Summary
This case report describes an outbreak and novel findings associated with a beta coronavirus (BCoV) infection that occurred on an American Miniature Horse (AMH) breeding farm in upstate New York, in January and February of 2013. Twenty‐nine AMH and one donkey were present on the farm when the outbreak occurred. One 10‐year‐old Quarter Horse mare, stabled at a separate location and owned by an employee of the farm, also tested positive. A polymerase chain reaction (PCR) assay for the detection of BCoV was performed at the Animal Health Diagnostic Center (AHDC) at Cornell on all faecal samples. The PCR assay used detects multiple beta coronaviruses, including, but not limited to, equine enteric coronavirus (ECoV). Novel findings regarding this BCoV infection in horses were recognised in this outbreak study. To the authors’ knowledge, this is the largest outbreak of BCoV described thus far in a closed herd on a single premise. The case fatality rate was 0% unlike that described in a previous outbreak of ECoV involving miniature horses and a miniature donkey (Fielding et al. 2015). The morbidity rate was lower in this outbreak than in previously described studies (Oue et al. 2013; Pusterla et al. 2013). This outbreak also demonstrated the potential for BCoV transmission via farm personnel. The duration of shedding of virus in the faeces among some asymptomatic horses in this outbreak was longer than previously described clinical cases of ECoV (Pusterla et al. 2013; Nemoto et al. 2014). This study suggests that asymptomatic animals may play a role in the maintenance of BCoV during an outbreak; therefore, the need for diagnostic testing of both clinically affected and apparently clinically normal horses on a premises followed by appropriate biosecurity and control measures.
Keywords: horse, diagnosis, clinical signs, beta coronavirus, American Miniature Horse
Introduction
Beta coronavirus (BCoV) was first detected and characterised from a foal with enterocolitis in 1999 (Davis et al. 2000). A more recent prevalence study demonstrated the equal likelihood of isolating ECoV from both gastrointestinal‐diseased and healthy foals (Slovis et al. 2014). As a co‐infecting agent, however, this same study showed a significant association between ECoV and gastrointestinal disease in foals (Slovis et al. 2014). Aside from gastrointestinal disease in foals, ECoV has also been associated with outbreaks in adult horses in racing facilities in Japan and in boarding facilities in the US (Oue et al. 2011, 2013; Pusterla et al. 2013). In these outbreaks of adult horses, the most common clinical signs associated with ECoV infection were anorexia, lethargy, fever and less commonly specific signs of gastrointestinal disease (Oue et al. 2011, 2013; Pusterla et al. 2013; Nemoto et al. 2014). ECoV has also rarely been associated with necrotising enteritis and hyperammonaemic encephalopathy in horses (Fielding et al. 2015; Giannitti et al. 2015). The purpose of this study is to describe an outbreak of BCoV that occurred on an American Miniature Horse (AMH) breeding farm in upstate New York. To the authors’ knowledge, this outbreak of BCoV constitutes the largest one described in a single, closed herd situation. The clinical presentation, faecal PCR results and faecal shedding of the virus for the animals involved in this outbreak are described. Possible transmission of BCoV via human movement from the outbreak site to one horse at a barn off the premises is also described. Aside from the size of this outbreak, several other factors also set it apart from other BCoV outbreaks previously described in the literature including the mortality rate, morbidity rate and duration of faecal shedding (Pusterla et al. 2013; Nemoto et al. 2014; Fielding et al. 2015).
Herd history
The BCoV outbreak reported here occurred at an American Miniature Horse farm in upstate New York from late January to early February of 2013. The outbreak of BCoV on this farm included 29 miniature horses and one donkey ranging in age from 6 months to 15 years (median 5.5 years). The horses were housed in individual pens within a single open barn, and the adult jennet was housed in a separate shed outside, disconnected from the main barn (Fig 1). The pens within the main barn allowed for physical contact between individuals in adjacent pens. The most recent addition to the farm arrived approximately 3 months prior to the onset of this outbreak. There was no recent travel history for any of the animals on the farm.
Figure 1.
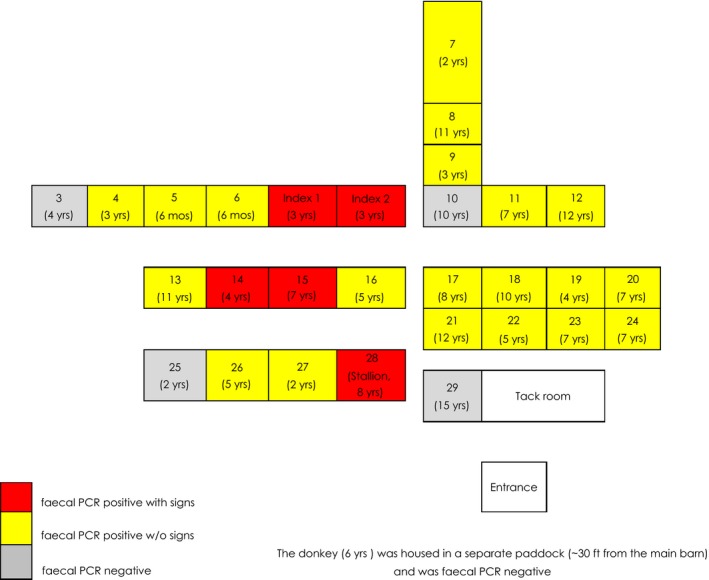
Barn layout demonstrating the location and age of all BCoV faecal PCR‐positive animals (with and without clinical signs) and BCoV faecal PCR‐negative animals.
Clinical findings
The outbreak began with the onset of fever, lethargy and mild colic signs (flank watching, laying down frequently, and anorexia) in two 3‐year‐old mares in adjacent pens (Fig 1, Index Cases 1 and 2). These two cases, beginning simultaneously, were considered the index cases of this outbreak. In total, five horses (all positive for BCoV on faecal PCR and ages ranging from 6 months to 12 years; median 5 years) developed clinical signs. The clinical signs of anorexia, lethargy and fever (ranging from 102.5 to 104.4°F; 39.2 to 40.2°C) were consistent among the five miniature horses who developed clinical illness over the course of the outbreak (Table 1). One infected horse also developed diarrhoea while two other infected horses developed mild colic signs and had decreased faecal production. Clinical signs in four of the five horses were self‐limiting and resolved in 2–5 days with nonsteroidal anti‐inflammatory drug (NSAID), flunixin meglumine, administration by the owner for febrile episodes. Two mares (the index cases) were given antibiotics at the onset of clinical signs, including trimethoprim sulphamethoxazole tablets orally and oxytetracycline intravenously. These treatments were administered prior to the diagnosis of BCoV. An 8‐year‐old stallion also required supportive care on the farm with intravenous fluids due to dehydration associated with diarrhoea. One of the index cases (Index Case 2) continued to be febrile, inappetent, had no manure production, and mild colic signs after 5 days of treatment on the farm and was referred for more intensive care to the Cornell University Equine Hospital (see hospitalised mare section). Once the diagnosis of BCoV infection was made via molecular detection of the virus in the faeces (PCR testing) from the hospitalised horse, faecal samples were collected from both clinically ill and healthy horses and the donkey on the premise for PCR detection of BCoV at various times over a 26‐day period beginning 9 days after the onset of clinical signs. All individuals on the farm had a minimum of one faecal PCR performed and a maximum of six faecal PCR tests for BCoV over that period. EDTA whole blood was also collected from six faecal BCoV PCR positive horses (three affected and three unaffected) and tested for BCoV by PCR. These blood samples were collected on Day 9 after clinical signs began in the index cases; Days 2, 4 and 9 after clinical signs developed in the three affected horses. Complete blood count (CBC) and serum chemistries were also performed on the index case who required hospitalisation.
Table 1.
Clinical and diagnostic (molecular) results from the 30 animals (29 horses and 1 donkey) involved in the BCoV outbreak in Upstate New York
| Number of animals (n = 30) | Percentage of total | |
|---|---|---|
| With any clinical signs | 5 | 17 |
| Anorexia | 5 | 17 |
| Fever | 5 | 17 |
| Colic | 2 | 7 |
| Soft formed faeces/diarrhoea | 1 | 3 |
| No clinical signs detected | 25 | 83 |
| Fatality | 0 | 0 |
| Faecal PCR‐positive sick | 5 | 17 |
| Faecal PCR‐negative sick | 0 | 0 |
| Faecal PCR‐positive normal | 20 | 67 |
| Faecal PCR‐negative normal | 5 | 17 |
| Number of animals (n = 6) | Percentage of total | |
|---|---|---|
| Blood PCR‐positive sick | 3 | 50 |
| Blood PCR‐negative sick | 0 | 0 |
| Blood PCR‐positive normal | 1 | 17 |
| Blood PCR‐negative normal | 2 | 33 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
One faecal sample was also collected for molecular detection of BCoV from a 10‐year‐old Quarter Horse mare who was owned by an employee of this farm, but housed at a separate location with additional horses. This horse developed a fever of unknown origin approximately 1 week after clinical signs were first detected in the two index cases at the primary farm.
Hospitalised mare
On arrival to the Cornell University Equine Hospital, Index Case 2 was lethargic and slightly underconditioned with a body condition score of 4/9. Her mucous membranes were dark red with a toxic line and her capillary refill time was about 3 s. Her sclera appeared mildly icteric and she was tachycardic (60 beats/min). Digital pulses were palpable in both front limbs and all four hooves were warm, but lameness was not noted. Her rectal temperature was 38.1°C (100.6°F). Significant CBC findings included an elevated HCT (47%, normal 34–46%) and low total solids (TS 49 g/L; normal 52–78 g/L) suggestive of both dehydration and protein loss. A leucopenia (4.7 ×109/L; normal 5.5–12.5 ×109/L) characterised by lymphopenia (1.5 ×109/L; normal 1.8–5.0 ×109/L) and neutropenia (2.8 ×109/L; normal 3.0–7.0 ×109/L) was present. Serum chemistry abnormalities included hyperlactataemia (3.56 mmol/L; normal 0.3–1.5 mmol/L), hyponatraemia (126 mmol/L; normal 128–142 mmol/L), hypochloraemia (93 mmol/L; normal 100–111 mmol/L) and elevated aspartate aminotransferase (AST) (834 U/L; normal 100–600 U/L). The mare was treated with supportive care including intravenous fluids (crystalloids and colloids), omeprazole, pentoxifylline and ice boots.
The mare continued to improve over the next few days: her temperature remained normal, clinical pathologic parameters normalised, appetite gradually increased and she finally passed faeces on Day 3 of hospitalisation (8 days after onset of clinical signs). A sample of that faeces was collected and tested positive for beta coronavirus by PCR. For infection control purposes, all animals admitted to Cornell University Equine Hospital are required to have a faecal Salmonella culture performed. This faecal sample was negative for Salmonella by culture. A faecal quantitative floatation was performed, and two strongyle eggs/gram were detected. Targeted diagnostic testing was performed on this case due to economic concerns. For this reason, along with season of the year (January in upstate New York), and the clinical signs present, other enteric pathogen testing was not performed.
Diagnostic test results
The aetiologic agent of the outbreak was determined to be a beta coronavirus based on molecular detection of virus in the faecal sample from the hospitalised mare initially, followed by molecular detection in additional faecal and whole blood samples from other miniature horses on the farm. The PCR assay used will also detect other members of the betacoronavirus family, including bovine enteric coronavirus, canine respiratory coronavirus, human coronavirus OC43 and equine enteric coronavirus. The primers and probe sequences target a conserved region within the nucleocapsid gene and were provided by a third party under a confidentiality agreement. The assay was originally validated using in vitro RNA transcripts containing the target region derived from bovine enteric coronavirus. Analytical sensitivity was determined to be 100 copies with a slope of 3.327 and an efficiency of 99.79%. All samples are run with an exogenous RNA control (encapsulated RNA phage) added at the samples lysis step to monitor for RT‐PCR inhibition.
Once the diagnosis of beta coronavirus was made on the hospitalised index case, faecal samples were collected from the remaining 29 animals at the farm (four of which also had current or previous clinical signs). Molecular diagnostic test results and clinical signs are summarised in Table 1. Fifteen of those animals sampled initially were positive via faecal PCR for BCoV. In total, 25 animals (83%) on the primary premise tested positive over the course of the 4‐week period during which faecal samples were collected. Five (20%) of the 25 animals who tested positive demonstrated clinical signs associated with BCoV, while 20 (80%) of the positive animals remained asymptomatic. No animals displaying clinical signs tested negative for BCoV. EDTA whole blood was also collected from six of the miniature horses that were PCR positive on faeces. These blood samples were collected one time, 9 days after the outbreak began on the farm. Four of these animals were PCR positive for BCoV on whole blood, three of which had previously (n = 1) or currently (n = 2) demonstrated clinical signs. Furthermore, virus isolation (VI) was performed on the EDTA whole blood samples and equine herpesvirus‐2 (EHV‐2) was isolated from two of the horses, one yearling without any clinical signs and one adult stallion with clinical signs. Isolation of this (EHV‐2) virus from EDTA whole blood in conjunction with molecular detection of BCoV in whole blood has not been reported previously and the role that EHV‐2 played in this outbreak, if any, is unclear. BCoV (including ECoV) is not routinely recovered by VI and most studies on ECoV prevalence rely on PCR assays (Pusterla et al. 2013, 2015; Miszczak et al. 2014; Hemida et al. 2017). To the authors’ knowledge, this was the first time that BCoV had been diagnosed in an adult horse in New York State and little was known about its role in disease in adult horses at the time. VI is not a pathogen‐specific testing modality, so it is often performed on whole blood samples from horses with fevers of unknown origin as a means of detecting viraemia due to unknown viral pathogens. VI was performed on the whole blood samples in this outbreak as part of an investigative workup for a fever of unknown origin and also because of the possibility, although unlikely, of isolating BCoV for further characterisation. The faecal sample collected from the farm employee's horse (housed in a separate location, approximately 6 miles from the primary premises) tested positive for BCoV by PCR. No additional samples were collected from this horse, so follow‐up testing was not performed. To the authors’ knowledge, no other horses present at this separate facility (6–8 total horses in the barn) became ill or were tested for BCoV.
Faecal samples were collected intermittently over the next several weeks from all AMH and the donkey on the primary premises. One horse tested positive initially and was still positive 25 days later on faecal PCR. Two other horses demonstrated BCoV shedding in faeces on two samples collected 22 and 18 days apart. There was no correlation seen between beta coronavirus cycle threshold (Ct) values in faecal samples and expression of clinical signs. Follow‐up samples beyond that time period were not collected and all three of these horses remained asymptomatic throughout the entire duration of the outbreak.
Discussion
The lack of mortality in this BCoV outbreak is in contrast to the case fatality rates reported in some previous outbreaks involving adult horses (Oue et al. 2011, 2013; Pusterla et al. 2013; Fielding et al. 2015). Previous studies have demonstrated that ECoV is a very common agent present in healthy foals <20 weeks of age (Slovis et al. 2014). For this reason, it has been hypothesised that there may be a protective effect of breeding farms against ECoV due to constant exposure of the mares to ECoV shed by the foals. This farm was not bringing in outside mares to be bred to their stallion. The stallion was only used for breeding the mares on this farm and the farm generally produced up to 6 foals per year. All mares and geldings were turned out together for exercise, so all had contact with each other daily and the stallion was turned out separately. Perhaps, the presence of foals on this farm helps explain the lack of mortality and the low morbidity seen in this outbreak compared with that described elsewhere. The exposure of adult horses to foals, could however, also explain the source of this outbreak as the two index cases were on one side of the barn, adjacent to the only two weanlings (6 months old) in the barn, who also tested positive for BCoV but never developed clinical illness. Ventilation of the barn, alone, does not offer much insight to disease spread of aerosolised viral particles as the barn had upward ventilation throughout. ECoV has been described in one outbreak among adult miniature horses and a miniature donkey originating from a single competition (Fielding et al. 2015). The case fatality rate of this outbreak was 27% among those animals who tested positive for ECoV in their faeces (Fielding et al. 2015). Other prior outbreaks have published case fatality rates ranging from 0 to 7% (Oue et al. 2011, 2013; Pusterla et al. 2013).
The published durations of faecal shedding of ECoV has ranged from 3 to 14 days among outbreaks and experimental infections in adult horses (Nemoto et al. 2014; Fielding et al. 2015). Over the course of this outbreak, BCoV PCR was performed on faecal samples from each animal as few as one time and as many as six times. The prolonged shedding periods of at least 25, 22 and 18 days duration occurred in three horses who failed to demonstrate any associated clinical signs throughout the outbreak period. To the authors’ knowledge, these durations of faecal detection are longer than previously described in animals lacking clinical signs of illness. In contrast, a European paper described one horse who shed ECoV in faeces for 35 days but subsequently died with severe diarrhoea (Miszczak et al. 2014). Similarly, faecal samples from a 28‐year‐old horse, submitted to the Animal Health Diagnostic Center (AHDC) at Cornell for BCoV faecal PCR testing, were positive at Days 1, 28, 63 and 99 after onset of disease. This horse originally demonstrated clinical signs of a fever 40°C (104°F), mild colic signs, lethargy, anorexia and developed soft‐formed faeces. Clinical illness lasted for a total of 7–10 days (A. Glaser, personal communication 2014). This information suggests that a potential for prolonged shedding, longer than the previously published 14 days, should be considered in any biosecurity plan following BCoV infection at a facility.
Horse 13 (Fig 1) was the most recent addition to this farm. This gelding was brought to the farm approximately 3 months prior to the onset of the outbreak. In this outbreak, Horse 13 tested positive for BCoV on faecal PCR during the outbreak but remained asymptomatic. While this may represent another possible source of this outbreak, it is difficult to explain why clinical disease would not have been seen sooner in the index cases if the new horse was shedding beta coronavirus upon arrival, as the incubation period for ECoV has been demonstrated to be 2–4 days post inoculation (Nemoto et al. 2014). If this horse was the source of the outbreak, it would represent a very prolonged shedding period (which is possible, as mentioned above) or else intermittent shedding of BCoV, which, to the authors’ knowledge, has not yet been documented.
An association has been demonstrated between viral load and mortality in ECoV outbreaks (Fielding et al. 2015). Although viral load was not measured in this outbreak, there was no correlation detected between beta coronavirus Ct values in faecal samples and expression of clinical signs, so it is unlikely that differences in viral load were solely responsible for the differences seen in disease expression in this outbreak. Other factors affecting mortality may include strain differences as well as environmental and host characteristics. The miniature horses of this report were all in excellent lean body condition. Such ideal condition may have offered protection from the common secondary complication, hepatic lipidosis, that may plague sick, inappetent miniature horses. The one animal which required care at a referral veterinary hospital had been treated for 5 days on the farm with nonsteroidal anti‐inflammatory medications, perhaps contributing significantly to the prolongation of clinical signs. Previously described outbreaks of ECoV demonstrated morbidity rates ranging from 20 to 67% (Pusterla et al. 2013; Fielding et al. 2015). The morbidity rate in this BCoV outbreak was only 17% (5 of 30 horses showing clinical signs), with 80% of the positive animals in this outbreak remaining asymptomatic. This is in contrast to only 33% asymptomatic, positive animals in a previously described ECoV outbreak among a similar population (Fielding et al. 2015). As mentioned previously, this discrepancy could also be explained by the hypothesis that exposure of adult horses to BCoV from foals may offer some protection against morbidity and mortality associated with this virus. This also suggests that there may be a greater likelihood than previously suspected of asymptomatic animals perpetuating an outbreak of BCoV. At the time of this outbreak, there was no equine‐specific antibody test available to detect horses with subclinical or previous ECoV infections. Currently, there is an ECoV S protein‐based ELISA that would have been useful to further characterise the role of asymptomatic shedders in this BCoV outbreak (Kooijman et al. 2016).
This outbreak also suggests that people can play a role in transmission of BCoV among horses. The horse who was housed at a separate facility, but owned by an employee of the primary premises, developed clinical signs compatible with BCoV and tested positive for BCoV on faecal PCR. Although an unrelated BCoV transmission and infection could have occurred on the second farm, a more likely explanation for the source of transmission was the farm employee as there were no other common contacts shared between the two farms. This highlights the need for stringent biosecurity efforts, including the limit of human movement to other farms during a BCoV outbreak.
Our findings suggest all individuals at risk for disease should be tested, not just those demonstrating clinical signs, prior to lifting quarantine measures. Further work is necessary to establish guidelines for BCoV quarantine timeframes as animals both with and without obvious clinical signs may shed virus for prolonged periods of time.
Authors’ declaration of interests
No conflicts of interest have been declared.
Ethical animal research
This investigation was focused on studying the animals affected by an outbreak of equine coronavirus. It involved only client‐owned animals. Client confidentiality was maintained and consent from the owner for all diagnostic testing was provided at the time of the investigation.
Sources of funding
The Animal Health Diagnostic Center at Cornell provided funding for the majority of the diagnostic testing involved with this outbreak investigation.
Authorship
E. Goodrich contributed to the study design, study execution, data analysis and interpretation and preparation of the manuscript. L. Mittel and S. Ness contributed to the study design and study execution. A. Glaser provided expertise regarding the molecular assay used in this study. R. Radcliffe contributed to the preparation of the manuscript. T. Divers contributed to the study design, study execution and preparation of the manuscript. All authors gave their final approval of the manuscript.
Acknowledgements
We thank Dr Edward Dubovi of the Animal Health Diagnostic Center, Cornell University, for providing technical expertise associated with the BCoV diagnostic testing.
References
- Davis, E. , Rush, B.R. , Cox, J. , DeBey, B. and Kapil, S. (2000) Neonatal enterocolitis associated with coronavirus infection in a foal: a case report. J. Vet. Diagn. Invest. 12, 153‐156. [DOI] [PubMed] [Google Scholar]
- Fielding, C.L. , Higgins, J.K. , Higgins, J.C. , McIntosh, S. , Scott, E. , Giannitti, F. and Pusterla, N. (2015) Disease associated with equine coronavirus infection and high case fatality rate. J. Vet. Intern. Med. 29, 307‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitti, F. , Diab, S. , Mete, A. , Stanton, J.B. , Fielding, L. , Crossley, B. , Sverlow, K. , Fish, S. , Mapes, S. , Scott, L. and Pusterla, N. (2015) Necrotizing enteritis and hyperammonemic encephalopathy associated with equine coronavirus infection in equids. Vet. Pathol. 52, 1148‐1156. [DOI] [PubMed] [Google Scholar]
- Hemida, M.G. , Chu, D.K.W. , Perera, R.A.P.M. , Ko, R.L.W. , So, R.T.Y. , Ng, B.C.Y. , Chan, S.M.S. , Chu, S. , Alnaeem, A.A. , Alhammadi, M.A. , Webby, R.J. , Poon, L.L.M. , Balasuriya, U.B.R. and Peiris, M. (2017) Coronavirus infections in horses in Saudi Arabia and Oman. Transbound Emerg. Dis. 64, 2093‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman, L.J. , Mapes, S.M. and Pusterla, N. (2016) Development of an equine coronavirus‐specific enzyme‐linked immunosorbent assay to determine serologic responses in naturally infected horses. J. Vet. Diagn. Invest. 28, 414‐418. [DOI] [PubMed] [Google Scholar]
- Miszczak, F. , Tesson, V. , Kin, N. , Dina, J. , Balasuriya, U.B.R. , Pronost, S. and Vabret, A. (2014) First detection of equine coronavirus (BCoV) in Europe. Vet. Microbiol. 171, 206‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto, M. , Oue, Y. , Morita, Y. , Kanno, T. , Kinoshita, Y. , Niwa, H. , Ueno, T. , Katayama, Y. , Bannai, H. , Tsujimura, K. , Yamanaka, T. and Kondo, T. (2014) Experimental inoculation of equine coronavirus into Japanese draft horses. Arch. Virol. 159, 3329‐3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oue, Y. , Ishihara, R. , Edamatsu, H. , Morita, Y. , Yoshida, M. , Yoshima, M. , Hatama, S. , Murakami, K. and Kanno, T. (2011) Isolation of an equine coronavirus from adult horses with pyrogenic and enteric disease and its antigenic and genomic characterization in comparison with the NC99 strain. Vet. Microbiol. 150, 41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oue, Y. , Morita, Y. , Kondo, T. and Nemoto, M. (2013) Epidemic of equine coronavirus at Obihiro Racecourse, Hokkaido, Japan in 2012. J. Vet. Med. Sci. 75, 1261‐1265. [DOI] [PubMed] [Google Scholar]
- Pusterla, N. , Mapes, S. , Wademan, C. , White, A. , Ball, R. , Sapp, K. , Burns, P. , Ormond, C. , Butterworth, K. , Bartol, J. and Magdesian, K.G. (2013) Emerging outbreaks associated with equine coronavirus in adult horses. Vet. Microbiol. 162, 228‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusterla, N. , Holzenkaempfer, N. , Mapes, S. and Kass, P. (2015) Prevalence of equine coronavirus in nasal secretions from horses with fever and upper respiratory tract infection. Vet. Rec. 177, 289. [DOI] [PubMed] [Google Scholar]
- Slovis, N.M. , Elam, J. , Estrada, M. and Leutenegger, C.M. (2014) Infectious agents associated with diarrhea in neonatal foals in central Kentucky: a comprehensive molecular study. Equine Vet. J. 46, 311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]


