Abstract
Introduction
Human coronavirus NL63 (HCoV‐NL63) is one of four common human respiratory coronaviruses. Despite high incidence, HCoV infections are grossly understudied. We here report a case of HCoV infection in a leukemia patient with fatal ARDS despite successful virus elimination by pegylated interferon‐alpha (PEG‐IFN‐α).
Case
The 27‐year‐old female pre‐T‐ALL patient was treated according to the German‐Multicenter Trial for Adult ALL protocol. No relevant infectious complications were seen until day 35 when the neutropenic patient developed fever without any clinical focus. Antibiotic prophylaxis was switched to meropenem. The patient deteriorated rapidly with respiratory failure due to ARDS. Anti‐infectious therapy was escalated to additional linezolid and liposomal amphotericine‐B. BAL revealed a significant viral load of HCoV‐NL63. Based on successful application of PEG‐IFN against the related SARS coronavirus in animal experiments, a single injection of 180 μg PEG‐IFN‐α2b was applied. Despite immediate initiation of treatment and elimination of virus in subsequent tests, the progressive lung failure led to death 7 d after onset of fever with massive lung bleeding as a consequence of diffuse alveolar hemorrhage.
Conclusion
This report emphasizes the fatal consequences of common respiratory virus infections in immunocompromised patients. HCoV‐NL63 replication can be reduced by treatment with peg‐IFN if diagnosed early.
Keywords: ALL, coronavirus, ARDS
Case report
Human coronavirus NL63 (HCoV‐NL63) is one of four common human respiratory coronaviruses 1. The emergence of SARS (severe acute respiratory syndrome) and MERS (Middle East Respiratory Syndrome) caused by two recently identified coronaviruses has brought the global attention to the clinical significance of coronaviruses 2. Clinical outcome data for non‐SARS/MERS coronavirus infections in immunocompromised patients are very rare as are potential treatment options for this type of infection. We here report a case of HCoV NL63 infection in a leukemia patient with fatal ARDS despite successful virus elimination by pegylated interferon‐alpha (PEG‐IFN‐α). The 27‐year‐old female pre‐T‐ALL patient was treated according to the German‐Multicenter Trial for Adult ALL (GMALL) 07‐03 protocol in January 2015. The patient experienced mild mucositis during therapy, but no other infectious complications (especially no coughing or respiratory tract infection signs) were seen until day 35. Infectious prophylaxis (fluoroquinolones, cotrimoxazole, aciclovir, and mycafungin) were applied according to our institutional recommendations since treatment initiation. In the morning of day 35, the patient developed fever (38.5°C), blood cultures were immediately taken, and the antibiotic prophylaxis was switched to broad spectrum antibiotics (meropenem). In the clinical examination, no infectious focus could be found, particularly no signs of respiratory tract infection. Two hours later, the patient collapsed in the bathroom, a positive shock index and decreased oxygen saturation immediately triggered ICU referral after stabilization of the patient by oxygen and additive volume supply. Antibiotic therapy was escalated to additional linezolid and antifungal therapy to liposomale amphotericine‐B due to the rapid clinical worsening as a consequence of suspected pneumonia with sepsis. During the night, the patient deteriorated rapidly because of respiratory failure (partial pressure of oxygen (paO2) 50 mmHg) even though immediate non‐invasive ventilation was started after ICU referral and the patient was intubated for mechanical ventilation in the following hours. Bronchoalveolar lavage (BAL) was carried out, and routine microbial analyses (cultures from urine and peripheral blood specimens) were repeatedly taken. Clinical instability with highest catecholamine requirement and highly invasive respiration parameters did not allow CT scan for further diagnostic work‐up of fulminant respiratory failure, but conventional X‐ray of the lungs showed typical signs of acute respiratory distress syndrome (ARDS) (Fig. 1). BAL revealed a significant viral load of human coronavirus NL63 (HCoV‐NL63, 9.3 × 104 copies/mL, real‐time RT‐PCR), no other respiratory viruses were detected. The blood cultures were positive for multisensible staphylococcus epidermidis triggering plastic change resulting in sterile follow‐up blood cultures. Of note, coronavirus was neither detectable in the peripheral blood nor pleural effusion. As lung organ failure clinically represented the leading problem, HCoV‐NL 63 infection was assumed to be the trigger for the ARDS.
Figure 1.
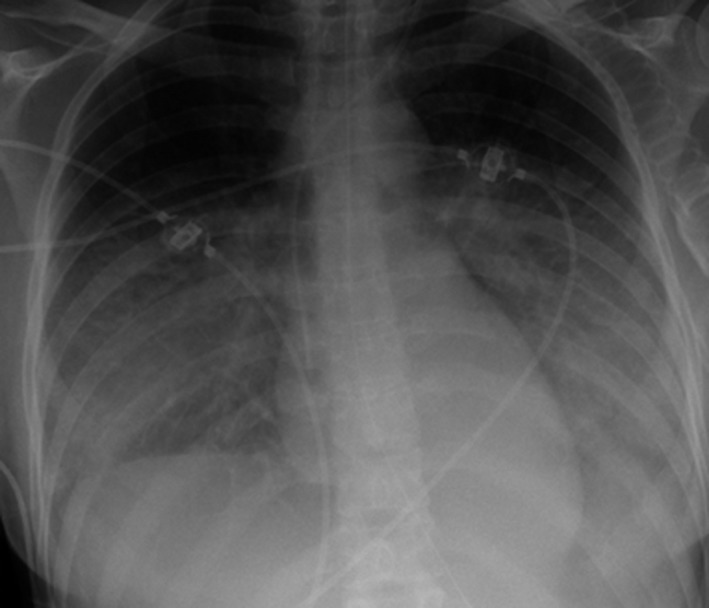
X‐ray of the lung with typical signs of acute respiratory distress syndrome (ARDS).
There is very limited literature for the treatment of HCoV NL63 infection as this viral infection in otherwise healthy individuals is usually cleared by the functional immune response. Based on the successful application of pegylated IFN against the related SARS coronavirus in animal experiments 3, a single injection of 180 μg PEG‐IFN‐α2b was applied. Intravenous immunoglobulins (1 g/kg) were given because of the wide prevalence of anti‐HCoV‐NL63 in the population. Steroids were provided to limit inflammation (2 mg/kg per day), based on experience with SARS‐CoV. Despite immediate initiation of treatment, the clinical picture significantly worsened throughout the next 48 h and mechanical ventilation had to maximally escalated (100% oxygen supply and highest tolerable pressures) to maintain sufficient oxygenation. The option for ECMO was rejected due to the excessive bleeding risk (thrombocytopenia and already beginning endotracheal bleeding). Of note, despite significant clinical worsening at this time point (48 h after PEG‐IFN‐α2b application), HCoV‐NL63 was no longer detectable in a BAL sample collected 4 d after the specimen testing positive for NL63 (Fig. 2). The clinical picture of septic shock with leading lung failure was progressive, and the patient died 7 d after the first fever episode due to massive and uncontrolled lung bleeding as a consequence of diffuse alveolar hemorrhage probably triggered by the coronaviral infection.
Figure 2.
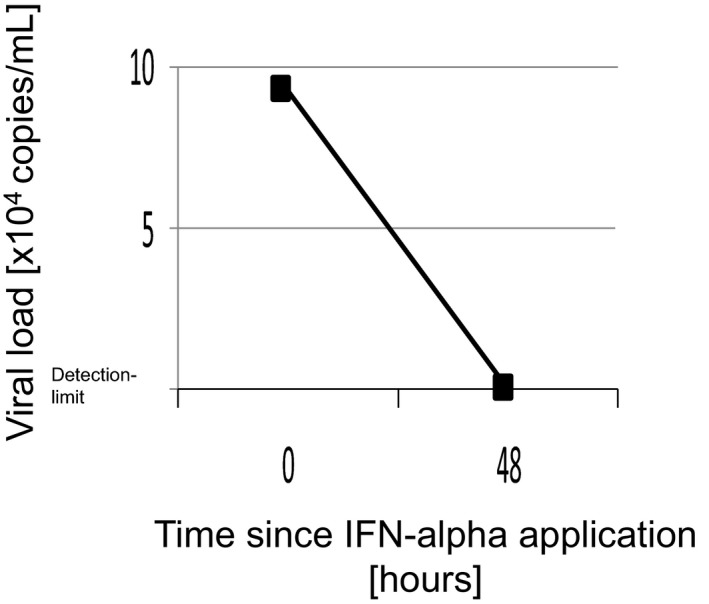
HCoV‐NL63 viral load in BAL before and 48 h after IFN‐alpha application.
To the best of our knowledge, this is the first report in the literature ascribing ARDS with subsequent diffuse alveolar hemorrhage in an immunocompromised patient to respiratory infection with HCoV‐NL63 despite successful viral clearance by pegylated IFN. The two novel coronaviruses SARS‐CoV and MERS‐CoV, similarly to our case, cause ARDS, which is associated with high mortality rates. So far, no clinically approved vaccines or antiviral drugs are available for either of these highly pathogenic infections, and the development of novel effective therapeutic and preventive strategies is urgently needed to reduce mortality. In contrast, HCoV‐NL63 is an alphacoronavirus that usually induces only mild to moderate upper respiratory tract infections and is associated with croup in children. No specific therapy exists so far and classical antiviral drugs, such as ribavirin, have only limited efficacy in other coronaviruses, such as SARS‐CoV and MERS‐CoV. Early reports emphasized the efficacy of IFN‐α in severe ARDS as prophylactic treatment with pegylated IFN‐α significantly reduced the viral load and protected rhesus macaques from SARS‐CoV infection in a prophylactic setting 4. The importance of IFN‐α is further underscored by the observation that both SARS‐CoV and MERS‐CoV infections induce an enhanced IFN type 1 secretion by human plasmacytoid dendritic cells 5 which also demonstrated therapeutic potential in murine CoV‐infection models. The efficacy of Peg‐IFN‐α is also supported by our case, as after single injection of Peg‐IFN‐α2b, HCoV‐NL63 could not further be detected in the BAL. However, rapid viral replication obviously severely damaged lung tissue (pneumocytes) and triggered severe inflammation which was not reversible even though the virus was successfully cleared. In the case presented here, the infection route remains elusive and excessive screening of the surrounding did not reveal any other infected individual. However, the case underlines the infection risk in hematological patients receiving chemotherapy and regularly steroids, which are known to be immunosuppressive. Therefore, in patients undergoing chemotherapy and clinical signs of respiratory tract infections appear, particular vigilance is required and clinical work‐up should include extensive screening for respiratory viruses to prevent excessive viral replication triggering irreversible end organ damage.
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis 2005;191:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drosten C, Günther S, Preiser W, et al Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967–76. [DOI] [PubMed] [Google Scholar]
- 3. Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H. Severe acute respiratory syndrome‐related coronavirus is inhibited by interferon‐alpha. J Infect Dis 2004;189:1164–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haagmans BL, Kuiken T, Martina BE, et al Pegylated interferon‐alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 2004;10:290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheuplein VA, Seifried J, Malczyk AH, et al High secretion of interferons by human plasmacytoid dendritic cells upon recognition of MERS‐CoV. J Virol 2005;89:3859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]


