Abstract
Nowadays, detecting the presence of bioterrorism and biohazard agents in environmental and food samples is of great concern, due to their toxicity, and because many of them are prone to be used in terrorism attacks. The use of functionalized magnetic beads (MBs) in the development of electrochemical immuno‐ and genosensors has resulted in innovative and powerful detection strategies that may be applied to environmental, food and clinical analysis. This review describes current research on the combination of functionalized MBs with electrochemical detection for the development of magnetobiosensors applied to rapid, sensitive and specific detection of bioterrorism and biohazard agents.
Keywords: Magnetic beads, Bioterrorism/biohazards, Electrochemical sensors
1 Introduction
The development and application of magnetic beads (MBs) in separation and detection methodologies has attracted strong interest in the last years. This is mainly due to the versatility, high surface area, chemical and physical stability, low toxicity and high biocompatibility exhibited by MBs 1. Their size, similar to that of molecules in nature, range from nm to a few µm, and the particles‐linked molecules can quickly agglomerate and be separated from a matrix or resuspended in an appropriate working medium without retaining any residual magnetism as a consequence of a change in an external magnetic force 2, 3.
MBs consist of a paramagnetic or superparamagnetic core surrounded by a polymeric outer layer suitable for the immobilization of biomolecules 4. The magnetic core is readily available in different iron oxide forms, among which magnetite (Fe3O4) and maghemite (γ‐Fe2O3, ferrimagnetic) stand out since their compatibility has been proven in bio‐labelling and bio‐separation 3, 5, 6. The development of coatings for the magnetic core of MBs was necessary to address their limitations including (a) high surface energies which lead to aggregation, (b) high chemical activity leading to their oxidation, loss of magnetic properties and dispersibility when exposed to air, and (c) biodegradation with subsequent changes in magnetic properties. Consequently, protective shells, mainly composed of agarose, cellulose, silica, silicone, porous glass, mica or polystyrene 7, were developed to protect and preserve the stability of iron oxide MBs. These shells also allowed further functionalization, thus promoting MBs performance as recognition elements in sensing and (bio)chemical arrays 3. Moreover, nowadays there are commercially available MBs modified with biomolecules that allow their use in different types of bioassays. So, MBs functionalized with: (a) streptavidin suitable for capturing biotinylated nucleic acids, aptamers, peptides, proteins, etc.; (b) protein A (protA) or protein G (protG), which specifically bind antibodies; (c) oligonucleotides; or (d) affinity ligands for specific capture of tagged recombinant proteins, etc. 7 can be purchased from different companies. MBs modification has resulted in important practical advantages from an analytical point of view, including (a) shorter reaction times between dissolved species and biomolecules immobilized on the surface of the beads which is also favoured by the easy dispersion of MBs into solution with only gentle shaking, (b) readily miniaturization of the assay system by using MBs as a mobile solid phase, (c) reduction of the required volumes of reagents and produced waste, and (d) obtaining lower detection limits with shorter assay times 1.
Owing to all these properties, MBs constitute an attractive platform for the design of electrochemical biosensors which also add their inherent advantages such as system miniaturization, low cost and ease of operation 2. In fact, electrochemical enzyme‐based biosensors, immunosensors and nucleic acid hybridization‐based sensors have been described in literature and cited in several reviews 1–5, 7–9.
Nowadays, there is a great concern on detecting the presence of toxic and infectious agents in environmental and food samples, not only due to their toxicity to human beings, animals or plants, but also because many of them are prone to be used in terrorism attacks. These agents can be classified in two main groups: one is constituted by chemical agents while the second one involves complex bioengineered microorganisms and pathogens.
Among chemical agents, insecticides and organophosphorous (OP) nerve gases can be highlighted. OP nerve gases are the most harmful chemical agents. They act in a similar way as insecticides, irreversibly inhibiting the catalytic active site of acetylcholinesterase (AchE), the enzyme that catalyzes the hydrolysis of the neurotransmitter acetylcholine. However, their mammalian toxicity is much higher 10. Examples include sarin, soman, fonofos and VX agent. The most widely used insecticides belong essentially to two families: organophosphorus (such as paraoxon, parathion, chlorpyriphos, ethyl oxon, or malathion) and carbamate (such as carbofuran, or carbaryl). Vesicants (mustards, lewisite) and pulmonary agents (chlorine, phosgene) are also used as chemical toxic agents.
Unlike a chemical agent attack, biological attacks do not cause an immediate reaction. However, biological agents are much more deadly than chemical ones 11. Bioterrorism agents are pathogenic organisms (bacteria, viruses) or biological toxins used to produce death and disease in humans, animals or plants for terrorist purposes. Although these agents are typically microorganisms found in nature, they can be modified to increase their virulence, to make them resistant to current antibiotics or vaccines, or to enhance the ability of these agents to be disseminated throughout the environment. While any germ, bacteria or virus could potentially be utilized by terrorists, there are a number of biological agents that have been recognized as being more likely to be used. This is due to their availability to terrorists and the ease by which these agents can be disseminated. Table 1 lists the main biological warfare agents (BWAs) together with the diseases they cause. The presence of microorganisms in food is also a natural and unavoidable occurrence. Cooking generally destroys most harmful bacteria, but undercooked foods, processed ready‐to‐eat foods, and minimally processed foods can contain harmful bacteria that are serious health threats.1
Table 1.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
To minimize the effects of natural outbreaks or deliberate attacks, near real‐time detection of infectious agents is an essential first step in mounting an appropriate response.
These infectious agents were traditionally detected and identified using standard microbiological and biochemical assays that are accurate but time‐consuming. These methods require isolation and/or culturing of large quantities of the infectious agents, and therefore the analysis requires several days to be completed. More recently, molecular approaches, such as the polymerase chain reaction (PCR) amplification and analysis of unique DNA sequences and/or rRNA, have supplanted traditional microbiological methods because they are highly accurate and sensitive, and take less time 12. However, these assays require specialized instruments and still take several hours to be performed. In addition, DNA‐based molecular techniques are limited to the detection of whole organisms and cannot detect toxins and other extracellular products or infectious agents.
Biosensor technology brings together the accuracy and sensitivity of standard approaches with improvement in rapidity of detection. Electrochemical sensors, in particular, offer several advantages compared to PCR and other molecular detection approaches, including a broad range of target analytes, minimal sample preparation, ability to analyze complex body fluids, high sensitivity, miniaturization, and compatibility with compact instrumentation. Biosensors also offer the possibility of continuous and real‐time monitoring of the environment for the presence of infectious agents to allow timely implementation of preventive and protective measurements. In fact, the majority of existing technologies for detecting biological agents rely on either antibodies as the recognition molecules, which bind with a surface feature of the BWA, or on the recognition of a nucleic acid sequence known to be found in the BWA being tested. A few other types of recognition molecules such as peptides, glycolipids and aptamers have also been used with similar transduction schemes 13. All these recognition elements can also be employed in the development of electrochemical biosensors.
The combination of functionalized MBs with electrochemical detection constitutes a powerful and efficient strategy for the development of magnetobiosensors which can be applied to the rapid and sensitive detection of many agents of environmental and clinical significance. This review focuses on current research regarding the use of MBs in the electrochemical sensing of bioterrorism and biohazard agents. Strategies involving methods based on the use of immuno‐ and nucleic acid‐MB‐based sensors by means of different amplification systems to achieve sensitive and specific electrochemical detection of these agents are discussed.
2 MBs‐Based Electrochemical Immunosensors
Several advantages have been claimed for immunoassays making use of MBs over conventional ones. They can be summarized as: (1) high reaction kinetics in a small volume, high surface area and high dispersion capability of MBs leading to an increase in surface‐to‐volume ratio, together with a decrease of matrix effects and, consequently, to a substantial shortening of the immunoreaction times; (2) versatile manipulation and optimization of experimental conditions, as a consequence of the separation between the electrochemical detection and the immunoreaction steps, as it will be illustrated below; (3) less time consuming due to decreased coating, competition and blocking times; (4) modification of MBs in numerous ways allowing different immobilization strategies; and (5) the immunocomplexes formed on the MBs surface can be easily detected without preconcentration or purification steps, which are normally required for standard immunoassays 1, 14, 15. In summary, the good analytical performance of the immunodevices using functionalized MBs can be attributed to the increased surface area and reactivity, high effectiveness of blocking reagents, and improved washing and separation steps.
2.1 Detection of Chemical Agents
Direct competitive immunoassay schemes, where the analyte and enzyme‐analyte conjugates (using mainly alkaline phosphatase (AP) and horseradish peroxidase (HRP) as enzyme labels) compete for a limited amount of antibodies‐coated MBs have been used to develop magnetoimmunosensors to detect 2,4‐dichlorophenoxiacetic acid (2,4‐D) 16, polychlorinated biphenyls (PCBs) 17–19, atrazine 20, 21, and the insecticide biomarker trichloropyridinol (TCP) 22. As an example, the schematic procedure for the determination of PCBs used by Centi et al. is displayed in Figure 1 17. Moreover, the characteristics and performance of these magnetoimmunosensors are summarized in Table 2. As can be seen, the performance of some of these immunosensing systems was successfully evaluated using spiked environmental samples (soils, marine sediment extracts, foods, and river and bottled water samples). The obtained results indicate that these convenient and sensitive methodologies offer great promise for decentralized environmental applications. In fact, they constitute interesting approaches which can be further used in the development of easy‐to‐use inexpensive tests for preliminary sample screening directly in‐field. Moreover, these strategies can be readily transferred to the detection of other environmental contaminants through the development of specific antibodies against these contaminants and are expected to open new opportunities for environmental monitoring and public health.1, 2
Figure 1.
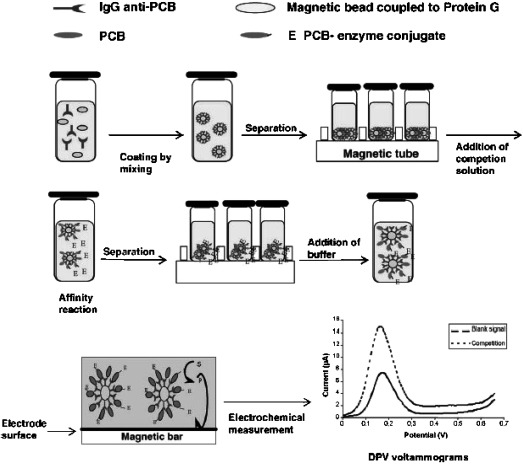
Schematic display of the experimental procedure used by Centi et al. for the determination of PCBs using MBs‐based immunosensors. Reprinted from [17] with permission. Copyright 2005 Elsevier.
Table 2.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
2.2 Detection of Viral, Bacterial and Antibacterial Agents
The development of magnetoimmunosensors for the detection of pathogen bacteria was firstly described by Kradtap et al. 23 and Che et al. 24. Kradtap et al. used an artificial microorganism called “Bugbead” as stimulant for the development of microbial sensors without introducing potential experimental hazards or other variables coming in when using real microorganisms 23. The applicability of these bugbeads in the development of immunosensors was demonstrated by the analysis of extracts containing serotype antigens of Escherichia coli O157 : H7 (E. coli O157 : H7). However, to our knowledge, these beads have never been used again in the development of electrochemical immunosensors. On the other hand, Che et al. developed an immunomagnetic separation (IMS) procedure to isolate the enteropathogen Campylobacter jejuni (C. jejuni) cells from the sample solution 24. The developed sensor that made use of a tyrosinase‐modified electrode was applied to the analysis of pure culture and poultry samples. The potential application of this immunosensor in the poultry industry was demonstrated by the rapidity of the assay (2.5 h), the detection limit at the lowest concentration level expected in these samples, and the simple system setup. Table 3 summarizes the main characteristics of these sensors.
Table 3.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
A fully‐automated fluidic system implying a bead‐based sandwich immunoassay with electrochemical detection was described for bacteriophage MS2 and ovalbumin (OVA) detection 25. The sandwich immunosensor was constructed by attaching a biotinylated antibody to streptavidin‐coated beads, capturing the antigen, and exposing the conjugate to a β‐galactosidase‐labelled antibody. The detection limits for MS2 and OVA were 990 and 470 ng mL−1, respectively.
Another magnetoimmunosensing sandwich approach was developed for the detection of Salmonella allowing, in 50 min and without any pretreatment, a detection limit of 7.5×103 colony forming units (cfu) mL−1 to be obtained in 10 times diluted milk samples 26. In this approach, the bacteria were captured and preconcentrated from milk samples with MBs through an immunological reaction. A polyclonal anti‐Salmonella antibody labelled with HRP was used as serological confirmation with electrochemical detection at a magnetoelectrode.
A disposable magnetoimmunosensor for the detection of Streptococcus pneumoniae (S. pneumoniae) was reported using a similar sandwich format where protA‐coated MBs were employed as a solid phase to immobilize specific S. pneumoniae capture antibodies. As can be seen in Figure 2, the same antibody but conjugated to HRP as enzyme label was used as the detection antibody to recognize the captured S. pneumoniae cells. The electrochemical detection of the enzyme reaction product was accomplished at disposable gold screen‐printed electrodes (Au/SPEs) modified with tetrathiafulvalene (TTF) as electron transfer mediator and using H2O2 as the enzyme substrate 27. The developed methodology was shown to be suitable for detecting 1.0×104 cfu of S. pneumoniae in inoculated urine samples without any sample pretreatment.2
Figure 2.
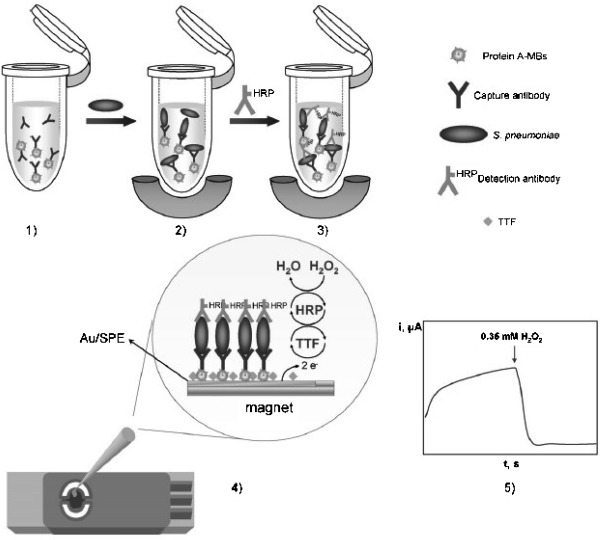
Immunosensor for the determination of S. pneumoniae: 1) immobilization of capture antibodies on protA‐modified MBs; 2) MB‐antibody–bacteria complexes separation and removing excess antibody; 3) incubation with labelled antibody; 4) MBs capture on TTF‐Au/SPEs; 5) amperometric detection of the mediated reduction of H2O2 with TTF. Reprinted from [27] with permission. Copyright 2010 Elsevier.
Some strategies have also been developed for the quantification of human antibodies against some bacterial agents or their specific toxins. Interesting examples of these approaches are the electrochemical magnetoimmunosensing assays developed for the rapid, selective and sensitive quantification of anti‐Helicobacter pylori 28, anti‐Clostridium tetani 14, and anti‐hepatitis B virus 29 IgG antibodies. The detection of these antibodies in serum was carried out by indirect antibody capture assays based on the use of purified antigens immobilized on magnetic microspheres. The IgG antibodies present in the serum samples were allowed to react immunologically with the immobilized antigens, and the bound antibodies were quantified by enzyme‐labelled 14, 28 or gold nanoparticles (AuNPs)‐conjugated 29 secondary antibodies specific to human IgG. These original approaches may be extended to on site applications dealing with the screening of antibody levels in complex samples.
Some novel strategies for the detection of viruses and bacteria based on magnetoimmunosensors have been described recently. A magnetic gold electrode was fabricated for the direct sensing of Japanese encephalitis virus (JEV) 30. Gold‐coated MBs were employed as the platforms for the immobilization and immunoreaction processes, and HRP was chosen as an enzymatic tracer. After the immunoreactions, multiwalled carbon nanotubes (MWCNTs) were mixed with the immunocomplex coated Au‐coated MBs to improve the sensitivity of the assay. A detection limit two orders of magnitude lower than that of immunochromatographic strip and similar to that obtained by reverse transcriptase polymerase chain reaction (RT‐PCR) was obtained (Table 3). The method was successfully applied in clinical samples (brain tissue of swine, mosquito and cerebrospinal fluid of human patients).
Also, novel magnetoimmunoassay‐based strategies for the detection of Plasmodium falciparum histidine‐rich protein 2 (HRP2), a malaria parasite biomarker, have been described. Two different kinds of magnetic supports were employed for this purpose: 1 µm tosyl‐activated MBs, and 300 nm active magnetic nanoparticles 31. This is the first report of a procedure based on a sandwich magnetoimmunoassay between the protein in the sample and two commercially available monoclonal antibodies that recognize two different epitopes of the antigen, one of them covalently coupled to MBs and the other labelled with the enzyme HRP. The electrochemical magnetoimunosensor showed better analytical performance than other methods in terms of limit of detection (see Table 3). Due to the high sensitivity achieved, this strategy offers great promise to implement rapid, simple, inexpensive and user‐friendly analytical methods for on‐site detection of falciparum malaria disease in patients, and also to screen out at‐risk blood samples for prevention of transfusion‐transmitted malaria, as well as for in vitro drug sensitivity testing.
Moreover, a novel electrochemical immunosensing assay for bacterial detection that combines a one‐step sandwich immunoassay, MBs target preconcentration, microfluidic technology, and amperometric detection using HRP as the label enzyme has recently been described 32. The enzymatic reaction takes place in an incubation micro‐chamber where the MBs are confined, upstream from the working electrode. The enzyme product is then pumped along a microchannel, where it is amperometrically detected by a set of microelectrodes. The system provided a significant improvement in terms of limit of detection and assay time (1 h) compared to classical ELISA detection. Also, it provides the possibility of addressing relatively complex sample matrices so that it could be potentially applicable to the food, pharmaceutical and medical fields if preconcentration and/or preenrichment strategies were implemented.
Table 3 summarizes the analytical characteristics of the reported methods for the detection of viral, bacterial and anti‐bacterial agents using electrochemical magnetoimmunosensors.3
2.3 Detection of Biological Toxins
Competitive magnetoimmunoassays have been described for the determination of several biological toxins. An electrochemical immunoassay for the determination of aflatoxin B1 (AFB1) in food has been reported. The approach was based on the use of multifunctional MBs as probes, which comprised magnetic CoFe2O4 and Prussian Blue nanoparticles and were used as an affinity support for the immobilization of the AFB1‐bovine serum albumin conjugate (AFB1‐BSA) 33. The concentrations of AFB1 were determined by means of a competitive‐type immunoassay using AuNPs modified with HRP‐labelled anti‐AFB1 as detection antibodies for the amplification of the electrochemical signal (Figure 3). This strategy gave rise to a high sensitivity, enhanced by the use of AuNPs, together with avoiding the need of adding an electron mediator in the detection solution due to the presence of Prussian Blue NPs, thus providing a convenient platform for clinical testing and drug screening.
Figure 3.
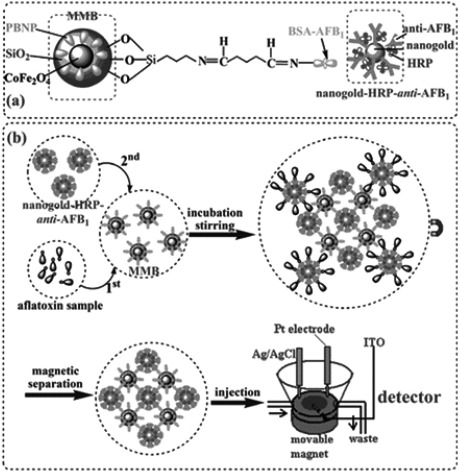
(a) Schematic illustration of the multifunctional MBs and the AuNP‐HRP‐anti‐AFB1, and (b) measurement process involved in the competitive immunoassay methodology. Reprinted from [33] with permission. Copyright 2009 RSC.
A related approach has been described for the detection of deoxynivalenol (DON), a mycotoxin produced by Fusarium fungi which represents a serious threat for the safety of cereal‐based food and feedstuffs 34. This methodology was based on the use of a novel anti‐DON Fab fragment and of immunomagnetic beads coupled with eight magnetized SPEs as electrochemical transducers. The experimental procedure consisted of coating MBs with the conjugated toxin and performing a competitive assay by adding the free toxin together with the Fab fragment, conjugated with a biotin group. In order to carry out the electrochemical detection, the immunological complex was linked to HRP via an avidin‐biotin binding. Although the developed system resulted in sensitivity and selectivity characteristics similar to those offered by ELISA or SPR methodologies, the employed multielectrode strip could be applied to multianalyte detection. Also, this kind of transducer is easily miniaturized in a cost‐effective and mass producible way thus opening a door to the development of a portable electrochemical instrument and miniaturized strip for lab‐on‐a‐chip approach.
Another example is that described for the determination of okadaic acid (OA), a lipophilic marine biotoxin ingested by various species of shellfish, involving the use of super paramagnetic nanobeads 15. Immobilized OA on streptavidin‐coated MBs competed with free OA in solution for the anti‐OA monoclonal antibody also in solution. A secondary antibody labelled with AP was used in the detection step which was carried out by DPV. The developed method was applied successfully to the determination of AO in mussels extracts demonstrating that the use of MBs reduced the analysis time and eliminated matrix effect and interferences.
Some electrochemical MBs‐based immunosensing approaches exhibiting successful integration in microfluidic devices have been also reported. One of them, based on a sandwich configuration, was developed for the detection of cholera toxin subunit B (CTB) 35. The sandwich format used comprised anti‐CTB antibody and ferri/ferrocyanide encapsulating ganglioside GM1 liposomes for signal amplification. Fluorescence and electrochemical detections were compared finding out that the electrochemical approach showed advantages in terms of flexibility and reliability of signal recording. A microfluidic device has been developed for a simplified detection of zearalenone (ZEA) on the basis of a competition scheme where this mycotoxin and an enzyme‐labelled derivative compete for the binding sites of a specific antibody immobilized on protG‐modified MBs.
Also, the possibility of using electrochemical detection coupled with a MBs‐based immunoassay for the detection of ZEA in baby food was demonstrated 36, 37. Subsequently, the developed system was integrated in microfluidic chips following the scheme displayed in Figure 4, where the simple channel layout of a double‐T microchip was used to perform sequentially the immunoreaction and the enzyme reaction by applying a program of electric fields 38. Another system for the determination of ZEA in feedstuffs samples has been recently reported using a microbiochip microfluidic immunosensor coupled with flow injection (FIA) with Au/SPEs as the working electrode showing good sensitivity and accuracy, and minimizing the use of expensive reagents 39.
Figure 4.
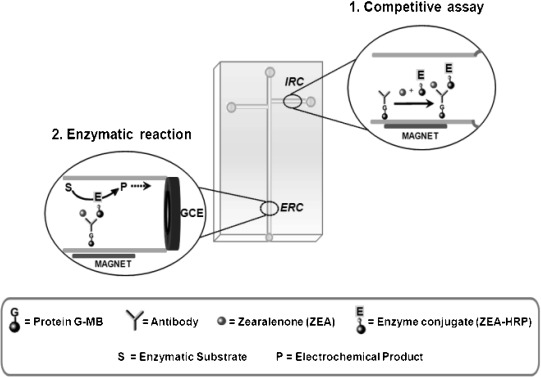
Schematic display of the microfluidic layout and immunoassay principle used for the determination of ZEA. IRC, immunological reaction chamber; ERC, enzymatic reaction chamber. Adapted from [38] with permission. Copyright 2011 RSC.
Table 4 summarizes the main characteristics reported for the detection of biological toxins using electrochemical magnetoimmunosensors.3, 4, 4
Table 4.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
3 MBs‐Based Electrochemical DNA Sensors
MBs have demonstrated to be versatile tools not only for the separation of nucleic acids but also as a platform for optimized DNA hybridization 7. The hybridization event is detected at a different surface, the working electrode, the detection being based either on the intrinsic DNA electroactivity or on DNA labelling in order to amplify the measured signals. Moreover, MBs have been used to discriminate genetic alleles and thus identify the presence of single nucleotide polymorphisms (SNPs), in the study of mutational status or genotyping of an organism, drug discovery, and genetic disorders associated with point mutations, as well as in the detection of viruses, bacteria and food‐borne pathogens 9.
LaGier et al. developed a label‐free electrochemical method based on the use of DNA probe‐coated MBs for the selective detection of E. coli 16S rRNA directly from lysed cells, by monitoring the guanine oxidation signal 40. Using this approach, 107 E. coli cells could be detected in 4 h. A sensitive and selective genomagnetic assay for the electrochemical detection of Salmonella spp. was developed based on a double labelling polymerase chain reaction (PCR) strategy that generated an amplicon of the IS200 insertion sequence with double labelling: with a biotinylated capture primer to achieve immobilization on streptavidin‐coated MBs, and with a digoxigenin signalling primer for electrochemical detection 41. A PCR reactor for real‐time electrochemical detection of Salmonella genome, in which the amplification and double‐labelling were directly performed on the streptavidin‐MBs by using magnetic primers, was also reported.
A highly sensitive assay for rapidly screening‐out Mycobacterium bovis’ (M. bovis) DNA in contaminated samples was also developed using electrochemical magnetogenosensing 42. The assay consisted on specific amplification and double‐tagging of the IS6110 DNA fragment, highly related to M. bovis, followed by electrochemical detection of the amplified product. PCR amplification, carried out using a labelled set of primers, resulted in a double‐tagged amplicon with both biotin and digoxigenin at each terminus. The double‐tagged amplicon was detected using a magnetosensor combined with streptavidin‐modified MBs (Figure 5). The assay allowed the detection of 10 fmol of PCR amplicon and showed promising features for the detection of M. bovis on dairy farms by screening for the presence of the bacterial DNA in milk samples.5
Figure 5.
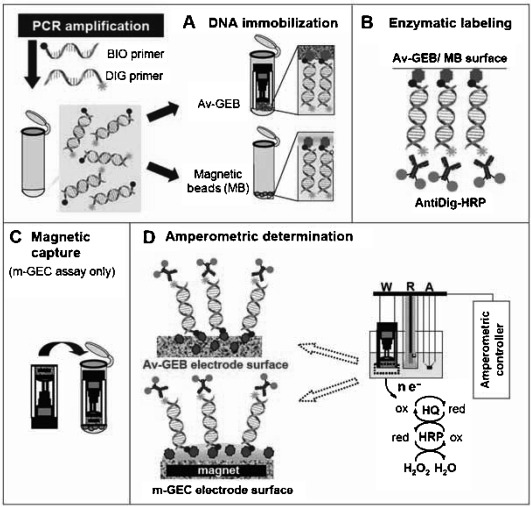
Schematic display of the methodology employed for the detection of M. bovis using MBs‐based electrochemical genosensors. Reprinted from [42] with permission. Copyright 2010 Spanish Society for Microbiology.
Integration of streptavidin‐coated paramagnetic microbeads modified with a biotinylated capture probe, with a commercially available microfluidic platform, resulted in a rapid and sensitive (0.2 nM detection limit) enzyme‐linked electrochemical genosensor used for the determination of a PCR amplified fragment of the Cor a 1.04 gene (182 bp) of hazelnut 43. A gravity‐driven microfluidic‐based electrochemical platform using micromagnetic beads as solid support for nucleic acid detection has been recently described and applied to the detection of specific DNA sequences of Legionella pneumophila with a detection limit of 0.33 nM 44. The developed device combined a special chip containing eight polymer microchannels with a portable, computer‐controlled, instrument where both hybridization and labelling events were performed on streptavidin‐coated paramagnetic microparticles functionalized with a biotinylated capture probe.
MBs covered with biotin‐labelled oligonucleotides have recently been used to isolate viral DNA sequences of human immunodeficiency virus (HIV) and influenza virus subtype H5N1 45. The viral nucleic acids were detected with carbon nanotubes (CNTs)‐modified SPEs coupled with a micro‐flow instrument. The described system could be useful for easy‐to‐use and rapid detection of viral nucleic acid.
The development of magnetoelectrochemical disposable DNA sensors for the detection of E. coli, based on the use of MBs and enzymatic signal amplification and the recognition of a characteristic region of the lacZ gene, was reported 46, 47. 326‐bp amplicons were obtained by asymmetric PCR (aPCR) using genomic DNA or directly from cell cultures (daPCR) and hybridized on streptavidin‐MBs modified with a specific biotinylated capture probe. After binding of a streptavidin‐HRP polymer to the resultant biotinylated hybrids, the modified MBs were captured by a magnetic field on the surface of TTF‐modified Au/SPEs. As low as 2.5 aM aPCR product could be detected with the developed methodology. A further approach involved the obtaining of the 326‐bp amplicons directly from cell cultures (daPCR) achieving E. coli detection at a concentration level of 1 cfu /100 mL with no need for culture preconcentration steps. These results demonstrated the usefulness of the developed sensors as screening or alarm devices indicating the possible presence of coliforms, together with their applicability to drinking water quality assurance (no coliform bacteria per 100 mL of water). The same research group employed similar strategies in the development of DNA sensors for the detection of a characteristic 235‐bp region of the gene coding for autolysin (LytA), a specific pneumococcus virulent factor 48. The obtained results demonstrated the usefulness of the developed methodology for rapid, simple, specific, quantitative, and sensitive detection of aPCR amplicons with a detection limit of 1.1 nM. Moreover, amplified daPCR products could be prepared with as few as 2 cfu from S. pneumoniae cultures, which make these devices very promising for the rapid identification of these bioagents. Under the experimental conditions employed, the assay was capable of distinguishing between S. pneumoniae and other streptococci of the mitis group also known to contain a lytA‐like gene.
An electrochemical competitive aptasensor for the detection of the mycotoxin ochratoxin A (OTA) has been described. The aptasensor was based on competition between the mycotoxin conjugated to the enzyme HRP and free OTA for streptavidin‐MBs functionalized with a biotinylated aptamer 49. This magnetic aptasensor showed a detection limit of 0.07 ng mL−1 and was accurately applied to extracts of certified and spiked wheat samples.
Table 5 summarizes the main analytical characteristics exhibited by electrochemical nucleic acid magnetosensors for the detection of bioterrorism and biohazard agents.5
Table 5.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
4 Magnetoimmuno‐PCR Approaches
The combination of IMS, PCR, and electrochemical magnetogenosensing of the resulting amplicons allows the implementation of rapid, sensitive, and selective assays for the detection of bacterial agents.
Salmonella was detected using a strategy based on capturing and preconcentrating the bacteria from milk samples by IMS 50.The bacteria attached to the MBs were then lysed by heating treatment to release the genomic DNA. Amplification of the genetic material with a double‐tagging set of primers was performed and the amplicons were detected by electrochemical measurement (Figure 6). Using this approach, a detection limit of 0.04 cfu mL−1 was obtained in skim milk samples preenriched for 6 h.
Figure 6.
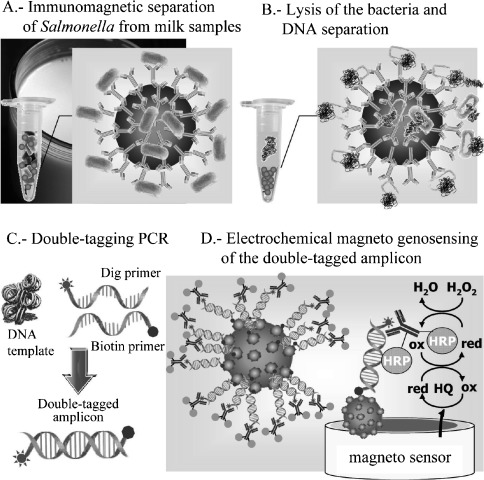
Schematic display of the electrochemical magnetoimmuno‐PCR approach for the detection Salmonella. Reprinted from [50] with permission. Copyright 2009 ACS.
An electrochemical magnetoimmuno‐PCR approach has been recently described for direct and highly sensitive detection of S. pneumoniae 51. The amplified products obtained by daPCR from bacteria attached to specific capture antibody‐modified MBs were detected similarly to that commented above using the electrochemical genosensor 48. The developed methodology allowed an easy discrimination between only 100 cfu mL−1 S. pneumoniae cultures and high concentrations (∼1.0×108 cfu mL−1) of other streptococci, thus demonstrating the reliability of the assay for the specific detection of low pneumococcus concentrations.6
5 Other Magnetosensor Approaches Employed in the Detection of Bioterrorism/Biohazard Agents
Some other approaches for the determination of bioterrorism/biohazard agents making use of other types of receptors and MBs have been described. A method for detecting wheat germ agglutininin (WGA) based on the use of glucosamine‐modified MBs and daunomycin‐labelled glucose as a probe was reported 52. When labelled glucose was held in the binding site of WGA to the sugar, the peak current decreased. Given that the binding between WGA and labelled glucose depended on the glucosamine concentration of glucosamine‐modified beads, the concentration was estimated from the change in the peak current. Other lectins not having the binding site to glucosamine or glucose did not produce any change of peak current. Therefore, this method would be powerful for evaluation of interaction between protein and sugar chain existing at cell surface. An ultrasensitive method to evaluate toxicity due to pesticides in a glass lab‐on‐a‐chip by means of enzymatic inhibition of acetylcholinesterase immobilised on MBs and amperometric detection has been developed and applied to the detection of carbofuram down to the nanomolar (sub‐ppb) level 53.
6 Conclusions
Micro‐and nano‐MBs play an important role in the development of electrochemical detection methodologies for biohazard and bioterrorism agents. MBs‐based electrochemical immunosensors have been widely employed with this purpose, making possible to analyze environmental and food samples as long as antibodies against specific bioagents are available. There are not so many examples on magnetic genosensors, although interesting approaches have also been developed in this field, where high sensitivities are obtained through different amplification systems, such as PCR or enzymatic signal amplification. Sensitivity and specificity can be improved in a high extent using magnetoimmuno‐PCR approaches, although very few examples have been described based on this innovative and combined new methodology. Future prospects should be focused on the extension of these magnetobioassays to develop highly demanded easy‐to‐use miniaturized and multiplexed bio‐platforms for the automated detection of multiple biohazard and/or bioterrorism agents on one shot. In this regard, combination of MBs‐based bioassays with microfluidic devices should facilitate implementing this kind of advanced bio‐platforms for the direct decentralized detection and with a short time from sample acquisition to readout. Another important challenge for analytical chemists is to validate the developed devices with real environmental, food and clinical samples. Moreover, integration of the biosensing platforms in the clothing of risk people, such as soldiers, constitutes also an exciting new field of work involving multidisciplinary strategies and economical interests.
Biographical Information
María Pedrero received her PhD in Chemical Sciences (Analytical Chemistry) in 1993 from the Universidad Complutense de Madrid. In 1994 she stayed at the New Mexico State University for her postdoctoral training with Prof. Joseph Wang. Since 1991, she has been working under various contracts in the Analytical Chemistry Department of the Chemistry Faculty of this University, where she is Professor of Analytical Chemistry since 2002. Her areas of interest include the development of enzymatic, immuno‐ and DNA‐electrochemical and impedimetric sensors, bioelectrocatalysis, and electrode modification for the ultrasensitive detection of bacteria and cardiac markers. She is also involved in the development of electrochemical arrays for multiplexed detection. She is co‐author of more than fifty research papers.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
Biographical Information
Susana Campuzano received her PhD in Analytical Chemistry from the Universidad Complutense de Madrid (Spain) in 2004. Since 2005 she works as Assistant Professor at the Analytical Chemistry Department of the Chemistry Faculty of the Universidad Complutense de Madrid where she collaborates in the “Electroanalysis and electrochemical (bio)sensors” research group leaded by Prof. J. M. Pingarrón. She worked as a Research Scholar in the research group of Prof. Joseph Wang at the Department of Nanoengineering in UCSD (USA) from January 2010 to July 2011. Her areas of interest include the development of enzymatic, immuno and DNA electrochemical sensors, and novel applications of advanced nanomachines in medical technology.
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
Biographical Information
José M. Pingarrón obtained his PhD in Sciences from Complutense University of Madrid. He is Professor of Analytical Chemistry at Complutense University of Madrid and head of the group “Electroanalysis and electrochemical (bio)sensors”. Current research includes the development of nanostructured electrochemical biosensors, including enzyme electrodes, immunosensors and genosensors for the ultrasensitive detection of bacteria, low molecule weight hormones and cancer markers as well as electrochemical arrays for multiplexed detection. He has authored over 240 research papers and several books and book chapters
The publisher did not receive permission from the copyright owner to include this object in this version of this product. Please refer either to the publisher's own online version of this product or the printed product where one exists.
Acknowledgements
This research was supported by the Spanish Ministerio de Ciencia e Innovación Research Project CTQ2009‐09351BQU, and the AVANSENS Program from the Comunidad de Madrid (S2009PPQ‐1642).
References
- 1. Kuramitz H., Anal. Bioanal. Chem. 2009, 394, 61. [DOI] [PubMed] [Google Scholar]
- 2. Hsing I. M., Xu Y., Zhao W., Electroanalysis 2007, 19, 755. [Google Scholar]
- 3. Simón de Dios A., Díaz‐García M. E., Anal. Chim. Acta 2010, 666, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albers J., Grunwald T., Nebling E., Piechtta G., Hintsche R., Anal. Bioanal. Chem. 2003, 377, 521. [DOI] [PubMed] [Google Scholar]
- 5. Andreescu S., Njagi J., Ispas C., Ravalli M. T., J. Environ. Monit. 2009, 11, 27. [DOI] [PubMed] [Google Scholar]
- 6. Teja A. S., Koh P. Y., Prog. Cryst. Growth Charact. Mater. 2009, 55, 22. [Google Scholar]
- 7. Palecek E., Fojta M., Talanta 2007, 74, 276. [DOI] [PubMed] [Google Scholar]
- 8. Aguilar‐Arteaga K., Rodriguez J. A., Barrado E., Anal. Chim. Acta, 2010, 674, 157. [DOI] [PubMed] [Google Scholar]
- 9. Dolatabadi J. E. N., Mashinchian O., Ayoubi B., Jamali A. A., Mobed A., Losic D., Omidi Y., de la Guardia M., Trends Anal. Chem. 2011, 30, 459. [Google Scholar]
- 10. Sadik O. A., Wanekaya A. K., Andreescu S., J. Environ. Monit. 2004, 6, 513. [DOI] [PubMed] [Google Scholar]
- 11. Shah J., Wilkins E., Electroanalysis 2003, 15, 157. [Google Scholar]
- 12. Deisingh A. K., Thompson M., Analyst 2002, 127, 567. [DOI] [PubMed] [Google Scholar]
- 13. Gooding J. J., Anal. Chim. Acta 2006, 559, 137. [Google Scholar]
- 14. Patris S., De Vriese C., Prohoroff F., Burgoa Calvo E., Arcos Martínez J., Kauffmann J. M., Electroanalysis 2010, 22, 41. [Google Scholar]
- 15. Hayat A., Barthelmebs L., Marty J. L., Anal. Chim. Acta 2011, 690, 248. [DOI] [PubMed] [Google Scholar]
- 16. Dequaire M., Degrand C., Limoges B., Anal. Chem. 1999, 71, 2571. [DOI] [PubMed] [Google Scholar]
- 17. Centi S., Laschi S., Fránek M., Mascini M., Anal. Chim. Acta 2005, 538, 205. [Google Scholar]
- 18. Centi S., Laschi S., Mascini M., Talanta 2007, 73, 394. [DOI] [PubMed] [Google Scholar]
- 19. Lin Y. Y., Liu G., Wai C. M., Lin Y., Anal. Chim. Acta 2008, 612, 23. [DOI] [PubMed] [Google Scholar]
- 20. Helali S., Martelet C., Abdelghani A., Maaref M. A., Jaffrezic‐Renault N., Electrochim. Acta 2006, 5182. [Google Scholar]
- 21.E. Zacco, R. Galve, J. Adrian, M. P Marco, M. I. Pividori, S. Alegret, Ibersensor 2006, 1. ISBN: 9974‐0‐0337‐7.
- 22. Liu G., Timchalk C., Lin Y., Electroanalysis 2006, 18, 1605. [Google Scholar]
- 23. Kradtap S., Wijayawardhana C. A., Schlueter K. T., Halsall H. B., Heineman W. R., Anal. Chim. Acta 2001, 444, 13. [Google Scholar]
- 24. Che Y., Li Y., Slavik M., Biosens. Bioelectron. 2001, 16, 791. [DOI] [PubMed] [Google Scholar]
- 25. Kuramitz H., Dziewatkoski M., Barnett B., Halsall H. B., Heineman W. R., Anal. Chim. Acta 2006, 561, 69. [Google Scholar]
- 26. Liébana S., Lermo A., Campoy S., Cortés M. P., Alegret S., Pividori M. I., Biosens. Bioelectron. 2009, 25, 510513. [DOI] [PubMed] [Google Scholar]
- 27. Campuzano S., Esteban‐Fernández de Ávila B., Yuste J., Pedrero M., García J. L., García P., García E., Pingarrón J. M., Biosens. Bioelectron. 2010, 26, 1225. [DOI] [PubMed] [Google Scholar]
- 28. Pereira S. V., Messina G. A., Raba J., J. Chromatogr. B 2010, 878, 253. [DOI] [PubMed] [Google Scholar]
- 29. de la Escosura‐Muñiz A., Maltez‐da Costa M., Sánchez‐Espinel C., Díaz‐Freitas B., Fernández‐Suarez J., González‐Fernández Á., Merkoci A., Biosens. Bioelectron. 2010, 26, 1710. [DOI] [PubMed] [Google Scholar]
- 30. Li F., Mei L., Li Y., Zhao K., Chen H., Wu P., Hu Y., Cao S., Biosensos. Bioelectron. 2011, 26, 4253. [DOI] [PubMed] [Google Scholar]
- 31. de Souza Castilho M., Laube T., Yamanaka H., Alegret S., Pividori M. I., Anal. Chem. 2011, 83, 5570. [DOI] [PubMed] [Google Scholar]
- 32. Laczka O., Maesa J. M., Godino N., Del Campo J., Fougt‐Hansen M., Kutter J. P., Snakenborg D., Muñoz‐Pascual F. X., Baldrich E., Biosens. Bioelectron. 2011, 3633. [DOI] [PubMed] [Google Scholar]
- 33. Tang D., Zhong Z., Niessner R., Knopp D., Analyst 2009, 134, 1554. [DOI] [PubMed] [Google Scholar]
- 34. Romanazzo D., Ricci F., Volpe G., Elliot C. T., Vesco S., Kroeger K., Moscone D., Stroka J., Egmond H. Van, Vehniäinen M., Palleschi G., Biosens. Bioelectron. 2010, 25, 2615. [DOI] [PubMed] [Google Scholar]
- 35. Bunyakul N., Edwards K. A., Promptmas C., Baeumner A. J., Anal. Bioanal. Chem. 2009, 393, 177. [DOI] [PubMed] [Google Scholar]
- 36. Hervás M., López M. A., Escarpa A., Anal. Chim. Acta 2009, 653, 167. [DOI] [PubMed] [Google Scholar]
- 37. Hervás M., López M. A., Escarpa A., Biosens. Bioelectron. 2010, 25, 1755. [DOI] [PubMed] [Google Scholar]
- 38. Hervás M., López M. A., Escarpa A., Analyst 2011, 136, 2131. [DOI] [PubMed] [Google Scholar]
- 39. Panini N. V., Salinas E., Messina G. A., Raba J., Food Chem. 2011, 125, 791. [Google Scholar]
- 40. LaGier M. J., Scholin C. A., Fell J. W., Wang J., Goodwin K. D., Marine Poll. Bull. 2005, 50, 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lermo A., Campoy S., Barbé J., Hernández S., Alegret S., Pividori M. I., Biosens. Bioelectron. 2007, 22, 2010. [DOI] [PubMed] [Google Scholar]
- 42. Lermo A., Liébana S., Campoy S., Fabiano S., García M. I., Soutullo A., Zumárraga M. J., Alegret S., Pividori M. I., Internat. Microb. 2010, 13, 91. [DOI] [PubMed] [Google Scholar]
- 43. Berti F., Laschi S., Palchetti I., Rossier J. S., Reymond F., Mascini M., Marrazza G., Talanta 2009, 77, 971. [DOI] [PubMed] [Google Scholar]
- 44. Laschi S., Miranda‐Castro R., González‐Fernández E., Palchetti I., Reymond F., Rossier J. S., Marrazza G., Electrophoresis 2010, 31, 3727. [DOI] [PubMed] [Google Scholar]
- 45. Adam V., Huska D., Hubalek J., Kizek R., Microfluid Nanofluid 2010, 8, 329. [Google Scholar]
- 46. Loaiza O. A., Campuzano S., Pedrero M., Pividori M. I., García P., Pingarrón J. M., Anal. Chem. 2008, 80, 8239. [DOI] [PubMed] [Google Scholar]
- 47. Loaiza O. A., Campuzano S., Pedrero M., García P., Pingarrón J. M., Analyst 2009, 134, 34. [DOI] [PubMed] [Google Scholar]
- 48. Campuzano S., Pedrero M., García J. L., Pingarrón J. M., García P., García E., Anal. Bioanal. Chem. 2011, 399, 2413. [DOI] [PubMed] [Google Scholar]
- 49. Bonel L., Vidal J. C., Duato P., Castillo J. R., Biosens. Bioelectron. 2011, 26, 3254. [DOI] [PubMed] [Google Scholar]
- 50. Liébana S., Lermo A., Campoy S., Barbé J., Alegret S., Pividori M. I., Anal. Chem. 2009, 81, 5812. [DOI] [PubMed] [Google Scholar]
- 51.S. Campuzano, B. Esteban, M. Pedrero, J. L. García, E. García, J. M. Pingarrón, P. García, Chem. Sens. 2011, 1, 8.
- 52. Sugawara K., Kamiya N., Hirabayashi G., Kuramitz H., Talanta 2007, 72, 1123. [DOI] [PubMed] [Google Scholar]
- 53. Llopis X., Pumera M., Alegret S., Merkoçi A., Lab Chip 2009, 9, 213. [DOI] [PubMed] [Google Scholar]


