Abstract
Meissner corpuscles are cutaneous mechanoreceptors that are usually located in the dermal papillae of human glabrous skin. Structurally, these sensory corpuscles consist of a mechanoreceptive sensory neuron surrounded by non‐myelinating lamellar Schwann‐like cells. Some authors have described a partially developed fibroblastic capsule of endoneurial or perineurial origin around Meissner corpuscles; however, others have noted that these structures are non‐encapsulated. As there is continuity between the periaxonic cells forming the sensory corpuscles and the cells of the nerve trunks, we used immunohistochemistry to examine the expression of endoneurial (CD34 antigen) or perineurial [Glucose transporter 1 (Glut1)] markers in human cutaneous Meissner corpuscles. We also investigated the immunohistochemical patterns of nestin and vimentin (the main intermediate filaments of the cytoskeleton of endoneurial and perineurial cells, respectively) in Meissner corpuscles. The most important finding from this study was that CD34‐positive cells formed a partial/complete capsule of endoneurial origin around most Meissner corpuscles, without signs of other perineurial Glut1‐positive elements. However, the cytoskeletal proteins of the capsular CD34‐positive cells did not include either nestin or vimentin, so the cytoskeletal composition of these cells remains to be established. Finally, the intensity of the immunoreactivity for CD34 in the capsule decreased with ageing, sometimes becoming completely absent in the oldest individuals. In conclusion, we report the first immunohistochemical evidence of the capsule of Meissner corpuscles in humans and demonstrate the endoneurial origin of the capsule.
Keywords: capsule, CD34 antigen, endoneurium, human, immunohistochemistry, Meissner corpuscles
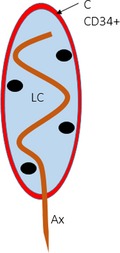
Meissner corpuscles are cutaneous mechanoreceptors located in the dermal papillae of human glabrous skin. Structurally they consist of one axon (Ax) surrounded by lamellar Schwann‐like cells (LC). The existence of a capsule surrounding it is controversial (C). Here we demonstrate that CD34‐positive cells form a partial/complete capsule of endoneurial origin.
Introduction
The periaxonic cells that form sensory corpuscles are continuous with the cells of nerve trunks, demonstrating a relationship between nerves and the components of Pacinian corpuscles (Vega et al. 2009); thus, the capsule and the outer core are related to the perineurium, the intermediate layer is related to the endoneurium, and the inner core is related to Schwann's cells (Feito et al. 2016; Garcia‐Piqueras et al. 2017).
Meissner corpuscles are cutaneous mechanoreceptors located in the human and primate glabrous skin that serve as rapidly adapting type I mechanoreceptors (Vega et al. 2012; Jones & Smith, 2014; Zimmerman et al. 2014). Structurally, Meissner corpuscles consist of the peripheral process of a mechanoreceptive sensory neuron and non‐myelinating lamellar Schwann‐like cells. Occasionally, a complete or partial capsule of connective tissue surrounding the corpuscle has been identified, and Meissner corpuscles have been described as non‐encapsulated, encapsulated and partially encapsulated (Vega et al. 2012; Piccinin & Schwartz, 2018). With regard to the putative capsule of human Meissner corpuscles, therefore, two main questions remain: whether or not the capsule exists and whether the capsule has an endoneurial and/or a perineural nature. The availability of specific markers for both the perineurium and the endoneurium (Weiss & Nickoloff, 1993; Khalifa et al. 2000; Hirose et al. 2003; Takebe et al. 2008; Richard et al. 2014) might help to answer these questions. The main cytoskeletal proteins in the cytoplasm of perineurial and endoneurial cells are vimentin and nestin, respectively (Vega et al. 2009; Richard et al. 2014). Recently, we demonstrated that the perineurial‐related cells of Pacinian corpuscles display epithelial membrane antigen and glucose transporter‐1 (Glut1) immunoreactivity, while endoneurial‐related cells are CD34‐positive (Garcia‐Piqueras et al. 2017; Feito et al. 2018).
The present study was designed to investigate whether human cutaneous Meissner corpuscles possess a complete or partially differentiated capsule and whether the capsules have endoneurial or perineurial characteristics. Furthermore, we analysed the occurrence of age‐dependent changes in the capsule, as these types of changes occur in other corpuscular constituents (Feito et al. 2018; Garcia‐Piqueras et al. 2019).
Methods
Skin samples were obtained from the palmar aspect of the distal phalanx of the first and second fingers during the autopsy of subjects who were free of known nerurological desease (n = 19), and from the incidental amputation of the fingertip (n = 6). The subjects' age ranged from 23 to 90 years. These materials were obtained from our laboratory collection (Registro Nacional de Biobancos, Sección colecciones, Ref. C‐0001627; responsible author O.G.‐S.), and the study was approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod. CElm, PAst: Proyecto 266/18). All materials were obtained in compliance with Spanish law (RD 1301/2006; Ley 14/2007; DR 1716/2011; Orden ECC 1414/2013).
Deparaffinized and rehydrated sections were processed for indirect immunohistochemistry using a Leica Bond™ Max automated stainer and a Leica Bond™ Polymer Refine Detection Kit (Leica Biosystems™, Newcastle, UK) according to the manufacturer's instructions. Table 1 summarizes the primary antibodies used in the study to examine the different constituents of Meissner corpuscles. Indirect immunohistochemistry included several negative and positive controls, as well as internal positive and negative controls.
Table 1.
Primary antibodies used in this study.
| Antigen | Origin | Dilution | Supplier |
|---|---|---|---|
| CD34 (clone QB‐END/10) | Mouse | Prediluted | Master Diagnostica* |
| Glut1 | Rabbit | 0.5 µg/mL | Cell Marque† |
| S100 protein (clone 4C4.9) | Mouse | 1 : 1000 | Thermo Scientific‡ |
| S100 protein | Rabbit | 1 : 1000 | Dako§ |
| Nestin | Rabbit | 1 : 200 | LifeSpan BioSciences¶ |
| Vimentin (clone 334) | Mouse | 1 µg/mL | Boehringer‐Mannheim** |
Glut1, glucose transporter 1.
Granada, Spain.
Rocklin, CA, USA.
Freemont, CA, USA.
Glostrup, Denmark.
Seattle, WA, USA.
Mannheim, Germany.
Furthermore, dual immunofluorescence was carried out to investigate the S100 protein, vimentin and nestin distribution, together with CD34 or Glut1. In deparaffinized and rehydrated sections, non‐specific binding was reduced (incubation for 30 min with a solution of 5% bovine serum albumin in Tris‐buffered saline, pH 7.4). Sections were then incubated overnight at 4°C in a humid chamber with a 1 : 1 (v/v) mixture of: anti‐CD34 or anti‐Glut1 and anti‐S100 protein; anti‐CD34 or anti‐Glut1 and anti‐vimentin; and anti‐CD34 or anti‐Glut1 and anti‐nestin. After rinsing, the sections were incubated for 1 h with Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (Serotec™, Oxford, UK, diluted 1 : 1000), rinsed again and incubated for another 1 h with a Cy3‐conjugated donkey anti‐mouse antibody (diluted 1 : 50; Jackson‐ImmunoResearch™, Baltimore, MD, USA). Both steps were performed at room temperature in a dark, humid chamber. Thereafter, sections were washed and mounted with Fluoromount Gold (ThermoFisher, Runcorn, UK) and finally, the sections were counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI; 10 ng/mL) to label the nuclei. Triple staining was detected using a Leica DMR‐XA automatic fluorescence microscope coupled with Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg GmbH, Germany), and the captured images were processed using the software ImageJ, version 1.43g, at Master Biophotonics Facility, McMaster University, Ontario (http://www.macbiophotonics.ca). As controls, representative sections were processed in the same way as described above, using non‐immune rabbit or mouse sera instead of primary antibodies or while omitting the primary antibodies during the incubation.
Results
Normal distribution patterns were observed for the investigated proteins Glut1, CD34 and vimentin in peripheral nerves and in Pacinian corpuscles, which served as positive controls. In peripheral nerves, the perineurium displayed strong immunoreactivity for Glut1, while the endoneurium showed immunoreactivity for CD34 (Fig. 1a–c). In Pacinian corpuscles, nestin and vimentin were detected in the inner core cells, while vimentin was the only intermediate filament found in the outer core and capsule (data not shown). In addition, intense Glut1 immunoreactivity was detected in the outer core and capsule, while CD34 was restricted to the intermediate layer (Fig. 1d–f). The expression of nestin and CD34 in Pacinian corpuscles is illustrated in the Supporting Information (Fig. S1).
Figure 1.
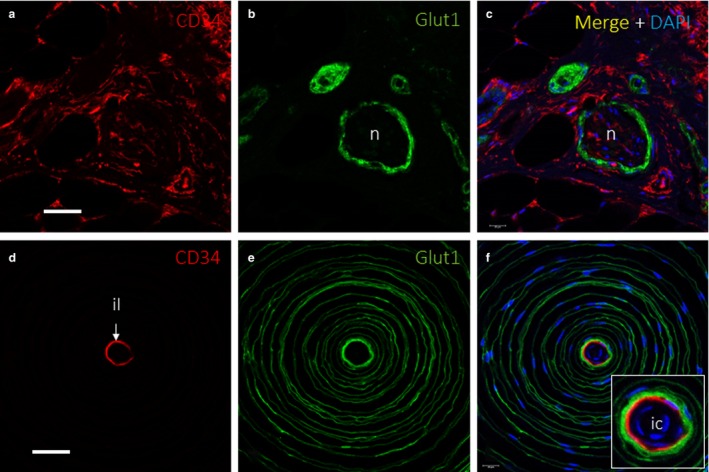
Extraneurial component in peripheral nerve and Pacinian corpuscles. Dual immunofluorescence for (a,c,d,f) CD34 and (b, c, e and f) Glucose transporter 1 (Glut1) showing endoneurium‐derived cells and perineurium‐derived cells, respectively, in a cross‐sectioned cutaneous peripheral nerve (a–c) and a Pacinian corpuscle (d–f). In the peripheral nerve, Glut1 identified the perineurium (b,c), and there was a fine, CD34‐positive network inside of the perineurium that corresponded to the endoneurium (a,c); some connective tissue, with CD34+ fibroblasts, is observed surrounding the nerve, which might correspond to epineurium. Additional findings in the figure are a very faint Glut1 expression corresponding to eccrine ducts (b) and several CD34+ connective tissue structures like blood vessels endothelia, fibroblasts and adipocytes (a,c). In the Pacinian corpuscle, CD34 identified a thin, intermediate layer (d,f), which was surrounded by several outer‐core lamellae (e,f). il, intermediate layer; n, nerve. Scale bar: 60 µm (a–c); 50 µm (d–f); ic, inner core.
Meissner corpuscles were identified based on the strong immunoreactivity of their constitutive lamellar cells (i.e. Schwann‐related cells) for the S100 protein and vimentin (Fig. 2a,b). Surrounding lamellar cells, a more or less continuous layer of vimentin‐positive fibroblasts, were observed in most corpuscles (Fig. 2c). When comparing serial sections processed for the detection of the S100 protein, vimentin and CD34, the immunoreactivity localization of the various proteins differed (Fig. 2d–f). These findings suggest that Meissner corpuscles are at least partially covered by a CD34‐positive capsule (thus, with an endoneurial nature), and cells did not exhibit simultaneous CD34 and vimentin immunoreactivity. These results were confirmed through dual immunolabelling (Fig. 3). In most cases (~75–80%), the lamellar cells of Meissner corpuscles were total or partially surrounded by a CD34‐positive layer (Fig. 4). Therefore, we explored the possibility that CD34 co‐localizes with nestin, as nestin is expressed occasionally by the lamellar cells (Calavia et al. 2012), and these proteins were not co‐localized in the same cells, confirming that Meissner corpuscles possess an endoneurial capsule (Fig. 5). To evaluate whether there were perineurial components, we performed single and dual immunolabelling for Glut1. In serial sections for CD34 and Glut1, the absence of Glut1 around Meissner corpuscles was observed (Fig. 6a–d), and these results were confirmed in sections processed for the simultaneous detection of Glut1 and CD34 (Fig. 6e–g).
Figure 2.
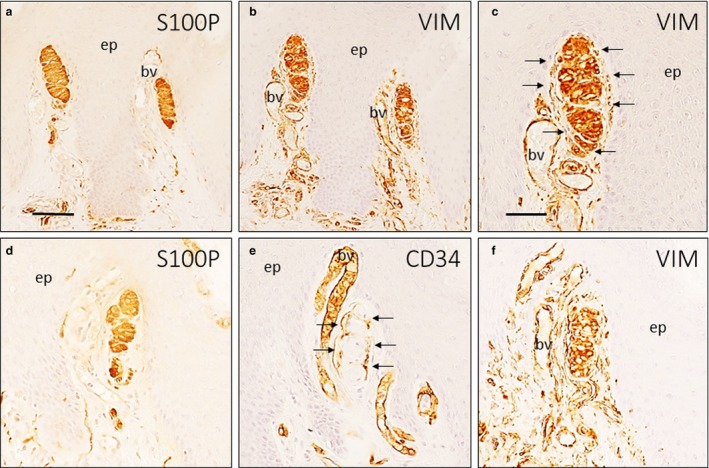
Simple immunohistochemistry of Meissner corpuscles. Approximately serial sections of two Meissner corpuscles (a–c, d–f; image c is a detail of image b), immunostained for the S100 protein (a,d), vimentin (b,c,f) and CD34 (e). Meissner corpuscles were identified by several flattened Schwann‐derived lamellar cells positive with S100 protein and vimentin (a,b). Several fibroblasts (arrows) were surrounding the corpuscle, but were not continuous with the corpuscle as they have no contact with it (c). In serial sections, several CD34‐positive cells, identified as fibroblasts (arrows), were observed that were continuous with corpuscular lamellar cells (d–f). bv, blood vessel; ep, epidermis; S100P, S100 protein; VIM, vimentin. Scale bar: 100 µm (a,b,d,e,f); 50 µm (c).
Figure 3.
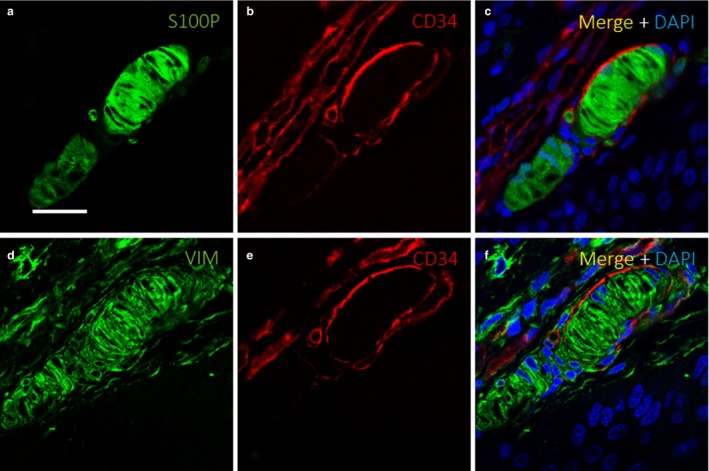
Evidence of Meissner corpuscle capsule. Dual immunofluorescence for S100 (a,c), CD34 (b–c, e–f) and vimentin (d,f) in consecutive sections of a Meissner corpuscle, showing a well‐defined capsule (b–c, e–f) surrounding S100‐positive (a,c) and vimentin‐positive lamellar cells that constitute the bulk of the corpuscle (d,f). The capsule was characteristically vimentin‐negative, as no yellow signal was observed (d–f); several vimentin‐positive structures are present surrounding the Meissner corpuscle, including dermal fibroblasts and vascular elements. S100P, S100 protein; VIM, vimentin. Scale bar: 30 µm.
Figure 4.
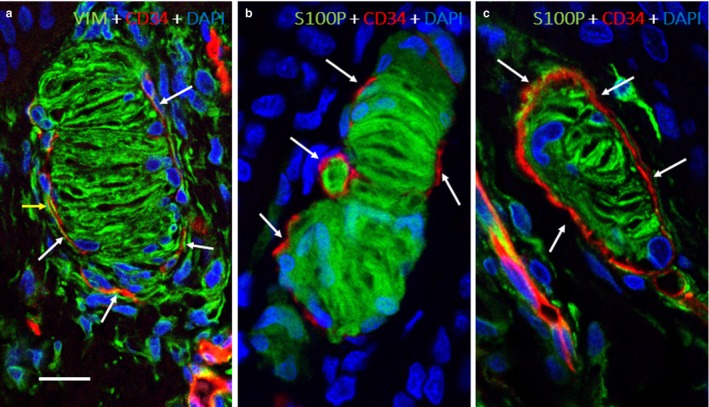
Cells surrounding the capsule. Dual immunofluorescence for vimentin and CD34 (a) and S100 and CD34 (b,c). Vimentin‐positive fibroblasts were distinctly separated from the flattened CD34‐positive fibroblasts (arrows) of the endoneurium‐derived capsule (a). The CD34‐positive capsule surrounding Meissner corpuscles was also sharply demarcated from S100‐positive lamellar cells (b,c). DAPI, 4′,6‐diamidino‐2‐phenylindole; S100P, S100 protein; VIM, vimentin. Scale bar: Scale bar: 30 µm.
Figure 5.
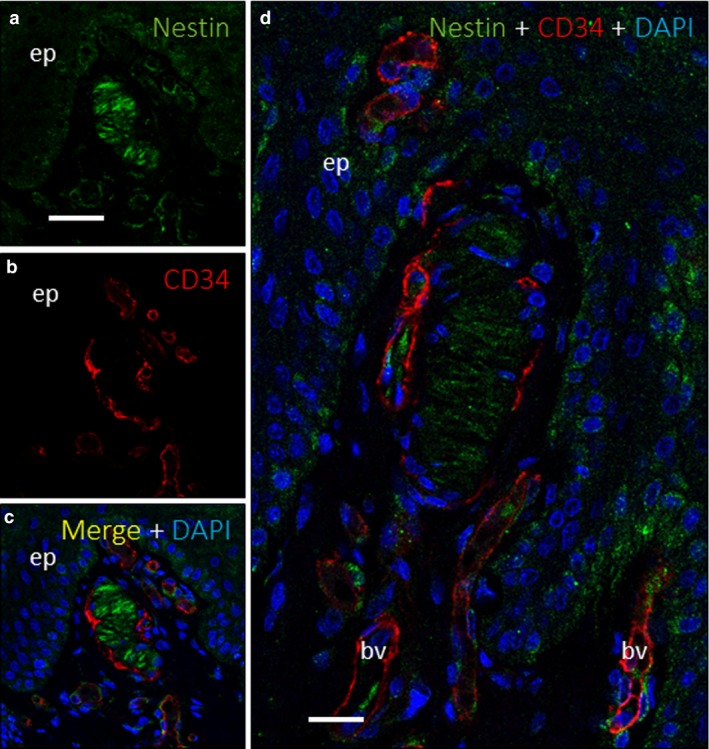
Nestin expression in Meissner corpuscles. Dual immunofluorescence for nestin (a,c–d) and CD34 (b–d) on a Meissner corpuscle. Nestin was detected in corpuscular lamellar cells with the same morphological pattern as S100 but with a lower intensity (a,c–d). CD34 (red) appeared as a discontinuous layer surrounding nestin‐positive cells (b–d, green), without any co‐expression, which would have appeared yellow (c,d). bv, blood vessel; DAPI, 4′,6‐diamidino‐2‐phenylindole; ep, epidermis. Scale bar: 110 µm (a–d); 20 µm (d).
Figure 6.
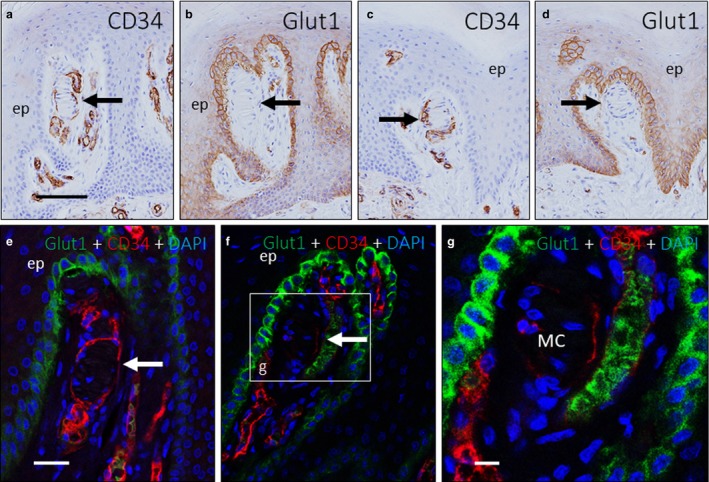
Absence of perineurial component in the capsule. Immunohistochemistry on approximately serial sections of two Meissner corpuscles (highlighted with arrows) (a–b and c‐d) immunostained for CD34 (a,c) and Glucose transporter 1 (Glut1; b,d); dual immunofluorescence for Glut1 and CD34 (e–g). There was no evidence of Glut1 expression in the stromal cells that were suggestive of perineurium‐derived fibroblasts (b,d–g) surrounding the CD34‐positive corpuscular capsule (a and c). Glut1 expression is found in the epidermal basal cell layer, as this protein is a glucose transporter (b,d–g). An inset in panel f shows a dilated blood vessel adjacent to a Meissner corpuscle that had both a CD34‐positive endothelial lining and several Glut1‐positive erythrocytes inside. DAPI, 4′,6‐diamidino‐2‐phenylindole; ep, epidermis; MC, Meissner's corpuscles. Scale bar: 110 µm (a–d); 50 µm (e–g).
Because Meissner corpuscles undergo developmental (Feito et al. 2018) and postnatal age‐dependent changes (Garcia‐Piqueras et al. 2019), we also investigated whether the CD34‐positive capsule that surrounds most Meissner corpuscles varied with age. Prenatal Meissner corpuscles lack a capsule, and the first evidence of a well‐defined capsule was at 23 years old. The capsule was present in adults, but the marker expression was less intense and fewer cells stained positively with age, until an age of 88 years, when CD34 expression disappeared (Fig. 7).
Figure 7.
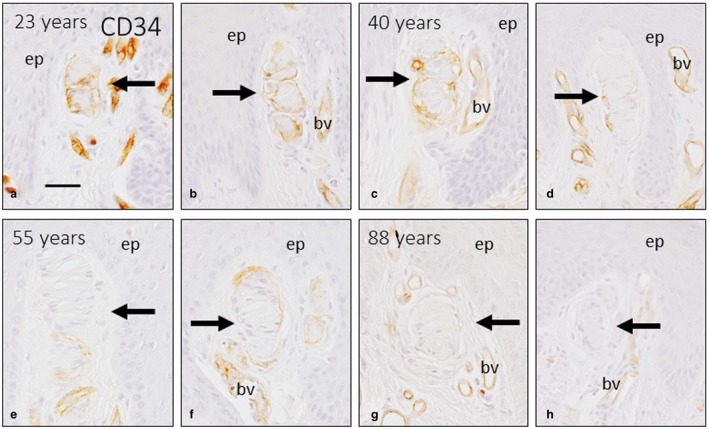
Ageing of the capsule of Meissner corpuscles. Images of representative Meissner corpuscles (arrows) from subjects of different ages, which included two corpuscles from a 22‐year‐old subject (a,b), two from a 40‐year‐old subject (c,d), two from a 55‐year‐old subject (e,f) and two from an 88‐year‐old subject (g,h), immunostained for CD34. Meissner corpuscles are highlighted with arrows. There was a decreasing gradient of CD34 expression from the 22‐year‐old subject, who had strong expression, to the 88‐year‐old subject, who did not have significant expression, suggesting the absence of Meissner corpuscle capsules at that age. The gradient of expression is attributable to a thicker and more continous capsule in the younger individuals in contrast to the discontinous and fine capsule of older corpuscles. Some blood vessels are found in proximity to Meissner corpuscles, with strong endothelial CD34 expression. bv, blood vessel; ep, epidermis. Scale bar: 50 µm.
Discussion
The present study was designed to analyse whether or not Meissner corpuscles have a differentiated capsule based on the continuity between endoneurial and perineurial nerve sheaths with periaxonic corpuscular covers (Malinovský, 1986). Glut1 and vimentin and CD34 and nestin were investigated to identify perineurial‐ and endoneurial‐related cells, respectively. The distribution of these antigens in human cutaneous Meissner corpuscles is well known (Vega et al. 2009; Richard et al. 2014; Feito et al. 2016; Garcia‐Piqueras et al. 2017). In addition, nestin‐positive potential neural stem cells have also been found adjacent to Meissner corpuscles in humans (Calavia et al. 2012) and mice (Widera et al. 2009).
CD34 is a glycoprotein cell–cell adhesion factor (da Silva et al. 2010) present in haematological precursors and blood vessels, as well as in mesenchymal cells that are able to differentiate (Sidney et al. 2014). In the skin, CD34 is localized in vascular endothelia, cells surrounding sweat glands, dermal dendritic cells, fibrocytes, mesenchymal stem cells and interstitial cells/telocytes (Narvaez et al. 1996; Sidney et al. 2014; Diaz‐Flores et al. 2016). In support of the present results, CD34 was also detected in the endoneurium (Weiss & Nickoloff, 1993; Khalifa et al. 2000; Hirose et al. 2003; Richard et al. 2014; Diaz‐Flores et al. 2016) and the so‐called intermediate endoneurial layer of Pacinian corpuscles (Garcia‐Piqueras et al. 2017).
With regard to Meissner corpuscles, in the present study, we provide immunohistochemical evidence of the existence of a CD34‐positive layer surrounding the Schwann‐derived lamellar cells. The endoneurial origin of this layer was supported by the presence of CD34 in the peripheral nerve endoneurium (Weiss & Nickoloff, 1993; Garcia‐Piqueras et al. 2017). Nestin expression was not found in our experience on endoneurial elements, neither Pacinian corpuscles capsule, Meissner corpuscles capsule or peripheral nerve; this partially agrees with the findings of Richard et al. (2014), who describe a faint nestin expression on cultured endoneurial fibroblasts. This endoneurium‐related layer had variable continuity, being more continuous and distinct in younger subjects. The immunoreactivity of this layer decreased with age and even disappeared in older subjects. Using the same arguments presented in the previous paragraph about the potential of CD34 cell for proliferation and differentiation (Sidney et al. 2014), it is very reasonable to hypothesize that this CD34‐positive layer outside the Meissner corpuscle has a role in the postnatal growth of Meissner corpuscles (Feito et al. 2016). In contrast to Pacinian corpuscles and in agreement with the literature, no perineurium‐derived elements were found surrounding the capsule of Meissner corpuscles. Despite there being no epineural markers, we cannot exclude the presence of the epineurium in Meissner corpuscles.
Based on the anatomical continuity between peripheral nerves and corpuscular structures (Malinovský, 1986) and the common immunohistochemical profiles of these structures (Vega et al. 1996, 2009), a relationship between the endoneurium, inner core of Pacinian corpuscles and the capsule of Meissner corpuscles can be established. Based on the previous experiments of Saxod (1996) with chimaeras, this CD34‐positive capsular structure surrounding Meissner corpuscles does not have a neural origin, and the components of this structure are recruited from resident mesenchymal cells by trophic factors released by the developing axon and/or the immature Schwann cells during development. If the mechanism of formation is the same between Pacinian and Meissner corpuscles, as described by Saxod (1996), the marked morphological variation between the two corpuscles is because of their asynchronous development (Feito et al. 2018).
In conclusion, we have described the presence of a defined layer surrounding the lamellar Schwann‐derived cells of Meissner corpuscles with variable continuity. This capsule was more apparent in 23‐year‐old subjects, and its immunoreactivity decreased with age, even disappearing in older subjects. No perineurium‐related structures were found in Meissner corpuscles.
Conflict of interest
None declared.
Supporting information
Fig. S1. Dual immunofluorescence for nestin (a, c‐d and f) and CD34 (b‐c and e‐f) on Pacinian corpuscles from a 22‐year‐old patient (a‐c) and a prenatal subject at 40 weeks of gestation (d‐f). Nestin was detected in the inner core cells of the corpuscle, with the same morphological pattern as S100, with very faint expression in the perineurium‐derived cells of the outer core and the absence of expression in the inner core in both samples (a, c‐d and f). CD34 appeared as a fine, intermediate layer surrounding nestin‐positive cells (b‐c) in the adult subject; CD34 appeared as a large, intermediate layer in the 40‐week fetus, interposed between the inner core and the outer core (d‐f). Scale bar: 50 µm.
Acknowledgements
This study was supported in part by a grant from Gerencia Regional de Salud de Castilla y León to J.F. and J.A.V. (GRS 1882/A/18).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Calavia M, Viña E, Menendez‐Gonzalez M, et al. (2012) Evidence of nestin‐positive cells in the human cutaneus meissner and pacinian corpuscles. CNS Neurol Disord Drug Targets 11, 869–877. [DOI] [PubMed] [Google Scholar]
- Diaz‐Flores L, Gutierrez R, Diaz‐Flores L Jr, et al. (2016) Behaviour of telocytes during physiopathological activation. Semin Cell Dev Biol 55, 50–61. [DOI] [PubMed] [Google Scholar]
- Feito J, Ramos‐Garcia JL, Gago A, et al. (2016) Pacinian corpuscles in a cervical chondrocutaneous remnant: a case report and update of pacinian corpuscles. Am J Dermatopathol 38, 231–235. [DOI] [PubMed] [Google Scholar]
- Feito J, García‐Suárez O, García‐Piqueras J, et al. (2018) The development of human digital meissner's and pacinian corpuscles. Ann Anat 219, 8–24. [DOI] [PubMed] [Google Scholar]
- Garcia‐Piqueras J, Garcia‐Suarez O, Rodriguez‐Gonzalez MC, et al. (2017) Endoneurial‐CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann Anat 211, 55–60. [DOI] [PubMed] [Google Scholar]
- Garcia‐Piqueras J, Garcia‐Mesa Y, Carcaba L, et al. (2019) Ageing of the somatosensory system at the periphery: age‐related changes in cutaneous mechanoreceptors. J Anat 234, 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Tani T, Shimada T, et al. (2003) Immunohistochemical demonstration of EMA/Glut1‐positive perineurial cells and CD34‐positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol 16, 293–298. [DOI] [PubMed] [Google Scholar]
- Jones LA, Smith AM (2014) Tactile sensory system: encoding from the periphery to the cortex. Wiley Interdiscip Rev Syst Biol Med 6, 279–287. [DOI] [PubMed] [Google Scholar]
- Khalifa MA, Montgomery EA, Ismiil N, et al. (2000) What are the CD34+ cells in benign peripheral nerve sheath tumors? Double immunostaining study of CD34 and S‐100 protein. Am J Clin Pathol 114, 123–126. [DOI] [PubMed] [Google Scholar]
- Malinovský L (1986) In: Mechanoreceptors and free nerve endings, In: Biology of the Integument. 2. Vertebrates (eds Bereiter‐Hahn J, Matoltsy AG, Richards KS.), pp. 535–560. Berlin: Springer‐Verlag. [Google Scholar]
- Narvaez D, Kanitakis J, Faure M, et al. (1996) Immunohistochemical study of CD34‐positive dendritic cells of human dermis. Am J Dermatopathol 18, 283–288. [DOI] [PubMed] [Google Scholar]
- Piccinin MA, Schwartz J (2018) Histology, Meissner Corpuscle. Treasure Island (FL): StatPearls Publishing. [PubMed] [Google Scholar]
- Richard L, Vedrenne N, Vallat JM, et al. (2014) Characterization of endoneurial fibroblast‐like cells from human and rat peripheral nerves. J Histochem Cytochem 62, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxod R (1996) Ontogeny of the cutaneous sensory organs. Microsc Res Tech 34, 313–333. [DOI] [PubMed] [Google Scholar]
- Sidney LE, Branch MJ, Dunphy SE, et al. (2014) Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32, 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CL, Goncalves R, Dos Santos F, et al. (2010) Dynamic cell‐cell interactions between cord blood haematopoietic progenitors and the cellular niche are essential for the expansion of CD34+, CD34+CD38‐ and early lymphoid CD7+ cells. J Tissue Eng Regen Med 4, 149–158. [DOI] [PubMed] [Google Scholar]
- Takebe K, Nio‐Kobayashi J, Takahashi‐Iwanaga H, et al. (2008) Histochemical demonstration of a monocarboxylate transporter in the mouse perineurium with special reference to GLUT1. Biomed Res 29, 297–306. [DOI] [PubMed] [Google Scholar]
- Vega JA, Haro JJ, Del Valle ME (1996) Immunohistochemistry of human cutaneous meissner and pacinian corpuscles. Microsc Res Tech 34, 351–361. [DOI] [PubMed] [Google Scholar]
- Vega JA, García‐Suárez O, Montaño JA, et al. (2009) The meissner and pacinian sensory corpuscles revisited new data from the last decade. Microsc Res Tech 72, 299–309. [DOI] [PubMed] [Google Scholar]
- Vega JA, Lopez‐Muniz A, Calavia MG, et al. (2012) Clinical implication of meissner`s corpuscles. CNS Neurol Disord Drug Targets 11, 856–868. [DOI] [PubMed] [Google Scholar]
- Weiss SW, Nickoloff BJ (1993) CD‐34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am J Surg Pathol 17, 1039–1045. [DOI] [PubMed] [Google Scholar]
- Widera D, Zander C, Heidbreder M, et al. (2009) Adult palatum as a novel source of neural crest‐related stem cells. Stem Cells 27, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD (2014) The gentle touch receptors of mammalian skin. Science 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Dual immunofluorescence for nestin (a, c‐d and f) and CD34 (b‐c and e‐f) on Pacinian corpuscles from a 22‐year‐old patient (a‐c) and a prenatal subject at 40 weeks of gestation (d‐f). Nestin was detected in the inner core cells of the corpuscle, with the same morphological pattern as S100, with very faint expression in the perineurium‐derived cells of the outer core and the absence of expression in the inner core in both samples (a, c‐d and f). CD34 appeared as a fine, intermediate layer surrounding nestin‐positive cells (b‐c) in the adult subject; CD34 appeared as a large, intermediate layer in the 40‐week fetus, interposed between the inner core and the outer core (d‐f). Scale bar: 50 µm.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


