adjusted for the overall size of the donor or related anatomical structures. Finally, we could not exclude the possibility that the advanced age of the donors affected our findings. Additional biomechanical studies or studies involving clinical case imaging are needed to validate our findings.
In conclusion, based on the morphology of the tendinous and aponeurotic connections, local capsular thickening and histological continuity, the transverse and descending parts of the iliofemoral ligament comprised the hip joint capsule itself, with fibers arranged according to the connection with the gluteus minimus tendon and the deep aponeurosis of iliopsoas, respectively. The so‐called iliofemoral ligament could therefore be regarded as the dynamic stabilizer. This anatomical knowledge provides a better understanding of the hip stabilization mechanism.
Conflict of interest
None declared.
Acknowledgements
This study was supported by JSPS KAKENHI (grant number JP 19K18488) and JA Kyosai Research Institute (Agricultural Cooperative Insurance Research Institute).
Data Availability Statement
Research data are not shared.
References
- Adams MA. (2016) Functional anatomy of the musculoskeletal system In: Gray's anatomy: the anatomical basis of clinical practice (eds. Standing S, Anand N, Birch R.), pp. 81–122. New York: Elsevier Limited. [Google Scholar]
- van Arkel RJ, Amis AA, Cobb JP, et al. (2015a) The capsular ligaments provide more hip rotational restraint than the acetabular labrum and the ligamentum teres : an experimental study. Bone Joint J 97‐B, 484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Arkel RJ, Amis AA, Jeffers JR (2015b) The envelope of passive motion allowed by the capsular ligaments of the hip. J Biomech 48, 3803–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, Bloom W (1986) A textbook of histology. Philadelphia: Saunders. [Google Scholar]
- Fick R (1904) Handbuch der Anatomie und Mechanik der Gelenke, unter Berücksichtigung der bewegenden Muskeln. Jena: Verlag von Gustav Fischer. [Google Scholar]
- Henle J (1855) Handbuch der systematischen Anatomie des Menschen. Braunschweig: Druck und Verlag von Friedrich Vieweg und Sohn. [Google Scholar]
- Myers CA, Register BC, Lertwanich P, et al. (2011) Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med 39, 85S–91S. [DOI] [PubMed] [Google Scholar]
- Neumann DA, et al. (2016) Hip In: Gray's anatomy: the anatomical basis of clinical practice (eds. Standing S, Anand N, Birch R.), pp. 1376–1382. New York: Elsevier Limited. [Google Scholar]
- Philippon MJ, Michalski MP, Campbell KJ, et al. (2015) A quantitative analysis of hip capsular thickness. Knee Surg Sports Traumatol Arthrosc 23, 2548–53. [DOI] [PubMed] [Google Scholar]
- Schäfer EA, Thane GD. (1894) Quain’s elements of anatomy. London; Longmans, Green, and Co. [Google Scholar]
- Tsutsumi M, Nimura A, Akita K (2019a) The gluteus medius tendon and its insertion sites: an anatomical study with possible implications for gluteus medius tears. J Bone Joint Surg Am 101, 177–184. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M, Nimura A, Honda E, et al. (2019b) An anatomical study of the anterosuperior capsular attachment site on the acetabulum. J Bone Joint Surg Am 101, 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FV, Negrao JR, Campos J, et al. (2012) Capsular ligaments of the hip: anatomic, histologic, and positional study in cadaveric specimens with MR arthrography. Radiology 263, 189–98. [DOI] [PubMed] [Google Scholar]
- Walters J, Solomons M, Davies J (2001) Gluteus minimus: observations on its insertion. J Anat 198, 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters BL, Cooper JH, Rodriguez JA (2014) New findings in hip capsular anatomy: dimensions of capsular thickness and pericapsular contributions. Arthroscopy 30, 1235–45. [DOI] [PubMed] [Google Scholar]
- Ward WT, Fleisch I‐D, Ganz R (2000) Anatomy of the iliocapsularis muscle: Relevance to surgery of the hip. Clin Orthop Relat Res 374, 278–285. [DOI] [PubMed] [Google Scholar]
- Wigley CB. (2016) Intergrating cell into tissues In: Gray's anatomy: the anatomical basis of clinical practice (eds. Standing S, Anand N, Birch R.), pp. 1376–1382. New York: Elsevier Limited. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


