Abstract
Vaccination is a principal and highly cost‐effective means of controlling infectious diseases, providing direct protection against pathogens by conferring long‐lasting immunological memory and inducing population‐level herd immunity. Despite rapid ongoing progress in vaccinology, there remain many obstacles to the development and deployment of novel or improved vaccines; these include the underlying science of how to induce and sustain appropriate protective immune responses as well as bureaucratic, logistic and socio‐political hurdles. The failure to distribute and administer existing vaccines to at‐risk communities continues to account for a large proportion of infant mortality worldwide: almost 20 million children do not have access to basic vaccines and several million still die each year as a result. While emerging epidemic or pandemic diseases pose a significant threat to global health and prosperity, there are many infectious diseases which provide a continuous or cyclical burden on healthcare systems which also need to be addressed. Gaps in knowledge of the human immune system stand in the way of developing technologies to overcome individual and pathogenic variation. The challenges in tackling infectious disease and directions that the field of preventive medicine may take to improve the current picture of global health are the focus of this review.
Keywords: Difficult pathogens, Emerging infectious diseases, Future vaccines, Immunisation programmes, Vaccine‐preventable disease
Infant mortality rates are declining worldwide due to successful immunisation programmes. If this trend is to continue, socio‐political issues impeding vaccine uptake and knowledge gaps hindering vaccine development for difficult pathogens and emerging infectious diseases must be addressed. Challenges posed to improving global health through immunisation are the focus of this review.
Introduction
According to World Health Organisation (WHO) estimates, between 2 and 3 million deaths are averted worldwide on an annual basis as a result of childhood vaccination 1. There is no doubt that vaccines as a medical intervention have had a profound effect on controlling disease and improving the status of global health. In the two centuries since Edward Jenner's pioneering work on the smallpox vaccine, smallpox has successfully been eradicated as a result of improvements made to the vaccine over time and effective immunisation programmes. The polio vaccine has been another of the great success stories of modern medicine; the disease has effectively been eliminated in most parts of the world and the global elimination of poliomyelitis seems possible, with very few new cases in the early part of 2017 2. However, logistical, scientific and cultural challenges and high cost of the polio eradication endeavours may reduce enthusiasm for efforts to eradicate other infectious diseases with focus remaining on control. Nevertheless, the repertoire of vaccine‐preventable diseases (VPDs) is expanding and existing vaccines can be improved to enhance tolerability and increase the duration of protection.
Just twenty years ago, the UK national immunisation schedule was far more sparsely populated than it has become in the present day (see Table 1). In the early 1990s, the infant vaccination programme in the UK consisted of three doses of the diphtheria, tetanus, whole cell pertussis (DTwP), and Haemophilus influenzae type b (Hib) vaccine, and oral polio vaccine, with booster doses of DT and oral polio given later in childhood and again in adolescence, the live‐attenuated measles, mumps and rubella (MMR) vaccine. In the space of the last two decades, the number of diseases against which children are immunised has greatly increased. The DTwP vaccine has been replaced by DTaP‐IPV‐Hib‐HBV which contains acellular pertussis and inactivated polio virus vaccines, improving the programme's safety profile with the addition of hepatitis B vaccine included in the combination during 2017. An acellular pertussis vaccine (DTaP) can also be given to pregnant mothers and has been shown to protect neonates against pertussis. Effective polysaccharide‐protein conjugate vaccines are now available for pneumococcus, Hib and capsular group A, C, W, and Y meningococcus. Rotavirus vaccine is now incorporated into the schedule and the HPV vaccine was introduced to protect girls against the major causes of cervical cancer. Vaccines against influenza, pneumococcus and shingles are now included in the programme to protect the elderly.
Table 1.
UK immunisation schedule: past and present. A. UK immunisation schedule in 1996 B. Current UK immunisation schedule. DTwP, diphtheria, tetanus, whole cell pertussis; MMR, measles, mumps, rubella; TIV, trivalent influenza vaccine; DTaP, diphtheria, tetanus, acellular pertussis; IPV, inactivated polio vaccine; Hib, Haemophilus influenzae type b; HepB, hepatitis B; PCV, pneumococcal conjugate vaccine; Rota, rotavirus; MenB, meningococcal group B; MenC, meningococcal group C; HPV, human papillomavirus; MenACWY, meningococcal group A, C, W, Y; PPV, pneumococcal polysaccharide vaccine
| A. UK immunisation schedule 1996 | |||||||
|---|---|---|---|---|---|---|---|
| 2 months | 3 months | 4 months | 13 months | 3 years | Teenagers | Elderly | |
| DTwP‐Hib + oral polio | DTwP‐Hib + oral polio | DTwP‐Hib + oral polio | MMR | DT+ oral polio | DT + oral polio | TIV | |
| MMR | |||||||
| B. Current UK immunisation schedule | |||||||
|---|---|---|---|---|---|---|---|
| Pregnant women | 2 months | 3 months | 4 months | 12–13 months | 3 years | Teenagers | Elderly |
| TIV DTaP | DTaP/IPV/Hib/HepB (6‐in‐1) | 6‐in‐1 | 6‐in‐1 | Hib/MenC | DTaP/Polio | D/T/Polio | TIV Shingles PPV |
| PCV | PCV | PCV | HPV (girls 12–13 years) | ||||
| Rota | Rota | MMR | MMR | MenACWY | |||
| MenB | MenB | MenB | |||||
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The ongoing decline in infant mortality can be partly attributed to the control of infectious disease through public health measures including immunisation (see Fig. 1A). The UK has benefited from a significant decline in the incidence of measles and rubella as a result of introduction of the MMR vaccine, estimated by the World Health Organisation (WHO) at 90% and 99%, respectively 3. However, the burden of VPD and infant mortality remains substantial in low‐ and middle‐income countries (see Fig. 1B). Recent figures show that diphtheria, tetanus and pertussis vaccine coverage has reached 86% worldwide 4, but it is estimated that, globally, as many as 19.4 million children do not have access to essential vaccines and up to 1.5 million more deaths could be avoided annually by improving vaccine coverage 1. Access to vaccines is clearly the greatest problem to be overcome in reducing infant mortality worldwide. However, there are other social factors which contribute to underuse of vaccines even in countries with full immunisation programmes in place. Scientifically, most of the remaining challenges in the field of vaccinology can be attributed to gaps in knowledge of the immune responses required to induce protection against specific pathogens, overcome pathogenic variation and enhance sub‐optimal immune responses in certain age groups. The main obstacles to progress in this field and reducing the global burden of infectious disease are the focus of this review.
Figure 1.
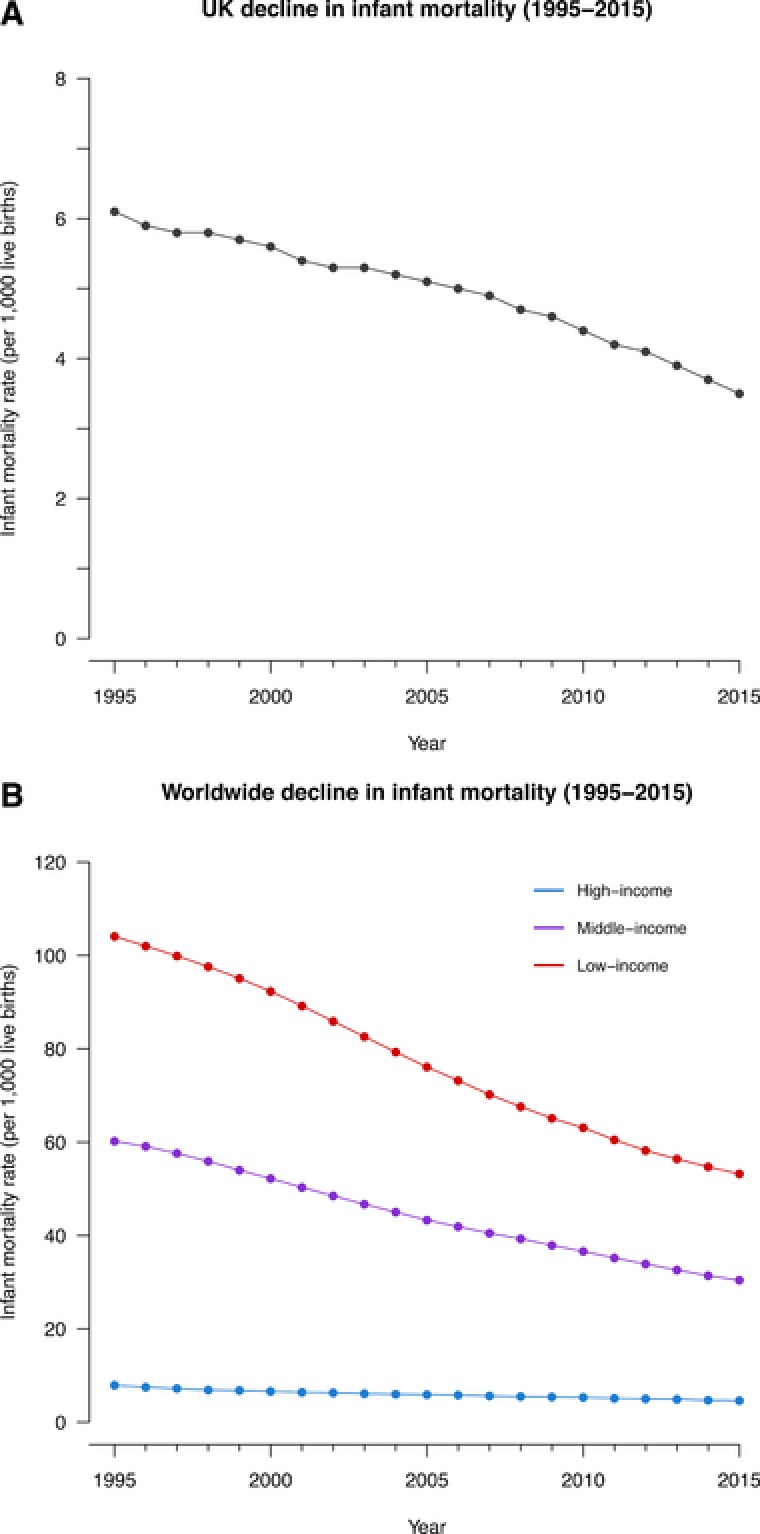
Decline in infant mortality rate per 1000 live births between 1995 and 2015. (A) Decline in infant mortality in the UK. (B) Comparison of the decline in infant mortality between low‐, middle‐, and high‐income countries worldwide. These data represent the number of infants dying before reaching one year of age, per 1000 live births in a given year and were estimated by the UN Inter‐agency Group for Child Mortality Estimation (UNICEF, WHO, World Bank, UN DESA Population Division).
Socio‐political challenges
Access
The greatest obstacle to improving global immunisation rates is poor access to vaccines for some individuals in all settings but especially in low‐income countries. The problem of access to vaccines in such countries is not limited to new vaccines; there is demand for basic existing vaccines such as DTP‐Hib‐HBV, PCV, rotavirus and MMR, yet access remains insufficient 5. Inaccessibility can be as a result of lack of proximity to health services; studies have highlighted lower rates of vaccine use and delay in vaccine uptake associated with rural communities in several programmes established to combat disease in Africa such as influenza in Kenya 6 and HPV in Nigeria 7. Rural living has also been associated with lower rates of primary immunisation in more economically advantaged countries such as Ireland 8. Even in urban environments engagement in immunisation programmes can be limited by factors such as poor living conditions and lack of education, particularly amongst migrant communities which suffer from economic disadvantages 9, 10.
Poliomyelitis stands on the precipice of global eradication but remains endemic in Pakistan and Afghanistan, despite elimination efforts in these populations. A recent study based on a survey conducted among Pakistani mothers found that lack of education and agency among women in society was associated with lower rates of vaccination 11. This highlights the link between the empowerment of women in society and childhood immunisation, with lower rates of immunisation frequently observed in countries where women experience greater societal and domestic oppression 12. Attacks on polio workers in these countries have also hindered vaccination campaigns 13 and engagement with local political, religious and community leaders is an important component of the strategy to improve access.
Safety concerns
Despite widespread access to basic and novel vaccines, vaccine uptake is still sub‐optimal in some high‐income countries. The association between unvaccinated individuals and VPD epidemics has been highlighted by a retrospective study of recent measles and pertussis outbreaks in the US which found that a significant proportion of cases in each outbreak affected those who had not received the relevant vaccine 14. There are concerns relating to the safety and necessity of vaccination among a small percentage of the population. While such hesitancy does not directly affect uptake, its influence on decision‐making and contribution to social uncertainty must not be underestimated. In the current era, medical and scientific authorities are under increased public scrutiny while individual choice in matters of healthcare has become more popular. The factors contributing to this are many, but controversies have had a substantial impact on attitudes towards vaccination. One recent example is the now refuted MMR claims over a proposed link with autism which was set in motion by a paper published in a medical journal in the UK and, as a result of media influence and advocacy groups, diffused across the globe 15. It took many years before public opinion reverted and MMR vaccine uptake was restored 16, though many parents still cite fear of autism as a concern 15. The best way to deal with these and other concerns that people may have is through education and public engagement but understanding the causes of hesitancy and specific interventions to address these concerns is crucial for effective management. The Vaccine Safety, Attitudes, Training and Communication Project is a European project which has shed light on many of the key issues surrounding parental attitudes to vaccination through national surveys 17. Improving understanding and acceptance of immunisation is of great importance for future national and global health.
Regulatory approval and implementation policy
Bringing a vaccine from discovery, through research and development to a licensed product is a lengthy and expensive process, taking an average of 11.9 years and costing between $500 million to $1 billion according to US estimates 18. Once a candidate has undergone rigorous preclinical studies required to enter the clinical testing, a vaccine undergoes phase 1 safety and immunogenicity trials followed by phase 2 proof‐of‐concept studies in a larger population, and then phase 3 trials to characterise the vaccine's efficacy and safety in the target population (see Fig. 2). After a vaccine has been shown to be safe and efficacious, manufacturers must obtain regulatory approval from the appropriate regulatory authorities. The Medicines and Healthcare Products Regulatory Authority is the authority responsible for approvals of vaccines in the UK and similar bodies exist for other geographical regions, for example the European Medicines Agency and the Food and Drug Administration regulate approvals for European and US medicines, respectively. Vaccines will often need to undergo additional local studies before approval is granted despite the availability of data from clinical studies carried out in other regions 5.
Figure 2.

Summary of the steps required to take a vaccine to licensure. Once a vaccine fulfils the criteria required of it at the preclinical stage of development it must then pass through three phases of clinical trials before it acquires regulatory approval. A Phase IV clinical trial can be conducted after licensure to assess the impact that the vaccine has had on a population.
In order for governments to implement a licensed vaccine in their national immunisation programme, cost‐effectiveness is often assessed as part of the decision‐making process. The typical metric applied to the analysis is ‘quality‐adjusted life years’ which serve as an indication of the number of reasonable quality years of life a person could acquire from medical intervention. In the UK, the National Institute for Health and Care Excellence has developed a methodology for assessing cost‐effectiveness and uses a cost of £20‐£30k for every quality‐adjusted life year as an upper limit for a public health intervention to be cost‐effective, though this is not the sole basis of acceptance or rejection 19. Risk of decision error, relative contribution of various sources of uncertainty and possible actions to reduce uncertainty must also be taken into consideration in order to maximise certainty of the net health benefit of a vaccine 20. The Joint Committee on Vaccination and Immunisation, an independent expert advisory committee, uses this methodology in making recommendations for the inclusion of a new vaccine in the immunisation schedule. Implementation policies vary between countries but all require the availability of sufficient data relating to disease burden, vaccine efficacy and economic and financial cost. Due to the multi‐disciplinary and region‐specific nature of implementation research, introduction of novel vaccines and escalation of vaccine coverage involves extensive collaboration between local research networks, industry and national government. This poses a challenge for countries which lack this infrastructure or financial capacity for implementation and will require the continued support of WHO initiatives.
Scientific challenges
Variation
Genetic variability of pathogens represents one of the greatest challenges to the design of novel effective vaccines. RNA viruses typically exhibit great genetic diversity and rapid evolution, making them particularly difficult targets for vaccination. This variation is partly attributable to the inherent error‐prone nature of RNA‐dependent RNA polymerase and its lack of proofreading activity. The rate of mutation of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) in vivo is astounding, estimated at 4.1 ± 1.7 × 10−3 per base per cell and 2.5 × 10⁻⁵ per base per replication cycle, respectively 21, 22. Bacterial pathogens also exhibit significant diversity in circulating strains within and between different populations. Capsular polysaccharides have been effectively targeted for pneumococcus and meningococcus, but protein antigens are less conserved between strains. For example, the immunogenic outer membrane protein, PorA, of Neisseria meningitidis exhibits phase variation through a combination of mutation, site‐specific recombination and epigenetic control of promoter regions, allowing the bacterium to evade bactericidal antibodies directed against this antigen 23. The protozoan parasite Plasmodium falciparum also exhibits antigenic diversity with polymorphisms in variable regions of its genome producing a range of antigen variants across endemic regions 24. Even when relatively conserved structural proteins are targeted in vaccine formulations, universal vaccine efforts will require advanced knowledge of molecular epidemiology and genetic variability between populations. Understanding the structural biology of antigen components of vaccine formulations is an important consideration when targeting multiple strains of a pathogen and extending vaccine strain coverage.
Genetic diversity in humans can have an effect on vaccine responses, most notably through variations associated with the human leukocyte antigen (HLA) alleles and thus helper T cell responses. Meta‐analyses have highlighted the link between several HLA allelic variants and other immune receptor single nucleotide polymorphisms (SNPs) and differential antibody responses to a number of vaccines 25. The strongest correlation between genotype and phenotype is seen in complete non‐responders to the hepatitis B vaccine whose HLA class II alleles are associated with antibody response to hepatitis B 26. A more complete understanding of the phenotypic effects of polymorphisms identified in genome‐wide studies through functional studies will be important in developing improved vaccines to overcome such variation within the population. Successful disease elimination is dependent on uniform responses to immunisation within a population if the effects of individual phenotypes cannot be overcome through herd immunity.
Outbreaks
The recent Ebola epidemic in parts of West Africa highlighted the importance of preparedness in dealing with emerging and outbreak pathogens. Despite the overwhelming global response to the crisis and the rapid mobilisation of candidate vaccines through trials, little could be done to control the spread of disease at the time. The Coalition for Epidemic Preparedness Innovations (CEPI) was established in the wake of this epidemic to fund the research and development of diagnostics, therapies and vaccines for epidemic infectious diseases (EIDs) to prevent a repeat of such a catastrophe 27. Its priorities include pathogens associated with recent outbreaks such as severe acute respiratory syndrome (SARS) coronavirus, Ebola and Zika virus, and several other emerging pathogens and neglected tropical diseases (NTDs). Many of the diseases deemed to be emergent threats represent significant knowledge gaps in immunology which will have to be overcome through multi‐disciplinary research endeavours.
The UK government pledged £120 million and established a UK Vaccine Network to address the problems of infectious diseases of epidemic potential which lack the market incentive for pharmaceutical industry investment. The UK Vaccine Network focuses on the development of vaccines against prioritised diseases through the cooperation of academic institutions, the National Health Service (NHS) and pharmaceutical companies 28. Collaborative efforts between scientists and between academia and industry will be vital for tackling future epidemics. There is much research to be done on known pathogens which represent possible threats and have not been as extensively characterised as those which have attracted the most funding in the past. This disparity can only be resolved by incentivising investment in research and development. On the other hand, the next threats to global health may be totally unknown. Recent outbreaks have increased awareness of the importance of scientific surveillance and potential pandemic control measures. Improving preparedness for the emergence of novel pathogens will rely more heavily on the mandatory reporting of surveillance data, the international flow of information to provide rapid and effective control, and the development of platform technologies into which components of new pathogens can be inserted in the rapid development of new vaccines.
Future vaccines
There are several diseases of varying prevalence and severity which represent a significant burden to health care systems in many countries and for which no licensed vaccine currently exists. As the leading cause of infant hospitalisation worldwide, respiratory syncytial virus (RSV) warrants particular attention. RSV is a significant cause of morbidity and mortality in infants worldwide and, considering its burden, is the most important infant disease which can be prevented by a vaccine for which no licensed vaccine exists 29. The virus causes respiratory tract infections (RTIs) which range from mild upper RTIs to severe lower RTIs, with up to half of children suffering from the latter developing recurrent wheezing or asthma 30. After malaria, RSV is the leading cause of infectious death in infants worldwide and is responsible for approximately 6.7% of all deaths in this age group 31. At the time of writing (June 2017) there were 59 vaccine candidates in various stages of development, 16 of which were undergoing clinical evaluation 32. However, the paucity of data relating to the epidemiology of RSV, transmission of the virus and the full health‐care burden, especially in low resource settings, will be an impediment to the decision‐making process for inclusion of a successful vaccine candidate in national immunisation programmes and must be addressed.
Maternal immunisation is being considered as a possible strategy for the prevention of RSV infection in early infancy, a time at which direct vaccination may be poorly effective 33. Indeed, one candidate has been demonstrated safe and immunogenic in pregnant women and a Phase III clinical trial has been initiated to determine its efficacy 34. Maternal immunisation has become an important approach to providing early protection in neonates and simultaneously offering a level of protection to pregnant women. During the natural course of a pregnancy, IgG antibodies are transferred from the mother to the developing foetus across the placental barrier via the neonatal Fc receptor (FcRn) present on syncytiotrophoblast cells, conferring passive immunity 35. For diseases such as pertussis which have peak incidence in the first months of life, maternal immunisation could provide protection for babies prior to receiving active routine immunisation in early infancy. Tetanus, diphtheria, pertussis (TDaP) vaccination in pregnant women has provided proof‐of‐principle that vaccination of pregnant women is safe for both mother and child and that antibodies can be protective in newborns 36. Such a strategy would also be effective in the prevention of group B streptococcal infection (GBS), which is transferred from mother to child at birth and can cause pneumonia, bacteraemia and meningitis in 2–3% of colonised neonates 37. A GBS candidate vaccine has also recently been shown to be safe and immunogenic in pregnant women 38. The global availability of an effective vaccine against GBS would relieve the burden of perinatal disease associated with this pathogen.
Cytomegalovirus (CMV) infection is extremely common, establishing latent infection in roughly half of the population in most high‐income countries 39. While adult CMV infection is usually mild and often asymptomatic, congenital acquisition of the virus, as a result of reactivation of the virus or primary infection in pregnancy, can lead to birth defects and long‐term sequelae 39. Congenital CMV infection affects 0.5–2% of newborns worldwide and it is the principal cause of cognitive impairment and loss of hearing in this cohort 40. The challenge to create a vaccine which could provide protection in utero is ongoing and deployment of such a vaccine prior to conception will likely require programmes aimed at childhood, and possibly prior to primary infection, i.e. in infancy. Group A streptococcus is another very common pathogen which has a diverse range of clinical manifestations, is associated with a significant global burden and disproportionately affects children of indigenous communities such as in Australia and New Zealand 41. Several vaccines are under development and data on efficacy are eagerly awaited 42. These are just some of the vaccines that may be developed and/or introduced to reduce infant mortality and alleviate morbidity over the next 20 years.
Another challenge is the growing problem of infection acquired in the hospital setting. Nosocomial infections, also referred to as health‐care associated infections (HCAIs), are an increasingly frequent cause of complications associated with hospitalisation, particularly among postoperative patients and those in intensive care units. HCAIs are responsible for extended hospitalisation at a significant cost to both patient and health systems. A comprehensive report published by the WHO in 2011 outlines in detail the global health burden caused by endemic HCAIs and makes a strong case for vaccine interventions 43. Infections caused by Clostridium difficile, norovirus, and antibiotic‐resistant Gram negative bacteria have become more frequent with the increasing use of antibiotics over the last few decades while Staphylococcus aureus remains the leading cause of HCAIs worldwide. The WHO recently published a list of a dozen families of bacteria which represent a serious threat to public health as a result of their acquisition of resistance genes 44. Among these pathogens, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacteriaceae were cited as being of highest priority. Research into developing vaccines against several of these bacteria is ongoing but is hindered by the lack of known correlates of protection and animal challenge models in which these can be demonstrated. The difficulties associated with targeting variable antigens also apply as many of these species exhibit substantial strain‐to‐strain variation. The threat of multidrug‐resistant bacterial strains is one that could be best circumvented by immunoprophylaxis of major risk groups.
Difficult pathogens
There are a number of challenges in vaccine development that have hampered development of vaccines against important pathogens such as HIV, malaria and tuberculosis (TB). These challenges arise largely because of the ability of these difficult pathogens to evade and manipulate the host immune response. In the case of HIV and HCV it is the considerable variability of protein sequence which develops during infection, which makes development of an inactivated vaccine based on a single sequence ineffective. Further understanding of the immune response to HIV may eventually provide the solution. One approach could be identification of broadly neutralising antibodies to prevent infection with the virus and cytotoxic T cell responses capable of eliminating infected CD4+ T cells. The induction of broadly‐neutralising antibody responses to HIV epitopes has been exemplified in recent studies of HIV envelope trimers in primates 45. Understanding the immunodominance of B cells to non‐neutralising epitopes and the mechanisms by which germinal centre responses can be optimised will be important for improving methods of generating neutralising antibodies to novel immunogens.
The high rates of sustained virologic response that can be achieved through direct‐acting antiviral therapies for HCV infection have called into question the necessity of a vaccine. However, these therapies are currently prohibitively expensive and it is still uncertain whether they protect patients from re‐infection. For these reasons, a preventive vaccine would be the most effective means of reducing the global burden of chronic liver disease associated with the virus. A consensus on the relative contribution of neutralising antibodies to viral epitopes and cytotoxic T cell responses directed against infected cells to resolving acute infection has not yet been established. The viral vector vaccine prime‐boost strategy currently undergoing clinical trials in an at‐risk population will test the current hypothesis that a T cell priming vaccine is sufficient to prevent acute HCV infection 46.
In addition to the antigenic variation of Plasmodium falciparum, the complexity of the parasite's life cycle presents a unique challenge to the identification of what constitutes a protective immune responses against the pathogen. The pre‐erythrocytic and blood stages of malaria infection still lack clear correlates of immunity, adding to the difficulty of pre‐clinical evaluation of candidate vaccines. To this end, challenge studies have been particularly informative for malaria vaccine development and had an important role to play in the optimisation of the malaria RTS,S vaccine 47. The establishment of phase I trials and human challenge studies in endemic regions is enhancing optimisation of malaria vaccines for target populations.
While Mycobacterium tuberculosis exhibits a much lower degree of variability than the other pathogens discussed in this section, the clinical manifestations of infection differ between age groups and successful intervention may require one vaccine for the pre‐exposed population and another for the exposed population to prevent disease progression. With regard to the former, efforts have been made to improve the existing Bacillus Calmette‐Guérin vaccine but have been hampered by lack of understanding for the variable efficacy of the vaccine against pulmonary TB 48. TB vaccine development would benefit greatly from the identification of new correlates of protection and animal models which more accurately reflect the progression of human TB. Indeed, it remains unknown which responses are important in prevention of infection, while the responses used to contain natural infection, such as polyfunctional CD4+ and CD8+ T‐cell responses, are better understood 49. Transcriptomic analyses often accompany vaccine efficacy studies and are an important tool for the identification of early gene “signatures” which may predict immunogenicity. Extensive characterisation of the yellow fever vaccine, YF‐17D, has provided proof‐of‐principle for the ability of such gene signatures to predict immune responses and protective immunity following immunisation 50, 51. This transcriptomics/ systems biology approach will be a useful tool to delineate new correlates of protection and will aid vaccine candidate selection in the future.
For most infections caused by pathogens which exhibit genetic variability, a vaccine which induces cross‐protective neutralising antibody responses is most desirable. This is particularly important for dengue virus given that during a natural infection an infected individual generates long‐lived, protective, neutralising antibodies against that serotype, but antibodies directed against other serotypes are cross‐reactive and non‐neutralising. This leads to an immunological phenomenon known as antibody‐dependent enhancement whereby dengue virus particles opsonised by these non‐neutralising antibodies infect Fc receptor‐expressing cells such as macrophages and monocytes 52. The possibility of exacerbation of a secondary heterologous dengue virus infection requires a quadrivalent vaccine that produces consistent and long‐lasting protection against all four serotypes to preclude this immunological enhancement.
The importance of basic immunology research in vaccine development is highlighted when prevailing hypotheses are rejected following failure in vaccine efficacy studies. This type of research is essential for addressing the knowledge gaps which continue to hinder vaccine development for difficult pathogens and for the formation of new scientific paradigms which will inform the development of the next generation of vaccines.
Immunosenescence
The increase in average life expectancy which has accompanied improvements made in public health and hygiene in high‐income countries over the last century has led to a growing elderly population. As adaptive responses decline in an ageing immune system due to the reduction in peripheral naïve T cells and B cells and the resultant shift towards memory phenotype and decrease in antigen‐specific responses, the elderly experience heightened morbidity and mortality from infections, particularly in those aged 80–100 years 53. These changes are described as immunosenescence and represent an issue of growing global concern for the maintenance of a healthy aging population. The current thinking is that in advanced age, the differentiation of haematopoietic stem cells into lymphoid and myeloid precursors skews in the direction of the latter, leading to fewer lymphocytes in the circulation, the causes of which are reviewed elsewhere 54. The result is an immune system that is more vulnerable to infection with certain pathogens but which is also less responsive to immunisation.
As mentioned earlier, the immunisation programme in the UK currently includes three vaccines for those aged 65 years and above. Several other vaccines from the immunisation schedule have been studied in this age group but of these, only the TDaP vaccine induces a protective immune response comparable to that induced when it is administered earlier in life 55. While the trivalent influenza vaccine, pneumococcal conjugate vaccine and herpes zoster vaccine are capable of mitigating their respective diseases to varying degrees, they do not induce a satisfactory immune response in the majority of the elderly population. Recent efforts to improve vaccine responses in the elderly have focused on increasing vaccine doses 56, exploring the use of alternative adjuvants to stimulate the innate response and engineering viral vectors to engage T cell responses 57. Some success has been achieved, for example, the zoster glycoprotein vaccine incorporating the AS01B adjuvant system was recently shown to reduce the risk of herpes zoster infection in adults greater than 70 years of age in a Phase III clinical trial 58. Greater understanding of the immunological factors that underlie the ageing adaptive response at the molecular and cellular level will be imperative to the development of restorative interventions that promote functional response to vaccination.
Conclusion
Immunisation rates have been steadily rising over the last two decades, and global infant mortality appears to be on a steady decline. However, this picture hides the patchy nature of progress, with infant mortality still representing approximately one third of deaths in low‐income countries, and vaccine‐preventable infectious disease constitutes a major proportion of these deaths 59. The GAVI Alliance has done a great deal to bring vaccines to those most at risk, but middle‐income countries do not meet the eligibility criteria for assistance. With the considerable expense of new vaccines such as the human papillomavirus and pneumococcal vaccines, the disparity of access between socioeconomic regions will take considerably more funding to overcome, especially as more countries graduate from Gavi eligibility.
Some of the current challenges to vaccine development have been summarised in this review, but rapid advances in the immunology that underpins vaccinology promises continuing progress in the development of novel, effective and safe vaccines. There are many new vaccines under development which target infections for which no vaccine currently exists and research into suitable antigens, live vectors and improved adjuvants continues to advance. The collaborative networks which have been established to deal with the threat of emerging infectious diseases provide testament to the effectiveness of partnerships between research institutes, industry and academia, allocating much needed resources to under‐funded areas of research. Recent UK‐based networks include those focused on maternal vaccines, vaccines for persistent intracellular infections, bacterial diseases and supporting the use of live infection challenge of volunteers to advance vaccinology 60. The infrastructure put in place by these projects will create platforms for further research and development, ensuring that global health will continue to improve over future decades.
Conflict of interest
A.J.P. has previously conducted clinical trials of vaccines on behalf of Oxford University funded by vaccine manufacturers but did not receive any personal payments from them. His department received unrestricted educational grants from Pfizer/GSK/Astra Zeneca in July 2016 for a course on Infection and Immunity in Children.
A.J.P. is chair of the UK Department of Health's (DH) Joint Committee on Vaccination and Immunisation (JCVI), and a member of WHOs Strategic Advisory Group of Experts, but the views expressed in this manuscript do not necessarily represent the views of JCVI or DH or WHO.
Acknowledgements
A.J.P. is a Jenner Investigator and James Martin Senior Fellow at the University of Oxford. A.J.P. is supported by the NIHR Oxford Biomedical Research Centre. PJMO is an NIHR Senior Investigator and President of the BSI. P.J.M.O. is supported by the Wellcome Trust and the NIHR Biomedical Research Centre at Imperial College NHS Healthcare Trust. D.S. receives funding from the Medical Research Council and the University of Oxford Scatcherd European Scholarship in the form of a DPhil studentship.
References
- 1. World Health Organization , Global Immunization Vision and Strategy 2006–2015 2016. Available at: http://www.who.int/mediacentre/factsheets/fs378/en/ [DOI] [PMC free article] [PubMed]
- 2. Polio Global Eradication Initiative , Polio today. 2017. Available at: http://polioeradication.org/polio-today/polio-now/this-week/
- 3. World Health Organization, Immunization Highlights 2013. 2014. Available at: http://www.euro.who.int/__data/assets/pdf_file/0007/248236/Immunization-Highlights-2013-E.pdf
- 4. World Health Organization, Immunization, vaccines and biologicals ‐ Diphtheria. 2015. http://www.who.int/immunization/monitoring_surveillance/burden/diphtheria/en/
- 5. Pagliusi, S. , Jain, R. and Suri, R. K. , Vaccines, our shared responsibility. Vaccine 2015. 33: 2197–2202. [DOI] [PubMed] [Google Scholar]
- 6. Otieno, N. A. , Nyawanda, B. O. , Audi, A. , Emukule, G. , Lebo, E. , Bigogo, G. , Ochola, R. et al, Demographic, socio‐economic and geographic determinants of seasonal influenza vaccine uptake in rural western Kenya, 2011. Vaccine 2014. 32: 6699–6704. [DOI] [PubMed] [Google Scholar]
- 7. Adepoju, E. G. , Ilori, T. , Olowookere, S. A. and Idowu, A. , Targeting women with free cervical cancer screening: challenges and lessons learnt from Osun state, southwest Nigeria. Pan Afr Med J 2016. 24: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jessop, L. J. , Kelleher, C. C. , Murrin, C. , Lotya, J. , Clarke, A. T. , O'Mahony, D. , Fallon, U. B. et al, Determinants of partial or no primary immunisations. Archives of Disease in Childhood 2010. 95: 603–605. [DOI] [PubMed] [Google Scholar]
- 9. Kusuma, Y. S. , Kumari, R. , Pandav, C. S. and Gupta, S. K. , Migration and immunization: determinants of childhood immunization uptake among socioeconomically disadvantaged migrants in Delhi, India. Trop Med Int Health 2010. 15: 1326–1332. [DOI] [PubMed] [Google Scholar]
- 10. Hu, Y. , Li, Q. , Chen, E. , Chen, Y. and Qi, X. , Determinants of childhood immunization uptake among socio‐economically disadvantaged migrants in East China. Int J Environ Res Public Health 2013. 10: 2845–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan, M. T. , Zaheer, S. and Shafique, K. , Maternal education, empowerment, economic status and child polio vaccination uptake in Pakistan: a population based cross sectional study. BMJ Open 2017. 7: e013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thorpe, S. , VanderEnde, K. , Peters, C. , Bardin, L. and Yount, K. M. , The influence of Women's empowerment on child immunization coverage in low, lower‐middle, and upper‐middle income countries: a systematic review of the literature. Matern Child Health J 2016. 20: 172–186. [DOI] [PubMed] [Google Scholar]
- 13. Walsh, D. and McNeil, D. G. , Gunmen in Pakistan kill women who were giving children polio vaccines. The New York Times 2012. [Accessed March 7, 2017] Available at: http://www.nytimes.com/2012/12/19/world/asia/attackers-in-pakistan-kill-anti-polio-workers.html. [Google Scholar]
- 14. Phadke, V. K. , Bednarczyk, R. A. , Salmon, D. A. and Omer, S. B. , Association between vaccine refusal and vaccine‐preventable diseases in the United States: a review of measles and pertussis. JAMA 2016. 315: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poland, G. A. and Spier, R. , Fear, misinformation, and innumerates: how the Wakefield paper, the press, and advocacy groups damaged the public health. Vaccine 2010. 28: 2361–2362. [DOI] [PubMed] [Google Scholar]
- 16. Smith, A. , Yarwood, J. and Salisbury, D. M. , Tracking mothers' attitudes to MMR immunisation 1996–2006. Vaccine 2007. 25: 3996–4002. [DOI] [PubMed] [Google Scholar]
- 17. Stefanoff, P. , Mamelund, S. E. , Robinson, M. , Netterlid, E. , Tuells, J. , Bergsaker, M. A. , Heijbel, H. et al, Tracking parental attitudes on vaccination across European countries: The Vaccine Safety, Attitudes, Training and Communication Project (VACSATC). Vaccine 2010. 28: 5731–5737. [DOI] [PubMed] [Google Scholar]
- 18. Pronker, E. S. , Weenen, T. C. , Commandeur, H. R. , Osterhaus, A. D. and Claassen, H. J. , The gold industry standard for risk and cost of drug and vaccine development revisited. Vaccine 2011. 29: 5846–5849. [DOI] [PubMed] [Google Scholar]
- 19. National Institute for Health and Care Excellence, Judging whether public health interventions offer value for money. 2013. Available at: https://www.nice.org.uk/advice/lgb10/chapter/judging-the-cost-effectiveness-of-public-health-activities-nices-approach-to-assessing-public-health-interventions
- 20. Joint Committee on Vaccination and Immunisation, Code of Practice. 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/224864/JCVI_Code_of_Practice_revision_2013_-_final.pdf
- 21. Cuevas, J. M. , Geller, R. , Garijo, R. , López‐Aldeguer, J. and Sanjuán, R. , Extremely High Mutation Rate of HIV‐1 In Vivo. PLoS Biology 2015. 13: e1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ribeiro, R. M. , Li, H. , Wang, S. , Stoddard, M. B. , Learn, G. H. , Korber, B. T. , Bhattacharya, T. et al, Quantifying the diversification of hepatitis C virus (HCV) during primary infection: estimates of the in vivo mutation rate. PLoS Pathog 2012. 8: e1002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tauseef, I. , Ali, Y. M. and Bayliss, C. D. , Phase variation of PorA, a major outer membrane protein, mediates escape of bactericidal antibodies by Neisseria meningitidis. Infect. Immun. 2013. 81: 1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Claessens, A. , Hamilton, W. L. , Kekre, M. , Otto, T. D. , Faizullabhoy, A. , Rayner, J. C. and Kwiatkowski, D. , Generation of antigenic diversity in Plasmodium falciparum by structured rearrangement of var genes during mitosis. PLOS Genetics 2014. 10: e1004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Posteraro, B. , Pastorino, R. , Di Giannantonio, P. , Ianuale, C. , Amore, R. , Ricciardi, W. and Boccia, S. , The link between genetic variation and variability in vaccine responses: systematic review and meta‐analyses. Vaccine 2014. 32: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 26. Li, Z. K. , Nie, J. J. , Li, J. and Zhuang, H. , The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta‐analysis. Vaccine 2013. 31: 4355–4361. [DOI] [PubMed] [Google Scholar]
- 27. Plotkin, S. A. , Mahmoud, A. A. and Farrar, J. , Establishing a global vaccine‐development fund. N Engl J Med 2015. 373: 297–300. [DOI] [PubMed] [Google Scholar]
- 28. British Society for Immunology, A proposal to create a “UK Vaccine Network”. 2015. Available at: https://www.immunology.org/sites/default/files/a-proposal-to-create-a-uk-vaccines-network-policy-briefing.pdf
- 29. Green, C. A. , Yeates, D. , Goldacre, A. , Sande, C. , Parslow, R. C. , McShane, P. , Pollard, A. J. et al, Admission to hospital for bronchiolitis in England: trends over five decades, geographical variation and association with perinatal characteristics and subsequent asthma. Arch Dis Child 2016. 101: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nenna, R. , Ferrara, M. , Nicolai, A. , Pierangeli, A. , Scagnolari, C. , Papoff, P. , Antonelli, G. et al, Viral Load in Infants Hospitalized for Respiratory Syncytial Virus Bronchiolitis Correlates with Recurrent Wheezing at Thirty‐Six‐Month Follow‐Up. Pediatr Infect Dis J 2015. 34: 1131–1132. [DOI] [PubMed] [Google Scholar]
- 31. Lozano, R. , Naghavi, M. , Foreman, K. , Lim, S. , Shibuya, K. , Aboyans, V. , Abraham, J. et al, Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012. 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins, D. , Trujillo, C. and Keech, C. , Advances in RSV vaccine research and development ‐ A global agenda. Vaccine 2016. 34: 2870–2875. [DOI] [PubMed] [Google Scholar]
- 33. Munoz, F. M. , Respiratory syncytial virus in infants: is maternal vaccination a realistic strategy? Curr Opin Infect Dis 2015. 28: 221–224. [DOI] [PubMed] [Google Scholar]
- 34. Novavax, Novavax Initiates Global Pivotal Phase 3 Trial of the RSV F Vaccine to Protect Infants via Maternal Immunization. 2015. Available at: http://ir.novavax.com/phoenix.zhtml?c=71178&p=irol-newsArticle&ID=2120087
- 35. Palmeira, P. , Quinello, C. , Silveira‐Lessa, A. L. , Zago, C. A. and Carneiro‐Sampaio, M. , IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012. 2012: 985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindsey, B. , Kampmann, B. and Jones, C. , Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr Opin Infect Dis 2013. 26: 248–253. [DOI] [PubMed] [Google Scholar]
- 37. Madhi, S. A. and Dangor, Z. , Prospects for preventing infant invasive GBS disease through maternal vaccination. Vaccine 2017. 35(35 Pt A): 4457–4460. [DOI] [PubMed] [Google Scholar]
- 38. Donders, G. G. G. , Halperin, S. A. , Devlieger, R. , Baker, S. , Forte, P. , Wittke, F. , Slobod, K. S. et al, Maternal immunization with an investigational trivalent group B streptococcal vaccine: a randomized controlled trial. Obstetrics Gynecology 2016. 127: 213–221. [DOI] [PubMed] [Google Scholar]
- 39. Manicklal, S. , Emery, V. C. , Lazzarotto, T. , Boppana, S. B. and Gupta, R. K. , The “Silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013. 26: 86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bialas, K. M. and Permar, S. R. , The March towards a Vaccine for Congenital CMV: Rationale and Models. PLOS Pathogens 2016. 12: e1005355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ralph, A. P. and Carapetis, J. R. , Group a streptococcal diseases and their global burden. Curr Top Microbiol Immunol 2013. 368: 1–27. [DOI] [PubMed] [Google Scholar]
- 42. Sheel, M. , Moreland, N. J. , Fraser, J. D. and Carapetis, J. , Development of Group A streptococcal vaccines: an unmet global health need. Exp. Rev. Vaccines 2016. 15: 227–238. [DOI] [PubMed] [Google Scholar]
- 43. World Health Organization , Report on the burden of endemic health care‐associated infection worldwide. 2011. Available at: http://apps.who.int/iris/bitstream/10665/80135/1/9789241501507_eng.pdf?ua=1
- 44. World Health Organization , WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. Available at: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/
- 45. Pauthner, M. , Havenar‐Daughton, C. , Sok, D. , Nkolola, J. P. , Bastidas, R. , Boopathy, A. V. , Carnathan, D. G. et al, Elicitation of robust tier 2 neutralizing antibody responses in nonhuman primates by HIV envelope trimer immunization using optimized approaches. Immunity 2017. 46: 1073–1088.e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. ClinicalTrials.gov, Staged Phase I/II Hepatitis C Prophylactic Vaccine. 2017. https://clinicaltrials.gov/ct2/show/NCT01436357
- 47. Sauerwein, R. W. , Roestenberg, M. and Moorthy, V. S. , Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol 2011. 11: 57–64. [DOI] [PubMed] [Google Scholar]
- 48. Davenne, T. and McShane, H. , Why don't we have an effective tuberculosis vaccine yet? Expert Rev Vaccines 2016. 15: 1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jasenosky, L. D. , Scriba, T. J. , Hanekom, W. A. and Goldfeld, A. E. , T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev 2015. 264: 74–87. [DOI] [PubMed] [Google Scholar]
- 50. Gaucher, D. , Therrien, R. , Kettaf, N. , Angermann, B. R. , Boucher, G. , Filali‐Mouhim, A. , Moser, J. M. et al, Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 2008. 205: 3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Querec, T. D. , Akondy, R. S. , Lee, E. K. , Cao, W. , Nakaya, H. I. , Teuwen, D. , Pirani, A. et al, Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 2009. 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wahala, W. and de Silva, A. M. , The human antibody response to dengue virus infection. Viruses 2011. 3: 2374–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weng, N.‐p. , Aging of the immune system: how much can the adaptive immune system adapt? Immunity 2006. 24: 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Van Zant, G. and Liang, Y. , Concise review: hematopoietic stem cell aging, life span, and transplantation. Stem Cells Transl Med 2012. 1: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weston, W. M. , Friedland, L. R. , Wu, X. and Howe, B. , Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix((R))): results of two randomized trials. Vaccine 2012. 30: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 56. DiazGranados, C. A. , Dunning, A. J. , Jordanov, E. , Landolfi, V. , Denis, M. and Talbot, H. K. , High‐dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009–2010 season. Vaccine 2013. 31: 861–866. [DOI] [PubMed] [Google Scholar]
- 57. Antrobus, R. D. , Lillie, P. J. , Berthoud, T. K. , Spencer, A. J. , McLaren, J. E. , Ladell, K. , Lambe, T. et al, A T cell‐inducing influenza vaccine for the elderly: safety and immunogenicity of MVA‐NP+M1 in adults aged over 50 years. PLoS One 2012. 7: e48322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cunningham, A. L. , Lal, H. , Kovac, M. , Chlibek, R. , Hwang, S.‐J. , Díez‐Domingo, J. , Godeaux, O. et al, Efficacy of the Herpes Zoster subunit vaccine in adults 70 years of age or older. N. England J. Med. 2016. 375: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 59. World Health Organization , The top 10 causes of death. 2017. http://www.who.int/mediacentre/factsheets/fs310/en/
- 60. Medical Research Council and Biotechnology and Biological Sciences Research Council, MRC/BBSRC Global Challenge Research Fund Networks for Vaccine R&D. 2016. https://www.mrc.ac.uk/documents/pdf/outcome-of-eoi-stage-2016/


