Abstract
Background
Capecitabine was previously used as a second‐line or salvage therapy for metastatic nasopharyngeal carcinoma (NPC) and has shown satisfactory curative effect as maintenance therapy in other metastatic cancers. This study aimed to explore the role of capecitabine as maintenance therapy in de novo metastatic NPC patients with different plasma Epstein‐Barr virus (EBV) DNA levels before treatment.
Methods
We selected de novo metastatic NPC patients treated with locoregional radiotherapy (LRRT) for this retrospective study. The propensity score matching (PSM) was applied to balance potential confounders between patients who underwent capecitabine maintenance therapy and those who did not with a ratio of 1:3. Overall survival (OS) was the primary endpoint. The association between capecitabine maintenance therapy and survival was assessed using the log‐rank test and a Cox proportional hazard model.
Results
Among all patients eligible for this study, 64 received capecitabine maintenance therapy after LRRT. After PSM, 192 patients were identified in the non‐maintenance group. In the matched cohort, patients treated with capecitabine achieved a higher 3‐year OS rate compared with patients in the non‐maintenance group (68.5% vs. 61.8%, P = 0.037). Multivariate analysis demonstrated that capecitabine maintenance therapy was an independent prognostic factor. In subgroup analysis, 3‐year OS rate was comparable between the maintenance and non‐maintenance groups in patients with high pretreatment EBV DNA levels (˃30,000 copies/mL) (54.8% vs. 45.8%, P = 0.835), whereas patients with low pretreatment EBV DNA levels (≤30,000 copies/mL) could benefit from capecitabine maintenance therapy in OS (90.0% vs. 68.1%, P = 0.003).
Conclusion
Capecitabine maintenance therapy may be superior to non‐maintenance therapy in prolonging OS for de novo metastatic NPC patients with pretreatment EBV DNA ≤ 30,000 copies/mL.
Keywords: capecitabine, de novo, Epstein‐Barr virus, locoregional, maintenance therapy, nasopharyngeal carcinoma, propensity score matching, radiotherapy, survival
Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
area under curve
- CCRT
concurrent chemoradiotherapy
- CI
confidence interval
- CT
computed tomography
- EBV
Epstein–Barr virus
- HR
hazard ratio
- IMRT
intensity‐modulated radiotherapy
- KPS
Karnofsky performance score
- LRRT
locoregional radiotherapy
- MRI
magnetic resonance imaging
- NPC
nasopharyngeal carcinoma
- OS
overall survival
- PCT
palliative chemotherapy
- PET‐CT
Positron emission tomography/computed tomography
- PSM
propensity score matching
- Qpcr
quantitative polymerase chain reaction
- ROC
receiver operating characteristic
1. BACKGROUND
Nasopharyngeal carcinoma (NPC) is an endemic malignancy in Southeastern Asia, especially Guangdong province in South China. Approximately 86,700 incident cases of NPC and 50,800 deaths have been reported in 2012 [1] Radiotherapy is the primary treatment of NPC because of its radiosensitive feature and deep anatomic location [2, 3]. For locally advanced NPC, concurrent chemoradiotherapy (CCRT) has been established as the standard treatment protocol according to findings from clinical trials [4, 5, 6].
With the development of radiotherapy, the survival rates of NPC patients have increased due to better locoregional control [7, 8]. Besides, induction therapy with gemcitabine and cisplatin was also verified to be more effective than standard CCRT [9]. Unfortunately, approximately 6%‐15% of NPC patients develop metastatic lesions at the time of initial diagnosis without any prior treatment, and the common metastatic sites tend to be the bones, lungs, and liver [3, 10, 11]. Once distant lesions have been detected, platinum‐based combination therapy is considered the standard treatment and has achieved satisfactory response rates [12, 13, 14, 15]. Furthermore, Chen et al. [16] have evaluated the impact of different treatment strategies on patients’ survival and confirmed the treatment value of locoregional radiotherapy (LRRT) following palliative chemotherapy (PCT) in patients with distant lesions at initial diagnosis.
Capecitabine is an oral fluoropyrimidine which has previously been used as a second‐line or salvage therapy in patients with metastatic NPC [17, 18]. According to a phase II trial, capecitabine plus cisplatin was an active first‐line combination in metastatic NPC and only required a short hospital stay with a response rate of 54% [19]. In other malignancies such as metastatic colorectal cancer, capecitabine maintenance therapy has been considered an appropriate option following induction chemotherapy based on the results of a randomized clinical trial [20]. However, research on de‐novo metastatic NPC patients receiving PCT and LRRT with or without capecitabine maintenance therapy has been poorly documented. The potential treatment efficacy of capecitabine prompted us to design this retrospective study and explore whether the application of capecitabine after LRRT could prolong the overall survival (OS) of these patients.
2. PATIENTS AND METHODS
2.1. Patient selection
Patients with newly diagnosed de novo metastatic NPCs treated at the Sun Yat‐sen University Cancer Center (SYSUCC) between July 1, 2006 and December 31, 2016 were selected. The eligibility criteria were as follows: (1) with histology‐confirmed NPC; (2) had distant metastasis at diagnosis; (3) age ≥ 18 years; (4) had complete treatment information; (5) received platinum‐based PCT; (6) received at least 3 cycles of capecitabine in the maintenance group; (7) received LRRT; (8) Karnofsky performance score (KPS) >70; (9) no previous malignancy; (10) normal renal (creatinine clearance ≥60 mL/min) and liver functions (alanine aminotransferase ≤ 2 times the upper limit of normal); (11) had pretreatment Epstein‐Barr virus (EBV) DNA serology results. The flow chart of patient inclusion is shown in Figure 1. All patients were restaged according to the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM staging system. Before treatment, each patient was assessed by routine inspection and received physical examination, fiber optic nasopharyngoscopy, magnetic resonance imaging (MRI) or computed tomography (CT) of the head and neck, chest radiography or CT, abdominal sonography or CT, bone scan, and hematologic examination. Positron emission tomography/computed tomography (PET/CT) was also applied selectively. This study was approved by the Research Ethics Committee of SYSUCC.
FIGURE 1.
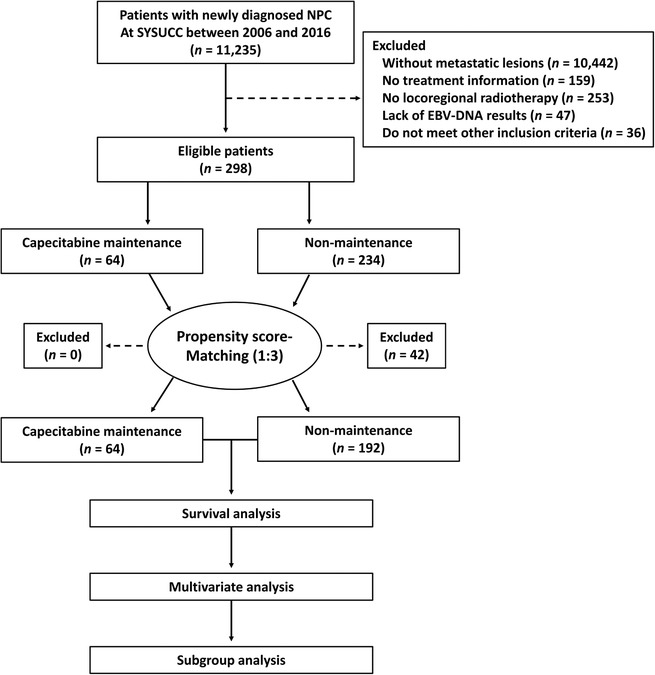
Flow chart of patient inclusion. NPC, nasopharyngeal carcinoma; EBV, Epstein‐Barr virus
2.2. Chemotherapy, radiotherapy, and capecitabine maintenance therapy
The common PCT regimens included TPF [intravenous administration of docetaxel (60 mg/m2) on day 1 and cisplatin (20‐25 mg/m2) on days 1‐3 plus 120‐hour continuous intravenous infusion of 5‐fluorouracil (0.5‐0.8 g/m2) on days 1‐5], PF [intravenous administration of cisplatin (20‐30 mg/m2) on days 1‐3 plus 120‐hour continuous intravenous infusion of 5‐fluorouracil (0.8‐1.0 g/m2) on days 1‐5], GP [intravenous administration of cisplatin (20‐30 mg/m2) on days 1‐3 and gemcitabine (0.8‐1.0 g/m2) on days 1 and 8], and TP [intravenous administration of docetaxel (75 mg/m2) on day 1 and cisplatin (20‐25 mg/m2) on days 1‐3]. All regimens were repeated every 3 weeks. All patients were treated with intensity‐modulated radiotherapy (IMRT) after PCT. The total dose of radiotherapy was 68‐70 Gy (1.8‐2.3 Gy/fraction, five daily fractions per week) for the primary tumor. IMRT was designed according to previous studies [7, 21]. Patients received oral administration of capecitabine (1.0 g/m2) twice daily on days 1‐14, every 3 weeks.
2.3. Quantification of plasma EBV DNA levels
EBV DNA has been demonstrated to be an important biomarker for NPC [22, 23, 24], and we used the level of EBV DNA to stratify risk in these patients. Pretreatment EBV DNA levels were measured with real‐time quantitative polymerase chain reaction (qPCR) as described in a previous study [25]. Receiver operating characteristic (ROC) curve analysis was used to establish the cut‐off value.
2.4. Outcome measurement and follow‐up
OS was the primary endpoint in this study, which was defined as the time from diagnosis to death of any cause or censored at the date of last follow‐up. Progression‐free survival (PFS) was the secondary endpoint, which was defined as the time from diagnosis to disease progression or death of any cause or censored at the date of last follow‐up. After treatment completion, patients were assessed every 3 months during the next 3 years and every 6 months thereafter until death. Fiber optic nasopharyngoscopy, MRI or CT of the head and neck, chest scan (radiography or CT), and abdominal scan (sonography or CT) were performed routinely or upon clinical indication of tumor progression. PET/CT was applied if clinically indicated. The last follow‐up date was January 15, 2019.
2.5. Statistical analysis
The propensity score matching (PSM) method was used to eliminate potential confounders that may influence treatment effects between patients treated with capecitabine and those who were not after PCT and IMRT. Propensity scores were calculated using logistic regression with a ratio of 1:3 to balance the covariates of gender, age, T stage, N stage, metastatic sites, and pretreatment EBV DNA levels. The Chi‐square test or Fisher's exact test was used to assess the differences between the two groups. We plotted the survival curves using the Kaplan‐Meier method and compared survival differences using the log‐rank test. Univariate and multivariate analyses using the Cox proportional hazard model was performed with the following variables: gender, age, T stage, N stage, metastatic site, pretreatment EBV DNA level, and maintenance therapy. All variables were included into the multivariate Cox model. Interaction analysis was performed between maintenance therapy and pretreatment EBV DNA level. The interaction analysis was conducted by means of a test of treatment‐by‐covariate interaction on the basis of the Cox proportional hazards model [26]. Adverse events (AEs) were evaluated according to Common Terminology Criteria for Adverse Events (version 4.0). Analyses were performed using the Statistical Package for Social Sciences, version 24.0 (IBM Corporation, Armonk, NY, USA) and R program (http://www.R-project.org). All statistical tests were two‐sided, and P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics and OS
Between July 1, 2006 and December 31, 2016, 298 de novo NPC patients were found eligible. The median age of all patients was 46 years (range, 18‐70 years); 248 (83.2%) were male, and 64 (21.5%) received capecitabine maintenance therapy. The median duration of PCT was 5 cycles (range, 2‐10 cycles) in the original cohort. Between PCT and IMRT, the median interval was 21 days (range, 10‐38 days). The median duration of capecitabine maintenance therapy was 6 cycles (range, 3‐18 cycles). The median interval between IMRT and capecitabine maintenance therapy was 30 days (range, 19‐45 days). After matching with the 64 patients in the maintenance group at a 1:3 ratio, 192 patients who did not receive capecitabine maintenance therapy were identified and classified into the non‐maintenance group. Table 1 includes the other salient characteristics. In terms of PCT cycles, concurrent chemotherapy, and radiotherapy, there were no significant differences between maintenance group and non‐maintenance group (Table S1). In the original cohort, the median follow‐up time was 33.1 months [interquartile range, 19.9‐53.4 months]. During follow‐up, 138 (46.3%) patients died. The 1‐, 3‐, and 5‐year OS rates were 91.4%, 63.3%, and 46.7%, respectively. The OS and PFS curves of the original cohort are shown in Figure S1.
TABLE 1.
Clinical characteristics of patients with de novo metastatic nasopharyngeal carcinoma in the capecitabine maintenance and non‐maintenance groups in the original cohort and the matched cohort
| The original cohort [cases (%)] | The matched cohort [cases (%)] | |||||
|---|---|---|---|---|---|---|
| Characteristic | Non‐maintenance | Maintenance | P value | Non‐maintenance | Maintenance | P value |
| Total | 234 | 64 | 192 | 64 | ||
| Gender | ||||||
| Male | 198 (84.6) | 50 (78.1) | 0.257 | 113 (81.3) | 50 (78.1) | 0.716 |
| Female | 36 (15.4) | 14 (21.9) | 79 (18.8) | 14 (21.9) | ||
| Age (years) | ||||||
| ≤46 | 114 (48.7) | 40 (62.5) | 0.066 | 113 (58.9) | 40 (62.4) | 0.660 |
| >46 | 120 (51.3) | 24 (37.5) | 79 (41.1) | 24 (37.5) | ||
| T stage# | ||||||
| T1 | 9 (3.8) | 2 (3.1) | 0.473 | 9 (4.7) | 2 (3.1) | 0.493 |
| T2 | 30 (12.8) | 13 (20.3) | 29 (15.1) | 13 (20.3) | ||
| T3 | 118 (50.3) | 28 (43.8) | 102 (53.1) | 28 (43.8) | ||
| T4 | 77 (32.9) | 21 (32.8) | 52 (27.1) | 21 (32.8) | ||
| N stage# | ||||||
| N0 | 9 (3.8) | 3 (4.7) | 0.333 | 8 (4.2) | 3 (4.7) | 0.730 |
| N1 | 43 (18.4) | 10 (15.6) | 39 (20.3) | 10 (15.6) | ||
| N2 | 89 (38.0) | 32 (50.0) | 82 (42.7) | 32 (50.0) | ||
| N3 | 93 (39.7) | 19 (29.7) | 63 (32.8) | 19 (29.7) | ||
| Metastatic sites | ||||||
| Bones | 122 (52.1) | 37 (57.8) | 0.657 | 105 (54.7) | 37 (57.8) | 0.574 |
| Lungs | 32 (13.7) | 5 (7.8) | 28 (14.6) | 5 (7.8) | ||
| Liver | 17 (7.3) | 6 (9.4) | 14 (7.3) | 6 (9.4) | ||
| Distant nodes | 22 (9.4) | 7 (10.9) | 14 (7.3) | 7 (9.7) | ||
| Multiple sites | 41 (17.5) | 9 (14.1) | 31 (16.1) | 9 (17.7) | ||
| Pretreatment EBV DNA (copies/mL) | ||||||
| ≤30,000 | 123 (52.6) | 31 (48.4) | 0.575 | 99 (51.6) | 31 (48.4) | 0.773 |
| ˃30,000 | 111 (47.4) | 33 (51.6) | 93 (48.4) | 33 (51.6) | ||
Abbreviations: EBV = Epstein‐Barr virus
According to the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC) TNM staging system
P values were calculated using the Pearson χ2 test
3.2. Cut‐off value of pretreatment EBV DNA levels
The median pretreatment EBV DNA level for the 298 patients was 24,500 (range, 0‐58,600,000) copies/mL. According to the ROC curve analysis, the cut‐off value was 29,350 copies/mL applied to discriminate OS curves of the two groups (sensitivity = 0.594, specificity = 0.604, area under curve [AUC] = 0.613) (Figure 2). To optimize the cut‐off value for its potential acceptance and clinical application, we rounded to the nearest integer of 30,000 copies/mL.
FIGURE 2.
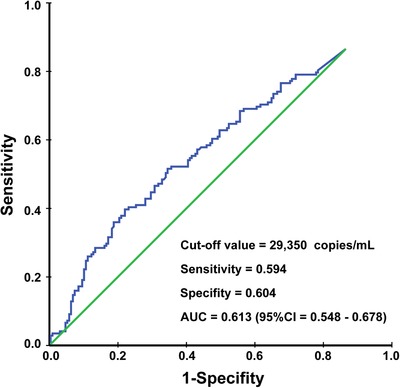
Receiver operating characteristic (ROC) curve analysis used to determine the cut‐off value of pretreatment EBV DNA levels to discriminate OS curves of the two groups. AUC, area under curve
3.3. Relationship between pre‐ and posttreatment EBV DNA levels
After treatment, a total of 262 patients had the data of posttreatment EBV DNA levels. In details, EBV DNA could not be detected (0 copy/mL) in 164 patients, whereas 98 patients had detectable EBV DNA. We further explored the relationship between pretreatment and posttreatment EBV DNA levels. As shown in Table S2, a high pretreatment EBV DNA level (>30,000 copies/mL) was significantly associated with a detectable EBV DNA level after treatment (>0 copies/mL) (P = 0.001).
3.4. Survival analysis based on the treatment strategies
We compared patients’ survival between the capecitabine maintenance and non‐maintenance groups in the matched cohort. In the univariate analysis, we found that the application of capecitabine maintenance therapy contributed to survival prolongation. The 3‐year OS rate was higher in the capecitabine maintenance group than in the non‐maintenance group (68.5% vs. 61.8%, P = 0.037) (Figure 3). Notably, there was no significant difference in OS between patients receiving different chemotherapy regimens, and there was no significant interaction effect between maintenance therapy and chemotherapy (all P > 0.05) (data not shown). We performed multivariate analyses in the matched cohort and found that the risk of death between the two groups was similar (hazard ratio [HR], 0.632; 95% confidence interval [CI], 0.399‐1.000; P = 0.050) (Table 2). More than one metastatic site was an independent risk factor of OS (HR, 2.736; 95% CI, 1.712‐4.372, P < 0.001). Additionally, patients with higher EBV DNA levels experienced worse survival outcome (HR, 1.451; 95% CI, 1.026‐2.051, P = 0.035).
FIGURE 3.
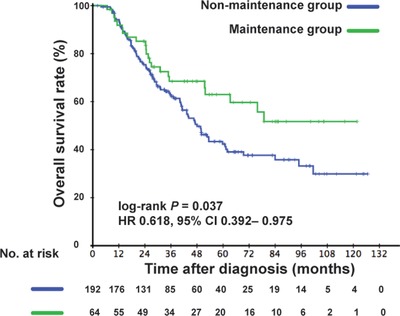
Kaplan‐Meier overall survival curves of patients with de novo metastatic nasopharyngeal carcinoma receiving capecitabine maintenance therapy or not in the matched cohort. HR, hazard ratio; CI, confidence interval
TABLE 2.
Univariate and multivariate analyses for prognostic factors of OS of patients with de novo metastatic nasopharyngeal carcinoma receiving capecitabine maintenance therapy or not in the matched cohort
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P value | HR | 95% CI | P value |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.679 | 0.415‐1.109 | 0.122 | 0.666 | 0.404‐1.098 | 0.111 |
| Age | ||||||
| ≤46 years | Reference | Reference | ||||
| >46 years | 0.982 | 0.683‐1.411 | 0.922 | 1.029 | 0.708‐1.495 | 0.881 |
| T stage | ||||||
| T1‐2 | Reference | Reference | ||||
| T3‐4 | 0.995 | 0.644‐1.536 | 0.980 | 0.797 | 0.507‐1.252 | 0.325 |
| N stage | ||||||
| N0‐1 | Reference | Reference | ||||
| N2‐3 | 1.213 | 0.789‐1.865 | 0.379 | 1.162 | 0.749‐1.803 | 0.503 |
| Metastatic site | ||||||
| Bones | Reference | Reference | ||||
| Lungs | 0.954 | 0.538‐1.659 | 0.843 | 1.023 | 0.577‐1.813 | 0.938 |
| Liver | 1.157 | 0.576‐2.326 | 0.682 | 1.189 | 0.588‐2.408 | 0.630 |
| Distant nodes | 0.494 | 0.179‐1.358 | 0.171 | 0.626 | 0.224‐1.750 | 0.372 |
| Multiple | 2.746 | 1.735‐4.347 | <0.001 | 2.736 | 1.712‐4.372 | <0.001 |
| Pretreatment EBV DNA level | ||||||
| ≤30,000 copies/mL | Reference | Reference | ||||
| ˃30,000 copies/mL | 1.851 | 1.259‐2.616 | 0.001 | 1.614 | 1.106‐2.353 | 0.013 |
| Treatment strategy | ||||||
| Non‐maintenance | Reference | Reference | ||||
| Maintenance | 0.618 | 0.392‐0.975 | 0.039 | 0.632 | 0.399‐1.000 | 0.050 |
Abbreviations: HR = hazard ratio; CI = confidence interval; EBV = Epstein‐Barr virus.
3.5. Subgroup analysis in the matched cohort
We further detected whether there was interaction effect between maintenance therapy and EBV DNA levels. After adjusting for gender, age, T stage, N stage, and metastatic site, we found that the curative effect of capecitabine was different in patients with different pretreatment EBV DNA levels (P = 0.035) (Table S3). Thus, we investigated the role of capecitabine maintenance therapy in patients with different EBV DNA levels. Among patients with EBV DNA ≤30,000 copies/mL, the 3‐year OS rate of the non‐maintenance group was significantly lower than that of the maintenance group (68.1% vs. 90.0%, P = 0.003). However, among patients with EBV DNA >30,000 copies/mL, the 3‐year OS rate was comparable in these two groups (54.8% vs. 45.8%, P = 0.835) (Figure 4). In multivariate analysis, maintenance therapy was identified as a protective factor for patients with low EBV DNA levels (HR, 0.277; 95% CI, 0.107–0.722; P = 0.009), but not for patients with high EBV DNA levels (HR, 0.896; 95% CI, 0.520–1.545; P = 0.693) (Table 3). We further analyzed the association of capecitabine maintenance therapy with survival in patients with different statuses of posttreatment EBV DNA (detectable and undetectable). Among the 262 patients who had the data of posttreatment EBV DNA levels, we found that only patients with undetectable post‐treatment EBV DNA could benefit from capecitabine maintenance therapy (P = 0.008), whereas patients with detectable posttreatment EBV DNA could not (P = 0.484) (Figure S2).
FIGURE 4.
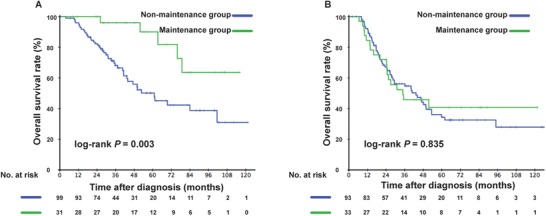
Kaplan‐Meier overall survival curves of de novo metastatic nasopharyngeal carcinoma patients with different pretreatment EBV DNA levels in the non‐maintenance and maintenance groups in the matched cohort. (A) Low‐risk subgroup with pretreatment EBV DNA ≤30,000 copies/mL. (B) High‐risk subgroup with pretreatment EBV DNA >30,000 copies/mL. P values were calculated using the log‐rank test. EBV, Epstein‐Barr virus
TABLE 3.
Cox proportional multivariate analysis for prognostic factors of OS of de novo metastatic nasopharyngeal carcinoma patients with different pretreatment EBV DNA levels in the matched cohort
| Low EBV DNA level | High EBV DNA level | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P value | HR | 95% CI | P value |
| Gender | ||||||
| Male | Reference | Reference | ||||
| Female | 0.517 | 0.229‐1.168 | 0.113 | 0.779 | 0.407‐1.492 | 0.452 |
| Age | ||||||
| ≤46 years | Reference | Reference | ||||
| >46 years | 1.128 | 0.604‐2.104 | 0.706 | 0.942 | 0.578‐1.535 | 0.810 |
| T stage | ||||||
| T1‐2 | Reference | Reference | ||||
| T3‐4 | 0.846 | 0.403‐1.776 | 0.658 | 0.731 | 0.399‐1.338 | 0.309 |
| N stage | ||||||
| N0‐1 | Reference | Reference | ||||
| N2‐3 | 1.863 | 0.904‐3.840 | 0.092 | 0.899 | 0.506‐1.597 | 0.717 |
| Metastatic site | ||||||
| Bones | Reference | Reference | ||||
| Lungs | 1.188 | 0.539‐2.620 | 0.669 | 0.876 | 0.369‐2.083 | 0.765 |
| Liver | 1.194 | 0.349‐4.086 | 0.777 | 1.265 | 0.527‐3.035 | 0.599 |
| Distant nodes | 0.667 | 0.153‐2.920 | 0.591 | 0.756 | 0.176‐3.251 | 0.707 |
| Multiple | 4.124 | 1.867‐9.109 | <0.001 | 2.180 | 1.188‐4.002 | 0.012 |
| Treatment strategy | ||||||
| Non‐maintenance | Reference | Reference | ||||
| Maintenance | 0.277 | 0.107‐0.722 | 0.009 | 0.896 | 0.520–1.545 | 0.693 |
Abbreviations: EBV = Epstein‐Barr virus; HR = hazard ratio; CI = confidence interval.
The cutoff of EBV DNA was 30,000 copies/mL.
3.6. Adverse events
The treatment‐related adverse events (AEs) of each group were also analyzed. A total of 37 treatment‐related grade 3‐4 AEs were reported in the maintenance group and 44 in the non‐maintenance group. As shown in Table S4, leukocytopenia, neutropenia, and hand‐foot syndrome were more common in the maintenance group (all P < 0.05). No toxicity‐associated deaths were observed.
4. DISCUSSION
Our results showed that the application of capecitabine after PCT and LRRT significantly prolonged the survival of patients with de novo metastatic nasopharyngeal carcinoma. Subgroup analysis further demonstrated that only patients with low EBV DNA levels (≤30,000 copies/mL) could benefit from capecitabine maintenance therapy, whereas patients with high EBV DNA levels (>30,000 copies/mL) could not benefit.
Capecitabine is an oral fluoropyrimidine with single‐agent activity. Several previous clinical trials have explored the efficacy and safety of capecitabine in metastatic NPC [17, 19, 27, 28, 29]. A phase II study in Chinese patients showed that the combination of capecitabine and cisplatin was effective and well‐tolerated as a first‐line treatment among metastatic NPC patients [27]. Similarly, Chua et al. [19] conducted a multicenter phase II study involving 39 patients with metastatic NPC and reported that the overall response rate was 53.8% and the median OS was 28.0 months. However, due to the limitation of the small sample size, it was difficult to get the exact conclusion on whether capecitabine could further prolong the survival of patients with metastatic NPC. Besides, patients with either distant metastases at diagnosis or relapse after primary treatment were all involved in these studies, which increased the heterogeneity of patients. In the present study, all the patients were confirmed to have metastasis before treatment. Based on a relatively large sample size, we could explore the curative effect of capecitabine as maintenance therapy for de novo metastatic NPC.
Used as a maintenance therapy regimen, capecitabine also showed treatment effects on other metastatic malignancies [20, 30, 31]. According to a randomized, open‐labeled, multicenter phase III trial, the application of capecitabine maintenance therapy significantly prolonged the survival of metastatic colorectal cancer patients and was considered to have acceptable toxicities [20]. The median OS was 25.63 months in the maintenance group and 23.30 months in the non‐maintenance group. Neutropenia, hand‐foot syndrome, and mucositis were the most common grade 3‐4 AEs. Another study assessing breast cancer indicated that maintenance with single‐agent capecitabine therapy was an effective and well‐tolerated treatment option for HER2‐negative metastatic breast cancer patients whose disease was controlled after 6 cycles of docetaxel plus capecitabine chemotherapy [31]. In the present study, the OS rate was higher in the maintenance group than in the non‐maintenance group. Multivariate analysis also showed that capecitabine maintenance therapy was an independent prognostic factor. Our results were consistent with the results of other metastatic malignancies, verifying that capecitabine maintenance therapy could also prolong the survival of metastatic NPC patients. Besides, the rates of AEs in the present study were similar to previous findings, and most AEs were mild and manageable, suggesting that the application of capecitabine was safe. Although some grade 3‐4 AEs (such as leukocytopenia and hand‐foot syndrome) were more common in the maintenance group than in the non‐maintenance group, the rate was still low. Notably, there were no toxicity‐associated deaths in the present study.
We further performed a subgroup analysis based on different EBV DNA levels. Interestingly, we noted that only patients with low pretreatment EBV DNA levels (≤30,000 copies/mL) benefited from capecitabine maintenance, whereas patients with high EBV DNA levels did not. This result might be explained by that plasma EBV DNA level was correlated with the tumor burden and prognosis of NPC [22, 23, 32, 33, 34, 35]. For patients with a low EBV DNA level, the distant lesions and primary tumor were better controlled after PCT and LRRT compared with patients with high EBV DNA level. The relationship between pre‐ and posttreatment EBV DNA levels also verified this point. Patients with low pretreatment EBV DNA levels were inclined to have undetectable EBV DNA levels after treatment and showed better disease control. Thus, capectabline maintenance therapy could help these patients achieve long‐term survival. However, patients with high EBV DNA levels experienced a more profound tumor burden and serious conditions. Their distant lesions were more difficult to eliminate compared with patients with low EBV DNA levels. Thus, the post‐LRRT capecitabine might be insufficient in inhibiting tumor progression. Intensive therapy such as the administration of targeted drugs or immunotherapy might be helpful in treating such patients [36, 37, 38].
Distant metastasis is the major cause of death in NPC patients, and platinum‐based PCT was established as the standard treatment. Previous study also demonstrated that LRRT could also benefit these patients in achieving longer OS compared with PCT alone. The present study put forward another view that capecitabine maintenance therapy following LRRT further prolonged the survival of de novo metastatic NPC patients with the safety profile and should be applied based on the disease condition. EBV DNA, which was the most important biomarker in NPC, could be used to select suitable patients for the therapy. Besides, capecitabine has the advantages of convenient administration. Therefore, patients do not need to stay in hospital in the duration of maintenance therapy. Because of the low rate of capecitabine‐related AEs, it was not strict in toxicity monitoring [20]. The monitoring hematologic examination can be easily conduced in local hospitals. Therefore, the use of capecitabine does not affect the qualities of patients’ life obviously. Considered as an appropriate option for metastatic NPC patients, maintenance therapy with oral capecitabine might play an important role in the management of metastatic NPC in future.
There are several limitations to this study. First, this was a retrospective study and selective bias was unavoidable. Therefore, the PSM method and multivariate analysis were applied to minimize the bias. Besides, capecitabine‐related AEs were hard to record accurately due to the retrospective design. Second, only 298 patients were eligible in the study duo to the low incidence of de novo metastatic NPC. Third, all patients were from one treatment center in an endemic area. Therefore, a multi‐institutional prospective study is required to validate our results in the future.
5. CONCLUSIONS
Pretreatment EBV DNA level is associated with the prognosis of de novo metastatic NPC. Capecitabine maintenance therapy can significantly prolong the OS of de novo metastatic NPC patients with pretreatment EBV DNA ≤ 30,000copies/mL.
COMPETING INTERESTS
The authors declare no competing interests.
AUTHORS' CONTRIBUTIONS
Study concepts: Hai‐Qiang Mai, Lin‐Quan Tang, Qiu‐Yan Chen.
Study design, statistical analysis, manuscript preparation, and manuscript editing: Xue‐Song Sun, Yu‐Jing Liang, Sai‐Lan Liu.
Manuscript review: Hai‐Qiang Mai, Lin‐Quan Tang, Qiu‐Yan Chen.
Supporting information
Supporting Information
Supporting Information
Supporting Information
DECLARATIONS
None to disclose
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective study was approved by the Clinical Research Committee of Sun Yat‐sen University Cancer Center, China. Patients were required to provide written informed consent before enrolling in the study (B2017‐072‐01).
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The key raw data have been deposited in Research Data Deposit (http://www.researchdata.org.cn), with the approval number of RDDA2019001137.
ACKNOWLEDGEMENTS
We thank all the patients who participated in this study.
FUNDING
This work was supported by grants from the National Key R&D Program of China (2016YFC0902003, 2017YFC1309003, 2017YFC0908500), the National Natural Science Foundation of China (No. 81425018, No. 81672868, No. 81602371), the Sun Yat‐sen University Clinical Research 5010 Program (201707020039, 2014A020212103, 16zxyc02), the Sci‐Tech Project Foundation of Guangzhou City (201707020039), the National Key Basic Research Program of China (No. 2013CB910304), the Special Support Plan of Guangdong Province (No. 2014TX01R145), the Sci‐Tech Project Foundation of Guangdong Province (No. 2014A020212103), the Health & Medical Collaborative Innovation Project of Guangzhou City (No. 201400000001), the National Science & Technology Pillar Program during the Twelfth Five‐year Plan Period (No. 2014BAI09B10), the PhD Start‐up Fund of Natural Science Foundation of Guangdong Province, China (2016A030310221), the cultivation foundation for the junior teachers in Sun Yat‐sen University (16ykpy28), the foundation for major projects and new cross subjects in Sun Yat‐sen University (16ykjc38), and the Fundamental Research Funds for the Central Universities Fundamental Research Funds for the Central Universities.
Sun X‐S, Liu S‐L, Liang Y‐J, et al. The role of capecitabine as maintenance therapy in de novo metastatic nasopharyngeal carcinoma: a propensity score matching study. Cancer Commun. 2020;40:32–42. 10.1002/cac2.12004
Xue‐Song Sun and Sai‐Lan Liu contributed equally to this work.
Contributor Information
Lin‐Quan Tang, Email: tanglq@sysucc.org.cn.
Hai‐Qiang Mai, Email: maihq@sysucc.org.cn.
REFERENCES
- 1. Torre L, Bray F, Siegel R, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. [DOI] [PubMed] [Google Scholar]
- 2. Chua M, Wee J, Hui E, Chan A. Nasopharyngeal carcinoma. Lancet (London, England). 2016;387 (10022):1012–24. [DOI] [PubMed] [Google Scholar]
- 3. Wei WI, JS Sham. Nasopharyngeal carcinoma. Lancet (London, England). 2005;365(9476):2041–54. [DOI] [PubMed] [Google Scholar]
- 4. Chan A, Teo P, Ngan R, Leung T, Lau W, Zee B, et al. Concurrent chemotherapy‐radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression‐free survival analysis of a phase III randomized trial. J Clin Oncol. 2002;20(8):2038–44. [DOI] [PubMed] [Google Scholar]
- 5. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression‐free survival. J Clin Oncol. 2003;21(4):631–7. [DOI] [PubMed] [Google Scholar]
- 6. Liao JF, Zhang Q, Du XJ, Lan M, Liu S, Xia YF, et al. Concurrent chemoradiotherapy with weekly docetaxel versus cisplatin in the treatment of locoregionally advanced nasopharyngeal carcinoma: a propensity score‐matched analysis. Cancer communications (London, England). 2019;39(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai S, Li W, Chen L, Luo W, Chen Y, Liu L, et al. How does intensity‐modulated radiotherapy versus conventional two‐dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–8. [DOI] [PubMed] [Google Scholar]
- 8. Zhang M, Li J, Shen G, Zou X, Xu J, Jiang R, et al. Intensity‐modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two‐dimensional radiotherapy: A 10‐year experience with a large cohort and long follow‐up. Eur J Cancer. 2015;51(17):2587–95. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Sun Y, Ma J. Induction gemcitabine and cisplatin in locoregionally advanced nasopharyngeal carcinoma. Cancer communications (London, England). 2019;39(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104(3):272–8. [DOI] [PubMed] [Google Scholar]
- 11. Tang L, Chen Q, Fan W, Liu H, Zhang L, Guo L, et al. Prospective study of tailoring whole‐body dual‐modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein‐Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31(23):2861–9. [DOI] [PubMed] [Google Scholar]
- 12. Ji J, Yun T, Kim S, Kang J, Park J, Cho I, et al. A prospective multicentre phase II study of cisplatin and weekly docetaxel as first‐line treatment for recurrent or metastatic nasopharyngeal cancer (KCSG HN07‐01). Eur J Cancer. 2012;48(17):3198–204. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open‐label, phase 3 trial. Lancet (London, England). 2016;388(10054):1883–92. [DOI] [PubMed] [Google Scholar]
- 14. Ngan R, Yiu H, Lau W, Yau S, Cheung F, Chan T, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13(8):1252–8. [DOI] [PubMed] [Google Scholar]
- 15. Chen C, Wang F, An X, Luo H, Wang Z, Liang Y, et al. Triplet combination with paclitaxel, cisplatin and 5‐FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71(2):371–8. [DOI] [PubMed] [Google Scholar]
- 16. Chen MY, Jiang R, Guo L, Zou X, Liu Q, Sun R, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum‐based chemotherapy. Oral oncology. 2003;39(4):361–6. [DOI] [PubMed] [Google Scholar]
- 18. Chua D, Wei WI, Sham JS, Au GK. Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol. 2008;38(4):244–9. [DOI] [PubMed] [Google Scholar]
- 19. Chua DT, Yiu HH, Seetalarom K, Ng AW, Kurnianda J, Shotelersuk K, et al. Phase II trial of capecitabine plus cisplatin as first‐line therapy in patients with metastatic nasopharyngeal cancer. Head Neck. 2012;34(9):1225–30. [DOI] [PubMed] [Google Scholar]
- 20. Luo HY, Li YH, Wang W, Wang ZQ, Yuan X, Ma D, et al. Single‐agent capecitabine as maintenance therapy after induction of XELOX (or FOLFOX) in first‐line treatment of metastatic colorectal cancer: randomized clinical trial of efficacy and safety. Ann Oncol. 2016;27(6):1074–81. [DOI] [PubMed] [Google Scholar]
- 21. Kam M, Teo P, Chau R, Cheung K, Choi P, Kwan W, et al. Treatment of nasopharyngeal carcinoma with intensity‐modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60(5):1440–50. [DOI] [PubMed] [Google Scholar]
- 22. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein‐Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461–70. [DOI] [PubMed] [Google Scholar]
- 23. Chan AT, Lo YM, Zee B, Chan LY, Ma BB, Leung SF, et al. Plasma Epstein‐Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Natl Cancer Inst. 2002;94(21):1614–9. [DOI] [PubMed] [Google Scholar]
- 24. Sun XS, Liu LT, Liu SL, Guo SS, Wen YF, Xie HJ, et al. Identifying optimal candidates for local treatment of the primary tumor among patients with de novo metastatic nasopharyngeal carcinoma: a retrospective cohort study based on Epstein‐Barr virus DNA level and tumor response to palliative chemotherapy. BMC cancer. 2019;19(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, et al. Comparison of plasma Epstein‐Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162–70. [DOI] [PubMed] [Google Scholar]
- 26. Fisher DJ, Copas AJ, Tierney JF, Parmar MK. A critical review of methods for the assessment of patient‐level interactions in individual participant data meta‐analysis of randomized trials, and guidance for practitioners. J Clin Epidemiol. 2011;64(9):949–67. [DOI] [PubMed] [Google Scholar]
- 27. Li YH, Wang FH, Jiang WQ, Xiang XJ, Deng YM, Hu GQ, et al. Phase II study of capecitabine and cisplatin combination as first‐line chemotherapy in Chinese patients with metastatic nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2008;62(3):539–44. [DOI] [PubMed] [Google Scholar]
- 28. Ciuleanu E, Irimie A, Ciuleanu TE, Popita V, Todor N, Ghilezan N. Capecitabine as salvage treatment in relapsed nasopharyngeal carcinoma: a phase II study. J BUON. 2008;13(1):37–42. [PubMed] [Google Scholar]
- 29. Chen SZ, Chen XM, Ding Y, Wang XC, Zhang F, Mo KL. Combined chemotherapy with cisplatin, docetaxel and capecitabine for metastatic nasopharyngeal carcinoma: a retrospective analysis. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(7):1114–8. [PubMed] [Google Scholar]
- 30. Ferrero JM, Hardy‐Bessard AC, Capitain O, Lortholary A, Salles B, Follana P, et al. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first‐line therapy for triple‐negative, metastatic, or locally advanced breast cancer: Results from the GINECO A‐TaXel phase 2 study. Cancer. 2016;122(20):3119–26. [DOI] [PubMed] [Google Scholar]
- 31. Surmeli ZG, Varol U, Cakar B, Degirmenci M, Arslan C, Piskin GD, et al. Capecitabine maintenance therapy following docetaxel/capecitabine combination treatment in patients with metastatic breast cancer. Oncol Lett. 2015;10(4):2598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang L, Li C, Li J, Chen W, Chen Q, Yuan L, et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst. 2016;108(1). [DOI] [PubMed] [Google Scholar]
- 33. An X, Wang FH, Ding PR, Deng L, Jiang WQ, Zhang L, et al. Plasma Epstein‐Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117(16):3750–7. [DOI] [PubMed] [Google Scholar]
- 34. Lo YM, Chan LY, Lo KW, Leung SF, Zhang J, Chan AT, et al. Quantitative analysis of cell‐free Epstein‐Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59(6):1188–91. [PubMed] [Google Scholar]
- 35. OuYang PY, You KY, Zhang LN, Xiao Y, Zhang XM, Xie FY. External validity of a prognostic nomogram for locoregionally advanced nasopharyngeal carcinoma based on the 8th edition of the AJCC/UICC staging system: a retrospective cohort study. Cancer communications (London, England). 2018;38(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Masmoudi A, Toumi N, Khanfir A, Kallel‐Slimi L, Daoud J, Karray H, et al. Epstein‐Barr virus‐targeted immunotherapy for nasopharyngeal carcinoma. Cancer treatment reviews. 2007;33(6):499–505. [DOI] [PubMed] [Google Scholar]
- 37. Gottschalk S, Heslop HE, Rooney CM. Adoptive immunotherapy for EBV‐associated malignancies. Leukemia Lymphoma. 2005;46(1):1–10. [DOI] [PubMed] [Google Scholar]
- 38. Chen Q, Tang L, Liu N, Han F, Guo L, Guo S, et al. Famitinib in combination with concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 1, open‐label, dose‐escalation Study. Cancer communications (London, England). 2018;38(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


