Abstract
The components of the tumor microenvironment (TME) in solid tumors, especially chemokines, are currently attracting much attention from scientists. C‐X‐C motif chemokine ligand 5 (CXCL5) is one of the important chemokines in TME. Overexpression of CXCL5 is closely related to the survival time, recurrence and metastasis of cancer patients. In TME, CXCL5 binds to its receptors, such as C‐X‐C motif chemokine receptor 2 (CXCR2), to participate in the recruitment of immune cells and promote angiogenesis, tumor growth, and metastasis. The CXCL5/CXCR2 axis can act as a bridge between tumor cells and host cells in TME. Blocking the transmission of CXCL5/CXCR2 signals can increase the sensitivity and effectiveness of immunotherapy and slow down tumor progression. CXCL5 and CXCR2 are also regarded as biomarkers for predicting prognosis and molecular targets for customizing the treatment. In this review, we summarized the current literature regarding the biological functions and clinical significance of CXCL5/CXCR2 axis in TME. The possibility to use CXCL5 and CXCR2 as potential prognostic biomarkers and therapeutic targets in cancer is also discussed
Keywords: biomarker, CXCL5, CXCR2, molecular targeted therapy, tumor microenvironment
Abbreviations
- ASC
Adipose tissue‐derived stem cell
- BMDC
bone marrow‐derived dendritic cell
- CEA
carcinoembryonic antigen
- CXCL1
C‐X‐C motif ligand 1
- CXCL2
C‐X‐C motif ligand 2
- CXCL3
C‐X‐C motif ligand 3
- CXCL5
C‐X‐C motif chemokine ligand 5
- CXCL6
C‐X‐C motif ligand 6
- CXCL7
C‐X‐C motif ligand 7
- CXCL8
C‐X‐C motif ligand 8
- CXCR1
C‐X‐C motif chemokine receptor 1
- CXCR2
C‐X‐C motif chemokine receptor 2
- DC
dendritic cell
- ENA‐78
neutrophil activating peptide 78
- FOXD1
Forkhead Box D1
- GPCR
G‐protein coupled receptor
- GSK‐3β
Glycogen synthase kinase‐3
- HER‐2
human epidermal growth factor receptor‐2
- IL‐17A
interleukin‐17A
- IL1β
interleukin‐1β
- IL‐8B
Interleukin‐8B
- LIX
lipopolysaccharide‐induced CXC chemokine
- MDSC
myeloid‐derived suppressor cell
- MMP
matrix metalloproteinase
- MSC
Mesenchymal stem cell
- NFκB
nuclear factor‐κappa B
- PAI‐1
plasminogen activator inhibitor‐1
- PD‐L1
programmed cell death protein 1
- SDF‐1
stromal cell‐derived factor‐1
- TME
tumor microenvironment
- TNF‐α
tumor necrosis factor‐α
- VEGFR
vascular endothelial growth factor receptor 1
1. INTRODUCTION
Even if the primary tumor is completely removed, it is still possible for the tumor to recur in distant organs including lung, brain, liver, and lymph nodes. This is a common feature of different types of malignant tumors. Therefore, researchers consider tumor development as an organic whole rather than merely genetic alterations and transformation of epithelial cells [1]. The tumor microenvironment (TME) facilitates tumor progression by affecting the immune surveillance and delaying the response of tumor cells to chemotherapy [2, 3]. The regulatory complexity of the interaction between tumors cells and host cells in TME affects the clinical application of many therapeutic targets [4]. In recent years, studies on C‐X‐C motif chemokine ligand 5 (CXCL5) in tumor progression have proved that it can be used as a potential indicator of tumor prognosis [5]. Previous studies have reported that CXCL5/C‐X‐C motif chemokine receptor 2 (CXCR2) axis can stimulate tumor growth and angiogenesis, and promote the infiltration and activation of host cells [6]. The recruited CXCR2+ cell populations play an essential role in malignant progression of tumors and act as novel targets for cancer treatment [7]. The importance of CXCL5/CXCR2 axis in various cancers had led to the development of several small molecule inhibitors that target this axis.
2. CXCL5/CXCR2 AXIS
2.1. CXCL5
CXC‐type chemokines are a class of small molecule peptides that function along with G‐protein coupled receptors (GPCRs) and participate in inflammatory response by attracting relevant immune cells [8]. CXCL5, also known as neutrophil activating peptide 78 (ENA‐78), belongs to the CXC‐type chemokine family with a specific amino acid sequence of glutamic acid‐leucine‐arginine which is shortly named as ELR motif. The ELR motif of CXC‐type chemokine family plays an important role in angiogenesis [8]. CXCL5 can recruit cells, such as T/B lymphocytes and eosinophils from the immune system to the corresponding regions during immune response [9]. Besides, it also participates in promoting the adhesion and remodeling of connective tissues [10].
CXCL5 is expressed by many immune cells, such as macrophages [11], eosinophils [12], as well as non‐immune cells including mesothelial cells [13], and fibroblasts [14]. TME involves various cells and factors complex composition in TME is connected with various sources of CXCL5. The sources of CXCL5 may be related to tumor autocrine and paracrine loop in the TME which is described in detailed below.
2.2. CXCR2
It is currently believed that CXCL5 transmits signals by binding to the Interleukin‐8B (IL‐8B) receptor, which was subsequently termed as CXCR2 [8]. CXCR2 is also highly expressed in many types of cancers (Table 1). CXCR2 abnormal expression combined with postoperative complications can be used as a predictor of tumor recurrence (Table 1). In addition to CXCL5, CXCR2 also binds to other ligands, including C‐X‐C motif ligand 1 (CXCL1), C‐X‐C motif ligand 2 (CXCL2), C‐X‐C motif ligand 3 (CXCL3), C‐X‐C motif ligand 6 (CXCL6), C‐X‐C motif ligand 7 (CXCL7) and C‐X‐C motif ligand 8 (CXCL8) [8]. Meanwhile, human CXCL6 binds to both C‐X‐C motif chemokine receptor 1 (CXCR1) and CXCR2. However, a study in mice demonstrated that there was only one protein that had good homology with CXCL5 and CXCL6, and was commonly referred to as murine CXCL5, or called murine lipopolysaccharide‐induced CXC chemokine (LIX) [8].
Table 1.
The clinical application prospects of CXCL5 and CXCR2
| Cancer types | Clinical relevance | |
|---|---|---|
| CXCL5 | CXCR2 | |
| Pancreatic cancer |
|
N/A |
| Glioma |
|
N/A |
| Hepatocellular carcinoma | ||
| Lung cancer | ||
| Colorectal cancer |
|
N/A |
| Osteosarcoma | ‐upregulate in tumor tissues than paracancerous tissues [57] | N/A |
| Gastric cancer | ‐be associated with T‐stage and distant metastasis [65] | |
| Nasopharyngeal carcinoma | N/A | |
| Prostate cancer |
|
N/A |
| Endometrial cancer |
|
N/A |
| Biliary tract cancer | N/A | |
| Breast cancer | N/A | |
| Bladder cancer |
|
N/A |
| Esophageal cancer | N/A | |
| Laryngeal squamous cell carcinoma |
|
|
| Astrocytic tumors | N/A |
|
| Renal cell carcinoma | N/A |
|
| Ovarian cancer | N/A |
|
Abbreviations: CXCL5, CXC motif ligand 5; CXCR2, C‐X‐C motif chemokine receptor 2; N/A, not applicable.
3. THE RELATIONSHIP BETWEEN CXCL5 AND TUMOR PROGRESSION
3.1. Overexpression of CXCL5 in tumors
According to the previous studies, more than 14 different malignant tumor types, including breast cancer [15], bladder cancer [16], non‐small cell lung cancer [17], gastric cancer [18], head and neck squamous cell carcinoma [19], cholangiocarcinoma [20], hepatocellular carcinoma [21], prostate cancer [22], pancreatic cancer [23], colorectal cancer [24], glioma [25], endometrial cancer [26], nasopharyngeal carcinoma [27], and squamous carcinoma of larynx [6], had high expression of CXCL5 when compared to para‐carcinoma or normal tissues (Table 1). By identifying and validating the genes regarding the development of gastric cancer, researchers have found that CXCL5, as one of the substances, was associated with early stages of the disease [28]. After the gastric cancer patients underwent radical surgery, their levels of CXCL5 in serum were found to be reduced [28]. The expression levels of CXCL5 in colorectal cancer tissues were also found to be higher than colon adenomas which the malignancy is lower than cancer [24, 29]. In colorectal cancer and hepatocellular carcinoma, CXCL5 expression in metastatic tissues was found to be significantly higher than in the adjacent tissues [24, 29]. The expression intensity of CXCL5 was also found to be consistent with the degree of malignancy, metastatic potential, and degree of inflammatory infiltration, which in turn bring new possibilities clinical applications [30].
3.2. The sources of CXCL5 in TME
Some scholars believed that CXCL5 in TME was predominantly produced by tumor cells in certain types of cancers, such as hepatocellular carcinoma [31], breast cancer [32], and intrahepatic cholangiocarcinoma [33]. Some research shows that CXCL5 is induced by interleukin‐17A (IL‐17A) or interleukin‐1β (IL1β) in TME and secreted by tumor cells, conferring it as an invasive phenotype of tumor cells [31, 32, 33]. These parallel with the views of Zhao et al. [34] that the primary source of CXCL5 in colorectal cancer is cancerous epithelial cells rather than fibroblasts in tumor stroma [34].
Some researchers have found that the host cells in the TME are also regarded as a non‐negligible source of CXCL5 (Figure 1A). CXCL5 was abnormal high expression in cancer‐associated fibroblasts in melanoma [35]. In addition, CXCL5 secreted by these fibroblasts promotes programmed cell death protein 1 (PD‐L1) expression in tumor cells, forming an immunosuppressive microenvironment [35]. Macrophages in gastric cancer are considered to be an important source of CXCL5. They can induce CXCL1 and CXCL5 expression via the STAT3 feedforward loop and thereby promote tumor cell migration [36]. Schwann cells in the peripheral nervous system are responsible for epithelial‐mesenchymal transition, invasion and metastasis of lung cancer cells through the CXCR2/PI3K/AKT/Glycogen synthase kinase‐3 (GSK‐3β)/Snail‐Twist signaling pathway by secreting CXCL5 [7]. Tumor‐associated dendritic cells (DC) interfere with functional DC maturation by secreting CXCL5 and IL‐1β [37]. Mesenchymal stem cells (MSCs) activated by acidic TME or tumor necrosis factor‐α (TNF‐α) can secrete CXCL5 and other pro‐inflammatory factors [37]. Besides, cancer‐associated mesothelial cells, which are generated by plasminogen activator inhibitor‐1 (PAI‐1), can secrete interleukin‐8 (IL‐8) and CXCL5 via the nuclear factor‐κappa B (NFκB) signaling pathway and thereby promoting peritoneal metastasis [13]. Adipose tissue‐derived stem cells (ASCs) secrete CXCL5 which subsequently promotes tumor growth and affects the development of breast tumors [38].
Figure 1.
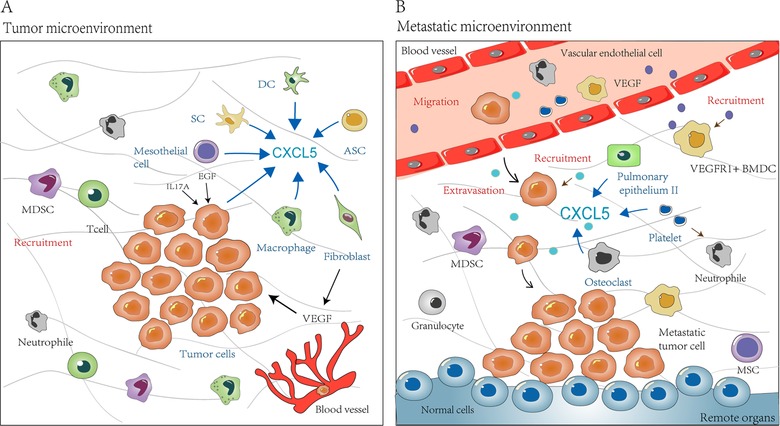
The sources and role of CXCL5 in TME and metastatic niche. (A) CXCL5 is derived from primary tumor cells and even tumor cells in the blood circulation. It is also secreted by some other host cells in TME, including fibroblasts, macrophages, mesothelial cells, SCs, DCs and ASCs. CXCL5 enriched in the microenvironment recruits immune‐related cells to tumors mass, such as neutrophils and MDSCs. Patients with high levels of CXCL5 have massive infiltration of T cells and macrophages, which in turn participates in immune responses. (B) Host cells in remote organs, such as pulmonary epithelium II, osteoclasts, and platelets, can secrete CXCL5 to help the formation of metastatic microenvironment, which is beneficial to tumorigenesis. In addition, in metastatic niche, CXCL5 can recruit tumor cells and immune cells, including VEGFR+ BMDCs, granulocytes, and MSCs, and thereby play an important role in tumor progression. Abbreviations: CXCL5, C‐X‐C motif chemokine ligand 5; SC, Schwann cells; DC, dendritic cell; ASC, Adipose tissue‐derived stem cell; MSC, Mesenchymal stem cell; MDSC, myeloid‐derived suppressor cell; BMDC, bone marrow‐derived dendritic cell; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor 1; EGF, epidermal growth factor; IL17A, interleukin‐17A
Studies on the relationship between tumors and distant organs have confirmed that the metastatic niche is also a link that cannot be ignored [39]. CXCL5 participates in pre‐metastatic niches formation (Figure 1B and Table 2). In nude mice bearing esophageal cancer xenograft with Id1 overexpression, vascular endothelial growth factor receptor 1 (VEGFR1)+ bone marrow‐derived dendritic cells (BMDCs) in the tumor macroenvironment form pre‐metastatic niches at distant sites. CXCL5 is upregulated in the metastatic lung tissues niches and cancer cells are attracted to the metastatic site via the CXCL5/CXCR2 axis [14]. Platelet‐derived CXCL5 are also reported to be involved in the recruitment of granulocytes into early metastatic niches [40]. In breast cancer, it has been observed that MSCs could significantly increase the metastatic rate of lung metastatic and bone metastatic events. This observation is related to CXCL5 secretion by MSCs and the recruitment of CXCR2+ neutrophils [37].
Table 2.
Functions of CXCL5 in TME and/or metastatic niche
| Functions of CXCL5 | References |
|---|---|
| Promote tumor angiogenesis and lymphangiogenesis in TME | [51, 52] |
| Regulate the function of neutrophil in TME | [45] |
| Positively related to the risk of metastasis | [62, 63, 72] |
| Recruit immune cells | |
| ‐ Recruit neutrophils in TME and metastatic niche | [30, 42, 43, 44, 45] |
| ‐ Recruit granulocytes to promote the formation of early metastatic microenvironment | [40, 49] |
| ‐ Promote MDSCs infiltration in TME | [47, 48] |
| ‐ Positively related to the extent of macrophage infiltration in TME | [50] |
| Promote cell proliferation of primary tumors | [26, 54, 55, 56, 57] |
Abbreviations: CXCL5, CXC motif ligand 5; TME, tumor microenvironment; MDSC, myeloid‐derived suppressor cell.
3.3. The role of CXCL5/CXCR2 axis in TME
In TME, CXCL5 can play an indirect role in promoting tumor progression by regulating the functions of various host cells. Zhou et al. [41] revealed that CXCL5 increased the possibility of proliferation and metastasis of intrahepatic cholangiocarcinoma in a mouse xenograft model. Interestingly, it does not exert any direct effect on intrahepatic cholangiocarcinoma cells in vitro. Neutrophils may be an important reason for the differences between in vivo and in vitro studies. CXCR2+ neutrophils are recruited by CXCL5 at tumor tissue, which then participate in tumor progression [30, 42]. Hepatocellular carcinoma stem cell‐like cells overexpress CXCL5, which thereafter recruits more tumor‐associated neutrophils penetration through CXCR2 activation [43]. The recruited neutrophils migrate through the lymphatic vessels and promote the lymphatic metastasis of tumor cells [44]. CXCL5‐overexpressing tumors reduce the potential for lung metastasis by recruiting neutrophils [45]. The results of multivariate regression analyses showed that the overexpression of CXCL5 combined with the degree of neutrophils infiltration in TME was related to advanced clinical stage (stage III or IV) [6], overall prognosis [29], and recurrence [30]. Moreover, CXCL5/CXCR2 axis not only contributes to the recruitment of neutrophils but also regulates the function of neutrophils in melanoma [45].
Patients with massive infiltration of myeloid‐derived suppressor cells (MDSCs) tumor tissues have significantly increased likelihood of developing advanced clinical stage disease, metastasis, drug resistance, and poor survival [46]. Polymorphonuclear MDSCs in renal cell carcinoma was found to be associated with tumor grade and demonstrated a positive correlation with the expression of inflammatory mediators such as CXCL5 [47]. In breast cancer and melanoma, the blockage of transforming growth factor‐β (TGF‐β) signaling promoted the secretion of CXCL5, CXCL1 and CXCL12, facilitating cancer progression and lung metastasis [48]. The process involves the recruitment of Gr‐1+ CD11b+ MDSCs to tumor tissues and the increasing flow of matrix metalloproteinases (MMPs) though stromal cell‐derived factor‐1 (SDF‐1)/CXCR4 and CXCL5/CXCR2 axes [48].
Previous experiments have shown that CXCL5 was positively associated with the degree of CD8+ T cells infiltration in colon cancer tissues [49]. CXCL5 also recruits CD11b+ MMP9+ Ly6G+ granulocytes to promote the formation of early metastatic niches via the CXCL5/CXCR2 axis [40]. A low expression of CXCL5 in tissues has been found to reduce the recruitment of tumor‐associated lymphocytes [9]. Also, CXCL5 has been associated with the degree of macrophage infiltration in primary organs, participating in innate immune responses and is considered important for immunotherapy [50].
3.4. The contributions of CXCL5/CXCR2 axis in tumor progression
Tumor progression is inseparable from tumor angiogenesis. The abundant blood vessels induced by overexpressed angiogenic factors supply more nutrient‐rich blood to the rapidly growing tumor tissues. CXCL5 has been reported to have chemotactic effects on vascular endothelium and is considered as an effective angiogenic factor (Figure 2 and Table 2). CXCL5 in patients with non‐small cell lung cancer and gastric cancer showed close association with vascular distribution of tumors [18]. In renal cell carcinoma and bladder cancer, CXCL5 is secreted by tumor cells and induces a strong synergistic effect on the proliferation and recruitment of endothelial cells in the pathway of CXCL5/CXCR2 axis [51, 52]. Recombinant human CXCL5 activates CXCR2 to promote angiogenesis and tube formation of blood vessels, and involves in the process of proliferation and migration of human umbilical vein endothelial cells via the CXCR2/AKT/NF‐κB/Forkhead Box D1 (FOXD1)/VEGF‐A signaling pathway [53]. Overexpression of CXCL5 in the subcutaneous xenograft tumor model increased the distribution density of micro vessels in vivo [53]. CXCL5/CXCR2 axis plays an important role in tumor angiogenesis.
Figure 2.
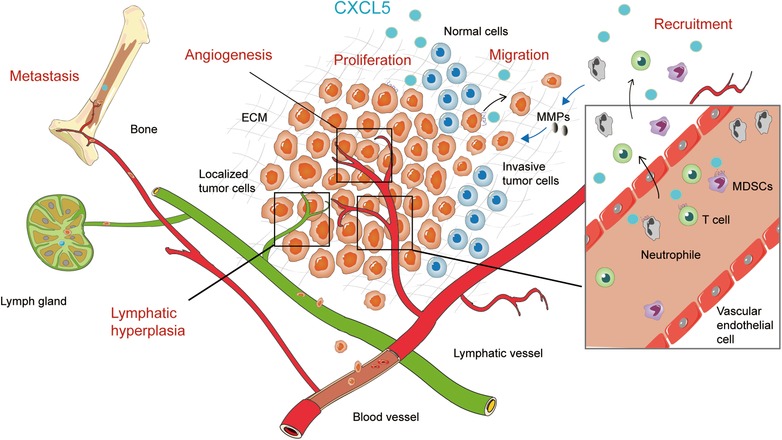
The contributions of CXCL5 to tumor progression. CXCL5 promotes proliferation of tumors in situ. CXCL5 promotes the migratory and invasive abilities of tumor cells to distant organs through blood vessels and lymphatic vessels. CXCL5 recruits more immune‐related cells, such as neutrophils, T cells and MDSCs, from the blood vessels to the tumor microenvironment. These cells play an essential role in the development of tumors via CXCL5/CXCR2 axis. In addition, CXCL5 promotes tumor‐associated angiogenesis and lymphangiogenesis, creating a more conducive environment for tumor growth and metastasis
CXCL5 has also been shown to be involved in many aspects of malignant progression of the tumor (Figure 2 and Table 2). CXCL5 promoted the proliferation of several types of tumor cells, such as the tumor cells in prostate cancer [54], cervical cancer [26], lung cancer [55], hepatoblastoma [56], and osteosarcoma [57]. The increasing expression of CXCL5 and IL‐8 induced by snail also promoted prostate cancer cell growth in vivo by CXCR2 activation [58]. However, the proliferation of prostate cancer cells stimulated by CXCL5 could not be replicated in vitro [58]. Similar results were observed from in vitro experiments conducted on intrahepatic cholangiocellular carcinoma, lung cancer, and colorectal cancer [41, 59]. In these studies, no statistical significance in the proliferation rate of cells with CXCL5 overexpression or low expression was observed. CXCL5 promotes cell proliferation only in some tumor types and the differences in cell proliferation might be related to the differential expression of receptors in various kinds of cells.
CXCL5 expression showed positive association with the risk of metastases in cancer patients. Some studies reported that the CXCL5 levels in metastatic tissues of lymph nodes were significantly higher than that in the primary tumor area in head and neck squamous cell carcinoma [19, 60]. The appearance of CXCL5 was reported to be closely related with the amount of neutrophil infiltration and the occurrence of local metastasis in T4 stage melanoma [44]. The content of CXCL5 in colorectal cancer liver metastasis tissue has been described to be higher than that in healthy liver [61]. In tumor tissues and blood of gastric cancer patients, high expression of CXCL5 indicated an increased disease risk and lymph node metastasis [60] but showed no close association with the T stages [18]. Circulating lung cancer cell lines (CTC‐TJH‐01) were constructed to find out that their metastatic ability was associated with CXCL5 overexpression [62]. CXCL5 allows tumor cells to acquire highly migrating and invasive phenotype. The expression of CXCL5 in high metastatic potential hepatocellular carcinoma cell lines are also stronger than the low invasive counterparts [63].
4. TARGETING CXCL5: PROSPECTS IN CLINICAL APPLICATION
4.1. CXCL5 as a potential prognostic biomarker
The overexpression of CXCL5 in tumors has been observed in many kinds of cancers, providing a powerful aid for individual treatment and prognosis assessment of cancer patients. The expression intensity of CXCL5 is closely related to the degree of tumor differentiation, clinical stage, metastasis, and survival time of cancer patients (Table 1). The results of multiple logistic regression analysis showed that CXCL5 expression level is a significant risk factor for N phase (N2, N3) in gastric cancer but is not associated with T phase [64]. According a recent meta‐analysis, CXCL5 expression level in tumor tissues was found to act as an independent prognostic factor for survival in cancer patients [5]. In postoperative survival analysis of patients after complete pancreatic cancer resection, the mean survival time of CXCL5 expression group is 25.5 months, shorter than the negative groups [31]. CXCL5 overexpression was also reported to be inversely associated with overall survival of patients with intrahepatic cholangiocarcinoma and hepatocellular carcinoma [5].
Many studies have accessed the predictive significance of CXCL5 in various cancer types. CXCL5 levels in serum assists in predicting the development and metastasis of gastric cancer. Compared with carcinoembryonic antigen (CEA), the detection of serum CXCL5 and matrix‐derived factor 1 (SDF‐1) could more accurately predict the prevalence of gastric cancer [65]. Combining CXCL5, SDF‐1 and CEA markers to predict gastric cancer metastasis demonstrated a specificity of 92.8% and a sensitivity of 75% [65]. CXCL5 levels were significantly increased in the serum of nasopharyngeal carcinoma patients than the healthy group [27]. The levels of CXCL5 in the serum of patients with metastatic prostate cancer were higher than patients with primary prostate cancer or controls [66]. But not all patients had high CXCL5 levels in their blood or tumor tissues. The content of CXCL5 in the peripheral circulation of patients with laryngeal squamous cell carcinoma or lung cancer has been reported to be significantly lower than that in the blood surrounding the tumor [6, 67]. Some researchers hypothesized that the main source of CXCL5 in the blood were the monocytes and platelets that are recruited to the tumor area and leading to decreased levels of CXCL5 in the blood. Consequently, it was reported that a high CXCL5 secretion in the TME could suppress the amount of CXCL5 in the blood.
4.2. Therapeutic potential of CXCL5 and CXCR2
Researchers have paid much attention to the potential of CXCL5 in cancer screening, personalized anticancer treatment. First, blocking CXCL5 seems as a new avenue in the process of anti‐tumor angiogenesis. Blocking CXCL5 effectively was found to reduce blood supply to the tumor, thereby slowing the process of disease progression. CXCR2 antagonist SCH‐527123 similarly reduced revascularization by blocking the NF‐κB/MAPK/AKT signaling pathway [68].
Blocking the CXCL5/CXCR2 signaling reduces tumor progression. The PI3K inhibitor (LY294002) blocks the phosphorylation of AKT and GSK‐3β in the CXCL5/CXCR2 axis signaling pathway and reduces the invasive ability of tumor cells [7]. Neutralization antibodies to CXCL5 decrease the probability of metastasis of breast cancer cells [69]. In addition, the drug sensitivity and thetreatment effects in different patients are vary widely. The CXCL5 inhibitor might also be a new direction to predicte the drug resistance. CXCL5 is up‐regulated in M‐RT4 cell line, which is a bladder cancer cell line and is resistant to mitomycin C. Reduced CXCL5 expression reduces the possibility of mitomycin C resistance [70]. Similarly, CXCL5 cytokine showed significant upregulation in sunitinib‐resistant cells [71]. CXCL5 can be used as a predictor of lysosomotropic drug resistance (sunitinib and lapatinib) which contributes to autophagy and inflammatory responses in breast and kidney cancer. Researchers believed that CXCL5 cannot predict the efficacy of bevacizumab guided by VEGF [71]. Second, CXCL5 was observed to be associated with end‐stage cancer and sunitinib recurrence rates [71]. Therefore, an examination to evaluate CXCL5 in the tissues should be conducted to guide further treatment. This is regarded as a potential asset for personalized medicine and more conducive to the efficacy of lysosomotropic drugs.
At the same time, CXCL5 acts as a potential combination therapeutic strategy for chemotherapy or immunotherapy. In lung cancer, neutralizing CXCL5 improved the efficacy of tyrosine kinase inhibitor gefitinib without increase of the associated side effects [72]. CXCL5 can be used as an adjuvant to chemotherapeutics and improve the efficacy of drugs.
The anti‐tumor impact of CXCR2 in immunotherapy has also received widespread attention. What remains to emphasize is not only whether CXCR2 inhibitor prevents the action of the CXCL5/CXCR2 axis but if it also affects the mediation of other ligands of CXCR2. CXCR2 acts as a potential target for regulating tumor immune escape. Blocking CXCR2 prevents the recruitment of inhibitory MDSCs and improves the utility of immunotherapy by enhancing the efficiency of anti‐PD‐1 in treating rhabdomyosarcoma [73]. Blocking CXCR2+PMN− MDSCs enhanced CD4+ and CD8+ T cells infiltration and reduced tumor weight [47]. CXCR2 blockage decreases colon cancer progression and increases the sensitivity to platinum‐based anticancer medications [68]. Inhibition of CXCR2 also reduces the recruitment of granulocytes to the primary tumor area to regulate the progression of pancreatic ductal adenocarcinoma [74]. Blockage of platelet‐derived CXCL5/7 signaling with CXCR2 blocker inhibits the establishment of early metastatic niches [40]. CXCR2 inhibitors demonstrated a good tolerance in most of the patients clinically, and relevant clinical trials are currently underway [75]. In human epidermalgrowth factor receptor‐2 (HER‐2)‐negative metastatic breast cancer patients, the non‐competitive CXCR1/2 antagonist Reparixin assisting paclitaxel is considered to be safe and has no mutual pharmacokinetic effects (NCT02001974) [75]. Relevant clinical applications are should be conducted to improve the survival time of patients (Table 3).
Table 3.
The clinical application prospects of CXCL5
5. CONCLUSIONS AND PERSPECTIVES
The interaction between TME and tumor cells has brought a new revolution in anti‐cancer immunotherapy. Current researches indicated that blocking the CXCL5/CXCR2 axis is an effective method to resist tumor cell growth and improve therapeutic sensitivity. Blocking the relationship between CXCL5 and tumor extracellular environment promoted improvement of treatment method. According to different expressions of CXCL5 in patients, the personality therapy was customized to improve the tumor prognosis effectively. We suggest that CXCL5 can be regarded as a new treatment paradigm for predicting tumor prognosis, assessing tumor progression, and impeding tumor development.
DECLARATIONS: ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This work was supported by the Project of Jiangsu Provincial Key medical talents in Jiangsu Province (ZDRCA2016034).
AUTHORS' CONTRIBUTIONS
All the authors made contributions to the conception, drafting, drawing and final revision.
ACKNOWLEDGEMENTS
Not applicable.
Zhang W, Wang H, Sun M, et al. CXCL5/CXCR2 axis in tumor microenvironment as potential diagnostic biomarker and therapeutic target. Cancer Communications. 2020;40:69–80. 10.1002/cac2.12010
Contributor Information
Qiang You, Email: qiangyou2013@163.com.
Lin Miao, Email: linmiao@njmu.edu.cn.
REFERENCES
- 1. Zhuang X, Zhang H, Hu G. Cancer and Microenvironment Plasticity: Double‐Edged Swords in Metastasis. Trends Pharmacol Sci. 2019;40(6):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun (Lond). 2019;39(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. [DOI] [PubMed] [Google Scholar]
- 5. Hu B, Fan H, Lv X, Chen S, Shao Z. Prognostic significance of CXCL5 expression in cancer patients: a meta‐analysis. Cancer Cell International. 2018;18:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang D, Zhou J, Tang D, Zhou L, Chou L, Chou KY, et al. Neutrophil infiltration mediated by CXCL5 accumulation in the laryngeal squamous cell carcinoma microenvironment: A mechanism by which tumour cells escape immune surveillance. Clinical immunology (Orlando, Fla). 2017;175:34–40. [DOI] [PubMed] [Google Scholar]
- 7. Zhou Y, Shurin GV, Zhong H, Bunimovich YL, Han B, Shurin MR. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018;78(20):5927–39. [DOI] [PubMed] [Google Scholar]
- 8. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 10. Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al. A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5. J Immunol. 2007;179(7):4849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123(3):1343–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Persson T, Monsef N, Andersson P, Bjartell A, Malm J, Calafat J, et al. Expression of the neutrophil‐activating CXC chemokine ENA‐78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003;33(4):531–7. [DOI] [PubMed] [Google Scholar]
- 13. Peng Y, Kajiyama H, Yuan H, Nakamura K, Yoshihara M, Yokoi A, et al. PAI‐1 secreted from metastatic ovarian cancer cells triggers the tumor‐promoting role of the mesothelium in a feedback loop to accelerate peritoneal dissemination. Cancer Lett. 2019;442:181–92. [DOI] [PubMed] [Google Scholar]
- 14. Xu WW, Li B, Guan XY, Chung SK, Wang Y, Yip YL, et al. Cancer cell‐secreted IGF2 instigates fibroblasts and bone marrow‐derived vascular progenitor cells to promote cancer progression. Nat Commun. 2017;8:14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, et al. CXC chemokines located in the 4q21 region are up‐regulated in breast cancer. Endocr Relat Cancer. 2007;14(4):1039–52. [DOI] [PubMed] [Google Scholar]
- 16. Gao Y, Guan Z, Chen J, Xie H, Yang Z, Fan J, et al. CXCL5/CXCR2 axis promotes bladder cancer cell migration and invasion by activating PI3K/AKT‐induced upregulation of MMP2/MMP9. Int J Oncol. 2015;47(2):690–700. [DOI] [PubMed] [Google Scholar]
- 17. Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, et al. Cyclooxygenase‐2‐dependent expression of angiogenic CXC chemokines ENA‐78/CXC Ligand (CXCL) 5 and interleukin‐8/CXCL8 in human non‐small cell lung cancer. Cancer Res. 2004;64(5):1853–60. [DOI] [PubMed] [Google Scholar]
- 18. Park JY, Park KH, Bang S, Kim MH, Lee JE, Gang J, et al. CXCL5 overexpression is associated with late stage gastric cancer. J Cancer Res Clin Oncol. 2007;133(11):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyazaki H, Patel V, Wang H, Ensley JF, Gutkind JS, Yeudall WA. Growth factor‐sensitive molecular targets identified in primary and metastatic head and neck squamous cell carcinoma using microarray analysis. Oral Oncol. 2006;42(3):240–56. [DOI] [PubMed] [Google Scholar]
- 20. Lee SJ, Kim JE, Kim ST, Lee J, Park SH, Park JO, et al. The Correlation Between Serum Chemokines and Clinical Outcome in Patients with Advanced Biliary Tract Cancer. Trans Oncol. 2018;11(2):353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakabe T, Azumi J, Umekita Y, Toriguchi K, Hatano E, Hirooka Y, et al. Expression of Cancer Stem Cell‐associated DKK1 mRNA Serves as Prognostic Marker for Hepatocellular Carcinoma. Anticancer Res. 2017;37(9):4881–8. [DOI] [PubMed] [Google Scholar]
- 22. Begley LA, Kasina S, Mehra R, Adsule S, Admon AJ, Lonigro RJ, et al. CXCL5 promotes prostate cancer progression. Neoplasia. 2008;10(3):244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li A, King J, Moro A, Sugi MD, Dawson DW, Kaplan J, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. Am J Pathol. 2011;178(3):1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rubie C, Frick VO, Wagner M, Schuld J, Graber S, Brittner B, et al. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dai Z, Wu J, Chen F, Cheng Q, Zhang M, Wang Y, et al. CXCL5 promotes the proliferation and migration of glioma cells in autocrine‐ and paracrine‐dependent manners. Oncol Rep. 2016;36(6):3303–10. [DOI] [PubMed] [Google Scholar]
- 26. Feng X, Zhang D, Li X, Ma S, Zhang C, Wang J, et al. CXCL5, the upregulated chemokine in patients with uterine cervix cancer, in vivo and in vitro contributes to oncogenic potential of Hela uterine cervix cancer cells. Biomed Pharm. 2018;107:1496–504. [DOI] [PubMed] [Google Scholar]
- 27. Qiu WZ, Zhang HB, Xia WX, Ke LR, Yang J, Yu YH, et al. 5.18 The CXCL5/CXCR2 axis contributes to the epithelial‐mesenchymal transition of nasopharyngeal carcinoma cells by activating ERK/GSK‐3beta/snail signalling. Cancer Cell Int. 2018;37(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rajkumar T, Vijayalakshmi N, Gopal G, Sabitha K, Shirley S, Raja UM, et al. Identification and validation of genes involved in gastric tumorigenesis. Cancer Cell Int. 2010;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dimberg J, Dienus O, Lofgren S, Hugander A, Wagsater D. Expression and gene polymorphisms of the chemokine CXCL5 in colorectal cancer patients. Int J Oncol. 2007;31(1):97–102. [PubMed] [Google Scholar]
- 30. Zhou SL, Dai Z, Zhou ZJ, Wang XY, Yang GH, Wang Z, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56(6):2242–54. [DOI] [PubMed] [Google Scholar]
- 31. Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, Masuda T, et al. Identification of CXCL5/ENA‐78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer‐associated fibroblasts. Int J Cancer. 2012;131(10):2234–41. [DOI] [PubMed] [Google Scholar]
- 32. Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, et al. IL‐17A produced by gammadelta T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74(7):1969–82. [DOI] [PubMed] [Google Scholar]
- 33. Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M, et al. TGF‐beta receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011;1(5):430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao J, Ou B, Han D, Wang P, Zong Y, Zhu C, et al. Tumor‐derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk‐1/Snail and AKT/GSK3beta/beta‐catenin pathways. Mol Cancer. 2017;16(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z, Zhou J, Zhang J, Li S, Wang H, Du J. Cancer‐associated fibroblasts promote PD‐L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer. 2019. 10.1002/ijc.32278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou Z, Xia G, Xiang Z, Liu M, Wei Z, Yan J, et al. A C‐X‐C Chemokine Receptor Type 2‐Dominated Cross‐talk between Tumor Cells and Macrophages Drives Gastric Cancer Metastasis. Clin Cancer Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu PF, Huang Y. TNFalpha‐activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene. 2017;36(4):482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y, Zhang X, Zhao H, Wang J, Zhang Q. CXCL5 secreted from adipose tissue‐derived stem cells promotes cancer cell proliferation. Oncol Lett. 2018;15(2):1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS. Breast cancer cells condition lymphatic endothelial cells within pre‐metastatic niches to promote metastasis. Nat Commun. 2014;5:4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou SL, Dai Z, Zhou ZJ, Chen Q, Wang Z, Xiao YS, et al. CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis. 2014;35(3):597–605. [DOI] [PubMed] [Google Scholar]
- 42. Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, et al. The Lineage‐Defining Transcription Factors SOX2 and NKX2‐1 Determine Lung Cancer Cell Fate and Shape the Tumor Immune Microenvironment. Immunity. 2018;49(4):764–79.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haider C, Hnat J, Wagner R, Huber H, Timelthaler G, Grubinger M, et al. Transforming Growth Factor‐beta and Axl Induce CXCL5 and Neutrophil Recruitment in Hepatocellular Carcinoma. Hepatology. 2019;69(1):222–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soler‐Cardona A, Forsthuber A, Lipp K, Ebersberger S, Heinz M, Schossleitner K, et al. CXCL5 Facilitates Melanoma Cell‐Neutrophil Interaction and Lymph Node Metastasis. J Invest Dermatol. 2018;138(7):1627–35. [DOI] [PubMed] [Google Scholar]
- 45. Forsthuber A, Lipp K, Andersen L, Ebersberger S, Grana C, Ellmeier W, et al. CXCL5 as Regulator of Neutrophil Function in Cutaneous Melanoma. J Invest Dermatol. 2019;139(1):186–94. [DOI] [PubMed] [Google Scholar]
- 46. Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–10. [DOI] [PubMed] [Google Scholar]
- 47. Najjar YG, Rayman P, Jia X, Pavicic PG, Jr. , Rini BI, Tannenbaum C, et al. Myeloid‐Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1beta, IL8, CXCL5, and Mip‐1alpha. Clinical Can Res. 2017;23(9):2346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr‐1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gil‐Bernabe AM, Ferjancic S, Tlalka M, Zhao L, Allen PD, Im JH, et al. Recruitment of monocytes/macrophages by tissue factor‐mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119(13):3164–75. [DOI] [PubMed] [Google Scholar]
- 50. Jiang SH, Zhu LL, Zhang M, Li RK, Yang Q, Yan JY, et al. GABRP regulates chemokine signalling, macrophage recruitment and tumour progression in pancreatic cancer through tuning KCNN4‐mediated Ca(2+) signalling in a GABA‐independent manner. Gut. 2019. 10.1136/gutjnl-2018-317479. [DOI] [PubMed] [Google Scholar]
- 51. Huang Z, Zhang M, Chen G, Wang W, Zhang P, Yue Y, et al. Bladder cancer cells interact with vascular endothelial cells triggering EGFR signals to promote tumor progression. Gut. 2019;54(5):1555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guan Z, Li C, Fan J, He D, Li L. Androgen receptor (AR) signaling promotes RCC progression via increased endothelial cell proliferation and recruitment by modulating AKT → NF‐kappaB → CXCL5 signaling. Sci Rep. 2016;6:37085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen C, Xu ZQ, Zong YP, Ou BC, Shen XH, Feng H, et al. CXCL5 induces tumor angiogenesis via enhancing the expression of FOXD1 mediated by the AKT/NF‐kappaB pathway in colorectal cancer. Cell Death and Disease. 2019;10(3):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qi Y, Zhao W, Li M, Shao M, Wang J, Sui H, et al. High C‐X‐C motif chemokine 5 expression is associated with malignant phenotypes of prostate cancer cells via autocrine and paracrine pathways. Int J Oncol. 2018;53(1):358–70. [DOI] [PubMed] [Google Scholar]
- 55. Wang L, Shi L, Gu J, Zhan C, Xi J. CXCL5 regulation of proliferation and migration in human non‐small cell lung cancer cells. J Physiol Biochem. 2018;74(2):313–24. [DOI] [PubMed] [Google Scholar]
- 56. Yang Y, Hou J, Shao M, Zhang W, Qi Y, E S, et al. CXCL5 as an autocrine or paracrine cytokine is associated with proliferation and migration of hepatoblastoma HepG2 cells. J Neurochem. 2017;14(6):7977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dang H, Wu W, Wang B, Cui C, Niu J, Chen J, et al. CXCL5 Plays a Promoting Role in Osteosarcoma Cell Migration and Invasion in Autocrine‐ and Paracrine‐Dependent Manners. Oncol Res. 2017;25(2):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, et al. Snail promotes CXCR2 ligand‐dependent tumor progression in non‐small cell lung carcinoma. Clinical Can Res. 2009;15(22):6820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Speetjens FM, Kuppen PJ, Sandel MH, Menon AG, Burg D, van de Velde CJ, et al. Disrupted expression of CXCL5 in colorectal cancer is associated with rapid tumor formation in rats and poor prognosis in patients. Clinical Can Res. 2008;14(8):2276–84. [DOI] [PubMed] [Google Scholar]
- 60. Miyazaki H, Patel V, Wang H, Edmunds RK, Gutkind JS, Yeudall WA. Down‐regulation of CXCL5 inhibits squamous carcinogenesis. Cancer Res. 2006;66(8):4279–84. [DOI] [PubMed] [Google Scholar]
- 61. Cui D, Zhao Y, Xu J. Activated CXCL5‐CXCR2 axis promotes the migration, invasion and EMT of papillary thyroid carcinoma cells via modulation of beta‐catenin pathway. Biochimie. 2018;148:1–11. [DOI] [PubMed] [Google Scholar]
- 62. Que Z, Luo B, Zhou Z, Dong C, Jiang Y, Wang L, et al. Establishment and characterization of a patient‐derived circulating lung tumor cell line in vitro and in vivo. Cancer Cell International. 2019;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu XJ, Yang BW, Gong FX, Huang PX, Wu WZ, Xia JL. [Expression and role of CXC chemokine 5 in liver cancer cells]. Zhonghua Yi Xue Za Zhi. 2012;92(38):2716–9. [PubMed] [Google Scholar]
- 64. Park Y, Kang MH, Seo HY, Park JM, Choi CW, Kim YH, et al. Bone morphogenetic protein‐2 levels are elevated in the patients with gastric cancer and correlate with disease progression. Med Oncol. 2010;27(4):1192–9. [DOI] [PubMed] [Google Scholar]
- 65. Lim JB, Chung HW. Serum ENA78/CXCL5, SDF‐1/CXCL12, and their combinations as potential biomarkers for prediction of the presence and distant metastasis of primary gastric cancer. Cytokine. 2015;73(1):16–22. [DOI] [PubMed] [Google Scholar]
- 66. Roca H, Jones JD, Purica MC, Weidner S, Koh AJ, Kuo R, et al. Apoptosis‐induced CXCL5 accelerates inflammation and growth of prostate tumor metastases in bone. J Clin Invest. 2018;128(1):248–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spaks A. Role of CXC group chemokines in lung cancer development and progression. J Thorac Dis. 2017;9(Suppl 3):S164–s71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, et al. The CXCR2 antagonist, SCH‐527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther. 2012;11(6):1353–64. [DOI] [PubMed] [Google Scholar]
- 69. Hsu YL, Hou MF, Kuo PL, Huang YF, Tsai EM. Breast tumor‐associated osteoblast‐derived CXCL5 increases cancer progression by ERK/MSK1/Elk‐1/snail signaling pathway. Oncogene. 2013;32(37):4436–47. [DOI] [PubMed] [Google Scholar]
- 70. Wang C, Li A, Yang S, Qiao R, Zhu X, Zhang J. CXCL5 promotes mitomycin C resistance in non‐muscle invasive bladder cancer by activating EMT and NF‐kappaB pathway. Cancer Immunol Immunother. 2018;498(4):862–8. [DOI] [PubMed] [Google Scholar]
- 71. Giuliano S, Dufies M, Ndiaye PD, Viotti J, Borchiellini D, Parola J, et al. Resistance to lysosomotropic drugs used to treat kidney and breast cancers involves autophagy and inflammation and converges in inducing CXCL5. Theranostics. 2019;9(4):1181–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kuo PL, Huang MS, Hung JY, Chou SH, Chiang SY, Huang YF, et al. Synergistic effect of lung tumor‐associated dendritic cell‐derived HB‐EGF and CXCL5 on cancer progression. Int J Cancer. 2014;135(1):96–108. [DOI] [PubMed] [Google Scholar]
- 73. Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2‐mediated MDSC tumor trafficking enhances anti‐PD1 efficacy. Sci Transl Med. 2014;6(237):237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ijichi H, Chytil A, Gorska AE, Aakre ME, Bierie B, Tada M, et al. Inhibiting Cxcr2 disrupts tumor‐stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest. 2011;121(10):4106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER‐2‐Negative Metastatic Breast Cancer. Clin. Cancer Res. 2017;23(18):5358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zou B, Liu X, Gong Y, Cai C, Li P, Xing S, et al. A novel 12‐marker panel of cancer‐associated fibroblasts involved in progression of hepatocellular carcinoma. Cancer Management and Research. 2018;10:5303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ, et al. CXCR2‐CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2015;34:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, et al. CXCR2/CXCL5 axis contributes to epithelial‐mesenchymal transition of HCC cells through activating PI3K/Akt/GSK‐3beta/Snail signaling. Cancer Lett. 2015;358(2):124–35. [DOI] [PubMed] [Google Scholar]
- 79. Wu K, Yu S, Liu Q, Bai X, Zheng X, Wu K. The clinical significance of CXCL5 in non‐small cell lung cancer. OncoTargets and Therapy. 2017;10:5561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kowalczuk O, Burzykowski T, Niklinska WE, Kozlowski M, Chyczewski L, Niklinski J. CXCL5 as a potential novel prognostic factor in early stage non‐small cell lung cancer: results of a study of expression levels of 23 genes. Tumour Biol. 2014;35(5):4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gold KA, Kim ES, Liu DD, Yuan P, Behrens C, Solis LM, et al. Prediction of survival in resected non‐small cell lung cancer using a protein expression‐based risk model: implications for personalized chemoprevention and therapy. Clinical Can Res. 2014;20(7):1946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saintigny P, Massarelli E, Lin S, Ahn YH, Chen Y, Goswami S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res. 2013;73(2):571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kawamura M, Toiyama Y, Tanaka K, Saigusa S, Okugawa Y, Hiro J, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. Eur J Cancer. 2012;48(14):2244–51. [DOI] [PubMed] [Google Scholar]
- 84. Wang Z, Liu H, Shen Z, Wang X, Zhang H, Qin J, et al. The prognostic value of CXC‐chemokine receptor 2 (CXCR2) in gastric cancer patients. BMC Cancer. 2015;15:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xiang Z, Zhou ZJ, Xia GK, Zhang XH, Wei ZW, Zhu JT, et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene. 2017;36(36):5122–33. [DOI] [PubMed] [Google Scholar]
- 86. Zhang H, Xia W, Lu X, Sun R, Wang L, Zheng L, et al. A novel statistical prognostic score model that includes serum CXCL5 levels and clinical classification predicts risk of disease progression and survival of nasopharyngeal carcinoma patients. PLoS One. 2013;8(2):e57830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS, Chan SC, et al. Identification of molecular markers and signaling pathway in endometrial cancer in Hong Kong Chinese women by genome‐wide gene expression profiling. Oncogene. 2007;26(13):1971–82. [DOI] [PubMed] [Google Scholar]
- 88. Zhu X, Qiao Y, Liu W, Wang W, Shen H, Lu Y, et al. CXCL5 is a potential diagnostic and prognostic marker for bladder cancer patients. Tumour Biol. 2016;37(4):4569–77. [DOI] [PubMed] [Google Scholar]
- 89. Nishi T, Takeuchi H, Matsuda S, Ogura M, Kawakubo H, Fukuda K, et al. CXCR2 expression and postoperative complications affect long‐term survival in patients with esophageal cancer. World J Surg Oncol. 2015;13:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liang B, Zhao H, Che JB, Wang HJ, Shi GN. Overexpression of interleukin‐8 receptor 2 (IL‐8R2) indicates better prognosis in esophageal adenocarcinoma and squamous cell carcinoma procession. Med Oncol. 2014;31(8):89. [DOI] [PubMed] [Google Scholar]
- 91. Sui P, Hu P, Zhang T, Zhang X, Liu Q, Du J. High expression of CXCR‐2 correlates with lymph node metastasis and predicts unfavorable prognosis in resected esophageal carcinoma. Med Oncol. 2014;31(2):809. [DOI] [PubMed] [Google Scholar]
- 92. Han L, Jiang B, Wu H, Wang X, Tang X, Huang J, et al. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Med Oncol. 2012;29(4):2466–72. [DOI] [PubMed] [Google Scholar]
- 93. Korkolopoulou P, Levidou G, El‐Habr EA, Adamopoulos C, Samaras V, Zisakis A, et al. Expression of interleukin‐8 receptor CXCR2 and suppressor of cytokine signaling‐3 in astrocytic tumors. Mol Med. 2012;18:379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. An H, Xu L, Chang Y, Zhu Y, Yang Y, Chen L, et al. CXC chemokine receptor 2 is associated with postoperative recurrence and survival of patients with non‐metastatic clear‐cell renal cell carcinoma. Eur J Cancer. 2015;51(14):1953–61. [DOI] [PubMed] [Google Scholar]
- 95. Rezakhaniha B, Dormanesh B, Pirasteh H, Yahaghi E, Masoumi B, Ziari K, et al. Immunohistochemical distinction of metastases of renal cell carcinoma with molecular analysis of overexpression of the chemokines CXCR2 and CXCR3 as independent positive prognostic factors for the tumorigenesis. IUBMB Life. 2016;68(8):629–33. [DOI] [PubMed] [Google Scholar]
- 96. Stofas A, Levidou G, Piperi C, Adamopoulos C, Dalagiorgou G, Bamias A, et al. The role of CXC‐chemokine receptor CXCR2 and suppressor of cytokine signaling‐3 (SOCS‐3) in renal cell carcinoma. BMC Cancer. 2014;14:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clinical Can Res. 2010;16(15):3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


