Summary
Equine coronavirus (ECoV) is an emerging virus associated clinically and epidemiologically with fever, depression, anorexia and less frequently colic and diarrhoea in adult horses. Sporadic cases and outbreaks have been reported with increased frequency since 2010 from Japan, the USA and more recently from Europe. A faeco‐oral transmission route is suspected and clinical or asymptomatic infected horses appear to be responsible for direct and indirect transmission of ECoV. A presumptive clinical diagnosis of ECoV infection may be suggested by clinical presentation, haematological abnormalities such as leucopenia due to lymphopenia and/or neutropenia. Confirmation of ECoV infection is provided by specific ECoV nucleic acid detection in faeces by quantitative PCR (qPCR) or demonstration of coronavirus antigen by immunohistochemistry or electron microscopy in intestinal biopsy material obtained ante or post mortem. The disease is generally self‐limiting and horses typically recover with symptomatic supportive care. Complications associated with disruption of the gastrointestinal barrier have been reported in some infected horses and include endotoxaemia, septicaemia and hyperammonaemia‐associated encephalopathy. Although specific immunoprophylactic measures have been shown to be effective in disease prevention for closely‐related coronaviruses such as bovine coronavirus (BCoV), such strategies have yet not been investigated for horses and disease prevention is limited to basic biosecurity protocols. This article reviews current knowledge concerning the aetiology, epidemiology, clinical signs, diagnosis, pathology, treatment and prevention of ECoV infection in adult horses.
Keywords: horse, equine coronavirus, clinical signs, diagnosis, treatment
Aetiology
Coronaviruses are members of the Coronaviridae family, all of which are single‐stranded, positive‐sense, nonsegmented, enveloped RNA viruses responsible for enteric, respiratory, hepatic or neurological disease in a variety of mammalian and avian species (Wege et al. 1982). The family Coronaviridae is subdivided into two subfamilies, Torovirinae and Coronavirinae, with the latter subfamily containing four genera defined on the basis of serological cross‐reactivity and genetic differences: Alphacoronavirus, Betacoronavirus, Deltacoronavirus and Gammacoronavirus (Woo et al. 2012). Equine coronavirus (ECoV) is classified within the Betacoronavirus genus, along with human OC43 and HKU1 coronavirus, bovine coronavirus, porcine haemagglutinating encephalomyelitis virus, canine respiratory coronavirus, mouse hepatitis virus and sialodacryoadenitis virus (Zhang et al. 2007).
The nucleocapsid protein of coronaviruses complexes with the genomic RNA to form a helical capsid structure found within the viral envelope. Trimers of the spike protein form peplomers embedded in the envelope giving the virion its corona or crown‐like morphology. In ECoV, the haemagglutinin esterase protein forms smaller spikes on the membrane. There are two fully transmembraneous structural proteins referred to as the membrane and small membrane proteins in the coronaviral virion (Weiss and Leibowitz 2011).
Epidemiology
Epidemiological information for ECoV is sparse at this time and either extrapolated from close‐related coronaviruses such as BCoV or largely based on nonpublished preliminary observations (Table 1).
Table 1.
Documented or observed epidemiological factors for BCoV and ECoV
| Epidemiological factors | BCoV (Winter dysentery) | ECoV |
|---|---|---|
| Case presentation | Epizootic | Sporadic and epizootic |
| Age of affected animals | Predominantly cattle | Predominantly adult horses |
| Seasonality | Usually during winter month (November to April) | Year around with increased reports during cold months (October to April) |
| Transmission route | Faeco‐oral | Suspected faeco‐oral for natural disease, naso‐oesophageal for experimental disease |
| Geographic distribution | More common in northern states | No geographic predilection |
| Morbidity rate | High (30–100%) | Variable (10–80%) |
| Mortality rate | Rare | Low |
| Incubation time | 2–8 days | 2–3 days |
| Outbreak duration | Less than 2 weeks | 2–3 weeks |
| Period of illness | Few days to 1 week | Few days to 1 week |
| Clinical signs | Explosive diarrhoea, anorexia, depression, reduced milk production | Anorexia, depression, fever, less frequently diarrhoea, colic, neurological signs |
| Diagnosis | Antigen‐capture ELISA, fluorescent antibody, qPCR, electron microscopy | qPCR |
| Recovery | Fast, generally self‐limiting disease | Fast, generally self‐limiting disease |
| Prevention | Vaccination and biosecurity | Biosecurity |
BCoV, bovine coronavirus; ECoV, equine coronavirus, quantitative PCR (qPCR).
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Clinical cases are either sporadic or epizootic. Collective data from three veterinary diagnostic laboratories in the USA (IDEXX Laboratories, Inc., West Sacramento; Animal Health Diagnostic Center (AHDC), Cornell University, Ithaca; Real‐time PCR Research and Diagnostics Core Facility, University of California, Davis) shows that the overall number of ECoV qPCR‐positive cases has steadily increased since 2010 (approximate time when ECoV testing by qPCR was introduced by molecular diagnostic laboratories) and that similar to BCoV, the case number is higher during the colder months of the year (October to April; Fig 1). The geographic origin of ECoV qPCR‐positive equids, based on zip codes of the submitting veterinary practices, is widespread across the lower 48 states of the USA (Fig 2). Clinical ECoV infection has predominantly been reported in adult horses (Oue et al. 2011, 2013; Pusterla et al. 2013; Fielding et al. 2015). Outbreaks have predominantly been reported in riding, racing and show horses and less frequently in breeding animals. Two breeding farms in New York state have experienced ECoV disease. On one farm, a yearling Standardbred filly died from an acute enteritis and on necropsy, faeces tested qPCR‐positive for ECoV and IHC was positive for coronavirus on small intestine. No additional cases were documented on this large farm even though this filly was pastured with other horses. Another breeding farm with 30 American miniature horses experienced an outbreak with a morbidity of 83% (L. Mittel, personal communication 2015). One hypothesis behind the lack of large numbers of documented clinical cases at larger breeding farms is the frequent circulation of ECoV between asymptomatic young animals and adult resident horses, conferring protection against clinical disease in adult horses.
Figure 1.
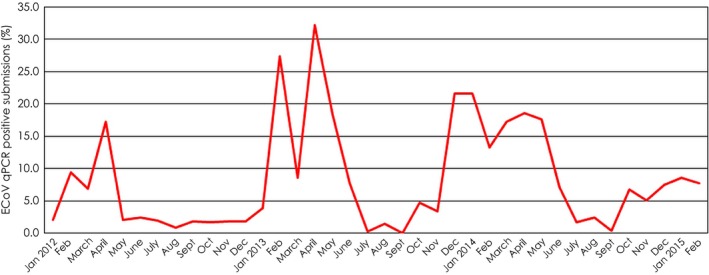
Percentage of ECoV PCR positive submissions amongst all equine enteric submissions processed from January 2012 to February 2015 at 3 commercial veterinary diagnostic laboratories in the USA (IDEXX Laboratories, Inc., West Sacramento; Animal Health Diagnostic Center, Cornell University, Ithaca; Real‐time PCR Research and Diagnostics Core Facility, University of California, Davis).
Figure 2.
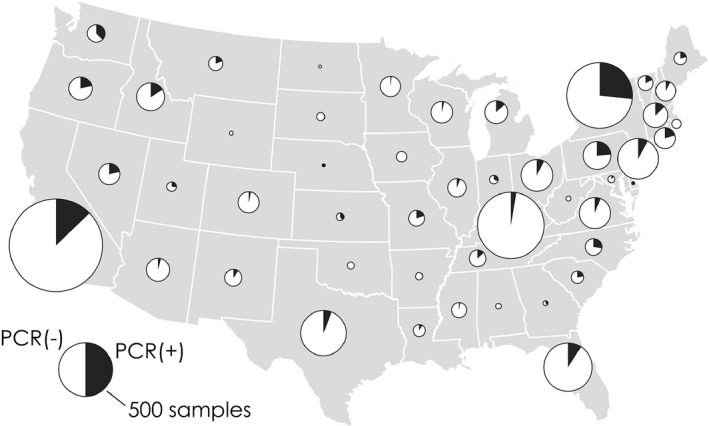
Relative proportion of ECoV qPCR‐positive (black) and qPCR‐negative (white) samples by states submitted from January 2012 to February 2015 to 3 commercial veterinary diagnostic laboratories in the USA (IDEXX Laboratories, Inc., West Sacramento; Animal Health Diagnostic Center, Cornell University, Ithaca; Real‐time PCR Research and Diagnostics Core Facility, University of California, Davis).
Preliminary epidemiological observations support a faeco‐oral route of transmission. This is supported by a recent experimental study in which three 9–10‐month‐old Japanese draught horses were successfully infected via naso‐oesophageal intubation using faecal material from a previously characterised ECoV infected horse (Nemoto et al. 2014) Two of the three draught horses developed clinical disease and all shed large amounts of ECoV in faeces. Bovine coronavirus (BCoV) is closely related to ECoV and considered a pneumoenteric virus, causing not only enteric disease but also mild upper respiratory signs (Saif et al. 1986; Tsunemitsu et al. 1999; Tråvén et al. 2001; O'Neill et al. 2014). Recently, a French group screened 395 faeces and 200 respiratory specimens submitted to a veterinary diagnostic laboratory for the presence of ECoV (Miszczak et al. 2014). The samples had been collected from foals and adult horses suffering from mild respiratory or enteric disease. In that study, the researchers found ECoV by qPCR in a total of 12 samples (11 faecal samples and one respiratory specimen). In a preliminary study testing nasal secretions from 2437 horses with signs of fever and/or acute onset of upper respiratory tract infection submitted to a commercial diagnostic laboratory in the USA from January 2013 to December 2014 for the detection of common respiratory pathogens, ECoV was detected by qPCR in only 17 (0.7%) horses (N. Pusterla, personal communication 2015). Collectively, both these studies show that ECoV is infrequently detected in nasal secretions from horses with infectious upper respiratory tract disease, suggesting lack of tropism for ECoV to the respiratory epithelium. In the three experimentally‐infected Japanese draught horses (Nemoto et al. 2014), nasal secretions were ECoV PCR positive during the time of peak faecal shedding but it could not be determined if this was due to nasal replication and shedding of the virus or from environmental contamination from the positive faeces or both.
Morbidity of ECoV in affected herds is variable and has been reported to range between 10 and 83% (Oue et al. 2011, 2013; Pusterla et al. 2013; Fielding et al. 2015). Mortality rates have been reported to be low (Oue et al. 2011, 2013; Pusterla et al. 2013), although a mortality rate of 27% was recently reported by Fielding et al. (2015) in American miniature horses. A recent outbreak investigated by the AHDC on an additional American miniature horse breeding farm found laboratory confirmed ECoV cases that developed serious but nonfatal illness (L. Mittel, personal communication 2015). The apparent increased susceptibility to ECoV disease in American miniature horses needs to be further investigated.
Mortality has been associated with endotoxaemia, septicaemia and hyperammonaemia‐associated encephalopathy (Pusterla et al. 2013; Fielding et al. 2015; Giannitti et al. 2015). A large number of documented clinical cases will be needed to determine the effect of various host, viral, treatment and environmental factors on outcome, as well as the identification of risk factors associated with risk of morbidity.
Similar to BCoV the incubation period is short and clinical disease develops between 48 and 72 h after either natural exposure or experimental infection (Nemoto et al. 2014; Fielding et al. 2015). Clinical signs persist for a few days to one week and generally resolve with minimal supportive care. Occasionally clinical signs can last longer as recently identified in two ECoV cases from Florida that experienced colic and systemic illness for 14 days (L. Mittel, personal communication 2015). Shedding post experimental infection as documented by qPCR ranged between 10 and 12 days (Nemoto et al. 2014). Faecal shedding of ECoV under natural conditions has been reported to range between 3 and 25 days (Pusterla et al. 2013; Fielding et al. 2015; L. Mittel, personal communication 2015). It is not clear how long ECoV will persist in the environment and potentially act as the source of infection. It is reasonable to draw comparisons from other coronaviral pathogens such as severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV) which has been shown in vitro to persist for 2 days in hospital wastewater, domestic sewage and dechlorinated tap water, while 3 days in faeces, 14 days in PBS and 17 days in urine at 20°C. However, at 4°C, the SARS‐CoV could persist for 14 days in wastewater and at least 17 days in faeces or urine (Wang et al. 2005). Enhanced replication of the virus in faeces in the environment during colder weather is one possible explanation for the apparent higher prevalence of test positive faecal samples and clinical disease during cooler weather. Equine coronavirus disease can occur in one or more horses on a farm with no recent exposure to new horses (T. Divers, personal communication 2015). Hypothetically, this might be explained by indirect ECoV transmission by nonequine animal species or that colder weather might allow increased faecal replication of the virus from a chronically infected horse.
Clinical presentation
Clinical information collected from 16 outbreaks during the time period of November 2011 to December 2014 on a total of 406 horses showed that 122 horses (30%) showed clinical signs (Pusterla et al. 2013; N. Pusterla, personal communication 2015). The main clinical signs reported were anorexia (98%), lethargy (89%) and fever (84%). The rectal temperature of febrile horses ranged from 38.6 to 41°C (median 39.9°C). Changes in faecal character, ranging from soft‐formed to watery consistency and colic were observed in 25 and 18% of horses with any clinical signs, respectively. Gastrointestinal signs are generally preceded by systemic signs of anorexia and fever (Fig 3). These results are in agreement with recent outbreaks from Japan in adult draught horses reporting that anorexia and pyrexia were the main clinical signs, while specific gastrointestinal signs were observed in about 10% of affected horses (Oue et al. 2011, 2013). In 3% of infected horses signs of encephalopathy including circling, head pressing, ataxia, proprioceptive deficits, nystagmus, recumbency and seizure have also been reported (Fig 4; Pusterla et al. 2013; Fielding et al. 2015).
Figure 3.
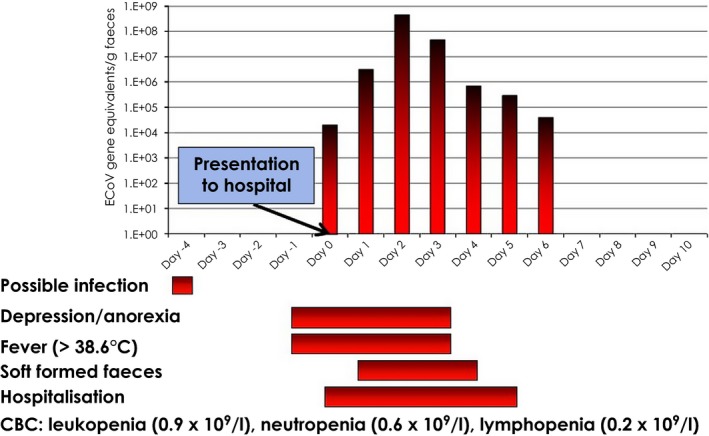
Diagram showing temporal clinical signs and faecal shedding of ECoV in an adult horse presented to a referring hospital because of anorexia, depression and fever. Whole blood for haematological analysis was collected on admission to the hospital.
Figure 4.

Head pressing in an adult American miniature horse with clinical ECoV infection.
Although clinical disease is apparent in 10–83% of ECoV infected horses, one needs to take into account that some horses remain asymptomatic after infection. Asymptomatic infection is defined as lack of clinical disease in a horse from which ECoV is detected in faeces by qPCR. The percentage of asymptomatic horses during an outbreak of ECoV has been observed to range between 11 and 83% (Pusterla et al. 2013; L. Mittel, personal communication 2015).
Diagnostic evaluation
The antemortem diagnosis of ECoV relies on the presence of clinical signs compatible with ECoV infection, neutropenia and/or lymphopenia, the exclusion of other infectious causes and molecular detection of ECoV in faeces.
The consistently observed haematological abnormalities observed with ECoV infection are leucopenia due to neutropenia and/or lymphopenia. The blood work from 73 clinical cases with suspected ECoV infection showed that the total nucleated cell count ranged 0.5–7.8×109/l (median 3.4×109/l; reference interval 5.0–11.6×109/l; Fig 5). The white cell blood count in the 73 diseased horses showed leucopenia in 25%, neutrophilia in 66% and lymphopenia in 72% of the horses. The neutrophil and lymphocyte counts in these horses ranged from 0.2–5.1×109/l (median 1.6×109/l; reference interval 2.6–6.8×109/l) and 0.8–3.6×109/l (median 1.1×109/l; reference interval 1.6–5.8×109/l), respectively. Both CBC and differential were unremarkable in only 11% of the horses. Additional, less consistent haematological abnormalities included the presence of band neutrophils and shifts in monocyte counts (most typically shifting from a low or a normal count to a high‐normal or rebound monocytosis). Occasional rebound leucocytosis due to neutrophilia and monocytosis during the disease course and recovery was also observed. Complete blood count abnormalities are expected to resolve within 5–7 days as long as no complications associated with the disruption of the gastrointestinal barrier occur. Biochemical parameters may be unremarkable but elevation of total and indirect bilirubin due to partial or complete anorexia, electrolyte changes consistent with enterocolitis, transient elevation of liver enzymes and renal parameters suggestive of prerenal azotaemia have been observed in some of the cases. It is judicious to measure blood ammonia in horses with suspected ECoV infection and concurrent signs of encephalopathy. Fielding et al. (2015) reported a case of severe hyperammonaemia (677 μmol/l; reference interval ≤60 μmol/l) with encephalopathic signs that subsequently died. Hyperammonaemia associated with ECoV infection is likely due to increased ammonia production within or absorption from the gastrointestinal tract due to gastrointestinal barrier breakdown. An increase in enteric ammonia production could also be the result of bacterial microbiome changes associated with EcoV infection.
Figure 5.
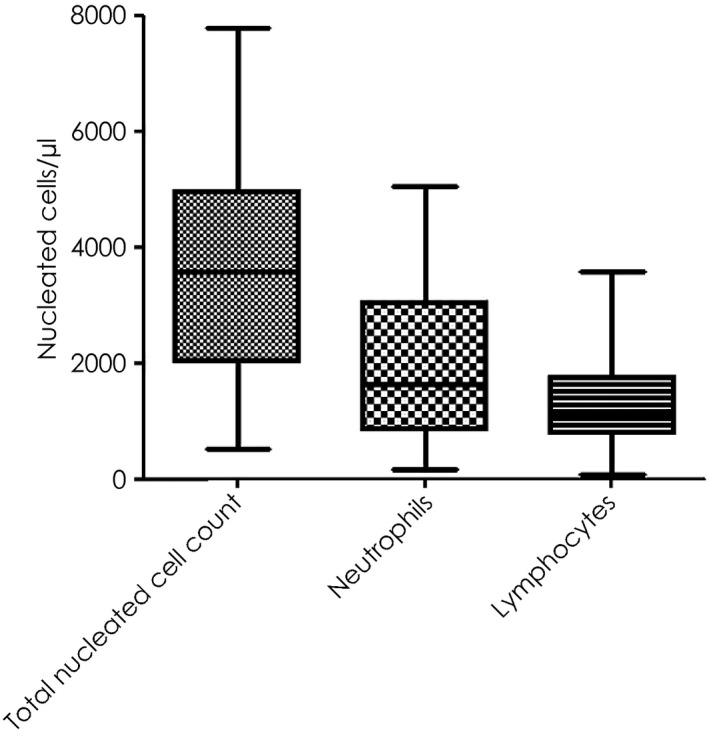
Haematological findings in 73 adult horses with laboratory confirmation of ECoV infection. The results are expressed as box and whiskers.
Isolation of ECoV in human rectal adenocarcinoma cells, although difficult has been previously reported (Guy et al. 2000; Oue et al. 2011). The need for cost‐effective and timely detection methods has restricted the diagnosis of ECoV infection to direct demonstration of coronavirus antigens or specific nucleic acids in biological samples. Historically, coronavirus detection in faeces had been based on negative‐stain EM and antigen‐capture ELISA (Davis et al. 2000; Guy et al. 2000). However, the sensitivity and specificity of these diagnostic modalities has not been evaluated and detection may be unsuccessful if viral particles are not present in sufficient numbers. Timely, cost‐effective and sensitive laboratory diagnosis of ECoV is through obtained faecal quantitative PCR (qPCR). A recent study evaluated the overall accuracy of qPCR and determined 90% accuracy between clinical status and PCR detection of ECoV in various outbreak populations (Pusterla et al. 2013). The authors have documented a few cases of ECoV infection which tested qPCR‐negative during early disease. These few horses ended testing qPCR‐positive on a 24–48 h recheck faecal sample. It is hypothesised that during peracute stages of infection, or when diseased horses experience gastrointestinal stasis due to colic, there are not enough viral particles in the faeces to be detected. The recommendation is to retest faecal matter in a suspected index case at a later time point or collect multiple samples for pooled testing. Viral kinetics of ECoV in faeces from experimentally‐infected horses showed that horses began to shed ECoV RNA in their faeces at 3 or 4 days post infection and continued shedding virus until 12 or 14 days post infection (Nemoto et al. 2014). Peak shedding is generally seen on Day 3–4 following the development of clinical signs (Fig 3) and qPCR detection of ECoV in naturally infected horses can last for 3–25 days (Pusterla et al. 2013; Fielding et al. 2015; L.D. Mittel, personal communication 2015).
Interestingly, faecal viral load measured by qPCR in foals (up to 6 months) appears to be lower compared with horses older than 12 months of age, although the difference is not statistically significant (P = 0.307). This observation suggests that viral replication in the gut is comparable (L. Leutenegger, personal communication 2015) between foals and older horses. In coronavirus infections in man, viral load is a strong prognostic indicator for clinical outcome and mortality (Hung et al. 2009). A similar observation has recently been reported for ECoV (Fielding et al. 2015). The viral load shed in human patients infected with coronavirus is associated with polymorphisms of genes involved in innate immunity, an individual's genetic make‐up and immunological host response to the virus (Chen et al. 2008). In addition, coronavirus strain variations can influence the replication ability and viral load as shown with feline coronavirus (FCoV) which exists in two genotypes: a benign feline enteric coronavirus genotype (FECV) with low replication competency in enterocytes and a highly virulent feline infectious peritonitis virus (FIPV) with an enhanced ability to replicate in different cell populations (Chang et al. 2012). Single point mutations on the spike gene are responsible for dramatic changes in cell tropism, replication competency and clinical manifestations.
Necropsy cases of suspected enteritis should have faeces or gastrointestinal content tested by qPCR for ECoV and other gastrointestinal infectious agents. Further, formalin‐fixed intestinal tissue samples can also be tested by immunochemistry and direct fluorescent antibody testing using BCoV reagents (Giannitti et al. 2015).
Pathogenesis
Enteritis caused by ECoV has been suspected in foals for many years but the direct pathogenicity of ECoV has only previously been described in one neonate (Davis et al. 2000). Further, the high and similar frequency of ECoV shedding detected in healthy foals and foals with gastrointestinal diseases from central Kentucky suggests that ECoV commonly circulates among young horses with subclinical disease (Slovis et al. 2014). An interesting observation is that all ECoV infections in foals with gastrointestinal disease were associated with coinfections, while most healthy foals infected with ECoV displayed a monoinfection (Slovis et al. 2014). This observation may indicate that in foals, pre‐existing ECoV infection may predispose to opportunistic secondary viral, bacterial or protozoal infections as shown for enteric and respiratory coronavirus in other species (Pakpinyo et al. 2003; Srikumaran et al. 2007; Brockmeier et al. 2008). Other hypothetical reasons for the lack of clinical signs in foals infected solely with ECoV is that host factors such as absence of specific receptor binding sites or presence of ECoV‐specific colostrally‐derived antibodies prevent the development of enteritis. However, in the vast majority of clinical adult horses, ECoV is a monoinfection, demonstrated by enteric panel qPCR testing. This suggests a unique pathogenicity, coinfection with still unknown pathogens or a distinct difference in immunological reaction between foals and adult horses towards the ECoV infection similar to FCoV. The development of a humoral, cell‐mediated or mixed immune response against the highly virulent FIPV version of FCoV distinctly influences the clinical outcome of the infection. Interestingly, the clinical outcome of FIP has been correlated with a severe suppression of NK cells and T regulatory cells confirming earlier reports that a weakened cell‐mediated immune response is associated with fatal FIPV infection (Vermeulen et al. 2013).
In adult horses, the pathology of ECoV has recently been described in three equids (Giannitti et al. 2015). The ECoV‐infected equids displayed severe diffuse necrotising enteritis with marked villus attenuation, epithelial cell necrosis in the tips of the villi, neutrophilic and fibrin extravasation into the small intestinal lumen (pseudomembrane formation), as well as crypt necrosis, microthrombosis and haemorrhage (Fig 6). Equine coronavirus was detected by qPCR in small intestinal tissue, gastrointestinal content and/or faeces and coronavirus antigen detected by immunohistochemistry and/or direct fluorescent antibody testing in the small intestine of all cases (Figs 7 and 8).
Figure 6.
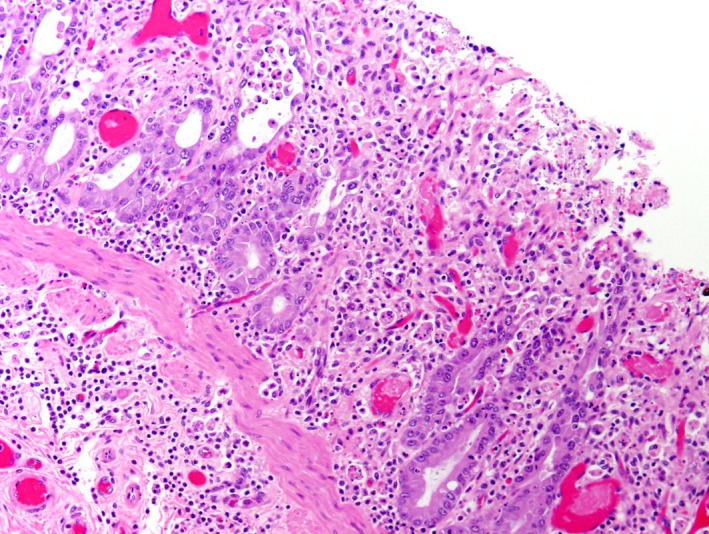
This illustration shows the H&E stain of jejunum from an adult horse with ECoV infection. There is loss of crypts and few remaining crypts are dilated, lined by attenuated epithelium and contain sloughed necrotic enterocytes. The lamina propria and superficial submucosa are expanded by inflammatory infiltrates. Capillaries and venules in the mucosa and submucosa are occluded by fibrin thrombi.
Figure 7.
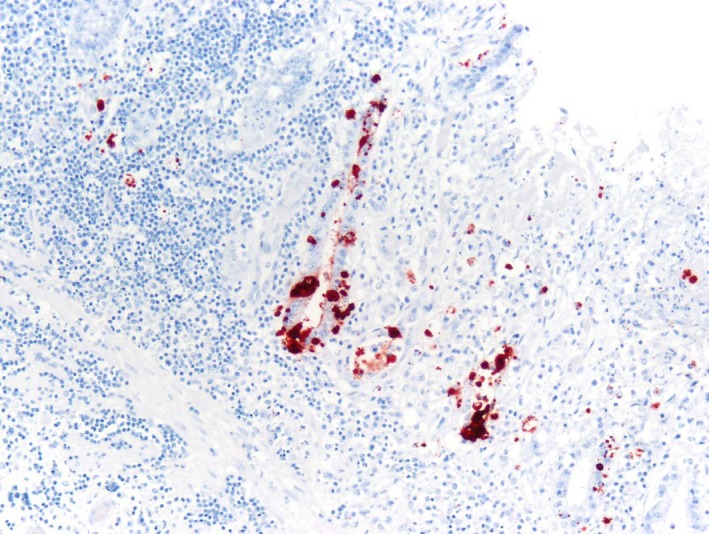
Bovine coronavirus immunohistochemistry and haematoxylin counterstain of jejunum from an adult horse with ECoV infection. Strong granular/globular immunoreactivity is seen in the cytoplasm of deep gland enterocytes.
Figure 8.
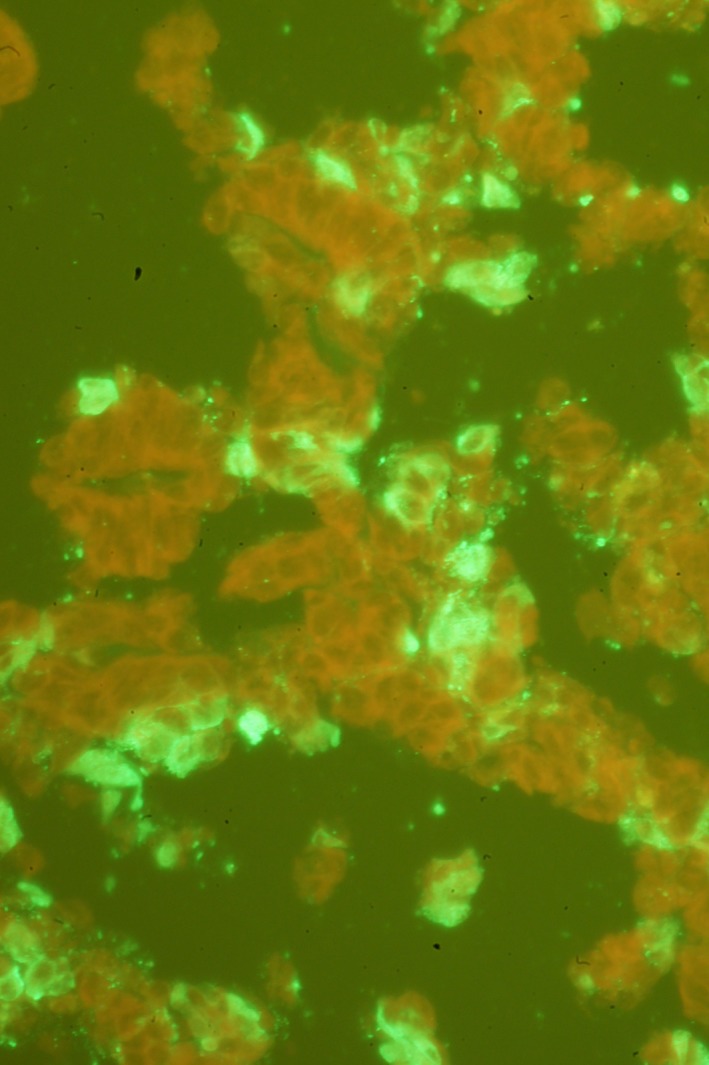
Direct fluorescent antibody test using bovine coronavirus antiserum in the jejunum of an adult horse with ECoV infection. Bright green immunofluorescent signal in the cytoplasm of enterocytes represents coronavirus particles.
Treatment and prevention
Most adult horses with clinical ECoV infection recover spontaneously in a few days without specific treatment. Horses with persistent elevated rectal temperature, anorexia and depression are routinely treated with nonsteroidal anti‐inflammatory drugs such as flunixin meglumine (0.5–1.1 mg/kg bwt q. 12–24 h intravenously (i.v.) or per os) or phenylbutazone (2–4 mg/kg bwt q. 12–24 h i.v. or per os) for 24–48 h, as long as their hydration status is believed normal. Horses with colic, persistent depression and anorexia and/or diarrhoea have been treated more intensively with fluid and electrolyte per nasogastric intubation or i.v. administration of polyionic fluids until clinical signs have resolved. Additionally, antimicrobials and gastrointestinal protectants should be considered in horses developing signs of endotoxaemia and/or septicaemia secondary to disruption of the gastrointestinal barrier. While hyperammonaemia‐associated encephalopathy only occurs in a small percentage of horses with ECoV infection, early recognition and treatment is associated with a positive outcome. Horses with suspected or documented hyperammonaemia are treated with oral lactulose (0.1–0.2 ml/kg bwt q. 6–12 h per os), neomycin sulfate (4–8 mg/kg bwt q. 8 h per os) or faecal transfaunation and crystalloid fluids.
Immunisation strategies have been best described in cattle for the prevention of winter dysentery infection using a modified‐live commercially available BCoV vaccine (Welter 1998). The use of BCoV vaccine in horses for the prevention of ECoV has, to the authors' knowledge, not been investigated and cannot be recommended at this time due to the lack of safety and efficacy data and sporadic incidence of disease. Cattle that recovered from winter dysentery after experimental infection with BCoV maintained a very long‐lasting BCoV‐specific serum (IgA and IgG) and local (IgA) antibody response (Tråvén et al. 2001).
Biosecurity recommendations
The prevention of ECoV infection should focus on the implementation of routine management practices aimed at reducing the likelihood of introducing and disseminating ECoV at any horse‐based premise (boarding facility, show ground, veterinary hospital). Due to the highly contagious nature of ECoV, any horse developing or presenting with significant fever, anorexia and depression with or without enteric signs (colic, diarrhoea) should be strictly isolated until a diagnosis is secured. Such an approach can prevent the quarantine of an entire horse population should ECoV infection be subsequently diagnosed. Once ECoV infection is confirmed, strict isolation procedures and secondary quarantine of the source stable of the particular horse should be employed. Post infection testing of clinical cases should be done to prevent viral spread to other horses. At stables and farms, all newly arrived horses should ideally be isolated for at least 3 weeks. Once an ECoV infection is suspected or confirmed, strict biosecurity measures including footbaths and the use of personal protective equipment should be provided and adequately maintained for sanitary purposes. Separate equipment, tack, bedding and feedstuffs should be used in the care of these animals. Grooms and other personnel should be instructed to work with these animals last in the course of their daily routine. Exercise periods should be confined to a time when other horses are not present in the training areas and riders should wear protective clothing and clean and thoroughly disinfect their boots, tack and hands after contact with such animals. Horses returning from shows or extended travelling events should be isolated according to their particular circumstances. All horse vans and trailers should be thoroughly cleaned and disinfected after use. Equine coronavirus is susceptible to common disinfectants including sodium hypochlorite, povidone iodine, chlorhexidine gluconate, phenols, quaternary ammonium compounds, accelerated hydrogen peroxide and peroxygen compounds. The examination of at risk horses for clinical signs of disease, including twice daily assessment of rectal temperature, remains the most effective tool in determining possible sources of virus introduction.
Conclusion
Equine coronavirus infection is an emerging disease in adult horses reported in Europe, Japan and the USA. Increased awareness of this disease in the field and availability of diagnostics for detecting ECoV in faeces of affected horses have resulted in increased reports of the disease. The epidemiology and pathogenesis of ECoV infection in horses is now being actively investigated.
Authors’ declaration of interests
Drs Pusterla and Divers have no conflicts of interest to declare. Drs Vin and Leutenegger work for IDEXX Laboratories and Dr Mittel works for the Animal Health Diagnostic Center at Cornell University.
Ethical animal research
Informed consent was not available and the laboratories do not use an admission form allowing owners to opt out of research studies.
Source of funding
None.
Authorship
All authors contributed equally to the preparation of the manuscript and manuscript review.
Acknowledgements
Dr Giannitti, Veterinary Diagnostic Laboratory, Veterinary Population Medicine Department, University of Minnesota, St Paul, Minnesota for the histopathological pictures. Karen Sverlow, California Animal Health and Food Safety Laboratory System, University of California, Davis, California, for the IFA picture. The US map was made by Nicholas Hollingshead from the AHDC at Cornell University. The authors would also like to thank all the veterinarians who have been involved in various ECoV outbreaks for their reliable documentation of clinical and laboratory findings.
References
- Brockmeier, S.L. , Loving, C.L. , Nicholson, T.L. and Palmer, M.V. (2008) Coinfection of pigs with porcine respiratory coronavirus and Bordetella bronchiseptica . Vet. Microbiol. 128, 36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. , Egberink, H.F. , Halpin, R. , Spiro, D.J. and Rottier, P.J.M. (2012) Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 18, 1089‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Yang, J. , Lin, J. , Fann, C. , Osyetrov, V. , King, C. , Chen, Y. , Chang, H. , Kuo, H. , Liao, F. and Ho, M. (2008) Nasopharyngeal shedding of severe acute respiratory syndrome‐associated coronavirus is associated with genetic polymorphisms. Clin. Infect. Dis. 42, 1561‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, E. , Rush, B.R. , Cox, J. , DeBey, B. and Kapil, S. (2000) Neonatal enterocolitis associated with coronavirus infection in a foal: a case report. J. Vet. Diagn. Invest. 12, 153‐156. [DOI] [PubMed] [Google Scholar]
- Fielding, C.L. , Higgins, J.K. , Higgins, J.C. , McIntosh, S. , Scott, E. , Giannitti, F. , Mete, A. and Pusterla, N. (2015) Disease associated with equine coronavirus infection and high case fatality rate. J. Vet. Intern. Med. 29, 307‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannitti, F. , Diab, S. , Mete, A. , Stanton, J.B. , Fielding, L. , Crossley, B. , Sverlow, K. , Fish, S. , Mapes, S. , Scott, L. and Pusterla, N. (2015) Necrotizing enteritis and hyperammonemic encephalopathy associated with equine coronavirus infection in equids. Vet. Pathol. doi: 10.1177/0300985814568683. [DOI] [PubMed] [Google Scholar]
- Guy, J.S. , Breslin, J.J. , Breuhaus, B. , Vivrette, S. and Smith, L.G. (2000) Characterization of a coronavirus isolated from a diarrheic foal. J. Clin. Microbiol. 38, 4523‐4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung, I.F. , Lau, S.K. , Woo, P.C. and Yuen, K.Y. (2009) Viral loads in clinical specimens and SARS manifestations. Hong Kong Med. J. 15, 20‐22. [PubMed] [Google Scholar]
- Miszczak, F. , Tesson, V. , Kin, N. , Dina, J. , Balasuriya, U.B. , Pronost, S. and Vabret, A. (2014) First detection of equine coronavirus (ECoV) in Europe. Vet. Microbiol. 171, 206‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto, M. , Oue, Y. , Morita, Y. , Kanno, T. , Kinoshita, Y. , Niwa, H. , Ueno, T. , Katayama, Y. , Bannai, H. , Tsujimura, K. , Yamanaka, T. and Kondo, T. (2014) Experimental inoculation of equine coronavirus into Japanese draft horses. Arch. Virol. 159, 3329‐3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, R. , Mooney, J. , Connaghan, E. , Furphy, C. and Graham, D.A. (2014) Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: a retrospective study. Vet. Rec. 175, 351. [DOI] [PubMed] [Google Scholar]
- Oue, Y. , Ishihara, R. , Edamatsu, H. , Morita, Y. , Yoshida, M. , Yoshima, M. , Hatama, S. , Murakami, K. and Kanno, T. (2011) Isolation of an equine coronavirus from adult horses with pyrogenic and enteric disease and its antigenic and genomic characterization in comparison with the NC99 strain. Vet. Microbiol. 150, 41‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oue, Y. , Morita, Y. , Kondo, T. and Nemoto, M. (2013) Epidemic of equine coronavirus at Obihiro Racecourse, Hokkaido, Japan in 2012. J. Vet. Med. Sci. 75, 1261‐1265. [DOI] [PubMed] [Google Scholar]
- Pakpinyo, S. , Ley, D.H. , Barnes, H.J. , Vaillancourt, J.P. and Guy, J.S. (2003) Enhancement of enteropathogenic Escherichia coli pathogenicity in young turkeys by concurrent turkey coronavirus infection. Avian Dis. 47, 396‐405. [DOI] [PubMed] [Google Scholar]
- Pusterla, N. , Mapes, S. , Wademan, C. , White, A. , Ball, R. , Sapp, K. , Burns, P. , Ormond, C. , Butterworth, K. , Bartol, J. and Magdesian, K.G. (2013) Emerging outbreaks associated with equine coronavirus in adult horses. Vet. Microbiol. 162, 228‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif, L.J. , Redman, D.R. , Moorhead, P.D. and Theil, K.W. (1986) Experimentally induced coronavirus infections in calves: viral replication in the respiratory and intestinal tracts. Am. J. Vet. Res. 47, 1426‐1432. [PubMed] [Google Scholar]
- Slovis, N.M. , Elam, J. , Estrada, M. and Leutenegger, C.M. (2014) Infectious agents associated with diarrhoea in neonatal foals in central Kentucky: a comprehensive molecular study. Equine Vet. J. 46, 311‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumaran, S. , Kelling, C.L. and Ambagala, A. (2007) Immune evasion by pathogens of bovine respiratory disease complex. Anim. Health Res. Rev. 8, 215‐229. [DOI] [PubMed] [Google Scholar]
- Tråvén, M. , Näslund, K. , Linde, N. , Linde, B. , Silván, A. , Fossum, C. , Hedlund, K.O. and Larsson, B. (2001) Experimental reproduction of winter dysentery in lactating cows using BCV – comparison with BCV infection in milk‐fed calves. Vet. Microbiol. 81, 127‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu, H. , Smith, D.R. and Saif, L.J. (1999) Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT‐PCR. Arch. Virol. 144, 167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen, B.L. , Devriendt, B. , Olyslaegers, D.A. , Dedeurwaerder, A. , Desmarets, L.M. , Favoreel, H.W. , Dewerchin, H.L. and Nauwynck, H.J. (2013) Suppression of NK cells and regulatory T lymphocytes in cats naturally infected with feline infectious peritonitis virus. Vet. Microbiol. 164, 46‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.W. , Li, J.S. , Jin, M. , Zhen, B. , Kong, Q.X. , Song, N. , Xiao, W.J. , Yin, J. , Wei, W. , Wang, G.J. , Si, B.Y. , Guo, B.Z. , Liu, C. , Ou, G.R. , Wang, M.N. , Fang, T.Y. , Chao, F.H. and Li, J.W. (2005) Study on the resistance of severe acute respiratory syndrome‐associated coronavirus. J. Virol. Methods 126, 171‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege, H. , Siddell, S. and Ter Meulen, V. (1982) The biology and pathogenesis of coronavirus. Curr. Top. Microbiol. Immunol. 99, 165‐200. [DOI] [PubMed] [Google Scholar]
- Weiss, S.R. and Leibowitz, J.L. (2011) Coronavirus pathogenesis. Adv. Virus Res. 81, 85‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter, M.W. (1998) Adaptation and serial passage of bovine coronavirus in an established diploid swine testicular cell line and subsequent development of a modified live vaccine. Adv. Exp. Med. Biol. 440, 707‐711. [DOI] [PubMed] [Google Scholar]
- Woo, P.C. , Lau, S.K. , Lam, C.S. , Lau, C.C. , Tsang, A.K. , Lau, J.H. , Bai, R. , Teng, J.L. , Tsang, C.C. , Wang, M. , Zheng, B.J. , Chan, K.H. and Yuen, K.Y. (2012) Discovery of seven novel mammalian and avian coronaviruses in the genus Deltacoronavirus supports bat coronaviruses as the gene source of Alphacoronavirus and Betacoronavirus and avian coronaviruses as the gene source of Gammacoronavirus and Deltacoronavirus. J. Virol. 86, 3995‐4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Guy, J.S. , Snijder, E.J. , Denniston, D.A. , Timoney, P.J. and Balasuriya, U.B. (2007) Genomic characterization of equine coronavirus. Virol. 369, 92‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]


