Key Points
Question
Is visual impairment associated with women’s risk for developing dementia?
Findings
In this cohort study of 1061 older women, baseline objectively measured visual impairment was associated with a 2- to 5-fold increased risk of dementia over a median 3.8 years of follow-up; 3.1% of participants without objective visual impairment developed dementia vs 8.2% of those with visual acuity of 20/40 or worse. More severe visual impairment was associated with increasingly elevated risk of incident dementia.
Meaning
These results suggest that visual impairment may be a risk factor for dementia; findings are limited by sample size, and more research is needed in a larger population.
Abstract
Importance
Dementia affects a large and growing population of older adults. Although past studies suggest an association between vision and cognitive impairment, there are limited data regarding longitudinal associations of vision with dementia.
Objective
To evaluate associations between visual impairment and risk of cognitive impairment.
Design, Setting, and Participants
A secondary analysis of a prospective longitudinal cohort study compared the likelihood of incident dementia or mild cognitive impairment (MCI) among women with and without baseline visual impairment using multivariable Cox proportional hazards regression models adjusting for characteristics of participants enrolled in Women’s Health Initiative (WHI) ancillary studies. The participants comprised community-dwelling older women (age, 66-84 years) concurrently enrolled in WHI Sight Examination (enrollment 2000-2002) and WHI Memory Study (enrollment 1996-1998, ongoing). The study was conducted from 2000 to the present.
Exposures
Objectively measured visual impairment at 3 thresholds (visual acuity worse than 20/40, 20/80, or 20/100) and self-reported visual impairment (determined using composite survey responses).
Main Outcomes and Measures
Hazard ratios (HRs) and 95% CIs for incident cognitive impairment after baseline eye examination were determined. Cognitive impairment (probable dementia or MCI) was based on cognitive testing, clinical assessment, and centralized review and adjudication. Models for (1) probable dementia, (2) MCI, and (3) probable dementia or MCI were evaluated.
Results
A total of 1061 women (mean [SD] age, 73.8 [3.7] years) were identified; 206 of these women (19.4%) had self-reported visual impairment and 183 women (17.2%) had objective visual impairment. Forty-two women (4.0%) were ultimately classified with probable dementia and 28 women (2.6%) with MCI that did not progress to dementia. Mean post–eye examination follow-up was 3.8 (1.8) years (range, 0-7 years). Women with vs without baseline objective visual impairment were more likely to develop dementia. Greatest risk for dementia was among women with visual acuity of 20/100 or worse at baseline (HR, 5.66; 95% CI, 1.75-18.37), followed by 20/80 or worse (HR, 5.20; 95% CI, 1.94-13.95), and 20/40 or worse (HR, 2.14; 95% CI, 1.08-4.21). Findings were similar for risk of MCI, with the greatest risk among women with baseline visual acuity of 20/100 or worse (HR, 6.43; 95% CI, 1.66-24.85).
Conclusions and Relevance
In secondary analysis of a prospective longitudinal cohort study of older women with formal vision and cognitive function testing, objective visual impairment appears to be associated with an increased risk of incident dementia. However, incident cases of dementia and the proportion of those with visual impairment were low. Research is needed to evaluate the effect of specific ophthalmic interventions on dementia.
This cohort study examines the visual levels of participants in the Women’s Health Initiative with vs without cognitive impairment.
Introduction
Alzheimer disease and related dementias currently affect approximately 20% of women aged 65 years and older, and the prevalence continues to grow.1 Better understanding of risk and ameliorating factors affecting dementia development, severity, and progression is necessary to address the growing population disease burden.
Visual and cognitive function may be interrelated, potentially reflecting greater cognitive load associated with low vision, underlying brain structural and functional changes, and/or social isolation from low vision. Cross-sectional studies imply a potential link between visual and cognitive impairment2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18; however, longitudinal evidence associating vision with incident dementia remains limited. A National Institute on Aging conference (under the National Institutes of Health Research Conference Cooperative Agreement U13Program) called for more research on the longitudinal effect of neurosensory impairment on cognitive function and effect of potential neurosensory interventions to improve cognitive function.19,20 However, it has not been thoroughly examined whether poor vision or eye disease are risk factors for cognitive impairment.
The Women’s Health Initiative (WHI), a study with more than 20 years of follow-up including detailed clinical information, presents an opportunity to investigate a longitudinal association between vision and cognitive impairment. Prior WHI research has demonstrated associations between retinopathy and cognitive impairment and brain ischemia identified on neuroimaging.21 In this study, we leveraged formal vision and longitudinal cognitive assessments to evaluate associations of baseline objective and subjective visual impairment with incident cognitive impairment. This large-scale, longitudinal analysis is particularly relevant for postmenopausal women, among whom visual impairment and eye disease are disproportionately prevalent.22,23
Methods
Participants
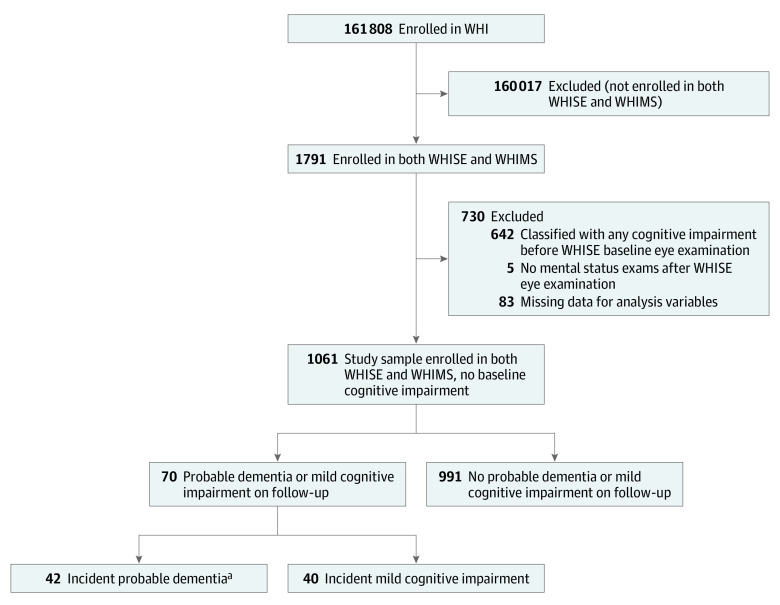
We evaluated participants enrolled in the Women’s Health Initiative Hormone Therapy Clinical Trials who participated in both Women's Health Initiative Sight Examination (WHISE) and Women’s Health Initiative Memory Study (WHIMS) ancillary studies, excluding those classified with any form of cognitive impairment (dementia or mild cognitive impairment [MCI]) before their baseline WHISE eye examination (Figure). All participants provided written informed consent; institutional review board approval was obtained by participating institutions. This analysis was approved by the Stanford University Institutional Review Board. The WHI study design and details have been described previously24,25 and are summarized in the eAppendix in the Supplement.
Figure. Sample Selection Strategy.
WHI indicates Women’s Health Initiative; WHIMS, Women’s Health Initiative Memory Study; WHISE, Women’s Health Initiative Sight Exam.
aIncludes 12 women classified with both mild cognitive impairment and subsequent probable dementia.
Women’s Health Initiative Memory Study
The WHIMS was a randomized placebo-controlled trial conducted to investigate the association between postmenopausal hormone therapy and risk for dementia and cognitive decline. The WHIMS initially enrolled 7427 participants from the WHI Hormone Therapy Clinical Trials between 1996 and 1998; 5835 of these participants continued to be followed up through 2007 in the WHIMS Extension. Women in WHIMS underwent annual screening with the Modified Mini-Mental State Examination26 (3MS) plus additional cognitive testing and clinical assessment for those scoring below a set threshold on the 3MS (eAppendix in the Supplement). Beginning in 2008, 2900 WHIMS participants were recruited for the WHI Epidemiology of Cognitive Health Outcomes study, which continued annual follow-up, transitioning to validated telephone-based cognitive assessment27 and proxy interviews of friends or family members who provided information on participants’ functional status. Data from WHIMS and WHIMS Epidemiology of Cognitive Health Outcomes were submitted for centralized adjudication to classify participants as having probable dementia, MCI, or no dementia (eAppendix in the Supplement).
Women's Health Initiative Sight Examination
The WHISE was designed to examine the effects of hormonal therapy on age-related maculopathy progression.28,29 Between April 2000 and June 2002, 4347 women recruited from the WHI Hormone Therapy Clinical Trials received a single eye examination, including visual acuity testing and fundus photography. Distance visual acuity was assessed with participants’ usual correction (glasses or contact lenses) using logMAR charts, evaluating left and right eyes separately. Participants unable to read at least 3 of 5 letters on the 20/40 line were tested for improvement with a pinhole occluder. Pinhole and nonpinhole visual acuity measurements were recorded separately. If unable to read any letters when positioned 4 m from the logMAR chart, participants were reassessed 2 m away, and if unable to read any letters at 2 m, they were reassessed at 1 m. Participants unable to read more than 2 letters at 1 m were assessed for ability to count fingers, see hand motion, light perception, or no light perception consistent with ophthalmic practice. At the initial study visit, WHISE staff also collected data on eye disease and self-reported visual functioning. Subsequent annual surveys were used to identify new eye disease diagnoses (eAppendix in the Supplement).
Risk Factors
Visual impairment was identified from WHISE visual acuity measurements (objective visual impairment) and questionnaires (self-reported visual impairment). We classified objective visual impairment at 3 threshold levels: nonpinhole visual acuity of 20/40 or worse, 20/80 or worse, and 20/100 or worse in at least 1 eye (because worse-seeing eye visual acuity is important for visual function).30,31,32,33,34,35,36 A 20/40 threshold is commonly used to evaluate visual acuity in clinical practice,8,35 and all states except Georgia, New Jersey, and Wyoming restrict driving privileges based on visual acuity of at least 20/40 in the better-seeing eye.37 Self-reported visual impairment was determined from composite visual function questionnaire responses (eTable 1 in the Supplement).
Ocular comorbidities were identified at the WHISE eye examination. Age-related macular degeneration, diabetic retinopathy, macular edema, retinal Hollenhorst plaque, and retinal vascular occlusion were identified for each participant via fundus photography interpretation by a trained examiner. Glaucoma was identified from self-report, large cup-disc ratio on fundus photography grading, and/or the presence of elevated intraocular pressure (>30 mm Hg). Cataract was identified from self-reported cataract or cataract surgery and/or the presence of aphakia, pseudophakia, or lens opacity on eye examination. We separately evaluated the presence of lens opacity alone. Other covariates included demographics (age at WHISE examination and race/ethnicity), educational level, physical activity, self-reported hearing loss, smoking, body mass index, and systemic comorbidities.
Outcome Variables
Our primary outcome was incident probable dementia centrally adjudicated after the WHISE eye examination. We additionally evaluated incident MCI, with or without progression to dementia, and a composite end point including incident cases of probable dementia or MCI (first event of either outcome).
Statistical Analysis
Participant characteristics were summarized using frequencies and percentages. We used separate Cox proportional hazards regression models to examine associations of objective and self-reported visual impairment, respectively, with risk of cognitive impairment. The proportional hazards assumption was met graphically. We defined the analysis index date as the date of a participant’s baseline WHISE examination with vision measurement. Participants who died or were lost to follow-up were right-censored from models at that time, and the follow-up period was treated as noninformative censored time for participants who did not develop dementia or MCI.
We constructed separate regression models to estimate hazard ratios (HRs) for (1) probable dementia, (2) MCI, and (3) probable dementia or MCI. Participants who developed incident MCI were considered still at risk for dementia, and thus were included in the population used to evaluate risk of probable dementia (model 1). For each respective outcome, vision was modeled as (1) self-reported visual impairment, (2) objective visual impairment 20/40 or worse, (3) objective visual impairment 20/80 or worse, and (4) objective visual impairment 20/100 or worse. All 12 regression models were adjusted for potential confounders, including age, race/ethnicity, educational level, physical activity, self-reported hearing loss, baseline 3MS score, smoking status, systemic comorbidities (depression, cardiovascular disease, congestive heart failure, hypertension, hyperlipidemia, chronic pulmonary disease, peptic ulcer disease, liver disease, leukemia/lymphoma, and diabetes), and the hormone therapy trial arm (estrogen supplementation or placebo). Effect modification from hormone therapy was evaluated via an interaction term between the hormone therapy trial arm and visual acuity. We also performed a stratified analysis for self-reported hearing loss and visual impairment.
We separately performed sensitivity analyses evaluating (1) objective visual impairment based on vision in the better-seeing eye, (2) objective visual impairment severity ranges instead of thresholds, using mutually exclusive categories of objective visual impairment (from 20/40 to better than 20/80, 20/80 to better than 20/100, and 20/100 or worse), and (3) subjective self-reported visual impairment based on questionnaires.
Associations between specific eye diseases (age-related macular degeneration, glaucoma, cataract or lens opacity, and diabetic retinopathy) and risk of dementia were examined using separate Cox proportional hazards regression models for each eye disease, adjusting for the same potential confounders plus presence of visual acuity of 20/40 or worse at the WHISE eye examination. We used a 2-sided P value of <.05 without adjustment for multiple comparisons to determine statistical significance. Statistical analyses were conducted using SAS, version 9.4 (SAS Institute Inc).
Results
Study Sample and Baseline Characteristics
Study inclusion criteria were met by 1061 participants (Figure), of whom 206 women (19.4%) self-reported visual impairment and 183 women (17.2%) had objective visual impairment 20/40 or worse. Mean (SD) follow-up post WHISE enrollment was 3.8 (1.8) years (range, 0-7 years). Mean (SD) age at WHISE examination was 73.8 (3.7) years (range, 66-84 years). Among 249 participants referred for additional testing, 42 women (16.9%) were ultimately classified as having probable dementia and 28 women (11.2%) were classified as having MCI that did not progress to dementia before censoring (Table 1).
Table 1. Participant Characteristics.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Objective vision measurement | Subjective visual impairment | |||||
| Better than 20/40 (n = 878) | 20/40 or worse (n = 183)a,b | 20/80 or worse (n = 31)a,c | 20/100 or worse (n = 21)a,d | No (n = 855) | Yes (n = 206) | |
| Age, mean (SD), y | 73.6 (3.7) | 74.6 (4.0)e | 75.7 (4.3)f | 74.91 (4.2) | 73.7 (3.7)g | 74.25 (4.0)g |
| ≤70 | 202 (23.0) | 29 (15.8)e | 5 (16.1)f | 3 (14.3) | 186 (21.8) | 45 (21.8) |
| >70 to ≤75 | 432 (49.2) | 76 (41.5)e | 11 (35.5)f | 10 (47.6) | 421 (49.2) | 87 (42.2) |
| >75 to ≤80 | 198 (22.6) | 62 (33.9)e | 11 (35.5)f | 5 (23.8) | 202 (23.6) | 58 (28.2) |
| >80 | 46 (5.2) | 16 (8.7)e | 4 (12.9)f | 3 (14.3) | 46 (5.4) | 16 (7.8) |
| Race/ethnicity | ||||||
| White | 791 (90.1) | 168 (91.8) | 30 (96.8) | 20 (95.2) | 785 (91.8) | 174 (84.5)e |
| Black | 52 (5.9) | 12 (6.6) | 0 | 0 | 43 (5.0) | 21 (10.2)e |
| Hispanic | 14 (1.6) | 1 (0.5) | 1 (3.2) | 1 (4.8) | 12 (1.4) | 3 (1.5)e |
| American Indian | 3 (0.3) | 0 | 0 | 0 | 2 (0.2) | 1 (0.5)e |
| Asian/Pacific Islander | 9 (1.0) | 0 | 0 | 0 | 4 (0.5) | 5 (2.4)e |
| Other | 9 (1.0) | 2 (1.1) | 0 | 0 | 9 (1.1) | 2 (1.0)e |
| Self-reported education al level | ||||||
| Less than high school diploma/GED | 47 (5.4) | 10 (5.5) | 0 | 0 | 32 (3.7)e | 25 (12.1)e |
| Some school after high school diploma | 207 (23.6) | 50 (27.3) | 7 (22.6) | 5 (23.8) | 210 (24.6)e | 47 (22.8)e |
| College degree or higher | 624 (71.1) | 123 (67.2) | 24 (77.4) | 16 (76.2) | 613 (71.7)e | 134 (65.0)e |
| Self-reported physical activityh | ||||||
| No activity | 188 (21.4) | 42 (23.0) | 8 (25.8) | 6 (28.6) | 182 (21.3) | 48 (23.3) |
| Some activity | 401 (45.7) | 88 (48.1) | 16 (51.6) | 12 (57.1) | 397 (46.4) | 92 (44.7) |
| Episodes/wk of moderate activity | ||||||
| 2 to <4 | 133 (15.1) | 30 (16.4) | 6 (19.4) | 3 (14.3) | 132 (15.4) | 31 (15.0) |
| ≥4 | 156 (17.8) | 23 (12.6) | 1 (3.2) | 0 | 144 (16.8) | 35 (17.0) |
| BMI | ||||||
| ≤25 | 261 (29.7) | 52 (28.4) | 11 (35.5) | 8 (38.1) | 262 (30.6) | 51 (24.8) |
| >25 to ≤30 | 286 (32.6) | 61 (33.3) | 10 (32.3) | 6 (28.6) | 281 (32.9) | 66 (32.0) |
| >30 to ≤35 | 204 (23.2) | 50 (27.3) | 7 (22.6) | 4 (19.0) | 194 (22.7) | 60 (29.1) |
| ≥35 | 127 (14.5) | 20 (10.9) | 3 (9.7) | 3 (14.3) | 118 (13.8) | 29 (14.1) |
| Self-reported hearing lossh | 126 (14.4) | 26 (14.2) | 6 (19.4) | 2 (9.5) | 115 (13.5) | 37 (18.0) |
| Smoking status (smoking years) | ||||||
| Never smoker | 482 (54.9) | 108 (59.0) | 20 (64.5) | 12 (57.1)g | 475 (55.6) | 115 (55.8) |
| Light (<10) | 96 (10.9) | 17 (9.3) | 4 (12.9) | 3 (14.3)g | 93 (10.9) | 20 (9.7) |
| Moderate (10-40) | 203 (23.1) | 35 (19.1) | 2 (6.5) | 1 (4.8)g | 191 (22.3) | 47 (22.8) |
| Heavy (>40, and current) | 97 (11.0) | 23 (12.6) | 5 (16.1) | 5 (23.8)g | 96 (11.2) | 24 (11.7) |
| Baseline systemic comorbiditiesh | ||||||
| Depression | 142 (16.2) | 26 (14.2) | 2 (6.5) | 2 (9.5) | 130 (15.2) | 38 (18.4) |
| Cardiovascular disease | 307 (35.0) | 64 (35.0) | 12 (38.7) | 7 (33.3) | 291 (34.0) | 80 (38.8) |
| Congestive heart failure | 28 (3.2) | 8 (4.4) | 2 (6.5) | 2 (9.5) | 27 (3.2) | 9 (4.4) |
| Hyperlipidemia | 157 (17.9) | 29 (15.8) | 5 (16.1) | 3 (14.3) | 146 (17.1) | 40 (19.4) |
| Hypertension | 339 (38.6) | 87 (47.5)f | 12 (38.7) | 4 (19.0)g | 330 (38.6) | 96 (46.6)f |
| Chronic pulmonary disease | 99 (11.3) | 24 (13.1) | 4 (12.9) | 2 (9.5) | 102 (11.9) | 21 (10.2) |
| Peptic ulcer disease | 80 (9.1) | 15 (8.2) | 1 (3.2) | 1 (4.8) | 66 (7.7) | 29 (14.1)e |
| Liver disease | 21 (2.4) | 4 (2.2) | 1 (3.2) | 0 | 22 (2.6) | 3 (1.5) |
| Diabetes | 87 (9.9) | 23 (12.6) | 6 (19.4) | 2 (9.5) | 77 (9.0) | 33 (16.0)e |
| Leukemia and/or lymphoma | 17 (1.9) | 4 (2.2) | 1 (3.2) | 0 | 20 (2.3) | 1 (0.5)g |
| Assignment to active hormone therapyi | 417 (47.5) | 93 (50.8) | 14 (45.2) | 9 (42.9) | 406 (47.5) | 104 (50.5) |
| Ocular comorbidities | ||||||
| Age-related macular degenerationj | 83 (9.5) | 35 (19.1)e | 8 (25.8)f | 6 (28.6)f | 91 (10.6) | 27 (13.1) |
| Glaucomak | 115 (13.1) | 18 (9.8) | 3 (9.7) | 3 (14.3) | 96 (11.2) | 37 (18.0)f |
| Cataractl | 610 (69.5) | 141 (77.0)f | 23 (74.2) | 16 (76.2) | 588 (68.8) | 163 (79.1)e |
| Lens opacity | 115 (13.1) | 41 (22.4)e | 6 (19.4) | 5 (23.8) | 123 (14.4) | 33 (16.0) |
| Diabetic retinopathyj | 81 (9.2) | 20 (10.9) | 3 (9.7) | 1 (4.8) | 70 (8.2) | 31 (15.0)e |
| Macular edemaj | 3 (0.3) | 0 | 0 | 0 | 1 (0.1) | 2 (1.0)g |
| Retinal Hollenhorst plaquej | 1 (0.1) | 0 | 0 | 0 | 1 (0.1) | 0 |
| Retinal vascular occlusionj | 2 (0.2) | 2 (1.1) | 2 (6.5)e | 1 (4.8)g | 4 (0.5) | 0 |
| Adjudicated dementia classificationm | ||||||
| No dementia | 829 (94.4) | 162 (88.5)e | 23 (74.2)e | 16 (76.2)e | 803 (93.9) | 188 (91.3) |
| Mild cognitive impairment | 28 (3.2) | 12 (6.6)g | 5 (16.1)e | 3 (14.3)f | 28 (3.3) | 12 (5.8) |
| Dementia | 27 (3.1) | 15 (8.2)e | 6 (19.4)e | 4 (19.0)e | 32 (3.7) | 10 (4.9) |
| 3MSE Score at baseline, mean (SD) | 96.9 (3.3) | 96.4 (3.1)g | 96.7 (2.7) | 97.1 (2.8) | 97.1 (2.8) | 95.4 (4.5)e |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared; 3MS, Modified Mini-Mental State Examination; WHISE, Women’s Health Initiative Sight Examination.
Classified as objective visual impairment.
Calculated by Fisher exact test comparison with better than 20/40.
Calculated by Fisher exact test comparison with better than 20/80.
Calculated by Fisher exact test comparison with better than 20/100.
P < .01.
P < .05.
P < .10.
eTable 6 in the Supplement.
Assignment to a hormone therapy trial arm in which the participant received estrogen supplementation vs placebo.
Determined based on fundus photo interpretation.
Presence of large cup-to-disc ratio on fundus photographs (trained reviewer assessment at preliminary photo grading), and/or elevated intraocular pressure (>30 mm Hg on examination), and/or self-reported glaucoma.
Presence of aphakia, intraocular lens, or lens opacity on examination, and/or self-reported cataract or cataract operation.
Based on each participant’s final reported dementia classification.
Mean baseline 3MS scores on t tests did not differ by visual impairment classification. However, in unadjusted analysis, more participants with objective visual impairment developed dementia during the study period. Six women (19.4%) with visual impairment 20/80 or worse and 15 women (8.2%) with visual acuity 20/40 or worse developed incident dementia, vs 27 women (3.1%) without objective visual impairment and 10 women (4.9%) with self-reported visual impairment (Table 1).
Baseline Visual Impairment and Risk of Incident Dementia
After adjusting for potential confounders (Table 2), objectively measured visual impairment was associated with a 2- to more than 5-fold higher risk of subsequent dementia, with stronger HRs at higher visual impairment thresholds. The risk of dementia was greatest among participants with visual acuity of 20/100 or worse (HR, 5.66; 95% CI, 1.75-18.37), followed by 20/80 or worse (HR, 5.20; 95% CI, 1.94-13.95) and 20/40 or worse (HR, 2.14; 95% CI, 1.08-4.21). Objective visual impairment was also associated with increased risk for incident MCI and the composite end point of incident dementia or MCI, similarly demonstrating greater HRs at higher levels of visual impairment. Findings were similar for risk of MCI, with the greatest risk among women with baseline visual acuity of 20/100 or worse (HR, 6.43; 95% CI, 1.66-24.85). Self-reported visual impairment was not associated with risk of dementia or MCI. Forty-one participants had both self-reported and objective visual impairment (≤20/40), of whom 4 women (9.8%) developed mild cognitive impairment and 6 women (14.6%) developed dementia.
Table 2. Adjusted Multivariable Cox Proportional Hazards Regression Models for Incidence of Dementia or Mild Cognitive Impairmenta.
| Characteristic | No. | HR (95% CI) | P value |
|---|---|---|---|
| Probable dementia | |||
| Objective visual impairment in either eye | |||
| 20/40 or worseb | 183 | 2.14 (1.08-4.21) | .03 |
| 20/80 or worsec | 31 | 5.20 (1.94-13.95) | .001 |
| 20/100 or worsed | 21 | 5.66 (1.75-18.37) | .004 |
| Subjective visual impairmente | 206 | 1.22 (0.56-2.66) | .61 |
| Mild cognitive impairment | |||
| Objective visual impairment in either eye | |||
| 20/40 or worseb | 183 | 1.84 (0.90-3.78) | .10 |
| 20/80 or worsec | 31 | 5.62 (1.94-16.33) | .002 |
| 20/100 or worsed | 21 | 6.43 (1.66-24.85) | .007 |
| Subjective visual impairmente | 206 | 1.30 (0.61-2.77) | .50 |
| Both mild cognitive impairment and dementia | |||
| Objective visual impairment in either eye | |||
| 20/40 or worseb | 183 | 1.74 (1.02-2.97) | .04 |
| 20/80 or worsec | 31 | 4.42 (1.95-10.03) | <.001 |
| 20/100 or worsed | 21 | 5.54 (2.02-15.16) | <.001 |
| Subjective visual impairmente | 206 | 1.27 (0.71-2.27) | .42 |
Abbreviations: HR, hazard ratio; 3MS, Modified Mini-Mental State Examination; WHISE, Women’s Health Initiative Sight Exam.
Twelve separate regression models were evaluated in total, assessing 3 outcomes of interest (probable dementia, MCI, and either probable dementia or MCI) and evaluating visual impairment as a risk factor defined in 4 different ways: objective visual impairment of 20/40 or worse, objective visual impairment of 20/80 or worse, objective visual impairment of 20/100 or worse, and presence of subjective visual impairment. Regression models adjusted for age, race/ethnicity, hormone therapy (based on trial arm), self-reported educational level, self-reported physical activity, self-reported hearing loss, smoking status, 3MS Score at WHISE baseline, and self-reported systemic comorbidities (depression, cardiovascular disease, congestive heart failure, hypertension, hyperlipidemia, chronic pulmonary disease, peptic ulcer disease, liver disease, leukemia or lymphoma, and diabetes).
Reference group: better than 20/40 visual acuity in either eye.
Reference group: better than 20/80 visual acuity in either eye.
Reference group: better than 20/100 visual acuity in either eye.
Reference group: no subjective visual impairment.
Variables other than age, including hormone therapy assignment, were not associated with dementia or MCI. However, visual impairment was associated with an even higher likelihood of dementia when combined with self-reported hearing loss (eTable 2 in the Supplement).
Although results from sensitivity analyses using visual impairment severity ranges instead of thresholds demonstrated greater risk for dementia or MCI with worse baseline visual impairment, results were not statistically significant (HR for probable dementia, 1.25; 95% CI, 0.58-2.71 among women with visual acuity of 20/40-20/80 and HR, 3.88; 95% CI, 0.74-20.28 among women with visual acuity of 20/80-20/100) (eTable 3 in the Supplement). Sensitivity analyses using visual impairment in the better-seeing eye were limited by insufficient sample size to evaluate an increasing association with visual impairment severity (eTable 4 and eTable 5 in the Supplement).
Eye Disease and Risk of Incident Dementia
We separately analyzed dementia likelihood among participants with evidence for age-related macular degeneration, glaucoma, cataract, or diabetic retinopathy—each a common eye disease causing visual impairment. Although some conditions were associated with increased hazard for incident dementia in unadjusted models, results were not statistically significant (HR 1.01-1.87; P > .05). None of these conditions was associated with dementia after adjusting for potential confounders; however, baseline visual impairment remained associated with increased risk for dementia in each model tested.
Discussion
Using data from 2 ancillary studies to WHI clinical trials, we evaluated 1061 non–cognitively impaired postmenopausal women who underwent comprehensive eye examinations and were subsequently followed up with annual cognitive assessments. We found that participants with objective visual impairment were more likely to develop incident dementia, even after adjusting for potential confounders, including demographics, systemic comorbidities, hearing impairment, educational level, physical activity, smoking, and hormone therapy, as well as baseline 3MS performance. This association was greater with more severe baseline visual impairment; risk of incident dementia increased 2-, 5-, and nearly 6-fold among participants who had 20/40 or worse, 20/80 or worse, and 20/100 or worse baseline vision, respectively. The association of visual and cognitive impairment was similar for MCI. These findings have important public health implications, given the need for early identification and mitigation of dementia risk factors.
Although many previous studies have reported associations between visual impairment and impaired cognition,3,4,7,11,12,14,17,18 evidence has been mixed.5,6,38 Most studies have been cross-sectional,3,4,5,11,12,14,17,18 and the few with 2 or more years’ follow-up lack sufficient repeat measures for longitudinal analyses.10,38,39 In addition, most analyses have used less detailed cognitive assessment protocols, considered visual impairment as a binary variable, and either did not evaluate visual impairment severity or had insufficient sample size to identify a statistically significant dose-response association.17,18,39,40,41,42,43,44,45 Our analysis builds on the existing literature, with participants having up to 7 years of follow-up, adjudicated cognitive assessment and classification protocol including both MCI and dementia, stratification of baseline objective visual impairment, and adjustment for specific conditions associated with visual or cognitive impairment.
Mechanisms underlying associations between visual and cognitive impairment are difficult to isolate; however, several hypotheses have been proposed. Under the common cause hypothesis, both cognitive and visual impairment (or neurosensory impairment considered broadly) are manifestations of central aging-related neurodegeneration.46 Alternatively, the sensory deprivation hypothesis suggests that persistently reduced neurosensory stimulation interferes with cognitive efficiency and potentially leads to neural injury and subsequent cognitive deterioration.46 In addition, individuals with poor vision may bear greater cognitive load when performing visual tasks, leading to an overtaxed brain, social disengagement, and worsening cognitive impairment. The association between cognition and visual function is likely bidirectional and multifactorial, with contributions from each of these factors. In this study, we found that visual impairment may precede the onset of clinical cognitive impairment by several years, suggesting that reduced visual acuity may be an early manifestation of central nervous system degeneration and/or that visual impairment may contribute to cognitive decline through greater cognitive burden and reduced cognitive input. Vision assessment is low-cost and noninvasive; it would be a valuable tool if able to identify individuals at higher risk for cognitive impairment and offer interventions to improve visual acuity, such as cataract surgery, that may have additional benefit in older individuals at risk for dementia. Older adults who undergo cataract surgery have been suggested to have lower risk of new-onset dementia, and other studies have suggested improved cognitive scores after cataract surgery.47,48,49,50,51,52,53 These findings suggest the potential value of providing older adults with regular vision screenings and interventions.
Results from previous studies on associations between specific eye diseases and cognitive impairment are mixed.6,8,9,54,55,56,57 After adjusting for the presence of objective visual impairment and other potential confounders, cataract, glaucoma, age-related macular degeneration, and diabetic retinopathy were not associated with dementia risk in our study. This lack of association most likely reflects asymmetric eye disease severity (worse in 1 eye), lack of correlation with visual significance (eg, visually significant cataract vs any lens opacity), and lower numbers of participants with each condition in our cohort. Associations between these conditions and dementia incidence may be mediated by decreased visual acuity or require larger sample size.
In contrast to prior cross-sectional studies,3 we did not find an association between self-reported visual impairment and dementia. Most women with self-reported visual impairment did not have objective visual impairment. However, studies may assess self-reported visual impairment differently and, being subjective, findings are inherently more prone to variation. In this analysis, we considered self-reported visual impairment to indicate at least moderate difficulty with visual tasks, such as reading street signs and driving. In addition, we evaluated self-reported visual impairment at only 1 time point—WHISE baseline eye examination. Objective visual impairment could have preceded self-reported visual impairment, and participants could still develop visual impairment after WHISE baseline but prior to developing dementia. It remains possible that self-reported visual impairment may be an early sign of dementia in a real-world setting or, alternatively, that self-reported visual impairment does not reliably reflect impending visual deterioration or cognitive decline.
Limitations
Our study has several limitations. Within the WHI study population, incident dementia and MCI cases were few, leading to wide 95% CIs. Although vision was formally measured by trained personnel in a clinical trial setting, assessment included only visual acuity at baseline. We were thus unable to evaluate vision as a time-varying covariate or include other visual function components, such as contrast sensitivity or depth perception. Also, the WHI Hormone Therapy Clinical Trials enrolled only women who fit specific eligibility criteria, so it is unclear whether our findings can be generalized to other populations, including other women or men with visual impairment.
Individuals with visual impairment may perform poorly on cognitive tests, especially tests with visual components. Although telephone-based cognitive assessment does not require good visual acuity, 3MS includes items that participants must read and follow instructions, write a short sentence, and copy 2 pentagons. However, survey scoring methods account for participants who are unable to perform these items owing to blindness or physical inability, and past studies have shown that the associations of visual impairment with falsely low true 3MS scores are negligible.58 Also, WHIMS participants who scored poorly on 3MS underwent additional cognitive testing, clinical assessment and, ultimately, adjudication for MCI or probable dementia (dementia type not specified). Reverse causation is also possible (ie, individuals with existing or incipient cognitive impairment may manifest visual impairment). However, our findings persisted even after adjusting for baseline cognitive performance. In addition, our results may underestimate the association between visual impairment and subsequent dementia. To achieve sufficient statistical power, we based our analyses on visual impairment in at least 1 eye, such that participants’ functional binocular visual acuity may have been better than the level used for analysis. The fact that we still found an association between visual impairment in the worse eye and subsequent dementia suggests that cognitive demand from even 1 eye with reduced vision may be sufficient to increase dementia risk. Furthermore, despite limitations, to our knowledge, this study is the first prospective analysis based on standardized, objective, visual acuity measurement and adjudicated cognitive assessment.
Conclusions
Dementia has been considered to be one of the greatest global health challenges of the 21st century.59 Prevention, early detection, and management are key priorities as population aging leads to rapid growth in dementia prevalence. In particular, identifying potentially modifiable risk factors is essential to ensure that patients have access to interventions and support when they are most able to benefit.
Based on this longitudinal analysis, objective visual impairment may be a potentially modifiable dementia risk factor. Postmenopausal women with objective visual impairment may be more than 5 times as likely to develop incident dementia, with a progressively greater likelihood among those with worse baseline vision, even after adjusting for other potential confounding factors. Regardless of mechanism—common cause, sensory deprivation, or a combination of factors—visual impairment could represent an early harbinger of risk for dementia. These findings suggest potential value for vision screening and vision-improving interventions. Further research is warranted to identify those at higher risk of developing cognitive impairment, investigate sex-related differences in eye disease and cognitive impairment, and evaluate the effect of ophthalmic interventions on dementia incidence and/or cognitive trajectories among patients with dementia.
eTable 1. Subjective Visual Impairment Classification Based on Visual Function Questionnaire Responses
eTable 2. Interaction for Hearing Loss and Visual Impairment
eTable 3. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Severity Ranges
eTable 4. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Thresholds in the Better-Seeing Eye
eTable 5. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Severity Ranges in the Better-Seeing Eye
eTable 6. Determination of Baseline Systemic Comorbidity Variables
eAppendix. Women’s Health Initiative, Women's Health Initiative Sight Exam Study, and Women’s Health Initiative Memory Study
References
- 1.Alzheimer’s Association 2020. Alzheimer’s disease facts and figures. Accessed March 11, 2020. https://www.alz.org/media/Documents/alzheimers-facts-and-figures_1.pdf
- 2.Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55(6):885-891. doi: 10.1111/j.1532-5415.2007.01093.x [DOI] [PubMed] [Google Scholar]
- 3.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135(9):963-970. doi: 10.1001/jamaophthalmol.2017.2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong SY, Cheung CY, Li X, et al. . Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study. Arch Ophthalmol. 2012;130(7):895-900. doi: 10.1001/archophthalmol.2012.152 [DOI] [PubMed] [Google Scholar]
- 5.Clemons TE, Rankin MW, McBee WL; Age-Related Eye Disease Study Research Group . Cognitive impairment in the age-related eye disease study: AREDS report no. 16. Arch Ophthalmol. 2006;124(4):537-543. doi: 10.1001/archopht.124.4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker ML, Wang JJ, Rogers S, et al. . Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol. 2009;127(5):667-673. doi: 10.1001/archophthalmol.2009.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen M, Edgar DF, Hancock B, et al. . The Prevalence of Visual Impairment in People with Dementia (the PrOVIDe study): a cross-sectional study of people aged 60–89 years with dementia and qualitative exploration of individual, career and professional perspectives. Health Services and Delivery Research; 2016. [PubMed] [Google Scholar]
- 8.Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52(6):353-358. doi: 10.1159/000094984 [DOI] [PubMed] [Google Scholar]
- 9.Woo SJ, Park KH, Ahn J, et al. . Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119(10):2094-2101. doi: 10.1016/j.ophtha.2012.04.026 [DOI] [PubMed] [Google Scholar]
- 10.Rait G, Fletcher A, Smeeth L, et al. . Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing. 2005;34(3):242-248. doi: 10.1093/ageing/afi039 [DOI] [PubMed] [Google Scholar]
- 11.Garin N, Olaya B, Lara E, et al. . Visual impairment and multimorbidity in a representative sample of the Spanish population. BMC Public Health. 2014;14:815. doi: 10.1186/1471-2458-14-815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Court H, McLean G, Guthrie B, Mercer SW, Smith DJ. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. doi: 10.1186/s12916-014-0181-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mangione CM, Seddon JM, Cook EF, et al. . Correlates of cognitive function scores in elderly outpatients. J Am Geriatr Soc. 1993;41(5):491-497. doi: 10.1111/j.1532-5415.1993.tb01883.x [DOI] [PubMed] [Google Scholar]
- 14.Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996;51(6):317-330. doi: 10.1093/geronb/51B.6.P317 [DOI] [PubMed] [Google Scholar]
- 15.Dupuis K, Pichora-Fuller MK, Chasteen AL, Marchuk V, Singh G, Smith SL. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22(4):413-437. doi: 10.1080/13825585.2014.968084 [DOI] [PubMed] [Google Scholar]
- 16.Lin MY, Gutierrez PR, Stone KL, et al. ; Study of Osteoporotic Fractures Research Group . Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52(12):1996-2002. doi: 10.1111/j.1532-5415.2004.52554.x [DOI] [PubMed] [Google Scholar]
- 17.Yamada Y, Denkinger MD, Onder G, et al. . Dual sensory impairment and cognitive decline: the results from the Shelter Study. J Gerontol A Biol Sci Med Sci. 2016;71(1):117-123. doi: 10.1093/gerona/glv036 [DOI] [PubMed] [Google Scholar]
- 18.Ward ME, Gelfand JM, Lui LY, et al. . Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann Neurol. 2018;83(4):730-738. doi: 10.1002/ana.25196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute on Aging; American Geriatrics Society Sensory impairment and cognitive decline. Geriatric Healthcare Professionals. Published 2017. Accessed April 11, 2019. https://www.americangeriatrics.org/programs/u13-conference-series/sensory-impairment-and-cognitive-decline
- 20.Whitson HE, Cronin-Golomb A, Cruickshanks KJ, et al. . American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: sensory impairment and cognitive decline in older adults. J Am Geriatr Soc. 2018;66(11):2052-2058. doi: 10.1111/jgs.15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haan M, Espeland MA, Klein BE, et al. ; Women’s Health Initiative Memory Study and the Women’s Health Initiative Sight Exam . Cognitive function and retinal and ischemic brain changes: the Women’s Health Initiative. Neurology. 2012;78(13):942-949. doi: 10.1212/WNL.0b013e31824d9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group . Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-485. doi: 10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- 23.Evans JR, Fletcher AE, Wormald RPL, et al. . Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2002;86(7):795-800. doi: 10.1136/bjo.86.7.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson G, Cummings S, Freedman LS, et al. ; The Women’s Health Initiative Study Group . Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61-109. doi: 10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- 25.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9)(suppl):S78-S86. doi: 10.1016/S1047-2797(03)00045-0 [DOI] [PubMed] [Google Scholar]
- 26.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 27.Rapp SR, Legault C, Espeland MA, et al. ; CAT Study Group . Validation of a cognitive assessment battery administered over the telephone. J Am Geriatr Soc. 2012;60(9):1616-1623. doi: 10.1111/j.1532-5415.2012.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haan MN, Klein R, Klein BE, et al. . Hormone therapy and age-related macular degeneration: the Women’s Health Initiative Sight Exam study. Arch Ophthalmol. 2006;124(7):988-992. doi: 10.1001/archopht.124.7.988 [DOI] [PubMed] [Google Scholar]
- 29.Klein R, Deng Y, Klein BEK, et al. . Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women’s Health Initiative Sight Exam ancillary study. Am J Ophthalmol. 2007;143(3):473-483. doi: 10.1016/j.ajo.2006.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirneiss C. The impact of a better-seeing eye and a worse-seeing eye on vision-related quality of life. Clin Ophthalmol. 2014;8:1703-1709. doi: 10.2147/OPTH.S64200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finger RP, Fenwick E, Hirneiss CW, et al. . Visual impairment as a function of visual acuity in both eyes and its impact on patient reported preferences. PLoS One. 2013;8(12):e81042. doi: 10.1371/journal.pone.0081042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamoureux EL, Chong E, Wang JJ, et al. . Visual impairment, causes of vision loss, and falls: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2008;49(2):528-533. doi: 10.1167/iovs.07-1036 [DOI] [PubMed] [Google Scholar]
- 33.Broman AT, Munoz B, Rodriguez J, et al. . The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: Proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(11):3393-3398. [PubMed] [Google Scholar]
- 34.Gray CS, Karimova G, Hildreth AJ, Crabtree L, Allen D, O’Connell JE. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. J Cataract Refract Surg. 2006;32(1):60-66. doi: 10.1016/j.jcrs.2005.07.040 [DOI] [PubMed] [Google Scholar]
- 35.West SK, Munoz B, Rubin GS, et al. . Function and visual impairment in a population-based study of older adults: the SEE project: Salisbury Eye Evaluation. Invest Ophthalmol Vis Sci. 1997;38(1):72-82. [PubMed] [Google Scholar]
- 36.Shekhawat NS, Stock MV, Baze EF, et al. . Impact of first-eye versus second-eye cataract surgery on visual function and quality of life. Ophthalmology. 2017;124(10):1496-1503. doi: 10.1016/j.ophtha.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 37.Steinkuller PG. Legal vision requirements for drivers in the United States. Virtual Mentor. 2010;12(12):938-940. doi: 10.1001/virtualmentor.2010.12.12.hlaw1-1012 [DOI] [PubMed] [Google Scholar]
- 38.Hong T, Mitchell P, Burlutsky G, Liew G, Wang JJ. Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the Blue Mountains Eye Study. PLoS One. 2016;11(1):e0147646. doi: 10.1371/journal.pone.0147646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elyashiv SM, Shabtai EL, Belkin M. Correlation between visual acuity and cognitive functions. Br J Ophthalmol. 2014;98(1):129-132. doi: 10.1136/bjophthalmol-2013-304149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes-Ortiz CA, Kuo YF, DiNuzzo AR, Ray LA, Raji MA, Markides KS. Near vision impairment predicts cognitive decline: data from the Hispanic Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc. 2005;53(4):681-686. doi: 10.1111/j.1532-5415.2005.53219.x [DOI] [PubMed] [Google Scholar]
- 41.Uhlmann RF, Larson EB, Koepsell TD, Rees TS, Duckert LG. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J Gen Intern Med. 1991;6(2):126-132. doi: 10.1007/BF02598307 [DOI] [PubMed] [Google Scholar]
- 42.Fischer ME, Cruickshanks KJ, Schubert CR, et al. . Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. 2016;64(10):1981-1987. doi: 10.1111/jgs.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N; Sense-Cog WP1 group . Visual and hearing impairments are associated with cognitive decline in older people. Age Ageing. 2018;47(4):575-581. doi: 10.1093/ageing/afy061 [DOI] [PubMed] [Google Scholar]
- 44.Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci. 2019;74(6):890-896. doi: 10.1093/gerona/gly264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naël V, Pérès K, Dartigues JF, et al. ; Sense-Cog consortium . Vision loss and 12-year risk of dementia in older adults: the 3C cohort study. Eur J Epidemiol. 2019;34(2):141-152. doi: 10.1007/s10654-018-00478-y [DOI] [PubMed] [Google Scholar]
- 46.Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12-21. doi: 10.1037/0882-7974.12.1.12 [DOI] [PubMed] [Google Scholar]
- 47.Lerner A, Debanne S, Belkin J, et al. . Visual and cognitive improvement following cataract surgery in subjects with dementia. Alzheimers Dement. 2014;10(4)(suppl):456-457. doi: 10.1016/j.jalz.2014.05.63024035058 [DOI] [Google Scholar]
- 48.Duffy M. Alzheimer research: cataract surgery for people with dementia improves vision and quality of life. VisionAware. Published 2014. Accessed September 25, 2018. https://www.visionaware.org/blog/visionaware-blog/alzheimer-research-cataract-surgery-for-people-with-dementia-improves-vision-and-quality-of-life/12
- 49.Tamura H, Tsukamoto H, Mukai S, et al. . Improvement in cognitive impairment after cataract surgery in elderly patients. J Cataract Refract Surg. 2004;30(3):598-602. doi: 10.1016/j.jcrs.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 50.Jefferis JM, Clarke MP, Taylor JP. Effect of cataract surgery on cognition, mood, and visual hallucinations in older adults. J Cataract Refract Surg. 2015;41(6):1241-1247. doi: 10.1016/j.jcrs.2014.09.044 [DOI] [PubMed] [Google Scholar]
- 51.Hall TA, McGwin G Jr, Owsley C. Effect of cataract surgery on cognitive function in older adults. J Am Geriatr Soc. 2005;53(12):2140-2144. doi: 10.1111/j.1532-5415.2005.00499.x [DOI] [PubMed] [Google Scholar]
- 52.ClinicalTrials.gov. Cataract Removal in Alzheimer’s Disease. NCT00921297. Published 2009. Accessed April 11, 2019. https://clinicaltrials.gov/ct2/show/study/NCT00921297.
- 53.Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N; SENSE-Cog WP1 group . Cataract surgery and age-related cognitive decline: a 13-year follow-up of the English Longitudinal Study of Ageing. PLoS One. 2018;13(10):e0204833. doi: 10.1371/journal.pone.0204833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding J, Strachan MWJ, Reynolds RM, et al. ; Edinburgh Type 2 Diabetes Study Investigators . Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59(11):2883-2889. doi: 10.2337/db10-0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamura H, Kawakami H, Kanamoto T, et al. . High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J Neurol Sci. 2006;246(1-2):79-83. doi: 10.1016/j.jns.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 56.Kessing LV, Lopez AG, Andersen PK, Kessing SV. No increased risk of developing Alzheimer disease in patients with glaucoma. J Glaucoma. 2007;16(1):47-51. doi: 10.1097/IJG.0b013e31802b3527 [DOI] [PubMed] [Google Scholar]
- 57.McGwin G, Hall TA, Searcey K, Modjarrad K, Owsley C. Cataract and cognitive function in older adults. [2]. J Am Geriatr Soc. 2005;53(7):1260-1261. doi: 10.1111/j.1532-5415.2005.53384_2.x [DOI] [PubMed] [Google Scholar]
- 58.Jefferis JM, Collerton J, Taylor JP, et al. . The impact of visual impairment on Mini-Mental State Examination Scores in the Newcastle 85+ study. Age Ageing. 2012;41(4):565-568. doi: 10.1093/ageing/afs042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673-2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Subjective Visual Impairment Classification Based on Visual Function Questionnaire Responses
eTable 2. Interaction for Hearing Loss and Visual Impairment
eTable 3. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Severity Ranges
eTable 4. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Thresholds in the Better-Seeing Eye
eTable 5. Multivariable Cox Regression Models for Incidence of Dementia or Mild Cognitive Impairment, Based on Visual Impairment Severity Ranges in the Better-Seeing Eye
eTable 6. Determination of Baseline Systemic Comorbidity Variables
eAppendix. Women’s Health Initiative, Women's Health Initiative Sight Exam Study, and Women’s Health Initiative Memory Study