Abstract
Objectives
T‐cell–replete haploidentical stem cell transplantation (Haplo‐SCT) with post‐transplant cyclophosphamide (PT‐Cy) is at high risk of invasive fungal infections (IFI), and anti‐mold–active drug is required for primary antifungal prophylaxis (PAP) according to international guidelines. No data are available on the efficacy of caspofungin as PAP in this setting.
Methods
Here, we report our retrospective experience with 103 consecutive patients treated with caspofungin as PAP after Haplo‐SCT. Caspofungin was administered only during the pre‐engraftment phase.
Results
Hundred‐day cumulative incidence of proven‐probable IFI (PP‐IFI) was 8.7% and median day of onset was 19 post‐SCT. No patient died of PP‐IFI, and overall survival (OS) and non‐relapse mortality (NRM) hazard ratio (HR) for patients experiencing IFI were 1.02 (P = 0.9) and 0.7 (P = 0.7), respectively. Three‐year overall survival (OS) and 1‐year non‐relapse mortality (NRM) were 55% and 19%, respectively. By univariate analysis, duration of neutropenic phase and partial remission pre‐transplant disease status were associated with increased incidence of IFI, but were not confirmed by multivariate analysis.
Conclusion
In summary, PAP with caspofungin is an effective strategy for preventing IFI in the context of Haplo‐SCT with PT‐Cy. Further efforts are required in order to identify more potent strategies able to avoid the occurrence of breakthrough infections.
Keywords: caspofungin, haploidentical stem cell transplant, primary antifungal prophylaxis
1. INTRODUCTION
Invasive fungal infections (IFIs) represent a significant cause of morbidity and mortality for patients undergoing allogeneic hematopoietic stem cell transplantation (Allo‐SCT). The cumulative incidence of proven‐probable IFI (PP‐IFI) ranges between 5% and 13% after Allo‐SCT1, 2, 3, 4, 5, 6 and varies according to several risk factors such as donor type, duration of neutropenia, and occurrence of graft‐versus‐host disease (GVHD) requiring systemic steroid treatment.
Among risk factors for IFI, donor type is the only pre‐transplant variable that is potentially useful to dictate the choice of primary antifungal prophylaxis (PAP). Both the prospective study from the Transnet database3 and the retrospective analysis from the Gruppo Italiano Trapianto Midollo Osseo (GITMO)7 have described that incidence of PP‐IFI is approximately twice as high after mismatched related donor (MMRD; 8.1%‐8.8%) compared with matched related donor (MRD; 4.6%‐5.8%). Other smaller retrospective studies, comprising different types of haploidentical transplant platforms, have consistently found a higher risk of IFI after haploidentical/MMRD transplant relative to MRD Allo‐SCT.8, 9 Antifungal prophylaxis was very heterogeneous in these studies ranging from fluconazole only to anti‐mold–active agents. All these reports support the urgent need for a more stringent PAP after haploidentical transplant in order to reduce the occurrence of IFI and of IFI‐related mortality that can be as high as 33%.9 In addition, epidemiologic studies on fungal infection and prophylaxis in the last years have shown a shift from Candida (that remains the second major cause, comprising albicans and non‐albicans) to Aspergillus species as the most frequent agents responsible for IFI after Allo‐SCT.1, 2, 3, 4, 5, 6, 7, 8, 9, 10
Given this background, the ECIL‐511 and GITMO12 recommended to employ anti‐mold prophylaxis for patients at higher risk of IFI based on donor type (haploidentical or cord blood), timing after transplant (pre‐ or post‐engraftment phase), and occurrence of GVHD. Itraconazole, voriconazole, and posaconazole received a BI‐BII level of recommendations by the last version of ECIL recommendation.13 No data are available either to recommend or to contraindicate echinocandin as PAP.
Based on the unmet needs of PAP in high‐risk patients, stating in November 2013 we employed caspofungin as anti‐mold agent in 103 consecutive patients undergoing haploidentical transplant (Haplo‐SCT) with post‐transplant cyclophosphamide (PT‐Cy) at our Institution. The aim of this study was to analyze the efficacy of caspofungin as PAP both in terms of IFI incidence in the first 100 days after Allo‐SCT and in terms of patients’ long‐term outcome.
2. PATIENTS AND METHODS
This is a retrospective study comprising 103 consecutive patients with a hematologic malignancy receiving a T‐cell–replete Haplo‐SCT with PT‐Cy at our Institution. A donor was defined haploidentical when donor and recipient had more than 2 HLA mismatch and share one haplotype. Our transplant strategy is based on the Baltimore study14 where donor graft was not manipulated (T‐cell–replete) and GVHD prophylaxis consisted of cyclosporine, mycophenolate, and high‐dose cyclophosphamide. Patients provided informed consent for the retrospective collection of their data. The study was approved from the Institutional Review Board at both institutions.
2.1. Conditioning regimens
Three main conditioning regimens were employed: (a) non‐myeloablative (NMA) regimen comprising Cy 14.5 mg/kg on days −6 and −5, fludarabine 30 mg/m2 from day −6 to day −2, and low‐dose total body irradiation (TBI) (2 Gy) on day −1; (b) reduced intensity (RIC) regimen comprising either thiotepa 10 mg/kg on day −5, cyclophosphamide 30‐60 mg/kg on day −4 and day −3, fludarabine 30 mg/m2 on day −4 and day −3, thiotepa 5 mg/kg on day −5, busulfan 3.2 mg/kg on day −4 and day −3, and fludarabine 50 mg/m2 from day −4 to day −2; and (c) myeloablative (MAC) conditioning comprising thiotepa 5 mg/kg on day −7 and day −6, busulfan 3.2 mg/kg/die from day −5 to day −3, and fludarabine 40 mg/m2 from day −5 to day −2.
2.2. Stem cell source
Potential family members were typed at the HLA‐A, HLA‐B, and HLA‐DRB1 loci at high level of resolution. Selected donors were also typed at the HLA‐C locus at a high‐resolution level. Graft source was represented either by bone marrow (BM) or by peripheral blood stem cells (PBSC). The target was a minimum of 4 × 106 CD34/kg. Unmanipulated BM and peripheral blood stem cells (PBSC) were used for stem cell support on day 0. Graft source at the beginning was mainly represented by bone marrow as in the Baltimore protocol. Thereafter, PBSC was mainly used due to the easier collection strategy.
2.3. Graft‐versus‐host disease (GVHD) prophylaxis
High‐dose cyclophosphamide (50 mg/kg) was administered on day +3 and day +4 after transplant. Cyclosporin A (CsA) was dosed at 3 mg/kg as a continuous infusion until discharge and was converted to an oral formulation thereafter. CSA dosages were adjusted based on respective range of activity, between 100 and 200 ng/mL. MMF was administered at 15 mg/kg po three times per day until day +35. CsA and MMF were started on day +5. CsA was tapered by day +100. G‐CSF was started on day +5 in all patients.
2.4. Engraftment and GVHD evaluation
Neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/L after transplantation. Platelet engraftment was defined as a platelet count of 20 × 109/L, with no transfusions during the preceding 7 days. Acute GVHD (aGVHD) was graded according to the Keystone criteria,15 and chronic GVHD (cGVHD) was retrospectively graded following the NIH criteria.16
2.5. Routine surveillance and antimicrobial prophylaxis
All patients were allocated in HEPA‐filtered rooms from the beginning of the conditioning until neutrophil engraftment. They received a low microbial diet. As blood vessel access, a CVC was placed. All patients underwent microbiological surveillance, including cultures from stool and urine and swabs from the anal area, starting before the conditioning and then weekly until discharge. In case of fever (defined when the temperature was >38°C), the following examinations were performed: duplicate blood cultures; high‐resolution thorax CT scan (HR‐CT); and bronchoalveolar lavage (BAL) when possible in case of any type of lung abnormality suspicious for infection. Microbiological investigations included microscopy and culture. On direct microscopy, samples were examined by preparing wet mount Gram stain to detect the presence of yeast cells, yeast with pseudohyphae, or septate hyphae for the mold. The samples for fungal culture were inoculated on Sabouraud's dextrose agar and incubated at 35°C. Yeast was further identified by MALDI TOF. The molds were identified using lactophenol cotton blue mount and slide culture. The samples were also investigated by culture for the presence of Staphylococcus aureus, Streptocooccus pneumoniae and pyogenes, enterobacteriaceae, Haemophilus influenzae, Moraxella catharralis, galactomannan (threshold for positivity:>1 optical density), Pneumocistis jroveci (immunohistochemistry and PCR), CMV, and respiratory virus (H1N1, parainfluenza, influenza A and B, adenovirus, respiratory syncytial virus, coronavirus, metapneumovirus, enterovirus, rhinovirus, and bocavirus). Antimicrobial prophylaxis was begun at the hospital during the conditioning regimen, consisted of acyclovir 800 mg per day, levofloxacin 500 mg per day, and sulfamethoxazole + trimethoprim administered as 2 tablets per day until day −1, and then resumed after hematologic reconstitution at a dosage of 1 tablet every other day. PAP consisted of caspofungin 70 mg/die on day −6 followed by caspofungin 50 mg/die from day −5 until neutrophil engraftment. Historical cohort treated with itraconazole received iv itraconazole PAP at the dosage of 200 mg/die from day −6 until neutrophil engraftment. Thereafter, PAP was performed by the means of posaconazole only in case of acute GVHD occurrence. PCR monitoring of cytomegalovirus was performed twice per week from day +15 until day +100. No routine screening for IFI was performed before transplant.
2.6. Fungal infections
Possible‐probable‐proven IFI (PPP‐IFI) and PP‐IFI were defined according to standard international criteria.17 Onset of PPP‐ and PP‐IFI was defined as the day of the first positive radiological examination, or positive culture or pathologic test. PPP‐ and PP‐IFIs were recorded during the first 100 days after Allo‐SCT.
2.7. Statistical methods
The primary endpoint of this study was to investigate cumulative incidence of IFI infections in patients receiving PAP with caspofungin. Secondary endpoints were long‐term outcome parameters such as overall survival (OS) and non‐relapse mortality (NRM). Categorical variables were expressed as proportions and continuous variables as medians with the respective range. The cumulative incidence of aGVHD was estimated considering death not related to aGVHD within a year post‐transplant as a competing event. cGVHD was estimated only for patients alive at day +100, considering death not related to cGVHD within 2 years post‐transplant as a competing event.18 For the calculation of NRM, disease relapse or progression was treated as a competing event, whereas NRM was the competing event for the calculation of cumulative incidence of relapse or progression. The Kaplan‐Meier method was used for the OS and progression‐free survival (PFS) analyses.19 Outcomes (and respective 95% confidence interval (CI)) were calculated from the date of transplantation. Comparisons between groups were made by log‐rank and Gray tests whenever indicated. The cumulative incidence of PPP‐ and PP‐IFI up to day 100 was estimated considering death for any other reason not related to IFI infection as a competing event. Univariate Cox regression models20 were used to identify significant moderators (independent covariates) on the occurrence of PP‐IFI. IFI was treated as a time‐varying explanatory covariate to account for its effect on OS and NRM. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. An HR >1 denotes an unfavorable effect. A P value <0.05 was considered significant. SPSS version 19.0 (IBM, Armonk, NY, USA) and EZR (“Easy R”; R Institute for Statistical Computing, Vienna, Austria) were used.
3. RESULTS
From November 2013 to December 2017, 103 consecutive patients received a Haplo‐SCT with PT‐Cy at our Institution and were treated with caspofungin as PAP. Patient characteristics are shown in Table 1. Median age was 52 (20‐72), and the main underlying diagnoses were represented by lymphoma and acute myeloid leukemia. Conditioning consisted mainly of RIC and NMA regimens (82%). Forty‐one percent of the patients received a graft from BM cells and 59% from PBSC. In the first 40 days after transplant (GITMO early phase), most patients (65%) were in the high‐risk category according to GITMO classification, mainly because they were treated with Haplo‐SCT plus the presence of another adverse variable, such as prolonged neutropenia or recurrent CMV infection. Between day 40 and day 100 (GITMO late phase), a minority of cases (35%) was in the high‐risk category (35%), mainly due to acute GVHD requiring systemic steroids, recurrent CMV infection, or disease.
Table 1.
Patients’ characteristics
| Characteristics | Total n = 103 |
|---|---|
| Age (median, range) | 52 (20‐72) |
| Sex | |
| Male/Female | 57/46 |
| Disease type | |
| Acute myeloid leukemia/MDS | 41 (40%) |
| Acute lymphoblastic leukemia | 5 (5%) |
| Hodgkin lymphoma | 33 (31%) |
| Non‐Hodgkin lymphoma | 22 (22%) |
| Multiple myeloma | 2 (2%) |
| Disease status before transplant | |
| CR | 62 (60%) |
| PR | 19 (19%) |
| SD/PD | 22 (21%) |
| DRI | |
| LR/IR | 76 (74%) |
| HR/VHR | 27 (26%) |
| Previous Auto‐Tx | N = 99 |
| No | 59 |
| Yes | 40 |
| Sex mismatch | |
| F→M | 20 (19%) |
| Others | 83 (81%) |
| HCT‐CI | N = 101 |
| 0‐1 | 27 (27%) |
| 2 | 26 (26%) |
| >3 | 48 (47%) |
| Conditioning | |
| NMA | 32 (31%) |
| RIC | 53 (51%) |
| MAC | 18 (18%) |
| Graft type | |
| BM | 42 (41%) |
| PBSC | 61 (59%) |
| CMV serostatus | N = 101 |
| Neg/Neg | 4 (4%) |
| Pos/Neg | 11 (11%) |
| Pos/Pos | 66 (65%) |
| Neg/Pos | 20 (20%) |
| Donor type | |
| Child | 39 (38%) |
| Sibling | 42 (41%) |
| Parent | 13 (12%) |
| Cousin/Nephew | 9 (9%) |
| GITMO early phase | |
| Standard Risk | 36 (35%) |
| High risk | 67 (65%) |
| GITMO late phase (%) | |
| Standard risk | 67 (65%) |
| High risk | 36 (35%) |
CR, complete remission; PR, partial remission; SD/PD, stable disease/progressive disease; DRI, Disease Risk Index; LR, low risk; IR, intermediate risk; HR, high risk; VHR, very high risk; Tx, transplant; F→M, female donor into male recipient; HCT‐CI, hematopoietic cell transplant‐comorbidity index; NMA, non‐myeloablative conditioning; RIC, reduced intensity conditioning; MAC, myeloablative conditioning; BM, bone marrow; PBSC, peripheral blood stem cells; CMV, cytomegalovirus; MDS, myelodysplastic syndrome; GITMO, Gruppo Italiano Trapianto Midollo Osseo.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.1. Invasive fungal infections and risk factors
During the first 100 days post‐Haplo‐SCT, cumulative incidence of PPP‐IFI was 11% (95% CI: 6‐17) (10 cases) (Figure 1A). PP‐IFI occurred only in 8 patients for a 100‐day cumulative incidence of 8.7% (95% CI: 4‐15) (Figure 1B). Among the 8 cases of PP‐IFI, the most common diagnosis was lung invasive aspergillosis (n = 7), while one patient had proven IFI without detection of Aspergillus at the time of lung resection. Median time of PP‐IFI was 18 days after Haplo‐SCT (range 15‐56), while most patients were still on caspofungin prophylaxis. No patient died of PP‐IFI in our cohort: By univariate analysis for OS and NRM, hazard ratio (HR) for patients with PP‐IFI relative to patients without IFI was 1.02 (95% CI: 0.3‐3.3, P = 0.97) and 0.73 (95% CI: 0.09‐5.56, P = 0.76), respectively.
Figure 1.
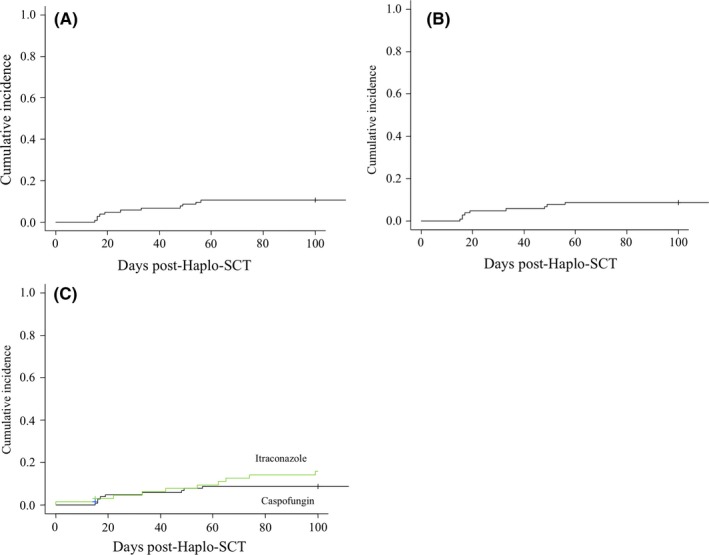
Cumulative incidence of invasive fungal infections (IFI) of patients receiving haploidentical stem cell transplantation (Haplo‐SCT) with post‐transplant cyclophosphamide (PT‐Cy): (A) day‐100 cumulative incidence of possible‐proven‐probable IFI of patients receiving caspofungin as primary antifungal prophylaxis; (B) day‐100 cumulative incidence of proven‐probable IFI of patients receiving caspofungin as primary antifungal prophylaxis; and (C) comparison of day‐100 cumulative incidence of proven‐probable IFI between patients receiving caspofungin or itraconazole as primary antifungal prophylaxis
When we analyzed risk factors commonly associated with fungal infection by univariate analysis, we found (Table 2) that the longer the post‐SCT neutropenic phase, the higher the chance of experiencing a PP‐IFI within the first 100 days. Because caspofungin prophylaxis was limited to the engraftment phase, and because most IFI occurred before day 40 (cumulative incidence 6%), we performed a subanalysis to study whether day 40‐IFI correlate with GITMO early‐phase risk category, but we could not find any significant difference (standard risk: 3% vs high risk: 7%, P = 0.4). Of note, by multivariate analysis only high/very high disease risk index (DRI) retained an independent value for increased risk of IFI (HR: 21.1, P = 0.047). However, the confidence intervals were very wide due to the small number of events; therefore, this result needs to be confirmed on a larger number of patients.
Table 2.
Univariate analysis of risk factors for PP‐IFI in pts receiving caspofungin as primary antifungal prophylaxis (PAP)
| Characteristics | HR (95% CI) | P |
|---|---|---|
| Sex | ||
| Female | 1 | 0.7 |
| Male | 0.7 (0.1‐3.1) | |
| Disease type | ||
| Myeloid | 1 | 0.9 |
| Lymphoid | 0.9 (0.2‐3.8) | |
| Disease status before transplant | ||
| CR | 1 | |
| PR | 3.4 (0.8‐13.7) | 0.08 |
| SD/PD | 0 | 0.9 |
| DRI | ||
| LR/IR | 1 | 0.3 |
| HR/VHR | 2.0 (0.5‐8.5) | |
| Sex mismatch | ||
| Others | 1 | 0.6 |
| F→M | 1.5 (0.3‐7.6) | |
| HCT‐CI | ||
| 0‐2 | 1 | 0.3 |
| ≥3 | 1.9 (0.4‐8.3) | |
| Conditioning | ||
| MAC | 1 | |
| RIC | NA | 0.9 |
| NMA | NA | 0.9 |
| Graft type | ||
| BM | 1 | 0.6 |
| PBSC | 0.7 (0.1‐2.8) | |
| CMV serostatus | ||
| Others | 1 | 0.7 |
| Neg/Pos | 1.3 (0.2‐6.8) | |
| Donor type | ||
| Parent | 1 | |
| Sibling | 0.6 (0.05‐6.9) | 0.7 |
| Child | 2.0 (0.2‐17.2) | 0.5 |
| Cousin/Nephew | 0 | 0.9 |
| GITMO early phase | ||
| Standard risk | 1 | 0.6 |
| High Risk | 1.4 (0.2‐7.2) | |
| GITMO late phase | ||
| Low risk | 1 | 0.4 |
| High risk | 0.5 (0.1‐2.6) | |
| aGVHD | ||
| No | 1 | |
| 2‐4 | 0.6(0.1‐3.1) | 0.6 |
| Recipient age | 1.03 (0.98‐1.08) | 0.2 |
| Donor age | 0.96 (0.91‐1.02) | 0.2 |
| Neutropenia | ||
| <=21 Days | 1 | 0.3 |
| >21 Days | 2.06 (0‐49‐8.63) | |
| Neutropenia duration | 1.04 (1.004‐1.09) | 0.03 |
HR, hazard ratio; CI, confident interval; CR, complete remission; PR, partial remission; SD/PD, stable disease/progressive disease; DRI, Disease Risk Index; LR, low risk; IR, intermediate risk; HR, high risk; VHR, very high risk; Tx, transplant; F→M, female donor into male recipient; HCT‐CI, hematopoietic cell transplant‐comorbidity index; NMA, non‐myeloablative conditioning; RIC, reduced intensity conditioning; MAC, myeloablative conditioning; BM, bone marrow; PBSC, peripheral blood stem cells; CMV, cytomegalovirus; aGVHD, acute graft‐versus‐host disease; GITMO, Gruppo Italiano Trapianto Midollo Osseo.
P values < 0.05 are highlighted in bold.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In addition, we compared the 100‐day cumulative incidence of PP‐IFI of our cohort with a historical group of patients (n = 66) that received iv itraconazole as PAP between April 2009 and October 2013 at our institution. The two groups differed for patients’ characteristics since the cohort receiving caspofungin was older and had a higher frequency of high‐risk HCT‐CI, previous autologous transplant, and more intensive conditioning (Table S1). Taking these limits into consideration, we observed a trend for reduced 100‐day cumulative incidence of PP‐IFI after caspofungin relative to itraconazole prophylaxis: 8.7% (95% CI: 4‐15) vs 16% (95% CI: 8‐26) (Figure 1C, P = 0.17).
3.2. Engraftment
Ninety‐four out of 103 patients (91%) were evaluable: 7 patients died before engraftment, and 2 experienced graft failure. The median time to ANC ≥0.5 × 109/mL was 21 days (range 15‐73), and the median time to platelet count ≥20 × 109/mL was 27 (range 12‐390). Of note, 45 patients (48%) had neutropenia lasting>21 days.
3.3. Survival and side effects
With a median follow‐up for alive patients of 19.5 months (range 4.5‐43), 53 patients are still alive. The 2‐year OS was 55% (95% CI: 44‐65) (Figure 2A), 2‐year PFS was 51% (95% CI: 39‐61), and the 1‐year NRM was 19% (95% CI: 13%‐27%) (Figure 2B). Cumulative incidence of grade 2‐4 and 3‐4 acute GVHD at 6 months was 31% (95% CI: 22‐40; Figure 2C) and 4% (95% CI: 1‐10), respectively. Median day of acute GVHD onset was 39 days (range 19‐185). 2‐year cumulative incidence of moderate‐severe chronic GVHD was 8% (95% CI: 3‐15) (Figure 2D), and median day of onset was 232 days (range 133‐430). For patients with a positive CMV serostatus, cumulative incidence of CMV reactivation requiring pre‐emptive therapy was 58% (48‐67).
Figure 2.
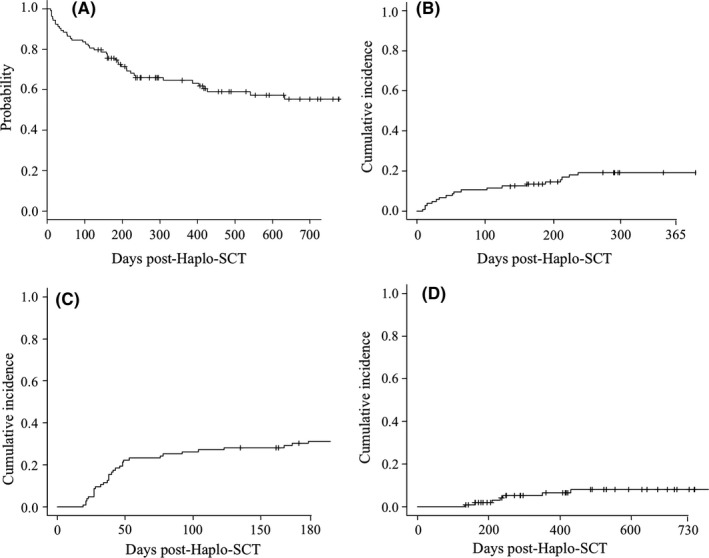
Outcome of patients receiving haploidentical stem cell transplantation (Haplo‐SCT) with post‐transplant cyclophosphamide (PT‐Cy) and caspofungin as primary antifungal prophylaxis: (A) Kaplan‐Meier estimate of 3‐year overall survival; (B) 1‐year cumulative incidence of non‐relapse mortality; (C) 6‐month cumulative incidence of grade 2‐4 acute GVHD; and (D) 2‐year cumulative incidence of moderate‐severe chronic GVHD
3.4. Characteristics and treatment of patients with pneumonitis
On Table 3, we reported all diagnosis of pneumonitis performed within the first 100 days post‐transplant in our cohort of 103 patients. Of relevance, we were able to perform bronchoalveolar lavage in 20 out of 25 (80%) cases of pneumonitis: This allowed to significantly reduce the percentage of PPP‐IFI in our cohort. It was not possible to perform bronchoalveolar lavage in 5 patients due to the severely compromised performance status. In details, the prevalent radiological pattern consisted of multiple areas of consolidation, with or without ground‐glass opacities, involving either the upper or the inferior lobes of the lung. When a PPP‐IFI was suspected, treatment of choice was to a shift of antifungal class from caspofungin to voriconazole or ambisome treatment (n = 10 cases). Moreover, results from BAL allowed to modify antibacterial treatment (n = 3 cases) or to initiate anti‐CMV therapy (n = 5 cases). Among all cases of pneumonitis, one died of CMV lung infection on day +178, while one patient, experiencing lung Aspergillosis on day +16, died because of septic shock on day +417.
Table 3.
Characteristics of patients developing pulmonary infection during the first 100 days after allo‐SCT
| Gender/Age | Radiological characteristics of the lung | Time from allo‐SCT | BAL (yes/no), result | EORTC classification of IFI | Therapy | Outcome |
|---|---|---|---|---|---|---|
| Day 0‐40 | ||||||
| M/70 | Areas of consolidation and interstitial thickening of the medium lobe of the right lung with pleural effusion | 15 | Yes: galactomannan positive | Probable IFI | Voriconazole | Alive (day+922) |
| F/68 | Area of consolidation of the superior lobe of the left lung | 19 | Yes: galactomannan positive | Probable IFI | Voriconazole | Dead (PD, day +427) |
| M/58 | Interstitial thickening and consolidation areas of the both lungs | 14 | No | NA | Piperacillin/tazobactam + linezolid | Dead (PD, day +22) |
| M/59 | Area of consolidation of the apex of the upper lobe of the left lung | 16 | Yes: galactomannan negative | Possible IFI.Lung resection on day +288: proven IFI (not Aspergillus) | Voriconazole followed by isavuconazole for persistent lung infection | Alive (day +296) |
| M/50 | Area of consolidation, with air bronchogram, of the right lung | 7 | Yes: Stenotrophomonas | NA | Tigecycline + ceftazidime | Dead (PD, day +14) |
| F/66 | Dysventilation areas of the bilateral lower lobes with pleural effusion and Interstitial thickening of the bilateral upper lobes. | 12 | Yes: CMV | NA | Ganciclovir | Dead (TMA; day +65) |
| F/57 | Areas of consolidation with surrounding ground glass of the upper lobe of the right lung | 10 | No for poor patient PS | NA | Piperacillin/tazobactam + daptomycin | Dead (PD, + 12) |
| M/49 | Consolidation areas of the bilateral lower lobes | 15 | Yes: negative | NA | Meropenem + vancomycin | Alive (day +1018) |
| F/42 | Areas of interstitial thickening of the medium right lobe with pleural effusion | 38 | Yes: P jiroveci | NA | Trimethoprim/sulfamethoxazole | Alive (day +889) |
| F/70 | Multiple areas of consolidation of both lungs, with the bigger one at the lingula with granulomatous characteristics | 16 | Yes: galactomannan and CMV positive | Probable IFI | Voriconazole | Dead (septic shock, day +417) |
| F/66 | Nodular opacities of the bilateral upper lobes | 25 | Yes: galactomannan negative | Possible IFI | Voriconazole | Dead (PD, day +94) |
| M/61 | Bilateral bronchial and peribronchial flogistic areas with pleuric fluid | 10 | Yes: CMV | NA | Ganciclovir | Alive (day +1134) |
| F/30 | Bilateral areas of consolidation with ground‐glass opacities of the medium lobes | 17 | Yes: galactomannan positive, | Probable IFI | Voriconazole | Alive (Day +465) |
| F/51 | Nodular opacity of the right upper lobe and bilateral pleural effusion | 36 | No | Possible IFI | Piperacillin/tazobactam + linezolid + voriconazole | Dead (septic shock, Day +54) |
| Day 41‐10 | ||||||
| F/56 | Nodular opacity of the medium and lower lobes of the left lung | 49 | Yes :galactomannan positive | Probable IFI | Ambisome, followed by voriconazole for persistence of lung infection | Dead (PD, day +109) |
| F/49 | Multiple bilateral areas of consolidation of both lungs and in particular located at the upper part of the lower left lobe | 65 | Yes: Psudomonas Aeruginosa | NA | Piperacillin/tazobactam + linezolid + amikacin+ambisome | Alive (day +916) |
| M/50 | Bilateral areas of consolidation and interstitial thickening | 48 | No | NA | Piperacillin/tazobactam + linezolid | Dead (septic shock, day +52) |
| M/55 | Area of consolidation of the lower left lobe and ground‐glass areas of the right lung | 80 | Yes: negative | NA | Meropenem | Alive (day +1060) |
| M/70 | Area of consolidation of the apex of the left upper lobe | 69 | No | NA | Piperacillin/tazobactam + voriconazole | Alive (day+922) |
| M/26 | Areas of consolidation with air bronchogram of the upper lobes and nodular opacities of both the lower lobes | 48 | Yes :galactomannan positive | Probable IFI | Piperacillin/tazobactam + voriconazole | Alive (Day +1126) |
| M/25 | Areas of consolidation of the upper right lobe | 76 | Yes: negative | NA | Ambisome | Dead (EBV LPD, Day +212) |
| M/70 | Area of consolidation of the lower right lobe, pleural effusion | 100 | Yes: negative | NA | Meropenem + vancomycin | Alive (day +485) |
| F/30 | Interstitial thickening of the both the upper lobes | 44 | Yes: CMV | NA | Ganciclovir | Dead (pneumonia, day +125) |
| M/51 | Ground‐glass area of the lower left lobe | 52 | Yes: CMV | NA | Ganciclovir | Alive (day +178) |
| M/72 | Interstitial thickening of the upper left lobe; area of dysventilation of the lower left lobe with pleural effusion | 56 | Yes: CMV and galactomannan positive | Probable IFI | Voriconazole + ganciclovir | Alive (day +184) |
F = female; M = male; BAL, bronchoalveolar lavage; SCT, stem cell transplant; IFI, invasive fungal infection; EORTC, European Organization for Research and Treatment of Cancer; NA, stands for no clinical criteria for possible IFI diagnosis as defined by EORTC criteria.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
This retrospective analysis brings evidence to the efficacy of caspofungin as primary antifungal prophylaxis in patients undergoing Haplo‐SCT with PT‐Cy that are at higher risk of mold infections relative to MRD counterparts. We found a 100‐day cumulative incidence of PP‐IFI of 8.7% that compares well with the ones reported in the literature in recipients of Haplo‐SCT.21
In the past few years, T‐cell–replete Haplo‐SCT has gradually replaced T‐cell–depleted (TCD) transplant because of supposed better immune reconstitution and low incidence of immunological complications and opportunistic infections. Ciurea et al described22 that the risk of IFI was 5.6 times higher after TCD transplant compared with T‐cell–replete Haplo‐SCT with PT‐Cy due to delayed immune reconstitution. The Beijing group, which utilized the GIAC platform for haploidentical‐SCT, found a cumulative incidence of IFI of 8.25% at 40 days and 13.1% at 1 year after SCT.23 Di Bartolomeo et al,24 who used a similar platform by adding basiliximab to the already intensified immunosuppressive regimen, described a higher incidence of IFI, 12.5% in the early phase followed by 7% in the late one. In the Baltimore platform, cumulative incidence of PP‐IFI varies between 8% and 16%.25 In the retrospective study by Raiola et al26 where fluconazole was used as PAP, 16% of Haplo‐SCT recipients experienced PP‐IFI, with Aspergillus as the most frequent agent followed by Candida non‐albicans and Fusarium species. In another report from the same group, where most of the patients received fluconazole and a minority caspofungin followed by itraconazole, cumulative incidence of PP‐IFI was 8%.27 In a recent experience, we reported that micafungin prophylaxis yielded a cumulative incidence of PP‐IFI of 12%.28 The present study compares well with all mentioned reports of IFI in recipients of Haplo‐SCT with PT‐Cy. 100‐day cumulative incidence of IFI of 8.7% stands in the lower range of IFI documented using the Baltimore platform, even if it is not possible to compare the present results with previous studies due to the great variability of conditioning regimen, disease type, and pre‐transplant disease status. It is important to point out that no patients died because of IFI in our cohort of patients. This result is quiet similar to the reports by Raiola et al,26, 27 but very different from the Beijing experience where 3‐year OS of patients with IFI was 24% vs 71% for patients without IFI.23 Whether these differences may be due to different GVHD prophylaxis regimens, with the one from Beijing being more intensified, or to the different hospital microbiological flora is difficult to interpret.
The optimal treatment for antifungal prophylaxis in Allo‐SCT recipients remains uncertain. This is mainly due to the lack of head‐to‐head randomized clinical trial that directly compares more than 2 different drug options in Allo‐SCT patients. The recent identification of risk factors for IFI and the following distinction of Allo‐SCT into low‐/standard‐ vs high‐risk categories make the results of previous randomized studies even more outdated because they are not enough powered to detect differences in drugs’ efficacy according to the IFI risk. A recent meta‐analysis of five randomized studies has shown that relative to fluconazole, PAP with itraconazole or voriconazole or posaconazole reduced the incidence of PP‐IFI, whereas posaconazole and voriconazole seemed superior to oral itraconazole in order to reduce the incidence of invasive aspergillosis.29 In details, cumulative incidence of PP‐IFI was very low in these phase 3 studies, ranging between 1% and 5%. Of note, these studies included only patients receiving a matched related or matched unrelated donor; therefore, it is not well known the efficacy of voriconazole and posaconazole in the Haplo‐SCT setting. In accordance with these findings, most recent updates of ECIL,11, 13 GITMO,12 German, and Australian guidelines30, 31 consistently proposed fluconazole as PAP for low‐/standard‐risk transplant and an anti‐mold agent of choice between posaconazole, voriconazole, and itraconazole for subjects at high risk. Caspofungin received a C level of recommendation by the Australian guidelines and a lack of data by ECIL‐511 and by the last ECIL recommendations.13 In this context, our study is the first report in adults providing evidence for efficacy of caspofungin at preventing IFI in Haplo‐SCT with PT‐CY that are at high risk of mold infections. One recent study in pediatric patients has compared amphotericin B (L‐AmB) with caspofungin as PAP after Allo‐SCT.32 In this study, caspofungin resulted in an excellent protection against IFI with only 1 out of 60 patients developing PP‐IFI. Another important observation of this study, besides similar efficacy of the two compounds, was the superior safety profile of caspofungin relative to L‐AmB. Consistently, we did not observe either kidney of liver toxicity and interaction with cyclosporine was very low, therefore avoiding the need for frequent cyclosporine dose adjustment as expected with fluconazole (data not shown). Therefore relative to other available antifungal agents, caspofungin provides both some advantages and disadvantages: It is probably more effective than fluconazole against mold infections and has less interactions with other drugs, but it is more expensive; relative to posaconazole and voriconazole, it is not clear whether it provides the same level of protection against mold infections, but it is less expensive and has a safer profile in terms of organ toxicity and drug interactions.
Risk factors for developing IFI in patients receiving haploidentical‐SCT are not very well known. Sun et al23 in the GIAC platform identified platelet engraftment time (>17 days), grade III‐IV acute GVHD, and high‐risk underlying disease as the most important risk factors for IFI. In our study, we have found by univariate analysis a direct correlation between risk of IFI and increasing number of days of neutropenia, but this was not confirmed by multivariate analysis where advanced DRI came out as the only variable affecting the probability of developing IFI. However, this result should be read with caution due to the relative low number of PP‐IFI (n = 8) events in our cohort.
Of course, this retrospective study is affected by several limitations. The fact that most patients received RIC or NMA transplant may have resulted in reduced incidence of PP‐IFI. Of note, we did not observe any increased incidence of PP‐IFI in the subgroup receiving MAC transplant (data not shown). Moreover, we restricted our analysis only to the first 100 days after transplant that may have induced us to lose a small part of events. We decided to do so because many factors other than antifungal prophylaxis, such as acute and chronic GVHD, are known to play a predominant role in the occurrence of IFI after day +100.7 Moreover, recent evidences such as the ones from GITMO showed that most PP‐IFI (76%) occurred in the first 100 days after allo‐SCT. Another limitation of this study concerns the comparison with itraconazole prophylaxis. Unfortunately, even if we observed a trend for reduced PP‐IFI in the cohort receiving caspofungin, no definitive conclusion can be taken probably because of the different characteristics of the 2 groups (patients with caspofungin had more adverse pre‐transplant features). Moreover, data on blood concentration of itraconazole are not available, even if all patients received iv itraconazole, thus reducing the risk of low blood levels as frequently observed with the oral formulation.
In conclusion, we found that PAP with caspofungin in the pre‐engraftment phase results in a manageable incidence of PP‐IFI in the platform of Haplo‐SCT with PT‐Cy. Larger and prospective studies are warranted in order to identify which agent may further reduce the cumulative incidence of proven/probable IFI in the context of Haplo‐SCT with PT‐Cy.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
Supporting information
Mariotti J, De Philippis C, Bramanti S, et al. Caspofungin for primary antifungal prophylaxis after T‐cell–replete haploidentical stem cell transplantation with post‐transplant cyclophosphamide. Eur J Haematol. 2019;102:357–367. 10.1111/ejh.13214
REFERENCES
- 1. Pagano L, Caira M, Nosari A, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B‐2004 study–Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161‐1170. [DOI] [PubMed] [Google Scholar]
- 2. Garcia‐Vidal C, Upton A, Kirby KA, Marr KA. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041‐1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant‐Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 4. Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009;48:265‐273. [DOI] [PubMed] [Google Scholar]
- 5. Kurosawa M, Yonezumi M, Hashino S, et al. Epidemiology and treatment outcome of invasive fungal infections in patients with hematological malignancies. Int J Hematol. 2012;96:748‐757. [DOI] [PubMed] [Google Scholar]
- 6. Nucci M, Garnica M, Gloria AB, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect. 2013;19:745‐751. [DOI] [PubMed] [Google Scholar]
- 7. Girmenia C, Raiola AM, Piciocchi A, et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: a prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20:872‐880. [DOI] [PubMed] [Google Scholar]
- 8. Gao L, Sun Y, Meng F, et al. Antifungal prophylaxis of patients undergoing allogenetic hematopoietic stem cell transplantation in China: a multicenter prospective observational study. J Hematol Oncol. 2016;9(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omer AK, Ziakas PD, Anagnostou T, et al. Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a single center experience. Biol Blood Marrow Transplant. 2013;19(8):1190‐1196. [DOI] [PubMed] [Google Scholar]
- 10. Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in haematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909‐917. [DOI] [PubMed] [Google Scholar]
- 11. http://www.kobe.fr/ecil/telechargements2013/ECIL5%20Antifungal%20Therapy.pdf.
- 12. Girmenia C, Barosi G, Piciocchi A, et al. Primary prophylaxis of invasive fungal diseases in allogeneic stem cell transplantation: revised recommendations from a consensus process by Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol Blood Marrow Transplant. 2014;20:1080‐1088. [DOI] [PubMed] [Google Scholar]
- 13. Maertens JA, Girmenia C, Brüggemann RJ, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 73(12), 3221–3230. [DOI] [PubMed] [Google Scholar]
- 14. Luznik L, O'Donnell PV, Symons HJ, et al. HLA‐haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high‐dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 16. Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft‐versus‐host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 17. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 19. Kaplan EL, Meier P. Non‐parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20. Cox D. Regression models and life tables. J R Stat Soc (B). 1972;34:187–220. [Google Scholar]
- 21. Fabricius WA, Ramanathan M. Review on haploidentical hematopoietic cell transplantation in patients with hematologic malignancies. Adv Hematol. 2016;2016:5726132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ciurea SO, Mulanovich V, Saliba RM, et al. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18(12):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun YQ, Xu LP, Liu DH, et al. The incidence and risk factors of invasive fungal infection after haploidentical haematopoietic stem cell transplantation without in vitro T‐cell depletion. Clin Microbiol Infect. 2012;18(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 24. Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G‐CSF‐primed bone marrow transplantation for patients with high‐risk hematologic malignancies. Blood. 2013;121(5):849–857. [DOI] [PubMed] [Google Scholar]
- 25. Aversa F, Prezioso L, Manfra I, Galaverna F, Spolzino A, Monti A. Immunity to infections after haploidentical hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis. 2016;8(1):e2016057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–122. [DOI] [PubMed] [Google Scholar]
- 27. Raiola A, Dominietto A, Varaldo R, et al. Unmanipulated haploidentical BMT following non‐myeloablative conditioning and post‐transplantation CY for advanced Hodgkin's lymphoma. Bone Marrow Transplant. 2014;49(2):190–194. [DOI] [PubMed] [Google Scholar]
- 28. Crocchiolo R, Bramanti S, Vai A, et al. Infections after T‐replete haploidentical transplantation and high‐dose cyclophosphamide as graft‐versus‐host disease prophylaxis. Transpl Infect Dis. 2015;17(2):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bow EJ, Vanness DJ, Slavin M, et al. Systematic review and mixed treatment comparison meta‐analysis of randomized clinical trials of primary oral antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. BMC Infect Dis. 2015;15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tacke D, Buchheidt D, Karthaus M, et al. Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. 2014 update of the recommendations of the Infectious Diseases Working Party of the German Society for Haematology and Oncology. Ann Hematol. 2014;2014(93):1449–1456. [DOI] [PubMed] [Google Scholar]
- 31. Fleming S, Yannakou CK, Haeusler GM, et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haematopoietic stem cell transplantation, 2014. Intern Med J. 2014;44:1283–1297. [DOI] [PubMed] [Google Scholar]
- 32. Döring M, Hartmann U, Erbacher A, Lang P, Handgretinger R, Müller I. Caspofungin as antifungal prophylaxis in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective analysis. BMC Infect Dis. 2012;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


