Abstract
The term “Big Black Brain” was first coined in 1993 to describe cases of abusive head trauma associated with subdural hematoma(s), brain swelling, and uni- or bilateral hypo-density involving the entire supratentorial compartment on CT scan imaging. This constellation of findings was invariably followed by extensive cerebral parenchymal destruction and a dismal neurological outcome or death. We describe two such cases and review the pathophysiology and differential diagnosis of this entity.
Keywords: Big black brain, Abusive head trauma, Cerebral edema, Cerebral hypodensity, Subdural hematoma, Brain damage
Approximately 80% of abusive head trauma (AHT) victims present with a subdural hematoma (SDH) on initial CT scan (Carré 2006; Case 2008; Kemp et al. 2011). Among those children, 35% will have no or mild clinical impairment, 50% will have moderate to severe neurologic sequelae, and 15% will die as a result of their injury (Barlow et al. 2004; Case 2008). The majority of SDH resulting from abuse are thin (<10 mm), do not exert significant mass effect on the underlying brain, and do not require neurosurgical intervention. The SDH itself is often the diagnostic marker of other associated pathological features such as diffuse axonal injury, brain edema, elevated intracranial pressure, and hypoxic-ischemic injury (Kemp et al. 2003).
In 1993, Duhaime et al. (1993) described cases of SDH associated with CT scan hypo-density and swelling involving the entire supratentorial compartment unilaterally (Fig. 1) or bilaterally; this constellation of findings was invariably followed by extensive cerebral parenchymal destruction and a dismal neurological outcome or death. Because of the typical dark-appearing, homogeneous, and extensively distributed low density seen on the CT scan of such children, the authors labeled this entity “The Big Black Brain” (BBB).
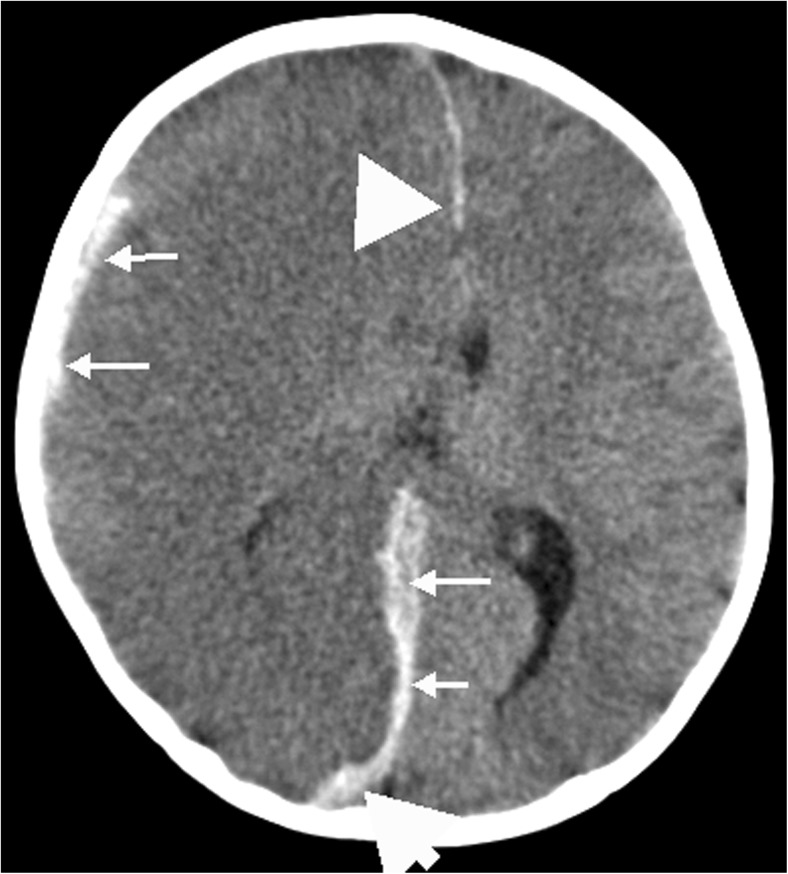
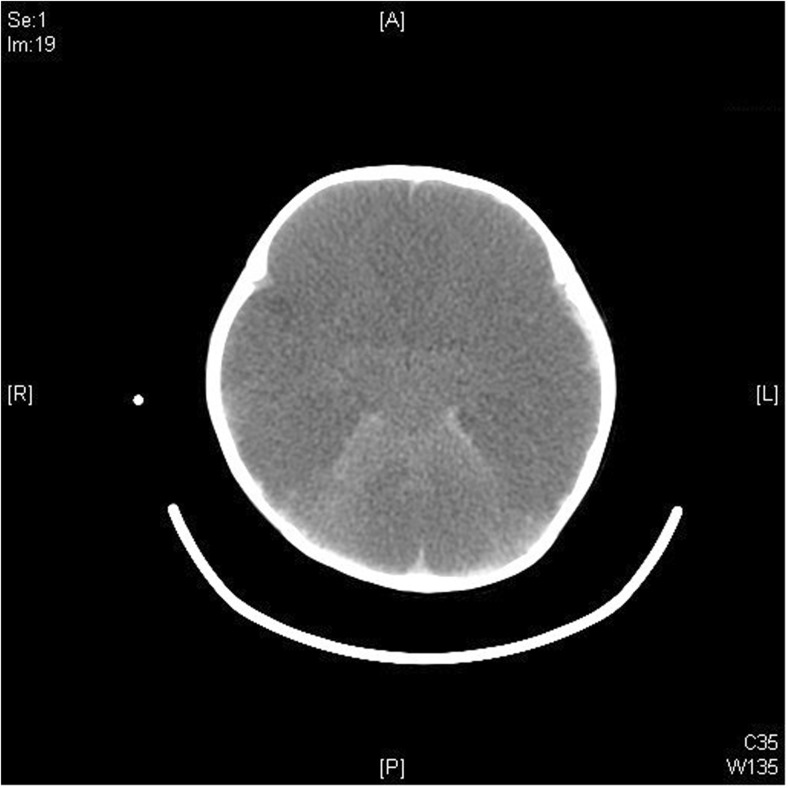
Fig. 1.
Unilateral big black brain in a 13-month-old AHT victim. CT: right-sided subdural hemorrhage with accompanying right brain swelling and hypo-density
Axial image from a noncontrast enhanced CT examination of the head in a 13 month old girl demonstrates diffuse decrease in attenuation of the right cerebral hemisphere with loss of grey-white differentiation. There is resultant leftward midline shift and mass effect upon the right lateral ventricle. Hyper-dense subdural hematoma is noted on the right, extending posteriorly along the falx (Courtesy of Kara G. Gill, MD).
Case Reports
Case 1
A 6 month-old male infant presented with new onset focal and generalized seizures without any history of trauma. His past history was benign, except for mildly falling weight percentiles. His current weight was at the 25th percentile and head size was at the 60th percentile on the Centers for Disease Control (CDC) Growth Curves. He had a history of 10 days of upper respiratory symptoms with intermittent fever. At the time of presentation, his fontanel was bulging, without evidence of external head injury. Initial head CT findings documented global left hemispheric hypo-density with loss of grey-white distinction and hemispheric swelling (Fig. 2a–c). A single bruise was present on his knee. He had profuse multi-layered retinal hemorrhages extending to the ora serrata and right-sided retinoschisis. His skeletal survey and 3-D skull reconstruction imaging were negative for fractures. Coagulation studies and urine screen for organic acids and amino-acids were normal. Nearly a month later, the child showed development of left hemispheric atrophy and left hypo-dense subdural effusion (Fig. 3).
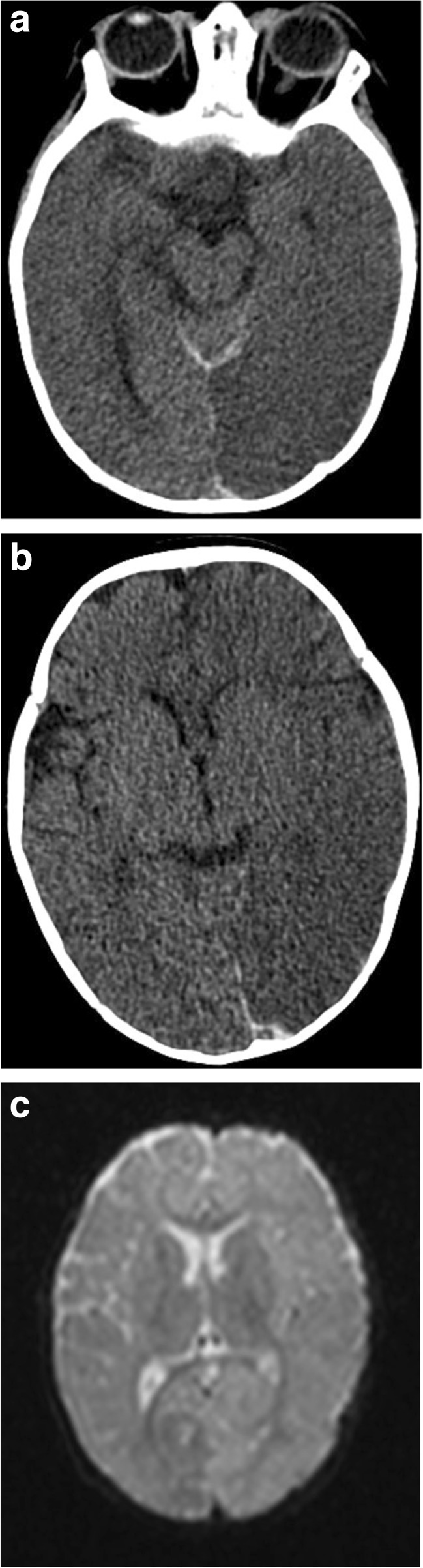
Fig. 2.
a–c. Unilateral big black brain in a 6-month old with AHT. a. Initial head CT scan demonstrates global left hemispheric hypo-density with loss of grey-white distinction and hemispheric swelling. b. Small posterior falx acute SDH and hypo-density of the left parietal-occipital brain. c. The next day the MRI demonstrates diffusion restriction involving the entire left hemisphere. Magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) failed to reveal vascular problems
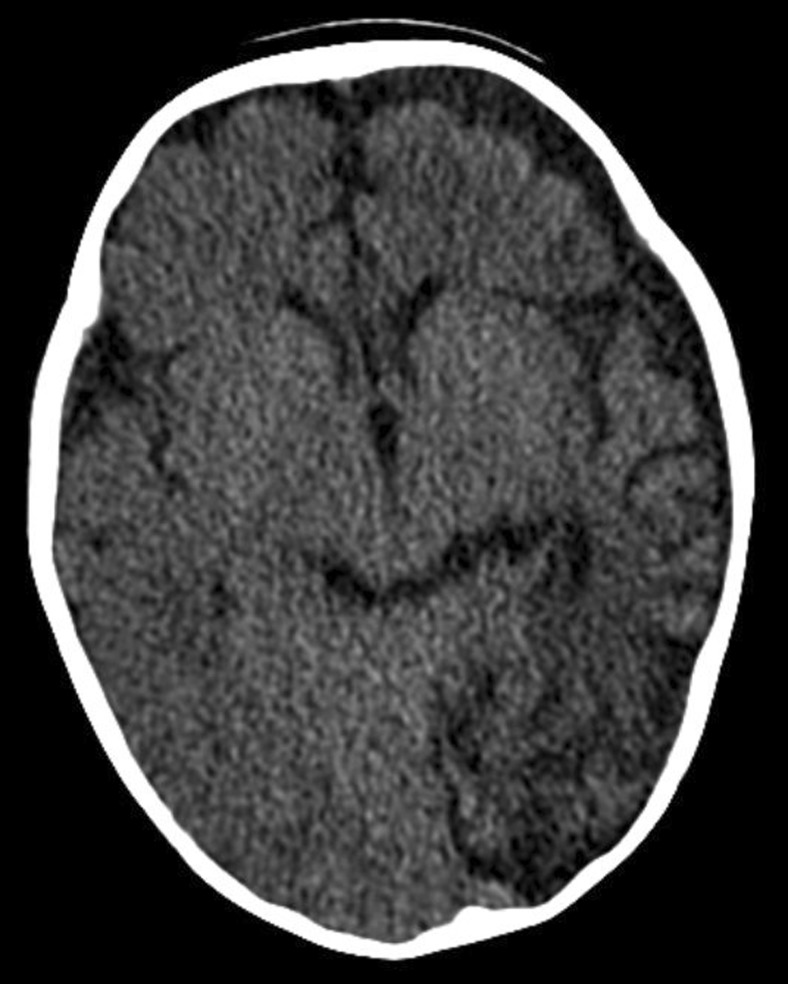
Fig. 3.
Sequelae of unilateral BBB
Nearly a month later, development of left hemispheric atrophy and left hypo-dense subdural effusion.
Case 2
A 6 month-old male infant was reported to be fussy. Father stated to medical personnel that while he was feeding the infant a bottle, the child coughed, and then became limp and unresponsive. Father stated the infant appeared pale with infrequent, gasping respirations. Father called emergency medical services and was coached in giving cardiopulmonary resuscitation (CPR) to the child. After only two mouth-to-mouth breaths and about 30 sternal compressions, the infant breathed more regularly, but remained unresponsive. The paramedics arrived about 3 min after the call and transported the child to an outside hospital where his pulse was 153 and oxygen saturation while being ventilated was 99%. The child remained unresponsive with fixed pupils and a Glasgow Coma Scale Score of 3. He had a mild anemia and mildly abnormal coagulation studies. The moderately prolonged prothrombin time for the infant was consistent with closed-head-injury associated coagulopathy (Peiniger et al. 2012). His initial head CT imaging in the receiving hospital showed significant cerebral edema and loss of grey-white distinction.
The infant was transferred to a tertiary referral center. There was no evidence of skin injuries, scalp swelling or skull fractures. A cranial CT obtained approximately seven hours after symptom onset documented hypo-density, brain swelling and loss of grey-white differentiation (Fig. 4a–c). Repeat head CT imaging 13 h after symptom onset documented global cerebral hypo-density (Fig. 5). A dilated ophthalmologic examination was conducted and documented multiple bilateral retinal hemorrhages involving all retinal layers, extending to the ora serrata and accompanied by bilateral retinoschisis. He was too unstable for a complete skeletal survey, but neck to pelvis CT scan failed to show other injuries or fractures. He was declared brain dead 24 h after symptom onset. Multiple rib fractures were found on high-detail post-mortem whole body CT scanning.
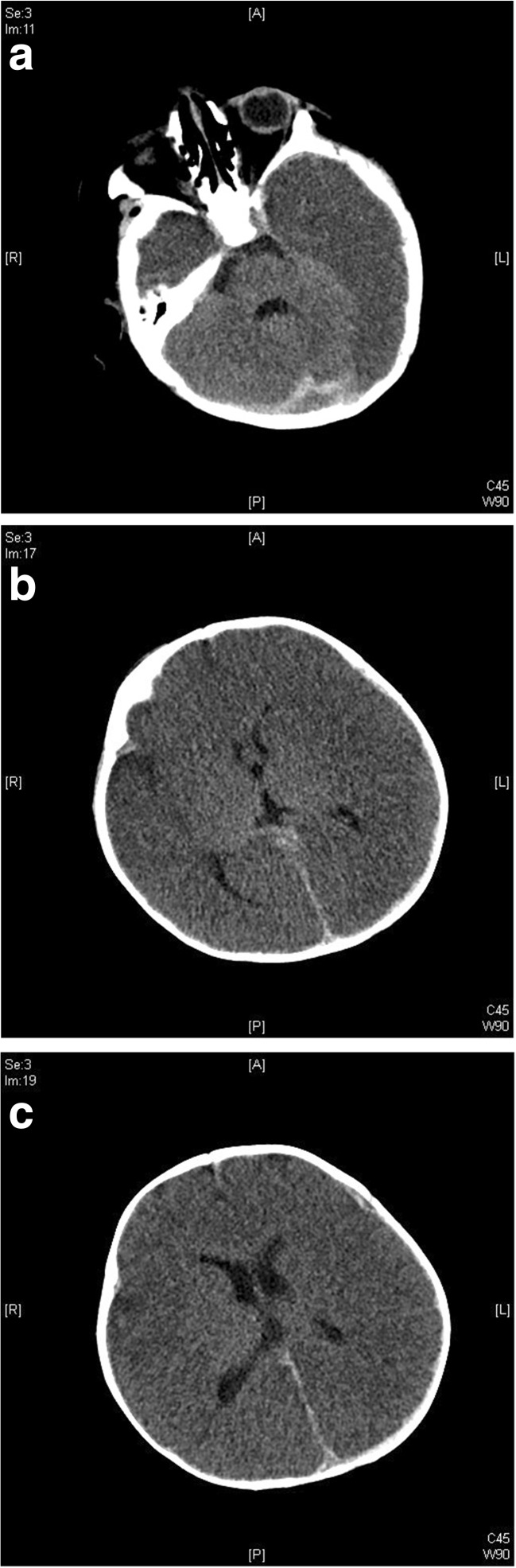
Fig. 4.
a–c. Bilateral BBB. Initial CT scan findings in a 6-month-old infant with AHT. a. Marked left greater than right temporal lobe hypo-density with preservation of cerebellar and brain stem density and blood layering along the falx. b. At the level of the 3rd ventricle, subdural blood is present along the posterior falx. Cortical density is decreased with brain swelling and loss of grey-white distinction. c. At the level of the lateral ventricles, the brain is dense, with partial loss of grey-white distinction, mild left to right midline shift with falcine and left anterior subdural blood
Fig. 5.
Bilateral BBB in a 6-month-old infant. Repeat Heat CT scan findings 13 h after initial presentation
Global cerebral hypo-density with relative cerebellar preservation.
Common Presentation
In the CT scans of two-thirds of the cases, the areas of low attenuation are present at the time of initial evaluation for AHT. In the remaining third of cases, they develop over several days (Dias et al. 1998). Graupman and Winston, describing the clinical presentation of 36 children who died of AHT reported, “Initial suspicion of a partial or subtle loss of gray–white matter differentiation evolved rapidly into strong evidence for massive hypoxia and/or edema, with the characteristic “black brain” appearance.” (Graupman and Winston 2006). When hypo-densities are present on the initial CT scan, the elapsed time between the reporting of the injury and the initial scan is approximately 3 h (Dias et al. 1998).
Among children with SDH due to AHT, about one-third will have areas of low attenuation on CT scan. Diffuse cerebral hypoattenuation consistent with BBB is seen in about half of those cases. In the other half, there is patchy cerebral hypoattenuation (Dias et al. 1998; Gilles and Nelson 1998). The initial signs and symptoms of children with BBB are similar to those of other victims of severe AHT and include:
Lethargy
Glasgow Coma Scale score of 7 or less after initial resuscitation (Duhaime et al. 1993) --Hypoventilation or apnea
Seizures
Shock
Retinal hemorrhages
It is worth noting that in AHT, the occurrence of clinical seizures after admission is associated with initial CT findings of midline shift, cerebral edema, and loss of gray-white differentiation (Goldstein et al. 2011).
Medical Imaging Features
The following features are typical of the BBB (Dias et al. 1998; Duhaime et al. 1993):
Subdural hematoma (which may be significant or visible only on MRI).
Loss of gray-white matter differentiation.
Uniform hemispheric low attenuation present on admission or within 72 h of admission. This extends across vascular territories. When unilateral, the low attenuation is seen on the side with the largest volume of SDH.
Additional features are:
Relative sparing of the posterior fossa structures.
Possible reversal sign: decreased density of the cortical gray and white matter on CT scan with relatively maintained density of the thalami, basal ganglia, brainstem and cerebellum.
Patent arterial circulation on MR angiography.
Swelling of the affected hemisphere, in unilateral cases this swelling causes a midline shift.
In survivors, development of rapid (within weeks) progressive parenchymal atrophy involving both the gray and white matter in the hypodense hemisphere. Dias reported that about 12% of cases of AHT had evidence of cerebral atrophy when the initial CT scan was obtained. This may have been caused by previous episodes of abuse (Dias et al. 1998).
There is no current literature reporting the MRI changes and pathological findings associated with the initial phase of BBB presentation. The subsequent extensive cerebral parenchymal destruction that invariably follows the acute phase is best demonstrated by MRI.
Subtypes
The BBB can be unilateral or bilateral. In very young infants, the bilateral pattern occurs twice as frequently, whereas older infants and toddlers more often develop the unilateral subtype (Duhaime et al. 1993). The bilateral form can occur from diffuse hypoxic-ischemic insults as well as in the setting of subdural hematomas.
The clinical status and long term prognosis is much worse in cases of bilateral involvement. One study reports that children who survive remain blind, non-ambulatory, nonverbal, and have profound developmental delay (Duhaime and Durham 2007). Unilateral involvement is followed by ipsilateral cortical atrophy, hemiplegia, seizure disorder and developmental delay (Gilles and Nelson 1998).
Pathophysiology
Emerging evidence suggests unique age-dependent responses following pediatric traumatic brain injury. Children appear to show more dramatic responses than adults (Huh and Raghupathi 2009). In a small percentage of children, mutation in the calcium channel gene CACNA1A predisposes children to early seizures and cerebral edema after trivial head trauma (Stam et al. 2009). Affected children usually have prior manifestations such as eye movement disorders and ataxia (Tantsis et al. 2016).
The rapid appearance of extensive, diffuse supratentorial low attenuation with loss of gray-white differentiation in unilateral or bilateral cases of BBB is seen routinely only in infants and toddlers. The pathophysiology leading to the BBB is poorly understood, but it might represent a mismatch between metabolic demand and substrate delivery (Duhaime and Durham 2007).
The clinical presentation of BBB is somewhat reminiscent of the rare second impact syndrome (SIS) affecting teenagers and young adults. It occurs when an athlete suffers a second, often trivial, head trauma before the symptoms of a prior concussion have resolved (McLendon et al. 2016). Within a few minutes after the second impact, uni- or bilateral brain edema leading to subtentorial herniation develops. During this period, the athlete, who is initially conscious yet stunned, precipitously collapses to the ground, semi-comatose, with rapidly dilating pupils, loss of eye movement, and respiratory failure. In the majority of cases, a small subdural hematoma ipsilateral to the brain swelling is present (Cantu and Gean 2010). In one case report, the subdural hematoma did not appear on a CT scan obtained after the first impact, but was present after the second impact (Weinstein et al. 2013). The rapid course of events in SIS has been attributed to a loss of autoregulation of the cerebral vasculature leading to hyperemic brain swelling (Cantu 2016). As opposed to the BBB, victims of SIS do not exhibit a loss of gray-white matter differentiation on CT scan.
Several factors could potentially be involved in the genesis of the BBB. These factors are expanded upon below.
Microvessel Vasospasm
In the area underlying the SDH creates a relative mismatch between substrate delivery and parenchymal demand, thereby leading to an area of brain damage. In an animal model of SDH, investigators have demonstrated decreased perfusion in spite of increased metabolism in the area of the brain adjacent to the hematoma (Kuroda and Bullock 1992).
Hypoxic-Ischemic Injury Due to Hypoventilation or Apnea
Hypoxia-ischemia is more common in infants and young children sustaining nonaccidental than accidental traumatic brain injury (Ichord et al. 2007). This is most likely related to the frequent occurrence of apnea in AHT. Roughly half of all AHT victims will present with apnea (Kemp et al. 2003). In a child with intracranial injury, a history of apnea is the feature most predictive of inflicted brain injury (PPV 93%, OR 17.06, p < 0.001) (Maguire et al. 2009). Gilles and Nelson reported on 14 AHT victims with SDH and either diffuse or focal cerebral hypoattenuation: apneas were present in 9 patients (64%) (Gilles and Nelson 1998). This may not explain why in one-third of cases the BBB is unilateral.
Seizures
This leads to increased metabolic demand and excitotoxic stress in the brain parenchyma (Liesemer et al. 2011). About 40% of all AHT victims suffer early post-traumatic seizures (EPTS) (Goldstein et al. 2011). In Gilles and Nelson’s report, 78% of the victims had EPTS (Gilles and Nelson 1998).
The increased frequency of both seizures and apnea in AHT cases associated with hypoattenuation (consistent with BBB) may simply reflect the severity of these cases or might have an effect in causing the BBB.
Diffuse Axonal Injury (DAI)
Shaking produces acceleration/deceleration forces, and the resultant shearing stress can lead to DA I (Case 2008; Oehmichen et al. 2008). Axonal damage can also be found at sites of significant impact (Case 2008). CT scan and MRI do not show direct evidence of DAI, but associated subdural and subarachnoid hemorrhage, as well as punctate hemorrhages, may be seen as markers of underlying brain injury.
Cerebral Edema and Increased Intracranial Pressure
Brain swelling is a crucial determinant of morbidity and mortality after head trauma. Trauma causes brain swelling in two stages. Initially, mechanical deformation leads to altered membrane permeability and disturbances in ionic fluxes which, if sustained, lead to edema (Marmarou 2007). The second stage evolves over hours to days, when the release of an entire cascade of neurotransmitters and neuropeptides promotes secondary tissue damage (Donkin et al. 2009). During this stage, trigeminal sensitization by subdural bleeding with release of trigeminal neuropeptides might be involved by causing vasodilation and increased blood flow in the ipsilateral hemisphere (Squier et al. 2012).
Strangulation
In 1987, Bird et al. reported three infants who presented with generalized seizures, bruises, subdural hematoma, and diffuse hypoattenuation of the ipsilateral hemisphere on CT scan (Bird et al. 1987). This was followed by cerebral infarction in the territories associated with the subdural hematoma. On the tenuous basis of unilateral small intramural hemorrhages in the common carotid artery in one of these patients, the authors concluded that these infants had been strangled. This theory has been widely criticized.
None of the potential etiologic factors in isolation can explain or reproduce the constellation of features present in the BBB. The most likely explanation is that in these cases, the brain is subjected to a combination of stresses that exceeds its capacity to compensate (Duhaime and Durham 2007).
Suggested Treatment
Treatment of children presenting with BBB is mainly supportive at this point, with intensive care maintenance of ventilation, oxygenation, circulation, blood pressure, osmotic agents, and relative hyperventilation for control of elevated intracranial pressure. Therapeutic hypothermia for neuroprotection has been studied in the treatment of hypoxic-ischemic encephalopathy (Adelson et al. 2005; Scholefield et al. 2013) but no study has been conducted solely on children with AHT. Decompressive craniectomy for control of increased intracranial pressure is also a consideration. It has been shown to decrease intracranial pressure and to improve brain tissue oxygenation, but there are no reports of successful long term benefits (Figaji et al. 2008). In view of the very dismal prognosis of bilateral BBB, consideration should be given to early withdrawal of life maintenance support.
Diagnostic Significance
The constellation of CT scan findings associated with the BBB is highly suspicious for AHT, especially in the unilateral form. The bilateral subtype has been described in cases of hypoxic-ischemic encephalopathy (Duhaime et al. 1993).
Recognizing this entity early is important. Because of its uniformly dismal prognosis and lack of effective treatment modalities, consideration should be given to the use of newer therapeutics such as controlled hypothermia and blockade of excitotoxic molecules.
Related Conditions and Potential Mimics
As mentioned above, diffuse axonal injury, cerebral edema, and hypoxic-ischemic encephalopathy are likely factors in the development of the BBB. Therefore, it can be expected that some of the clinical and radiological manifestations of these entities will overlap with features of the BBB.
Diffuse Axonal Injury
On computed tomography, diffuse axonal injury can present as cerebral edema with loss of gray-white differentiation; but often, the radiologic findings are limited to the presence of SDH or subarachnoid hemorrhage as markers of underlying brain injury (Grant 2015; Topal et al. 2008).
Cerebral Edema
Cerebral edema is also associated with loss of grey-white matter differentiation on CT scan, but other features are present as well: effacement of the basal cisterns, ventricular compression, and flattened gyral pattern with effacement of cortical sulci.
Hypoxic-Ischemic Encephalopathy (HIE)
Many CT scan features of the BBB and HIEare similar (Kemp et al. 2003; McKinney et al. 2008): Loss of gray-white matter differentiation; Areas of low attenuation; Possible reversal sign; and in survivors, development of progressive parenchymal atrophy.
Some features seem to be more typical of the BBB: the area of low attenuation is severe, uniform, and lacks predilection for arterial border zones. HIE can be more accurately diagnosed by diffusion weighted MRI (DW-MRI): in the acute phase (<3 days), diffuse cortical restriction is present, whereas in the subacute phase (> 3 days), there are more extensive findings, including white matter restricted diffusion (McKinney et al. 2008; Parizel et al. 2003).
Unfortunately, there is no current literature reporting the MRI changes associated with the initial phase of BBB presentation; so, at this point, it is unclear whether BBB is a separate condition, or just a severe form of HIE with some distinctive CT scan features. In cases of bilateral involvement, it is difficult if not impossible to separate these two entities.
Conclusion
AHT is often results in subdural hemorrhage accompanied by severe primary brain injury. Secondary insults, such as cardiovascular and respiratory compromise and seizures, potentiate this brain injury. Such insults can cause the imaging picture of the “Big black brain”, which represents regional or global brain swelling and hypoxic-ischemic injury. The long term consequences are often permanent regions of brain atrophy with its accompanying functional impairments.
Acknowledgements
The authors would like to thank Kara G. Gill, MD for contributing radiologic imaging to this manuscript.
Compliance with ethical standards
Disclosure of Interest
Drs. Knox and Feldman both testify in civil and criminal trials. Monies earned are given to their respective institutions and to Dr. Feldman. Drs. Knox and Luyet are currently authoring a book on child torture as a form of child abuse.
Ethical Standards and Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation [institutional and national] and with the Helsinki Declaration of 1975, as revised in 2000. This manuscript discusses two cases of children with big black brain presentations. Both the University of Wisconsin Health Sciences IRB and the University of Washington IRB did not require IRB approval for these case reports. Additionally, no informed consent of patients was required.
Footnotes
Drs. Knox and Feldman both testify in civil and criminal trials. Monies are given to their respective institutions and to Dr. Feldman. Drs. Knox and Luyet are currently writing a book on child torture as a form of child abuse.
References
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56(4):740–754. doi: 10.1227/01.NEU.0000156471.50726.26. [DOI] [PubMed] [Google Scholar]
- Barlow K, Thompson E, Johnson D, Minns RA. The neurological outcome of non-accidental head injury. Pediatric Rehabilitation. 2004;7(3):195–203. doi: 10.1080/13638490410001715331. [DOI] [PubMed] [Google Scholar]
- Bird CR, McMahan JR, Gilles FH, Senac MO, Apthorp JS. Strangulation in child abuse: CT diagnosis. Radiology. 1987;163(2):373–375. doi: 10.1148/radiology.163.2.3562816. [DOI] [PubMed] [Google Scholar]
- Cantu RC. Dysautoregulation/Second impact syndrome with recurrent athletic head injury. World Neurosurgery. 2016 doi: 10.1016/j.wneu.2016.04.056. [DOI] [PubMed] [Google Scholar]
- Cantu RC, Gean AD. Second-impact syndrome and a small subdural hematoma: an uncommon catastrophic result of repetitive head injury with a characteristic imaging appearance. Journal of Neurotrauma. 2010;27(9):1557–1564. doi: 10.1089/neu.2010.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré, M. (2006). Neuroradiology. In K. Rauth-Farley, L. Frasier, R. Alexander, & R. Parrish (Eds.), Abusive head trauma in infants and children: A medical, legal, and forensic reference (pp. 73–98). St. Louis: GW Publishing.
- Case ME. Inflicted traumatic brain injury in infants and young children. Brain Pathology. 2008;18(4):571–582. doi: 10.1111/j.1750-3639.2008.00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MS, Backstrom J, Falk M, Li V. Serial radiography in the infant shaken impact syndrome. Pediatric Neurosurgery. 1998;29(2):77–85. doi: 10.1159/000028694. [DOI] [PubMed] [Google Scholar]
- Donkin JJ, Nimmo AJ, Cernak I, Blumbergs PC, Vink R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. Journal of Cerebral Blood Flow and Metabolism. 2009;29(8):1388–1398. doi: 10.1038/jcbfm.2009.63. [DOI] [PubMed] [Google Scholar]
- Duhaime AC, Bilaniuk L, Zimmerman R. The “big black brain”: radiographic changes after severe inflicted head injury in infancy. Journal of Neurotrauma. 1993;10:S59. [Google Scholar]
- Duhaime AC, Durham S. Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”) Progress in Brain Research. 2007;161:293–302. doi: 10.1016/S0079-6123(06)61020-0. [DOI] [PubMed] [Google Scholar]
- Figaji AA, Fieggen AG, Argent AC, Le Roux PD, Peter JC. Intracranial pressure and cerebral oxygenation changes after decompressive craniectomy in children with severe traumatic brain injury. Acta Neurochirurgica Supplement. 2008;102:77–80. doi: 10.1007/978-3-211-85578-2_15. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Nelson MD., Jr Cerebral complications of nonaccidental head injury in childhood. Pediatric Neurology. 1998;19(2):119–128. doi: 10.1016/S0887-8994(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Leonhardt D, Kmytyuk N, Kim F, Wang D, Wainwright MS. Abnormal neuroimaging is associated with early in-hospital seizures in pediatric abusive head trauma. Neurocritical Care. 2011;15(1):63–69. doi: 10.1007/s12028-010-9468-5. [DOI] [PubMed] [Google Scholar]
- Grant P. Abusive head trauma: parenchymal injury. In: Kleinman P, editor. Diagnostic imaging of child abuse. Cambridge UK: Cambridge University Press; 2015. [Google Scholar]
- Graupman P, Winston KR. Nonaccidental head trauma as a cause of childhood death. Journal of Neurosurgery. 2006;104(4 Suppl):245–250. doi: 10.3171/ped.2006.104.4.245. [DOI] [PubMed] [Google Scholar]
- Huh JW, Raghupathi R. New concepts in treatment of pediatric traumatic brain injury. Anesthesiology Clinics. 2009;27(2):213–240. doi: 10.1016/j.anclin.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichord RN, Naim M, Pollock AN, Nance ML, Margulies SS, Christian CW. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. Journal of Neurotrauma. 2007;24(1):106–118. doi: 10.1089/neu.2006.0087. [DOI] [PubMed] [Google Scholar]
- Kemp AM, Jaspan T, Griffiths J, Stoodley N, Mann MK, Tempest V, Maguire SA. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Archives of Disease in Childhood. 2011;96(12):1103–1112. doi: 10.1136/archdischild-2011-300630. [DOI] [PubMed] [Google Scholar]
- Kemp AM, Stoodley N, Cobley C, Coles L, Kemp KW. Apnoea and brain swelling in non-accidental head injury. Archives of Disease in Childhood. 2003;88(6):472–476. doi: 10.1136/adc.88.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Bullock R. Local cerebral blood flow mapping before and after removal of acute subdural hematoma in the rat. Neurosurgery. 1992;30(5):687–691. [PubMed] [Google Scholar]
- Liesemer K, Bratton SL, Zebrack CM, Brockmeyer D, Statler KD. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. Journal of Neurotrauma. 2011;28(5):755–762. doi: 10.1089/neu.2010.1518. [DOI] [PubMed] [Google Scholar]
- Maguire S, Pickerd N, Farewell D, Mann M, Tempest V, Kemp AM. Which clinical features distinguish inflicted from non-inflicted brain injury? A systematic review. Archives of Disease in Childhood. 2009;94(11):860–867. doi: 10.1136/adc.2008.150110. [DOI] [PubMed] [Google Scholar]
- Marmarou A. A review of progress in understanding the pathophysiology and treatment of brain edema. Neurosurgical Focus. 2007;22(5):E1. doi: 10.3171/foc.2007.22.5.2. [DOI] [PubMed] [Google Scholar]
- McKinney AM, Thompson LR, Truwit CL, Velders S, Karagulle A, Kiragu A. Unilateral hypoxic-ischemic injury in young children from abusive head trauma, lacking craniocervical vascular dissection or cord injury. Pediatric Radiology. 2008;38(2):164–174. doi: 10.1007/s00247-007-0673-0. [DOI] [PubMed] [Google Scholar]
- McLendon LA, Kralik SF, Grayson PA, Golomb MR. The controversial second impact syndrome: a review of the literature. Pediatric Neurology. 2016;62:9–17. doi: 10.1016/j.pediatrneurol.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Oehmichen M, Schleiss D, Pedal I, Saternus KS, Gerling I, Meissner C. Shaken baby syndrome: re-examination of diffuse axonal injury as cause of death. Acta Neuropathologica. 2008;116(3):317–329. doi: 10.1007/s00401-008-0356-4. [DOI] [PubMed] [Google Scholar]
- Parizel PM, Ceulemans B, Laridon A, Ozsarlak O, Van Goethem JW, Jorens PG. Cortical hypoxic-ischemic brain damage in shaken-baby (shaken impact) syndrome: value of diffusion-weighted MRI. Pediatric Radiology. 2003;33(12):868–871. doi: 10.1007/s00247-003-1025-3. [DOI] [PubMed] [Google Scholar]
- Peiniger, S., Nienaber, U., Lefering, R., Braun, M., & Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. (2012). Glasgow coma scale as a predictor for hemocoagulative disorders after blunt pediatric traumatic brain injury. Pediatr Crit Care Med, 13(4), 455–460. doi:10.1097/PCC.0b013e31823893c5. [DOI] [PubMed]
- Scholefield B, Duncan H, Davies P, Gao Smith F, Khan K, Perkins GD, Morris K. Hypothermia for neuroprotection in children after cardiopulmonary arrest. Cochrane Database of Systematic Reviews. 2013;2:CD009442. doi: 10.1002/14651858.CD009442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier W, Mack J, Green A, Aziz T. The pathophysiology of brain swelling associated with subdural hemorrhage: the role of the trigeminovascular system. Childs Nervous System. 2012;28(12):2005–2015. doi: 10.1007/s00381-012-1870-1. [DOI] [PubMed] [Google Scholar]
- Stam AH, Luijckx GJ, Poll-The BT, Ginjaar IB, Frants RR, Haan J, van den Maagdenberg AM. Early seizures and cerebral oedema after trivial head trauma associated with the CACNA1A S218L mutation. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(10):1125–1129. doi: 10.1136/jnnp.2009.177279. [DOI] [PubMed] [Google Scholar]
- Tantsis EM, Gill D, Griffiths L, Gupta S, Lawson J, Maksemous N, Menezes MP. Eye movement disorders are an early manifestation of CACNA1A mutations in children. Developmental Medicine and Child Neurology. 2016;58(6):639–644. doi: 10.1111/dmcn.13033. [DOI] [PubMed] [Google Scholar]
- Topal NB, Hakyemez B, Erdogan C, Bulut M, Koksal O, Akkose S, Korfali E. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurological Research. 2008;30(9):974–978. doi: 10.1179/016164108X323799. [DOI] [PubMed] [Google Scholar]
- Weinstein E, Turner M, Kuzma BB, Feuer H. Second impact syndrome in football: new imaging and insights into a rare and devastating condition. Journal of Neurosurgery Pediatrics. 2013;11(3):331–334. doi: 10.3171/2012.11.PEDS12343. [DOI] [PubMed] [Google Scholar]