Abstract
Selected disulfide bonds in membrane proteins are labile and are thus susceptible to changes in redox potential and/or the presence of thiol isomerase enzymes. Modification of these disulfide bonds can lead to conformational changes of the protein that in turn may alter protein activity and function. This occurs in the entry of several enveloped viruses into their host cells, e.g. HIV, hepatitis C virus and Newcastle disease virus. Labile disulfide bonds are also important in platelet activation, cytokine signalling and in a variety of diseases including cancer and arthritis. In this review we will concentrate on recent advances in understanding the conditions that lead to disulfide bond reduction in membrane proteins and their effects in regulating immune function.
Keywords: Disulfide redox, Immunoregulation, Membrane protein, Virus fusion
Labile disulfide bonds
A role in regulating membrane protein function
Cysteine is a unique aa in that it contains a sulfydryl group (–SH) that can covalently bond with a sulfydryl group of a separate cysteine residue to form a disulfide bond. Disulfide bonds can form between cysteines within the same polypeptide chain (intramolecular disulfide bond) or between cysteines on different polypeptide chains (intermolecular disulfide bond). The formation of disulfide bonds requires oxidation of the constituent cysteine residues. Once formed disulfide bonds are not chemically inert, they can be reduced back to their constituent cysteine residues. Although disulfide bonds are clearly important structurally, there is increasing evidence that they can be reduced under certain physiological conditions and that this can affect the activity of the protein itself with downstream functional consequences. In this review we discuss how these redox events may be important in regulating the immune system and we review recent data on the characterisation of membrane proteins that contain labile disulfide bonds.
Structure and modification
The disulfide bonds formed between cysteine residues in proteins play an important structural role such as stabilising Ig‐like domains in the harsh extracellular environment by bridging the beta sheets at the core of the fold, stabilising dimer formation for example, bridging between the light and heavy chains of Ig. Some disulfide bonds have a catalytic role notably in thioredoxin and protein disulfide isomerases (PDIs). In addition other disulfide bonds can be reduced and these can lead to changes in protein structure and have been termed ‘allosteric’. The disulfide bonds themselves are heterogeneous in geometry and this is discussed in detail by Schmidt et al. 1 who studied the geometry of about 7000 disulfide bonds from known structures. In this review we use term labile to describe those disulfide bonds that can be reduced under physiological conditions. It seems likely that they will cause conformational changes but, in the absence of such data, we use the simple term ‘labile’ meaning easy to break. Recent screening studies have shown that a surprisingly large number of different membrane proteins have labile disulfide bonds that can be reduced by mild reducing conditions in vitro and that many are reduced in vivo during inflammation 2. The proteins identified include integrins, adhesion proteins, cytokine and chemokine receptors, Ag receptors and transporters.
PDI is an enzyme involved in catalysing disulfide bond formation, reduction and isomerization 3, 4, 5. As a member of the thioredoxin superfamily, it contains regions with high aa sequence identity to the thioredoxin active site, which is comprised of a double‐cysteine motif (CXXC, C refers to cysteine and X any aa). Disulfide bond reduction by PDI and thioredoxin superfamily members is a catalytic process where one molecule of enzyme can reduce many disulfide bonds. However co‐enzymes such as thioredoxin reductase are needed to supply electrons to PDI to allow continuous turnover. This is controlled by the NADPH pathway 6.
Reducing conditions at the cell surface and extracellularly
The cytoplasm of cells is maintained under reducing conditions that are highly controlled, for example, in disease situations and in processes such as the oxidative burst. This area is beyond the scope of this review and is extensively reviewed elsewhere 7. The extracellular space is generally oxidising but decreases in redox potential occur in inflammation and immune activation. For example, activation of DCs leads to the production of free extracellular cysteine 8, in vivo immunisation leads to increased free thiol production and induction of thioredoxin expression in lymphoid organs 9 and levels of extracellular thioredoxin are increased in lung injury 10. Enzymes such as the PDI members are generally associated with the ER where they assist in the proper folding of proteins but they are also found in other locations such as the cell surface, the extracellular space, the cytosol and the nucleus 4. Localisation of PDI and related proteins on the cell surface or the extracellular space can cause the reduction of labile disulfide bonds in membrane proteins, which may be important for viral infection, disease or cell signalling 4, 5. Thus the presence of thioredoxin and PDI provides the mechanism to alter disulfide bonds in membrane proteins as discussed later in a variety of different situations.
Viruses
HIV
Enveloped viruses infect host cells by firstly binding to specific host cell receptors and then fusing with the host cell membrane. Virus‐cell membrane fusion is a consequence of conformational changes in the envelope (ENV) proteins. These changes can be triggered by acidification, as in influenza virus infection 11, or by rearrangement of disulfide bonds by PDI family members as shown in HIV and other viruses such as, HCV, Newcastle disease virus and rotavirus as discussed in this and subsequent sections.
HIV is a major human pathogen that causes Acquired Immune Deficient Syndrome (AIDS). During HIV entry into the host cell, HIV ENV gp 120 (gp120) binds the CD4 receptor on T cells or macrophages 12. HIV uptake can be blocked by preventing disulfide bond reduction by several agents including the PDI inhibitor bacitracin, the membrane‐impermeable sulfhydryl reagent 5,5’‐dithiobis (2‐nitrobenzoic acid) (DTNB) and Abs against PDI (reviewed in 13). Various disulfide bonds in gp120 have been implicated as being labile using thioredoxin, PDI and glutaredoxin 14, 15, 16, 17. A recent comparison of PDI, thioredoxin and DTT indicates reduction to some extent of five disulfide bonds in gp120 18, 19, and complexes of PDI and gp120 have been identified 16. It is proposed that the conformational change that occurs due to disulfide bond reduction leads to structural changes in gp41 which, in turn lead to the fusion of the viral envelope with the host cell 20.
CD4, the receptor for HIV, also contains a labile disulfide bond in domain 2 of the four extracellular Ig‐like domains 21. There is evidence that CD4 may exist as disulfide‐linked multimers on the T‐ and myeloid‐cell surfaces and that multimer formation increases upon activation of a myeloid cell line with PMA 22. It is proposed that HIV has a preference for binding to the monomeric, reduced form of CD4 23. Interestingly thioredoxin but not PDI was effective in reducing the labile disulfide bond of CD4 whereas PDI is clearly involved in HIV uptake 13. The role of disulfide exchange in the various stages of HIV uptake and shedding together with possibilities for therapy has been extensively studied and is reviewed in 13.
HCV
HCV, which primarily infects the liver, is the causative agent of hepatitis C. In severe cases patients develop chronic liver infections and subsequent liver cirrhosis, carcinoma, hepatitis and liver failure 24. Entry of HCV is a slow and complex multi‐step process that involves initial attachment of the virion to the host cell, interaction of the envelope proteins with the host cell surface receptors and subsequent internalisation by membrane fusion (reviewed in 25). The two viral envelope proteins E1 and E2 are expressed as inactive precursors 26 which associate as large covalent complexes 27. The two envelope proteins E1 and E2, contain 8 and 18 cysteine residues, respectively 28. Alkyl‐ation of free cysteine residues on viral particles containing E1 and E2 has been shown to block the uptake of the virus 28. This process, however, could not be rescued by addition of reducing agents as is the case for other retroviruses 28. Recent mutagenesis studies on E2 showed that all nine disulfide pairs were strictly required for infection. The role of cysteines is complex as muta‐genesis of individual cysteines had differential effects on assembly with E1 and binding to the tetraspanin membrane protein CD81 29.
Newcastle disease virus
Newcastle disease virus infection is extremely rare in humans and usually only occurs in people in close contact with infected birds. Entry into the host cell is mediated by viral haemagglutinin neur‐aminidase (HN) and fusion (F) gp attachment to the host cell. Free thiols in the F gp, which are required for virus entry into the cell 30, are only present after binding of the virus to the target cell surface 31. This suggests that labile disulfide bonds are reduced before major conformational changes in the F protein occur and before activation by HN. How the appearance of free thiols influences F protein activation or conformational changes is as yet unknown 30. The enzyme responsible for catalysing the reduction of disulfide bonds in F protein is likely to be PDI due to the observation that membrane‐impermeable inhibitors of PDI (DTNB, bacitracin and anti‐PDI Ab) could suppress the formation of free thiols and inhibit virus entry 30. Further support for this is the observation that over‐expression of PDI resulted in significantly increased cell–cell fusion mediated by F and HN proteins 32.
Other viruses
Labile disulfide bonds are likely to be important in other viruses. For instance in the rotaviruses that are a major cause of diarrhoea, in addition to proteolytic cleavage of the surface proteins, labile disulfide bonds are implicated in virus entry by the blocking of virus uptake by reagents such as DTNB, bacitracin and Abs to PDI 33. DTNB also inhibited the uptake of the Sindbis alphavirus that causes fever in humans and, interestingly, virus fusion could be enhanced by exogenous reducing agent 34. Although the SARS (severe acute respiratory syndrome) coronavirus contains free cysteine and labile disulfides, cysteine blocking reagents such as DTNB and bacitracin had no effect on virus uptake 35.
The extracellular redox state affects immune regulation, disease and clotting
Immune regulation
A reducing environment is important in the generation of immune responses as naive T cells do not express a cystine transporter and require a source of extracellular cysteine. XC – is a major transporter for cystine that is comprised of a common CD98 chain and a specific chain for cysteine, xCT. The DCs provide the source of cysteine for T cells. Although it was originally thought that, on activation, DCs secrete thioredoxin that in turn can reduce cystine to cysteine (Fig. 1) 8, further analysis suggests this is not the main cysteine source for T cells 36. Blocking the XC – transporter, for example, prevents cysteine accumulation in DCs indicating the importance of cystine uptake by the DCs and secretion of cysteine 36. When Tregs act on DCs, this process is inhibited 36. This inhibition depends on cell contact between the DC and the Treg and the modulation of glutathione metabolism in the DC 37.
Figure 1.
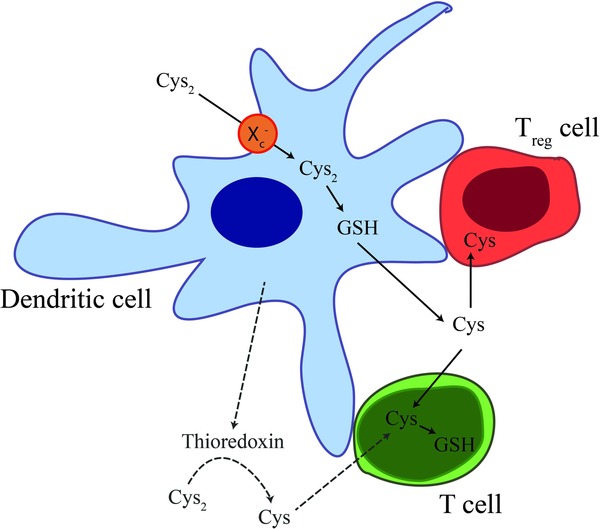
Tregs can limit redox changes. Resting T cells require a source of extracellular cysteine (Cys) as they lack the cystine (Cys2) transporter XC – 8, 36, 37. This transporter is, however, expressed by DCs meaning that these cells can generate glutathione (GSH) (cystine is rate‐limiting for glutathione production); this, in turn, leads to the secretion of Cys that can be utilised by T cells. Thioredoxin secretion from DCs can be induced by activation but in the absence of a source of electrons, which are required for conversion of cysteine from cystine by thioredoxin, may not be a major source of extracellular Cys for T cells. The interaction of Tregs with DCs leads to inhibition of extracellular Cys 37.
The importance of the cystine transporter system for the immune system is illustrated in the gut, where lamina propria macrophages in mice do not normally express the XC – transporter which is, in contrast, expressed in peripheral blood monocytes. Gut lamina propria macrophages are therefore unable to take up cystine and hence cannot provide cysteine for the neighbouring T cells 38. This may be important for maintaining an environment in the healthy gut that is more tolerant than that found in inflammatory bowel disease, a disease state in which there is increased expression of the XC – transporter and higher T‐cell activity 38.
In addition to the metabolic effects of cystine transport and reduction, the reducing environment has effects on membrane and secreted proteins. This is illustrated by the breadth of membrane proteins on leukocytes that have labile disulfide bonds that are susceptible to the reducing conditions that are typical of both T‐cell activation and innate activation in an LPS model of inflammation 2. In one case a labile disulfide bond was identified in CD132 (see Fig. 2), the common gamma chain of several cytokine receptors, which on reduction prevented IL‐2 signalling 39. Signalling of other cytokine receptors seems likely to be affected 40 with possible additional effects on cytokines as well as IL‐4 has a labile disulfide that leads to loss of activity upon reduction 41. The implication is that the effects of the pro‐inflammatory cytokines may be ameliorated by the redox changes resulting from the inflammatory response itself – a feedback control mechanism. It seems likely that other cytokine/chemokine interactions are affected by redox conditions. For example, thioredoxin affects the chemokine‐induced chemotaxis of eosinophils although the molecular basis for this is unclear 42. A different type of cytokine is the high mobility group box 1 (HMGB1) that is both a nuclear protein that regulates transcription and a secreted protein demonstrating extracellular inflammatory cytokine activity. The latter activity is dependent on one free cysteine and a disulfide bond that can be reduced by mild reducing conditions 43.
Figure 2.
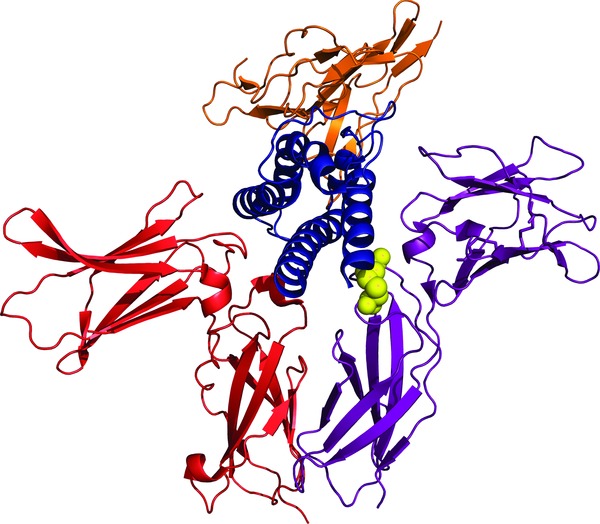
One of the disulfide bonds in CD132 is labile and lies at the interface with IL‐2. Crystal structure of the IL‐2/IL‐2 receptor complex (PDB code 2ERJ). The labile disulfide bond (yellow spheres) in CD132 (purple) is in direct contact with the cytokine, IL‐2 (blue). The IL‐2 receptor alpha chain (CD25) is shown in orange and the beta chain (CD122) is shown in red.
Redox changes, such as secretion of thioredoxin, occur to balance ROS production. For example, the induction of IL‐1β processing in monocytes involves firstly an ROS response and then an anti‐oxidant response but studies with inhibitors show that both are necessary for production of IL‐1β 44. As the effects of ROS on the immune system have recently been reviewed 45, this shall not be discussed further here.
Cancer
An important aspect of cancer resistance to chemotherapy is the up‐regulation of anti‐oxidant systems such as the cysteine/cystine redox cycle, PDI, thioredoxin, glutathione 46 and the XC – cystine transporter 47. A possible link to the cell surface is indicated by a CD44 variant (CD44v) that interacts with the xCT component of the cysteine transporter; ablation of CD44v leads to inhibition of xCT and subsequent suppression of tumour growth 48.
The analysis of differential expression of proteins in invasive glioma cells and angiogenic glioma cells revealed that PDI is over‐expressed in the invasive phenotype 49. Interestingly, PDI was found in the tumour periphery but not in the angiogenic core. This led to the hypothesis that PDI has a functional role in glioma cell migration, which is supported by the observation that tumour cell migration is inhibited by bacitracin, a non‐selective PDI inhibitor, and by a PDI Ab 49. PDI can reduce disulfide bonds in many proteins, but one class of particular interest with respect to cancer is the metalloproteinase family of enzymes that can modulate the extracellular environment and is important in metastasis; PDI had recently been shown to regulate the activity and secretion of MMP‐9 50. A key element in these enzymes is that the catalytic site contains a Zn ion coordinated to histidine residues and one cysteine; the site is in an inactive state until the cysteine coordination is broken – the ‘Cys Switch’ – by changes in the conformation of the protein that exposes the Zn ion to solvent 51, 52. The potential for redox involvement in the immune regulation of cancer is large as illustrated by recent data indicating that chronic lymphocytic leukemia cells have high levels of surface PDI and thioredoxin that are associated with receptors for TNF, and blocking PDI and thioredoxin activity inhibits the production of the autocrine TNF 53.
Rheumatoid arthritis
Rheumatoid arthritis is a chronic inflammatory disease that is triggered by both environmental and genetic factors. A link between rheumatoid arthritis regulation and the ROS system is provided by the finding that neutrophil cytosolic factor 1 (Ncf1) is a regulator of rheumatoid arthritis 54. Ncf1, is a component of the NADPH oxidase complex which catalyses the reduction of oxygen to ROS (reviewed in 55, 56, 57). With regard to membrane proteins in particular, a decreased level of ROS has been shown to increase the number of reduced thiol groups (–SH) on the T‐cell surface 58. This resulted in increased activation and proliferation of T cells, as well as arthritis incidence and severity 58.
Platelet activation is associated with redox changes at the cell surface
Platelets are involved in haemostasis, wound healing and atherosclerosis. There are extensive data to suggest that platelet activation is associated with an increase in the extracellular redox potential and several PDI family members come to the cell surface 59, 60, 61. A key protein that can be modulated by reduction is the fibrinogen receptor, integrin αIIbβ3. This is the most abundant integrin on the platelet surface and its activation leads to increased binding of adhesive ligands such as fibrinogen, fibronectin and von Willebrand factor which in turn promote thrombus formation 59. The β subunit of αIIbβ3 contains 56 highly conserved cysteine residues which all form disulfide bonds 62. Interestingly, the active state of αIIbβ3 seems to be attained by the reduction of cysteines within the EGF‐like domains αIIbβ3 63. Mutagenesis of a single disulfide in the β3 chain leads to constitutively active αIIbβ3 64 whereas a more extensive mutagenesis of specific disulfide bonds in β3 gave differential affects on the function of αIIb and αV integrins 65. Integrins are unusual in containing disulfide bonds not only in the extracellular domains but also in their intracellular part and mutagenesis of the latter can also affect ligand binding 66. The binding capacity of integrins can be modulated by various stimuli and the underlying concept is that the disulfide bonds maintain the integrin in a less active form (Fig. 3). This is evidenced by the finding that cysteine mutations and the addition of reducing agents result in increased ligand binding 67, 68, 69 and, more recently, a PDI inhibitor was shown to block thrombus formation 70. In addition to the fibrinogen receptor integrin αIIbβ3, other platelet surface proteins are known to have increased levels of free thiols, such as the P2Y12 ADP receptor, gp Ib adhesion receptor α (GPIbα) and the gp VI collagen receptor (GPVI) on platelet activation 59. Thus, redox changes at the surface of platelets seem likely to be important in regulating clotting.
Figure 3.
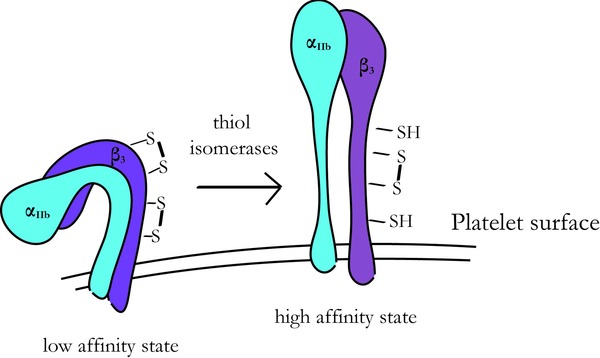
Scheme showing the redox regulation of the αIIbβ3 integrin on platelets. The resting state of αIIbβ3 has a low affinity for fibrin‐ogen, αIIbβ3 being present in its oxidized state. The high affinity state is reached by reducing disulfide bonds on αIIbβ3.
Concluding remarks
Although the role of disulfide bonds in stabilising protein structures is well known, we have only recently started to understand the role of redox labile disulfide bonds. By changing from an oxidized to a reduced state, these bonds can control protein function. Labile disulfide bonds in membrane proteins play a key role in a number of viral infections, as well as in rheumatoid arthritis, cancer, platelet activation and cytokine signalling. Given the large numbers of membrane proteins with labile disulfides, it is likely that they may be important in immune responses, a variety of diseases and inflammation. These molecular mechanisms could be important for therapy based on four types of strategy: (i) prevention of the reduction of labile disulfide bonds by Abs that block PDI access to specific targets; (ii) the use of small molecular inhibitors or Abs to target specific PDIs; (iii) targeting the pathways that lead to activation of the cell surface PDIs; and (iv) targeting the transporters involved in the redox system.
Conflict of interest
The authors declare no financial or commercial conflicts of interest.
Abbreviations
- DTNB
5,5’‐dithiobis (2‐nitrobenzoic acid)
- HN
haem‐agglutinin neuraminidase
- PDI
protein disulfide isomerase
Acknowledgements
This work was supported by the Medical Research Council (reference G9826026). MS was supported by a Scatcherd European Scholarship. We are grateful for helpful comments from Marion H. Brown and Lisa‐Marie Holbrook.
References
- 1. Schmidt, B. , Ho, L. and Hogg, P. J. , Allosteric disulfide bonds. Biochemistry 2006. 45: 7429–7433. [DOI] [PubMed] [Google Scholar]
- 2. Metcalfe, C. , Cresswell, P. , Ciaccia, L. , Thomas, B. and Barclay, A. N. , Labile disulfide bonds are common at the leucocyte cell surface. Open Biol. 2011. 1: 110010–110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freedman, R. B. , Hirst, T. R. and Tuite, M. F. , Protein disulphide isomerase: building bridges in protein folding. Trends Biochem. Sci. 1994. 19: 331–336. [DOI] [PubMed] [Google Scholar]
- 4. Benham, A. M. , The protein disulfide isomerase family: key players in health and disease. Antioxid. Redox Signal. 2012. 16: 781–789. [DOI] [PubMed] [Google Scholar]
- 5. Hatahet, F. and Ruddock, L. W. , Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid. Redox Signal. 2009. 11: 2807–2850. [DOI] [PubMed] [Google Scholar]
- 6. Arner, E. S. and Holmgren, A. , Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000. 267: 6102–6109. [DOI] [PubMed] [Google Scholar]
- 7. Kemp, M. , Go, Y. M. and Jones, D. P. , Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic. Biol. Med. 2008. 44: 921–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angelini, G. , Gardella, S. , Ardy, M. , Ciriolo, M. R. , Filomeni, G. , Di Trapani, G. , Clarke, F. et al., Antigen‐presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. USA 2002. 99: 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castellani, P. , Angelini, G. , Delfino, L. , Matucci, A. and Rubartelli, A. , The thiol redox state of lymphoid organs is modified by immunization: role of different immune cell populations. Eur. J. Immunol. 2008. 38: 2419–2425. [DOI] [PubMed] [Google Scholar]
- 10. Callister, M. E. , Burke‐Gaffney, A. , Quinlan, G. J. , Nicholson, A. G. , Florio, R. , Nakamura, H. , Yodoi, J. et al., Extracellular thioredoxin levels are increased in patients with acute lung injury. Thorax 2006. 61: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Colman, P. M. and Lawrence, M. C. , The structural biology of type I viral membrane fusion. Nature Rev. Mol. Cell Biol. 2003. 4: 309–319. [DOI] [PubMed] [Google Scholar]
- 12. Wyatt, R. and Sodroski, J. , The HIV‐1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 1998. 280: 1884–1888. [DOI] [PubMed] [Google Scholar]
- 13. Fenouillet, E. , Barbouche, R. and Jones, I. M. , Cell entry by enveloped viruses: redox considerations for HIV and SARS‐coronavirus. Antioxid. Redox Signal. 2007. 9: 1009–1034. [DOI] [PubMed] [Google Scholar]
- 14. Azimi, I. , Matthias, L. J. , Center, R. J. , Wong, J. W. H. and Hogg, P. J. , Disulfide bond that constrains the HIV‐1 gp120 V3 domain is cleaved by thioredoxin. J. Biol. Chem. 2010. 285: 40072–40080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Go, E. P. , Zhang, Y. , Menon, S. and Desaire, H. , Analysis of the disulfide bond arrangement of the HIV‐1 envelope protein CON‐S gp140 DeltaCFI shows variability in the V1 and V2 regions. J. Prot. Res. 2011. 10: 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ou, W. and Silver, J. , Role of protein disulfide isomerase and other thiol‐reactive proteins in HIV‐1 envelope protein‐mediated fusion. Virology 2006. 350: 406–417. [DOI] [PubMed] [Google Scholar]
- 17. Auwerx, J. , Isacsson, O. , S√∂derlund, J. , Balzarini, J. , Johansson, M. and Lundberg, M. , Human glutaredoxin‐1 catalyzes the reduction of HIV‐1 gp120 and CD4 disulfides and its inhibition reduces HIV‐1 replication. Int. J. Biochem. Cell Biol. 2009. 41: 1269–1275. [DOI] [PubMed] [Google Scholar]
- 18. Reiser, K. , Francois, K. O. , Schols, D. , Bergman, T. , Jornvall, H. , Balzarini, J. , Karlsson, A. et al., Thioredoxin‐1 and protein disulfide isomerase catalyze the reduction of similar disulfides in HIV gp120. Int. J. Biochem. Cell Biol. 2012. 44: 556–562. [DOI] [PubMed] [Google Scholar]
- 19. Papandreou, M. J. , Barbouche, R. , Guieu, R. , Rivera, S. , Fantini, J. , Khrestchatisky, M. , Jones, I. M. et al., Mapping of domains on HIV envelope protein mediating association with calnexin and protein‐disulfide isomerase. J. Biol. Chem. 2010. 285: 13788–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ashkenazi, A. , Viard, M. , Wexler‐Cohen, Y. , Blumenthal, R. and Shai, Y. , Viral envelope protein folding and membrane hemifusion are enhanced by the conserved loop region of HIV‐1 gp41. FASEB J. 2011. 25: 2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthias, L. J. , Yam, P. T. W. , Jiang, X.‐M. , Vandegraaff, N. , Li, P. , Poumbourios, P. , Donoghue, N. et al., Disulfide exchange in domain 2 of CD4 is required for entry of HIV‐1. Nat. Immunol. 2002. 3: 727–732. [DOI] [PubMed] [Google Scholar]
- 22. Lynch, G. W. , Sloane, A. J. , Raso, V. , Lai, A. and Cunningham, A. L. , Direct evidence for native CD4 oligomers in lymphoid and monocytoid cells. Eur. J. Immunol. 1999. 29: 2590–2602. [DOI] [PubMed] [Google Scholar]
- 23. Matthias, L. J. , Azimi, I. , Tabrett, C. A. and Hogg, P. J. , Reduced monomeric CD4 is the preferred receptor for HIV. J. Biol. Chem. 2010. 285: 40793–40799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoofnagle, J. H. , Course and outcome of hepatitis C. Hepatology 2002. 36: S21–S29. [DOI] [PubMed] [Google Scholar]
- 25. Zeisel, M. B. , Barth, H. , Schuster, C. and Baumert, T. F. , Hepatitis C virus entry: molecular mechanisms and targets for antiviral therapy. Front. Biosci. 2009. 14: 3274–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartosch, B. and Cosset, F. L. , Cell entry of hepatitis C virus. Virology 2006. 348: 1–12. [DOI] [PubMed] [Google Scholar]
- 27. Vieyres, G. , Thomas, X. , Descamps, V. R. , Duverlie, G. , Patel, A. H. and Dubuisson, J. , Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J. Virol. 2010. 84: 10159–10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fraser, J. , Boo, I. , Poumbourios, P. and Drummer, H. E. , Hepatitis C virus (HCV) envelope glycoproteins E1 and E2 contain reduced cysteine residues essential for virus entry. J. Biol. Chem. 2011. 286: 31984–31992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCaffrey, K. , Boo, I. , Tewierek, K. , Edmunds, M. L. , Poumbourios, P. and Drummer, H. E. , Role of conserved cysteine residues in hepatitis C virus glycoprotein e2 folding and function. J. Virol. 2012. 86: 3961–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jain, S. , McGinnes, L. W. and Morrison, T. G. , Thiol/disulfide exchange is required for membrane fusion directed by the Newcastle disease virus fusion protein. J. Virol. 2007. 81: 2328–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jain, S. , McGinnes, L. W. and Morrison, T. G. , Role of thiol/disulfide exchange in newcastle disease virus entry. J. Virol. 2009. 83: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jain, S. , McGinnes, L. W. and Morrison, T. G. , Overexpression of thiol/disulfide isomerases enhances membrane fusion directed by the Newcastle disease virus fusion protein. J. Virol. 2008. 82: 12039–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calderon, M. N. , Guerrero, C. A. , Acosta, O. , Lopez, S. and Arias, C. F. , Inhibiting rotavirus infection by membrane‐impermeant thiol/disulfide exchange blockers and antibodies against protein disulfide isomerase. Intervirology 2012. 55: 451–464. [DOI] [PubMed] [Google Scholar]
- 34. Abell, B. A. and Brown, D. T. , Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J. Virol. 1993. 67: 5496–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavillette, D. , Barbouche, R. , Yao, Y. , Boson, B. , Cosset, F. L. , Jones, I. M. and Fenouillet, E. , Significant redox insensitivity of the functions of the SARS‐CoV spike glycoprotein: comparison with HIV envelope. J. Biol. Chem. 2006. 281: 9200–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yan, Z. , Garg, S. K. , Kipnis, J. and Banerjee, R. , Extracellular redox modulation by regulatory T cells. Nat. Chem. Biol. 2009. 5: 721–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan, Z. , Garg, S. K. and Banerjee, R. , Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J. Biol. Chem. 2010. 285: 41525–41532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sido, B. , Lasitschka, F. , Giese, T. , Gassler, N. , Funke, B. , Schr√∂der‐Braunstein, J. , Brunnemer, U. et al., A prominent role for mucosal cystine/cysteine metabolism in intestinal immunoregulation. Gastroenterology 2008. 134: 179–191. [DOI] [PubMed] [Google Scholar]
- 39. Metcalfe, C. , Cresswell, P. and Barclay, A. N. , Interleukin‐2 signalling is modulated by a labile disulfide bond in the CD132 chain of its receptor. Open Biology 2012. 2: 110036–110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang, X. , Lupardus, P. , Laporte, S. L. and Garcia, K. C. , Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 2009. 27: 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curbo, S. , Gaudin, R. l. , Carlsten, M. , Malmberg, K.‐J. , Troye‐Blomberg, M. , Ahlborg, N. , Karlsson, A. et al., Regulation of interleukin‐4 signaling by extracellular reduction of intramolecular disulfides. Biochem. Biophys. Res. Commun. 2009. 390: 1272–1277. [DOI] [PubMed] [Google Scholar]
- 42. Kobayashi, N. , Yamada, Y. , Ito, W. , Ueki, S. , Kayaba, H. , Nakamura, H. , Yodoi, J. et al., Thioredoxin reduces C‐C chemokine‐induced chemotaxis of human eosinophils. Allergy 2009. 64: 1130–1135. [DOI] [PubMed] [Google Scholar]
- 43. Yang, H. , Lundback, P. , Ottosson, L. , Erlandsson‐Harris, H. , Venereau, E. , Bianchi, M. E. , Al‐Abed, Y. et al., Redox modification of cysteine residues regulates the cytokine activity of high mobility group box‐1 (HMGB1). Mol. Med. 2012. 18: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Tassi, S. , Carta, S. , Vene, R. , Delfino, L. , Ciriolo, M. R. and Rubartelli, A. , Pathogen‐induced interleukin‐1beta processing and secretion is regulated by a biphasic redox response. J. Immunol. 2009. 183: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 45. Kesarwani, P. , Murali, A. K. , Al‐Khami, A. A. and Mehrotra, S. , Redox regulation of T‐cell function: from molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vene, R. , Castellani, P. , Delfino, L. , Lucibello, M. , Ciriolo, M. R. and Rubartelli, A. , The cystine/cysteine cycle and GSH are independent and crucial antioxidant systems in malignant melanoma cells and represent druggable targets. Antioxid. Redox Signal. 2011. 15: 2439–2453. [DOI] [PubMed] [Google Scholar]
- 47. Lo, M. , Ling, V. , Wang, Y. Z. and Gout, P. W. , The xc‐ cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer 2008. 99: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishimoto, T. , Nagano, O. , Yae, T. , Tamada, M. , Motohara, T. , Oshima, H. , Oshima, M. et al., CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(‐) and thereby promotes tumor growth. Cancer Cell 2011. 19:387–400. [DOI] [PubMed] [Google Scholar]
- 49. Goplen, D. , Wang, J. , Enger, P. Ò. , Tysnes, B. B. , Terzis, A. J. A. , Laerum, O. D. and Bjerkvig, R. , Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res. 2006. 66: 9895–9902. [DOI] [PubMed] [Google Scholar]
- 50. Khan, M. M. , Simizu, S. , Suzuki, T. , Masuda, A. , Kawatani, M. , Muroi, M. , Dohmae, N. et al., Protein disulfide isomerase‐mediated disulfide bonds regulate the gelatinolytic activity and secretion of matrix metalloproteinase‐9. Exp. Cell Res. 2012. 318: 904–914. [DOI] [PubMed] [Google Scholar]
- 51. Van Wart, H. E. and Birkedal‐Hansen, H. , The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 1990. 87: 5578–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tallant, C. , Marrero, A. and Gomis‐Ruth, F. X. , Matrix metalloproteinases: fold and function of their catalytic domains. Biochim. Biophys. Acta 2010. 1803: 20–28. [DOI] [PubMed] [Google Scholar]
- 53. Soderberg, A. , Hossain, A. and Rosen, A. , A protein‐disulfide isomerase/thioredoxin‐1 complex is physically attached to exofacial membrane TNF‐receptors: overexpression in chronic lymphocytic leukemia cells. Antioxid. Redox Signal. 2012. [DOI] [PubMed] [Google Scholar]
- 54. Olofsson, P. , Holmberg, J. , Tordsson, J. , Lu, S. , Akerstr√∂m, B. and Holmdahl, R. , Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat. Genet. 2003. 33: 25–32. [DOI] [PubMed] [Google Scholar]
- 55. Hultqvist, M. , Olsson, L. M. , Gelderman, K. A. and Holmdahl, R. , The protective role of ROS in autoimmune disease. Trends Immunol. 2009. 30: 201–208. [DOI] [PubMed] [Google Scholar]
- 56. Olsson, L. M. , Nerstedt, A. , Lindqvist, A. K. , Johansson, S. C. , Medstrand, P. , Olofsson, P. and Holmdahl, R. , Copy number variation of the gene NCF1 is associated with rheumatoid arthritis. Antioxid. Redox Signal. 2012. 16: 71–78. [DOI] [PubMed] [Google Scholar]
- 57. Hultqvist, M. , Olofsson, P. , Gelderman, K. A. , Holmberg, J. and Holmdahl, R. , A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006. 3: 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gelderman, K. A. , Hultqvist, M. , Holmberg, J. , Olofsson, P. and Holmdahl, R. , T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc. Natl. Acad. Sci. USA 2006. 103: 12831–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Essex, D. W. , Redox control of platelet function. Antioxid. Redox Signal. 2009. 11: 1191–1225. [DOI] [PubMed] [Google Scholar]
- 60. Holbrook, L.‐M. , Watkins, N. A. , Simmonds, A. D. , Jones, C. I. , Ouwehand, W. H. and Gibbins, J. M. , Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br. J. Haematol. 2010. 148: 627–637. [DOI] [PubMed] [Google Scholar]
- 61. Holbrook, L. M. , Sasikumar, P. , Stanley, R. G. , Simmonds, A. D. , Bicknell, A. B. and Gibbins, J. M. , The platelet‐surface thiol isomerase enzyme ERp57 modulates platelet function. J. Thromb. Haemost. 2012. 10: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Calvete, J. J. , Henschen, A. and González‐Rodríguez, J. , Assignment of disulphide bonds in human platelet GPIIIa. A disulphide pattern for the beta‐subunits of the integrin family. Biochem. J. 1991. 274: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kamata, T. , Ambo, H. , Puzon‐McLaughlin, W. , Tieu, K. K. , Handa, M. , Ikeda, Y. and Takada, Y. , Critical cysteine residues for regulation of integrin alphaIIbbeta3 are clustered in the epidermal growth factor domains of the beta3 subunit. Biochem. J. 2004. 378: 1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sun, Q. H. , Liu, C. Y. , Wang, R. , Paddock, C. and Newman, P. J. , Disruption of the long‐range GPIIIa Cys(5)‐Cys(435) disulfide bond results in the production of constitutively active GPIIb‐IIIa (alpha(IIb)beta(3)) integrin complexes. Blood 2002. 100: 2094–2101. [DOI] [PubMed] [Google Scholar]
- 65. Mor‐Cohen, R. , Rosenberg, N. , Einav, Y. , Zelzion, E. , Landau, M. , Mansour, W. , Averbukh, Y. et al., Unique disulfide bonds in epidermal growth factor (EGF) domains of beta3 affect structure and function of alphaIIbbeta3 and alphavbeta3 integrins in different manner. J. Biol. Chem. 2012. 287: 8879–8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Butta, N. , Arias‐Salgado, E. G. , Gonzalez‐Manchon, C. , Ferrer, M. , Larrucea, S. , Ayuso, M. S. and Parrilla, R. , Disruption of the beta3 663–687 disulfide bridge confers constitutive activity to beta3 integrins. Blood 2003. 102: 2491–2497. [DOI] [PubMed] [Google Scholar]
- 67. Chigaev, A. , Zwartz, G. J. , Buranda, T. , Edwards, B. S. , Prossnitz, E. R. and Sklar, L. A. , Conformational regulation of alpha 4 beta 1‐integrin affinity by reducing agents. “Inside‐out” signaling is independent of and additive to reduction‐regulated integrin activation. J. Biol. Chem. 2004. 279: 32435–32443. [DOI] [PubMed] [Google Scholar]
- 68. Nolan, S. M. , Mathew, E. C. , Scarth, S. L. , Al‐Shamkhani, A. and Law, S. K. , The effects of cysteine to alanine mutations of CD18 on the expression and adhesion of the CD11/CD18 integrins. FEBS Lett. 2000. 486: 89–92. [DOI] [PubMed] [Google Scholar]
- 69. Smagghe, B. J. , Huang, P. S. , Ban, Y. E. , Baker, D. and Springer, T. A. , Modulation of integrin activation by an entropic spring in the {beta}‐knee. J. Biol. Chem. 2010. 285: 32954–32966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jasuja, R. , Passam, F. H. , Kennedy, D. R. , Kim, S. H. , van Hessem, L. , Lin, L. , Bowley, S. R. et al., Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J. Clin. Invest. 2012. 122: 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]


